- 1Department of Gastroenterology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 2Institute of Hepatobiliary Pancreatic Intestinal Diseases, North Sichuan Medical College, Nanchong, China
- 3Department of General Surgery, Chengdu XinHua Hospital Affiliated to North Sichuan Medical College, Chengdu, China
Bariatric surgery stands as the most potent treatment for achieving substantial weight reduction and alleviating the complications associated with obesity. However, it is not the treatment of choice for patients with obesity combined with type 2 diabetes mellitus, and the benefit of bariatric surgery varies widely among individuals. There is a noticeable inconsistency in the outcomes following these procedures. The ability to predict how an individual will respond to bariatric surgery is a valuable asset in clinical practice. And the importance of postoperative interventions should not be underestimated. Proactive measures targeting both pre- and post-operative eating habits and lifestyle adjustments are of greater significance than the investigation into pre-operative factors alone. The judicious application of medication, endoscopic intervention and conversional surgeries after bariatric surgery can yield superior outcomes in managing recurrent weight regain and the recurrence of diabetes, albeit with consideration for the associated complication rates.
1 Introduction
Obesity represents a complex and widespread chronic condition, ranking among the most prevalent health issues globally (1). Bariatric surgery (BS) has become the most effective intervention for the treatment of obesity. However, many patients yet suffer from the postoperative complication of recurrent weight gain (RWG). It is important for those patients to increase their moderate to vigorous physical activity (MVPA) to mitigate RWG (2). And dietary interventions are significant since irregular eating habits are linked to RWG (3). Anti-obesity drugs can act as a potent supplementary treatment following BS, aiding in enhancing weight reduction after the procedure or averting the RWG (4, 5). Furthermore, laparoscopy intervention is a minimally invasive and safe treatment for RWG which provides sustainable weight loss (6). Conversion of previous BS may be necessary in instances of RWG and the presence of complications while conversional surgery has greater morbidity than primary surgery (7).
Furthermore, bariatric surgery is also capable of alleviate type 2 diabetes mellitus (T2DM). A range of predictive models have been utilized to forecast the remission of T2DM, including Individualized Metabolic Surgery (IMS) (8), ABCD score (9), the Diabetes Remission (DiaRem) and the Advanced Diabetes Remission (Ad-DiaRem) (10). However, some patients may present complication of T2DM recurrence (11). Regarding the management of this postoperative complication, exercise is connected with the decrease of blood glucose (12) and the American Diabetes Association (ADA) advocates for individualized eating plans in nutrition therapy (13). Currently, there is no standardized pharmaceutical treatment for managing recurring T2DM, but emerging data may assist physicians in selecting appropriate medications (14). Additionally, conversional surgery was found to markedly control blood glucose with higher risks of complications compared to the primary surgery.
Our study provides a narrative review of the factors that can predict weight loss, remission of diabetes, and strategies for managing the post-BS RWG and diabetes recurrence.
2 Predictors of postoperative outcomes
2.1 Predictors of postoperative weight loss
The extent of weight loss variation is likely influenced more by biological elements that affect neuro-endocrine processes, as well as psychological factors that impact eating habits (15). It is probable that a sophisticated interaction between biological and mental processes plays a significant role (15). An increase in age has often been identified as a factor that predicts a reduced weight loss outcome post-surgery (16–19). Elder people often exhibit a decreased basal metabolic rate coupled with a reduction in physical activity (20). Additionally, as age advances, there is a growing challenge to alter established dietary and lifestyle habits, which leads to a less significant reduction in weight (21). A higher starting BMI typically correlates with a more substantial absolute weight loss (22). However, when weight loss is measured relatively, those with a higher initial BMI may experience a smaller percentage reduction (23–26). Specifically, patients with T2DM tend to achieve less weight loss (17, 23).
A majority of research on preoperative weight loss has yielded either negative or inconclusive findings. In a randomized controlled trial, Kalarchian et al. (27) compared patients who underwent a 6-month behavioral lifestyle intervention with those who received standard pre-surgical care. They found no significant discrepancy in post-operative weight reduction between the two groups. Similarly, Krimpuri et al. (28) have noted that the predictive power of pre-operative weight loss diminishes gradually by the one-year mark. Several retrospective studies have also failed to find compelling evidence that pre-operative weight loss is reliable for predicting post-operative weight loss (29–31). The American Society for Metabolic and Bariatric Surgery (ASMBS) has concluded that few medical evidence can support the notion that preoperative weight loss offers any advantage in terms of bariatric surgery outcomes (32). Meanwhile, Mocanu et al. (33) supposed that prroperative weight loss is needed since they verified that a reduction in weight prior to surgery correlates with better chances of survival within 30 days and a decreased likelihood of postoperative leaks. Tolvanen et al. (34) discovered that individuals who have made efforts to lose weight demonstrated enhanced cognitive restraint in their dietary habits which highlights the critical need for pharmacological and psychological assessments before BS.
2.2 Predictors of postoperative T2DM remission
T2DM remission is characterized by a spontaneous or intervention-induced return of hemoglobin A1c (HbA1c) levels to below 6.5% (or less than 48 mmol/mol), which is maintained for a minimum of 3 months without the diabetes medications (35). Effectiveness of Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) in decreasing blood sugar levels in obese patients with uncontrolled T2DM had been proven (36). The likelihood of remission can differ based on the specific surgical procedure performed. Furthermore, factors such as the duration of T2DM, pre-operative C-peptide levels as well as HbA1c levels have been identified as predictors of T2DM remission (8, 37).
Additionally, various scoring systems have been devised to estimate the likelihood of diabetes remission for individual patients. Aminian et al. (8) utilized a dataset comprising T2DM patients who accepted RYGB and SG procedures to create a nomogram that generated an Individualized Metabolic Surgery (IMS) score which was used to evaluate the effectiveness of different surgical techniques in achieving diabetes remission rates across various stages of T2DM severity. Lee et al. (9) introduced the Diabetes Surgery Score, also known as the ABCD score, which takes the age, C-peptide levels, BMI, and T2DM duration of patients into account. The ABCD score demonstrated prominent specificity and accurant predictivity, while it only targeted the Asian group. The Diabetes Remission (DiaRem) score incorporated preoperative clinical variables to predict the probability of T2DM remission over a five-year period. However, Aron-Wisnewsky et al. (10) discovered that the DiaRem score had limited predictive power for lower scores. As a result, the DiaBetter score was introduced, incorporating factors such as T2DM duration and glycated hemoglobin levels. Both the DiaBetter and DiaRem scores were found to have comparable predictive value for two-year T2DM remission rates following both RYGB and SG procedures (38). Ultimately, the DiaRem score’s precision and predictive capabilities were enhanced by taking into account the duration of T2DM and the dosage of hypoglycemic medications used. This led to the development of the Advanced Diabetes Remission (Ad-DiaRem) score (10). Within a cohort of Israeli individuals with five-year post-operative diabetes status data, the Ad-DiaRem score demonstrated a slight improvement over the DiaRem score in the prediction of long-term T2DM remission following RYGB surgery (39). As seen above, a highly predictive and accurate diabetes scoring system is important in predicting remission of type 2 diabetes after bariatric surgery.
3 Management of RWG
3.1 RWG after BS
In the latest meeting held by International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) (40), an agreement was established to utilize the term “recurrent weight gain (RWG)” for individuals who undergo substantial weight increase following their initial weight reduction post-surgery. This term is defined as a weight gain exceeding 30% or an exacerbation of an obesity-related complication that was a pivotal reason for undergoing surgery. Given the different efficacy of each BS procedures (41, 42) and variable effects in different patient groups, these criteria should be personalized, complemented by the expertise of clinical judgment.
3.2 Behavioral interventions
Empirical studies endorse the significance of MVPA, in curbing post-bariatric surgery weight regain (43). Notably, the numerous evidences are based on objective daily assessments of patients’ MVPA, which minimizes the bias associated with self-reported activity levels. As highlighted in a previous review (44), it is essential for future research to detail exercise adherence rates to guide the formulation of effective exercise programs. Consequently, interventions should focus on imparting behavioral strategies—such as self-monitoring of exercise, setting achievable goals, and scheduling exercise routines—to ensure the long-term maintenance of physical activity habits (45).
Ongoing and regular consultation with a dietitian specializing in BS is linked to enhanced outcomes in terms of weight loss success (46). Continued dietary guidance post-surgery is highly advantageous for the majority of patients (47). Patients’ capacity to digest solid foods is restricted, which calls for a gradual dietary progression from liquids to solids (48). The primary objective of dietary counseling following BS is to ensure an adequate intake of high-quality protein and recommended daily protein intake ranges from 60 to 120 grams, contingent upon the specific surgical procedure performed (49). Furthermore, it is crucial to ensure patients comply with the prescribed regimen of vitamin and mineral supplements (50). The dietary guidelines for post-BS recommends such as restricting meal portion sizes to 125 grams every 30 min and choosing foods that are rich in protein and high in fiber. This includes a variety of options like eggs, poultry, fruits, lean meats, fish, vegetables, low-fat dairy products, legumes, and whole grains (51).
3.3 Anti-obesity medicine
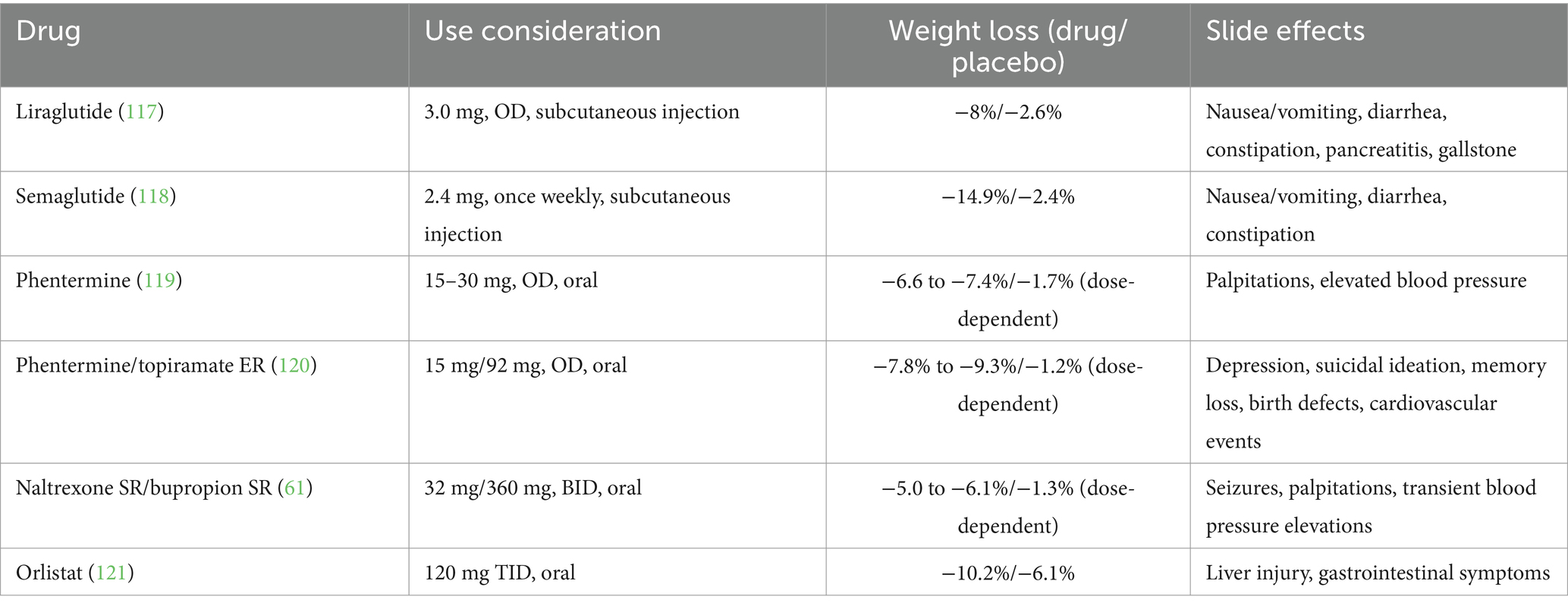
Anti-obesity medicine (AOM) options that are currently approved by the US Food and Drug Administration (FDA) include liraglutide, phentermine, phentermine/topiramate extended release (ER), naltrexone sustained release (SR) /bupropion sustained release (SR) and orlistat (52).
Liraglutide, an injectable glucagon-like peptide-1 (GLP-1) receptor agonist, has received FDA approval as an AOM agent at a dosage of 3.0 mg. It is believed to control appetite via both peripheral and central nervous system pathways and has demonstrated efficacy in bariatric surgery patients with RWG (53). Furthermore, preliminary evidence hints that GLP-1 agonists might offer a therapeutic advantage in addressing hypoglycemia (54). In addition to liraglutide, another, semaglutide, a GLP-1 agonist approved for T2DM treatment, has shown efficacy in promoting weight loss (55) and potentially surpassing liraglutide (56). The mechanism of GLP-1 (Figure 1A) and the effects of GLP-1 on the body (Figure 1B) are illustrated in Figure 1. Phentermine functions as a sympathomimetic amine, which stimulates the release of catecholamines in the hypothalamus, resulting in the suppression of appetite. When administered as a standalone treatment or in conjunction with topiramate, it has been demonstrated a reduced RWG (57) and facilitated weight reduction in cases where the outcomes of BS have been suboptimal (58). Topiramate, a medication primarily used to treat epilepsy and migraines, has not been officially approved for monotherapy in obesity treatment. However, it is frequently prescribed off-label due to its appetite-suppressing properties. It has been utilized both as a solo treatment and in combination with phentermine, as well as for the management of binge eating disorder (59). Notably, topiramate has turned into a highly effective option for managing weight regain in a subset of postoperative patients in a specific study (60). Naltrexone SR/Bupropion SR represents another FDA-approved dual-drug AOM. Each of these medications targets the central nervous system (CNS) reward pathways, and there is a theorized synergistic impact on human appetite regulation. This effect is suggested by animal studies, which propose that the combination acts on the specific receptor to enhance gorged feelings and prevent inhibited feedback. A number of randomized, double-blind, placebo-controlled trials have substantiated the efficacy of this medication pairing in combating RWG (61). Orlistat functions as a lipase inhibitor, which leads to the reduced absorption of 25–30% of the dietary fat consumed through the gastrointestinal tract (62). Current therapeutic options are reviewed in Table 1.
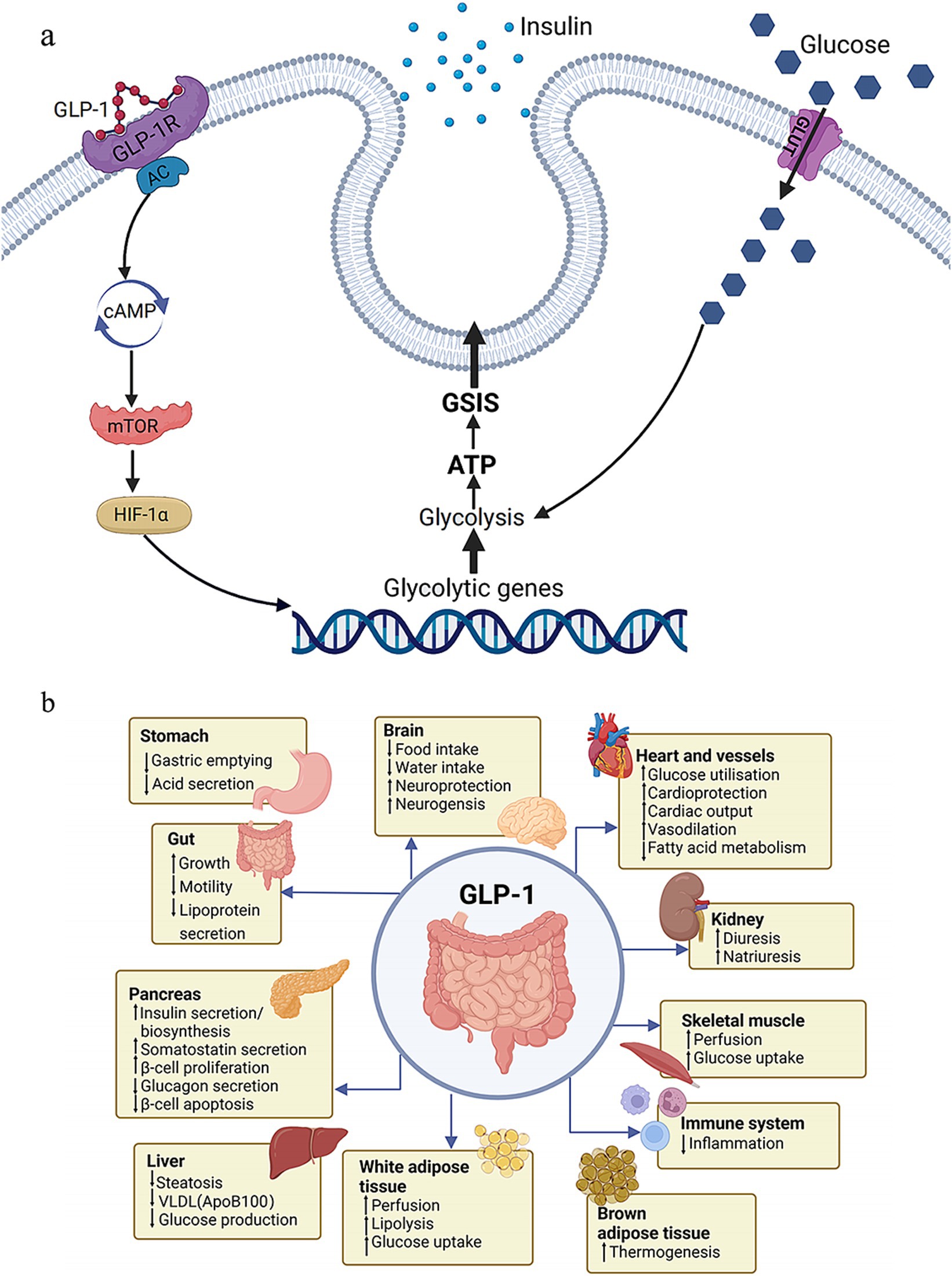
Figure 1. Function of GLP-1. (A) Molecular mechanism of GLP-1. (B) Effects of GLP-1 on the body. GLP-1, glucagon-like peptide-1; glucose-stimulated insulin secretion.
3.4 Endoscopic interventions
Endoscopic sleeve gastroplasty (ESG) involved creating a two-row plication, effectively reducing the size beginning from the gastroesophageal joint to the prepyloric antrum by forming a narrow sleeve-like structure (63). The first instance of a revisional ESG following SG was documented by Sharaiha and colleagues, resulting in 9-kilogram decrease (64). Across a retrospective investigation of five individuals who received a revisional ESG due to an enlarged gastric sleeve, a consistent TWL ranging from 6.7 to 17.2% was noted at the 12-month mark (65). A subsequent report detailed the revisional ESG as a “sleeve-in-sleeve” process, which involved creating additional applications in the stomach based on a special approach. The patient in this case experienced a favorable post-procedure outcome, with a weight loss of 7 kilograms, equating to an 8% TWL, reported at the three-month follow-up (66).
Transoral Outlet Reduction (TORe) after RYGB operates by constricting the gastrojejunal anastomosis (GJA) diameter with the aid of endoscopic tools and platforms that are commercially accessible. The TORe procedure diminishes the GJA’s size, thereby facilitating weight loss through a mechanical limitation that curtails hunger and enhances satiety (67). The execution of TORe can be varied, encompassing full-thickness endoscopic suturing, plications, and hybrid techniques that may include the ablation or resection of the GJA’s mucosal layer (68). The follow-up results indicated that patients who underwent TORe achieved a 3.5% total weight loss (TWL), a statistically significant improvement over the 0.4% TWL observed in the control group that received a sham procedure (p = 0.02) after 1 year (69). Subsequent advancements in the TORe approach have been made to boost its effectiveness (70). Argon Plasma Coagulation (APC) is a noncontact method of electrocoagulation, leading to a gradual reduction in diameter (71). The use of APC in the context of the GJA was primarily showcased in 2006 as a supplementary step during the standard TORe flow. Patients who received APC prior to suturing exhibited greater weight loss compared to those who underwent suturing without this preliminary step (72).
Restorative obesity surgery endoluminal (ROSE) is an alternative process to tackle RWG after RYGB (71). A multicenter registry reported on the outcomes of a cohort of patients who employed a non-invasive revision method to reduce the dimensions of their stoma and pouch. The study demonstrated that, at 6 months post-procedure (with data from 96 patients), there was an average weight reduction equivalent to 32% of the weight regained from the lowest weight point (73).
3.5 Conversional surgery
3.5.1 Conversional surgery after SG
Conversional surgeries for RWG after SG include endoscopic sleeve gastroplasty (ESG), re-sleeve gastrectomy (RSG), RYGB, one-anastomosis gastric bypass (OAGB), single-anastomosis duodeno-ileal bypass (SADI), and duodenal switch (DS) (74). Efficacy of different conversional surgeries on BMI during follow-up are showed in Figure 2.
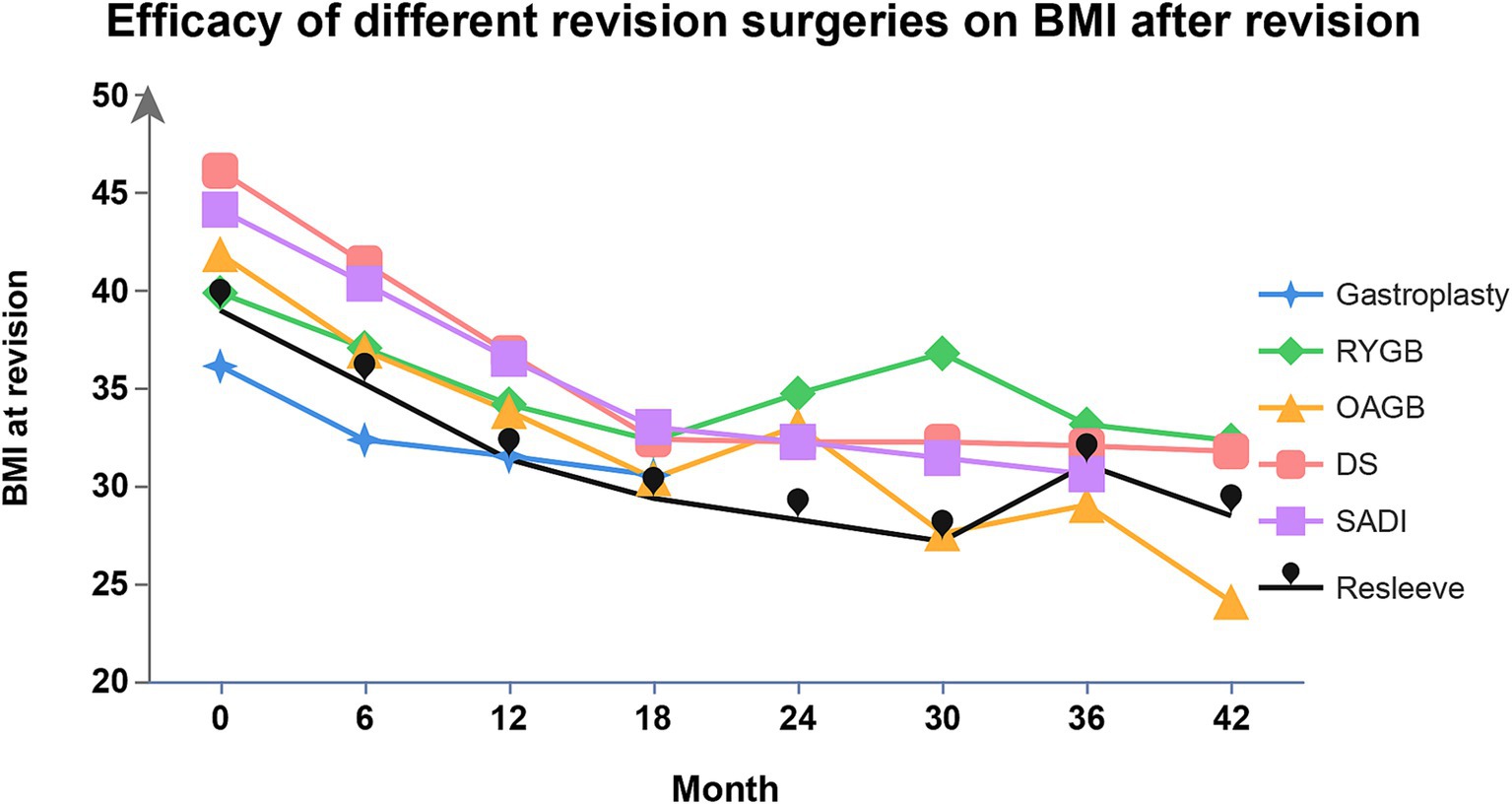
Figure 2. Efficacy of different conversional surgeries after SG on BMI. BMI, body mass index; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; OAGB, one-anastomosis gastric bypass; SADI, single-anastomosis duodeno-ileal bypass; DS, duodenal switch.
ESG is a non-invasive, incisionless procedure that reshapes the stomach by applying full-thickness sutures, thereby decreasing its capacity and slowing down gastric emptying (75). An observational study conducted by Sharaiha and colleagues (76) has demonstrated that ESG can lead to positive metabolic changes and improvements in obesity-related comorbidities. In cases where RWG is experienced following a SG, RSG may be considered, particularly if the stomach’s dilation exceeds 4 cm in diameter (77). RSG involves the reshaping of the remaining stomach volume. It has been suggested under circumstances where the stomach volume, as measured by a gastroscanner, surpasses 250 cc, as proposed by a French research team in 2014 (78). However, due to the high rates of postoperative gastroesophageal reflux disease (GERD), RSG is typically recommended only for select individuals with a significantly excessed the gastric fundus or antrum (79). A conversional OAGB involves the construction of a gastric pouch and the creation of a gastrojejunal anastomosis with a relatively broadened biliary limb (80). The absence of a jejuno-jejunal anastomosis in OAGB reduces the potential for future complications (81), and this procedure is also believed to decrease operative time as it necessitates the formation of only one anastomosis (82). The SADI-S was devised as a streamlined version of the biliopancreatic diversion/duodenal switch, aiming to reduce operating time and postoperative complications while retaining the same principles and effectiveness (83). The separation of the ileum results in the formation of two separate segments, which are then rejoined using a surgical connection, establishing the helpful channel that facilitates digestive absorption post-conversion from SG to DS. While DS is recognized for reaching the most significant weight reduction outcomes following an unsuccessful SG, it comes with the trade-offs of a higher risk of developing complications and the complexity inherent in the surgery (84, 85).
3.5.2 Conversional surgery after RYGB
Conversional surgeries after RYGB include the conversion of the GJA and/or pouch, gastric band around the upper pouch with laparoscope (LGB), a band with laparoscope combined with pouch resizing, distalization-RYGB (D-RYGB) and a duodenal switch (DS). Franken et al. (86) estimated the function and safety of those clinical techniques following RYGB for RWG. Efficacy of various conversional operations on BMI in the follow-up assay is shown in Figure 3.
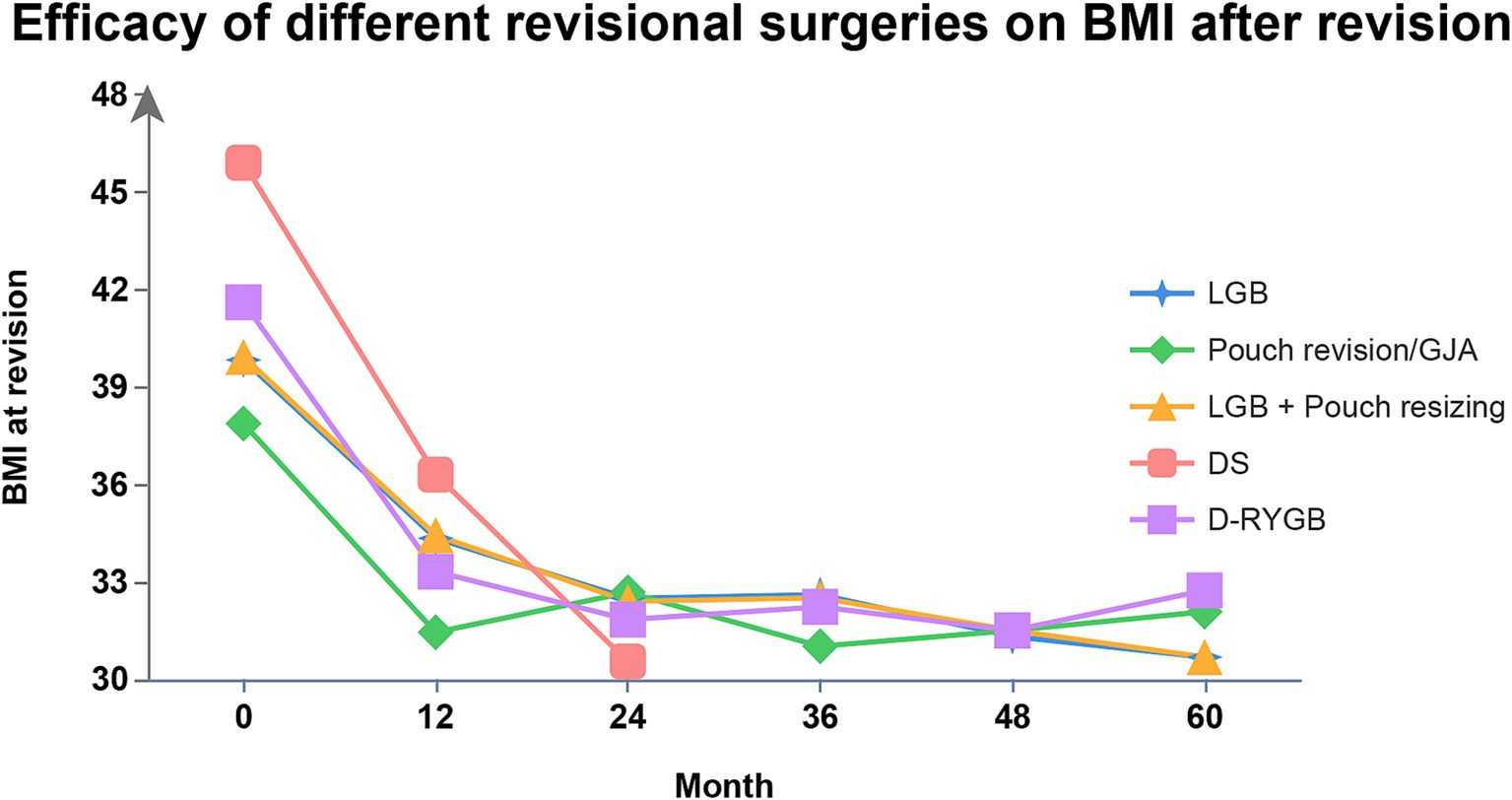
Figure 3. Efficacy of different conversional surgeries after RYGB on BMI. BMI, body mass index; RYGB, Roux-en-Y gastric bypass; LGB, laparoscopic gastric band; D-RYGB, distalization- Roux-en-Y gastric bypass; GJA, gastrojejunal anastomosis; DS, duodenal switch.
GJA or pouch conversion can be performed by either reshaping these structures or by removing part of the GJA followed by a reconstruction (87). LGB is a restrictive procedure that involves placing an artificial band near the gastrojejunostomy (88). DS is a more complex operation that includes creating a partial sleeve gastrectomy while keeping the pylorus intact, forming a Roux limb, extending the biliopancreatic limb, and establishing a short useful channel (89). D-RYGB is achieved by reducing the channel length, thereby enhancing the malabsorptive effect of the RYGB. There are two main methods of distalization: either by reconfiguring the Roux limb (Type 1) or the Y limb (Type 2). Shin et al. (90) suggested that an optimal total length of the alimentary limb should be around 300 centimeters to minimize the risk of malnutrition and reduce the occurrence of diarrhea, thereby improving the overall quality of life for patients undergoing these complex conversional procedures.
4 Management of T2DM recurrence
4.1 T2DM recurrence after BS
BS is typically linked to substantial enhancements or even the remission of diseases related to obesity, with a particularly notable impact on T2DM. T2DM is a multifaceted hormonal and metabolic condition characterized by varying levels of insulin resistance and impairment of the pancreatic β-cells (91). In the Swedish Obese Subjects (SOS) trial, a significant proportion of participants with T2DM at the outset—72%—were observed to be in remission after a 2-year post-bariatric surgery follow-up period. However, a noteworthy 50% of these individuals saw a return of T2DM symptoms by the 10-year check-up (92). The STAMPEDE was a randomized trial evaluating and comparing RYGB versus sleeve gastrectomy SG in obese patients with T2DM. The trial’s data indicated a decline in the remission rates of T2DM; after RYGB, the percentage dropped from 78% in the initial year to 45% at the 5th year, and following SG, it decreased from 51 to 25% over the same period (93). Definition of baseline T2DM and the recurrence within a 10- or 15-year period is defined as an HbA1c level of 48 mmol/mol or higher, a blood glucose level of 6.1 mmol/L or above (plasma glucose of 7 mmol/L or above), or the use of T2DM medications (11) (Table 2).
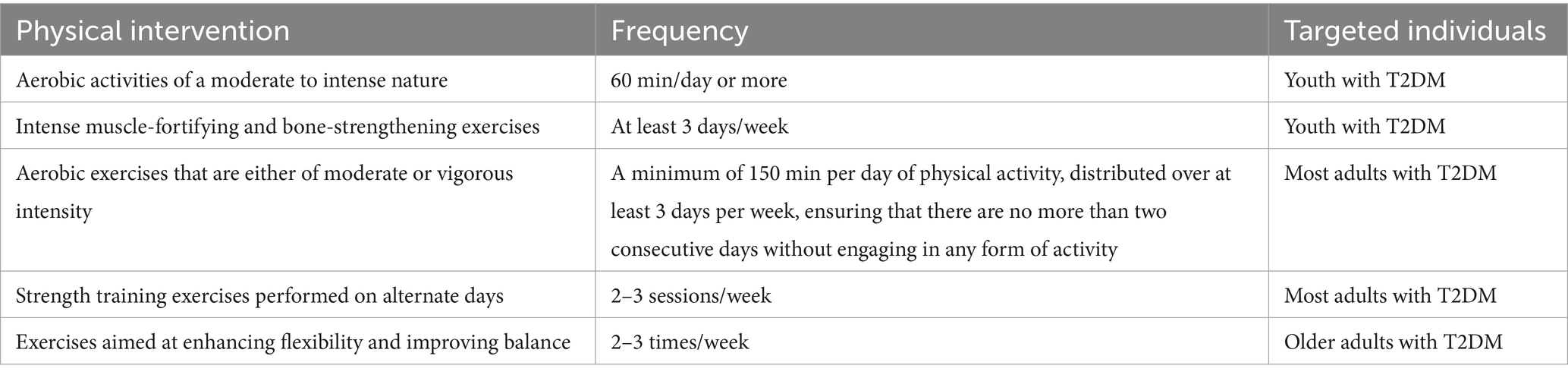
Table 2. Physical interventions which ADA recommends and its corresponding frequency and targeted individuals.
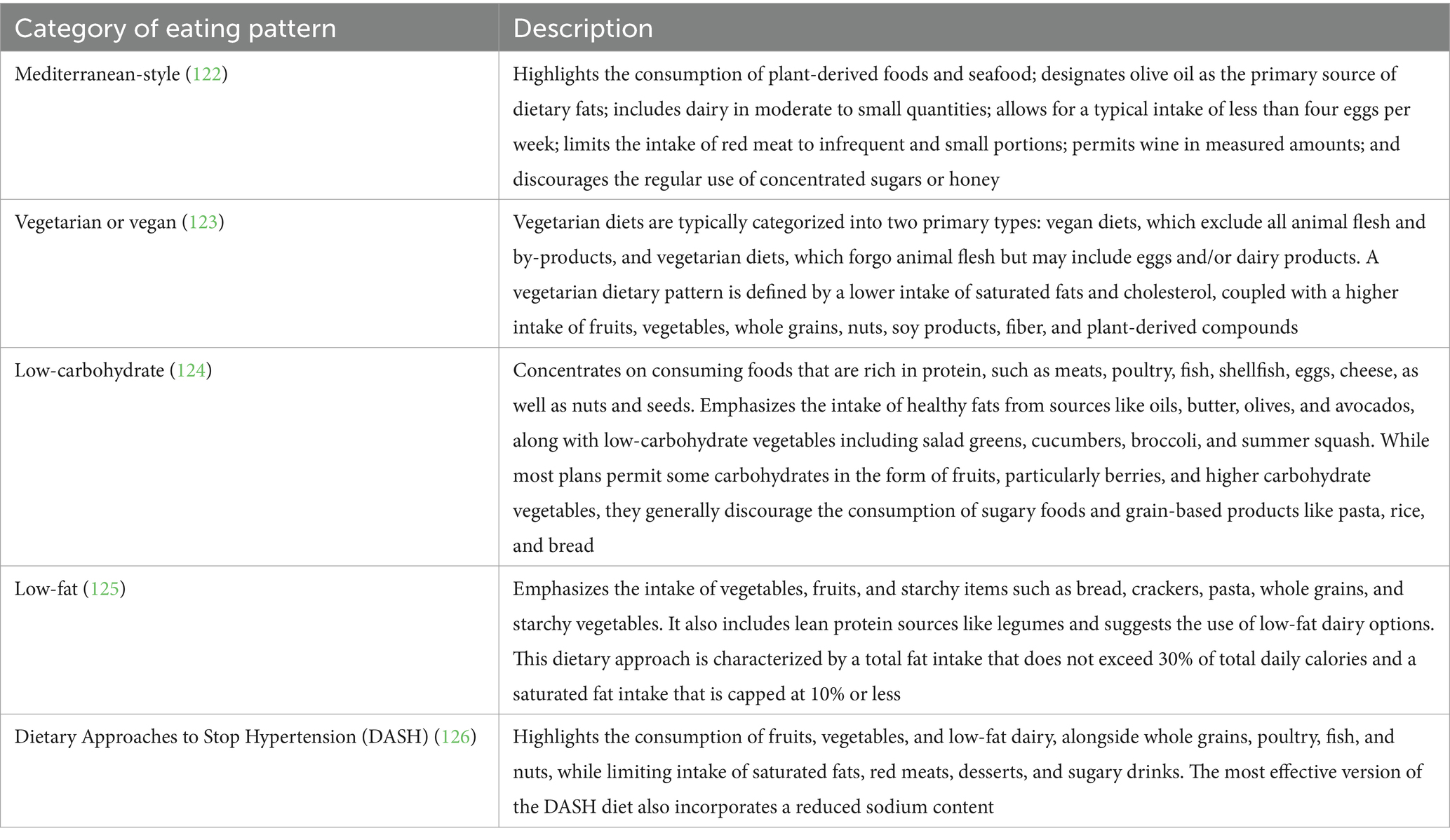
The ADA holds the stance that there is not a uniform dietary approach suitable for all individuals with diabetes. Traditionally, the ADA has endorsed a personalized dietary plan developed through a collaborative effort tailored to the specific necessities and preferences of the diabetes populations (13). The following eating patterns are listed in Table 3. Various dietary patterns for specific individuals have been shown to achieve varying levels of health benefits as evidence accumulates. Healthcare providers should concentrate on the fundamental elements shared across these patterns, which include reducing the consumption of added sugars, prioritizing the intake of nonstarchy vegetables and refined grains, and opting for whole foods over their highly processed counterparts whenever feasible (94).
4.2 Hypoglycemic agents
The ADA lists first-line hypoglycemic agents in its latest guidelines, including GLP-1 receptor agonists (GLP-1RA), dipeptidyl peptidase 4 (DPP-4) inhibitors (DPP-4i), sodium–glucose cotransporter 2 (SGLT-2) inhibitors (SGLT-2i), metformin, thiazolidinediones (TZDs), sulfonylureas and insulin (95). GLP-1 RAs target the pancreas to stimulate the release of insulin and curb the production of glucagon with the function within the gastrointestinal tract to slow down the process of gastric emptying (96). DPP-4i elevate endogenous incretin levels by inhibiting the activity of DPP-4 (96). SGLT-2i decrease renal glucose reabsorption (97). Metformin might target the liver to suppress gluconeogenesis and skeletal muscles to improve the utilization of glucose (98). It may also play a role in the gut by increasing the levels of GLP-1 (99). TZDs enhance insulin sensitivity in the skeletal muscles, adipose tissue, and liver. Sulfonylureas stimulate the pancreas to augment the secretion of insulin (100). Figure 4 shows the mechanism and target organ of hypoglycemic agents. Factors that are specific to the individual and influence the selection of treatment encompass personalized blood sugar targets (101), the person’s susceptibility to hypoglycemia, and their medical history or risk factors associated with cardiovascular, renal, hepatic, and other comorbidities and complications related to diabetes (102–104). Potential therapeutic options are reviewed in Table 4.
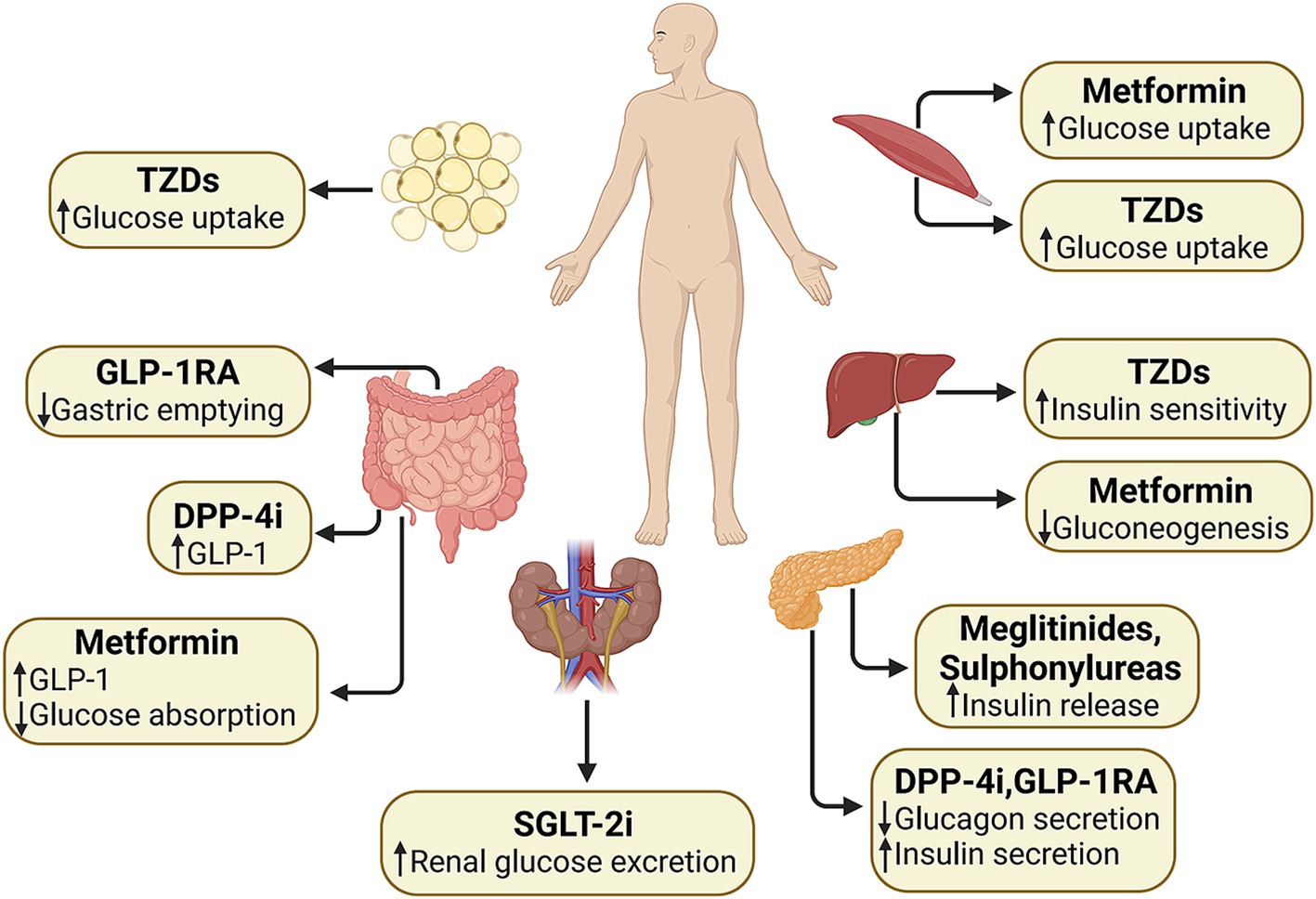
Figure 4. Effects of different types of hypoglycemic agents on the body. GLP-1, glucagon-like peptide-1; GLP-1 RA, glucagon-like peptide-1 receptor agonists; DPP-4i, dipeptidyl peptidase-4 inhibitors; SGLT-2i, sodium–glucose cotransporter 2 inhibitors; TZDs, thiazolidinediones.
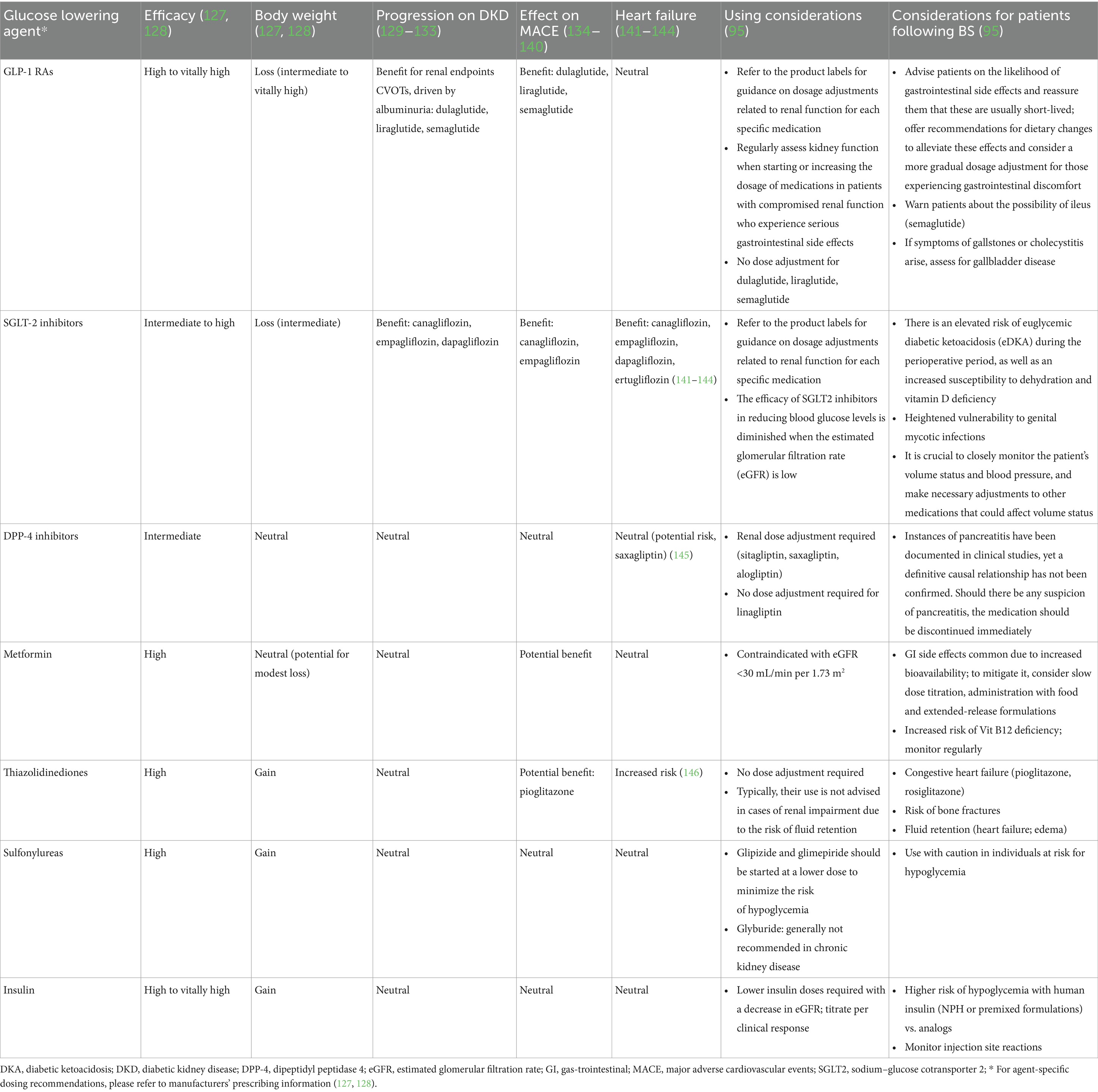
Table 4. Overview of blood glucose-lowering medications and the pertinent clinical factors to consider.
4.3 Conversional surgeries
Yan et al. (105) has evaluated the influence of conversional surgery on T2DM. Aleassa EM et al. corroborated findings that the overall improvement in T2DM can vary from 65 to 100%, contingent upon the specific indices and types of reconstructive surgery performed (106). Below, we will review types of conversional operations undergone: vertical banded gastroplasty (VBG) to Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB) conversions, sleeve gastrectomy (SG) conversions, and conversion of pouch/stoma after RYGB.
Challenges such as band erosion, dysphagia, and staple line failure have diminished the use of VBG. However, due to the anatomical changes induced by the procedure, converting VBG to RYGB has been shown to offer metabolic benefits for individuals with T2DM (107–109). Gagné et al. (110) examined data from patients under this conversion from July 1999 to April 2010 and discovered that T2DM improved or resolved in 90% of cases. Sarhan et al. (111) reviewed records of 102 patients who had a conversional RYGB following an unsuccessful VBG from April 2014 to January 2018, noting a T2DM improvement rate of 75.7% with complete remission and a 24.3% partial remission. Ngiam et al. (112) demonstrated the effectiveness of these conversional surgeries in resolving diabetes compared to AGB. Vidal et al. (113) reported comparable resolution rates for T2DM after SG and RYGB (51.4% vs. 62.0%) at the four-month mark post-surgery. Yeung et al. (114) observed little significant discrepancy in medication reduction for diabetes and hypertension at 12 months after RYGB (33% reduction, no cessation of diabetes medication) and SG (60% reduction, 40% off diabetes medication). Lee et al. (115) divided T2DM into three assessed stages at the initial time, enabling selection of procedures from evidence-based practices. Both procedures significantly improved T2DM in mild (IMS score ≤ 25) and severe cases (IMS score > 95), but RYGB was notably more effective in intermediate cases due to its more pronounced neurohormonal impact. Conversion of pouch/stoma after RYGB in cohort demonstrated by Aleassa et al. (106) resulted in further significant weight reduction and controlling T2DM better. Rawlins et al. (116) reviewed cases from 2002 to 2009 involving the conversion of RYGB to a distal gastric bypass, revealing that patients who underwent this distalization to 100-cm distal common channel experienced improvements in diabetes management.
5 Conclusion
BS remains the most potent treatment for weight reduction and alleviating the T2DM. However, the postoperative outcomes can vary significantly among patients. Factors after the surgery have a more substantial influence on predicting postoperative weight loss compared to those assessed before the procedure. Despite this, there is a scarcity of holistic predictive models that anticipate weight loss outcomes post-surgery. Therefore, there is a need for scoring systems that can amalgamate various factors and accurately forecast weight loss outcomes. In this context, certain models, notably the Ad-DiaRem, demonstrate a relatively strong ability to predict the remission of T2DM following BS. It is essential to recognize that enhanced diabetes management is a significant achievement, even if it does not result in complete remission. Some patients might experience RWG and a recurrence of diabetes. Given that most postoperative behavioral factors are modifiable, proactive measures to influence postoperative eating habits and lifestyle changes are crucial. Furthermore, recommendations for behavioral interventions should be tailored to meet the specific needs of each patient. In terms of pharmaceutical treatment, healthcare providers should adhere to the same principles for dose initiation and titration as they would for patients who have not undergone surgery. The treatment should commence with the lowest possible dose, with subsequent adjustments made based on individual requirements. In cases where monotherapy proves insufficient, combination therapies can be considered. Endoscopic management, recognized for its minimally invasive nature, has predominantly been realized through standard or modified TORe in patients who have undergone RYGB. Concurrently, ESG is gaining traction as a secure and effective method for those who have had SG. However, the sustainability of these procedures is uncertain without concurrent dietary and lifestyle interventions. There is an evident need for a prospective, randomized study to evaluate this innovative technique. It is crucial to acknowledge that the advantageous outcomes of conversional surgeries are often coupled with an increased risk and complexity of complications. Therefore, referral centers should be considered the most suitable venues for conducting conversional surgeries, and stringent postoperative surveillance is imperative.
Author contributions
HX: Conceptualization, Writing – original draft. YD: Investigation, Writing – original draft. YT: Software, Writing – original draft. YR: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82370601).
Acknowledgments
We thank the reviewers for their comments on the manuscript and the English editor for improving the manuscript’s language.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Blüher, M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
2. Busetto, L, Dicker, D, Azran, C, Batterham, RL, Farpour-Lambert, N, Fried, M, et al. Obesity management task force of the European Association for the Study of obesity released "practical recommendations for the post-bariatric surgery medical management". Obes Surg. (2018) 28:2117–21. doi: 10.1007/s11695-018-3283-z
3. Livhits, M, Mercado, C, Yermilov, I, Parikh, JA, Dutson, E, Mehran, A, et al. Patient behaviors associated with weight regain after laparoscopic gastric bypass. Obes Res Clin Pract. (2011) 5:e169–266. doi: 10.1016/j.orcp.2011.03.004
4. Istfan, NW, Lipartia, M, Anderson, WA, Hess, DT, and Apovian, CM. Approach to the patient: Management of the Post-Bariatric Surgery Patient with Weight Regain. J Clin Endocrinol Metab. (2021) 106:251–63. doi: 10.1210/clinem/dgaa702
5. Horváth, L, Mráz, M, Jude, EB, and Haluzík, M. Pharmacotherapy as an augmentation to bariatric surgery for obesity. Drugs. (2024) 84:933–52. doi: 10.1007/s40265-024-02029-0
6. Vargas, EJ, Bazerbachi, F, Rizk, M, Rustagi, T, Acosta, A, Wilson, EB, et al. Transoral outlet reduction with full thickness endoscopic suturing for weight regain after gastric bypass: a large multicenter international experience and meta-analysis. Surg Endosc. (2018) 32:252–9. doi: 10.1007/s00464-017-5671-1
7. Karmali, S, Brar, B, Shi, X, Sharma, AM, de Gara, C, and Birch, DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. (2013) 23:1922–33. doi: 10.1007/s11695-013-1070-4
8. Aminian, A, Brethauer, SA, Andalib, A, Nowacki, AS, Jimenez, A, Corcelles, R, et al. Individualized metabolic surgery score: procedure selection based on Diabetes severity. Ann Surg. (2017) 266:650–7. doi: 10.1097/SLA.0000000000002407
9. Lee, WJ, Hur, KY, Lakadawala, M, Kasama, K, Wong, SK, Chen, SC, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. (2013) 9:379–84. doi: 10.1016/j.soard.2012.07.015
10. Aron-Wisnewsky, J, Sokolovska, N, Liu, Y, Comaneshter, DS, Vinker, S, Pecht, T, et al. The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia. (2017) 60:1892–902. doi: 10.1007/s00125-017-4371-7
11. Sjöholm, K, Svensson, PA, Taube, M, Jacobson, P, Andersson-Assarsson, JC, Carlsson, LMS, et al. Evaluation of prediction models for type 2 Diabetes relapse after post-bariatric surgery remission: a post hoc analysis of 15-year follow-up data from the Swedish obese subjects (SOS) study. Obes Surg. (2020) 30:3955–60. doi: 10.1007/s11695-020-04763-2
12. Piercy, KL, Troiano, RP, Ballard, RM, Carlson, SA, Fulton, JE, Galuska, DA, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
13. Inzucchi, SE, Bergenstal, RM, Buse, JB, Diamant, M, Ferrannini, E, Nauck, M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2012) 35:1364–79. doi: 10.2337/dc12-0413
14. Chee, YJ, and Dalan, R. Novel therapeutics for type 2 Diabetes mellitus-a Look at the past decade and a glimpse into the future. Biomedicines. (2024) 12:1386. doi: 10.3390/biomedicines12071386
15. Belligoli, A, Bettini, S, Segato, G, and Busetto, L. Predicting responses to bariatric and metabolic surgery. Curr Obes Rep. (2020) 9:373–9. doi: 10.1007/s13679-020-00390-1
16. Busetto, L, Segato, G, De Marchi, F, Foletto, M, De Luca, M, Caniato, D, et al. Outcome predictors in morbidly obese recipients of an adjustable gastric band. Obes Surg. (2002) 12:83–92. doi: 10.1381/096089202321144649
17. Hatoum, IJ, Stein, HK, Merrifield, BF, and Kaplan, LM. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring, MD). (2009) 17:92–9. doi: 10.1038/oby.2008.507
18. Busetto, L, Angrisani, L, Basso, N, Favretti, F, Furbetta, F, and Lorenzo, M. Safety and efficacy of laparoscopic adjustable gastric banding in the elderly. Obesity (Silver Spring, MD). (2008) 16:334–8. doi: 10.1038/oby.2007.85
19. Steinbeisser, M, McCracken, J, and Kharbutli, B. Laparoscopic sleeve gastrectomy: preoperative weight loss and other factors as predictors of postoperative success. Obes Surg. (2017) 27:1508–13. doi: 10.1007/s11695-016-2520-6
20. Pannemans, DL, and Westerterp, KR. Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr. (1995) 73:571–81. doi: 10.1079/BJN19950059
21. Pfefferkorn, U, Hort, S, Beluli, M, La Vista, M, and Züger, T. Weight loss after bariatric surgery in different age groups. Obes Surg. (2023) 33:1154–9. doi: 10.1007/s11695-023-06488-4
22. Larsen, JK, Geenen, R, Maas, C, de Wit, P, van Antwerpen, T, Brand, N, et al. Personality as a predictor of weight loss maintenance after surgery for morbid obesity. Obes Res. (2004) 12:1828–34. doi: 10.1038/oby.2004.227
23. Ma, Y, Pagoto, SL, Olendzki, BC, Hafner, AR, Perugini, RA, Mason, R, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. (2006) 16:1227–31. doi: 10.1381/096089206778392284
24. Lutfi, R, Torquati, A, Sekhar, N, and Richards, WO. Predictors of success after laparoscopic gastric bypass: a multivariate analysis of socioeconomic factors. Surg Endosc. (2006) 20:864–7. doi: 10.1007/s00464-005-0115-8
25. Chau, WY, Schmidt, HJ, Kouli, W, Davis, D, Wasielewski, A, and Ballantyne, GH. Patient characteristics impacting excess weight loss following laparoscopic adjustable gastric banding. Obes Surg. (2005) 15:346–50. doi: 10.1381/0960892053576811
26. Dixon, JB, Dixon, ME, and O'Brien, PE. Pre-operative predictors of weight loss at 1-year after lap-band surgery. Obes Surg. (2001) 11:200–7. doi: 10.1381/096089201321577884
27. Kalarchian, MA, Marcus, MD, Courcoulas, AP, Cheng, Y, and Levine, MD. Preoperative lifestyle intervention in bariatric surgery: a randomized clinical trial. Surg Obes Relat Dis. (2016) 12:180–7. doi: 10.1016/j.soard.2015.05.004
28. Krimpuri, RD, Yokley, JM, Seeholzer, EL, Horwath, EL, Thomas, CL, and Bardaro, SJ. Qualifying for bariatric surgery: is preoperative weight loss a reliable predictor of postoperative weight loss? Surg Obes Relat Dis. (2018) 14:60–4. doi: 10.1016/j.soard.2017.07.012
29. Giordano, S, and Victorzon, M. The impact of preoperative weight loss before laparoscopic gastric bypass. Obes Surg. (2014) 24:669–74. doi: 10.1007/s11695-013-1165-y
30. Sherman, WE, Lane, AE, Mangieri, CW, Choi, YU, and Faler, BJ. Does preoperative weight change predict postoperative weight loss after laparoscopic sleeve gastrectomy? Bariatric Surg Pract Patient Care. (2015) 10:126–9. doi: 10.1089/bari.2015.0023
31. McNickle, AG, and Bonomo, SR. Predictability of first-year weight loss in laparoscopic sleeve gastrectomy. Surg Endosc. (2017) 31:4145–9. doi: 10.1007/s00464-017-5467-3
32. Kim, JJ, Rogers, AM, Ballem, N, and Schirmer, B. ASMBS updated position statement on insurance mandated preoperative weight loss requirements. Surg Obes Relat Dis. (2016) 12:955–9. doi: 10.1016/j.soard.2016.04.019
33. Mocanu, V, Marcil, G, Dang, JT, Birch, DW, Switzer, NJ, and Karmali, S. Preoperative weight loss is linked to improved mortality and leaks following elective bariatric surgery: an analysis of 548,597 patients from 2015-2018. Surg Obes Relat Dis. (2021) 17:1846–53. doi: 10.1016/j.soard.2021.06.021
34. Tolvanen, L, Christenson, A, Eke, H, Bonn, SE, and Lagerros, YT. Weight loss history and its Association with self-esteem and eating behaviors in adolescents and young adults with obesity. Obes Facts. (2023) 16:293–300. doi: 10.1159/000529267
35. Riddle, MC, Cefalu, WT, Evans, PH, Gerstein, HC, Nauck, MA, Oh, WK, et al. Consensus report: definition and interpretation of remission in type 2 Diabetes. Diabetes Care. (2021) 44:2438–44. doi: 10.2337/dci21-0034
36. Schauer, PR, Kashyap, SR, Wolski, K, Brethauer, SA, Kirwan, JP, Pothier, CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. (2012) 366:1567–76. doi: 10.1056/NEJMoa1200225
37. Brethauer, SA, Aminian, A, Romero-Talamás, H, Batayyah, E, Mackey, J, Kennedy, L, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. (2013) 258:628–636; discussion 636–637. doi: 10.1097/SLA.0b013e3182a5034b
38. Pucci, A, Tymoszuk, U, Cheung, WH, Makaronidis, JM, Scholes, S, Tharakan, G, et al. Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: the role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet Med. (2018) 35:360–7. doi: 10.1111/dme.13532
39. Dicker, D, Golan, R, Aron-Wisnewsky, J, Zucker, JD, Sokolowska, N, Comaneshter, DS, et al. Prediction of long-term Diabetes remission after RYGB, sleeve gastrectomy, and adjustable gastric banding using DiaRem and advanced-DiaRem scores. Obes Surg. (2019) 29:796–804. doi: 10.1007/s11695-018-3583-3
40. Haddad, A, Suter, M, Greve, JW, Shikora, S, Prager, G, Dayyeh, BA, et al. Therapeutic options for recurrence of weight and obesity related complications after metabolic and bariatric surgery: an IFSO position statement. Obes Surg. (2024) 34:3944–62. doi: 10.1007/s11695-024-07489-7
41. Peterli, R, Wölnerhanssen, BK, Peters, T, Vetter, D, Kröll, D, Borbély, Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. (2018) 319:255–65. doi: 10.1001/jama.2017.20897
42. Salminen, P, Helmiö, M, Ovaska, J, Juuti, A, Leivonen, M, Peromaa-Haavisto, P, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. (2018) 319:241–54. doi: 10.1001/jama.2017.20313
43. Josbeno, DA, Kalarchian, M, Sparto, PJ, Otto, AD, and Jakicic, JM. Physical activity and physical function in individuals post-bariatric surgery. Obes Surg. (2011) 21:1243–9. doi: 10.1007/s11695-010-0327-4
44. Baillot, A, St-Pierre, M, Bernard, P, Burkhardt, L, Chorfi, W, Oppert, JM, et al. Exercise and bariatric surgery: a systematic review and meta-analysis of the feasibility and acceptability of exercise and controlled trial methods. Obes Rev. (2022) 23:e13480. doi: 10.1111/obr.13480
45. Bond, DS, Thomas, JG, Vithiananthan, S, Unick, J, Webster, J, Roye, GD, et al. Intervention-related increases in preoperative physical activity are maintained 6-months after bariatric surgery: results from the bari-active trial. Int J Obes. (2017) 41:467–70. doi: 10.1038/ijo.2016.237
46. Freire, RH, Borges, MC, Alvarez-Leite, JI, and Correia, MITD. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition (Burbank, Los Angeles County, Calif). (2012) 28:53–8. doi: 10.1016/j.nut.2011.01.011
47. Rusch, MD, and Andris, D. Maladaptive eating patterns after weight-loss surgery. Nutr Clin Pract. (2007) 22:41–9. doi: 10.1177/011542650702200141
48. Bosnic, G. Nutritional requirements after bariatric surgery. Crit Care Nurs Clin North Am. (2014) 26:255–62. doi: 10.1016/j.ccell.2014.02.002
49. Mechanick, JI, Youdim, A, Jones, DB, Timothy Garvey, W, Hurley, DL, Molly McMahon, M, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & bariatric surgery. Surg Obes Relat Dis. (2013) 9:159–91. doi: 10.1016/j.soard.2012.12.010
50. Magallares, A, and Schomerus, G. Mental and physical health-related quality of life in obese patients before and after bariatric surgery: a meta-analysis. Psychol Health Med. (2015) 20:165–76. doi: 10.1080/13548506.2014.963627
51. Parkes, E. Nutritional management of patients after bariatric surgery. Am J Med Sci. (2006) 331:207–13. doi: 10.1097/00000441-200604000-00007
52. Srivastava, G, and Apovian, CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. (2018) 14:12–24. doi: 10.1038/nrendo.2017.122
53. Miras, AD, Pérez-Pevida, B, Aldhwayan, M, Kamocka, A, McGlone, ER, Al-Najim, W, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diab Endocrinol. (2019) 7:549–59. doi: 10.1016/S2213-8587(19)30157-3
54. Abrahamsson, N, Engström, BE, Sundbom, M, and Karlsson, FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. (2013) 169:885–9. doi: 10.1530/EJE-13-0504
55. Ahrén, B, Atkin, SL, Charpentier, G, Warren, ML, Wilding, JPH, Birch, S, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. (2018) 20:2210–9. doi: 10.1111/dom.13353
56. Murvelashvili, N, Xie, L, Schellinger, JN, Mathew, MS, Marroquin, EM, Lingvay, I, et al. Effectiveness of semaglutide versus liraglutide for treating post-metabolic and bariatric surgery weight recurrence. Obesity (Silver Spring, Md). (2023) 31:1280–9. doi: 10.1002/oby.23736
57. Istfan, NW, Anderson, WA, Hess, DT, Yu, L, Carmine, B, and Apovian, CM. The mitigating effect of phentermine and Topiramate on weight regain after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring, Md). (2020) 28:1023–30. doi: 10.1002/oby.22786
58. Toth, AT, Gomez, G, Shukla, AP, Pratt, JS, Cena, H, Biino, G, et al. Weight loss medications in young adults after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Children (Basel, Switzerland). (2018) 5:116. doi: 10.3390/children5090116
59. Grilo, CM, Reas, DL, and Mitchell, JE. Combining pharmacological and psychological treatments for binge eating disorder: current status, limitations, and future directions. Curr Psychiatry Rep. (2016) 18:55. doi: 10.1007/s11920-016-0696-z
60. Stanford, FC, Alfaris, N, Gomez, G, Ricks, ET, Shukla, AP, Corey, KE, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis. (2017) 13:491–500. doi: 10.1016/j.soard.2016.10.018
61. Greenway, FL, Fujioka, K, Plodkowski, RA, Mudaliar, S, Guttadauria, M, Erickson, J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). (2010) 376:595–605. doi: 10.1016/S0140-6736(10)60888-4
62. Avenell, A, Robertson, C, Skea, Z, Jacobsen, E, Boyers, D, Cooper, D, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the REBALANCE mixed-methods systematic review and economic evaluation. Health Technol Assessment (Winchester, England). (2018) 22:1–246. doi: 10.3310/hta22680
63. Abu Dayyeh, BK, Rajan, E, and Gostout, CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. (2013) 78:530–5. doi: 10.1016/j.gie.2013.04.197
64. Sharaiha, RZ, Kedia, P, Kumta, N, Aronne, LJ, and Kahaleh, M. Endoscopic sleeve plication for revision of sleeve gastrectomy. Gastrointest Endosc. (2015) 81:1004. doi: 10.1016/j.gie.2014.06.008
65. Eid, G. Sleeve gastrectomy revision by endoluminal sleeve plication gastroplasty: a small pilot case series. Surg Endosc. (2017) 31:4252–5. doi: 10.1007/s00464-017-5469-1
66. Jirapinyo, P, de Moura, DTH, and Thompson, CC. Sleeve in sleeve: endoscopic revision for weight regain after sleeve gastrectomy. VideoGIE. (2019) 4:454–7. doi: 10.1016/j.vgie.2019.07.003
67. Jirapinyo, P, Dayyeh, BK, and Thompson, CC. Gastrojejunal anastomotic reduction for weight regain in roux-en-y gastric bypass patients: physiological, behavioral, and anatomical effects of endoscopic suturing and sclerotherapy. Surg Obes Relat Dis. (2016) 12:1810–6. doi: 10.1016/j.soard.2016.09.036
68. Kumar, N, and Thompson, CC. Comparison of a superficial suturing device with a full-thickness suturing device for transoral outlet reduction (with videos). Gastrointest Endosc. (2014) 79:984–9. doi: 10.1016/j.gie.2014.02.006
69. Thompson, CC, Chand, B, Chen, YK, DeMarco, DC, Miller, L, Schweitzer, M, et al. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology. (2013) 145:129–37.e3. doi: 10.1053/j.gastro.2013.04.002
70. Schulman, AR, Kumar, N, and Thompson, CC. Transoral outlet reduction: a comparison of purse-string with interrupted stitch technique. Gastrointest Endosc. (2018) 87:1222–8. doi: 10.1016/j.gie.2017.10.034
71. Bulajic, M, Vadalà di Prampero, SF, Boškoski, I, and Costamagna, G. Endoscopic therapy of weight regain after bariatric surgery. World J Gastrointest Surg. (2021) 13:1584–96. doi: 10.4240/wjgs.v13.i12.1584
72. Thompson, CC, Slattery, J, Bundga, ME, and Lautz, DB. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc. (2006) 20:1744–8. doi: 10.1007/s00464-006-0045-0
73. Horgan, S, Jacobsen, G, Weiss, GD, Oldham, JS Jr, Denk, PM, Borao, F, et al. Incisionless revision of post-Roux-en-Y bypass stomal and pouch dilation: multicenter registry results. Surg Obes Relat Dis. (2010) 6:290–5. doi: 10.1016/j.soard.2009.12.011
74. Franken, RJ, Sluiter, NR, Franken, J, de Vries, R, Souverein, D, Gerdes, VEA, et al. Treatment options for weight regain or Insufcient weight loss after sleeve gastrectomy: a systematic review and Meta-analysis. Obes Surg. (2022) 32:2035–46. doi: 10.1007/s11695-022-06020-0
75. de Moura, DTH, de Moura, EGH, and Thompson, CC. Endoscopic sleeve gastroplasty: from whence we came and where we are going. World J Gastrointest Endoscopy. (2019) 11:322–8. doi: 10.4253/wjge.v11.i5.322
76. Sharaiha, RZ, Kumta, NA, Saumoy, M, Desai, AP, Sarkisian, AM, Benevenuto, A, et al. Endoscopic sleeve Gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol. (2017) 15:504–10. doi: 10.1016/j.cgh.2016.12.012
77. Langer, FB, Bohdjalian, A, Felberbauer, FX, Fleischmann, E, Reza Hoda, MA, Ludvik, B, et al. Does gastric dilatation limit the success of sleeve gastrectomy as a sole operation for morbid obesity? Obes Surg. (2006) 16:166–71. doi: 10.1381/096089206775565276
78. El Khoury, L, Catheline, JM, Taher, M, Roussel, J, Bendacha, Y, Romero, R, et al. Re-sleeve gastrectomy is a safe and sensible intervention in selected patients: retrospective cohort study. Int J Surg (London, England). (2023) 109:4145–50. doi: 10.1097/JS9.0000000000000743
79. Bonaldi, M, Rubicondo, C, Giorgi, R, Cesana, G, Ciccarese, F, Uccelli, M, et al. Re-sleeve gastrectomy: weight loss, comorbidities and gerd evaluation in a large series with 5 years of follow-up. Updat Surg. (2023) 75:959–65. doi: 10.1007/s13304-023-01471-1
80. Chakhtoura, G, Zinzindohoué, F, Ghanem, Y, Ruseykin, I, Dutranoy, JC, and Chevallier, JM. Primary results of laparoscopic mini-gastric bypass in a French obesity-surgery specialized university hospital. Obes Surg. (2008) 18:1130–3. doi: 10.1007/s11695-008-9594-8
81. Jia, D, Tan, H, Faramand, A, and Fang, F. One anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity: a systematic review and Meta-analysis of randomized clinical trials. Obes Surg. (2020) 30:1211–8. doi: 10.1007/s11695-019-04288-3
82. Robert, M, Espalieu, P, Pelascini, E, Caiazzo, R, Sterkers, A, Khamphommala, L, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet (London, England). (2019) 393:1299–309. doi: 10.1016/S0140-6736(19)30475-1
83. Wysocki, M, Borys, M, Budzyńska, D, Pisarska-Adamczyk, M, Małczak, P, Rajtar, A, et al. Initial experience with laparoscopic revisional single anastomosis duodeno-ileal bypass (SADI-S) after failed sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne. (2023) 18:298–304. doi: 10.5114/wiitm.2023.128683
84. Homan, J, Betzel, B, Aarts, EO, van Laarhoven, KJ, Janssen, IM, and Berends, FJ. Secondary surgery after sleeve gastrectomy: Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. (2015) 11:771–7. doi: 10.1016/j.soard.2014.09.029
85. Casillas, RA, Um, SS, Zelada Getty, JL, Sachs, S, and Kim, BB. Revision of primary sleeve gastrectomy to Roux-en-Y gastric bypass: indications and outcomes from a high-volume center. Surg Obes Relat Dis. (2016) 12:1817–25. doi: 10.1016/j.soard.2016.09.038
86. Franken, RJ, Franken, J, Sluiter, NR, de Vries, R, Euser, S, Gerdes, VEA, et al. Efficacy and safety of revisional treatments for weight regain or insufficient weight loss after Roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Rev. (2023) 24:e13607. doi: 10.1111/obr.13607
87. Razzak, FA, Kerbage, A, Brunaldi, VO, Mrad, R, Mahmoud, T, Gala, K, et al. Correlation between Gastrojejunal anastomosis diameter, Distensibility index, and weight regain after Roux-en-Y gastric bypass. Obes Surg. (2023) 33:4042–8. doi: 10.1007/s11695-023-06918-3
88. Arica, PC, Aydin, S, Zengin, U, Kocael, A, Orhan, A, Zengin, K, et al. The effects on obesity related peptides of laparoscopic gastric band applications in morbidly obese patients. J Investigat Surg. (2018) 31:89–95. doi: 10.1080/08941939.2017.1280564
89. Ng, PC, Sharp, LS, and Bermudez, DM. Duodenal switch: fully stapled technique. Surg Obes Relat Dis. (2019) 15:512. doi: 10.1016/j.soard.2018.12.031
90. Shin, RD, Goldberg, MB, Shafran, AS, Shikora, SA, Majumdar, MC, and Shikora, SA. Revision of Roux-en-Y gastric bypass with limb Distalization for inadequate weight loss or weight regain. Obes Surg. (2019) 29:811–8. doi: 10.1007/s11695-018-03635-0
91. Liao, EP. Management of type 2 diabetes: new and future developments in treatment. Am J Med. (2012) 125:S2–3. doi: 10.1016/j.amjmed.2012.05.008
92. Sjöström, L. Review of the key results from the Swedish obese subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. (2013) 273:219–34. doi: 10.1111/joim.12012
93. Schauer, PR, Bhatt, DL, Kirwan, JP, Wolski, K, Aminian, A, Brethauer, SA, et al. Bariatric surgery versus intensive medical therapy for Diabetes - 5-year outcomes. N Engl J Med. (2017) 376:641–51. doi: 10.1056/NEJMoa1600869
94. Gardner, CD, Trepanowski, JF, Del Gobbo, LC, Hauser, ME, Rigdon, J, Ioannidis, JPA, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the Association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. (2018) 319:667–79. doi: 10.1001/jama.2018.0245
95. Pharmacologic Approaches to Glycemic Treatment. Standards of Care in Diabetes-2024. Diabetes Care. (2024) 47:S158–78. doi: 10.2337/dc24-S009
96. Verspohl, EJ. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. (2009) 124:113–38. doi: 10.1016/j.pharmthera.2009.06.002
97. Bailey, CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. (2011) 32:63–71. doi: 10.1016/j.tips.2010.11.011
98. Miller, RA, Chu, Q, Xie, J, Foretz, M, Viollet, B, and Birnbaum, MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. (2013) 494:256–60. doi: 10.1038/nature11808
99. Mulherin, AJ, Oh, AH, Kim, H, Grieco, A, Lauffer, LM, and Brubaker, PL. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. (2011) 152:4610–9. doi: 10.1210/en.2011-1485
100. Tian, YA, Johnson, G, and Ashcroft, SJ. Sulfonylureas enhance exocytosis from pancreatic beta-cells by a mechanism that does not involve direct activation of protein kinase C. Diabetes. (1998) 47:1722–6. doi: 10.2337/diabetes.47.11.1722
101. Glycemic Goals and Hypoglycemia. Standards of Care in Diabetes-2024. Diabetes Care. (2024) 47:S111–s25.
102. Comprehensive Medical Evaluation and Assessment of Comorbidities. Standards of medical Care in Diabetes-2022. Diabetes Care. (2022) 45:S46–s59.
103. Cardiovascular Disease and Risk Management. Standards of Care in Diabetes-2024. Diabetes Care. (2024) 47:S179–s218.
104. ElSayed, NA, Aleppo, G, Aroda, VR, Bannuru, RR, Brown, FM, Bruemmer, D, et al. Chronic kidney disease and risk management: standards of Care in Diabetes-2023. Diabetes Care. (2023) 46:S191–s202. doi: 10.2337/dc23-S011
105. Yan, J, Cohen, R, and Aminian, A. Reoperative bariatric surgery for treatment of type 2 diabetes mellitus. Surg Obes Relat Dis. (2017) 13:1412–21. doi: 10.1016/j.soard.2017.04.019
106. Aleassa, EM, Hassan, M, Hayes, K, Brethauer, SA, Schauer, PR, and Aminian, A. Effect of revisional bariatric surgery on type 2 diabetes mellitus. Surg Endosc. (2019) 33:2642–8. doi: 10.1007/s00464-018-6541-1
107. Yale, CE. Conversion surgery for morbid obesity: complications and long-term weight control. Surgery. (1989) 106:474–80.
108. Linner, JH, and Drew, RL. Reoperative surgery—indications, efficacy, and long-term follow-up. Am J Clin Nutr. (1992) 55:606s–10s. doi: 10.1093/ajcn/55.2.606s
109. Behrns, KE, Smith, CD, Kelly, KA, and Sarr, MG. Reoperative bariatric surgery. Lessons learned to improve patient selection and results. Ann Surg. (1993) 218:646–53. doi: 10.1097/00000658-199321850-00010
110. Gagné, DJ, Dovec, E, and Urbandt, JE. Laparoscopic revision of vertical banded gastroplasty to Roux-en-Y gastric bypass: outcomes of 105 patients. Surg Obes Relat Dis. (2011) 7:493–9. doi: 10.1016/j.soard.2010.10.014
111. Sarhan, MD, AbdelSalam, NM, Mostafa, MS, Yehia, A, Anwar, I, and Fathy, E. Laparoscopic Roux-en-Y gastric bypass after failed vertical banded Gastroplasty: 2-year follow-up of 102 patients. Obes Surg. (2021) 31:2717–22. doi: 10.1007/s11695-021-05328-7
112. Ngiam, KY, Khoo, VY, Kong, L, and Cheng, AK. Laparoscopic adjustable gastric banding revisions in Singapore: a 10-year experience. Obes Surg. (2016) 26:1069–74. doi: 10.1007/s11695-015-1852-y
113. Vidal, J, Ibarzabal, A, Romero, F, Delgado, S, Momblán, D, Flores, L, et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg. (2008) 18:1077–82. doi: 10.1007/s11695-008-9547-2
114. Yeung, L, Durkan, B, Barrett, A, Kraft, C, Vu, K, Phillips, E, et al. Single-stage revision from gastric band to gastric bypass or sleeve gastrectomy: 6- and 12-month outcomes. Surg Endosc. (2016) 30:2244–50. doi: 10.1007/s00464-015-4498-x
115. Lee, WJ, Almulaifi, A, Tsou, JJ, Ser, KH, Lee, YC, and Chen, SC. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg Obes Relat Dis. (2015) 11:991–6. doi: 10.1016/j.soard.2014.12.027
116. Rawlins, ML, Teel, D 2nd, Hedgcorth, K, and Maguire, JP. Revision of Roux-en-Y gastric bypass to distal bypass for failed weight loss. Surg Obes Relat Dis. (2011) 7:45–9. doi: 10.1016/j.soard.2010.08.013
117. Pi-Sunyer, X, Astrup, A, Fujioka, K, Greenway, F, Halpern, A, Krempf, M, et al. A randomized, controlled trial of 3.0 mg of Liraglutide in weight management. N Engl J Med. (2015) 373:11–22. doi: 10.1056/NEJMoa1411892
118. Wilding, JPH, Batterham, RL, Calanna, S, Davies, M, Van Gaal, LF, Lingvay, I, et al. Once-weekly Semaglutide in adults with overweight or obesity. N Engl J Med. (2021) 384:989–1002. doi: 10.1056/NEJMoa2032183
119. Aronne, LJ, Wadden, TA, Peterson, C, Winslow, D, Odeh, S, and Gadde, KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring, Md). (2013) 21:2163–71. doi: 10.1002/oby.20584
120. Garvey, WT, Ryan, DH, Look, M, Gadde, KM, Allison, DB, Peterson, CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. (2012) 95:297–308. doi: 10.3945/ajcn.111.024927
121. Sjöström, L, Rissanen, A, Andersen, T, Boldrin, M, Golay, A, Koppeschaar, HP, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European multicentre orlistat study group. Lancet (London, England). (1998) 352:167–72. doi: 10.1016/S0140-6736(97)11509-4
122. Martínez-González, MA, Montero, P, Ruiz-Canela, M, Toledo, E, Estruch, R, Gómez-Gracia, E, et al. Yearly attained adherence to Mediterranean diet and incidence of diabetes in a large randomized trial. Cardiovasc Diabetol. (2023) 22:262. doi: 10.1186/s12933-023-01994-2
123. Craig, WJ, and Mangels, AR. Position of the American dietetic Association: vegetarian diets. J Am Diet Assoc. (2009) 109:1266–82. doi: 10.1016/j.jada.2009.05.027
124. Wheeler, ML, Dunbar, SA, Jaacks, LM, Karmally, W, Mayer-Davis, EJ, Wylie-Rosett, J, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. (2012) 35:434–45. doi: 10.2337/dc11-2216
125. Krauss, RM, Eckel, RH, Howard, B, Appel, LJ, Daniels, SR, Deckelbaum, RJ, et al. AHA dietary guidelines: revision 2000: a statement for healthcare professionals from the nutrition Committee of the American Heart Association. Stroke. (2000) 31:2751–66. doi: 10.1161/01.STR.31.11.2751
126. Harsha, DW, Lin, PH, Obarzanek, E, Karanja, NM, Moore, TJ, and Caballero, B. Dietary approaches to stop hypertension: a summary of study results. DASH collaborative research group. J Am Diet Assoc. (1999) 99:S35–9. doi: 10.1016/S0002-8223(99)00414-9
127. Tsapas, A, Avgerinos, I, Karagiannis, T, Malandris, K, Manolopoulos, A, Andreadis, P, et al. Comparative effectiveness of glucose-lowering drugs for type 2 Diabetes: a systematic review and network Meta-analysis. Ann Intern Med. (2020) 173:278–86. doi: 10.7326/M20-0864
128. Tsapas, A, Karagiannis, T, Kakotrichi, P, Avgerinos, I, Mantsiou, C, Tousinas, G, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. (2021) 23:2116–24. doi: 10.1111/dom.14451
129. Botros, FT, Gerstein, HC, Malik, R, Nicolay, C, Hoover, A, Turfanda, I, et al. Dulaglutide and kidney function-related outcomes in type 2 Diabetes: a REWIND post hoc analysis. Diabetes Care. (2023) 46:1524–30. doi: 10.2337/dc23-0231
130. Herrington, WG, and Haynes, R. Diabetic kidney disease - Semaglutide flows into the mainstream. N Engl J Med. (2024) 391:178–9. doi: 10.1056/NEJMe2406408
131. Perkovic, V, Jardine, MJ, Neal, B, Bompoint, S, Heerspink, HJL, Charytan, DM, et al. Canagliflozin and renal outcomes in type 2 Diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
132. Lu, Q, Yang, L, Xiao, JJ, Liu, Q, Ni, L, Hu, JW, et al. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic Biol Med. (2023) 195:89–102. doi: 10.1016/j.freeradbiomed.2022.12.088
133. Wang, YH, Chang, DY, Zhao, MH, and Chen, M. Dapagliflozin alleviates diabetic kidney disease via hypoxia inducible factor 1α/Heme oxygenase 1-mediated Ferroptosis. Antioxid Redox Signal. (2024) 40:492–509. doi: 10.1089/ars.2022.0169
134. Verma, S, David, JP, Leiter, LA, Michelsen, MM, Rasmussen, S, and Bhatt, DL. Semaglutide reduces the risk of major adverse cardiovascular events consistently across baseline triglyceride levels in patients with type 2 diabetes: post hoc analyses of the SUSTAIN 6 and PIONEER 6 trials. Diabetes Obes Metab. (2023) 25:2388–92. doi: 10.1111/dom.15081
135. Verma, S, Bain, SC, Buse, JB, Idorn, T, Rasmussen, S, Ørsted, DD, et al. Occurence of first and recurrent major adverse cardiovascular events with Liraglutide treatment among patients with type 2 Diabetes and high risk of cardiovascular events: a post hoc analysis of a randomized clinical trial. JAMA Cardiol. (2019) 4:1214–20. doi: 10.1001/jamacardio.2019.3080
136. Stenberg, E, and Näslund, E. Major adverse cardiovascular events among patients with type-2 diabetes, a nationwide cohort study comparing primary metabolic and bariatric surgery to GLP-1 receptor agonist treatment. Int J Obes. (2023) 47:251–6. doi: 10.1038/s41366-023-01254-z
137. Weir, MR, Gogate, J, Damaraju, CV, Correa-Rotter, R, and Mahaffey, KW. Effects of canagliflozin on major adverse cardiovascular events by baseline estimated glomerular filtration rate: pooled Hispanic subgroup analyses from the CANVAS program and CREDENCE trial. Diabetes Obes Metab. (2022) 24:12–20. doi: 10.1111/dom.14539
138. Htoo, PT, Tesfaye, H, Schneeweiss, S, Wexler, DJ, Everett, BM, Glynn, RJ, et al. Cardiorenal effectiveness of empagliflozin vs. glucagon-like peptide-1 receptor agonists: final-year results from the EMPRISE study. Cardiovasc Diabetol. (2024) 23:57. doi: 10.1186/s12933-024-02150-0
139. Roumie, CL, Chipman, J, Min, JY, Hackstadt, AJ, Hung, AM, Greevy, RA Jr, et al. Association of Treatment with Metformin vs sulfonylurea with major adverse cardiovascular events among patients with Diabetes and reduced kidney function. JAMA. (2019) 322:1167–77. doi: 10.1001/jama.2019.13206
140. Wilcox, R, Kupfer, S, and Erdmann, E. Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: results from PROspective pioglitAzone clinical trial in macro vascular events (PROactive 10). Am Heart J. (2008) 155:712–7. doi: 10.1016/j.ahj.2007.11.029
141. Rådholm, K, Figtree, G, Perkovic, V, Solomon, SD, Mahaffey, KW, de Zeeuw, D, et al. Canagliflozin and heart failure in type 2 Diabetes mellitus: results from the CANVAS program. Circulation. (2018) 138:458–68. doi: 10.1161/CIRCULATIONAHA.118.034222
142. Anker, SD, Butler, J, Filippatos, G, Ferreira, JP, Bocchi, E, Böhm, M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
143. McMurray, JJV, Solomon, SD, Inzucchi, SE, Køber, L, Kosiborod, MN, Martinez, FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
144. Cannon, CP, Pratley, R, Dagogo-Jack, S, Mancuso, J, Huyck, S, Masiukiewicz, U, et al. Cardiovascular outcomes with Ertugliflozin in type 2 Diabetes. N Engl J Med. (2020) 383:1425–35. doi: 10.1056/NEJMoa2004967
145. Scirica, BM, Bhatt, DL, Braunwald, E, Steg, PG, Davidson, J, Hirshberg, B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. (2013) 369:1317–26. doi: 10.1056/NEJMoa1307684
Keywords: bariatric surgery, type 2 diabetes mellitus, recurrent weight gain, glucagon-like peptide-1, conversional surgery
Citation: Xiao H, Du Y, Tan Y and Ren Y (2024) Prognosticating post-bariatric surgery outcomes and management of postoperative recurrent weight gain and diabetes recurrence. Front. Nutr. 11:1510403. doi: 10.3389/fnut.2024.1510403
Edited by:
Mohamed Hany, Alexandria University, EgyptReviewed by:
Qianyi Wan, Sichuan University, ChinaFelipe Chaim, State University of Campinas, Brazil
Mohamed Hossameldin Hussein Rushdy Zidan, Alexandria University, Egypt
Copyright © 2024 Xiao, Du, Tan and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yixing Ren, eWl4aW5ncmVuQG5zbWMuZWR1LmNu
†These authors have contributed equally to this work
 He Xiao
He Xiao Yudie Du1,2†
Yudie Du1,2† Yixing Ren
Yixing Ren