- 1Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Department of Nutrition and Food Hygiene, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 3Department of Preventive Medicine, School of Public Health, Guangdong Medical University, Dongguan, Guangdong, China
Background and objective: Previous studies have shown positive associations of waist circumference (WC) and waist-to-height ratio (WHtR) with left ventricular hypertrophy (LVH) among children and adolescents. However, most of these studies were cross-sectional or limited to only two time points. We aim to estimate the association of trajectories in WC and WHtR with LVH during childhood.
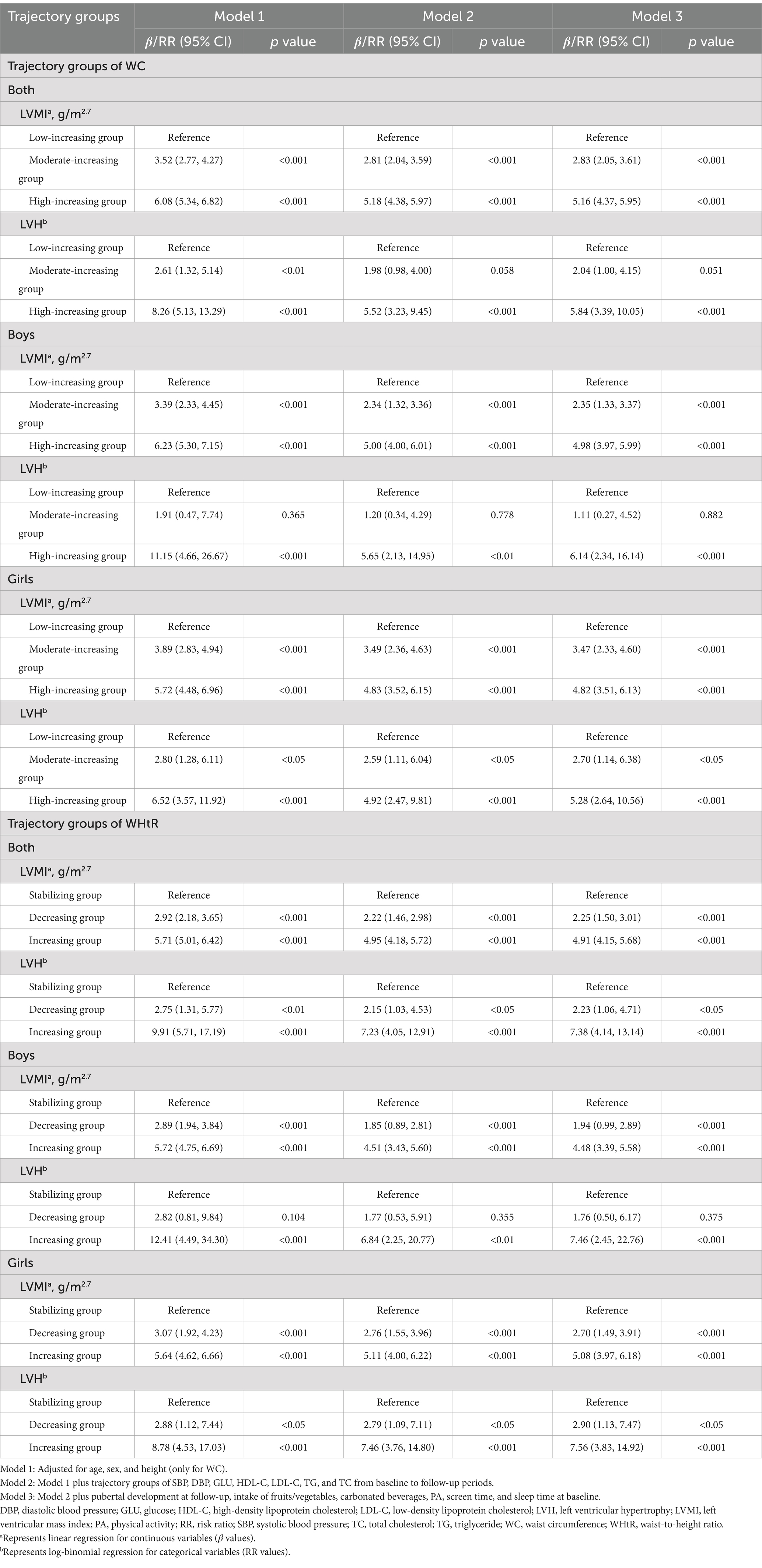
Methods: Data were from the prospective “Huantai Childhood Cardiovascular Health Cohort Study” conducted from 2017 to 2023 in Huantai County, Zibo City, Shandong Province. Group-based trajectory modeling was used to categorize WC into three groups: low-increasing, moderate-increasing, and high-increasing trajectories. Similarly, WHtR was categorized into three groups: stabilizing, decreasing, and increasing trajectories. Linear and log-binomial regression analyses were used to examine the associations of WC and WHtR trajectories with increased left ventricular mass index (LVMI) and LVH.
Results: A total of 946 children were included, with 51.9% being boys and an average age of 8 years at baseline. After adjustment for potential covariates, children in the high-increasing WC group and the increasing WHtR trajectory group had increased LVMI (β = 5.16 g/m2.7, 95% confidence interval (CI): 4.37, 5.95 and β = 4.91 g/m2.7, 95% CI: 4.15, 5.68) and a higher risk of LVH [risk ratio (RR) = 5.84, 95% CI: 3.39, 10.05 and RR = 7.38, 95% CI: 4.14, 13.14] compared to the low-increasing WC group and stabilizing WHtR group, respectively. Interestingly, the moderate-increasing WC and decreasing WHtR trajectory groups still have an increased LVMI (β = 2.83 g/m2.7, 95% CI: 2.05, 3.61 and β = 2.25 g/m2.7, 95% CI: 1.50, 3.01) and a higher risk of LVH (RR = 2.04, 95% CI: 1.00, 4.15 and RR = 2.23, 95% CI: 1.06, 4.71) compared to the low-increasing WC group and stabilizing WHtR group, respectively. Similar results were found when stratified by sex.
Conclusion: We found the risk of LVH was not fully eliminated among children with a decreasing WHtR trajectory. These findings underscore the need for early prevention and continuous monitoring of WC and WHtR to help prevent future sub-clinical cardiovascular damage in childhood.
Introduction
Left ventricular hypertrophy (LVH), an extreme increase in left ventricular mass (LVM), is a significant risk factor and predictor for cardiovascular morbidity and mortality (1, 2). The identification of cardiovascular risk factors associated with LVH is important, particularly for children and adolescents, because early organ damage may already occur in this age group (3, 4). Fortunately, evidence indicates that adverse ventricular remodeling can be reversed with early intervention (5, 6). Therefore, it is necessary to identify the risk factors that contribute to LVH regression, thereby implementing targeted preventive and therapeutic strategies.
Numerous studies have shown that general obesity, defined by body mass index (BMI), was associated with LVH in children and adolescents (7–10). However, BMI fails to effectively distinguish the distribution between fat mass and fat-free mass. Studies have indicated that waist circumference (WC) and waist-to-height ratio (WHtR), as markers of central obesity, were superior to BMI and more closely associated with cardio-metabolic risk (11, 12). Several studies have shown that central obesity, defined by WC or WHtR, is associated with LVH among children and adolescents (13–21). However, most previous research was cross-sectional or limited to only two time points, which may overestimate or underestimate the true associations because participants who experienced significant WC or WHtR changes over time may eventually return to their initial status. Therefore, it is necessary to capture the full changes in trajectories of WC or WHtR to better understand their associations with LVH, ultimately leading to more effective and targeted interventions.
In this study, we aimed to clarify the association between different trajectories of WC or WHtR measured at four time points and the risk of LVH based on a population-based prospective study. In addition, we aim to explore the effects of gender differences in WC or WHtR trajectories on cardiac damage.
Methods
Data were from the prospective “Huantai Childhood Cardiovascular Health Cohort Study” conducted in Huantai County, Zibo City, Shandong Province, China (22). Participants were recruited in a large local primary school using a convenient cluster sampling design. The baseline survey was conducted between November 2017 and January 2018 where 1,515 children aged 6–11 years were recruited at baseline and followed up every 2 years (i.e., 2017, 2019, 2021, and 2023). After excluding children and adolescents with missing information on anthropometric measurements, lifestyle behaviors, blood biochemical markers, ultrasound measurements, or WC/WHtR measurements at any time points, 946 children were finally included in the study (Supplementary Figure S1). Supplementary Table S1 presents a comparison of the baseline characteristics between included and excluded participants. The study was approved by the Ethics Committee of the School of Public Health, Shandong University (Approval No. 20160308). All participants voluntarily joined the study, with informed consent provided by their guardians.
Height, weight, and WC were measured twice using standardized calibrated instruments, and the mean values were used for analysis. Blood pressure (BP, HEM 7012, Omron, Osaka, Japan) was measured three times, with differences of less than 10 mmHg between any two measurements, and the mean values of the last two readings were used for data analysis. These methods have been reported in detail previously (22). BMI (kg/m2) was calculated as weight (kg) divided by height (m) squared. WHtR was calculated as WC (cm) divided by height (cm). We categorize WC into three groups: low-increasing, moderate-increasing, and high-increasing trajectories. Similarly, we classified WHtR into three groups: stabilizing, decreasing, and increasing trajectories.
A structured self-reported questionnaire was used to collect data on demographics (e.g., age and sex), pubertal development (e.g., menarche for girls or spermatogenesis for boys), and lifestyle behaviors (e.g., intake of fruits and vegetables, intake of carbonated beverages, physical activity (PA), screen time, and sleep duration). The questionnaires were carried out in classrooms by investigators with standardized training, and respondents were personally informed by instructions. The forms were filled out by parents who assisted the children and once completed, they were independently reviewed and verified by two staff members. To ensure reliability, a random selection of 13 students retested the questionnaires 1 week later. The results demonstrated strong consistency between the initial and retested measurements, as evidenced by kappa values greater than 0.8 for each item related to dietary condition (22). The pubertal development variable was categorized as “yes” if boys experienced seminal emission or girls experienced menstruation, and “no” if they did not. Insufficient intake of fruits/vegetables was defined as consuming less than 5 servings per day (23). Frequent intake of carbonated beverages was defined as consuming more than once per week (23). Insufficient PA was defined as less than 1 h of combined vigorous and moderate exercise per day (24). Excessive screen time was defined as more than 2 h of screen exposure per day (25). Insufficient sleep was defined as less than 9 h (6–12 years old) or less than 8 h (13–18 years old) per day (26). An automatic analyzer (Beckman Coulter, AU480) was used to measure blood biochemical markers [e.g., glucose (GLU), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)].
Before the study, 20 subjects were selected and their measurements were taken by two sonographers: one for quality control and the other for testing. The correlation coefficient of the measured values was 0.94, demonstrating a high level of agreement (27). During the formal study, left ventricular indicators were measured using a validated and calibrated color Doppler ultrasound machine (Philips, CX30, United States) by one experienced sonographer. All procedures strictly adhered to the Pediatric Cardiac Measurement Guidelines of the American Society of Echocardiography (28). The left ventricular end-diastolic diameter (LVDD), interventricular septal thickness (IVST), and left ventricular posterior wall thickness (LVPWT) were assessed using a 2e4 MHz convex array transducer. Based on these measurements and height, the LVMI was calculated using the following formulas (16): LVM (g) = 0.8*1.04*[(IVST+LVDD+LVPWT)3 − (LVDD)3] + 0.6; LVMI (g/m2.7) = LVM/height2.7. LVH was determined as the LVMI higher than the age- and sex-specific 90th percentile of this study population (10, 29).
T-tests and χ2-tests were, respectively, used to assess the differences in continuous and categorical variables between boys and girls at baseline. Group-based trajectory modeling (GBTM) was used to analyze the trajectories of WC and WHtR with the optimal number of groups determined based on the Bayesian information criterion (BIC), average posterior probability (AvePP), and the proportion of individuals in each trajectory group. Analysis of variance (ANOVA) and χ2-test were used to assess the differences in continuous and categorical variables across trajectory groups, respectively. We used linear regression to examine the associations of WC or WHtR trajectories from 2017 to 2023 with LVMI at the end of follow-up in three models and used log-binomial regression to assess their associations with LVH. Model 1 adjusted for age, sex, and height (only for WC); Model 2 further adjusted for trajectory groups of systolic BP (SBP), diastolic BP (DBP), GLU, HDL-C, LDL-C, TG, and TC from baseline to follow-up periods; Model 3 additionally adjusted for pubertal development at follow-up, intake of fruits/vegetables, intake of carbonated beverages, PA, screen time, and sleep time at baseline. A two-sided p value <0.05 indicated statistical significance. All statistical analyses were performed using R (version 4.3.3).
Results
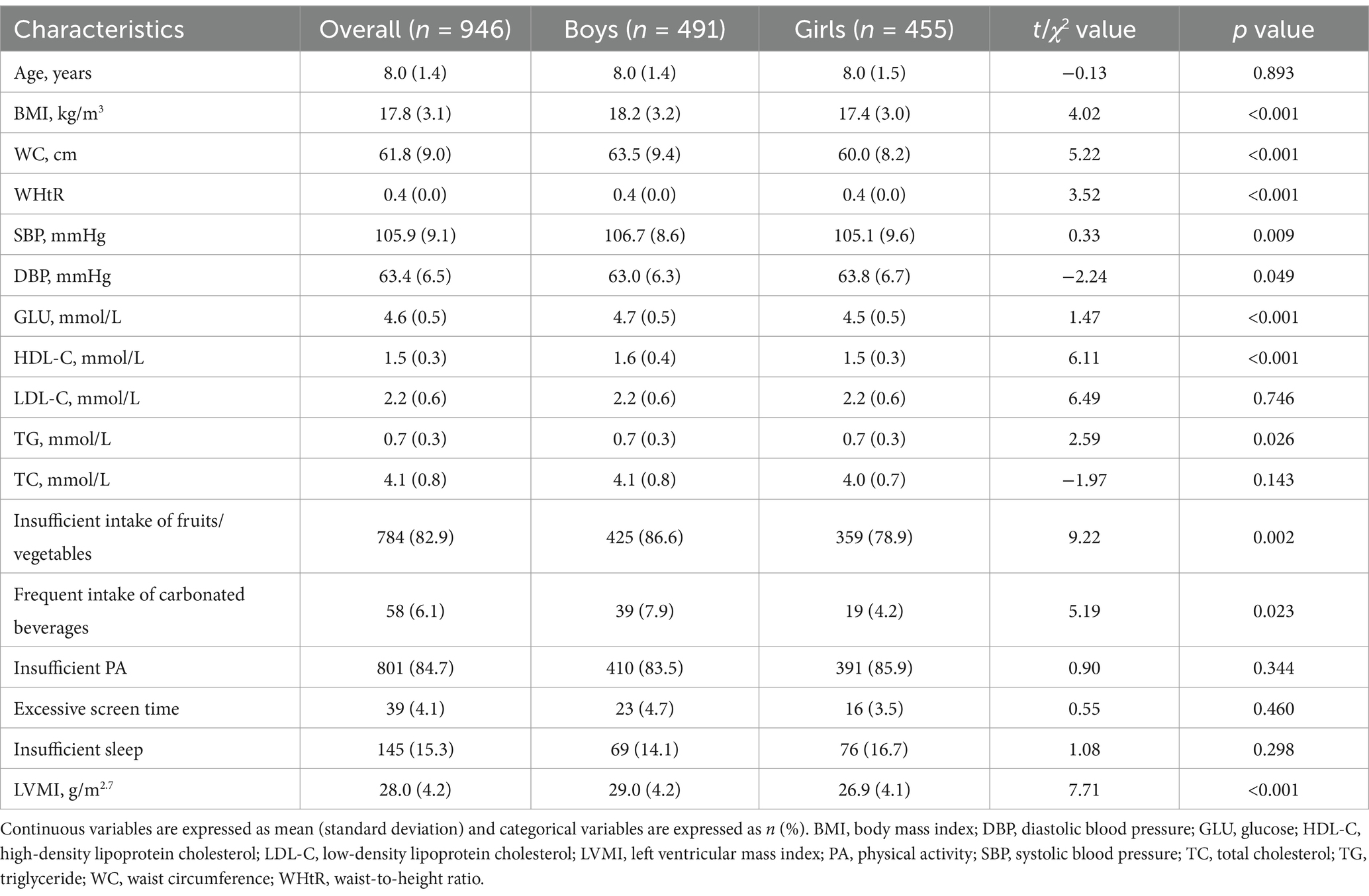
In this study, a total of 946 children were included, with 51.9% being boys and an average age of 8 years at baseline (Table 1). At baseline, boys had higher values of BMI, WC, WHtR, SBP, GLU, HDL-C, insufficient intake of fruits/vegetables, frequent intake of carbonated beverages, and LVMI compared to girls (all p values <0.05).
Supplementary Table S2 presents the details of the GBTM of trajectory groupings. Based on the BIC, the proportion of AvePP>0.7, and the proportion of individuals in each trajectory group, we selected a three-trajectory grouping for both WC and WHtR. From 2017 to 2023, 54.2, 18.7, and 27.1% of children exhibited low-increasing, moderate-increasing, and high-increasing WC trajectory patterns, respectively (Figure 1A). The moderate-increasing and high-increasing WC trajectory groups had a significantly higher proportion of boys, as well as higher baseline values of BMI, WC, WHtR, SBP, DBP, GLU, LDL-C, TG, LVMI, and LVH compared to the low-increasing group (all p values <0.05, Table 2). During the same period, 52.9, 19.0, and 28.1% of children exhibited stabilizing, decreasing, and increasing WHtR trajectory patterns, respectively (Figure 1B). The decreasing and increasing WHtR groups had a significantly higher proportion of boys, as well as higher baseline values of BMI, WC, WHtR, SBP, DBP, GLU, LDL-C, TG, LVMI, and LVH compared to the stabilizing group (all p values <0.05, Table 3). At the end of follow-up, similar differences in characteristics across trajectory groups were found (Tables 2, 3).
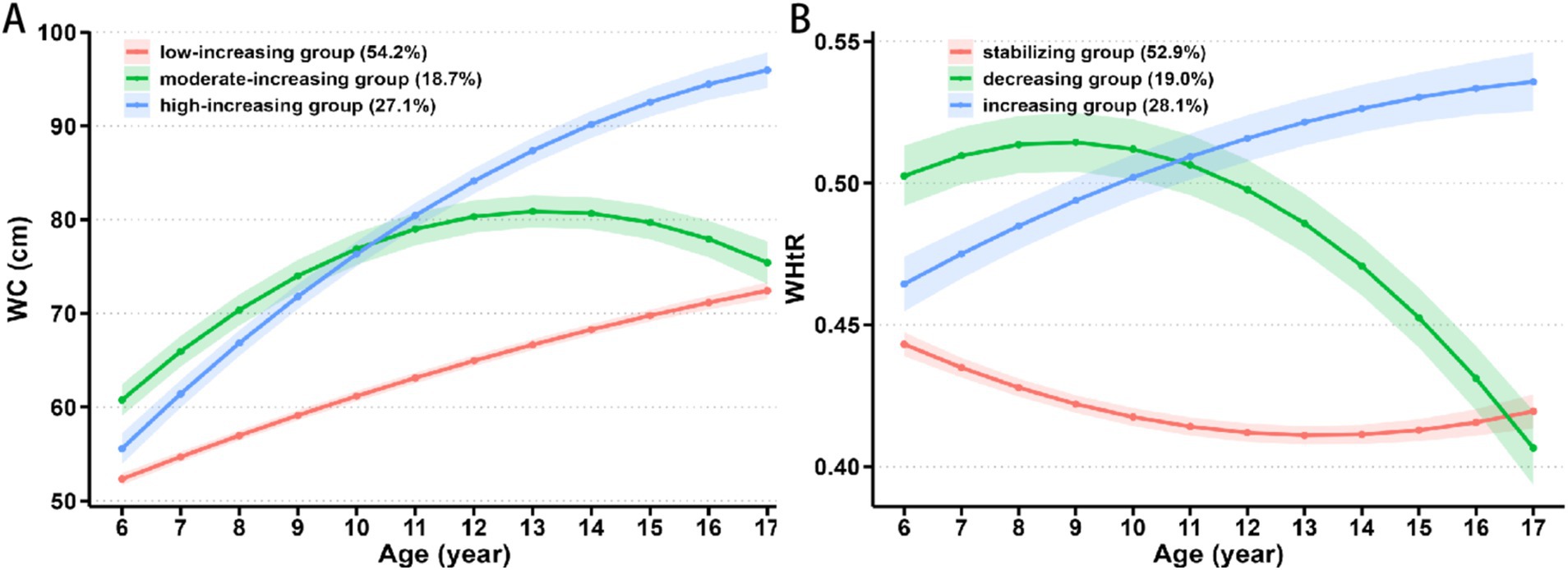
Figure 1. Change in trajectories of (A) WC and (B) WHtR with age. WC, waist circumference; WHtR, waist-to-height ratio.

Table 2. Characteristics of baseline (2017) and follow-up (2023) periods of study participants in different WC trajectory subgroups.
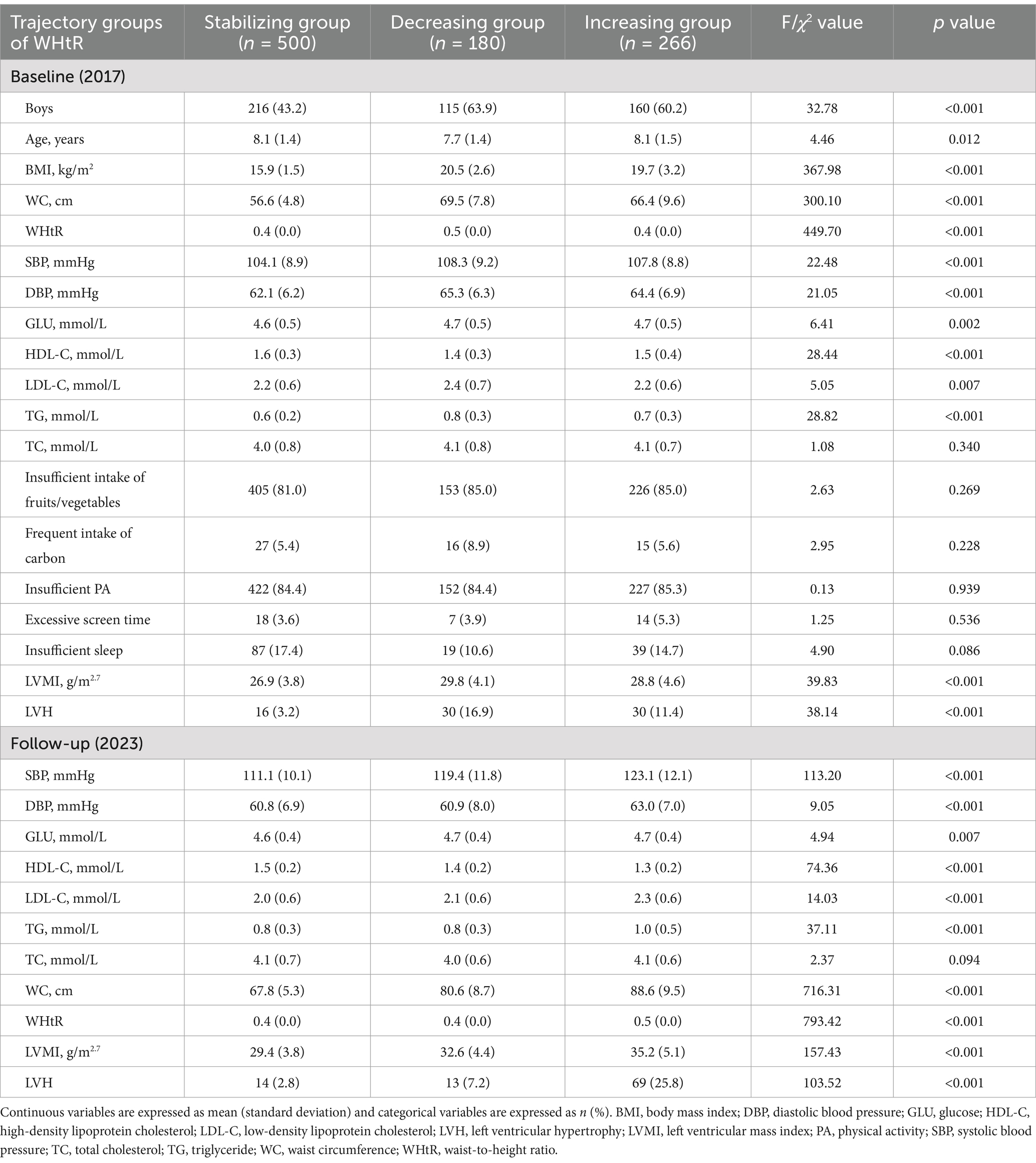
Table 3. Characteristics of baseline (2017) and follow-up (2023) periods of study participants in different WHtR trajectory subgroups.
Children in the moderate-increasing and high-increasing WC trajectory groups had a higher LVMI [β = 2.83 g/m2.7, 95% confidence interval (CI): 2.05, 3.61 and β = 5.16 g/m2.7, 95% CI: 4.37, 5.95] and a higher risk of LVH [risk ratio (RR) = 2.04, 95% CI: 1.00, 4.15 and RR = 5.84, 95% CI: 3.39, 10.05] compared to the low-increasing group (Table 4). Similarly, in the WHtR trajectory groups, the decreasing group and increasing group had a higher LVMI (β = 2.25 g/m2.7, 95% CI: 1.50, 3.01 and β = 4.91 g/m2.7, 95% CI: 4.15, 5.68) and a higher risk of LVH (RR = 2.23, 95% CI: 1.06, 4.71 and RR = 7.38, 95% CI: 4.14, 13.14) compared to the stabilizing group. Similar results were found when stratified by gender (almost all p values <0.05, Table 4).
Discussion
To the best of our knowledge, this is the first study to estimate the association of WC and WHtR trajectories with LVH during childhood. We found that rapid increases in either WC or WHtR during childhood significantly increase the subsequent risk of LVH. However, the risk of LVH was not fully eliminated among children who had a decreasing WHtR trajectory. These findings not only enrich the current understanding of the impact of childhood central obesity on cardiac damage but also aid in the precise identification of high-risk individuals, thereby enabling more targeted prevention strategies.
Previous studies have evaluated the association between central obesity and LVMI (13, 15–17, 19, 21, 30). For example, an observational study of Italian children aged 7–16 years (n = 63) showed that high WC and WHtR were associated with an increased risk of LVH (13). Similarly, another Italian cohort study of 459 obese children with a mean age of 10.6 years demonstrated a positive association between WHtR and higher LVMI (15). Our previous two-year follow-up study on Chinese children aged 6–11 years also demonstrated that persistently high WC at baseline and follow-up had higher LVMI and higher odds of LVH (16). However, prior studies have been cross-sectional or limited to two time points, often limited by small sample sizes and inadequate control of covariates such as dietary habits, PA, changes in blood lipids, and blood glucose, on fluctuations in WC or WHtR, potentially leading to overestimation or underestimation of the true associations (6, 31). Therefore, prospective studies with multiple time points and adequate control of covariates are needed to better understand the temporal changes of central obesity and the risk of cardiac damage.
In this study, we examined the association between trajectory changes in WC or WHtR during childhood with four time points and subsequent LVH with the adjustment of full covariates. As expected (16, 32, 33), we found that both the high-increasing WC and the increasing WHtR trajectory groups were associated with a higher risk of LVH. However, we observed that the risk of LVH was reduced but not fully eliminated in the moderate-increasing WC and the decreasing WHtR trajectory groups, regardless of sex. Two prospective longitudinal cohort studies similarly showed that a moderate increase in WC and a decrease in WHtR during childhood were associated with an increased risk of carotid intima-media thickness and Type-2 diabetes in adulthood (34, 35). Inconsistent with this study, a previous two-year follow-up study showed that the increased odds of LVH could be eliminated among children who had high WC but achieved normal WC 2 years later (16). A 12-month intervention study of 86 children and adolescents aged 5–17 years with essential hypertension found that reductions in WHtR and WC predicted a decrease in LVMI (36). A cohort study of 2,898 Portuguese adolescents showed that decreasing WC trajectory from 13 to 21 years was associated with increased risk of SBP, DBP, insulin resistance, and TG among males, but not females (37). This discrepancy may be mainly attributed to differences in population, outcomes, and classifications of WC or WHtR (i.e., the previous study classified central obesity versus normal status based on two time points, which might have ignored weight regain vs. reduced WC trajectories in this study). Additionally, factors such as dietary and PA habits, as well as changes in blood lipids, glucose, and BP, might affect LVH (38). After adjusting for these covariates, we still found an increased risk of LVH among those with a moderate-increasing WC trajectory or a decreasing WHtR trajectory. These suggest that an increase in WC or WHtR can independently increase the risk of LVH among children and persist even if these measures are later reduced. These findings emphasize the importance of maintaining a healthy WC from an early age to prevent the development of sub-clinical cardiovascular damage. Adopting a healthy diet, increasing PA, reducing sedentary time, and ensuring adequate sleep can aid in maintaining a healthy WC (39, 40).
To the best of our knowledge, this is the first study examining the association of WC and WHtR trajectories with LVMI in childhood. However, several limitations must be acknowledged. First, it used convenience sampling by selecting students from a specific elementary school, therefore limiting the generalizability of the findings. Second, the influence of unmeasured confounders, such as family history of cardiovascular disease and air pollutants might have influenced both adiposity and cardiac structural changes over time. However, the aforementioned and other unmeasured confounders could have attenuated the associations between adiposity and cardiac structural changes toward the null. Third, covariates such as lifestyle behaviors were obtained through self-report, potentially introducing information bias. Fourth, the analysis was limited to gender stratification due to the small sample size.
In conclusion, this study demonstrated that rapid growth in WC and WHtR during childhood can predict subsequent LVH, and the risk persists even if WC and WHtR are later reduced or reversed. These findings underscore the need for early prevention and continuous monitoring of WC and WHtR, along with long-term management of central obesity in children, to help prevent future sub-clinical cardiovascular damage.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the School of Public Health, Shandong University (Approval No. 20160308). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
XJ: Formal analysis, Methodology, Software, Writing – original draft. MZ: Data curation, Writing – review & editing. JS: Conceptualization, Resources, Writing – review & editing. BX: Conceptualization, Data curation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1506191/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Study flowchart. WC, waist circumference; WHtR, waist-to-height ratio.
References
1. Gardin, JM, and Lauer, MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. (2004) 292:2396–8. doi: 10.1001/jama.292.19.2396
2. Levy, D, Garrison, RJ, Savage, DD, Kannel, WB, and Castelli, WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. New Engl J Med. (1990) 322:1561–6. doi: 10.1056/NEJM199005313222203
3. Mangner, N, Scheuermann, K, Winzer, E, Wagner, I, Hoellriegel, R, Sandri, M, et al. Childhood obesity: impact on cardiac geometry and function. J Am Coll Cardiol Img. (2014) 7:1198–205. doi: 10.1016/j.jcmg.2014.08.006
4. Di Bonito, P, Moio, N, Scilla, C, Cavuto, L, Sibilio, G, Forziato, C, et al. Preclinical manifestations of organ damage associated with the metabolic syndrome and its factors in outpatient children. Atherosclerosis. (2010) 213:611–5. doi: 10.1016/j.atherosclerosis.2010.09.017
5. Kim, GH, Uriel, N, and Burkhoff, D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. (2018) 15:83–96. doi: 10.1038/nrcardio.2017.139
6. Martin, TG, Juarros, MA, and Leinwand, LA. Regression of cardiac hypertrophy in health and disease: mechanisms and therapeutic potential. Nat Rev Cardiol. (2023) 20:347–63. doi: 10.1038/s41569-022-00806-6
7. Bastien, M, Poirier, P, Lemieux, I, and Després, JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. (2014) 56:369–81. doi: 10.1016/j.pcad.2013.10.016
8. Sorof, J, and Daniels, S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. (2002) 40:441–7. doi: 10.1161/01.HYP.0000032940.33466.12
9. Pieruzzi, F, Antolini, L, Salerno, FR, Giussani, M, Brambilla, P, Galbiati, S, et al. The role of blood pressure, body weight and fat distribution on left ventricular mass, diastolic function and cardiac geometry in children. J Hypertens. (2015) 33:1182–92. doi: 10.1097/HJH.0000000000000552
10. Hietalampi, H, Pahkala, K, Jokinen, E, Rönnemaa, T, Viikari, JS, Niinikoski, H, et al. Left ventricular mass and geometry in adolescence: early childhood determinants. Hypertension. (2012) 60:1266–72. doi: 10.1161/HYPERTENSIONAHA.112.194290
11. Czernichow, S, Kengne, AP, Stamatakis, E, Hamer, M, and Batty, GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. (2011) 12:680–7. doi: 10.1111/j.1467-789X.2011.00879.x
12. Zong, X, Kelishadi, R, Hong, YM, Schwandt, P, Matsha, TE, Mill, JG, et al. Establishing international optimal cut-offs of waist-to-height ratio for predicting cardiometabolic risk in children and adolescents aged 6–18 years. BMC Med. (2023) 21:442. doi: 10.1186/s12916-023-03169-y
13. Vizzuso, S, Del Torto, A, Fiore, G, Carugo, S, Zuccotti, G, and Verduci, E. Tri-ponderal mass index and left ventricular hypertrophy in a cohort of caucasian children and adolescents with obesity. Ital J Pediatr. (2024) 50:75. doi: 10.1186/s13052-024-01634-9
14. Wang, H, Zhao, M, Magnussen, CG, and Xi, B. Utility of three adiposity indices for identifying left ventricular hypertrophy and geometric remodeling in Chinese children. Front Endocrinol (Lausanne). (2021) 12:762250. doi: 10.3389/fendo.2021.762250
15. Genovesi, S, Tassistro, E, Giussani, M, Lieti, G, Patti, I, Orlando, A, et al. Association of obesity phenotypes with left ventricular mass index and left ventricular hypertrophy in children and adolescents. Front Endocrinol (Lausanne). (2022) 13:1006588. doi: 10.3389/fendo.2022.1006588
16. Wang, H, Zhao, M, Magnussen, CG, and Xi, B. Change in waist circumference over 2 years and the odds of left ventricular hypertrophy among Chinese children. Nutr Metab Cardiovasc Dis. (2021) 31:2484–9. doi: 10.1016/j.numecd.2021.04.027
17. Zhang, Y, Zhao, M, Bovet, P, and Xi, B. Association of abdominal obesity and high blood pressure with left ventricular hypertrophy and geometric remodeling in Chinese children. Nutr Metab Cardiovasc Dis. (2021) 31:306–13. doi: 10.1016/j.numecd.2020.09.007
18. Mehta, SK. Waist circumference to height ratio and left ventricular mass in children and adolescents. Cardiol Young. (2016) 26:658–62. doi: 10.1017/S1047951115000803
19. Trandafir, LM, Russu, G, Moscalu, M, Miron, I, Lupu, VV, Leon Constantin, MM, et al. Waist circumference a clinical criterion for prediction of cardio-vascular complications in children and adolescences with overweight and obesity. Medicine (Baltimore). (2020) 99:e20923. doi: 10.1097/MD.0000000000020923
20. Mehta, SK. Left ventricular mass in children and adolescents with elevated body mass index and normal waist circumference. Am J Cardiol. (2014) 113:1054–7. doi: 10.1016/j.amjcard.2013.11.068
21. Di Bonito, P, Moio, N, Sibilio, G, Cavuto, L, Sanguigno, E, Forziato, C, et al. Cardiometabolic phenotype in children with obesity. J Pediatr. (2014) 165:1184–9. doi: 10.1016/j.jpeds.2014.08.007
22. Yang, LL, Zhang, Q, Zhang, YQ, Sun, JH, Zhao, M, and Xi, B. Design of Huantai Childhood Cardiovascular Health Cohort Study. Chin J Prev Med. (2020) 54:1461–4. doi: 10.3760/cma.j.cn112150-20200610-00857
23. Wang, H, Sun, J, Zhang, Z, Yang, L, Zhao, M, Bovet, P, et al. Waist circumference change and risk of high carotid intima-media thickness in a cohort of Chinese children. J Hypertens. (2021) 39:1901–7. doi: 10.1097/HJH.0000000000002881
24. World Health Organization. (2024) Global recommendations on physical activity for health. Available at: https://wwwwhoint/publications/i/item/9789241599979 (accessed August 5, 2024).
25. Council on Communications and Media. Children, adolescents, and the media. Pediatrics. (2013) 132:958–61. doi: 10.1542/peds.2013-2656
26. Paruthi, S, Brooks, LJ, D'Ambrosio, C, Hall, WA, Kotagal, S, Lloyd, RM, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of sleep medicine. J Clin Sleep Med. (2016) 12:785–6. doi: 10.5664/jcsm.5866
27. Ma, CW, Yang, L, Zhao, M, and Xi, B. Association of abdominal obesity and obesity types with carotid intima-media thickness in children in China. Chin J Epidemiol. (2020) 41:1450–4. doi: 10.3760/cma.j.cn112338-20200225-00171
28. Lang, RM, Bierig, M, Devereux, RB, Flachskampf, FA, Foster, E, Pellikka, PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. (2005) 18:1440–63. doi: 10.1016/j.echo.2005.10.005
29. Geng, Y, Zhang, Q, Zhang, YQ, Yang, LL, Zhao, M, and Xi, B. Association between parental education level and left ventricular hypertrophy in childhood. Chin J Prev Med. (2021) 55:667–71. doi: 10.3760/cma.j.cn112150-20200610-00854
30. Rodicio, MM, Domenech de Miguel, V, Guinda Jiménez, M, Cigarrán Guldrís, S, López Franco, MM, Estany Gestal, A, et al. Early cardiac abnormalities in obese children and their relationship with adiposity. Nutrition. (2018) 46:83–9. doi: 10.1016/j.nut.2017.09.001
31. Dang, K, Wang, X, Hu, J, Zhang, Y, Cheng, L, Qi, X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. (2024) 23:8. doi: 10.1186/s12933-023-02115-9
32. Wang, L, Lee, Y, Wu, Y, Zhang, X, Jin, C, Huang, Z, et al. A prospective study of waist circumference trajectories and incident cardiovascular disease in China: the Kailuan cohort study. Am J Clin Nutr. (2021) 113:338–47. doi: 10.1093/ajcn/nqaa331
33. Klingberg, S, Mehlig, K, Lanfer, A, Björkelund, C, Heitmann, BL, and Lissner, L. Increase in waist circumference over 6 years predicts subsequent cardiovascular disease and total mortality in nordic women. Obesity (Silver Spring). (2015) 23:2123–30. doi: 10.1002/oby.21203
34. Seyedhoseinpour, A, Barzin, M, Mahdavi, M, Valizadeh, M, Azizi, F, and Hosseinpanah, F. Association between BMI trajectories from childhood to early adulthood and the carotid intima-media thickness in early adulthood: Tehran lipid and glucose study. BMC Public Health. (2023) 23:2233. doi: 10.1186/s12889-023-17184-4
35. Carli, MM, Sabo, RT, and Sun, SS. Childhood waist growth curves and adult diabetes. J Dev Orig Health Dis. (2022) 13:656–62. doi: 10.1017/S2040174421000544
36. Litwin, M, Niemirska, A, Sladowska-Kozlowska, J, Wierzbicka, A, Janas, R, Wawer, ZT, et al. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr Nephrol. (2010) 25:2489–99. doi: 10.1007/s00467-010-1626-7
37. Araújo, J, Barros, H, Ramos, E, and Li, L. Trajectories of total and central adiposity throughout adolescence and cardiometabolic factors in early adulthood. Int J Obes. (2016) 40:1899–905. doi: 10.1038/ijo.2016.170
38. Falkner, B, DeLoach, S, Keith, SW, and Gidding, SS. High risk blood pressure and obesity increase the risk for left ventricular hypertrophy in African-American adolescents. J Pediatr. (2013) 162:94–100. doi: 10.1016/j.jpeds.2012.06.009
39. Lister, NB, Baur, LA, Felix, JF, Hill, AJ, Marcus, C, Reinehr, T, et al. Child and adolescent obesity. Nat Rev Dis Primers. (2023) 9:24. doi: 10.1038/s41572-023-00435-4
Keywords: left ventricular hypertrophy, childhood, central obesity, waist circumference, waist-to-height ratio
Citation: Jin X, Zhao M, Sun J and Xi B (2024) Trajectories in waist circumference and waist-to-height ratio with left ventricular hypertrophy in childhood. Front. Nutr. 11:1506191. doi: 10.3389/fnut.2024.1506191
Edited by:
Mariacristina Siotto, IRCCS Don Carlo Gnocchi Firenze, ItalyReviewed by:
Vesna Herceg-Čavrak, Libertas University, CroatiaFeitong Wu, Baker Heart and Diabetes Institute, Australia
Copyright © 2024 Jin, Zhao, Sun and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Xi, eGlibzIwMDdAMTI2LmNvbQ==; Jiahong Sun, amlhaG9uZ3N1bjE5OTBAMTI2LmNvbQ==
 Xuli Jin
Xuli Jin Min Zhao
Min Zhao Jiahong Sun
Jiahong Sun Bo Xi
Bo Xi