- 1Department of Endoscopy, Shijiazhuang Traditional Chinese Medicine Hospital, Shijiazhuang, China
- 2Department of Tuberculosis, Shandong Public Health Clinical Center, Jinan, Shandong, China
Objectives: Gout is associated with hyperuricemia, and serum magnesium levels are negatively correlated with uric acid levels. Magnesium intake is also associated with a reduced risk of hyperuricemia. However, the relationship between the magnesium depletion score (MDS), which represents the systemic magnesium status, and gout is unclear. This study was conducted to investigate the association between MDS and gout as well as explore the impact of dietary magnesium intake on this relationship.
Methods: We analyzed 18,039 adults with gout data who participated in the National Health and Nutrition Examination Survey between 2007 and 2016. Magnesium deficiency status was assessed using the MDS, a comprehensive scoring tool. Considering the possible effects of dietary magnesium intake, weighted multivariable logistic regression and subgroup analyses were used to assess the correlation between MDS and gout.
Results: The overall prevalence of gout among adults in the United States between 2007 and 2016 was 4.7%. After adjusting for confounders, MDS and gout risk showed a significant positive correlation. Individuals with an MDS of 2 and ≥ 3 had higher odds of gout than those with an MDS of 0 (MDS = 2, odds ratio: 1.86 [1.18–2.93], p = 0.008; MDS = 3, odds ratio: 2.17 [1.37–3.43], p = 0.001; p for trend <0.001). Dietary magnesium intake did not moderate the correlation between MDS and gout risk.
Conclusion: A positive correlation exists between magnesium deficiency, as quantified using the MDS, and gout risk among adults in the United States. Additionally, dietary magnesium intake did not alter this association.
1 Introduction
Gout is caused by the deposition of monosodium urate crystals in non-joint and joint structures (1). This disease is characterized by severe joint pain, swelling, warmth, and functional impairment, particularly of the metatarsophalangeal joint of the big toe (2). Depending on the population studied and methodologies employed, reports on the global prevalence and incidence of gout vary widely. The prevalence of gout ranges from <1 to 6.8%, and its incidence ranges from 0.58 to 2.89 cases per 1,000 person-year (3). The prevalence and incidence of gout have increased in recent years (4–6), making it an important public health issue.
Magnesium is an essential element in the human body with a crucial role in supporting and maintaining health and life, and should be continuously replenished through dietary intake and water consumption (7). Magnesium is vital for stabilizing the tertiary structure of DNA and RNA; participating in various enzymatic reactions including energy metabolism and protein synthesis; regulating cellular Mg2+ handling; and influencing cell signaling and proliferation (8–13). However, previous research primarily focused on the effects of diet and serum magnesium on gout. Serum magnesium levels are negatively correlated with uric acid levels (14), and increased magnesium intake is associated with a reduced risk of hyperuricemia (15). Notably, serum Mg2+ levels reflect only approximately 1% of total magnesium content in the body, as most magnesium is stored in the bones, muscles, and soft tissues (7). Moreover, the magnesium content in the body is subject to dynamic fluctuations, which are primarily absorbed through the gastrointestinal tract and excreted via the kidneys (16). Therefore, serum magnesium levels may not accurately represent the overall magnesium status of the body.
The magnesium depletion score (MDS) is a comprehensive scoring tool designed to assess overall magnesium deficiency by aggregating four established risk factors and considering the pathophysiological factors that affect the renal reabsorption capacity, including proton pump inhibitor (PPI) use, diuretic use, alcohol consumption, and renal disease (17). Compared with serum magnesium, the MDS is a more sensitive and reliable measure of an individual’s magnesium deficiency or depletion status (17). Current studies indicate that MDS is significantly associated with various conditions, including systemic inflammation, cardiovascular diseases, renal diseases, metabolic syndrome, hypertension, and diabetes (17–22).
Considering that the association between MDS and gout is unclear, we performed a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES). Our aim was to analyze the association between MDS and gout, thereby establishing a stronger link between magnesium status and gout risk, and to explore whether dietary magnesium moderates this relationship.
2 Materials and methods
2.1 Study population and design
The research data were sourced from the NHANES, conducted by the Centers for Disease Control and Prevention. The NHANES employs a robust, multistage, and stratified sampling method gathering detailed information through interviews and assessments as well as provide valuable insights into demographic and health-related factors of the United States population.
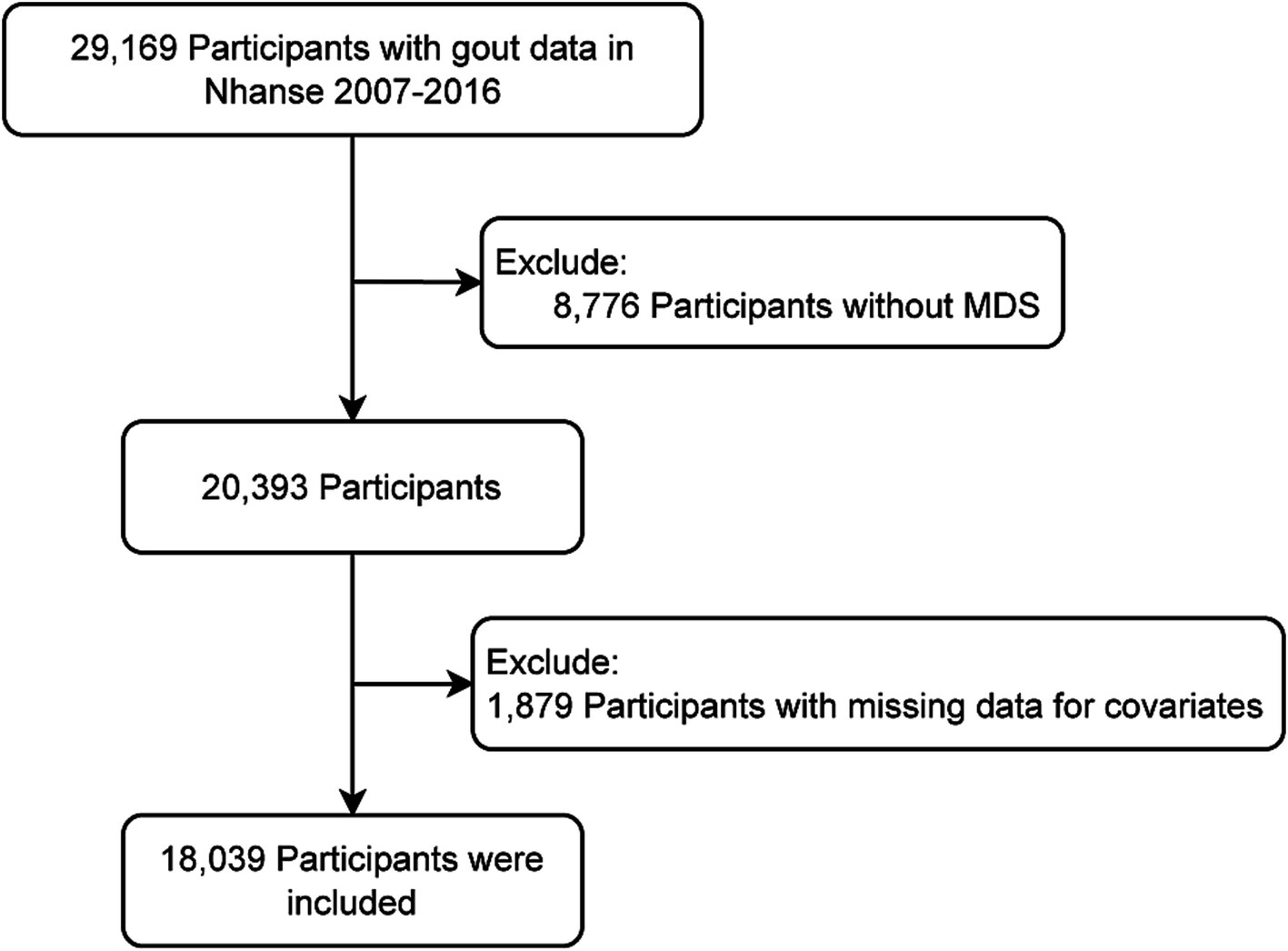
Based on the availability of MDS and gout information, 29,169 participants were initially included from five cycles of NHANES data (2007–2016). Participants who could not have their MDS determined (n = 8,776) or had missing covariates data (n = 2,354) were excluded. Ultimately, 18,039 participants were included (Figure 1).
This study was approved by the Research Ethics Review Committee of the National Center for Health Statistics. Each participant provided written informed consent. The NHANES dataset, along with accompanying documentation and agreements, was obtained from the website.
2.2 MDS assessment
Magnesium depletion score was calculated by summing the scores of the following entries: one point each for current use of diuretics and PPIs, one point for an estimated glomerular filtration rate of 60–90 mL/(min·1.73 m2), two points for an estimated glomerular filtration rate < 60 mL/(min·1.73 m2), and one point for heavy drinking (>2 drinks/day for men, >1 drink/day for women) (17). Using the Chronic Kidney Disease (CKD) Epidemiology Collaboration equation, serum creatinine was used to determine the estimated glomerular filtration rate (23). MDS was classified into four groups: MDS = 0, 1, 2, and ≥ 3.
2.3 Definition of gout
The presence of gout was determined through self-reported response in a medical condition questionnaire (24).
2.4 Covariate assessment
Baseline data included age, sex, race, body mass index (BMI), marital status, smoking status, educational attainment, poverty-to-income ratio, physical activity, dietary magnesium intake, and chronic conditions such as coronary heart disease, diabetes, stroke, and hypertension. Smoking status was categorized into never, former, and current smokers based on whether the participants had smoked <100 cigarettes in their lifetime and their current smoking status. Adequate physical activity was defined as: 75 min per week of vigorous-intensity aerobic activity or at least 150 min per week of moderate-intensity (25). Otherwise, physical activity was inadequate. Magnesium intake was collected from the total nutrient intake provided by the first 24-h dietary recall interview obtained from the Mobile Examination Center. Diabetes was determined by: (1) self-report or medication use; (2) HbA1c level ≥ 6.5%; (3) fasting blood sugar level ≥ 7.0 mmol/L; or (4) 2-h plasma glucose ≥200 mg/dL (26). Hypertension was determined by: (1) self-report or medication use and (2) the average of three systolic blood pressure readings ≥140 mmHg or diastolic blood pressure readings ≥90 mmHg (27). Coronary heart disease and stroke were determined based on self-reported information.
2.5 Statistical analysis
Continuous variables were presented as weighted medians and interquartile ranges or weighted means and standard deviations, whereas categorical variables were expressed as frequencies and weight percentages. To detect differences in baseline characteristics between the gout and control groups, we compared continuous variables using Student’s t-test and categorical variables using Chi-square test. Multivariable adjusted logistic regression and subgroup analyses were used to assess the relationship between MDS and gout. Confounders were selected based on previous research or a change-in-effect estimate of >10%. Three models were assessed: Model 1 was unadjusted; Model 2 included sex and age; and Model 3 was additionally adjusted for race, smoking status, educational attainment, physical activity, BMI, poverty income ratio, dietary magnesium intake, coronary heart disease, diabetes, stroke, and hypertension. All analyses took into account the complex survey design and incorporated sample weights. Sensitivity analysis was performed using complete case analysis, supplemented by multiple imputations (five iterations) to address any missing data. The results were presented as odds ratios with 95% confidence intervals (CIs). A p value <0.05 was considered to indicate statistically significant differences. All data computations were performed using R 4.2.2 and Free Statistics software version 2.0 (28).
3 Results
3.1 Baseline characteristics
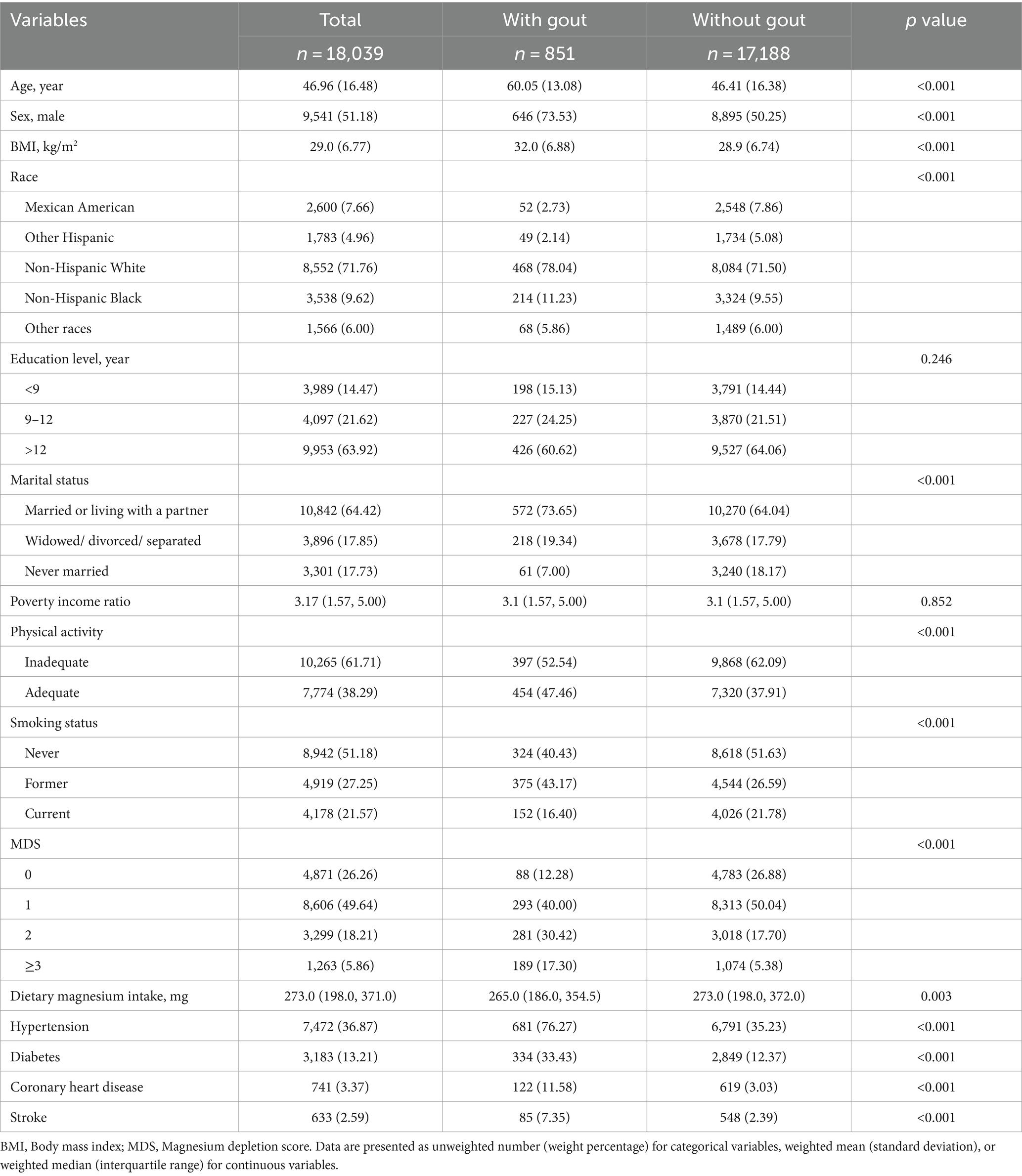
In total, 18,039 participants were included in this study, with 851 and 17,188 participants in the gout and control groups, respectively. The two groups exhibited significant differences in baseline characteristics including age, sex, race, BMI, marital status, physical activity, smoking status, MDS, magnesium intake, and comorbidities (Table 1). Participants with gout were older, more likely to be male, married or living with a partner, have ever smoked, be physically active, and have a higher MDS compared with participants without gout. In addition, participants with gout had a higher risk of diabetes, stroke, hypertension, and coronary heart disease than those without gout did.
3.2 Relationship between MDS and gout
Table 2 shows the association between MDS and gout risk using weighted multivariable logistic regression. After adjusting for confounders, individuals with MDS 1, 2, and ≥ 3 had a 1.45- (95% CI, 0.93–2.26), 1.86- (95% CI, 1.18–2.93), and 2.17-fold (95% CI, 1.37–3.43) increased risk of gout, respectively, compared with participants with an MDS of 0 (p for trend <0.001). Thus, adults with a higher MDS were more likely to develop gout.
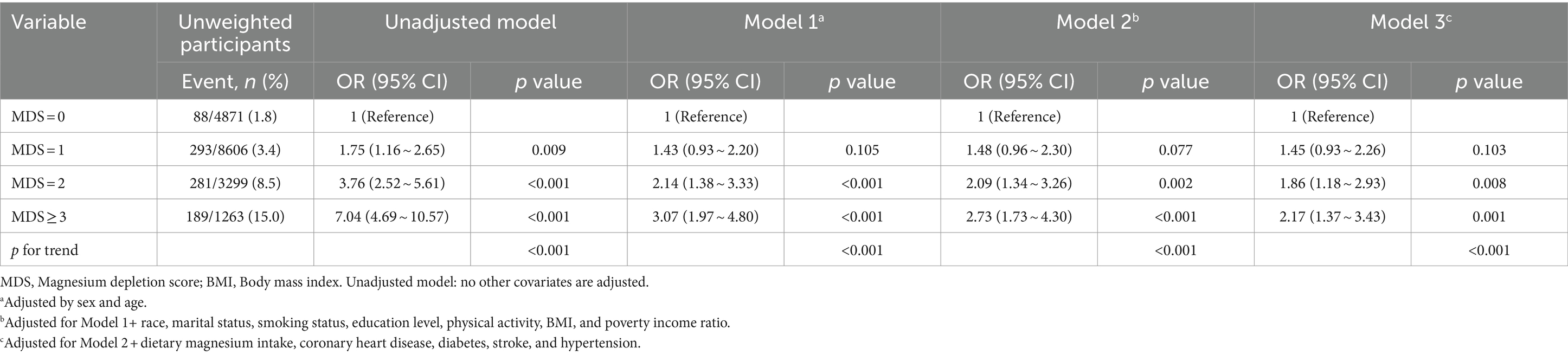
Table 2. Multivariable logistics regression analysis of the association between magnesium depletion score and gout, weighted.
3.3 Sensitivity analysis
To evaluate the stability of the association between MDS and gout within various subgroups, we conducted a subgroup analysis. Interaction tests indicated no statistically significant differences in the association between MDS and gout among different subgroups (Figure 2, p for interaction >0.05), suggesting that these factors did not significantly influence this positive association. Sensitivity analysis revealed that the relationship between MDS and gout was not significantly affected by multiple imputations of missing data (Supplementary Table S1).
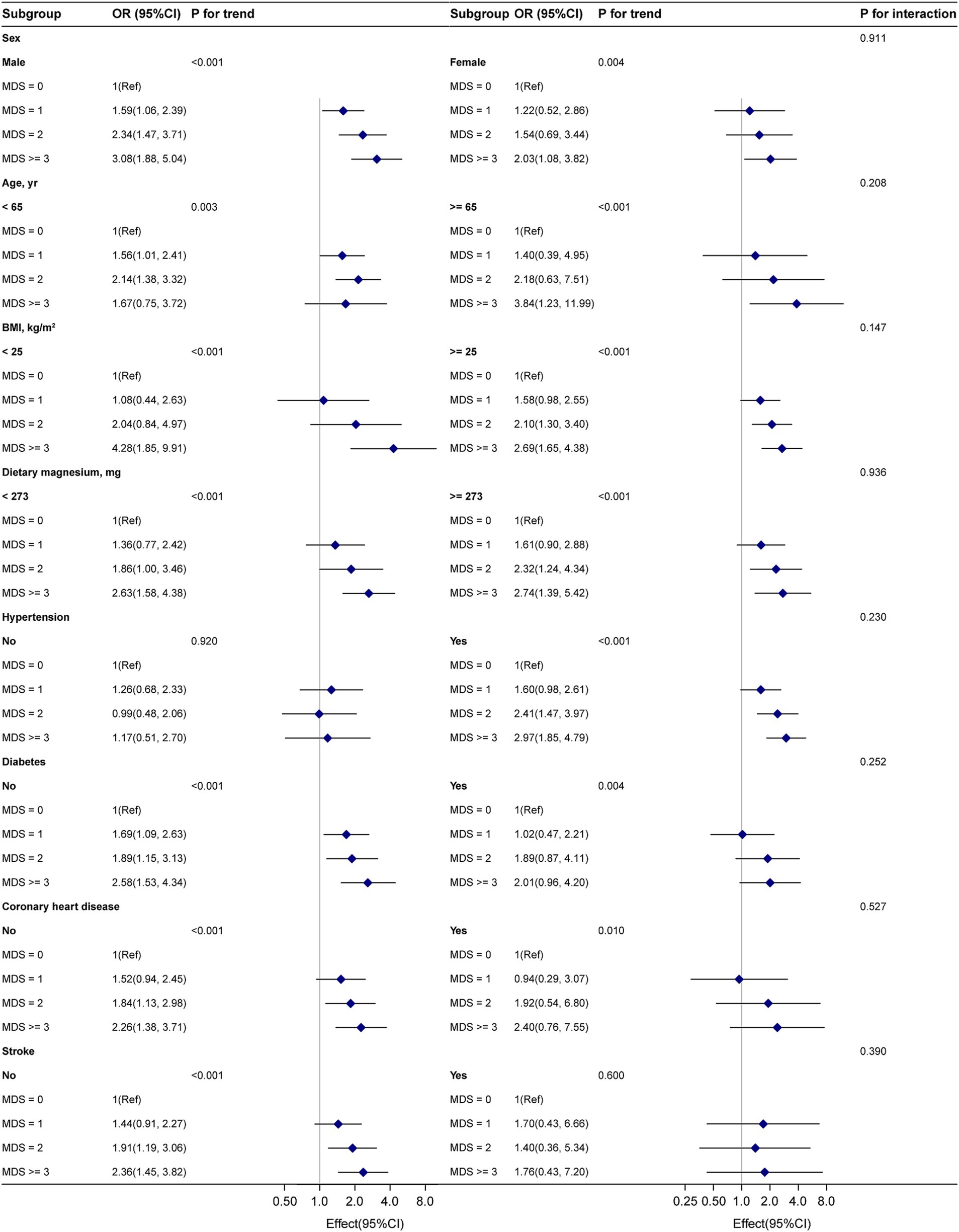
Figure 2. Subgroup analyses of MDS and gout. Adjustment by sex, age, BMI, race, smoking status, education level, physical activity, poverty income ratio, dietary magnesium intake, coronary heart disease, diabetes, stroke, and hypertension.
4 Discussion
We utilized NHANES data from five cycles (2007–2016) to investigate the association between MDS and gout risk in adults in the United States. The estimated overall prevalence of gout among adults in the United States during this period was 4.7%. After adjusting for confounders, we observed a significant positive correlation between MDS and gout risk. Subgroup analysis confirmed the stability of this association. Dietary magnesium intake did not moderate the positive correlation between MDS and gout risk.
Individuals with normal serum magnesium levels may experience magnesium deficiencies and respond to supplementation (18). The magnesium tolerance test is more accurate but impractical because of the need for 24-h urine collection and intravenous infusion (29). Fan et al. developed an MDS by combining four factors (alcohol consumption, PPI use, diuretic use, and chronic kidney disease) and validated its accuracy compared with that of the magnesium tolerance test (17). The evaluated factors reduce magnesium reabsorption. Alcohol consumption leads to rapid magnesium excretion in the urine (30). Loop-blocking diuretics cause magnesium loss by altering the renin-angiotensin-aldosterone system as well as calcium and parathyroid hormone concentrations (31). PPIs reduce renal magnesium reabsorption by downregulating the activity of the epithelial magnesium channel transient receptor potential melastatin 6 (32, 33). Under certain pathological conditions such as chronic kidney disease, large changes in renal magnesium reabsorption can occur. Chronic kidney disease is associated with increased urinary magnesium excretion (16).
Magnesium has unique antioxidant and anti-inflammatory properties. Research suggests that increased dietary magnesium intake is associated with a reduced risk of hyperuricemia (34), prompting the exploration of its interaction with MDS in gout risk. Our results confirm that dietary magnesium intake does not moderate the correlation between MDS and gout incidence. This finding aligns with those of previous studies, indicating a minimal impact of dietary magnesium on the association between MDS and chronic obstructive pulmonary disease (35), congestive heart failure (36), and cardiovascular diseases (18). Our results underscore the importance of considering the systemic magnesium status in gout risk reduction. When the magnesium status is suboptimal, relying solely on dietary intake may not effectively mitigate the risk of gout, similar to in other related diseases.
Given that gout patients often present with hyperuricemia and both acute or chronic inflammation (1), we hypothesize that magnesium deficiency, as indicated by MDS, may contribute to gout development through these pathways. Firstly, magnesium is involved in a wide range of biochemical reactions, particularly intracellular phosphorylation, which is crucial for initiating DNA synthesis and cell proliferation (37, 38). Additionally, low magnesium levels can affect oxidative stress, leading to oxidative DNA modifications and impaired DNA repair (39). This may result in significant DNA damage and the release of purine nucleotides, whose catabolism ultimately produces uric acid (40). Secondly, experimental studies have shown that magnesium deficiency is associated with acute inflammatory responses mediated by calcium, N-methyl-D-aspartate, and tumor necrosis factor-alpha (41), along with increases in C-reactive protein, interleukin-6, and fibrinogen (42–44), all of which are sensitive biomarkers of inflammation. Although further mechanistic studies are needed, the available evidence partially supports the possibility of the proposed mechanism.
Our research has found that high MDS significantly increases the risk of gout among American adults. Therefore, addressing the four key factors involved in MDS—alcohol consumption, PPI use, diuretic use, and chronic kidney disease—by adopting strategies to reduce magnesium depletion could help lower the risk of gout. Specific measures include reducing alcohol intake, appropriately using PPIs and diuretics, and managing chronic kidney disease. Reducing alcohol consumption can lower urinary magnesium excretion (45); PPIs are commonly prescribed for gastrointestinal issues, but their overuse has exceeded the actual number of necessary cases (46–48); diuretics are used to manage conditions such as hypertension and heart failure, but they can also lead to magnesium loss in urine, highlighting the risk of overuse (49). Monitoring and regulating the use of PPIs and diuretics is crucial, and healthcare providers should balance the benefits of these treatments against the potential risk of magnesium depletion. Lastly, CKD is a progressive, incurable condition related to magnesium metabolism abnormalities (50). Patients with CKD need to be particularly cautious about alcohol and medication usage to avoid exacerbating magnesium depletion, which could increase the risk of gout.
This study had some limitations. First, as a cross-sectional study, it inherently cannot establish causality. Although the association between MDS and gout may be explained by hyperuricemia and inflammatory mechanisms, further mechanistic studies are required to confirm this hypothesis. Second, cross-sectional studies inherently face issues with missing data. We addressed this by performing multiple imputations for missing covariate data, and the results remained stable. Third, gout data from self-reports may have been affected by recall bias. Fourth, dietary magnesium intake was assessed through a single 24-h recall, which may not accurately reflect long-term dietary habits or magnesium status over time. Fifth, while our results are based on NHANES, which uses a complex multistage probability sampling design to obtain a nationally representative sample of non-institutionalized United States adults, the generalizability of these findings to other geographic regions or racial/ethnic groups requires further validation. Finally, although we adjusted for some confounders, other factors such as genetics, lifestyle, and environment may have influenced the results.
5 Conclusion
According to the MDS, a positive correlation exists between magnesium deficiency and gout risk among adults in the United States. Additionally, dietary magnesium intake did not alter this association.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: NHANES is a public dataset that can be freely accessed at http://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by Research Ethics Review Committee of the National Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XC: Conceptualization, Writing – original draft, Writing – review & editing. HF: Data curation, Formal analysis, Investigation, Writing – original draft. HW: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1485578/full#supplementary-material
References
1. Dalbeth, N, Gosling, AL, Gaffo, A, and Abhishek, A. Gout Lancet. (2021) 397:1843–55. doi: 10.1016/s0140-6736(21)00569-9
2. Cohen-Rosenblum, AR, Somogyi, JR, Hynes, KK, and Guevara, ME. Orthopaedic management of gout. J Am Acad Orthop Surg Glob Res Rev. (2022) 6:e22.00216. doi: 10.5435/JAAOSGlobal-D-22-00216
3. Dehlin, M, Jacobsson, L, and Roddy, E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16:380–90. doi: 10.1038/s41584-020-0441-1
4. Roddy, E, and Doherty, M. Epidemiology of gout. Arthritis Res Ther. (2010) 12:223. doi: 10.1186/ar3199
5. Kuo, CF, Grainge, MJ, Zhang, W, and Doherty, M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. (2015) 11:649–62. doi: 10.1038/nrrheum.2015.91
6. Rai, SK, Aviña-Zubieta, JA, McCormick, N, De Vera, MA, Shojania, K, Sayre, EC, et al. The rising prevalence and incidence of gout in British columbia, Canada: population-based trends from 2000 to 2012. Semin Arthritis Rheum. (2017) 46:451–6. doi: 10.1016/j.semarthrit.2016.08.006
7. Fiorentini, D, Cappadone, C, Farruggia, G, and Prata, C. Magnesium: biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients. (2021) 13:1136. doi: 10.3390/nu13041136
8. Chiu, TK, and Dickerson, RE. 1 a crystal structures of b-DNA reveal sequence-specific binding and groove-specific bending of DNA by magnesium and calcium. J Mol Biol. (2000) 301:915–45. doi: 10.1006/jmbi.2000.4012
9. Green, L, Kim, CH, Bustamante, C, and Tinoco, I Jr. Characterization of the mechanical unfolding of rna pseudoknots. J Mol Biol. (2008) 375:511–28. doi: 10.1016/j.jmb.2007.05.058
10. Caspi, R, Altman, T, Dreher, K, Fulcher, CA, Subhraveti, P, Keseler, IM, et al. The metacyc database of metabolic pathways and enzymes and the biocyc collection of pathway/genome databases. Nucleic Acids Res. (2012) 40:D742–53. doi: 10.1093/nar/gkr1014
11. Chaigne-Delalande, B, Li, FY, O'Connor, GM, Lukacs, MJ, Jiang, P, Zheng, L, et al. Mg2+ regulates cytotoxic functions of nk and cd8 t cells in chronic ebv infection through nkg2d. Science (New York, NY). (2013) 341:186–91. doi: 10.1126/science.1240094
12. Li, FY, Chaigne-Delalande, B, Kanellopoulou, C, Davis, JC, Matthews, HF, Douek, DC, et al. Second messenger role for mg2+ revealed by human t-cell immunodeficiency. Nature. (2011) 475:471–6. doi: 10.1038/nature10246
13. Weber, JD, and Gutmann, DH. Deconvoluting mtor biology. Cell Cycle. (2012) 11:236–48. doi: 10.4161/cc.11.2.19022
14. Krishna, SP, Akhter, S, Nessa, A, Hoque, MR, Saha, BK, Faysal, MR, et al. Status of serum magnesium and uric acid in patients with chronic obstructive pulmonary disease. Mymensingh Med J. (2023) 32:927–32.
15. Ray, EC. Evolving understanding of cardiovascular protection by sglt2 inhibitors: focus on renal protection, myocardial effects, uric acid, and magnesium balance. Curr Opin Pharmacol. (2020) 54:11–7. doi: 10.1016/j.coph.2020.06.001
16. de Baaij, JH, Hoenderop, JG, and Bindels, RJ. Magnesium in man: implications for health and disease. Physiol Rev. (2015) 95:1–46. doi: 10.1152/physrev.00012.2014
17. Fan, L, Zhu, X, Rosanoff, A, Costello, RB, Yu, C, Ness, R, et al. Magnesium depletion score (mds) predicts risk of systemic inflammation and cardiovascular mortality among us adults. J Nutr. (2021) 151:2226–35. doi: 10.1093/jn/nxab138
18. Ye, L, Zhang, C, Duan, Q, Shao, Y, and Zhou, J. Association of magnesium depletion score with cardiovascular disease and its association with longitudinal mortality in patients with cardiovascular disease. J Am Heart Assoc. (2023) 12:e030077. doi: 10.1161/jaha.123.030077
19. Yin, S, Zhou, Z, Lin, T, and Wang, X. Magnesium depletion score is associated with long-term mortality in chronic kidney diseases: a prospective population-based cohort study. J Nephrol. (2023) 36:755–65. doi: 10.1007/s40620-022-01489-5
20. Wang, X, Zeng, Z, Wang, X, Zhao, P, Xiong, L, Liao, T, et al. Magnesium depletion score and metabolic syndrome in us adults: analysis of nhanes 2003-2018. J Clin Endocrinol Metab. (2024). doi: 10.1210/clinem/dgae075
21. Tan, MY, Mo, CY, and Zhao, Q. The association between magnesium depletion score and hypertension in us adults: evidence from the national health and nutrition examination survey (2007-2018). Biol Trace Elem Res. (2023) 202:4418–30. doi: 10.1007/s12011-023-04034-y
22. Tian, Z, Qu, S, Chen, Y, Fang, J, Song, X, He, K, et al. Associations of the magnesium depletion score and magnesium intake with diabetes among us adults: an analysis of the national health and nutrition examination survey 2011-2018. Epidemiol Health. (2024) 46:e2024020. doi: 10.4178/epih.e2024020
23. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
24. McCormick, N, Lu, N, Yokose, C, Joshi, AD, Sheehy, S, Rosenberg, L, et al. Racial and sex disparities in gout prevalence among us adults. JAMA Netw Open. (2022) 5:e2226804. doi: 10.1001/jamanetworkopen.2022.26804
25. Razouki, ZA, Zhang, X, Hwang, JP, and Heredia, NI. Clinical factors associated with non-obese nonalcoholic fatty liver disease detected among us adults in the nhanes 2017-2018. J Clin Med. (2022) 11:4260. doi: 10.3390/jcm11154260
26. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43:S14–s31. doi: 10.2337/dc20-S002
27. Zheng, Y, Wang, J, Wang, Y, Xu, K, and Chen, X. The hidden dangers of plant-based diets affecting bone health: a cross-sectional study with u.S. national health and nutrition examination survey (nhanes) data from 2005–2018. Nutrients. (2023) 15:1794. doi: 10.3390/nu15071794
28. Ruan, Z, Lu, T, Chen, Y, Yuan, M, Yu, H, Liu, R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient us adults. JAMA Dermatol. (2022) 158:745–53. doi: 10.1001/jamadermatol.2022.1609
29. Arnaud, MJ. Update on the assessment of magnesium status. Br J Nutr. (2008) 99:S24–36. doi: 10.1017/S000711450800682X
30. Rylander, R, Mégevand, Y, Lasserre, B, Amstutz, W, and Granbom, S. Moderate alcohol consumption and urinary excretion of magnesium and calcium. Scand J Clin Lab Invest. (2001) 61:401–5. doi: 10.1080/003655101316911459
32. Nijenhuis, T, Vallon, V, van der Kemp, AW, Loffing, J, Hoenderop, JG, and Bindels, RJ. Enhanced passive ca2+ reabsorption and reduced mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. (2005) 115:1651–8. doi: 10.1172/jci24134
33. William, JH, and Danziger, J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. (2016) 5:152–7. doi: 10.5527/wjn.v5.i2.152
34. Zhang, Y, and Qiu, H. Dietary magnesium intake and hyperuricemia among us adults. Nutrients. (2018) 10:296. doi: 10.3390/nu10030296
35. Wang, KJ, Chen, H, Wang, J, and Wang, Y. Association between magnesium depletion score and chronic obstructive pulmonary disease risk: a secondary data analysis from nhanes. BMJ Open. (2024) 14:e083275. doi: 10.1136/bmjopen-2023-083275
36. Zhao, D, Chen, P, Chen, M, Chen, L, and Wang, L. Association of magnesium depletion score with congestive heart failure: results from the nhanes 2007–2016. Biol Trace Elem Res. (2024) 202:454–65. doi: 10.1007/s12011-023-03697-x
37. Rubin, H. Central role for magnesium in coordinate control of metabolism and growth in animal cells. Proc Natl Acad Sci USA. (1975) 72:3551–5. doi: 10.1073/pnas.72.9.3551
38. Rubin, H. Central roles of mg2+ and mgatp2- in the regulation of protein synthesis and cell proliferation: significance for neoplastic transformation. Adv Cancer Res. (2005) 93:1–58. doi: 10.1016/s0065-230x(05)93001-7
39. Wolf, FI, Maier, JA, Nasulewicz, A, Feillet-Coudray, C, Simonacci, M, Mazur, A, et al. Magnesium and neoplasia: from carcinogenesis to tumor growth and progression or treatment. Arch Biochem Biophys. (2007) 458:24–32. doi: 10.1016/j.abb.2006.02.016
40. Cao, J, Zhang, J, Zhang, Y, Li, H, Jiang, C, Lin, T, et al. Plasma magnesium and the risk of new-onset hyperuricaemia in hypertensive patients. Br J Nutr. (2020) 124:156–63. doi: 10.1017/S0007114520001099
41. Nielsen, FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev. (2010) 68:333–40. doi: 10.1111/j.1753-4887.2010.00293.x
42. Kim, DJ, Xun, P, Liu, K, Loria, C, Yokota, K, Jacobs, DR Jr, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. (2010) 33:2604–10. doi: 10.2337/dc10-0994
43. Dibaba, DT, Xun, P, and He, K. Dietary magnesium intake is inversely associated with serum c-reactive protein levels: Meta-analysis and systematic review. Eur J Clin Nutr. (2014) 68:971. doi: 10.1038/ejcn.2014.111
44. Song, Y, Li, TY, van Dam, RM, Manson, JE, and Hu, FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. (2007) 85:1068–74. doi: 10.1093/ajcn/85.4.1068
45. Baj, J, Flieger, W, Teresiński, G, Buszewicz, G, Sitarz, R, Forma, A, et al. Magnesium, calcium, potassium, sodium, phosphorus, selenium, zinc, and chromium levels in alcohol use disorder: a review. J Clin Med. (2020) 9:1901. doi: 10.3390/jcm9061901
46. Friedenberg, FK, Hanlon, A, Vanar, V, Nehemia, D, Mekapati, J, Nelson, DB, et al. Trends in gastroesophageal reflux disease as measured by the national ambulatory medical care survey. Dig Dis Sci. (2010) 55:1911–7. doi: 10.1007/s10620-009-1004-0
47. Grigg, E, Davis, I, Williams, D, and Schade, R. Can intervention reduce abuse of proton pump inhibitors?: 1114. Am J Gastroenterol. (2010) 105:S404. doi: 10.14309/00000434-201010001-01114
48. Shalaby, E. #4068 evaluation of chronic toxic effect of proton pump inhibitors (ppis) abuse on magnesium level in hemodialysis patients. Nephrol Dial Transplant. (2023) 38:gfad063c_4068. doi: 10.1093/ndt/gfad063c_4068
49. Bartoli, E, Rossi, L, Sola, D, Castello, L, Sainaghi, PP, and Smirne, C. Use, misuse and abuse of diuretics. Eur J Intern Med. (2017) 39:9–17. doi: 10.1016/j.ejim.2017.01.016
Keywords: gout, magnesium depletion score, cross-sectional study, National Health and Nutrition Examination Survey, association
Citation: Cao X, Feng H and Wang H (2024) Magnesium depletion score and gout: insights from NHANES data. Front. Nutr. 11:1485578. doi: 10.3389/fnut.2024.1485578
Edited by:
Mariacristina Siotto, IRCCS Don Carlo Gnocchi Firenze, ItalyReviewed by:
Azam Doustmohammadian, Iran University of Medical Sciences, IranHarlan Sayles, University of Nebraska Medical Center, United States
Minghao Liang, Shandong University of Traditional Chinese Medicine, China
Pengfei Wang, Fujian Provincial Hospital, China
Qishu Li, Twelfth Guangzhou City People’s Hospital, China
Copyright © 2024 Cao, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijie Wang, d2FuZ19odWlqaWVAb3V0bG9vay5jb20=
†These authors have contributed equally to this work and share first authorship
 Xu Cao
Xu Cao Haixia Feng2†
Haixia Feng2† Huijie Wang
Huijie Wang