
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 21 November 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1464414
Background: Osteoarthritis (OA) is the most prevalent form of arthritis worldwide. Inflammation and nutrition status play crucial roles in the development and progression of OA. The advanced lung cancer inflammation index (ALI) serves as a composite indicator for evaluating inflammation and nutritional status, while the systemic immune inflammation index (SII) is a novel marker for assessing immune-related inflammation. The study aimed to investigate the associations of the ALI and SII with all-cause and cardiovascular mortality among US adults with OA.
Methods: A total of 2,602 individuals aged 20 years and above with OA were included in the study from the National Health and Nutrition Examination Survey (NHANES) spanning from 1999 to 2018. Participants were categorized into higher or lower ALI and SII groups using cut-off values determined by the maximally selected rank statistics method. The Kaplan-Meier analysis, Cox proportional hazards models, and Fine Gray competing risk regression models were employed to assess the associations between the ALI/SII and mortality in OA patients. Additionally, stratified and subgroup analyses were conducted to enhance the robustness of the findings. Furthermore, time-dependent receiver operating characteristic (ROC) analysis was used to evaluate the predictive capacity of ALI and SII for mortality.
Results: Higher SII levels were associated with a 2-fold increase in the risk of all-cause mortality (HR: 2.00, 95% CI: 1.59–2.52, p < 0.001), whereas individuals with higher ALI in the OA group exhibited a significantly reduced risk of all-cause mortality (HR: 0.49, 95% CI: 0.39–0.60, p < 0.001). Notably, in Model 3, individuals with higher ALI demonstrated a substantially lower risk of cardiovascular mortality (HR: 0.60, 95% CI: 0.44–0.82, p < 0.001). Conversely, in fully adjusted models, those with higher SII experienced a significantly higher risk (HR: 1.83, 95% CI: 1.29–2.60, p < 0.001). The RCS analysis revealed a J-shaped non-linear relationship between SII levels and all-cause mortality (p overall < 0.001; p non-linear < 0.001), and an L-shaped non-linear association between ALI levels and all-cause mortality (p overall < 0.001; p non-linear = 0.002). The time-dependent ROC curves illustrated that ALI and SII displayed a reasonably good and consistent predictive performance for both short- and long-term mortality in OA patients.
Conclusions: Lower ALI and higher SII values were correlated with increased risks of all-cause and cardiovascular mortality among US adults with OA.
Osteoarthritis (OA) is an age-related joint condition impacting articular cartilage and the overall joint structure, causing joint pain, swelling, stiffness, joint deformity, and significant functional limitations, often leading to high disability. The main pathological features included cartilage deterioration, bone resorption, and osteophyte formation (1). OA is becoming increasingly prevalent globally, with substantial impacts on various health outcomes in the US and globally (2). According to data from the National Health Interview Survey, approximately 14 million Americans suffer from symptomatic knee osteoarthritis (KOA) (3). As one of the most common chronic illnesses, OA significantly affects both the physical and mental wellbeing of individuals, while also imposing a substantial financial and medical burden (4). Individuals diagnosed with OA experience a heightened risk of both all-cause and cardiovascular mortality compared to the general population (5, 6). The importance of the management of OA as part of the care of patients with multiple chronic diseases cannot be overlooked, given its impact on disability and functional limitations, as well as high all-cause and cardiovascular mortality rates (7). Therefore, this study intends to provide strategies for the long-term management of patients with arthritis by investigating the correlation between simple and easily accessible biomarkers and mortality in OA and to provide a key reference for the development of targeted interventions in the future.
Low-grade chronic inflammation is believed to be essential in the development of OA, despite its exact pathophysiology remaining unclear (8). In OA, the innate immune system plays a critical role in sustaining persistent, low-grade inflammation (8). Local joint inflammation can directly harm the joint, aggravate the severity of joint symptoms, and increase the loss of cartilage (9). Furthermore, systemic inflammation stimulates local joint inflammation, ultimately resulting in OA (10). Previous research has demonstrated that a close relationship between nutritional status indicators like albumin and body mass index (BMI) and the prognosis of OA patients (11–13). A meta-analysis found that obesity not only increases the risk of osteoarthritis (OA) but also contributes to disease progression and complications, including mortality, particularly when related to cardiovascular comorbidities. The high BMI is a leading factor in OA-related mortality and overall disease burden (14). Albumin level is a useful indicator of nutritional status in clinical populations (15). Low serum albumin was associated with increased mortality and several additional major perioperative complications after total knee arthroplasty for OA (16). Metabolic syndrome and obesity are well-known risk factors for osteoarthritis likely due to increased joint load and the promotion of chronic systemic inflammation (8). Hence, both inflammation and nutritional status impact the prognosis of OA patients. Consequently, the use of a single indicator to assess inflammation or nutritional status for prognosis evaluation may not be comprehensive. There is a critical need for novel predictive indicators that simultaneously consider inflammation and nutritional status to provide a thorough prognosis evaluation for OA patients. This study aims to identify new predictive markers that concurrently evaluate inflammation and nutritional status to comprehensively assess the outcome of OA patients.
The advanced lung cancer inflammation index (ALI) is an innovative composite measure that combines indicators of inflammation and nutritional status by considering parameters such as albumin, BMI, and the neutrophil-to-lymphocyte ratio (NLR). Initially developed to assess the outcome of lung cancer patients, ALI has been expanded for use in various other malignancies like esophageal, colorectal, pancreatic, and gastric cancers (17–22). Due to its capability to comprehensively evaluate both inflammation and nutritional status, ALI's application can be extended to chronic inflammatory conditions such as hypertension, diabetes, heart failure, stroke, and even rheumatoid arthritis (23–27). The systemic immune-inflammation index (SII) is a novel composite marker of systemic inflammation that incorporates neutrophil, lymphocyte, and platelet counts to reflect both pro-inflammatory and anti-inflammatory responses. Initially, it was designed to project longevity and the return of cancer (28, 29). Nonetheless, it is currently uncertain how the ALI and SII relate to the risk of mortality in OA patients.
The study represented a pioneering investigation that explores the associations between the innovative integrated inflammatory and nutritional status markers ALI and SII, and the mortality rates among patients with OA.
The study utilized public data from the National Health and Nutrition Examination Survey (NHANES) 1999–2018. The NHANES study was approved by the National Center for Health Statistics (NCHS) ethics review board, and all participants gave written informed permission. The study followed the principles of the 1975 Declaration of Helsinki. The study's inclusion criteria were as follows: (1) Participants diagnosed with osteoarthritis; (2) age over 20 years. Some participants were excluded for the following reasons: (1) The data on the level of survival and keep up time are inadequate; (2) Lack of information regarding albumin, BMI, lymphocytes, and neutrophils; (3) Missing details on other covariates. The diagnosis of OA was based on self-reports from participants. Figure 1 displays the detailed screening procedure for this study.
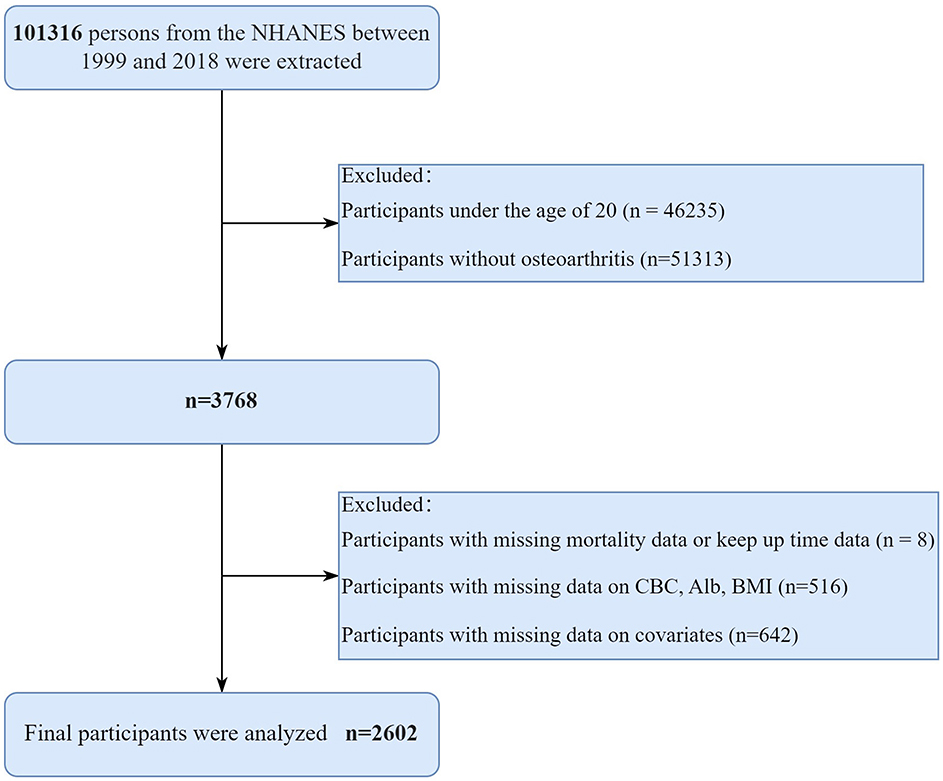
Figure 1. The detailed screening procedure for this study. NHANES, National Health and Nutrition Examination Survey; CBC, complete blood count; Alb, albumin; BMI, Body mass index.
The ALI was calculated using BMI, albumin level (g/dL), and NLR with the following equation: BMI (kg/m2) * albumin/NLR. The NLR was determined by dividing the neutrophil count by the lymphocyte count (30). The SII was calculated by multiplying the platelet count by the neutrophil count and then dividing by the lymphocyte count (28, 29). These values were extracted from peripheral complete blood count (CBC). Thresholds of 32.43 for ALI and 991.43 for SII were determined using a data-driven approach by the maximally selected rank statistics method (MSRSM) (Figure 2). Participants were categorized into two groups based on these cutoff points.
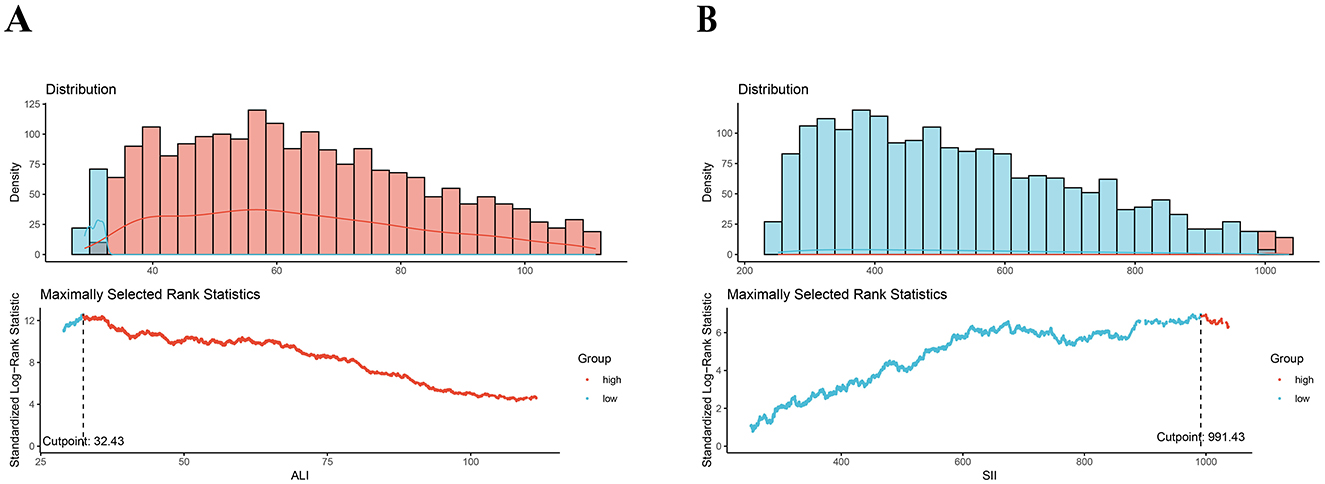
Figure 2. Maximally selected rank statistics method for the ideal cutoff points for the ALI (A) and SII (B). SII, systemic immune-inflammation index; SIRI, systemic inflammation response index.
Participant mortality status in the NHANES database was ascertained by accessing the NHANES public-use linked mortality files (LMFs) via a unique personal-level serial number (SEQN) identifier. The most recent update was on December 31, 2019. Specifically, cardiovascular mortality was identified through ICD-10 codes ranging from I00 to I078, and cancer-related mortality was verified for deaths resulting from malignant neoplasms (C00–C97).
The covariates in this study considered demographic characteristics, socioeconomic status, and health conditions. Demographic features comprised age, gender, and race. Socioeconomic status included educational background and poverty-to-income ratio (PIR). Health status included smoking and alcohol consumption status as well as the presence of hypertension or diabetes. Moreover, laboratory data included measurements of total cholesterol and high-density lipoprotein levels.
Due to the complexity of data collection in the NAHNES database, sampling weights were incorporated to ensure the data representativeness, following NHANES recommendations. The specific practice involved combining the MEC examination weights of each cycle (WTMEC4YR, WTMEC2YR, and WTMEPRP). Data analysis was carried using R software version 4.3.2 (R Project for Statistical Computing, Vienna, Austria). Statistical significance level for the tests was set at p < 0.05 (two-tailed). For the description of baseline features, persistent variables were presented as mean ± standard deviation, and group differences were compared using analysis of variance (ANOVA). Categorical variables were depicted as frequencies and percentages. Wilcoxon rank-sum test was employed for ordinal categorical variables, while chi-square test was utilized for nominal categorical variables.
Kaplan–Meier (KM) method and univariate Fine Gray competing-risk regression models were conducted to assess the cumulative incidence of all-cause mortality and cardiovascular mortality rate among the higher and lower ALI and SII groups, accounting for the competing risk of non-cardiovascular mortality (27). Hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality were estimated using Cox proportional hazards models and multivariate Fine-Gray models. Three models were employed in this study: Model 1 was without adjustments. Model 2 was modified for age, gender, race, educational background, marital status, and poverty-income ratio. Model 3 was further modified for total cholesterol, high-density lipoprotein, smoking status, drinking status, history of cancer/malignancy, and coronary heart disease. ALI and SII were natural log-transformed for normalization... Restricted cubic spline (RCS) analysis was performed to explore non-linear associations between ALI, SII and mortality in OA patients.
To enhance the robustness of the study, sensitivity and subgroup analyses were conducted. Finally, ROC analyses were employed to evaluate the accuracy of ALI/SII in forecasting short- and long-term survival outcomes at different time points (3-, 5-, 10-, and 15-years).
Initially, a total of 101,316 individuals from the NHANES between 1999 and 2018 were included in the analysis. According to the standards for inclusion and exclusion, a total of 2,602 patients with OA from the United States were enrolled in the final study. The detailed flow chart is presented in Figure 1. Among the 2,602 individuals, the average age was 60.26 years, with 63.65% being women. The average ALI was 66.25, the average SII was 620.04, and the average BMI was 30.08 kg/m2. Approximately half of the participants (53.88%) were current or former smokers, while more than half (86.99%) were current or former alcohol drinkers. A total of 351 patients (13.49%) had a lower ALI, whereas 294 patients (11.30%) exhibited a higher SII. In comparison to participants with a higher ALI, those with a lower ALI were older, more women, more smokers, had a lower poverty-income ratio, had a lower BMI, and more patients had cancer and cardiovascular disease (CVD) (all p < 0.05). However, there were no major distinctions in age, gender, PIR, BMI, smoking status, drinking status and the incidence of cancer and CVD (all p > 0.05). Details are provided in Table 1.
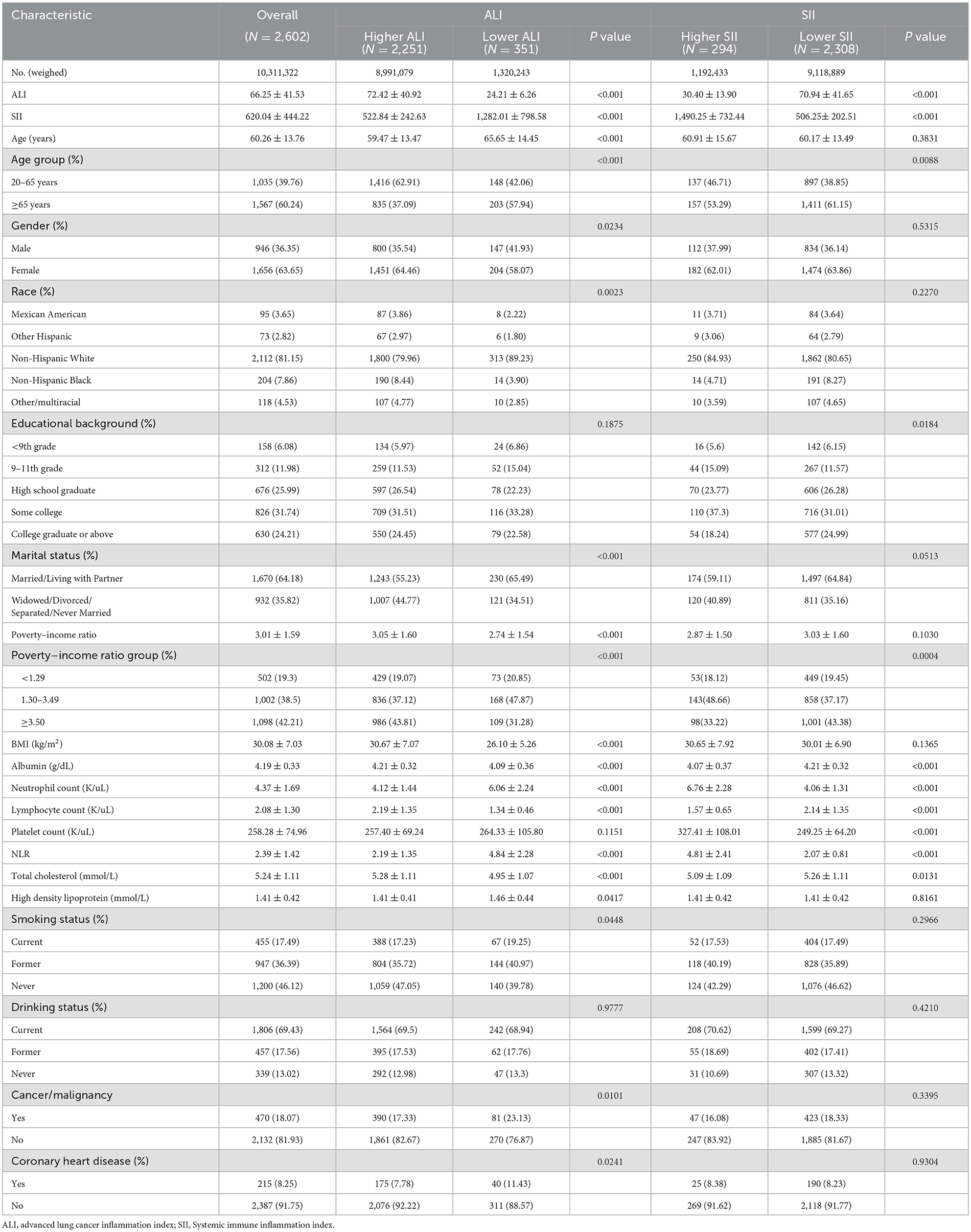
Table 1. Baseline characteristics of osteoarthritis patients according to the ALI and SII (weighted).
Among the 2,602 patients with osteoarthritis, 636 all-cause deaths (28.82% of the population) and 226 cardiovascular-related deaths (8.89% of the sample) were recorded over an average follow-up period of 9.42 years (IQR 4.94–12.58 years). The KM curves and univariate Fine–Gray models based on ALI and SII values among adults with OA are presented in Figure 3. Individuals with lower ALI and higher SII levels demonstrated significantly higher all-cause mortality compared to those with higher ALI and lower SII values (both p < 0.001). Similarly, lower ALI values and higher SII values were significantly associated with an increased cumulative probability of cardiovascular mortality during the follow-up period, compared to higher ALI and lower SII values (both p < 0.001).

Figure 3. Cumulative incidence curves demonstrating how the ALI/SII affects mortality results. Kaplan-Meier curves for all-cause mortality, stratified by ALI (A) and SII (B). Competing risk analysis for cardiovascular mortality and competing risk of death for the ALI (C) and SII (D) groups. OA, osteoarthritis; ALI, Advanced Lung Cancer Inflammation Index; SII, systemic immune–inflammation index.
Table 2 presents the results of four Cox models that illustrate the associations between inflammation and nutrition status and both all-cause and cardiovascular mortality. Model 3 included a broader range of covariates to better mitigating account for confounding variables, with a focus on the results derived from the model. The subgroup with higher ALI exhibited significantly lower all-cause mortality rates compared to those with low ALI (HR: 0.49, 95% CI: 0.39–0.60, p < 0.001). Higher SII levels were associated with a 2-fold increased risk of all-cause mortality (HR: 2.00, 95% CI: 1.59–2.52, p < 0.001). In the multivariate Fine-Gray models of model 3, the higher-ALI group had a considerably reduced risk of cardiovascular mortality (HR: 0.60, 95% CI: 0.44–0.82, p < 0.001) while the higher-SII group exhibited a considerably higher risk in fully adjusted models (HR: 1.83, 95% CI: 1.29–2.60, p < 0.001).
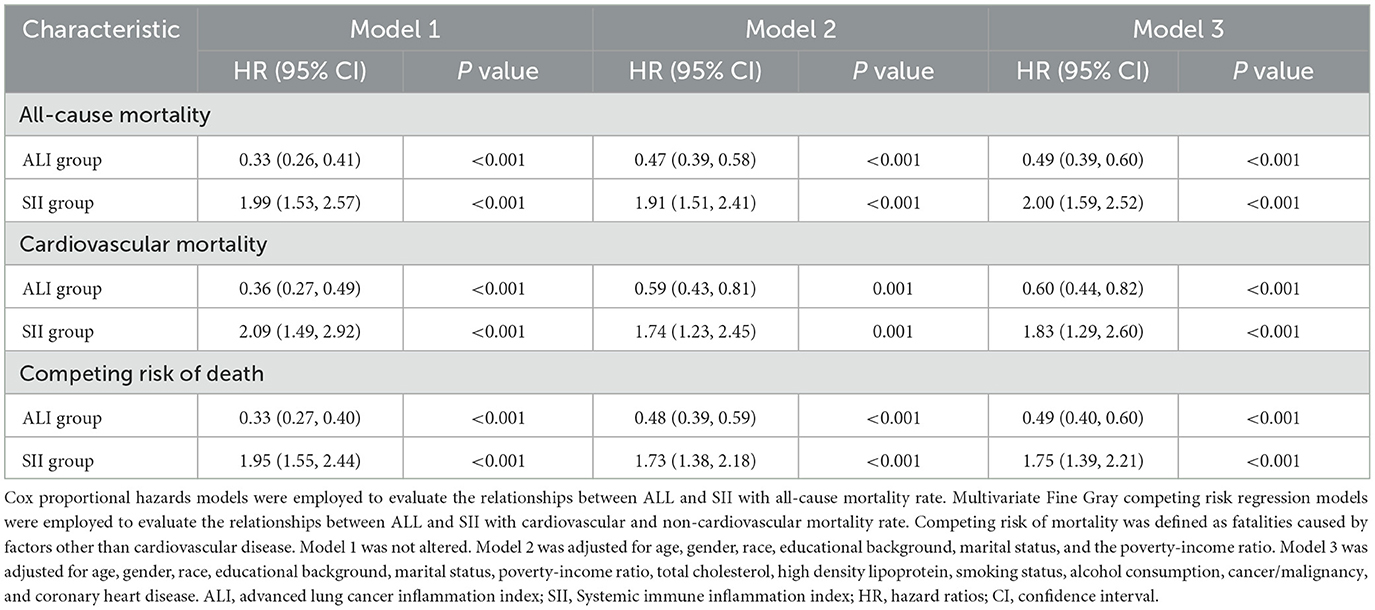
Table 2. Hazard ratios of all-cause mortality and cardiovascular mortality according to the ALI and SII among patients with osteoarthritis.
There existed an L-shaped non-linear correlation among ALI and all-cause mortality (p overall < 0.001; p non-linear = 0.002) (Figure 4A), while a J-shaped non-linear association was observed between SII levels and mortality (p overall < 0.001; p non-linear < 0.001) (Figure 4B). Conversely, a linear correlation was found between ALI, the SII, and cardiovascular mortality (p overall > 0.05; p non-linear > 0.05) (Figures 4C, D).
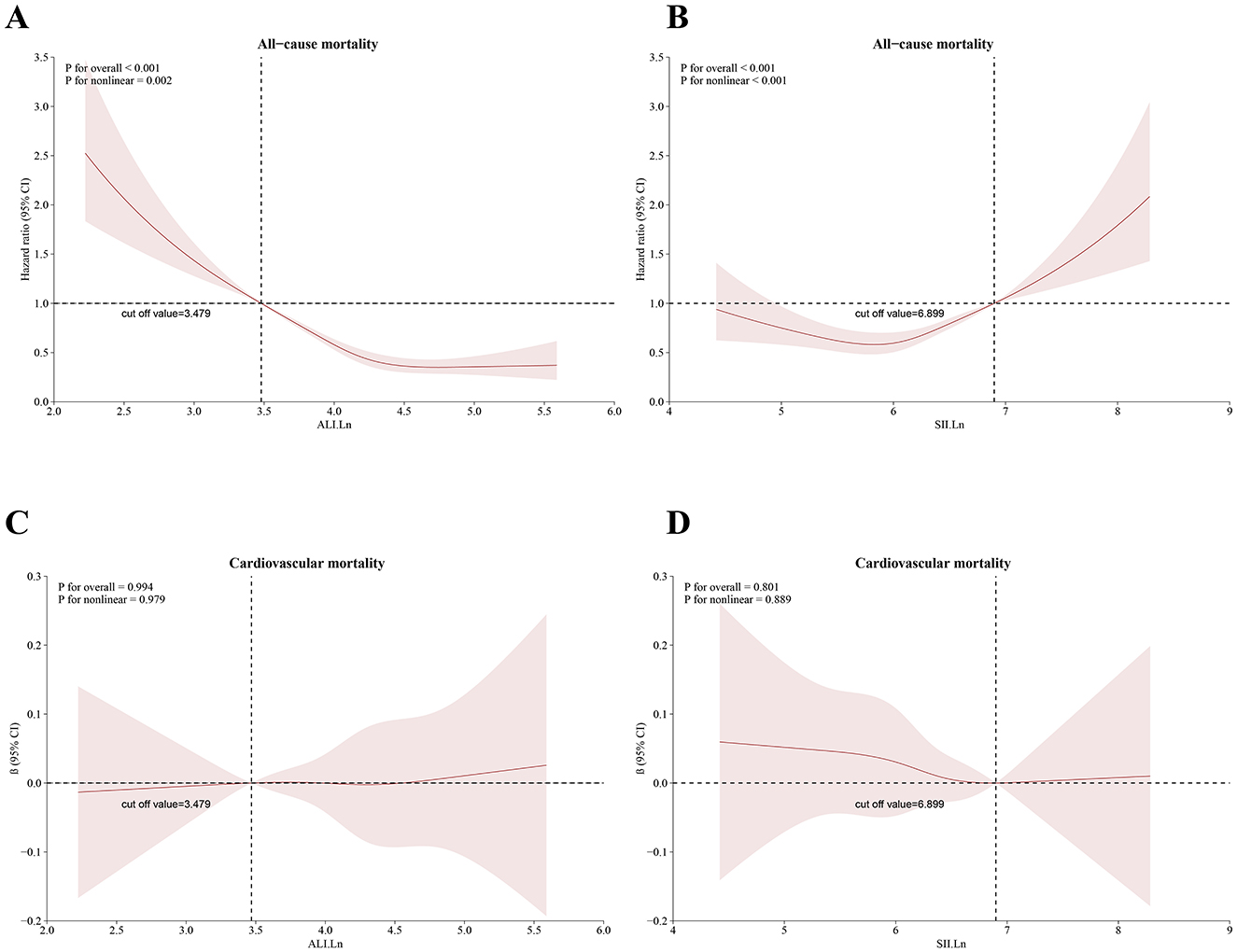
Figure 4. Restricted cubic spline for the relationship between death and the ALI and SII. According to the ALI and SII, (A, B) represent all-cause mortality; (C, D) represent cardiovascular mortality. The 95% CI is shown by the transparent area, while the HR is indicated by the red line. Model 3 was used to alter these analyses. ALI, Advanced Lung Cancer Inflammation Index; SII, systemic immune–inflammation index.
Table 3 displays the associations of the ALI and SII with all-cause mortality in subgroup analysis. The results indicated that low ALI and high SII were associated with an increased risk of all-cause mortality in most subgroups. Notably, the relationship between SII and all-cause mortality was insignificant in individuals with coronary heart disease. The relationships between cardiovascular rates and SII were observed across subgroups (Table 4). However, with regards to cardiovascular mortality, significant interactions were only found with age and PIR subgroups, while other subgroups did not show any notable interactions. Furthermore, sensitivity analysis demonstrated no significant changes in results when analyzing individuals who died from cancer (n = 2,451) (Supplementary Table S1), deaths due to other/unspecified causes (n = 2,414) (Supplementary Table S2), individuals under 40 years of age (n = 2,446) (Supplementary Table S3), or those with < 3 years of follow-up (n = 2,230) (Supplementary Table S4).
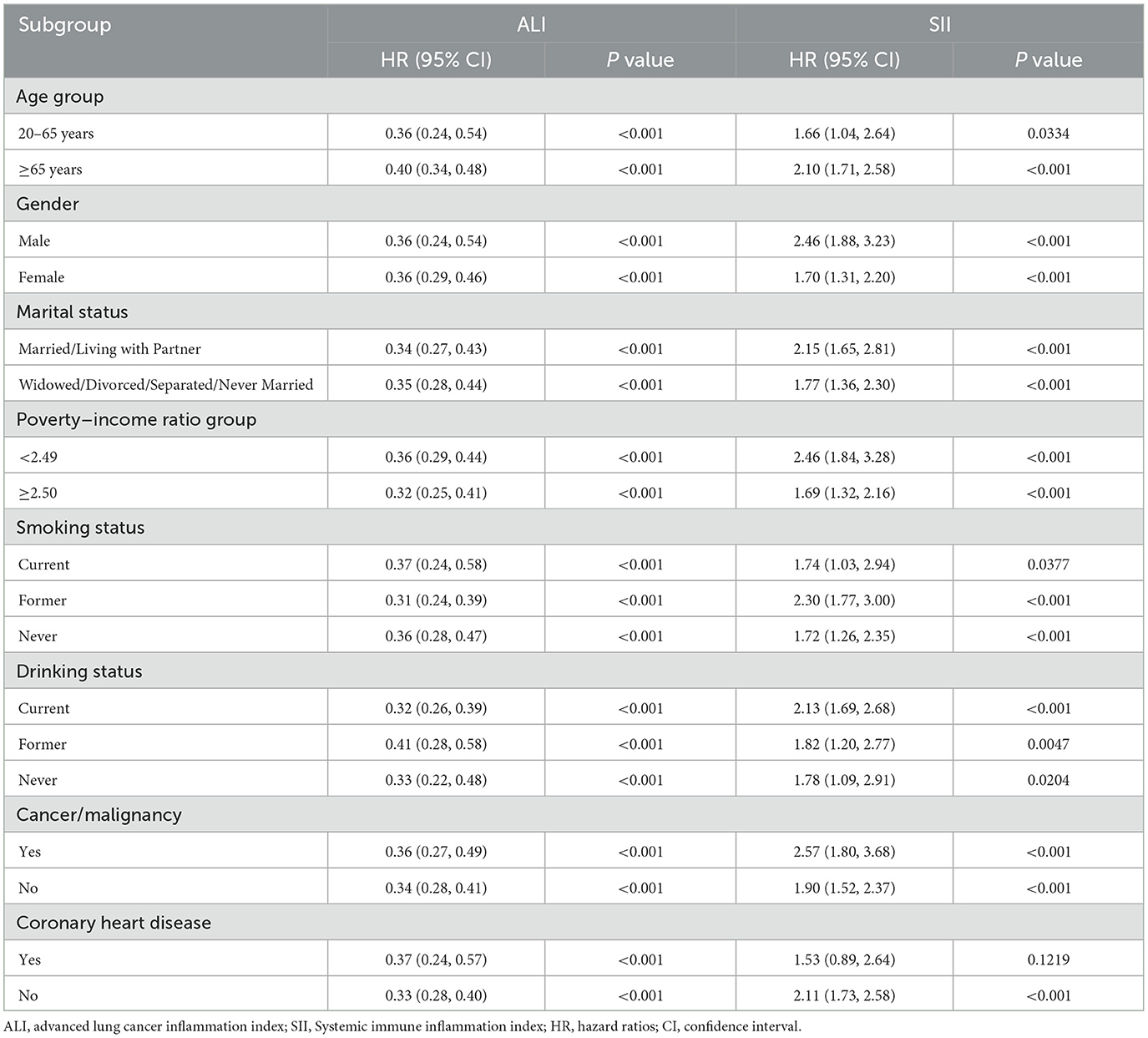
Table 3. Subgroup analysis investigation of the relationships between the ALI/SII and all-cause mortality among osteoarthritis patients.
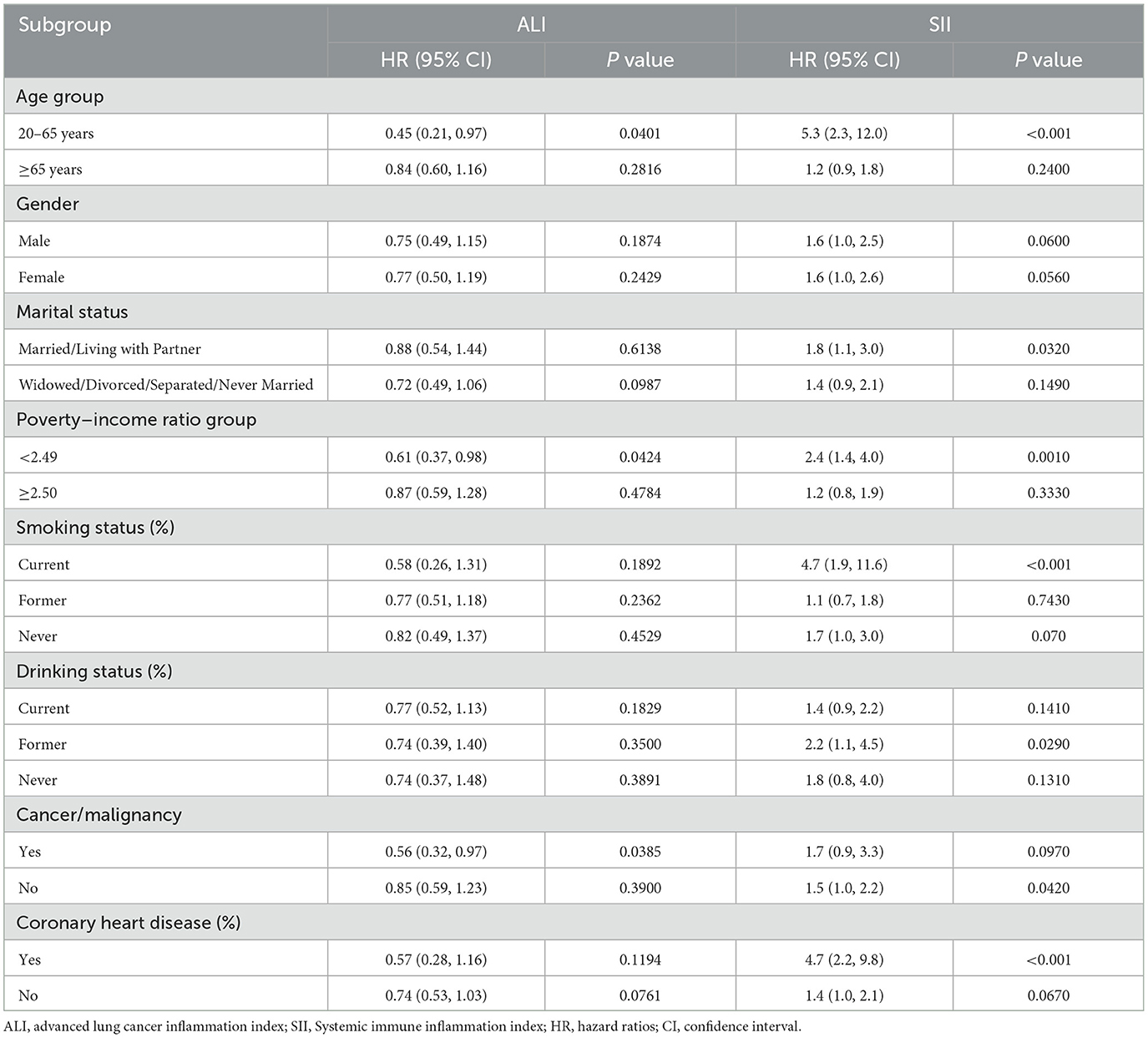
Table 4. Subgroup analysis of the relationships between the ALI/SII and cardiovascular mortality among osteoarthritis patients.
To evaluate the predictive performance of ALI and SII in patients with OA, time-dependent ROC (Figure 5) and AUC curves (Supplementary Figure S1). were generated based on the results of the model 3 Cox regression analysis. The AUCs for the ALI were 0.8072, 0.7996, 0.8262, and 0.8566 at 3-, 5-, 10-, and 15-years of all-cause mortality, respectively. Similarly, the SII showed AUCs values of 0.8026, 0.7998, 0.8248, and 0.8549 for the corresponding time points. For cardiovascular mortality predictions, the ALI had AUCs of 0.7037, 0.6989, 0.7151, and 0.7918 at 3-, 5-, 10-, and 15-years intervals, respectively. Conversely, the SII demonstrated AUC values of 0.7019, 0.7118, 0.7246, and 0.7793 at the same time intervals (Figure 5). The time-dependent ROC and AUC curves demonstrated that both ALI and SII exhibited moderate and consistent performance in predicting mortality.
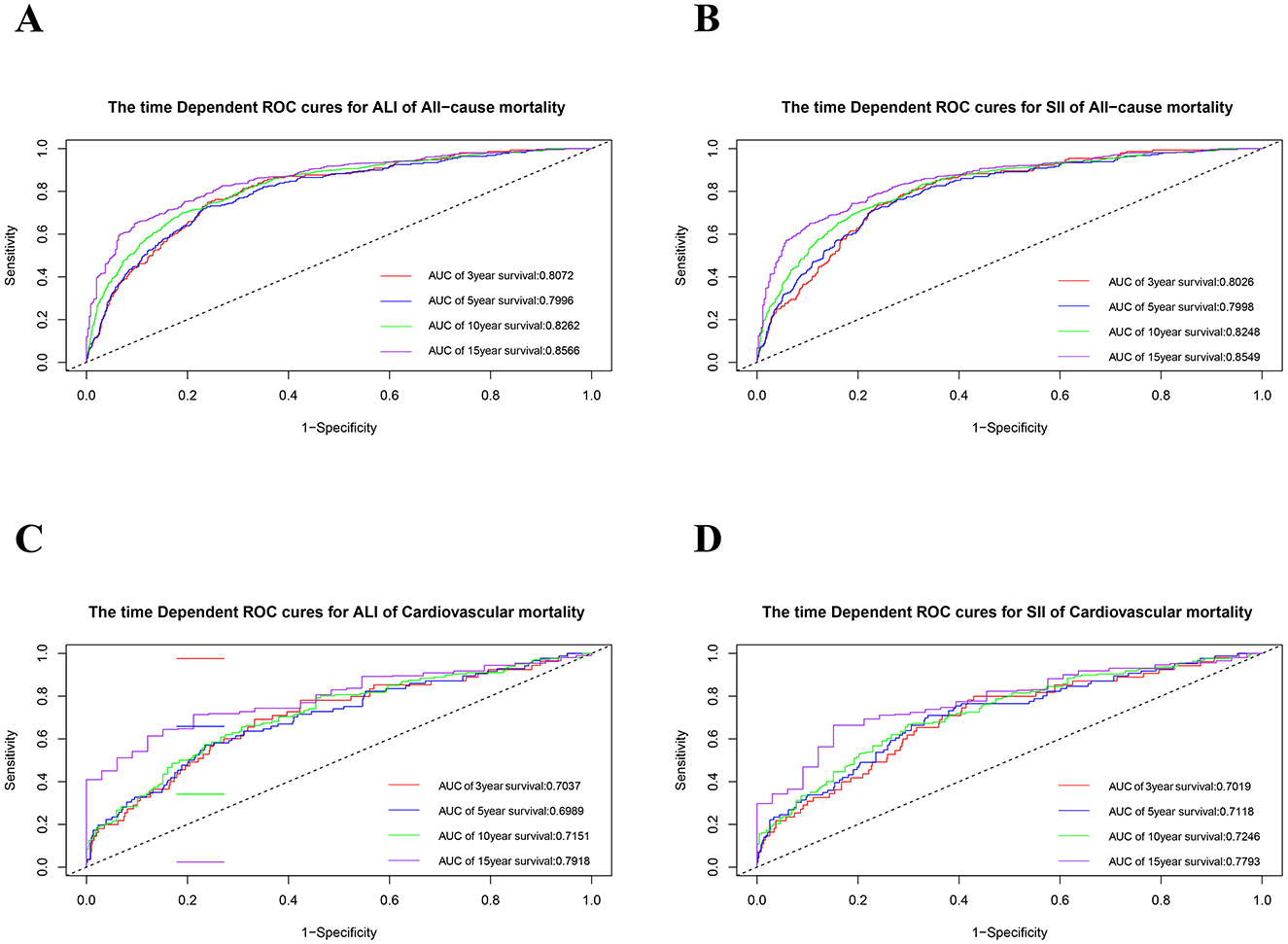
Figure 5. The time-dependent ROC curves for 3-, 5-, 10-, and 15- year survival predictions. (A) ALI for all-cause mortality; (B) SII for all-cause mortality; (C) ALI for cardiovascular mortality; (D) SII for cardiovascular mortality. ROC, receiver operating characteristic; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index.
This is a large sample study aimed at providing easy and accessible monitoring markers for the long-term management of OA and providing evidence-based evidence for policy makers and clinicians. Based on a sizable cohort, this investigation was the first to explore the connections between ALI and SII and cardiovascular as well as all-cause mortality in OA patients. An increase in ALI was associated with reduced all-cause and cardiovascular mortality after multivariate adjustments. On the other hand, there was a strong correlation between an increase SII and increased cardiovascular and all-cause mortality. The data also revealed a J-shaped non-linear association between SII levels and all-cause mortality, as well as an L-shaped non-linear link between ALI levels and all-cause mortality. These results highlight the significance of ALI and SII in assessing the risk and predicting the prognosis of osteoarthritis (OA) patients. Furthermore, ROC analysis confirmed the predictive capability of ALI and SII in forecasting survival outcomes in OA patients. The AUC values for predicting all-cause mortality at 3-, 5-, 10-, and 15-years were 0.8072, 0.7996, 0.8262, and 0.8566 for ALI, and 0.8026, 0.7998, 0.8248, and 0.8549 for SII, respectively.
Patients with OA experience higher all-cause death rates than the general population, especially if they have a history of cardiovascular disease, which is a major risk factor (31). Recent evidence suggests that OA increases the risk of developing CVD (32, 33). While OA was traditionally believed to result only from cartilage deterioration causing joint damage, a more modern perspective emphasizes the role of both systemic and local inflammation in causing cartilage damage (34). OA is now understood as a joint disease involving inflammation of the synovium (synovitis), with inflammatory cell infiltration being a key characteristic (35–37). Additionally, synovitis in OA is associated with more severe joint symptoms, greater cartilage loss, reduced mobility, and higher radiographic grades (38, 39). Obesity and metabolic syndrome are significant modifiable risk factors for both the onset and advancement of OA (40, 41).
As an index integrating inflammation and nutrition, the prognostic value of ALI has been demonstrated in patients with various types of cancer (17, 19, 21). Previous research has established ALI as a reliable marker for predicting outcomes in operable non-small cell lung cancer patients (42). Recent studies have indicated the utility of ALI in assessing prognosis and the protective effects in patients with rheumatoid arthritis (27), which is consistent with our findings. The L-shaped non-linear link between ALI levels and all-cause mortality in OA patients can be explained from several perspectives. Firstly, the NLR plays a crucial role. High levels of neutrophils indicated non-specific inflammation, while low levels of lymphocytes indicated impaired immune function (43). Therefore, a higher NLR may reflect immune activity during chronic inflammation (44). In OA, the inflammation is typically chronic and low-grade, involving primarily innate immune responses with some adaptive involvement (45). The infiltration of immune cells into the synovial membrane leads to synovitis in the early stages of OA. Neutrophils are the first immune cells to post the synovium following joint damage, promoting tissue degradation through neutrophil elastase (NE) action, osteophyte formation, and the production of inflammatory cytokines and chemokines (46). Meanwhile, lymphocytes also accumulate in the synovium with impaired T and B cell function observed in OA patients compared to healthy individuals, indicating a dysfunctional immune response that may contribute significantly to OA pathogenesis (47–49). Secondly, serum albumin serves as a widely used marker for assessing nutritional status. Research has shown higher levels of serum albumin in OA patients (50). Evidence from a Mendelian randomization (MR) study supports that high levels of retinol and albumin may have a preventive impact on the incidence of osteoarthritis (12). Serum albumin has been implicated in pain reduction and effective OA management by promoting anti-inflammatory prostaglandin production, assisting in inflammation resolution, supporting cartilage healing, and aiding in symptom improvement (51). Low albumin levels may predict poorer health outcomes due to its role as a marker for systemic inflammation and nutritional status, which are crucial factors in managing OA and its comorbidities, such as cardiovascular diseases (52). Thirdly, obesity has been linked to unfavorable outcomes in OA patients. Enhanced BMI significantly increases the risk of developing radiographic and clinical hand OA, as indicated in recent meta-analyses (14, 53). Obesity is a major cause of OA, driven by multiple factors beyond biomechanical changes. Additionally, adipose tissue induces the production of inflammatory mediators and cartilage degradation factors, which contribute to cartilage deterioration and the progression of OA (54, 55). A study on elderly patients revealed that obesity not only poses a risk for OA but also for cardiovascular disease (13). Research also suggested that extreme BMIs—both low and high—are linked to worse outcomes, including higher mortality (56).
The SII has been seen associated with higher mortality rates in individuals with various diseases (57, 58). Osteoarthritis is becoming regarded as a low-grade inflammatory condition that can be impacted by platelets. Elevated platelet levels, which frequently reflect systemic inflammation, are associated with OA severity and may imply a higher risk of mortality, particularly from cardiovascular problems, which are common in OA patients (59). There is a growing understanding of the significant role platelets play in inflammatory and immune responses. Platelet activation results in increasing immune responses, contributing to the degradation of cartilage and bone in OA (60). Platelet counts outside the normal range (either low or high) predicted increased all-cause and cardiovascular mortality in the general population and the elderly, which may explain the J-shaped association between SII and all-cause mortality of OA (61). The SII integrates three distinct types of inflammatory cells, demonstrating their interconnected impacts and cumulative influence. The approach avoids the limitations of single blood cell measures, which can be influenced by factors affecting cell volume, thereby providing a more comprehensive insight into the overall inflammatory condition. Based on the results, ALI and SII are expected to be predictors for risk stratification in patients with osteoarthritis. Our results showed that elevated ALI levels (>32.23) and lower SII values (< 991.43) are associated with consistently lower mortality rates among OA patients. Therefore, more attention and management should be strengthened for this population. The study revealed that the relationships between ALI, SII and mortality outcomes in individuals with OA varied by subgroup and were not totally consistent. In terms of all-cause events, the association with lower ALI levels and higher SII values was most pronounced in individuals aged 20–65 years, males, former smokers, and current drinkers. This could be explained by the higher prevalence of OA in females compared to and in older individuals compared to younger ones (62). Notably, the inflammatory and nutritional status of patients with osteoarthritis changes over time, and these changes may lead to anti-inflammatory and pro-inflammatory imbalances, comorbidities, and increased resistance to medications (63). Therefore, dynamic monitoring of ALI and SII and assessing their impact on OA mortality may offer deeper insights.
This research has several important implications. Firstly, an important advantage of this research is the use of a substantial, nationwide cohort of US people that was tracked over time. Secondly, we enhanced the robustness of our results by conducting extensive subgroup and sensitivity analyses. Particularly, our findings highlight the prospective value of the ALI and SII as predictive factors for assessing the likelihood of death in OA patients. These variables, which can be quite easily obtained from CBC, liver function tests, and basic body measurements without additional expense, could assist healthcare professionals in identifying OA patients at a heightened risk of mortality, enabling targeted interventions to reduce risks. In order to lessen confounding effects, we included total cholesterol and HDL as factors since hyperlipidemia is a risk factor for OA.
Nonetheless, there are several limitations. Firstly, the impact of dynamic changes in ALI and SII on the mortality rate of OA were not evaluated in this study. Secondly, while we adjusted for the majority of possible variables, residual confounding due to unmeasured factors may still be present. Genetic predisposition, psychological stress, and occupational factors can all influence inflammatory nutritional status, but could not be adjusted in our study. The effects of these confounding factors should be attended to in future database development and clinical studies to improve the accuracy of the model estimates. Thirdly, potential bias could have arisen due to the reliance on self-reported OA diagnoses by patients. The concordance rate between self-reported OA and clinically well-defined OA was 81%, so the OA diagnosis can be confirmed by combining clinical assessment and imaging data, such as bone density and digital radiography. Fourthly, the data extracted from the NHANES database are mainly from household interviews and questionnaires, then there may be a selection bias by excluding individuals with severe disability who may not participate in NHANES. Therefore, it is imperative to validate our discoveries in varied cohorts and explore the fundamental mechanisms further.
In conclusion, our research showed that lower ALI levels and higher SII values, reflecting increased systemic inflammation and poor nutritional status, are independently associated with higher risks of all-cause and cardiovascular mortality in US adults with osteoarthritis. There was a J-shaped non-linear link between SII values and all-cause mortality and an L-shaped non-linear relationship between ALI levels and all-cause mortality in OA patients. However, to better understand the complex interactions between inflammatory and nutritional biomarkers and survival outcomes in people with OA, more research is necessary.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/data-linkage/mortality.htm.
The studies involving humans were approved by the NHANES study was approved by the National Center for Health Statistics (NCHS) ethics review board, and all participants gave written informed permission. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
QG: Writing – original draft. YS: Writing – review & editing. FW: Writing – review & editing. WZ: Writing – review & editing. XD: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Grants from the National Key Research and Development Program of China (2021YFC2501304) and the Science and Technology Program of Jiangxi Province's Department of Health (20204254) were used to support this study.
The authors express their gratitude to the NHANES personnel and participants for their invaluable contributions. We are very grateful to Ph.D. Chen Yan for the English language improvement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1464414/full#supplementary-material
ALI, Advanced Lung Cancer Inflammation Index; Alb, albumin; AUC, Area under the curve; BMI, Body mass index; CBC, complete blood count; CI, Confidence interval; HR, Hazard ratios; KOA, knee osteoarthritis; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; NLR, Neutrophil–lymphocyte ratio; OA, Osteoarthritis; PIR, poverty-to-income ratio; RCS, Restricted cubic spline; ROC, Receiver-operator characteristic curve; SII, Systemic immune inflammation index.
1. Osteoarthritis Research Society International. Osteoarthritis: a serious disease, submitted to the U.S. Food and Drug Administration. Available at: https://www.oarsi.org/research/oa-serious-disease (accessed 3 December, 2020).
2. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. (2018) 30:160–7. doi: 10.1097/BOR.0000000000000479
3. Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. (2016) 68:1743–50. doi: 10.1002/acr.22897
4. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. (2014) 10:437–41. doi: 10.1038/nrrheum.2014.44
5. Barbour KE, Lui L-Y, Nevitt MC, Murphy LB, Helmick CG, Theis KA, et al. Hip osteoarthritis and the risk of all-cause and disease-specific mortality in older women: a population-based cohort study. Arthr Rheumatol. (2015) 67:1798–805. doi: 10.1002/art.39113
6. Liu Q, Niu J, Huang J, Ke Y, Tang X, Wu X, et al. Knee osteoarthritis and all-cause mortality: the Wuchuan Osteoarthritis Study. Osteoarthr Cartil. (2015) 23:1154–7. doi: 10.1016/j.joca.2015.03.021
7. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S bone and joint initiative. Semin Arthr Rheumat. (2014) 43:701–12. doi: 10.1016/j.semarthrit.2013.11.012
8. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2016) 12:580–92. doi: 10.1038/nrrheum.2016.136
9. Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. (2011) 70:805–11. doi: 10.1136/ard.2010.139618
10. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. (2013) 25:114–8. doi: 10.1097/BOR.0b013e32835a9414
11. Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engström G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. (2009) 68:490–6. doi: 10.1136/ard.2008.089748
12. Tang Y, Xu X, Zhang S, Kong W, Zhang W, Zhu T. Genetic liability for diet-derived circulating antioxidants, oxidative stress, and risk of osteoarthritis: a Mendelian randomization study. Front Nutr. (2023) 10:1233086. doi: 10.3389/fnut.2023.1233086
13. Hawker GA, King LK. The burden of osteoarthritis in older adults. Clin Geriatr Med. (2022) 38:181–92. doi: 10.1016/j.cger.2021.11.005
14. Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open. (2015) 5:e007568. doi: 10.1136/bmjopen-2014-007568
15. Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: review and meta analysis. Maturitas. (2015) 81:17–27. doi: 10.1016/j.maturitas.2015.02.009
16. Nelson CL, Elkassabany NM, Kamath AF, Liu J. Low albumin levels, more than morbid obesity, are associated with complications after TKA. Clin Orthop Relat Res. (2015) 473:3163–72. doi: 10.1007/s11999-015-4333-7
17. Mountzios G, Samantas E, Senghas K, Zervas E, Krisam J, Samitas K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO open. (2021) 6:100254. doi: 10.1016/j.esmoop.2021.100254
18. Song M, Zhang Q, Song C, Liu T, Zhang X, Ruan G, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:2504–14. doi: 10.1002/jcsm.13032
19. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kimura K, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer. (2019) 19:241. doi: 10.1186/s12885-019-5468-9
20. Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther. (2014) 7:1811–5. doi: 10.2147/OTT.S68084
21. Topkan E, Mertsoylu H, Ozdemir Y, Sezer A, Kucuk A, Besen AA, et al. Prognostic usefulness of advanced lung cancer inflammation index in locally-advanced pancreatic carcinoma patients treated with radical chemoradiotherapy. Cancer Manag Res. (2019) 11:8807–15. doi: 10.2147/CMAR.S222297
22. Huo C, Liu Y, Xie F, Zhao L, Huang H, Feng Q. Advanced lung cancer inflammation index predicts the outcomes of patients with non-metastatic gastric cancer after radical surgical resection. J Gastrointest Oncol. (2023) 14:1653–4. doi: 10.21037/jgo-23-315
23. Tu J, Wu B, Xiu J, Deng J, Lin S, Lu J, et al. Advanced lung cancer inflammation index is associated with long-term cardiovascular death in hypertensive patients: national health and nutrition examination study, 1999-2018. Front Physiol. (2023) 14:1074672. doi: 10.3389/fphys.2023.1074672
24. Fan W, Zhang Y, Liu Y, Ding Z, Si Y, Shi F, et al. Nomograms based on the advanced lung cancer inflammation index for the prediction of coronary artery disease and calcification. Clin Appl Thromb Hemost. (2021) 27:10760296211060455. doi: 10.1177/10760296211060455
25. Chen Y, Guan M, Wang R, Wang X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999-2018. Front Endocrinol. (2023) 14:1298345. doi: 10.3389/fendo.2023.1298345
26. Chen X, Hong C, Guo Z, Huang H, Ye L. Association between advanced lung cancer inflammation index and all-cause and cardiovascular mortality among stroke patients: NHANES, 1999-2018. Front Public Health. (2024) 12:1370322. doi: 10.3389/fpubh.2024.1370322
27. Ma Z, Wu S, Guo Y, Ouyang S, Wang N. Association of advanced lung cancer inflammation index with all-cause and cardiovascular mortality in US patients with rheumatoid arthritis. Front Nutr. (2024) 11:1397326. doi: 10.3389/fnut.2024.1397326
28. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
29. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
30. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. (2019) 8:886–94. doi: 10.21037/tlcr.2019.11.16
31. Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. (2011) 342:d1165. doi: 10.1136/bmj.d1165
32. Schieir O, Tosevski C, Glazier RH, Hogg-Johnson S, Badley EM. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis. (2017) 76:1396–404. doi: 10.1136/annrheumdis-2016-210275
33. Courties A, Sellam J, Maheu E, Cadet C, Barthe Y, Carrat F, et al. Coronary heart disease is associated with a worse clinical outcome of hand osteoarthritis: a cross-sectional and longitudinal study. RMD open. (2017) 3:e000344. doi: 10.1136/rmdopen-2016-000344
34. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartilage. (2013) 21:16–21. doi: 10.1016/j.joca.2012.11.012
35. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthr Rheum. (2012) 64:1697–707. doi: 10.1002/art.34453
36. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. (2007) 213:626–34. doi: 10.1002/jcp.21258
37. Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthr Rheum. (2010) 62:647–57. doi: 10.1002/art.27290
38. Krasnokutsky S, Belitskaya-Lévy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. (2011) 63:2983–91. doi: 10.1002/art.30471
39. Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. (2011) 63:391–400. doi: 10.1002/art.30137
40. Lane NE, Shidara K, Wise BL. Osteoarthritis year in review 2016: clinical. Osteoarthr Cartilage. (2017) 25:209–15. doi: 10.1016/j.joca.2016.09.025
41. Allen KD, Golightly YM. State of the evidence. Curr Opin Rheumatol. (2015) 27:276–83. doi: 10.1097/BOR.0000000000000161
42. Ma S, Li Z, Wang L. The advanced lung cancer inflammation index (ALI) predicted the postoperative survival rate of patients with non-small cell lung cancer and the construction of a nomogram model. World J Surg Oncol. (2024) 22:158. doi: 10.1186/s12957-024-03432-3
43. Azab B, Daoud J, Naeem FB, Nasr R, Ross J, Ghimire P, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study). Ren Fail. (2012) 34:571–6. doi: 10.3109/0886022X.2012.668741
44. Adane T, Melku M, Worku YB, Fasil A, Aynalem M, Kelem A, et al. The association between neutrophil-to-lymphocyte ratio and glycemic control in type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. (2023) 2023:3117396. doi: 10.1155/2023/3117396
45. Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. (2013) 146:185–96. doi: 10.1016/j.clim.2012.12.011
46. Chaney S, Vergara R, Qiryaqoz Z, Suggs K, Akkouch A. The involvement of neutrophils in the pathophysiology and treatment of osteoarthritis. Biomedicines. (2022) 10:1604. doi: 10.3390/biomedicines10071604
47. Ponchel F, Burska AN, Hensor EMA, Raja R, Campbell M, Emery P, et al. Changes in peripheral blood immune cell composition in osteoarthritis. Osteoarthr Cartilage. (2015) 23:1870–8. doi: 10.1016/j.joca.2015.06.018
48. Zhu W, Zhang X, Jiang Y, Liu X, Huang L, Wei Q, et al. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin Rheumatol. (2020) 39:523–32. doi: 10.1007/s10067-019-04768-y
49. Shan Y, Qi C, Liu Y, Gao H, Zhao D, Jiang Y. Increased frequency of peripheral blood follicular helper T cells and elevated serum IL-21 levels in patients with knee osteoarthritis. Mol Med Rep. (2017) 15:1095–102. doi: 10.3892/mmr.2017.6132
50. Shabestari M, Shabestari YR, Landin MA, Pepaj M, Cleland TP, Reseland JE, et al. Altered protein levels in bone marrow lesions of hip osteoarthritis: Analysis by proteomics and multiplex immunoassays. Int J Rheum Dis. (2020) 23:788–99. doi: 10.1111/1756-185X.13843
51. Bar-Or D, Thomas GW, Rael LT, Gersch ED, Rubinstein P, Brody E. Low molecular weight fraction of commercial human serum albumin induces morphologic and transcriptional changes of bone marrow-derived mesenchymal stem cells. Stem Cells Transl Med. (2015) 4:945–55. doi: 10.5966/sctm.2014-0293
52. Hawker GA, King LK. Furthering the links between osteoarthritis and other health conditions. Rheumatology. (2021) 60:3964–5. doi: 10.1093/rheumatology/keab376
53. Jiang L, Xie X, Wang Y, Wang Y, Lu Y, Tian T, et al. Body mass index and hand osteoarthritis susceptibility: an updated meta-analysis. Int J Rheum Dis. (2016) 19:1244–54. doi: 10.1111/1756-185X.12895
54. Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, Van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. (2010) 69:761–5. doi: 10.1136/ard.2008.106930
55. Conde J, Scotece M, Gómez R, Lopez V, Gómez-Reino JJ, Gualillo O. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis. (2011) 2011:203901. doi: 10.1155/2011/203901
56. Qi Y, Zhang B, Yang H. Associations between body mass index and all-cause and CVD mortality in agriculture, forestry, and fishing occupations: A prospective cohort study using NHANES data (1999-2014). PLoS ONE. (2024) 19:e0305922. doi: 10.1371/journal.pone.0305922
57. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (SII), system inflammation response Index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12:1128. doi: 10.3390/jcm12031128
58. Zhu D, Wang C, Zhou Y, Che H, Wang R, Cheng L, et al. The associations of two novel inflammation biomarkers, SIRI and SII, with mortality risk in patients with chronic heart failure. J Inflamm Res. (2024) 17:1255–64. doi: 10.2147/JIR.S451190
59. Pan H, Lin S. Association of hemoglobin, albumin, lymphocyte, and platelet score with risk of cerebrovascular, cardiovascular, and all-cause mortality in the general population: results from the NHANES 1999-2018. Front Endocrinol. (2023) 14:1173399. doi: 10.3389/fendo.2023.1173399
60. Tramś E, Malesa K, Pomianowski S, Kamiński R. Role of platelets in osteoarthritis-updated systematic review and meta-analysis on the role of platelet-rich plasma in osteoarthritis. Cells. (2022) 11:1080. doi: 10.3390/cells11071080
61. Song PS, Ahn KT, Jeong JO, Jeon KH, Song YB, Gwon HC, et al. Association of baseline platelet count with all-cause mortality after acute myocardial infarction. Eur Heart J. (2021) 10:176–83. doi: 10.1177/2048872620925257
62. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. (2016) 59:134–8. doi: 10.1016/j.rehab.2016.01.006
63. Voloshyna LO, Smiyan SI, Doholich OI. Evolution of pro-and anti-inflammatory cytokines rates as well as inflammatory markers in patients with osteoarthritis depending on the age comorbidity rate and on the treatment. Pharma Innov. (2017) 6:89. Available at: https://www.proquest.com/docview/1924453870/E1AF8C19B2AB4AF6PQ/1?sourcetype=Scholarly%20Journals
Keywords: systemic immune inflammation index (SII), advanced lung cancer inflammation index (ALI), mortality, osteoarthritis, National Health and Nutrition Examination Survey (NHANES), biomarker
Citation: Guo Q, Shao Y, Wang F, Zhou W and Duan X (2024) Association of inflammation and nutrition status with all-cause and cardiovascular mortality in individuals with osteoarthritis: NHANES, 1999–2018. Front. Nutr. 11:1464414. doi: 10.3389/fnut.2024.1464414
Received: 19 July 2024; Accepted: 05 November 2024;
Published: 21 November 2024.
Edited by:
I-Shiang Tzeng, National Taipei University, TaiwanReviewed by:
Mohamed Hsairi, Ministry of Public Health, TunisiaCopyright © 2024 Guo, Shao, Wang, Zhou and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinwang Duan, ZHh3X2VmeWZzbXlrQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.