
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 12 November 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1462789
Background and objective: Albuminuria is an important early marker of kidney damage and progression of chronic kidney disease and is also linked to several chronic systemic diseases. The Prognostic Nutritional Index (PNI) is widely used in the assessment of multiple diseases. However, research dealing with the relationship between PNI and albuminuria remains scarce. This research project aims to examine this association.
Methods and materials: The present study employed data from the National Health and Nutrition Examination Survey (NHANES) between 2017 and 2020, including 7,737 adult participants who met the study criteria. PNI was analyzed as a quartile-categorized variable. Multivariable regression models and smoothing curve fitting were adopted to examine the relationship between PNI and albuminuria. In order to ascertain the stability of the association across different populations, subgroup analyses were performed.
Results: The study found a statistically significant inverse relationship between higher PNI levels and the prevalence of albuminuria. The fully adjusted model indicates that a one-unit increase in PNI is associated with a 4% reduced odds of albuminuria prevalence [0.96 (0.93, 0.98)]. Quartile analysis showed a stable inverse relationship, with the highest PNI quartile having the significantly lower odds of albuminuria prevalence [0.76 (0.62, 0.94), p for trend = 0.0004]. Smooth curve fitting and two-piecewise linear regression models indicated a nonlinear relationship between PNI and albuminuria, with a turning point at 42. Subgroup analysis confirmed the reliability of the inverse relationship between PNI and albuminuria across all groups.
Conclusion: The findings of this study indicated that higher PNI levels are significantly inversely related to the odds prevalence of albuminuria. PNI could serve as an important predictor for the occurrence of albuminuria. Further prospective studies are needed to validate this association.
Albuminuria refers to the presence of abnormal amounts of albumin in the urine, typically expressed as an albumin-to-creatinine ratio (ACR) exceeding 30 mg/g (1). It acts as an early indicator of kidney damage and a significant predictor of the progression of chronic kidney disease (CKD) (2). Persistent high levels of albuminuria often indicate further deterioration of kidney function (3, 4). Albuminuria is also a complication of systemic chronic diseases such as diabetes and hypertension (5). Recent research indicates that persistent albuminuria is significantly correlated with adverse cardiovascular consequences, thereby establishing it not merely as a marker of renal health but also as an important indicator of systemic well-being. The prevalence of microalbuminuria figures between 5 and 19% in the general population, with rates as high as 23% observed in hypertensive patients and 40% in diabetic patients (6). The close association between albuminuria and CKD highlights its significance as a public health issue, emphasizing the importance of early clinical screening and intervention, especially in at-risk populations (7, 8).
.The Prognostic Nutritional Index (PNI) is a calculated value based on two variables: serum albumin levels and lymphocyte counts, and it evaluates an individual’s nutritional, immune, and inflammatory status. Initially, PNI was used to assess the prognosis of patients with gastrointestinal malignancies (9). Increasing evidence suggests that PNI is widely used in the assessment of various diseases, including mortality in coronary artery disease (10), survival rates in patients undergoing continuous renal replacement therapy (11), prognosis in cervical cancer (12), migraine (13), diabetic nephropathy in type 2 diabetes (14), and cognitive function in the elderly (15). Consequently, the potential clinical applications of PNI have attracted considerable interest from the medical community. The growing body of evidence lends support to the view that PNI has the potential to serve as a valuable biomarker in clinical practice.
Prior research has demonstrated a correlation between declining kidney function and the presence of systemic inflammation and nutritional status. A cohort study found that higher levels of some inflammatory markers (16–18) are associated with CKD. Notably, polymorphisms in proinflammatory cytokine genes have been associated with albuminuria in the Japanese general population, as evidenced by the Takahata study (19). This study suggests that genetic variations influencing inflammatory responses may contribute to the development of albuminuria. Similarly, the Framingham Offspring cohort study demonstrated a significant relationship between inflammation, kidney function, and albuminuria, reinforcing the notion that inflammatory processes play a crucial role in kidney health (20). Furthermore, recent findings indicate that podocyte-specific activation of the NOD-like receptor family, pyrin domain containing 3 inflammasome is a key contributor to diabetic kidney disease, highlighting the role of inflammation at the cellular level in the progression of renal complications (21). Malnutrition is a prevalent issue among individuals with renal insufficiency, and there is a significant association between malnutrition and elevated levels of inflammatory markers in these patients (22, 23). A correlation has been identified between micronutrient deficiencies and an increase in albuminuria. One such deficiency, vitamin D, has been demonstrated to promote albuminuria via its influence on inflammatory responses and immune functions (24, 25). Furthermore, evidence from studies indicates that administration of these micronutrients can mitigate kidney damage and improve albuminuria levels (26). However, there are few studies investigating the relationship between PNI and albuminuria. This study endeavors to investigate the relationship between PNI and albuminuria through the analysis of data derived from the National Health and Nutrition Examination Survey (NHANES). By addressing this gap in the literature, we hope to contribute valuable insights into the role of nutritional status in kidney health, thereby informing clinical interventions aimed at mitigating CKD risks.
NHANES is a national cross-sectional survey using a multi-stage probability sampling design to provide representative data on the health and nutritional status of the non-institutionalized US population. The NHANES study was approved by the Ethics Review Board of the National Center for Health Statistics (NCHS), and all participants provided written informed consent prior to enrollment. Our analysis focused on adults aged 20 years and older, utilizing data collected during the NHANES cycles from 2017 to 2020.
Initially, a total of 15,560 eligible participants were recruited for the study. However, we excluded participants who were under 20 years of age (6,328 individuals), those with incomplete ACR data (913 individuals), and those with incomplete PNI data (582 individuals). As a result, 7,737 participants met the final study criteria (Figure 1).
In this study, the exposure variable was PNI, which was calculated using the following formula: PNI = 10 × albumin (g/dL) + 0.005 × absolute lymphocyte count (103cells/μL). PNI was analyzed as a quartile-categorized variable. Albumin concentration was measured using bromocresol purple dye, and lymphocyte counts were obtained through the use of a Beckman Coulter DxH 800 analyzer.
Albuminuria was defined based on ACR, with a threshold of >30 mg/g, consistent with previous studies (27). Urinary albumin was determined via solid-phase fluorescence immunoassay, while urinary creatinine levels were quantified via modified Jaffe kinetic analysis.
In order to gain a deeper understanding of the relationship between PNI and albuminuria, we conducted a thorough review of existing cross-sectional studies and considered a range of potential confounding factors (26, 28, 29). These included demographic variables (age, gender, race, poverty income ratio, education level, and marital status), lifestyle factors (drinking status, smoking status, and moderate work activity status), and clinical measurements (white blood cell count, low-density lipoprotein cholesterol, total cholesterol, platelet count, high-density lipoprotein cholesterol, waist circumference, triglycerides, Body Mass Index (BMI), blood urea nitrogen, serum creatinine, uric acid, glomerular filtration rate, systolic blood pressure, diastolic blood pressure). Additionally, the presence of comorbid conditions such as diabetes, hypertension, hyperlipidemia, and kidney stones was recorded. All data generated by NHANES are freely accessible to the public via the CDC website.1
In light of the intrinsic complexity of the NHANES sampling design, we employed the use of appropriate sampling weights. A weighted ANOVA was employed to assess between-group differences based on PNI quartiles for continuous variables, while a weighted chi-square test was applied for categorical variables. The objective was to ascertain whether there was a correlation between PNI and albuminuria by utilizing multivariable regression models. In Model 1, no covariates were included in the adjustments. Model 2 included adjustments for the covariates of age, gender, and race. In Model 3, all the covariates previously mentioned were included as adjustment variables including demographic variables, lifestyle factors, and clinical measurements. Sensitivity analysis was performed using PNI as a quartile-categorized variable. Smooth curve fitting was employed to evaluate the non-linear correlation between PNI and urinary protein. In the event of a nonlinear relationship being identified, a two-piecewise linear regression model was adopted for comparison with the single-line model (non-segmented). This involved a log-likelihood ratio test to assess the performance of the two models. The threshold effect size was calculated and tested for significance. Finally, subgroup analysis was carried out with the aim of idenfitying the stability of the relationship between PNI and albuminuria across different populations, with stratification by gender, age, race, BMI, diabetes, and hypertension. A p-value <0.05 was deemed to be statistically significant. All analyses were conducted utilizing R version 4.1.3 and Empower software.
Table 1 presented the weighted distribution of all clinical characteristics of the included participants, stratified by PNI quartiles. A total of 7,737 adult participants were included, with a 48.27 ± 17.22-year-old average age, 48.34% male and 51.66% female. The prevalence of hypertension and diabetes mellitus was significantly higher in the lower percentile PNI group and decreased with increasing PNI levels. Furthermore, age demonstrated a decreasing trend in PNI quartiles (p < 0.0001). No significant differences in BMI, diastolic blood pressure, and triglycerides were observed at varying PNI levels. The PNI quartile ranges were 21–38, 38–41, 41–43, and 43–54. The overall prevalence of albuminuria was 10.23%; the prevalence in quartiles 1, 2, 3, and 4 was 14.11, 11.06, 8.8, and 8.1%, respectively.

Table 1. Baseline characteristics of the study population (Location: United States, Years: 2017–2020).
Table 2 demonstrated a negative correlation between PNI and the probability of albuminuria. In the unadjusted model [0.92 (0.90, 0.94)], the demographic-adjusted model [0.93 (0.91, 0.95)], and the fully adjusted model [0.96 (0.93, 0.98)], a negative correlation between PNI and albuminuria was observed. In the completely adjusted Model 3, a 4% decrease in the odds of albuminuria prevalence was observed with each unit increase in PNI, although the effect was slightly diminished, a significant negative correlation was still observed between PNI and albuminuria. When PNI was categorized into quartiles, a stable negative association was observed, which was statistically significant. The evidence provided thus far lends further support to the robustness of this correlation. A comparison of the lowest and highest PNI quartiles revealed a 24% lower prevalence of albuminuria in the latter [0.76 (0.62, 0.94), p for trend = 0.0004]. Although the data for the Q4 group demonstrated a robust association in both Models 1 and 2 (p < 0.0001), the observed association exhibited a slight reduction in strength but remained statistically significant following the adjustment for additional confounding variables in Model 3 (p = 0.0004). Nonlinear relationships between PNI and albuminuria were demonstrated by the smooth curve fitting analysis (Figure 2). The two-piecewise linear regression model showed a turning point at 42, with a log-likelihood ratio < 0.001 (Table 3).
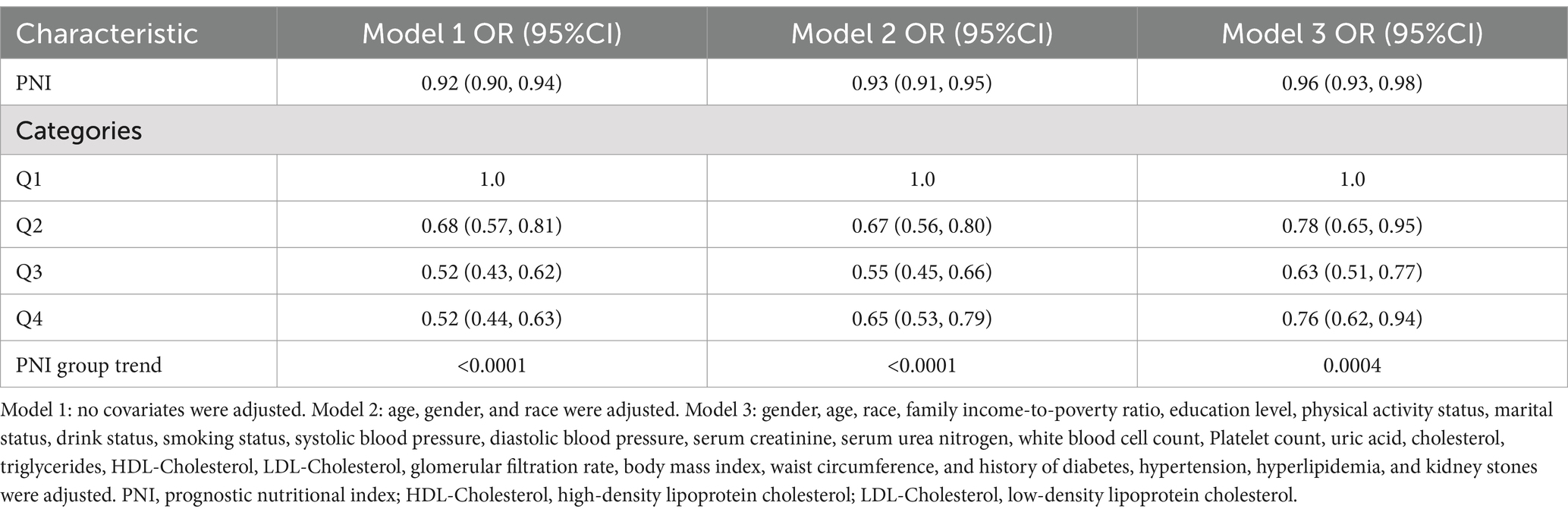
Table 2. Associations between prognostic nutritional index and albuminuria (Location: United States, Years: 2017–2020).
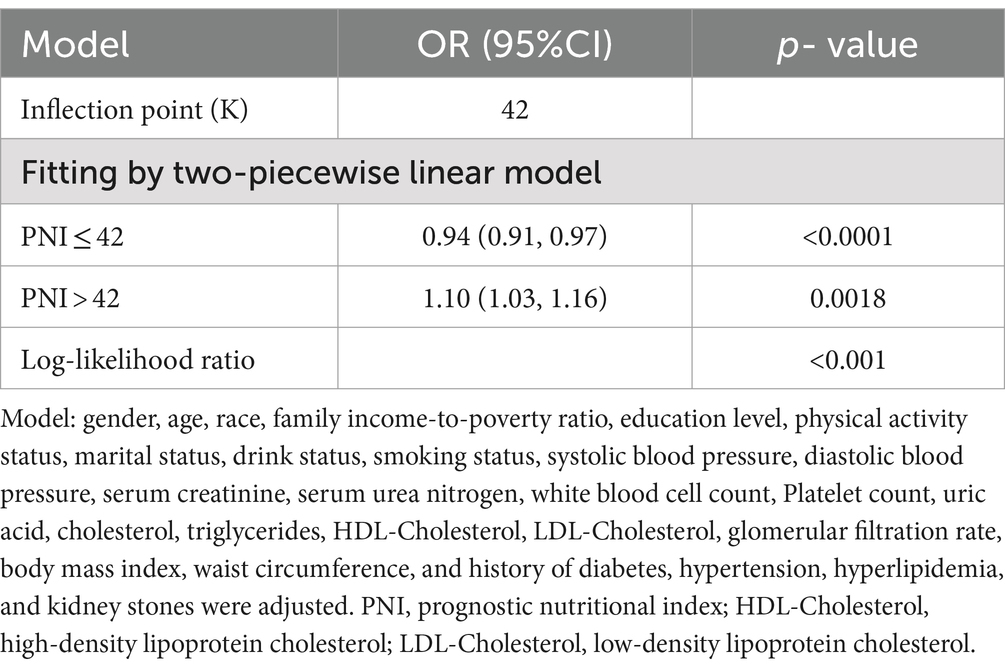
Table 3. Threshold effect analysis of prognostic nutritional index on albuminuria using a two-piecewise linear regression model (Location: United States, Years: 2017–2020).
Subgroup analyses showed that PNI was inversely related to albuminuria in all subgroups. Although some differences in the association between PNI and albuminuria were observed between the various subgroups, the interaction analyses demonstrated that these differences did not reach statistical significance (p-value >0.05 for all interactions). This suggests that the correlation between PNI and albuminuria was largely consistent, although the impact may have been more pronounced in certain subgroups. For instance, the correlation was less pronounced in female subjects in the sex-stratified analysis and did not attain statistical significance (p = 0.1691). Furthermore, the correlation between PNI and albuminuria was also not statistically significant in the 20–40 age group, individuals with normal weight, overweight individuals, individuals with diabetes mellitus, and certain racial categories (non-Hispanic whites and other races; Table 4).
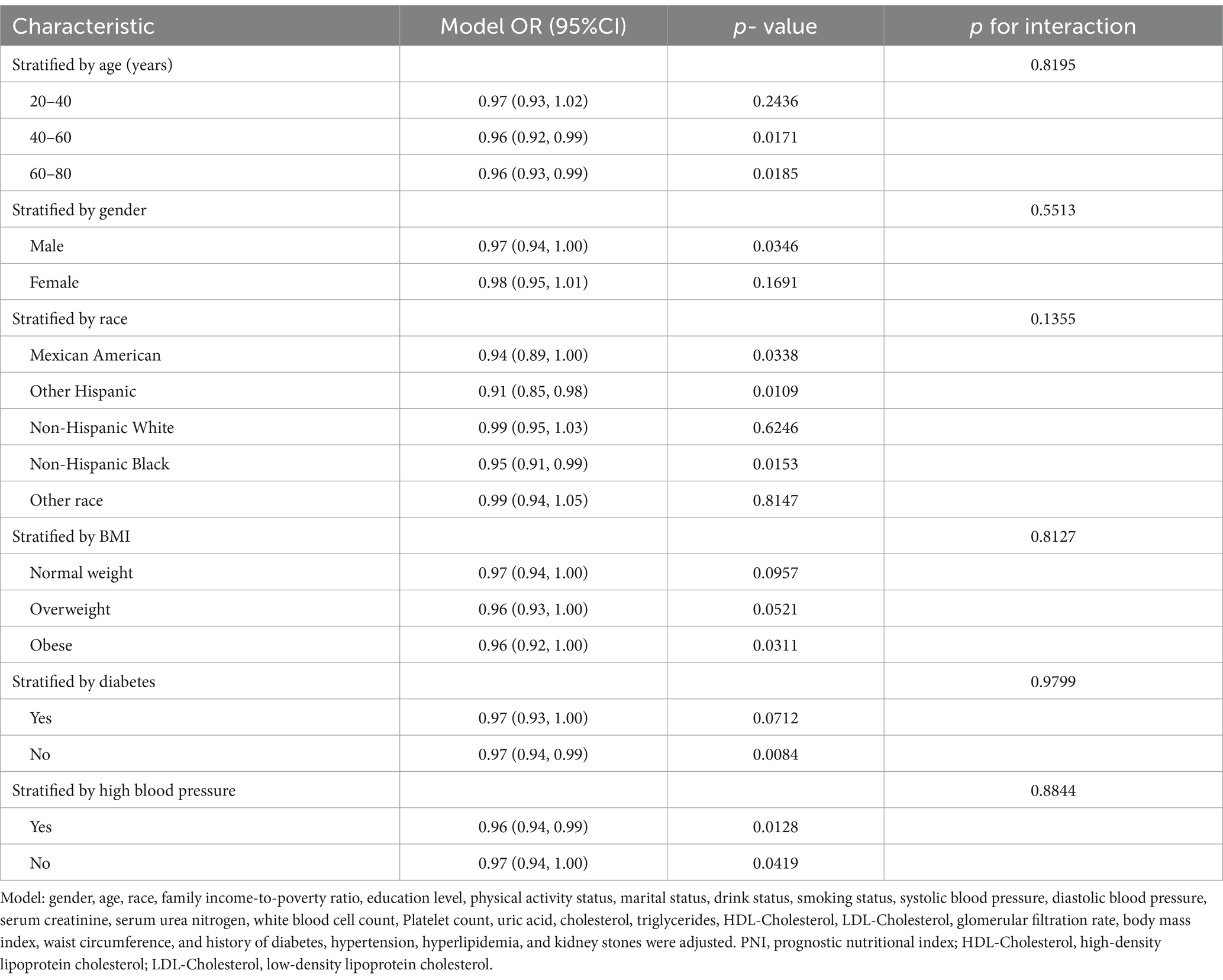
Table 4. Subgroup analysis of the association between prognostic nutritional index and albuminuria (Location: United States, Years: 2017–2020).
This study, which included 7,737 adult participants from the NHANES, aimed to assess the association between PNI and albuminuria. We observed that higher PNI levels were related to a lower odds prevalence of albuminuria. This negative association was still found to be stable in the completely adjusted model. Non-linear association between PNI and albuminuria suggested by smooth curve fitting, with a turning point at 42. No significant interaction effects were found in different subgroups, indicating that this inverse relationship was consistent across different populations. Our findings suggest that PNI can predict the occurrence of albuminuria. For individuals with low PNI levels, early management and intervention in nutrition and inflammation may reduce the incidence of albuminuria and slow the progression of kidney damage.
PNI is a composite index that is simple and easy to obtain, with broad clinical applications. PNI levels are associated with the risk and progression of kidney damage, according to existing studies. A meta-analysis conducted by Chen et al. revealed that PNI is a valuable predictive marker for acute kidney injury (AKI) in critically ill patients, with higher sensitivity in CKD patients compared to non-CKD patients (30). A retrospective cohort study by Zhang et al. involving 14,349 participants demonstrated that PNI serves as an independently prognostic factor for all-cause mortality in diabetic nephropathy patients. The study found that increased PNI levels were associated with a reduced risk of mortality [0.64, (0.459,0.892), p = 0.01]. PNI can also be employed as an efficacious indicator for the prediction of AKI resulting from the administration of contrast media in patients diagnosed with coronary artery disease (31, 32). Another retrospective study including 3,360 CKD patients, showed that elevated PNI were inversely correlated with mortality risk, with mortality decreasing in an “L” shape as PNI levels increased (33). These studies suggest that PNI has potential clinical value in predicting kidney damage.
PNI is composed of two critical factors: albumin and lymphocytes, which assess the body’s inflammation, nutrition, and immune status. The mechanisms underlying the relationship between PNI and albuminuria remain unclear. Existing studies suggest that inflammation and nutritional status are closely related to kidney function, which may explain the potential mechanisms between PNI and albuminuria. A 15-year follow-up cohort study including 4,926 participants demonstrated that in vitro inflammatory markers, including C-reactive protein, interleukin-6(IL-6), tumor necrosis factor-α (TNF-α), and white blood cell, were positively associated with a heightened risk of CKD (17). Furthermore, animal studies have indicated that inflammation is associated with declining kidney function. In IL-6 transgenic mice, overexpression of IL-6 promotes inflammation, mediates tubulointerstitial lesions, potentially affects glomerular damage, and increases oxidative stress and apoptosis, leading to progressive kidney injury and multiple myeloma kidney disease development (34). Another study in an inflammatory rat model showed that elevated TNF and interleukin-1 (IL-1) levels increased the severity of glomerular damage in nephritis (35). Pro-inflammatory cytokines accelerate protein degradation while inhibiting protein synthesis, leading to an imbalance that increases protein consumption and malnutrition (36). Serum albumin, another component of PNI, reflects an individual’s nutritional status. When glomerular filtration function is impaired, large amounts of protein, especially albumin, are lost in the urine, leading to decreased serum albumin levels. Continuous protein loss can cause malnutrition, decreased immune function, increased risk of infections, and other complications, further damaging kidney function (22). Malnutrition not only reduces protein synthesis but also affects kidney repair and regeneration (37). Serum albumin also has antioxidant and anti-inflammatory properties (38). A reduction in serum albumin levels is associated with an increase in inflammatory markers (39), with inflammatory cytokines including IL-1, IL-6, and TNF-α linked to decreased serum albumin levels (40). Normal physiological levels of albumin can inhibit the expression of vascular cell adhesion molecules induced by TNF-α, thereby reducing inflammation (41). Low albumin levels in diabetic patients may also accelerate oxidative stress and inflammatory responses, worsening kidney function (42, 43). A retrospective study by Zhang et al. indicated that diabetic nephropathy patients with low serum albumin levels were more likely to progress to end-stage renal disease (39). Similarly, albuminuria is an important predictor of poor prognosis in CKD patients (39, 44, 45). In summary, inflammation can cause malnutrition, and malnutrition can lead to decreased immune function, exacerbating inflammation in a vicious cycle, which in turn causes renal insufficiency and albuminuria.
A significant negative correlation was observed between PNI and albuminuria. Nevertheless, subgroup analyses demonstrated discrepancies in the magnitude and statistical significance of the association across gender, age, and ethnic groups. To illustrate, a significant correlation was identified between reduced PNI and elevated albuminuria in male subjects, whereas this association was not statistically significant in female subjects. This may be attributable to physiological differences between men and women and their disparate nutritional intake and health behaviors. Furthermore, no significant association was observed within the age group 20–40 years, which may be attributed to the fact that this age group typically exhibits superior health status, thereby attenuating the impact of PNI on albuminuria. Additionally, no significant associations were identified for individuals with normal weight, overweight status, diabetes mellitus, or among certain racial groups (non-Hispanic whites, other race). Further investigation into the underlying biological and socioeconomic factors may be necessary to elucidate these observations.
The study’s notable strengths include a large and representative sample size and the adjustment of potential confounders to provide accurate and reliable results. Nevertheless, a causal relationship between PNI and albuminuria cannot be established due to the cross-sectional design of the study. Secondly, despite the inclusion of numerous confounders, there are still some significant influences that remain uncontrolled for, such as medications and diseases that affect albumin levels. These unincluded confounders may have an impact on the association between PNI and albuminuria. Furthermore, PNI, as a static indicator, reflects the current nutritional status of an individual, but it does not capture its changes over time. Given that PNI can fluctuate in response to an individual’s health status and lifestyle habits, data obtained at a single point in time may not fully reflect the long-term nutritional status and its impact on health.
Our study indicated that higher PNI levels are associated with reduced albuminuria excretion. Our study lends support to the hypothesis that nutritional status may play a role in the progression of kidney injury. However, to gain a more comprehensive understanding of this relationship and to incorporate additional influencing factors, it would be beneficial to conduct prospective studies and explore the causal mechanisms of this association. Such studies should aim to identify the role of nutritional status and inflammatory markers in influencing PNI and albuminuria. Additionally, it would be beneficial to investigate the potential clinical applications of PNI in managing nutrition and inflammation in patients with CKD and other related conditions. By incorporating a diverse range of populations and considering various confounding factors, future research can provide more generalized findings. Furthermore, interventions aimed at improving nutritional status and reducing inflammation should be evaluated for their effectiveness in lowering albuminuria levels, potentially leading to new strategies for preventing CKD progression.
Publicly available datasets were analyzed in this study. This data can be found at: www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
ZJ: Writing – review & editing, Writing – original draft. XZ: Writing – review & editing, Writing – original draft. HJ: Writing – original draft. DZ: Writing – original draft. FS: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Technology Promotion Program of the Chinese People’s Liberation Army Air Force Medical Center (no. 2022ZTYB14) and Beijing Natural Science Foundation (no. 7232174).
We would like to thank all participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PNI, Prognostic Nutritional Index; NHANES, National Health and Nutrition Examination Survey; ACR, Albumin-to-Creatinine Ratio; CKD, Chronic Kidney Disease; AKI, Acute Kidney Injury; TNF-α, Tumor Necrosis Factor-α; IL-6, Interleukin-6; IL-1, Interleukin-1; BMI, Body Mass Index; CDC, Centers for Disease Control and Prevention.
1. Matsushita, K, van der Velde, M, Astor, BC, Woodward, M, Levey, AS, de Jong, PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/S0140-6736(10)60674-5
2. Molitoris, BA, Sandoval, RM, Yadav, SPS, and Wagner, MC. Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications. Physiol Rev. (2022) 102:1625–67. doi: 10.1152/physrev.00014.2021
3. Heerspink, HJL, Greene, T, Tighiouart, H, Gansevoort, RT, Coresh, J, Simon, AL, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. (2019) 7:128–39. doi: 10.1016/S2213-8587(18)30314-0
4. Vestergaard, AHS, Jensen, SK, Heide-Jørgensen, U, Frederiksen, LE, Birn, H, Jarbøl, DE, et al. Risk factor analysis for a rapid progression of chronic kidney disease. Nephrol Dial Transplant. (2024) 39:1150–8. doi: 10.1093/ndt/gfad271
5. Mottl, AK, and Nicholas, SB. KDOQI Commentary on the KDIGO 2022 update to the clinical practice guideline for diabetes management in CKD. Am J Kidney Dis. (2022) 83:277–87. doi: 10.1053/j.ajkd.2023.09.003
6. Thoenes, M, Bramlage, P, Khan, BV, Schieffer, B, Kirch, W, and Weir, MR. Albuminuria: pathophysiology, epidemiology and clinical relevance of an emerging marker for cardiovascular disease. Futur Cardiol. (2007) 3:519–24. doi: 10.2217/14796678.3.5.519
7. Hahn, KM, and Strutz, F. The early diagnosis and treatment of chronic renal insufficiency. Dtsch Arztebl Int. (2024) 121:428–35. doi: 10.3238/arztebl.m2024.0072
8. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
9. Onodera, T, Goseki, N, and Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
10. Wu, TT, Pan, Y, Zhi, XY, Deng, CJ, Wang, S, Guo, XX, et al. Association between extremely high prognostic nutritional index and all-cause mortality in patients with coronary artery disease: secondary analysis of a prospective cohort study in China. BMJ Open. (2024) 14:e079954. doi: 10.1136/bmjopen-2023-079954
11. Lee, YF, Lin, PR, Wu, SH, Hsu, HH, Yang, SY, and Kor, CT. Impact of the prognostic nutritional index on renal replacement therapy-free survival and mortality in patients on continuous renal replacement therapy. Ren Fail. (2024) 46:2365394. doi: 10.1080/0886022X.2024.2365394
12. Chen, JL, Huang, CY, Shih, IL, Liou, YM, Tai, YJ, Chiang, YC, et al. Prognostic nutritional index and neutrophil-lymphocyte ratio predict toxicities and prognosis in patients with cervical cancer treated with curative radiochemotherapy. J Formos Med Assoc. (2024) 123:671–8. doi: 10.1016/j.jfma.2023.10.022
13. Peng, C, Gao, L, Wu, K, Jiang, X, Chen, X, Li, C, et al. Association between the prognostic nutritional index and severe headache or migraine: a population-based study. Nutr Neurosci. (2023) 26:1202–11. doi: 10.1080/1028415X.2022.2143958
14. Zhang, J, Chen, Y, Zou, L, and Gong, R. Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. (2023) 60:235–45. doi: 10.1007/s00592-022-01985-x
15. Zhou, J, Ma, L, Zhao, L, Sheng, J, Xu, Y, Chen, J, et al. Association between the prognostic nutritional index and cognitive function among older adults in the United States: a population-based study. J Alzheimers Dis. (2021) 83:819–31. doi: 10.3233/JAD-210141
16. Panichi, V, Migliori, M, De Pietro, S, Taccola, D, Bianchi, AM, Giovannini, L, et al. C-reactive protein and interleukin-6 levels are related to renal function in predialytic chronic renal failure. Nephron. (2002) 91:594–600. doi: 10.1159/000065018
17. Shankar, A, Sun, L, Klein, BE, Lee, KE, Muntner, P, Nieto, FJ, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. (2011) 80:1231–8. doi: 10.1038/ki.2011.283
18. Descamps-Latscha, B, Herbelin, A, Nguyen, AT, Roux-Lombard, P, Zingraff, J, Moynot, A, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. (1995) 154:882–92. doi: 10.4049/jimmunol.154.2.882
19. Mashima, Y, Konta, T, Kudo, K, Suzuki, K, Ikeda, A, Ichikawa, K, et al. Polymorphism of proinflammatory cytokine genes and albuminuria in the Japanese general population: the Takahata study. Nephrol Dial Transplant. (2011) 26:3902–7. doi: 10.1093/ndt/gfr105
20. Upadhyay, A, Larson, MG, Guo, CY, Vasan, RS, Lipinska, I, O'Donnell, CJ, et al. Inflammation, kidney function and albuminuria in the Framingham offspring cohort. Nephrol Dial Transplant. (2011) 26:920–6. doi: 10.1093/ndt/gfq471
21. Shahzad, K, Fatima, S, Khawaja, H, Elwakiel, A, Gadi, I, Ambreen, S, et al. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. (2022) 102:766–79. doi: 10.1016/j.kint.2022.06.010
22. Kalantar-Zadeh, K, Ikizler, TA, Block, G, Avram, MM, and Kopple, JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. (2003) 42:864–81. doi: 10.1016/j.ajkd.2003.07.016
23. Kalantar-Zadeh, K, Stenvinkel, P, Bross, R, Khawar, OS, Rammohan, M, Colman, S, et al. Kidney insufficiency and nutrient-based modulation of inflammation. Curr Opin Clin Nutr Metab Care. (2005) 8:388–96. doi: 10.1097/01.mco.0000172578.56396.9e
24. Damasiewicz, MJ, Magliano, DJ, Daly, RM, Gagnon, C, Lu, ZX, Ebeling, PR, et al. 25-Hydroxyvitamin D levels and chronic kidney disease in the AusDiab (Australian diabetes, obesity and lifestyle) study. BMC Nephrol. (2012) 13:55. doi: 10.1186/1471-2369-13-55
25. Liu, M, Wang, J, and He, Y. Serum 25-Hydroxyvitamin D were associated with higher risk of both albuminuria and impaired GFR incidence: a cohort study based on CLHLS study. BMC Nephrol. (2019) 20:20. doi: 10.1186/s12882-019-1202-8
26. Li, YC. Renoprotective effects of vitamin D analogs. Kidney Int. (2010) 78:134–9. doi: 10.1038/ki.2009.175
27. Cho, YT, Chen, CW, Chen, MP, Hu, JL, Su, H, Shiea, J, et al. Diagnosis of albuminuria by tryptic digestion and matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. Clin Chim Acta. (2013) 420:76–81. doi: 10.1016/j.cca.2012.12.016
28. Yu, X, Pu, X, Xi, Y, Li, X, Li, H, and Zheng, D. Association between the lipid accumulation product and chronic kidney disease among adults in the United States. Sci Rep. (2024) 14:21423. doi: 10.1038/s41598-024-71894-2
29. Pi, Y, Liao, X, Song, X, Cao, Y, Tang, X, Lin, G, et al. Association between dietary intake of selenium and chronic kidney disease in US adults: a cross-sectional study of NHANES 2015-2018. Front Nutr. (2024) 11:1396470. doi: 10.3389/fnut.2024.1396470
30. Chen, JJ, Lee, TH, Lai, PC, Chang, CH, Wu, CH, and Huang, YT. Prognostic nutritional index as a predictive marker for acute kidney injury in adult critical illness population: a systematic review and diagnostic test accuracy meta-analysis. J Intensive Care. (2024) 12:16. doi: 10.1186/s40560-024-00729-z
31. Sertdemir, AL, İcli, A, Aribas, A, Tatar, S, Akilli, NB, Alsancak, Y, et al. Prognostic nutritional index and the risk of acute kidney injury in patients with acute coronary syndrome. Rev Assoc Med Bras. (1992) 67:1124–9. doi: 10.1590/1806-9282.20210460
32. Han, M, Lee, HW, Lee, HC, Kim, HJ, Seong, EY, and Song, SH. Impact of nutritional index on contrast-associated acute kidney injury and mortality after percutaneous coronary intervention. Sci Rep. (2021) 11:7123. doi: 10.1038/s41598-021-86680-7
33. Yu, JH, Chen, Y, and Yin, MG. Association between the prognostic nutritional index (PNI) and all-cause mortality in patients with chronic kidney disease. Ren Fail. (2023) 45:2264393. doi: 10.1080/0886022X.2023.2264393
34. Fattori, E, Della Rocca, C, Costa, P, Giorgio, M, Dente, B, Pozzi, L, et al. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood. (1994) 83:2570–9. doi: 10.1182/blood.V83.9.2570.2570
35. Tomosugi, NI, Cashman, SJ, Hay, H, Pusey, CD, Evans, DJ, Shaw, A, et al. Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J Immunol. (1989) 142:3083–90. doi: 10.4049/jimmunol.142.9.3083
36. Ahmed, G. An overview of inflammation: mechanism and consequences. Front Biol. (2011) 6:1123. doi: 10.1007/s11515-011-1123-9
37. Kopple, JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. (2001) 37:S66–70. doi: 10.1053/ajkd.2001.20748
38. Arques, S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. (2018) 52:8–12. doi: 10.1016/j.ejim.2018.04.014
39. Zhang, J, Zhang, R, Wang, Y, Li, H, Han, Q, Wu, Y, et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res. (2019) 2019:7825804. doi: 10.1155/2019/7825804
40. Moshage, HJ, Janssen, JA, Franssen, JH, Hafkenscheid, JC, and Yap, SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. (1987) 79:1635–41. doi: 10.1172/JCI113000
41. Zhang, WJ, and Frei, B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. (2002) 55:820–9. doi: 10.1016/S0008-6363(02)00492-3
42. Michelis, R, Kristal, B, Zeitun, T, Shapiro, G, Fridman, Y, Geron, R, et al. Albumin oxidation leads to neutrophil activation in vitro and inaccurate measurement of serum albumin in patients with diabetic nephropathy. Free Radic Biol Med. (2013) 60:49–55. doi: 10.1016/j.freeradbiomed.2013.02.005
43. Michelis, R, Kristal, B, Snitkovsky, T, and Sela, S. Oxidative modifications impair albumin quantification. Biochem Biophys Res Commun. (2010) 401:137–42. doi: 10.1016/j.bbrc.2010.09.027
44. Hou, FF, Zhang, X, Zhang, GH, Xie, D, Chen, PY, Zhang, WR, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. (2006) 354:131–40. doi: 10.1056/NEJMoa053107
Keywords: albuminuria, cross-sectional study, inflammation, kidney injury, nutrition, prognostic nutritional index
Citation: Jiang Z, Zhu X, Jiang H, Zhao D and Su F (2024) Prognostic nutritional index and albuminuria in adults aged 20 years and above: a cross-sectional analysis in the United States. Front. Nutr. 11:1462789. doi: 10.3389/fnut.2024.1462789
Received: 01 August 2024; Accepted: 30 October 2024;
Published: 12 November 2024.
Edited by:
Ayenew Negesse, Debre Markos University, EthiopiaReviewed by:
Xianfeng Wu, Shanghai Jiao Tong University, ChinaCopyright © 2024 Jiang, Zhu, Jiang, Zhao and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feifei Su, c3VmZWlmZWlCSjIwMjRAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.