
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 08 November 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1456091
This article is part of the Research Topic Assessment of Nutritional Status in Chronic Diseases View all 21 articles
Introduction: Malnutrition remains common in adults over 60 years old. Although there are screening and diagnostic tools for malnutrition, there is no globally used approach to diagnosing malnutrition in older adults admitted to the hospital. In this study, we verified the agreement between the Global Leadership Initiative on Malnutrition (GLIM) and the Mini Nutritional Assessment (MNA) and the ability of the instruments to identify frailty and sarcopenia associated with malnutrition.
Methods: For adults over 60 years old, malnutrition diagnosis was performed using the Mini Nutritional Assessment Full Form (MNA-FF) tool and the GLIM criteria, which included calf circumference and fat-free mass index to assess muscle mass, with and without the Mini Nutritional Assessment Short Form (MNA-SF) screening. Health conditions were assessed in older adults, and the association of these conditions with malnutrition was analyzed using both tools.
Results: A total of 432 adults over 60 years old were investigated with a mean age of 71.14 ± 8 years. The GLIM criteria with the nutritional screening tool identified 61–63% of older adults as malnourished. Of these, 63–64% were severely malnourished. The MNA-FF tool classified 20% of those assessed as malnourished. The agreement between the MNA-FF and GLIM was better with the use of screening, with a kappa (K) value of −0.10 and − 0.11. Sarcopenia was associated with malnutrition as identified by the MNA-FF (OR: 3.08, 95% CI: 1.84–5.14) and only by the GLIM ANTHRO (OR: 1.66, 95% CI: 1.05–2.63). Frailty was associated with the MNA-FF (OR: 15.99, 95% CI: 2.16–118.36), GLIM ANTHRO (OR: 2.21, 95% CI: 1.31–3.71), and GLIM BIA (OR: 2.45, 95% CI: 1.45–4.12).
Conclusion: It is possible to verify that divergent conceptual frameworks are used to understand malnutrition by the MNA-FF and GLIM and that the GLIM obtained a greater number of malnutrition diagnoses. Both the GLIM ANTHRO and the MNA-FF associated malnutrition with frailty and sarcopenia, with higher hazard ratios for the MNA-FF.
Malnutrition remains common in older populations around the world. Current estimates indicate that around a quarter of older adults (65 years and older) are malnourished or at risk of malnutrition (1). This number is likely to increase along with the rapid increase in the aging population. The United Nations (UN) predicts that, between 2019 and 2050, the population aged 65 and older will double in many regions (2). To reduce malnutrition in older populations, a timely and concerted effort is needed to prioritize, prevent, recognize, and adequately manage malnutrition in this age group (3).
There are no simple and unequivocal criteria to diagnose malnutrition mainly those associated with nutritional and clinical conditions with high specificity and sensitivity (4). The Mini Nutritional Assessment (MNA) is considered one of the most validated tools for identifying malnutrition or risk of malnutrition in older adults (5, 6). However, the Mini Nutritional Assessment Full Form (MNA-FF) has disadvantages, including subjective questions that are not appropriate for older adults who are hospitalized, difficulty in administering it to older adults with cognitive impairment, and a completion time of 10 to 15 min (7). Even so, it is validated as a good nutritional screening tool (8). Thus, various tools have been applied to quickly identify malnutrition in hospitalized older adults, each with its own strengths and weaknesses (7).
The Global Leadership Initiative on Malnutrition (GLIM) published a proposal to facilitate malnutrition diagnoses with more specific and objective criteria in an approach that begins with nutritional risk screening followed by two steps, one of which consists of a more in-depth assessment to diagnose malnutrition. GLIM consists of three phenotypic criteria (reduced body mass index (BMI), unintentional weight loss, and reduced muscle mass) and two etiological criteria (reduced dietary intake/impaired nutrient assimilation and inflammation or disease burden). The presence of at least one of each type of criterion contributes to establishing a malnutrition diagnosis, and phenotypic criteria can be used to classify severity (4).
Clinical practice will benefit from a validated nutritional assessment instrument in adults over 60 years of age admitted to the hospital. Thus, this research aimed to verify the malnutrition diagnosis agreement using the GLIM criteria compared to the MNA-FF in adults over 60 years of age admitted to the hospital, checking which of the two tools is best associated with the clinical conditions presented by adults.
Participants in this study, aged 60 years or older, were recruited from a university hospital and admitted to the geriatric or medical clinic. They were included based on specific criteria: both genders, age 60 or over, with appropriate physical and clinical conditions as per the data collected from their medical records, and the ability to answer the required questions. Exclusion criteria included: bedridden individuals, which made anthropometric assessment (weight and height) impossible; the presence of edema in the extremities, anasarca, or ascites that could lead to weight overestimation; limb amputations; individuals ineligible for disease-modifying therapies based on their medical records; those with spinal cord injuries or compression; individuals with pacemakers, heart valves, or metal prostheses; corticotherapy treatment; individuals with severe liver or kidney disease; and individuals with consumptive syndromes. This study was conducted between June 2021 and June 2023. The surveys were carried out by the research team duly trained within 48 h after hospital admission and other information came from hospital records. The study was carried out in accordance with the ethical guidelines for human research, and informed consent was obtained from all participants. The study was approved by the Ethics Committee (REC Protocol Approval 4.949.371/September/2021) and steered under the Declaration of Helsinki.
Body weight and height were measured or estimated (9, 10), and arm circumference (AC), calf circumference (CC) (11), and body mass index (BMI) were calculated. For handgrip strength, a hydraulic hand dynamometer was used, and the maximum value of three measurements of the dominant hand was used (12).
Bioelectrical impedance analysis (BIA) was carried out using Sanny® equipment, specifically the tetrapolar BioSanny4 1,010 model. All measurements were performed under standardized conditions in accordance with the manufacturer’s protocol. The calculations were made using regression equations (13, 14) incorporated into the BIA software. Fat mass (FM), skeletal muscle mass (SMM), and fat-free mass (FFM) were assessed, with FFM being the final measure used to calculate the fat-free mass index (FFMI).
In step 0 of the GLIM approach, the Mini Nutritional Assessment Short Form (MNA-SF) screening tool was used to screen the risk of malnutrition. The MNA is divided into screening and global assessment, and both parts were used in the research (15). Recent unintentional weight loss and reduced food intake were self-reported.
For stages 1 and 2 of the GLIM, which involve diagnosing and classifying the severity of malnutrition, all the GLIM criteria were applied (4). For step 1, phenotypic criteria, the BMI was categorized into moderately low, if BMI <20 kg/m2 (<22 if over 70 years old), and severely low, if BMI <18.5 kg /m2 (<20 if over 70 years old). For weight loss in the last 6 months, a cutoff point of 10% was used. Low body muscle mass, as measured by BIA, was defined as a FFMI (FFM (kg)/height (m2)) <15 kg/m2 for women and < 17 kg/m2 for men, indicating moderate malnutrition, according to the FFM cutoff values (12, 16). No additional FFMI cutoffs were used to define severe malnutrition as this is not specified in the original GLIM publication (4).
For the etiological criteria assessing participants’ disease/inflammation burden, the following were considered: acute disease, acute chronic disease, infection, or injury, all of which are generally associated with inflammatory activity. This criterion was supported by C-reactive protein (CRP) assessment (> 5 mg/L, when the contribution of inflammatory components was uncertain) (17, 18). The other etiological criteria were obtained through interviews, and malabsorptive intestinal conditions were collected from the patients’ medical records. For step 2 of the GLIM process, malnutrition severity was categorized as moderate by BMI and/or weight loss of 5 to 10% and/or reduced FFMI (19), using cutoff points CC < 33 cm for women and < 34 cm for men [21]. Participants were classified as severely malnourished by BMI and/or weight loss >10% [4] and/or CC < 31 cm for women and < 32 cm for men (20).
To assess the risk of malnutrition and the presence of malnutrition, MNA, (15) was applied. Isolated screening was used in the initial part of the GLIM tool. It consists of six items pertaining to food intake, involuntary weight loss, mobility, acute disease or psychological stress, neuropsychological problems, and BMI. A score of 12 or greater indicates that the individual was well nourished and needs no further intervention. A score of 8 to 11 indicates that the person was at risk of malnutrition, and a score of 7 or less indicates that the individual was malnourished. The complete MNA-FF was also used for nutritional diagnosis, as it is considered the gold standard for older adults. The first part of MNA-SF (short form) comprises six questions. The second part consists of 12 questions with a maximum score of 16 points. At the end, the scores were added, and patients were classified as having normal nutritional status (score > 23.5), and at risk of malnutrition (score 17–23.5) or malnourished (score < 17).
Sarcopenia diagnosis was conducted according to the algorithm suggested in the European consensus on sarcopenia. Individuals at risk of sarcopenia were assessed using the SARC-F muscular strength assessment via dynamometry. If the measured strength was reduced (< 27 KgF for men and < 16 KgF for women), the test result was considered positive for probable sarcopenia, indicating the need for further confirmation of low muscle quantity and quality (21). This was carried out using BIA appendicular skeletal muscle mass (ASMM) values, being considered reduced if <20 kg for men and < 15 kg for women, resulting in a positive diagnosis for sarcopenia (12).
Frailty syndrome was screened using the self-referred frailty instrument developed by Nunes et al. (2015) (22). Participants were classified as frail if they exhibited three or more of the five components, pre-frail with one or two components present, and not frail if none of the components were met.
For statistical analysis, the Statistical Package for the Social Sciences (SPSS) version 21.0 (SPSS Inc., Chicago, IL, USA) was used. The Kolmogorov–Smirnov test was used for continuous variables. Student’s t-test was then applied to continuous data with normal distribution. For categorical variables, the chi-square test was performed. When expected cell values were less than 5, Fisher’s exact test was considered. Pearson’s correlation was used for normal distribution.
The statistical analyses recommended by Van Der Schueren were used to validate the GLIM criteria (23), using individuals with any combination of phenotypic and etiological criteria. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) they were calculated using the association of the MNA-SF screening instrument with the other GLIM criteria versus MNA-FF. Sensitivity and specificity of >80% were interpreted as acceptable for malnutrition diagnosis, and agreement between instruments was calculated using Cohen’s kappa. The level of agreement was interpreted as almost perfect if Cohen’s kappa (k) was >0.91, strong if k = 0.81–0.90, moderate if k = 0.60–0.80, weak if k = 0.40–0.59, and minimum if k < 0.40. Binomial logistic regression analysis was performed to assess relationships between sarcopenia, frailty, and malnourished by MNA-FF, GLIM ANTHRO, and GLIM BIA. Adjustments were made in adjustment for sex and age. The results were expressed with odds ratios (ORs) and 95% confidence intervals (95%CIs). The results that presented a significance level lower than 0.05 were considered significant.
A total of 432 individuals aged 60 or over were eligible, and their sociodemographic, clinical, and anthropometric characteristics are described in Table 1. The results of the total GLIM process are reported in Figure 1. Using MNA-SF as a screening tool in step 0, 81% were observed to be at risk of malnutrition. Depending on the methodology in step 1, it was observed that 36–37% of participants were categorized as mildly/moderately malnourished, and 63–64% of participants were categorized as severely malnourished. Meanwhile, MNA-FF found only 20% malnourished. When applying GLIM independent of screening, the proportion of malnourished adults over 60 years old was higher for step 1 and lower than GLIM with screening only for severe malnutrition.
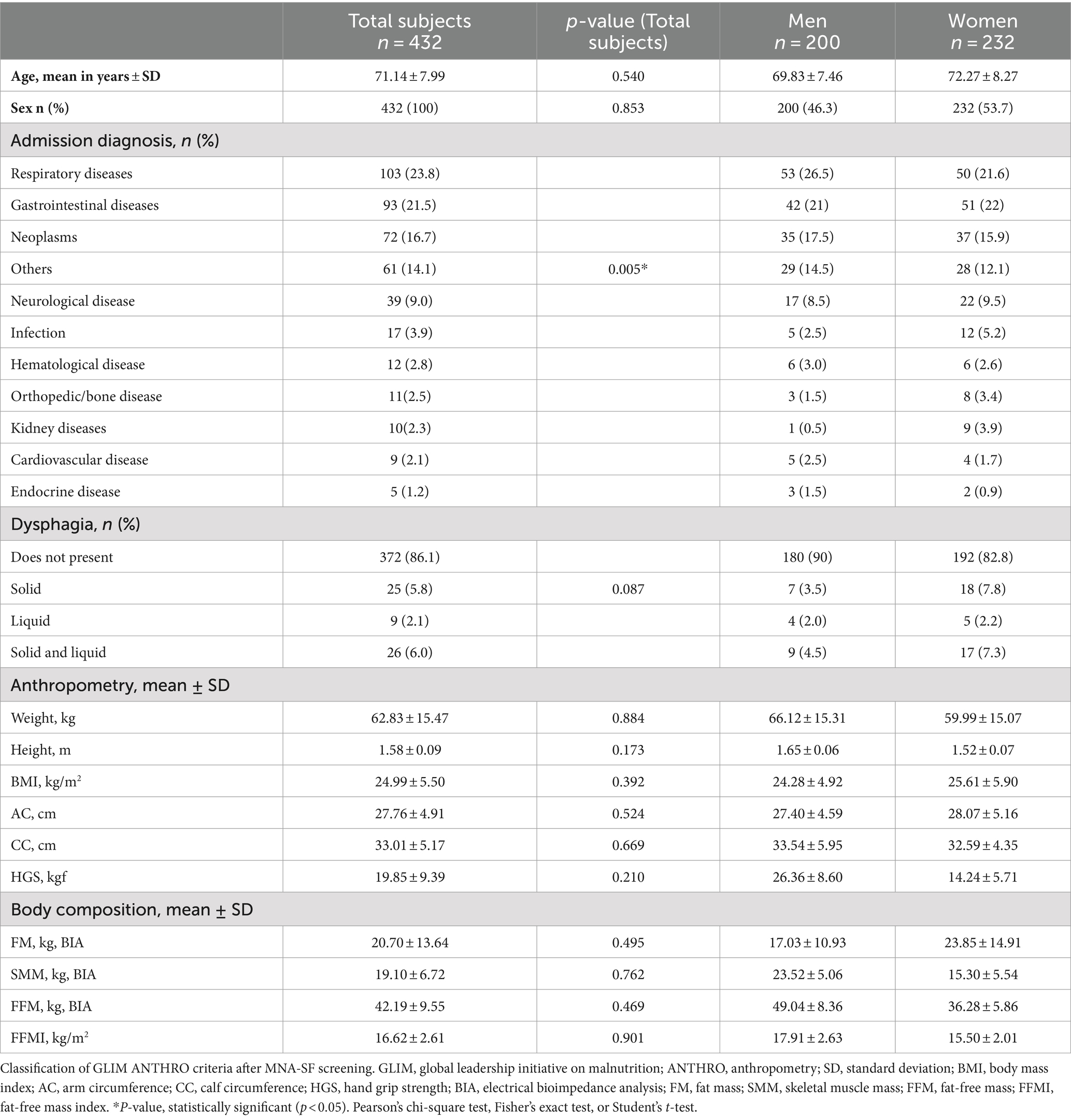
Table 1. Characterization and association of sociodemographic, clinical, and anthropometric variables with malnutrition using GLIM ANTHRO.
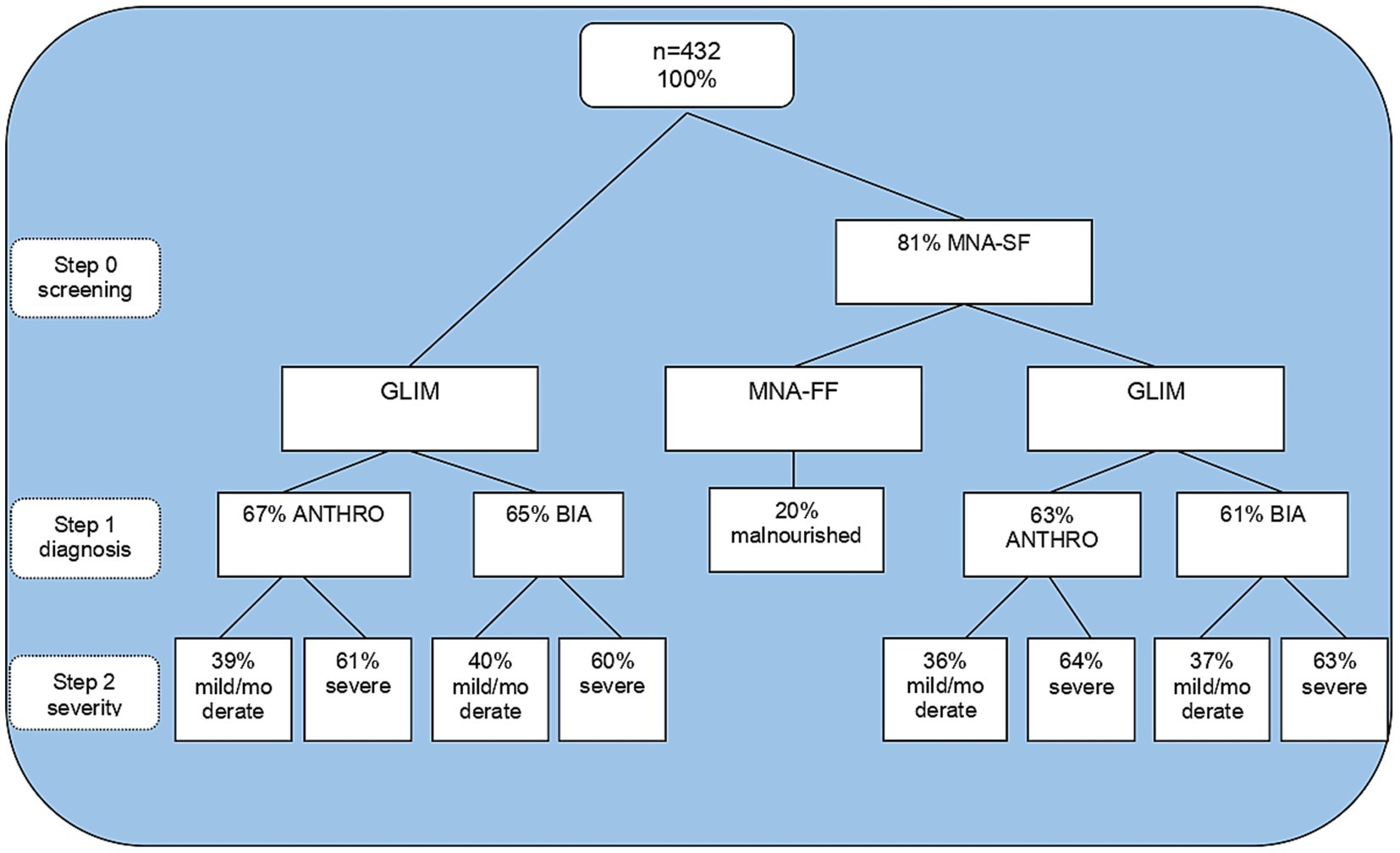
Figure 1. Steps of malnutrition diagnosis by GLIM and MNA. GLIM, global leadership initiative on malnutrition; MNA-SF, mini nutritional assessment short form; ANTHRO, anthropometry; BIA: bioelectrical impedance analysis.
Data relating to the GLIM criterion in steps 1 and 2 (without applying step 0) demonstrated that by anthropometry, 67% of the assessed population was diagnosed as malnourished. Of these, 39% were moderately malnourished, and 61% were severely malnourished.
Using bioelectrical impedance, the occurrence of 65% of malnutrition was found. Of these, 40% were moderately malnourished and 60% severely malnourished. Weight loss and reduced food intake were the most frequent combination that led to malnutrition diagnosis, followed by weight loss and inflammation for both GLIM methodologies. The combination of low BMI and inflammation was also the least common for both methods according to Table 2.
The agreement between the GLIM criteria, both with and without screening, and MNA-FF is presented in Table 3. The GLIM process identified more individuals than the MNA-FF for both GLIM ANTHRO and GLIM BIA. When considering the MNA-FF as a reference method, GLIM sensitivity reached acceptable levels, with and without screening, regardless of the methodology. However, specificity was significantly reduced, though it increased slightly when screening tools were employed compared to GLIM without screening. The agreement between the MNA-FF and GLIM ANTHRO criteria with screening was minimal (kappa −0.11) as well as using the GLIM BIA with screening (kappa −0.11), and those without screening obtained a kappa of −0.10 for GLIM ANTHRO and kappa of −0.10 for GLIM BIA. NPV was acceptable for both GLIM methodologies with and without screening, increasing with the use of screening, indicating that GLIM had a high probability of assessing individuals as not malnourished and that they actually did not have a malnutrition diagnosis, unlike PPV.
Table 4 presents the association, determined through logistic regression, between malnutrition assessed using the GLIM tools (both methods) and the MNA-FF, as well as the health conditions of the individuals at the time of hospital admission. It was possible to observe that both in the unadjusted analysis and in the analysis adjusted for sex and age, individuals with malnutrition assessed by the MNA-FF and GLIM ANTHRO had a greater chance of sarcopenia and frailty, which differed from the assessment using the GLIM BIA, which showed an increased risk only for frailty.
This study shows that the frequency of malnutrition diagnosed by the GLIM method was higher than the MNA-FF and that this tool does not identify malnutrition severity. GLIM anthropometry and GLIM bioimpedance had very similar implications, suggesting that, when using GLIM references to assess muscle mass, regardless of the method, the findings are analogous and the values agree with the prevalence of malnutrition in the studied population (24). The disagreement between the MNA-FF and GLIM methods was generally very high, being greater when the MNA-SF tool was not used.
When comparing our results with the literature, it was possible to verify that the percentage of malnourished individuals using the MNA-SF and all GLIM criteria was higher than that found by other authors (25, 26). Although all studies use the MNA-SF as a screening step, divergences in the prevalence of malnutrition are understandable due to population differences, the number of GLIM criteria adopted, and the measurement of muscle mass, which should follow the proposed guidelines (19).
Specificity, PPV, and NPV increased when comparing the GLIM and MNA-FF when screening was applied. The MNA-FF identified a relatively high proportion of individuals “at risk of malnutrition,” confirming its high sensitivity (25). In this study, the number of malnourished diagnoses without screening was only 4% higher than those who used this step. This suggests that the use of screening resulted in a reduced number of malnourished individuals who could not be identified, another study found an even higher number of malnourished people who were not identified using screening (27). Other authors also found that, even though they were classified as malnourished by the GLIM, some older adults did not present nutritional risk according to the MNA-SF (28, 29).
GLIM sensitivity was maintained without screening, demonstrating that GLIM can verify malnourished older adults regardless of screening. This can be supported by the fact that none of the existing screening tools are able to detect low muscle mass (30). In a study using the GLIM and MNA-FF, a sensitivity of 76% was observed (25). A similar sensitivity of 75% was also verified when analyzing older adults in the community with the MNA (31). Therefore, when comparing the tools, given the sensitivity and specificity of screening, as well as better identification of malnutrition severity, it is suggested that the screening tool be used in GLIM.
The kappa value showed insignificant variation when comparing the MNA-FF method to the GLIM with and without screening. This agreement could be explained since MNA-FF does not assess the reduction in FFM, inflammation, or weight loss prior to 3 months, in the same way as the GLIM, which goes much less in-depth into nutritional assessment, such as when a body composition index is measured using the GLIM. In addition, the MNA-SF uses a different BMI scaling system and a lower cutoff point. Thus, although the MNA is suitable for risk and malnutrition, the GLIM may be more efficient for detecting hidden malnutrition, including sarcopenic obesity.
It was observed that one study compared the GLIM with the MNA-FF (28), and only a few had applied the MNA-SF followed by the GLIM to diagnose malnutrition in hospitalized older adults (25, 26, 28, 29, 32). It was found that the GLIM without screening identified more than three times the number of malnourished individuals as the MNA-FF. Other authors, using FFMI, observed a disparity in malnutrition rates: 36% identified as malnourished by GLIM compared to 15% by PG SGA (33). Similar values were found without using FFMI (34), in contrast to other studies that reported lower malnutrition prevalence with GLIM (27) and Ref. (35).
GLIM sensitivity was greater than 90% in analysis without screening, being higher than the results presented in the literature (33, 34, 36). The PPV without screening was close to some studies, at 34% (36) and 29% (33), but lower when compared to PPV of 83% (36). The kappa value without the screening step indicated a very low agreement between the GLIM and MNA-FF, being lower than the kappa of 0.32 (34) and 0.45 (35).
Discrepancies in comparisons can be partially explained by differences in the criteria used in each tool. Many studies do not use all the GLIM criteria and focus on populations from different regions and socioeconomic conditions. Additionally, tools, such as the MNA-FF do not use the same diverse criteria for identifying malnutrition as GLIM does.
Malnutrition identified by GLIM ANTHRO and MNA-FF was associated with sarcopenia, an association already reported by a systematic review with meta-analysis (37), confirming the premise that ignoring malnutrition can lead to sarcopenia and that the concomitance of these conditions is defined as malnutrition-sarcopenia syndrome (38). Older adults admitted with the syndrome have twice the risk of death as malnourished or sarcopenic adults alone (39). However, some individuals with sarcopenia, in this and another study, did not show malnutrition at screening, which further emphasizes the need to make a complete diagnosis for both malnutrition and sarcopenia (40).
Regarding frailty, an association with malnutrition was found by GLIM and MNA-FF, with a much higher risk when using the MNA-FF, as observed in hospitalized frail older adults (26) and adults over 60 years old (41). In homes of older adults, the coexistence of three conditions was also observed, such as malnutrition, frailty, and physical dysfunction (42).
MNA-FF and GLIM ANTHRO had a better association with health conditions than GLIM BIA, possibly because BIA is more affected by clinical conditions in hospitalized patients, which can alter tissue physiology, than muscle assessment by calf circumference. BIA estimates total body water and other body compartments using predictive equations that assume constant tissue hydration in individuals (43). However, this assumption may not always occur during hospitalization. In addition, anthropometry and bioimpedance methods express different aspects and levels of nutritional deficiency (44). However, it was also possible to observe that MNA-FF was a better predictor of frailty and sarcopenia in individuals than GLIM.
It was possible to verify that the MNA-FF and GLIM result in low agreement as they use divergent conceptual frameworks to understand malnutrition, which subsequently leads to different prevalences of malnutrition. However, although the GLIM ANTHRO obtained a greater number of malnutrition diagnoses and showed a good association with frailty and sarcopenia, more studies are needed to support its use in the diagnosis of malnutrition in hospitalized populations over the age of 60, since the MNA-FF was able to predict sarcopenia and frailty with a higher hazard ratio.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Hospital Complex Research Ethics Committee – Hospital Universitário Oswaldo Cruz and Cardiological Emergency Room of Pernambuco (REC/HUOC/PROCAPE), under Opinion 4.949.371, September 2, 2021. The studies were conducted in accordance with the local legislation and institutional requirements. The participants or participants’ legal guardians provided their written informed consent to participate in this study.
GS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AB: Data curation, Investigation, Writing – review & editing. MC: Data curation, Investigation, Writing – review & editing. AS: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001 and Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE) (BIC 0940–4.05/22 and BIC 0728–4.05/23).
We would like to thank the study participants and Oswaldo Cruz University Hospital (University of Pernambuco) for allowing data collection and project execution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1456091/full#supplementary-material
1. Leij-Halfwerk, S, Verwijs, MH, Van Houdt, S, Borkent, JW, Guaitoli, PR, Pelgrim, T, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas. (2019) 126:80–9. doi: 10.1016/j.maturitas.2019.05.006
2. United Nations Department of Economic and Social Affairs Population Division World population prospects 2019 . OMS. (2019). Available at: https://population.un.org/wpp/ (Accessed February 1, 2022.
3. Volkert, D, Beck, AM, Cederholm, T, Cruz-Jentoft, A, Hooper, L, Kiesswetter, E, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr. (2022) 41:958–89. doi: 10.1016/j.clnu.2022.01.024
4. Cederholm, T, Jensen, GL, Correia, MITD, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. GLIM Core leadership committee; GLIM working group. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
5. Guigoz, Y . The Mini nutritional assessment (MNA) review of the literature--what does it tell us? J Nutr Health Aging. (2006) 10:466–85.
6. Ghazi, L, Fereshtehnejad, SM, Abbasi Fard, S, Sadeghi, M, Shahidi, GA, and Lökk, J. Mini nutritional assessment (MNA) is rather a reliable and valid instrument to assess nutritional status in Iranian healthy adults and elderly with a chronic disease. Ecol Food Nutr. (2015) 54:342–57. doi: 10.1080/03670244.2014.994743
7. Dent, E, Hoogendijk, EO, Visvanathan, R, and Wright, ORL. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging. (2019) 23:431–41. doi: 10.1007/s12603-019-1176-z
8. Vellas, B, Villars, H, Abellan, G, Soto, ME, Rolland, Y, Guigoz, Y, et al. Overview of the MNA--Its history and challenges. J Nutr Health Aging (2006) 10:456–463.
9. Chumlea, WC, Guo, S, Roche, AF, and Steinbaugh, ML. Prediction of body weight for the nonambulatory elderly from anthropometry. J Am Diet Assoc. (1988) 88:564–8.
10. Chumlea, WC, Roche, AF, and Steinbaugh, ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc. (1985) 33:116–20. doi: 10.1111/j.1532-5415.1985.tb02276.x
12. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Writing Group for the European Working Group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
13. Sun, SS, Chumlea, WC, Heymsfield, SB, Lukaski, HC, Schoeller, D, Friedl, K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. (2003) 77:331–40. doi: 10.1093/ajcn/77.2.331
14. Gray, DS, Bray, GA, Gemayel, N, and Kaplan, K. Effect of obesity on bioelectrical impedance. Am J Clin Nutr. (1989) 50:255–60. doi: 10.1093/ajcn/50.2.255
15. Zhang, X, Zhang, X, Zhu, Y, Tao, J, Zhang, Z, Zhang, Y, et al. Predictive value of nutritional risk screening 2002 and Mini nutritional assessment short form in mortality in Chinese hospitalized geriatric patients. Clin Interv Aging. (2020) 20:441–9. doi: 10.2147/CIA.S244910
16. Cederholm, T, Bosaeus, I, Barazzoni, R, Bauer, J, Van Gossum, A, Klek, S, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
17. Brito, JE, Burgel, CF, Lima, J, Chites, VS, Saragiotto, CB, Rabito, EI, et al. GLIM criteria for malnutrition diagnosis of hospitalized patients presents satisfactory criterion validity: a prospective cohort study. Clin Nutr. (2021) 40:4366–72. doi: 10.1016/j.clnu.2021.01.009
18. Cederholm, T, Barazzoni, R, Austin, P, Ballmer, P, Biolo, G, Bischoff, SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
19. Barazzoni, R, Jensen, GL, Correia, M, Gonzalez, MC, Higashiguchi, T, Shi, HP, et al. Guidance for assessment of the muscle mass phenotypic criterion for the global leadership initiative on malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. (2022) 41:1425–33. doi: 10.1016/j.clnu.2022.02.001
20. Gonzalez, MC, Mehrnezhad, A, Razaviarab, N, Barbosa-Silva, TG, and Heymsfield, SB. Calf circumference: cutoff values from the NHANES 1999–2006. Am J Clin Nutr. (2021) 113:1679–87. doi: 10.1093/ajcn/nqab029
21. Sánchez-Rodríguez, D, Annweiler, C, and Cederholm, T. A translational approach for the clinical application of recently updated definitions of malnutrition (GLIM) and sarcopenia (EWGSOP2). Maturitas. (2019) 122:89–90. doi: 10.1016/j.maturitas.2018.11.013
22. Nunes, DP, Duarte, YAO, Santos, JLF, and Lebrão, ML. Screening for frailty in older adults using a self-reported instrument. Rev Saúde Pública. (2015):49. doi: 10.1590/S0034-8910.2015049005516
23. de van der Schueren, MAE, Keller, H, Cederholm, T, Barazzoni, R, Compher, C, Correia, MITD, et al. Global leadership initiative on malnutrition (GLIM): guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin Nutr. (2020) 39:2872–80. doi: 10.1016/j.clnu.2019.12.022
24. Gonçalves, TJM, Horie, LM, Gonçalves, SLAB, Bacchi, MK, Bailer, MC, Barbosa-Silva, TG, et al. Diretriz BRASPEN de terapia nutricional no envelhecimento, Brazilian Society of Parenteral and Enteral Nutrition-BRASPEN. J Inter Des. (2019) 34:2–58.
25. Muñoz, FSS, Garcez, FB, Alencar, JCG, Cederholm, T, Aprahamian, I, Morley, JE, et al. Applicability of the GLIM criteria for the diagnosis of malnutrition in older adults in the emergency ward: a pilot validation study. Clin Nutr. (2021) 40:5447–56. doi: 10.1016/j.clnu.2021.09.024
26. Pourhassan, M, Rommersbach, N, Lueg, G, Klimek, C, Schnatmann, M, Liermann, D, et al. The impact of malnutrition on acute muscle wasting in frail older hospitalized patients. Nutrients. (2020) 12:1387. doi: 10.3390/nu12051387
27. Rosnes, KS, Henriksen, C, Høidalen, A, and Paur, I. Agreement between the GLIM criteria and PG-SGA in a mixed patient population at a nutrition outpatient clinic. Clin Nutr. (2021) 40:5030–7. doi: 10.1016/j.clnu.2021.07.019
28. Kaluźniak-Szymanowska, A, Krzymińska-Siemaszko, R, Wieczorowska-Tobis, K, and Deskur-Śmielecka, E. Optimal assessment of nutritional status in older subjects with the chronic obstructive pulmonary disease-a comparison of three screening tools used in the GLIM diagnostic algorithm. Int J Environ Res Public Health. (2022) 19:1025. doi: 10.3390/ijerph19031025
29. Sobrini, P, Sánchez-Castellano, C, and Cruz-Jentoft, AJ. MNA-SF as a screening tool for malnutrition diagnosed with the glim criteria in older persons with cancer. Eur Geriatr Med. (2021) 12:653–6. doi: 10.1007/s41999-020-00442-8
30. Ræder, H, Henriksen, C, Bøhn, SK, Henriksen, HB, Kværner, AS, Rolid, K, et al. Agreement between PG-SGA category and fat-free mass in colorectal cancer patients. Clin Nutr ESPEN. (2018) 27:24–31. doi: 10.1016/j.clnesp.2018.07.005
31. Xu, JY, Zhu, MW, Zhang, H, Li, L, Tang, PX, Chen, W, et al. A cross-sectional study of GLIM-defined malnutrition based on new validated calf circumference cut-off values and different screening tools in hospitalised patients over 70 years old. J Nutr Health Aging. (2020) 24:832–8. doi: 10.1007/s12603-020-1386-4
32. Stefani, GP, Crestani, MS, Scott, LM, Soares, CH, and Steemburgo, T. Complementarity of nutritional assessment tools to predict prolonged hospital stay and readmission in older patients with solid tumors: a secondary analysis of a cohort study. Nutrition. (2023) 113:112089. doi: 10.1016/j.nut.2023.112089
33. Henriksen, C, Paur, I, Pedersen, A, Kværner, AS, Ræder, H, Henriksen, HB, et al. Agreement between GLIM and PG-SGA for diagnosis of malnutrition depends on the screening tool used in GLIM. Clin Nutr. (2022) 41:329–36. doi: 10.1016/j.clnu.2021.12.024
34. De Groot, LM, Lee, G, Ackerie, A, and van der Meij, BS. Malnutrition screening and assessment in the Cancer care ambulatory setting: mortality predictability and validity of the patient-generated subjective global assessment short form (PG-SGA SF) and the GLIM criteria. Nutrients. (2020) 12:2287. doi: 10.3390/nu12082287
35. Zhang, Z, Wan, Z, Zhu, Y, Zhang, L, Zhang, L, and Wan, H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: a multi-center study. Nutrition. (2021) 83:111072. doi: 10.1016/j.nut.2020.111072
36. Allard, JP, Keller, H, Gramlich, L, Jeejeebhoy, KN, Laporte, M, and Duerksen, DR. GLIM criteria has fair sensitivity and specificity for diagnosing malnutrition when using SGA as comparator. Clin Nutr. (2020) 39:2771–7. doi: 10.1016/j.clnu.2019.12.004
37. Ligthart-Melis, GC, Luiking, YC, Kakourou, A, Cederholm, T, Maier, AB, and de van der Schueren, MAE. Frailty, sarcopenia, and malnutrition frequently (co-)occur in hospitalized older adults: a systematic review and Meta-analysis. J Am Med Dir Assoc. (2020) 21:1216–28. doi: 10.1016/j.jamda.2020.03.006
38. Kaluźniak-Szymanowska, A, Krzymińska-Siemaszko, R, Deskur-Śmielecka, E, Lewandowicz, M, Kaczmarek, B, and Wieczorowska-Tobis, K. Malnutrition, sarcopenia, and malnutrition-sarcopenia syndrome in older adults with COPD. Nutrients. (2021) 14:44. doi: 10.3390/nu14010044
39. Hu, X, Zhang, L, Wang, H, Hao, Q, Dong, B, and Yang, M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep. (2017) 7:3171–9. doi: 10.1038/s41598-017-03388-3
40. Fashho, E, Ahmed, T, Garden, G, Readman, D, Storey, L, Wilkinson, L, et al. Investigating the prevalence of malnutrition, frailty and physical disability and the association between them amongst older care home residents. Clin Nutr ESPEN. (2020) 40:231–6. doi: 10.1016/j.clnesp.2020.09.014
41. Ji, T, Li, Y, Liu, P, Zhang, Y, Song, Y, and Ma, L. Validation of GLIM criteria on malnutrition in older Chinese inpatients. Front Nutr. (2022) 15:01–9. doi: 10.3389/fnut.2022.969666
42. Yeung, SSY, Chan, RSM, Kwok, T, Lee, JSW, and Woo, J. Malnutrition according to GLIM criteria and adverse outcomes in community-dwelling Chinese older adults: a prospective analysis. J Am Med Dir Assoc. (2021) 22:1953–1959.e4. doi: 10.1016/j.jamda.2020.09.029
43. Gomes, TLM, Peixoto, MVM, Azevedo, ZM, Fonseca, VM, and Ramos, EG. Association of electrical bioimpedance vectors with the nutritional classification of vulnerable multiethnic children. Clin Nutr ESPEN. (2021) 46:453–8. doi: 10.1016/j.clnesp.2021.09.011
Keywords: calf circumference, fat-free mass index, GLIM, muscle mass, nutritional assessment, nutritional screening
Citation: da Silva GD, Batista AVDA, Costa MCRDA and dos Santos ACO (2024) The ability of GLIM and MNA-FF to diagnose malnutrition and predict sarcopenia and frailty in hospitalized adults over 60 years of age. Front. Nutr. 11:1456091. doi: 10.3389/fnut.2024.1456091
Received: 28 June 2024; Accepted: 17 October 2024;
Published: 08 November 2024.
Edited by:
Mariacristina Siotto, IRCCS Don Carlo Gnocchi Firenze, ItalyReviewed by:
Irena Keser, University of Zagreb, CroatiaCopyright © 2024 da Silva, Batista, Costa and dos Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriella D. da Silva, Z2FicmllbGxhLmRpYXNzQHVwZS5icg==; Ana C. O. dos Santos, YW5hLm9saXZlaXJhQHVwZS5icg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.