
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 23 October 2024
Sec. Nutritional Epidemiology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1453062
This article is part of the Research Topic Assessment of Nutritional Status in Chronic Diseases View all 21 articles
Background: The combined effect of depression and nutritional-inflammatory status on mortality in the chronic kidney disease (CKD) population is unclear.
Methods: We prospectively analyzed 3,934 (weighted population: 22,611,423) CKD participants from the National Health and Nutrition Examination Survey (2007–2018). Depression and nutritional-inflammatory status were assessed with Patient Health Questionnaire 9 (PHQ-9) and Advanced Lung Cancer Inflammation Index (ALI), respectively. Weighted multivariate COX regression models, restricted cubic splines (RCS) models, and stratified analyses were used to investigate the association of PHQ-9 scores and ALI with all-cause mortality.
Results: During a median follow-up of 5.8 years (interquartile range 3.4–8.6 years), a total of 985 patients died (25.0%). Each point increase in a patient’s PHQ-9 score increased the risk of all-cause mortality by 4% (HR, 1.04; 95% CI, 1.02–1.06; p < 0.001), in the full adjusted model. However, an increase in ALI levels was associated with a decreased risk. HRs (95% CI) of 0.76 (0.65–0.90), 0.70 (0.57–0.86), and 0.51 (0.41–0.64) in the Q2, Q3, and Q4 of ALI compared with the Q1 of ALI, respectively. In addition, the joint analysis showed that CKD patients without depression and with higher ALI were associated with a reduced risk of all-cause mortality. Namely, patients in the highest ALI group (Q4) without depression had the lowest risk (HR, 0.32; 95% CI, 0.21–0.48). Furthermore, this combined effect was consistent across all subgroups, and no significant interaction was found (p > 0.05 for interaction).
Conclusion: In a nationally representative sample of US patients with CKD, coexisting depression and poorer nutrition-inflammation were associated with a significantly increased risk of all-cause mortality.
Chronic kidney disease (CKD) is a worldwide health problem that is widespread and serious, directly leading to the global burden of incidence and mortality and is also an essential risk factor for cardiovascular disease (CVD) (1, 2). The prevalence of CKD among adults in the US was about 15 percent in 2015–2018, and that number appears to be increasing (3, 4). Notably between 1990 and 2017, the global all-age mortality rate for CKD increased by 41.5% (2). The long-term prognosis of patients with CKD is related to a variety of factors, including nutritional status, degree of inflammation, and psychological factors (5–7). Interestingly, they are interrelated and interact with each other (8–10). Therefore, identifying these modifiable prognostic factors is urgent for the survival of CKD patients.
Recently, multiple nutritional/inflammatory indicators have been increasingly shown to serve as valid predictors of prognosis in chronic kidney disease (6, 11–13). The advanced lung cancer inflammation index (ALI) is a new indicator of systemic inflammation level and nutritional status, which consists of neutrophil to lymphocyte ratio (NLR), serum albumin, and body mass index (BMI), and it showed superior predictive prognostic ability for cancer patients relative to other metrics (14–16). Notably, the predictive properties of ALI for prognosis in CKD patients have not been explored.
In addition, psychological factors also have a greater impact on the prognosis of CKD. Depression is among the most widespread psychological disturbances in patients with CKD, affecting an estimated one in four patients (17, 18). Interestingly, there is a bidirectional association between depression and CKD, which may lead to the disease occurring in conjunction with each other (19). Depressed CKD patients tend to have worse outcomes (20, 21). The relationship between depressive symptoms and nutrient-inflammatory states is close and complex (8, 22–25). The causal relationship between them is controversial, and some studies have suggested a bidirectional link (8, 22, 25–27). A recent meta-analysis of cross-sectional associations found significantly higher levels of pro-inflammatory biomarkers, C-reactive protein, interleukin 6 (IL-6), and tumor necrosis factor-alpha, and significantly lower levels of the anti-inflammatory cytokine IL-10 in CKD and end-stage renal failure patients with depressive symptoms relative to CKD and end-stage renal failure patients without depressive symptoms (28). Although many studies explored the relationship between nutritional-inflammatory status and depression, no studies have examined the significance of the combination of nutritional-inflammatory status and depression on the prognosis of CKD patients.
We conducted a prospective study through a nationally representative sample of CKD in the US. First, to examine the performance of ALI for mortality prediction and compare it with other indicators of nutritional/inflammation (based on blood counts). Subsequently, it was used to represent the nutritional-inflammatory status, and we then explored the independent and joint effects of this indicator and depression on the risk of all-cause mortality.
This prospective cohort study used data from a nationally representative sample of six consecutive cycles of the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2018. The Ethics Review Board of the National Center for Health Statistics approved all NHANES protocols, and written informed consent was obtained from all participants. The survey has been conducted every 2 years since 1999 to monitor the health and nutritional status of the US population through interviews and physical examinations.
NHANES uses stratified, multistage random sampling to collect data from a nationally representative, noninstitutionalized US sample. Participants were considered to have CKD when they presented with an estimated glomerular filtration rate (eGFR) < 60 mL /min/1.73 m2 or urinary albumin/creatinine ratio (UACR) ≥ 30 mg/g (29). The eGFR was calculated by the CKD-EPI equation (30). Among the 59,482 participants from 2007 to 2018, we excluded those (a) with incomplete Patient Health Questionnaire-9 (PHQ-9) (n = 28,022); (b) who were pregnant (n = 323); (c) less than 20 years old (n = 1,616); (d) without CKD (n = 24,222); (e) with missing mortality data (n = 6); (f) with missing data on nutritional/inflammatory indicators (n = 342); (g) any missing covariate information (n = 1,017). A sample of 3,934 (weighted population: 22,611,423) eligible CKD patients were finally included in this study.
We used PHQ-9, which includes nine items (lack of interest, depressed mood, trouble sleeping, fatigue, appetite problems, worthlessness, lack of concentration, psychomotor agitation or retardation, and suicidal thoughts), to determine the depression in the CKD population. The score for each item is based on how often the participant has experienced a particular symptom in the past 2 weeks. Each project has four answer categories, “not at all,” “several days,” “more than half the days,” and “nearly every day,” corresponding to 0, 1, 2, and 3 points, respectively. As a result, the total score on the PHQ-9 ranges from 0 to 27, with higher scores representing more severe depressive symptoms. Based on extensive research on the accuracy of the PHQ-9, we defined participants with a total score ≥ 10 as having depression (31, 32).
ALI was obtained by multiplying BMI (kg/m2) by serum albumin (g/dL) divided by NLR. A higher ALI represents better nutritional status and lower levels of inflammation in the participants. Detailed calculations of other nutritional/inflammatory indicators were shown in Supplementary Table S1.
The study endpoint for this cohort was all-cause mortality, meaning death due to any cause. Participants’ vital status and the length of follow-up were determined by cross-referencing with the National Death Index until December 31, 2019.1 The follow-up period was counted from initial participation in the NHANES program to the date of death or December 31, 2019.
The covariates in this study consisted mainly of demographic characteristics (age, sex, race, education level, income-poverty ratio [PIR], and marital status), medical history information (diabetes mellitus [DM], CVD, hyperlipidemia, hypertension, cancer, smoking status, drinking status, and sleep duration) and laboratory blood indicators (high-density lipoprotein cholesterol [HDL-C], alanine aminotransferase [ALT], aspartate aminotransferase [AST], total cholesterol [TC], and eGFR). Specific groupings of categorical variables and units for all variables can be found in Table 1. Participants were considered to have a history of CVD when they self-reported angina, congestive heart failure, coronary heart disease, heart attack, and stroke. When participants were asked, “Have you ever been told by a doctor or other health professional that you have cancer or any malignant tumor?,” patients were considered to have a history of cancer if they answered yes. All other variable definitions are available in this study (33). Exhaustive measurement techniques for all variables in this study are available at https://www.cdc.gov/nchs/nhanes/.
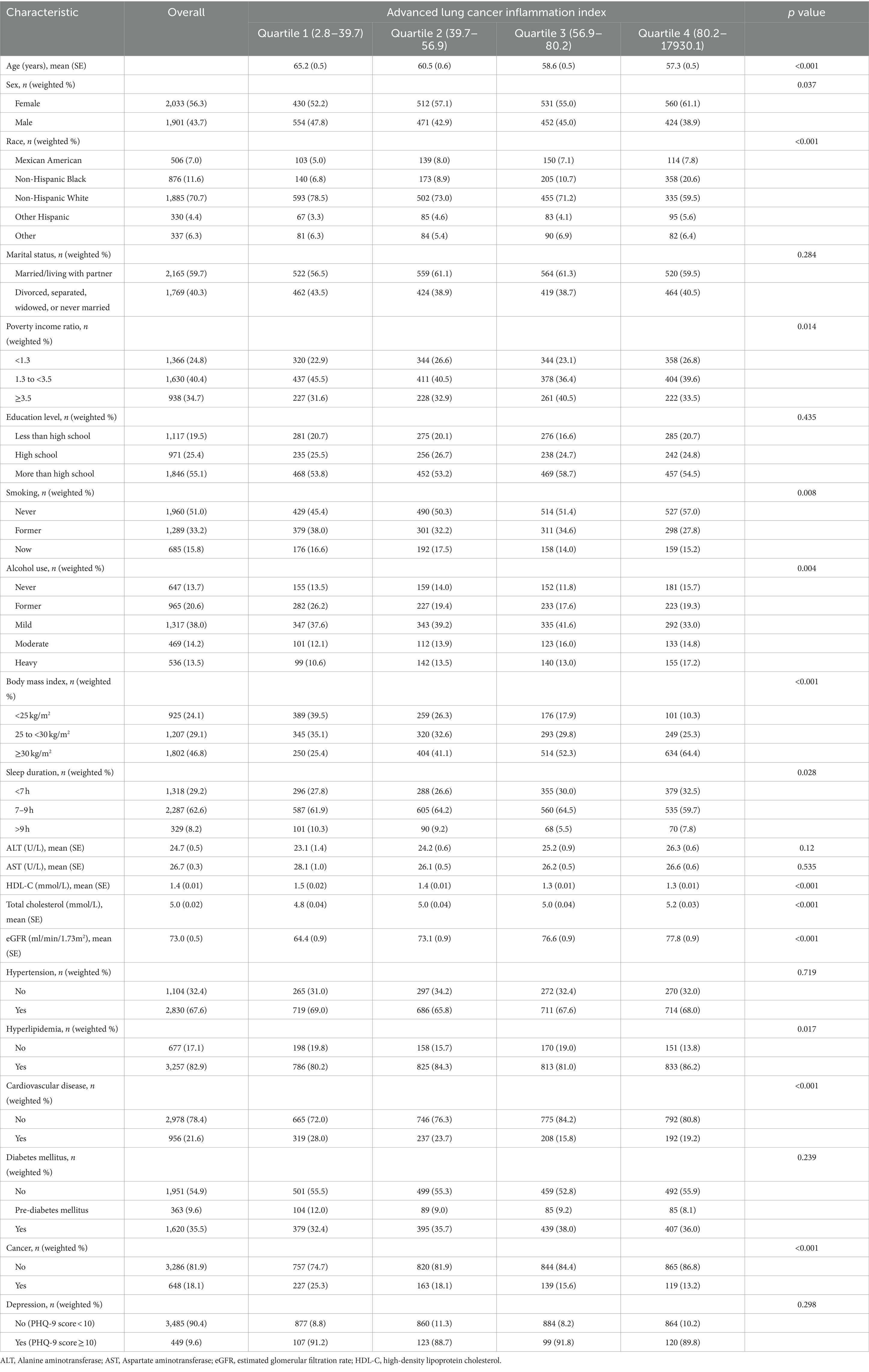
Table 1. Baseline characteristics of chronic kidney disease participants stratified by quartiles of advanced lung cancer inflammation index.
Sample weighting, clustering, and stratification were applied to the analysis in this study due to the complexity of the NHANES sampling design. Baseline characteristics were expressed as quartiles (Q1–Q4) of the ALI. Continuous and categorical variables were, respectively, expressed as weighted means ± standard error (SE) and unweighted frequencies (weighted percentages) and were compared using weighted linear regression and chi-square tests, respectively. We used time-dependent receiver operating characteristic curves (time-ROC) to examine the performance of ALI for mortality prediction and compare it with other indicators of nutritional/inflammation (based on blood counts).
Hazard ratios (HRs) and 95% confidence interval (CI) for the effects of depression and ALI on all-cause mortality were assessed using weighted multivariate Cox proportional hazards models, respectively. Subsequently, we grouped participants in the four ALI levels into eight subgroups based on the presence or absence of depression, to investigate the impact of depression in combination with ALI on the prognosis of patients with CKD. This effect was assessed by weighted multivariate Cox proportional hazards modeling using Q1 ALI participants with depression as controls. A total of three models were constructed. Model 1 was not adjusted. Model 2 was adjusted for demographic characteristics. Model 3 was adjusted for medical history information, and laboratory blood indicators based on Model 2. Harrell et al. concluded that the model fits better when the number of knots is four, i.e., it takes into account the smoothness of the curves while avoiding the reduction in accuracy caused by overfitting (34). Therefore, in this study, the knots of the RCS model are set to four (5th, 35th, 65th, and 95th percentiles) to further explore potential nonlinear associations of PHQ-9 scores and ALI with all-cause mortality.
We repeated the main analyses stratified by sex, age (<65 vs. ≥65 years), PIR (<1.3 vs. 1.3 to <3.5 vs. ≥3.5) and DM (yes vs. pre-DM vs. no). We also used stratification analysis to assess potential interactions. In the stratified analysis of joint influence, due to the small sample size of some groups, we transformed ALI into a binary variable (≤49.98 and >49.98) based on the optimal cut-off of the ROC curve ALI. Subsequently, we performed two sensitivity analyses. In the first analysis, participants who died during the first year of follow-up were excluded from the analysis to reduce the possibility of reverse causality bias. For the second analysis, we unweighted the analyses to compare whether weighted analyses had a large effect on the results.
All statistical analyses were performed using R software version 4.4.0 when a two-sided p < 0.05 was considered the threshold for statistical significance.
A total of 3,934 patients with CKD (weighted population: 22,611,423; weighted mean age [SE] 60.4 [0.3] years; weighted female proportion 56.3%). Of these participants, 506 (7.0%) were Mexican American, 1,885 (70.7%) were Non-Hispanic White, 876 (11.6%) were Non-Hispanic Black, 330 (4.4%) were Other Hispanic, and 337 (6.3%) were of Other races. Participants were categorized into 4 groups based on quartiles of the ALI: Q1 (2.8–39.7), Q2 (39.7–56.9), Q3 (56.9–80.2), and Q4 (80.2–17930.1). Their detailed baseline data characteristics were presented in Table 1. Participants with lower ALI were more likely to be older, male, Non-Hispanic White, PIR between 1.3 and 3.5, former alcohol use, former smoking, BMI between 25 and 30 kg/m2, sleep duration > 9 h, higher HDL-C, lower total cholesterol, lower eGFR, non-hyperlipidemia, and history of CVD and cancer.
The effectiveness of 14 nutritional/inflammatory indicators for the prediction of all-cause mortality was assessed using time-ROC. The area under the curve (AUC) for all-cause mortality at 1, 3, 5, and 10 years for ALI was 0.672, 0.669, 0.648, and 0.642, respectively, which was the largest relative to any of the other nutritional/inflammatory indicators (Figure 1).
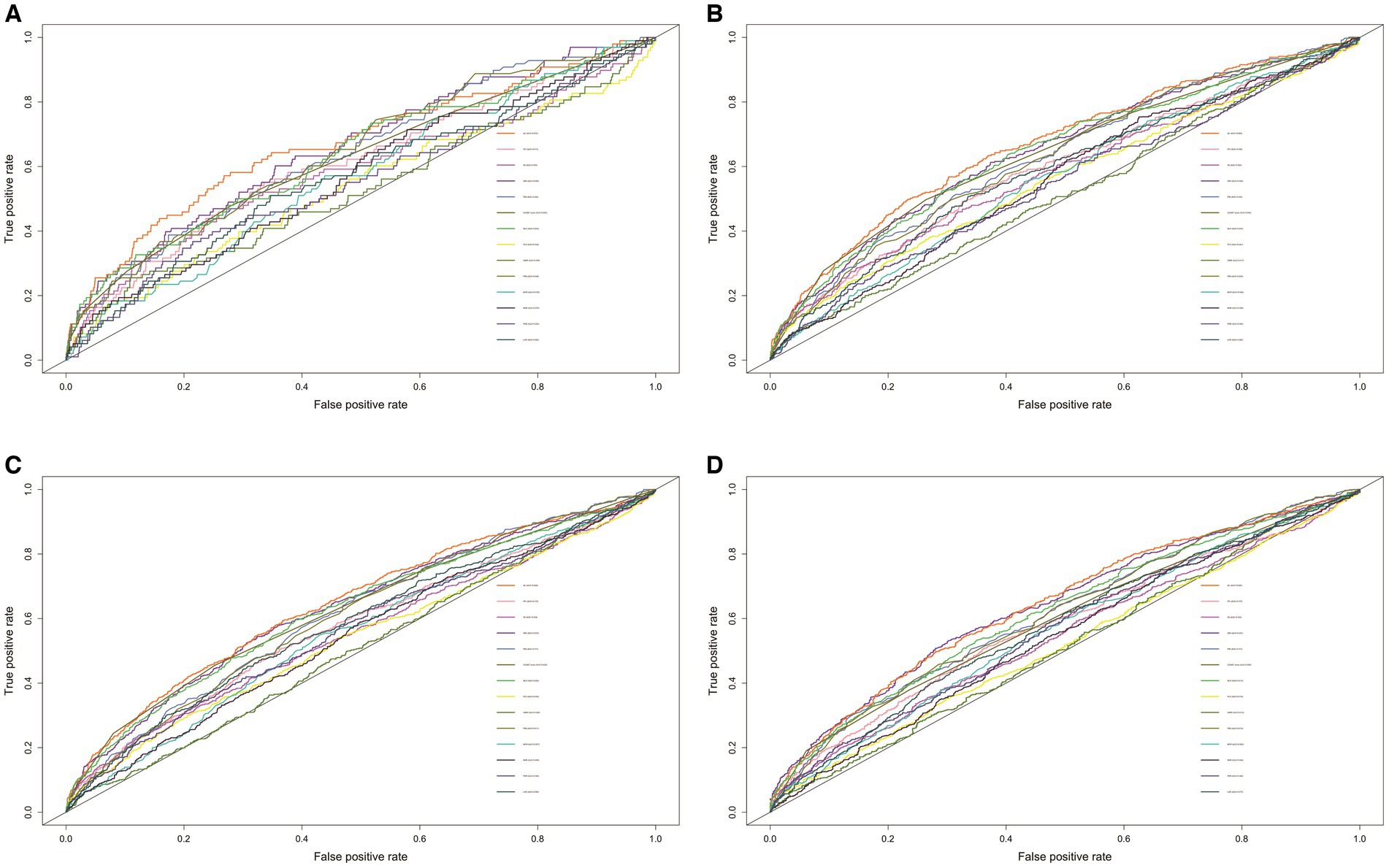
Figure 1. The ROC of nutritional/inflammatory indicators in predicting 1- (A), 3- (B), 5- (C), and 10- (D) years all-cause mortality in patients with chronic kidney disease. ROC, receiver operating characteristics curve; AUC, area under the curve; ALI, advanced lung cancer inflammation index; NLR, neutrophil to lymphocyte ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; PIV, pan-immune-inflammation value; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; GNRI, geriatric nutritional risk index; PINI, prognostic immune nutritional index; NHR, neutrophil to high-density lipoprotein-cholesterol ratio; MHR, Monocyte to high-density lipoprotein-cholesterol ratio; PHR, platelet to high-density lipoprotein-cholesterol ratio; LHR, lymphocyte to high-density lipoprotein-cholesterol ratio; COUNT score, controlling nutritional status score.
During a median follow-up of 5.8 years (interquartile range 3.4–8.6 years), a total of 985 patients died (25.0%). Weighted multivariate Cox proportional hazards regression model (Model 3) showed that the risk of all-cause mortality was elevated in CKD patients with depression compared to CKD patients without it, with HR of 1.55 (95% CI, 1.24–1.95) (Table 2). In addition, the risk increased by 4% (p < 0.001) for each point increase in participants’ PHQ-9 scores. Furthermore, elevated ALI levels were associated with a significantly lower risk (Table 2). Multivariable-adjusted model (Model 3) showed HRs (95% CI) of 0.76 (0.65–0.90), 0.70 (0.57–0.86), and 0.51 (0.41–0.64) in the Q2, Q3, and Q4 of ALI compared with the Q1 of ALI, respectively.
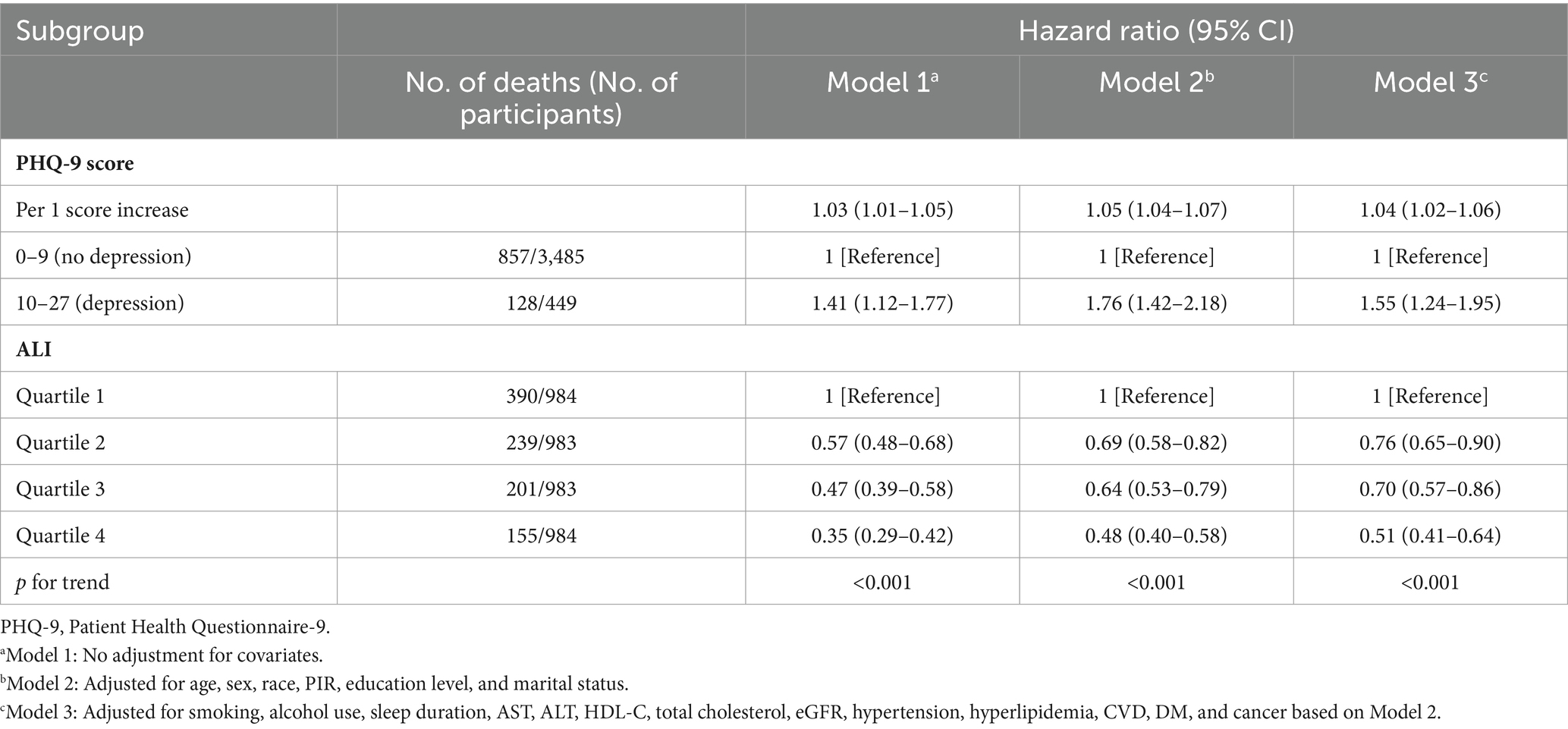
Table 2. Association of advanced lung cancer inflammation index (ALI) and PHQ-9 score with all-cause mortality among chronic kidney disease patients.
We observed a nonlinear relationship between PHQ-9 score (p for non-linear = 0.019) and ALI (p for non-linear<0.001) and the risk of all-cause mortality using the RCS model (adjusted for all covariates) (Figure 2). As the PHQ-9 score increased, the risk first increased sharply and then leveled off. In addition, there was an “L” shaped correlation between the risk and the level of ALI, with the risk initially decreasing significantly with increasing ALI and then leveling off.
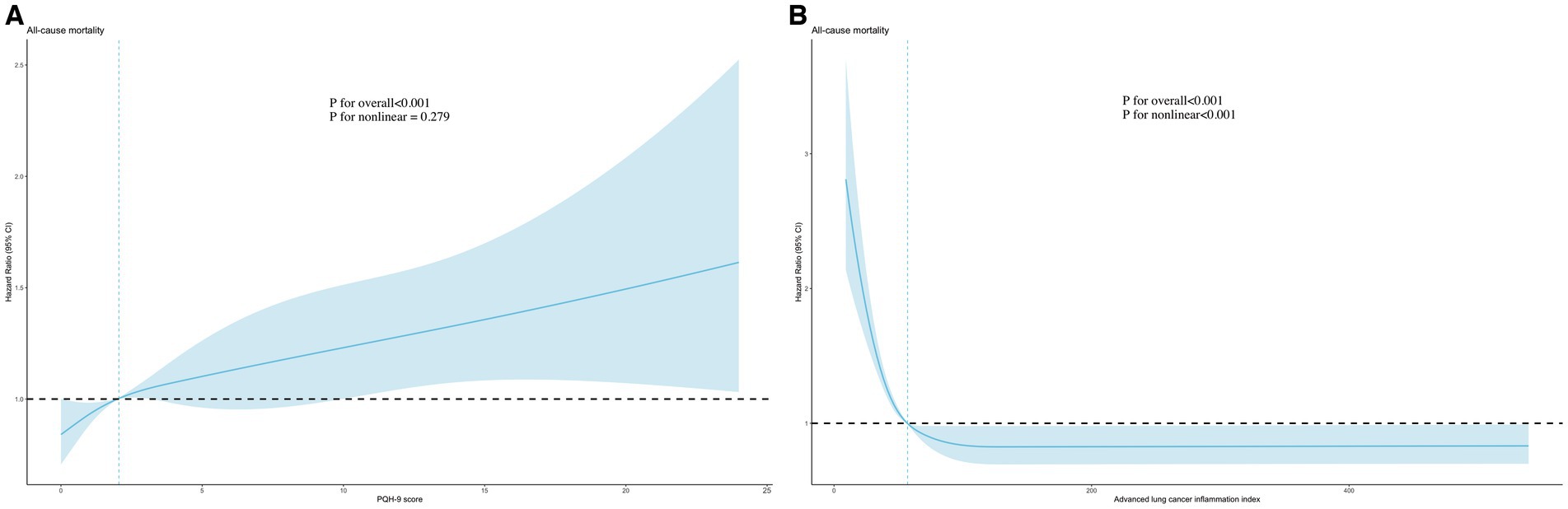
Figure 2. Association of PHQ-9 score (A) and advanced lung cancer inflammation index (B) with all-cause mortality using RCS models. Adjusted for age, sex, race, PIR, education level, marital status, smoking, alcohol use, sleep duration, AST, ALT, HDL-C, total cholesterol, eGFR, hypertension, hyperlipidemia, CVD, DM, and cancer. PHQ-9, Patient Health Questionnaire-9; RCS, restricted cubic spline.
The potential joint effect of depression and ALI on all-cause mortality was further explored by combining the presence/absence of the depression group and the ALI group into 8 categories of variables to indicate joint exposure. We observed an increased risk in participants with depression and lower ALI levels (Table 3; Figure 3). In the fully adjusted model, participants with both no depression and the highest levels (Q4) had the lowest risk mortality (HR, 0.32; 95% CI, 0.21–0.48) compared with participants with both depression and low levels of ALI (Q1).
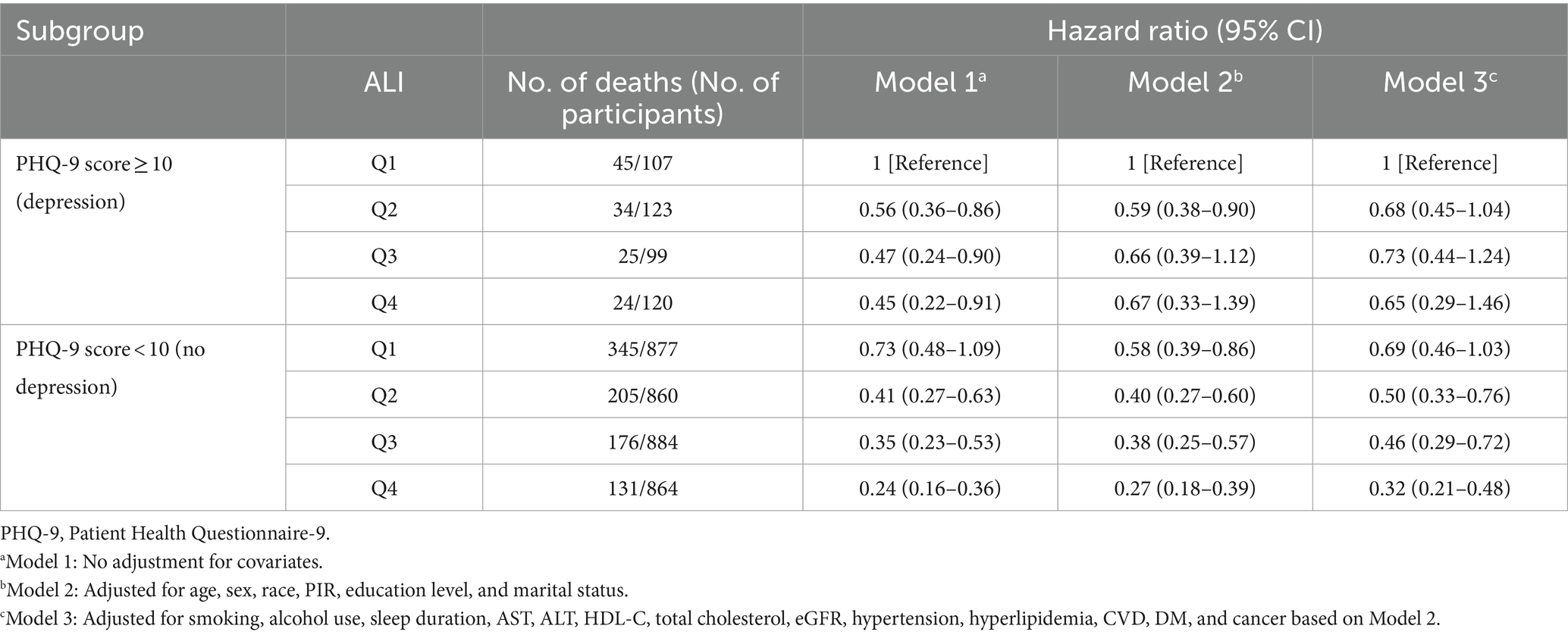
Table 3. Joint association of advanced lung cancer inflammation index (ALI) and PHQ-9 score with all-cause mortality among chronic kidney disease patients.
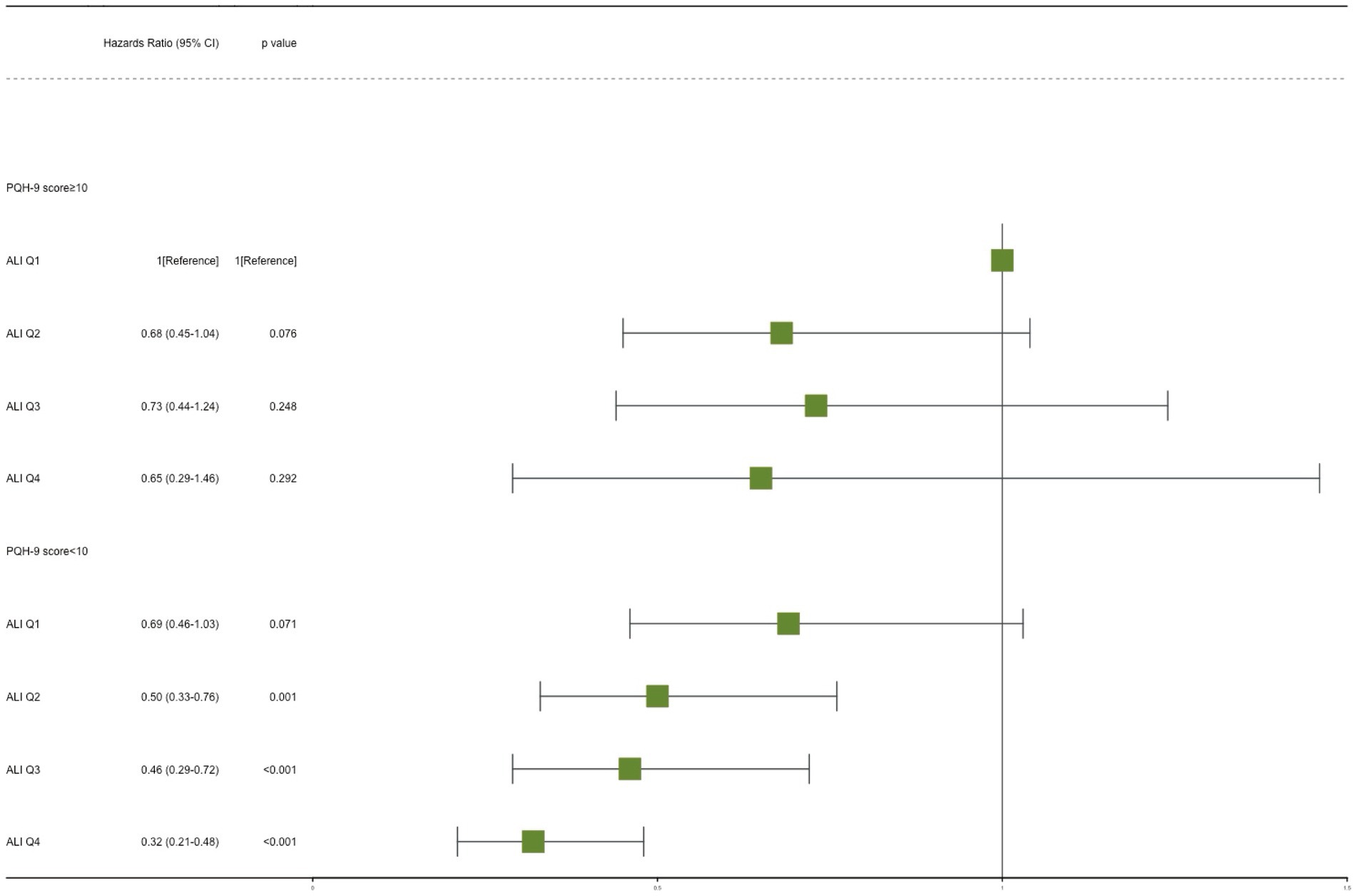
Figure 3. Joint effect analysis of depression and advanced lung cancer inflammation index (ALI) with risks of all-cause mortality. Adjusted for age, sex, race, PIR, education level, marital status, smoking, alcohol use, sleep duration, AST, ALT, HDL-C, total cholesterol, eGFR, hypertension, hyperlipidemia, CVD, DM, and cancer. PHQ-9, Patient Health Questionnaire-9.
In the subgroup analyses, we performed the main analyses stratified by age (<65 vs. ≥65 years), PIR (<1.3 vs. 1.3 to <3.5 vs. ≥3.5) and DM (yes vs. pre-DM vs. no). The joint effect was consistent in all subgroups, and no significant interaction was found (Supplementary Tables S2–S5).
Two sensitivity analyses were then performed to show that the combined effect remained robust. First, deaths with less than 1 year of follow-up were excluded (Supplementary Table S6). Second, no weighting was applied to the analyses (Supplementary Table S7). However, the joint effect remained robust.
This study investigated the impact of depression and nutritional-inflammatory status (ALI) on survival in a CKD population using a prospective nationally representative cohort. First, a comparison of the ALI with the 14 indicators of nutritional/inflammation (based on blood counts) demonstrated its superior performance in predicting mortality. Second, we found that PHQ-9 score and ALI were independently associated with the risk of all-cause mortality. In addition, our findings suggest that patients with both depression and low levels of ALI (Q1) have the highest risk relative to the rest of the population. Finally, this joint effect was consistent in all subgroups, and this main association also proved robust in sensitivity analyses. This is the first study to investigate the combined impact of nutritional-inflammatory status and depression on the risk of death in patients with CKD.
Several studies have demonstrated that depressive symptoms in CKD patients are inextricably linked to the immune-inflammatory state (8–10, 35–39). These studies all concluded that depression in CKD patients tends to be associated with malnutrition and higher levels of inflammation. One of the most basic comprehensive management and dietary treatments for the CKD population is a low-protein diet (LPD) (40). Although this management approach slows the progression of CKD, it cannot be ignored that it increases the risk of malnutrition (41, 42). LPD is usually accompanied by high carbohydrate intake and acute muscle loss leading to increased levels of inflammation and insulin resistance (43–45). In addition, patients have decreased levels of certain amino acids (e.g., tyrosine and tryptophan) and micronutrients (e.g., vitamin D and zinc) (44, 45). All of these will lead to an increased risk of depression (45–47). Chronic inflammation is often present in people with CKD, and this is an important route to depression (26, 48). For example, inflammation can contribute to depression by affecting the activation of the hypothalamic-pituitary-adrenal axis and disrupting the two-way communication system between the gut microbiota and the brain (49–51).
Interestingly, depression can exacerbate levels of malnutrition and inflammation leading to a vicious cycle of disease. When depression occurs in CKD patients it can lead to loss of appetite and reduced dietary compliance, which can cause malnutrition in the organism (52, 53). It is also an indisputable fact that depression leads to increased peripheral inflammation through various mechanisms (54). For example, patients with depression have an increased release of adrenocorticotropic hormones from the central system leading to excessive activation of the hypothalamic-pituitary-adrenal axis ultimately exacerbating systemic inflammation (19). In short, depressive symptoms, malnutrition, and inflammation interact and influence each other. Nevertheless, only two studies to date have explored the combined effects of inflammation/malnutrition and depression on mortality risk (15, 55). The study populations for these two cohorts were derived from representative samples of ≥50-year-old community residents in England and ≥40-year-old cancer survivors in the US, respectively. They confirmed that the combined effects were associated with a significantly increased risk of all-cause and CVD death in men and a significantly increased risk of all-cause and non-cancer mortality in cancer survivors. Notably, similar studies have not been explored in CKD.
Most studies have shown that depression not only increases the incidence of CKD but also accelerates the progression of CKD leading to a higher risk of death (19, 56–58). Although our findings are consistent with this, this conclusion is also somewhat controversial. A recent Mendelian randomization study found that depressive symptoms are causally associated with decreased eGFR and are an important causative factor in impairing renal function, however, it is not correlated with the risk of incident of CKD (59). Furthermore, a cohort study of Chinese peritoneal dialysis patients concluded that depression was not an independent prognostic indicator of their predicted one-year mortality rate (60). Interestingly, Saglimbene et al. confirmed that depressive symptoms were only associated with the risk of non-CVD death in CKD patients on hemodialysis, but not with the risk of CVD death (61). These differences may be due to the short follow-up period, small sample size, and differences in the characteristics of the population. Moreover, depression has been associated with several comorbidities in CKD, for example, it is associated with infection in dialysis patients (62), low bone mineral density in elderly non-dialysis patients (63), and sarcopenia in patients with different stages of CKD (64). The exact mechanism by which depression are associated with a poor prognosis in CKD is unclear. According to previous studies (19, 56), this may be related to behavioral and biological factors in depressed patients. Depressed patients are more prone to many adverse health behaviors, such as obesity, decreased treatment adherence, and lack of physical activity, which are risk factors for CKD prognosis (19, 65). Depression is commonly associated with higher levels of low-grade systemic inflammation, endothelial dysfunction, abnormal activation of the hypothalamic-pituitary-adrenal axis, and overactivation of the sympathetic nervous system, which persistently interferes with the intrarenal microcirculation and perfusion distribution, leading to further renal damage in patients with CKD (66, 67).
There is a wide variety of nutritional/inflammatory markers and most studies have only explored their relationship with CKD prognosis separately (6, 11–13, 68). However, no study has compared their predictive performance in a comprehensive manner to select the best predictor. We included as many previous nutritional/inflammatory indicators as possible in this study and subsequently confirmed that ALI had the strongest predictive performance by time-ROC. On the other hand, this is the first study to investigate the association between ALI and prognosis in the CKD population. ALI was first developed to assess the prognosis of lung cancer patients and was found to be the best inflammatory biomarker of overall survival in lung cancer patients (14). Subsequently, a growing number of studies have found it to have superior predictive performance in multiple types of cancer, which include gastric, colorectal, head and neck squamous cell carcinoma, melanoma, thymic epithelial tumors, hepatocellular carcinoma, nasopharyngeal carcinoma, and diffuse large B-cell lymphoma (15, 69, 70). Interestingly, in recent years ALI has been found to have excellent predictive performance in certain non-tumor diseases as well, which include stroke, type 2 diabetes, heart failure, and hypertension (71–74). Alarmingly in this study, ALI was found to be the strongest predictor of all-cause mortality in CKD patients relative to other nutritional/inflammatory markers. This is mainly since low ALI reflects a combination of malnutrition and high levels of inflammation, which are important factors in the poor prognosis of CKD.
ALI was calculated from BMI, serum albumin level, and NLR. This is even though higher BMI is a risk factor for developing CKD (75–77). Interestingly, higher BMI was associated with a decreased risk of death in the CKD population (78, 79). CKD is a chronic inflammation-related disease prone to malnutrition leading to cachexia (79). Cachexia is commonly associated with a worsening prognosis, and BMI is one of the criteria used to diagnose cachexia (79). Serum albumin levels are also a key indicator of the body’s nutritional status, and hypoalbuminemia is a key predictor of death in patients with CKD (80). Hypoalbuminemia in the CKD population is commonly caused by chronic inflammation, dialysis treatment, catabolic alterations, and protein restriction. In addition, albumin is one of the most important antioxidants in the blood (81), and its decline exacerbates the systemic inflammatory response in CKD. Notably, when combined with the degree of systemic inflammation serum albumin can better predict the risk of death in CKD patients (82). This partly explains the superior performance of ALI predictions. NLR, derived from neutrophil and lymphocyte counts, is an important indicator of systemic inflammation and is strongly associated with the prognosis of CKD patients (83, 84). Neutrophils can cause renal fibrosis directly or indirectly by releasing reactive oxygen species, granular material, inflammatory mediators, collagen1, and pro-fibrotic inflammatory cytokines, leading to CKD progression (85). Whereas many types of lymphocytes attenuate inflammation and fibrosis in the kidney, such as IL-33R+ and IL-2Ra + regulatory T cells, INF-γ-producing CD8+ T cells, and CD11c+ CD8+ T cells in obstructed kidney (86, 87). In overview, it is significant to explore the combined effects of depression and ALI on the prognosis of patients with CKD.
The major strength of our study is that it used a representative sample of CKD patients in the US, as well as a multiethnic sample so that the findings can be generalized to the larger CKD population. Furthermore, reliable and exhaustive data were included in NHNAES so that we could control as much as possible for well-known confounders such as demographic characteristics, lifestyle, and history of chronic disease. We also performed some sensitivity analyses at the end to assess the robustness of the findings.
Nevertheless, some limitations must be taken into account when explaining our findings. Firstly, indicators of depression and nutritional-inflammatory status in the CKD population were measured only at the time of baseline data collection; therefore, we were unable to obtain the dynamics of these conditions during the follow-up period. Secondly, although as much information on confounders as possible was obtained, there was still residual confounding information that could not be obtained, such as medication adherence, the cycle and duration of dialysis treatments, renal transplant history, and medication use. For example, decreased medication adherence is common in depressed patients and this can have a serious impact on the prognosis of CKD patients. One study found that depression in hemodialysis patients was significantly associated with blood pressure medication nonadherence (88). This has led to confusion about whether depression itself has an impact on CKD prognosis. In addition, a portion of depressed patients may be receiving sertraline. Of note, one study found that sertraline use in CKD patients was associated with downregulation of certain otherwise high inflammatory biomarkers (89). So the inflammatory markers we measured may have been affected by these drugs as well. Therefore, the absence of above information may lead to biased study results. Thirdly, CKD populations with major depressive symptoms may disproportionately choose not to participate in the NHANES survey (90); therefore, a small proportion of major depressive populations may be absent in this study.
In this cohort study of CKD patients in the US, findings showed that both depression and nutritional-inflammatory status were associated with their all-cause mortality. Notably, patients with poorer nutritional-inflammatory status and depression had the worst prognosis relative to the other populations. These results emphasize that special attention should be paid to depression and nutrient-inflammatory status when developing individualized intervention strategies to improve the prognosis of patients with CKD and that they should be targeted for treatment and prevention.
Publicly available datasets were analyzed in this study. This data can be found here: Publicly available datasets were analyzed in this study. This data can be found here: NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm).
The studies involving humans were approved by the studies involving human participants were reviewed and approved by the studies involving human participants were reviewed and approved by the NCHS Ethic Review Board. The patients/participants provided their written informed consent to participate in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. WL: Data curation, Methodology, Investigation, Funding acquisition, Software,Writing – original draft. XL: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. JW: Resources, Writing – original draft. YC: Data curation, Methodology, Supervision, Project administration, Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital (ZXY-YJJ2023-2).
The authors are sincerely grateful to the NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1453062/full#supplementary-material
1. Jha, V, Garcia-Garcia, G, Iseki, K, Li, Z, Naicker, S, Plattner, B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
2. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
3. Johansen, KL, Chertow, GM, Foley, RN, Gilbertson, DT, Herzog, CA, Ishani, A, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. (2021) 77:A7–a8. doi: 10.1053/j.ajkd.2021.01.002
4. Bowe, B, Xie, Y, Li, T, Mokdad, AH, Xian, H, Yan, Y, et al. Changes in the US burden of chronic kidney disease from 2002 to 2016: An analysis of the global burden of disease study. JAMA Netw Open. (2018) 1:e184412. doi: 10.1001/jamanetworkopen.2018.4412
5. Hedayati, SS, Minhajuddin, AT, Afshar, M, Toto, RD, Trivedi, MH, and Rush, AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. (2010) 303:1946–53. doi: 10.1001/jama.2010.619
6. Chen, Y, Nie, Y, Wu, J, Li, C, Zheng, L, Zhu, B, et al. Association between systemic inflammatory indicators with the survival of chronic kidney disease: a prospective study based on NHANES. Front Immunol. (2024) 15:1365591. doi: 10.3389/fimmu.2024.1365591
7. Graterol Torres, F, Molina, M, Soler-Majoral, J, Romero-González, G, Rodríguez Chitiva, N, Troya-Saborido, M, et al. Evolving concepts on inflammatory biomarkers and malnutrition in chronic kidney disease. Nutrients. (2022) 14:4297. doi: 10.3390/nu14204297
8. Guenzani, D, Buoli, M, Caldiroli, L, Carnevali, GS, Serati, M, Vezza, C, et al. Malnutrition and inflammation are associated with severity of depressive and cognitive symptoms of old patients affected by chronic kidney disease. J Psychosom Res. (2019) 124:109783. doi: 10.1016/j.jpsychores.2019.109783
9. Gencer, F, Yıldıran, H, and Erten, Y. Association of Malnutrition Inflammation Score with Anthropometric Parameters, depression, and quality of life in hemodialysis patients. J Am Coll Nutr. (2019) 38:457–62. doi: 10.1080/07315724.2018.1550371
10. Alshogran, OY, Khalil, AA, Oweis, AO, Altawalbeh, SM, and Alqudah, MAY. Association of brain-derived neurotrophic factor and interleukin-6 serum levels with depressive and anxiety symptoms in hemodialysis patients. Gen Hosp Psychiatry. (2018) 53:25–31. doi: 10.1016/j.genhosppsych.2018.04.003
11. Gu, L, Xia, Z, Qing, B, Wang, W, Chen, H, Wang, J, et al. Systemic inflammatory response index (SIRI) is associated with all-cause mortality and cardiovascular mortality in population with chronic kidney disease: evidence from NHANES (2001-2018). Front Immunol. (2024) 15:1338025. doi: 10.3389/fimmu.2024.1338025
12. Yu, JH, Chen, Y, and Yin, MG. Association between the prognostic nutritional index (PNI) and all-cause mortality in patients with chronic kidney disease. Ren Fail. (2023) 45:2264393. doi: 10.1080/0886022X.2023.2264393
13. Huang, P, Mai, Y, Zhao, J, Yi, Y, and Wen, Y. Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: observational study of 40,937 adults. Inflamm Res. (2024) 73:655–67. doi: 10.1007/s00011-024-01861-0
14. Song, M, Zhang, Q, Song, C, Liu, T, Zhang, X, Ruan, G, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:2504–14. doi: 10.1002/jcsm.13032
15. Yao, J, Chen, X, Meng, F, Cao, H, and Shu, X. Combined influence of nutritional and inflammatory status and depressive symptoms on mortality among US cancer survivors: findings from the NHANES. Brain Behav Immun. (2024) 115:109–17. doi: 10.1016/j.bbi.2023.10.002
16. Kusunoki, K, Toiyama, Y, Okugawa, Y, Yamamoto, A, Omura, Y, Kusunoki, Y, et al. The advanced lung cancer inflammation index predicts outcomes in patients with Crohn's disease after surgical resection. Color Dis. (2021) 23:84–93. doi: 10.1111/codi.15248
17. Palmer, S, Vecchio, M, Craig, JC, Tonelli, M, Johnson, DW, Nicolucci, A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. (2013) 84:179–91. doi: 10.1038/ki.2013.77
18. Bautovich, A, Katz, I, Smith, M, Loo, CK, and Harvey, SB. Depression and chronic kidney disease: a review for clinicians. Aust N Z J Psychiatry. (2014) 48:530–41. doi: 10.1177/0004867414528589
19. Liu, M, Zhang, Y, Yang, S, Wu, Q, Ye, Z, Zhou, C, et al. Bidirectional relations between depression symptoms and chronic kidney disease. J Affect Disord. (2022) 311:224–30. doi: 10.1016/j.jad.2022.05.104
20. Palmer, SC, Vecchio, M, Craig, JC, Tonelli, M, Johnson, DW, Nicolucci, A, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. (2013) 62:493–505. doi: 10.1053/j.ajkd.2013.02.369
21. Hedayati, SS, Bosworth, HB, Briley, LP, Sloane, RJ, Pieper, CF, Kimmel, PL, et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. (2008) 74:930–6. doi: 10.1038/ki.2008.311
22. Czira, ME, Lindner, AV, Szeifert, L, Molnar, MZ, Fornadi, K, Kelemen, A, et al. Association between the malnutrition-inflammation score and depressive symptoms in kidney transplanted patients. Gen Hosp Psychiatry. (2011) 33:157–65. doi: 10.1016/j.genhosppsych.2011.01.012
23. Chae, WR, Nübel, J, Baumert, J, Gold, SM, and Otte, C. Association of depression and obesity with C-reactive protein in Germany: a large nationally representative study. Brain Behav Immun. (2022) 103:223–31. doi: 10.1016/j.bbi.2022.04.024
24. Lamers, F, Milaneschi, Y, Smit, JH, Schoevers, RA, Wittenberg, G, and Penninx, B. Longitudinal association between depression and inflammatory markers: results from the Netherlands study of depression and anxiety. Biol Psychiatry. (2019) 85:829–37. doi: 10.1016/j.biopsych.2018.12.020
25. Abdulan, IM, Onofriescu, M, Stefaniu, R, Mastaleru, A, Mocanu, V, Alexa, ID, et al. The predictive value of malnutrition for functional and cognitive status in elderly hemodialysis patients. Int Urol Nephrol. (2019) 51:155–62. doi: 10.1007/s11255-018-2000-0
26. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
27. Miller, AH, and Raison, CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
28. Jayakumar, S, Jennings, S, Halvorsrud, K, Clesse, C, Yaqoob, MM, Carvalho, LA, et al. A systematic review and meta-analysis of the evidence on inflammation in depressive illness and symptoms in chronic and end-stage kidney disease. Psychol Med. (2023) 53:5839–51. doi: 10.1017/S0033291722003099
29. KDIGO . 2021 clinical practice guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:S1–s276. doi: 10.1016/j.kint.2021.05.021
30. Delgado, C, Baweja, M, Crews, DC, Eneanya, ND, Gadegbeku, CA, Inker, LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. (2021) 32:2994–3015. doi: 10.1681/ASN.2021070988
31. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
32. Levis, B, Benedetti, A, and Thombs, BD. Accuracy of patient health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. (2019) 365:l1476. doi: 10.1136/bmj.l1476
33. Wang, M, Huang, ZH, Zhu, YH, He, P, and Fan, QL. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011-2018. Food Funct. (2023) 14:9279–86. doi: 10.1039/D3FO01157G
35. Ibrahim, S, and El Salamony, O. Depression, quality of life and malnutrition-inflammation scores in hemodialysis patients. Am J Nephrol. (2008) 28:784–91. doi: 10.1159/000131101
36. Markaki, AG, Charonitaki, A, Psylinakis, E, Dimitropoulakis, P, and Spyridaki, A. Nutritional status in hemodialysis patients is inversely related to depression and introversion. Psychol Health Med. (2019) 24:1213–9. doi: 10.1080/13548506.2019.1612074
37. Yuan, D, Kuan, T, Ling, H, Wang, H, Feng, L, Zhao, Q, et al. Serum metabolomics of end-stage renal disease patients with depression: potential biomarkers for diagnosis. Ren Fail. (2021) 43:1479–91. doi: 10.1080/0886022X.2021.1994995
38. Brys, ADH, Di Stasio, E, Lenaert, B, Sanguinetti, M, Picca, A, Calvani, R, et al. Serum interleukin-6 and endotoxin levels and their relationship with fatigue and depressive symptoms in patients on chronic haemodialysis. Cytokine. (2020) 125:154823. doi: 10.1016/j.cyto.2019.154823
39. Tutan, D, Erdoğan Kaya, A, and Eser, B. The relationship between neutrophil lymphocyte ratio, platelet lymphocyte ratio, and depression in dialysis patients. Medicine (Baltimore). (2023) 102:e35197. doi: 10.1097/MD.0000000000035197
40. Lee, DY, Han, SY, Lee, K, Lee, Y, Phan, L, Mansur, RB, et al. Association of a low protein diet with depressive symptoms and poor health-related quality of life in CKD. J Psychiatr Res. (2023) 161:282–8. doi: 10.1016/j.jpsychires.2023.02.032
41. Yue, H, Zhou, P, Xu, Z, Liu, L, Zong, A, Qiu, B, et al. Effect of low-protein diet on kidney function and nutrition in nephropathy: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2020) 39:2675–85. doi: 10.1016/j.clnu.2019.11.039
42. Ko, GJ, Obi, Y, Tortorici, AR, and Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care. (2017) 20:77–85. doi: 10.1097/MCO.0000000000000342
43. Soczynska, JK, Kennedy, SH, Woldeyohannes, HO, Liauw, SS, Alsuwaidan, M, Yim, CY, et al. Mood disorders and obesity: understanding inflammation as a pathophysiological nexus. NeuroMolecular Med. (2011) 13:93–116. doi: 10.1007/s12017-010-8140-8
44. Zhang, Y, Liu, C, Zhao, Y, Zhang, X, Li, B, and Cui, R. The effects of calorie restriction in depression and potential mechanisms. Curr Neuropharmacol. (2015) 13:536–42. doi: 10.2174/1570159X13666150326003852
45. Siew, ED, and Ikizler, TA. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial. (2010) 23:378–82. doi: 10.1111/j.1525-139X.2010.00763.x
46. Rao, TS, Asha, MR, Ramesh, BN, and Rao, KS. Understanding nutrition, depression and mental illnesses. Indian J Psychiatry. (2008) 50:77–82. doi: 10.4103/0019-5545.42391
47. Lyra, ESNM, Lam, MP, Soares, CN, Munoz, DP, Milev, R, and De Felice, FG. Insulin resistance as a shared pathogenic mechanism between depression and type 2 diabetes. Front Psych. (2019) 10:57. doi: 10.3389/fpsyt.2019.00057
48. Kadatane, SP, Satariano, M, Massey, M, Mongan, K, and Raina, R. The role of inflammation in CKD. Cells. (2023) 12:1581. doi: 10.3390/cells12121581
49. Zhu, F, Tu, H, and Chen, T. The microbiota-gut-brain Axis in depression: the potential pathophysiological mechanisms and microbiota combined Antidepression effect. Nutrients. (2022) 14:2081. doi: 10.3390/nu14102081
50. Dziurkowska, E, and Wesolowski, M. Cortisol as a biomarker of mental disorder severity. J Clin Med. (2021) 10:5204. doi: 10.3390/jcm10215204
51. van Dooren, FE, Schram, MT, Schalkwijk, CG, Stehouwer, CD, Henry, RM, Dagnelie, PC, et al. Associations of low grade inflammation and endothelial dysfunction with depression - the Maastricht study. Brain Behav Immun. (2016) 56:390–6. doi: 10.1016/j.bbi.2016.03.004
52. Bossola, M, Ciciarelli, C, Di Stasio, E, Panocchia, N, Conte, GL, Rosa, F, et al. Relationship between appetite and symptoms of depression and anxiety in patients on chronic hemodialysis. J Ren Nutr. (2012) 22:27–33. doi: 10.1053/j.jrn.2011.02.005
53. Ibrahim, S, Hossam, M, and Belal, D. Study of non-compliance among chronic hemodialysis patients and its impact on patients' outcomes. Saudi J Kidney Dis Transpl. (2015) 26:243–9. doi: 10.4103/1319-2442.152405
54. Pariante, CM . Increased inflammation in depression: a little in all, or a lot in a few? Am J Psychiatry. (2021) 178:1077–9. doi: 10.1176/appi.ajp.2021.21101043
55. Lawes, S, Demakakos, P, Steptoe, A, Lewis, G, and Carvalho, LA. Combined influence of depressive symptoms and systemic inflammation on all-cause and cardiovascular mortality: evidence for differential effects by gender in the English longitudinal study of ageing. Psychol Med. (2019) 49:1521–31. doi: 10.1017/S003329171800209X
56. Ahola, AJ, Harjutsalo, V, Forsblom, C, Pouwer, F, and Groop, PH. Depression is associated with progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. (2021) 44:174–80. doi: 10.2337/dc20-0493
57. Chilcot, J, Guirguis, A, Friedli, K, Almond, M, Day, C, Da Silva-Gane, M, et al. Depression symptoms in Haemodialysis patients predict all-cause mortality but not kidney transplantation: a cause-specific outcome analysis. Ann Behav Med. (2018) 52:1–8. doi: 10.1007/s12160-017-9918-9
58. Tsai, YC, Chiu, YW, Hung, CC, Hwang, SJ, Tsai, JC, Wang, SL, et al. Association of symptoms of depression with progression of CKD. Am J Kidney Dis. (2012) 60:54–61. doi: 10.1053/j.ajkd.2012.02.325
59. Park, S, Lee, S, Kim, Y, Lee, Y, Kang, MW, Kim, K, et al. Causal effects of positive affect, life satisfaction, depressive symptoms, and neuroticism on kidney function: a Mendelian randomization study. J Am Soc Nephrol. (2021) 32:1484–96. doi: 10.1681/ASN.2020071086
60. Chan, GC, Ng, JK, Chow, KM, Kwan, BC, Kwong, VW, Pang, WF, et al. Depression does not predict clinical outcome of Chinese peritoneal Dialysis patients after adjusting for the degree of frailty. BMC Nephrol. (2020) 21:329. doi: 10.1186/s12882-020-01994-4
61. Saglimbene, V, Palmer, S, Scardapane, M, Craig, JC, Ruospo, M, Natale, P, et al. Depression and all-cause and cardiovascular mortality in patients on haemodialysis: a multinational cohort study. Nephrol Dial Transplant. (2017) 32:377–84. doi: 10.1093/ndt/gfw016
62. Wu, PH, Lin, MY, Huang, TH, Lin, YT, Hung, CC, Yeh, YC, et al. Depression amongst patients commencing maintenance dialysis is associated with increased risk of death and severe infections: a nationwide cohort study. PLoS One. (2019) 14:e0218335. doi: 10.1371/journal.pone.0218335
63. Lee, DY, Yoo, DK, Han, SY, Lee, K, Lee, Y, Teopiz, KM, et al. Association between depressive symptoms and bone density in elderly patients with non-dialysis dependent chronic kidney disease. J Affect Disord. (2022) 319:549–54. doi: 10.1016/j.jad.2022.09.014
64. Kurita, N, Wakita, T, Fujimoto, S, Yanagi, M, Koitabashi, K, Suzuki, T, et al. Hopelessness and depression predict sarcopenia in advanced CKD and Dialysis: a multicenter cohort study. J Nutr Health Aging. (2021) 25:593–9. doi: 10.1007/s12603-020-1556-4
65. Jeon, HO, Kim, J, and Kim, O. Factors affecting depressive symptoms in employed hemodialysis patients with chronic renal failure. Psychol Health Med. (2020) 25:940–9. doi: 10.1080/13548506.2019.1702218
66. Bivanco-Lima, D, Souza Santos, I, Vannucchi, AM, and Almeida Ribeiro, MC. Cardiovascular risk in individuals with depression. Rev Assoc Med Bras (1992). (2013) 59:298–304. doi: 10.1016/j.ramb.2012.12.006
67. Mihai, S, Codrici, E, Popescu, ID, Enciu, AM, Albulescu, L, Necula, LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. (2018) 2018:1–16. doi: 10.1155/2018/2180373
68. Lai, W, Xie, Y, Zhao, X, Xu, X, Yu, S, Lu, H, et al. Elevated systemic immune inflammation level increases the risk of total and cause-specific mortality among patients with chronic kidney disease: a large multi-center longitudinal study. Inflamm Res. (2023) 72:149–58. doi: 10.1007/s00011-022-01659-y
69. Ruan, GT, Yang, M, Zhang, XW, Song, MM, Hu, CL, Ge, YZ, et al. Association of Systemic Inflammation and Overall Survival in elderly patients with Cancer Cachexia - results from a multicenter study. J Inflamm Res. (2021) 14:5527–40. doi: 10.2147/JIR.S332408
70. Wen, YZ, Liu, GM, Liao, JP, and Xu, JW. Advanced lung cancer inflammation index predicts overall survival of hepatocellular carcinoma after hepatectomy. Front Oncol. (2024) 14:1294253. doi: 10.3389/fonc.2024.1294253
71. Yuan, X, Huang, B, Wang, R, Tie, H, and Luo, S. The prognostic value of advanced lung cancer inflammation index (ALI) in elderly patients with heart failure. Front Cardiovasc Med. (2022) 9:934551. doi: 10.3389/fcvm.2022.934551
72. Zhang, Y, Pan, Y, Tu, J, Liao, L, Lin, S, Chen, K, et al. The advanced lung cancer inflammation index predicts long-term outcomes in patients with hypertension: national health and nutrition examination study, 1999-2014. Front Nutr. (2022) 9:989914. doi: 10.3389/fnut.2022.989914
73. Chen, X, Hong, C, Guo, Z, Huang, H, and Ye, L. Association between advanced lung cancer inflammation index and all-cause and cardiovascular mortality among stroke patients: NHANES, 1999-2018. Front Public Health. (2024) 12:1370322. doi: 10.3389/fpubh.2024.1370322
74. Chen, Y, Guan, M, Wang, R, and Wang, X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999-2018. Front Endocrinol (Lausanne). (2023) 14:1298345. doi: 10.3389/fendo.2023.1298345
75. Tsur, AM, Akavian, I, Landau, R, Derazne, E, Tzur, D, Vivante, A, et al. Adolescent body mass index and early chronic kidney disease in young adulthood. JAMA Pediatr. (2024) 178:142–50. doi: 10.1001/jamapediatrics.2023.5420
76. Wang, T, Zhou, Z, Ren, L, Shen, Z, Li, J, and Zhang, L. Prediction of the risk of 3-year chronic kidney disease among elderly people: a community-based cohort study. Ren Fail. (2024) 46:2303205. doi: 10.1080/0886022X.2024.2303205
77. Kanbay, M, Copur, S, Siriopol, D, Yildiz, AB, Berkkan, M, Tuttle, KR, et al. The risk for chronic kidney disease in metabolically healthy obese patients: a systematic review and meta-analysis. Eur J Clin Investig. (2023) 53:e13878. doi: 10.1111/eci.13878
78. Zimmermann, S, Mathew, A, Schöppe, R, Mangova, G, Biemann, R, Surov, A, et al. Fat tissue quantity, waist circumference or waist-to-hip ratio in patients with chronic kidney disease: a systematic review and meta-analysis. Obes Res Clin Pract. (2024) 18:81–7. doi: 10.1016/j.orcp.2024.03.007
79. Ishida, Y, Maeda, K, Nonogaki, T, Shimizu, A, Ueshima, J, Nagano, A, et al. Body mass index and weight loss predict mortality in older patients with chronic kidney disease. Geriatr Gerontol Int. (2022) 22:984–5. doi: 10.1111/ggi.14488
80. Lai, KJ, Hsieh, YP, Chiu, PF, and Lin, PR. Association of Albumin and Globulin with mortality risk in incident peritoneal dialysis patients. Nutrients. (2022) 14:2850. doi: 10.3390/nu14142850
81. Roche, M, Rondeau, P, Singh, NR, Tarnus, E, and Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. (2008) 582:1783–7. doi: 10.1016/j.febslet.2008.04.057
82. Alves, FC, Sun, J, Qureshi, AR, Dai, L, Snaedal, S, Bárány, P, et al. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One. (2018) 13:e0190410. doi: 10.1371/journal.pone.0190410
83. Zhao, WM, Tao, SM, and Liu, GL. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail. (2020) 42:1059–66. doi: 10.1080/0886022X.2020.1832521
84. Ao, G, Wang, Y, Qi, X, Wang, F, and Wen, H. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: a meta-analysis. Clin Exp Nephrol. (2021) 25:157–65. doi: 10.1007/s10157-020-01975-9
85. Liu, X, Li, X, Chen, Y, Liu, X, Liu, Y, Wei, H, et al. Systemic immune-inflammation index is associated with chronic kidney disease in the U.S. population: insights from NHANES 2007-2018. Front Immunol. (2024) 15:1331610. doi: 10.3389/fimmu.2024.1331610
86. Gao, M, Wang, J, Zang, J, An, Y, and Dong, Y. The mechanism of CD8(+) T cells for reducing myofibroblasts accumulation during renal fibrosis. Biomol Ther. (2021) 11:990. doi: 10.3390/biom11070990
87. Do Valle Duraes, F, Lafont, A, Beibel, M, Martin, K, Darribat, K, Cuttat, R, et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight. (2020) 5:e130651. doi: 10.1172/jci.insight.130651
88. Kauric-Klein, Z . Depression and medication adherence in patients on hemodialysis. Curr Hypertens Rev. (2017) 13:138–43. doi: 10.2174/1573402113666171129182611
89. Gregg, LP, Carmody, T, Le, D, Toto, RD, Trivedi, MH, and Hedayati, SS. Inflammation and response to sertraline treatment in patients with CKD and major depression. Am J Kidney Dis. (2020) 75:457–60. doi: 10.1053/j.ajkd.2019.09.007
Keywords: inflammatory, nutritional, chronic kidney disease, depression, mortality, NHANES
Citation: Zhou J, Liu W, Liu X, Wu J and Chen Y (2024) Independent and joint influence of depression and advanced lung cancer inflammation index on mortality among individuals with chronic kidney disease. Front. Nutr. 11:1453062. doi: 10.3389/fnut.2024.1453062
Received: 22 June 2024; Accepted: 03 September 2024;
Published: 23 October 2024.
Edited by:
Olivia Di Vincenzo, University of Naples Federico II, ItalyReviewed by:
Hacer Erdem Tilki, Ondokuz Mayıs University, TürkiyeCopyright © 2024 Zhou, Liu, Liu, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Chen, eWluZy5jaGVuQHRtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.