
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 19 September 2024
Sec. Nutrition, Psychology and Brain Health
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1451386
Background: Previous studies have reported the association between dietary total antioxidant capacity (TAC) and risk of stroke, but these findings have been inconsistent. We therefore performed this systematic review and dose–response meta-analysis of observational studies to evaluate the association between dietary TAC and risk of stroke.
Methods: A systematic literature search was carried out through PubMed, ISI Web of Science, EBSCO, Scopus and China National Knowledge Infrastructure (CNKI) databases, to find the relevant articles published up to 31 May, 2024. Random-effects or fixed-effects models were used to pool the relative risks (RRs) and their 95% confidence intervals (CIs) where appropriate. Heterogeneity across studies were determined using the Cochran’s Q test and I-square (I2) statistics.
Results: Eight observational studies (six cohort and two case–control studies) were included in the final analysis. The pooled results showed that higher intake of dietary TAC was associated with a lower risk of stroke (RR = 0.88; 95%CI: 0.81–0.95, p = 0.002). Additionally, dose–response analysis of cohort studies demonstrated a linear association between dietary TAC intake and risk of stroke (RR = 0.994; 95%CI: 0.990–0.999, Pnon-linearity = 0.329, Pdose–response = 0.014). Subgroup analyses showed the inverse association between dietary TAC intake and risk of stroke in the studies with mean age < 50 (RR = 0.82, 95%CI: 0.67–0.99, p = 0.044), and there was no evidence of heterogeneity (p = 0.360; I2 = 0.0%).
Conclusion: Our findings indicated that higher intake of dietary TAC was inversely associated with the risk of stroke. Future studies in particular of longitudinal design are needed to confirm this inverse relationship.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024547706.
Stroke is a prevalent global disease, with an estimated 12.2 millions new cases and 101 million prevalent cases in 2019 (1). It ranks as the second leading cause of death and the third leading cause of death and disability combined worldwide, posing a serious threats to public health (2). The Global Burden of Disease Study (GBD) 2017 estimated that stroke-related deaths in China reached approximately 2 million in 2017, making stroke as a leading cause of death (3). The well-established risk factors for stroke included smoking, physical inactivity, obesity, hypertension, diabetes, cardiovascular disease and unhealthy diet (4). Therefore, the implementation of effective prevention strategies is essential to mitigate the burden of this disease.
Over the past few decades, it has been suggested that dietary factors have an essential role in preventing stroke (5). Notably, evidence has shown that some kinds of dietary patterns, minerals and vitamins play an important role in preventing various chronic diseases, such as cardiovascular diseases and diabetes (6, 7). However, there was not sufficient literature examining the benefits of dietary antioxidants intake on cardiovascular diseases, particularly stroke. A previous meta-analysis found that increased consumption of fruit and vegetables was associated with a reduced risk of stroke (8). Researchers hypothesized that phytochemicals and vitamins with antioxidant properties present in fruit and vegetables might play a pivotal role in this favorable effect (9). Indeed, dietary antioxidants have been known to scavenge free radicals and reduce oxidative stress (10). Previous epidemiological studies have mainly focused on the effects of the intake of individual antioxidants or a group of antioxidants on the risk of stroke (11–13). For example, in an earlier meta-analysis of prospective studies, dietary intake of vitamin C was significantly associated with a lower risk of stroke (14). However, due to the synergistic effects of different dietary antioxidants, an assessment of individual antioxidant intake May not fully reflect the total antioxidant capacity of the diet (15). To consider this, dietary total antioxidant capacity (TAC) has been developed as a suitable tool for studying the potential beneficial effects of the overall dietary antioxidants, taking into account the cumulative/synergistic effects of various antioxidants and their interactions with each other (16).
Recently, dietary TAC has garnered considerable attention in nutritional research (17). Accumulating evidence suggests that dietary TAC is inversely associated with adverse health outcomes, such as cardiovascular diseases, cancer and mortality (16, 18, 19). To date, only a few epidemiological studies have evaluated the association between dietary TAC and risk of stroke (9, 20–26). However, findings of these previous studies were inconsistent. While some studies reported an inverse association between dietary TAC and risk of stroke (21, 23), others found a non-significant association (24–26). Furthermore, to our best knowledge, no systematic review and dose–response meta-analysis has so far been conducted to assess the association between dietary TAC and risk of stroke. Therefore, to ascertain the association between dietary TAC and risk of stroke, we conducted a systematic review and dose–response meta-analysis to summarize the findings from observational studies published up to May 2024.
This study complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (27). The protocol of this meta-analysis has been registered in the International Prospective Register of Systematic reviews (PROSPERO), and its registration number is CRD42024547706.
A systematic search using PubMed, ISI Web of Science, EBSCO, Scopus, and CNKI databases was performed to find the studies that evaluated the association between dietary TAC and stroke up to 31 May, 2024. The search strategy included the following keywords: “dietary antioxidant capacity,” “dietary total antioxidant capacity,” “dietary TAC,” “non-enzymatic antioxidant capacity,” “dietary antioxidant index,” “antioxidant capacity of diet” and “stroke.” Moreover, hand-searching from reference lists of all relevant articles, previous reviews and meta-analyses was performed to identify relevant studies. Meanwhile, unpublished studies or gray literature were not included in this meta-analysis. The complete search strategy could be found in the Supplementary Table S1. Two authors (Y.-Q.H and L.S.) independently screened and crosschecked each article from the literature search, and a third author (X.-Z.H) was consulted to resolve any discrepancies.
In the initial search, two authors (Y.-Q.H and X.-Z.H.) independently screened the titles and abstracts of the retrieved articles and excluded duplicates and irrelevant articles. Then, the full-text versions of the articles were reviewed basing on the inclusion and exclusion criteria of the current systematic review and meta-analysis. To be included in our analyses, studies must meet all of the following eligibility criteria: (1) observational studies, e.g., cohort, case–control or cross-sectional studies; (2) those studies were published in English or Chinese languages; (3) the exposure of interest was dietary TAC; (4) the outcome of interest was stroke, including any fatal/non-fatal ischemic stroke, hemorrhagic stroke, or other cerebrovascular accidents; (5) studies providing the relative risks (RRs), odds ratios (ORs) and hazards ratios (HRs) along with their corresponding 95% confidence intervals (CIs) of stroke (or sufficient data to calculate them); (6) If the original data in the retrieved studies lacked sufficient detail, the corresponding author of this study would be contacted by email twice. Besides, studies were excluded if they met one of the following criteria: (1) animal, cell culture, and in vitro studies; (2) non-observational studies, including conference abstracts, reviews, editorials, case reports, book chapters, and letters; (3) did not provide the HRs, RRs or ORs with corresponding 95% CIs; (4): the exposure of interest was single antioxidant, such as vitamin C; (5) Irrelevant articles. In case of multiple published reports on the same dataset, we selected the most recent study; Otherwise, the one with the most number of cases was selected. When results of a study for men and women were reported separately, we treated each analysis as a separate study.
Two independent authors (L.Y. and Y.-J.N) independently extracted the following information from all selected article: the first author’s last name, publication year, study design, study region, sample size, number of participants and stroke cases, mean age/age range, duration of follow-up in cohort studies, methods used for assessing dietary TAC, confounding variables adjusted in the multivariate analyses, and reported risk estimates with their corresponding 95% CIs of stroke across categories of dietary TAC.
The authors (L.S. and Q.Z) independently assessed the quality of included studies using the Newcastle-Ottawa Scale(NOS), which was designed for non-randomized studies (28). This scale is composed of eight items in three domains: study selection, comparability of participants, and assessment of outcome/exposure of interest, with a maximum score of 9. Studies with NOS scores ≥7 points were classified as high quality (29). Any discrepancies between two authors were resolved by a third author to reach a consensus.
Dietary TAC, also known as the non-enzymatic antioxidant capacity (NEAC), has been developed to assess the overall antioxidant activity from foods and beverages using different chemical assays, such as the ferric reducing antioxidant potential (FRAP), oxygen radical absorbance capacity (ORAC), Trolox equivalent antioxidant capacity (TEAC), vitamin C equivalent antioxidant capacity (VCEAC) and 2,2′-azino-bis (3-ethylbenzthiazoline) 6-sulfonic acid (ABTS), and the total radical-trapping antioxidant parameter (TRAP) (30).
In this study, we used RRs and 95%CIs as the risk estimate for the main analysis. Also, we assumed that the HR was approximately equal to the RR (31). For the ORs, they were converted into RRs using the following formula: RR = OR/[(1-P0) + (P0*OR)], in which P0 shows the incidence of stroke in the non-exposed group (32). Log-transformed RRs and their corresponding standard errors (SEs) were obtained using risk ratios (full adjusted ORs, HRs and RRs and corresponding 95% CIs), which were previously extracted for the association between dietary TAC and stroke risk. We performed a pairwise meta-analysis by pooled the RRs and 95% CIs for the highest versus the lowest categories of dietary TAC in relation to the risk of stroke. Heterogeneity across studies was evaluated by the Cochran’s Q test and quantified by I2 statistics. In our analyses, if p-values of Cochran’s Q test <0.10 or I2 > 50% demonstrated the high heterogeneity, and a DerSimonnian and Laird random-effects model was used to pool the RRs. Conversely, a p value of Q-test >0.10 or I2 < 50% indicated an absence of heterogeneity among studies, and a fixed-effects model was used to calculate the pooled RRs (33). If the results indicated significant heterogeneity among studies, sensitivity and subgroup analyses were used to explore potential sources of heterogeneity. Subgroup analyses were performed based on study design (cohort or case–control studies), study region (Western countries or other), mean age (≥50 or <50), sample size (<5,000 or ≥5,000), study quality (≥7 or <7), and methods for dietary assessment (FFQ or 24 h dietary records). Sensitivity analysis was conducted to confirm whether the pooled RRs were robust or sensitive to the impact of a certain study. Publication bias was assessed via visual inspection of funnel plots and quantified by both Begg’s and Egger’s tests (34). If publication bias was found, the trim and fill method was used to re-calculate our results (35). Finally, we also performed a dose–response meta-analysis to estimate the trend from the correlated log RRs across the categories of dietary TAC scores. A two-stage GLST model based on generalized least squares was applied to examine the linear or non-linear dose–response relationship between dietary TAC and risk of stroke (36). We used dietary TAC modelling and restricted cubic splines with three knots at fixed percentiles (10, 50 and 90%) of the distribution. All statistical analyses were carried out using STATA/SE, version 12.0 (StataCorp, College Station, Texas, USA). We considered a p-value were ≤ 0.05 (two -sided) to be statistically significant unless otherwise specified.
The flow chart of literature search process is shown in Figure 1. In the initial literature search, a total of 1,058 relevant articles were retrieved for this study. After eliminating 139 duplicates, 919 articles were selected. Afterward, 901 articles were excluded based on the review of the titles and abstracts of retrieved articles. Eighteen full-text articles were independently reviewed in details. Out of the remaining 20 articles, 12 were excluded because of the following reasons: did not use the stroke as the outcome of interest (n = 3), reported the association between composite dietary antioxidant index and stroke (n = 6), assessed the association between single antioxidant intake and risk of stroke (n = 2), and reported the same participants (n = 1). Finally, eight articles were considered eligible for inclusion in this systematic review and meta-analysis (9, 20–26). The PICO for this meta-analysis is shown in Table 1.
The characteristics of the eligible studies are listed in Table 2. A total of eight articles was included, with sample size of included studies ranging from 237 to 45,882. Of all the included studies, six were cohort studies (9, 22–26) and two were case–control studies (20, 21). With regards to the origin of the studies, three studies were carried out in Swedish (9, 23, 26), two in Iran (20, 21), one in the United States (22), one in Netherlands (23), and one in Italy (24). All of the included studies were published between 2011 and 2024. Two studies included women only (23, 26), and other six studies included both men and women (9, 20, 21, 24, 25). The follow-up duration for the cohort studies ranged from 7.9 to 20.2 years. The age of participants across studies ranged from ages 18 to above. Seven of included studies used FFQs to collect dietary data (9, 20, 21, 23–26), and one study used 24-h dietary recall (22). To measure the dietary TAC, five studies used the FRAP assay (9, 20, 21, 24, 26), one study used the ORAC assay (23), one study used VCEAC and ABTS assays (22), and one study used the TEAC assay (25). Based on the NOS, from eight included studies, seven studies were high quality (9, 21–26), and the remaining one study was medium quality (20). The quality assessment of included studies bases on NOS criteria is shown in Table 3.
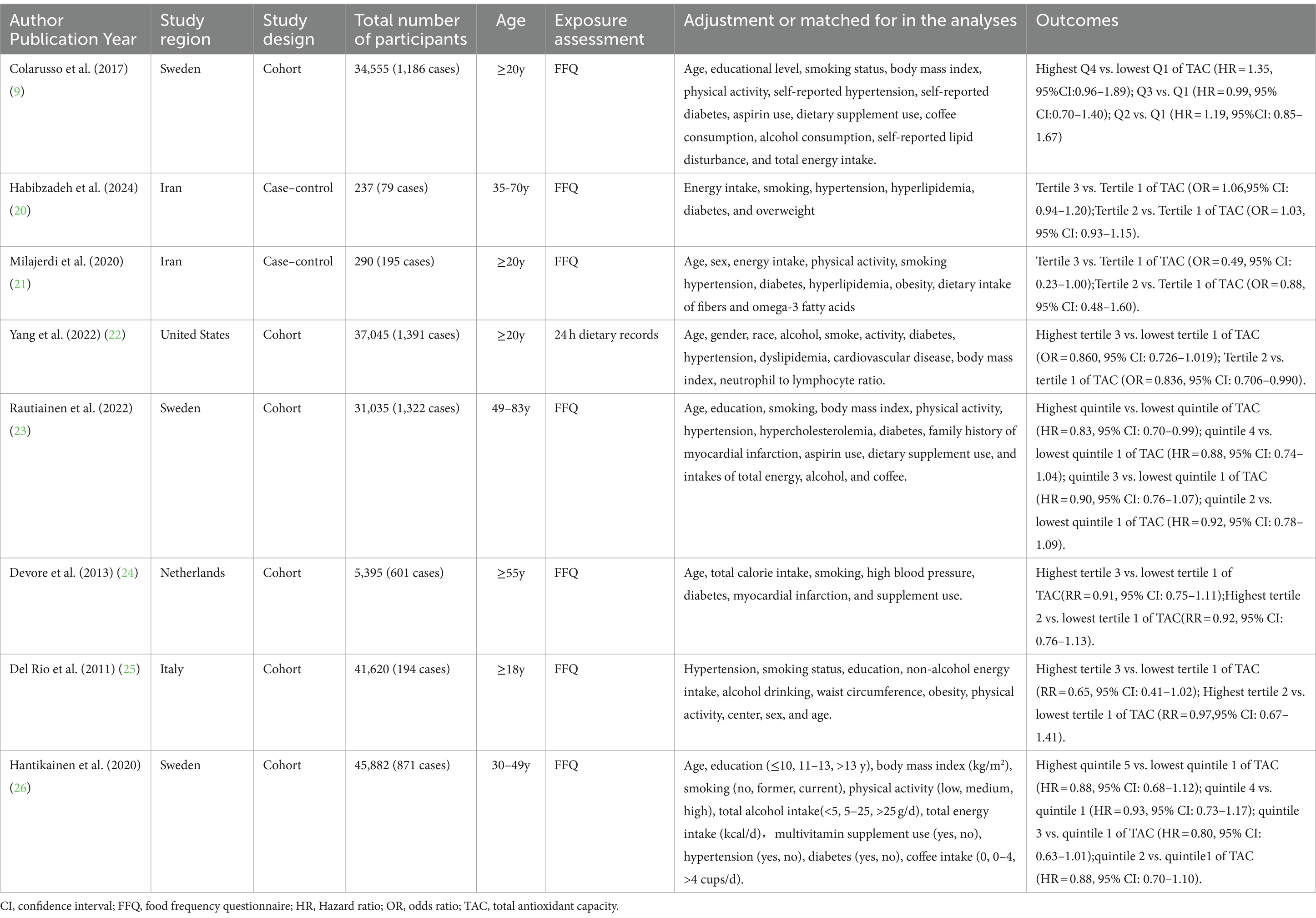
Table 2. Characteristics of the included studies on the relationship between dietary total antioxidant capacity and risk of stroke.
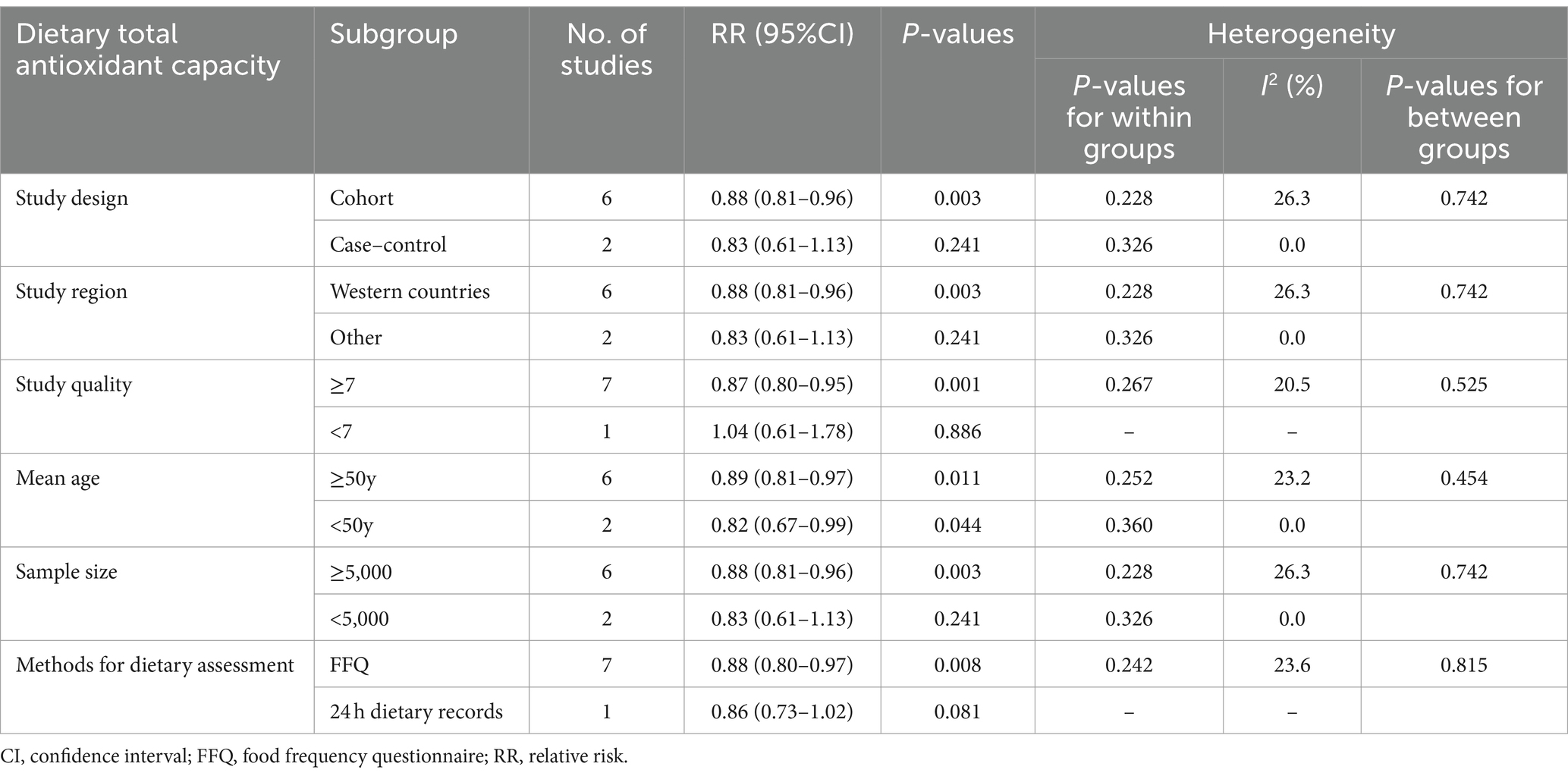
Table 3. Subgroup analyses of stroke for the highest versus lowest category of dietary total antioxidant capacity intake.
Eight studies involving 196,059 participants and 5,839 stroke cases, were included to evaluate the association between dietary TAC and stroke risk. Combining nine effect sizes from eight studies, Figure 2 shows the evidence of a 12% lower risk of stroke in the highest compared with the lowest categories of dietary TAC scores (RR = 0.88; 95%CI: 0.81–0.95, p = 0.002). The low heterogeneity was observed in the included studies (I2 = 13.1%; p = 0.325), thus a fixed-effects model was used to calculate the pooled RRs. Given the low heterogeneity of this meta-analysis, subgroup analyses were not performed to explore the potential sources of heterogeneity across studies.
Five cohort studies (9, 23–26) were included in the dose–response analysis for the relationship between dietary TAC and risk of stroke (Figure 3). The dose–response meta-analysis indicated a linear association between dietary TAC and risk of stroke in the analysis of cohort studies (RR = 0.994; 95%CI:0.990–0.999, Pdose–response = 0.014, Pnon-linearity = 0.329).
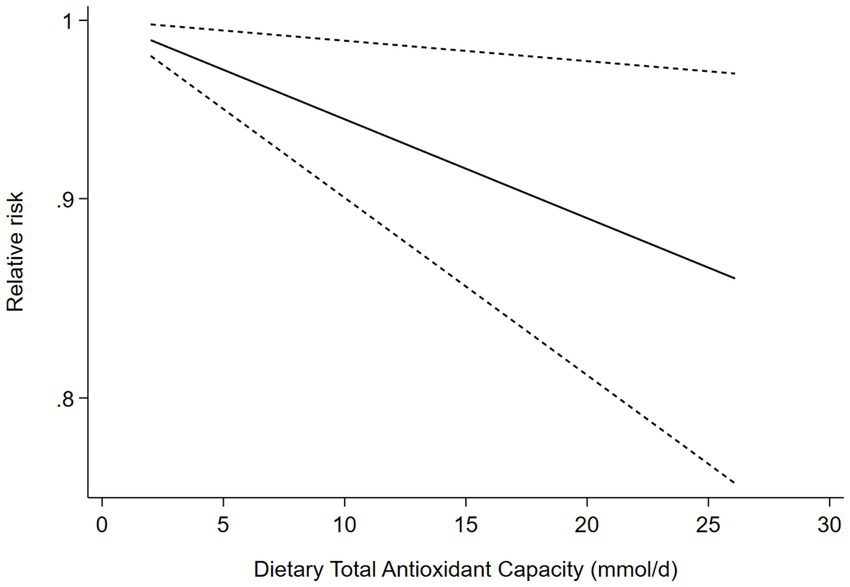
Figure 3. Dose-response association between dietary TAC and risk of stroke in the analysis of five cohort studies.
To further assess the probable sources of heterogeneity across included studies, we conducted subgroup analyses basing on study design, study region, mean age, sample size, study quality, and methods for dietary assessment (Table 3). The results of subgroup analyses showed that dietary TAC was statistically significant in the studies with mean age < 50 (RR = 0.82, 95%CI: 0.67–0.99, p = 0.044), and there was no evidence of heterogeneity (p = 0.360; I2 = 0.0%). Moreover, there was an inverse association between dietary TAC intake and risk of stroke in cohort studies, Western countries and sample size≥5,000 (RR = 0.88; 95%CI: 0.81–0.96, p = 0.003), with less evidence of heterogeneity (p = 0.228; I2 = 26.3%).
As shown in Supplementary Figure S1, inspection of funnel plots revealed little evidence of asymmetry. Begg’s test for publication bias had no statistical significance (highest compared with lowest categories of dietary TAC: p = 0.602). In addition, Egger’s test for publication bias had no statistical significance (p = 0.559).
In sensitivity analysis (Supplementary Figure S2), the results showed that the association between dietary TAC and stroke risk was robust and not affected by any single study or a couple of studies.
The quality of included studies using NOS criteria is shown in Table 4. Seven out of eight included studies received NOS scores ≥7 points, and were classified as of high- quality (9, 21–26). In addition, the remaining one article was classified as of medium-quality (20).
As far as we know, this study is the first systematic review and dose–response meta-analysis to exclusively and systematically evaluate the association between dietary TAC and risk of stroke. The results showed that higher dietary TAC was associated with a reduced risk of stroke. Additionally, the dose–response meta-analysis revealed a significant linear association between dietary TAC and risk of stroke in cohort studies. Sensitivity analysis showed that the pooled results were robust and not affected by any single study or a couple of studies. Our findings corroborate previous research and underscore the important role of dietary TAC in the prevention of stroke.
Although the incidence of stroke has decreased in some Western countries, it remains as the second leading cause of death worldwide (20). Given the global public health concern, it is crucial to clarify effective prevention strategies for stroke. Dietary factors have consistently been recognized as an important and modifiable risk factors for stroke (37). Notably, previous epidemiological studies have primarily focused on the intake of single antioxidants or a group of antioxidants in relation to stroke risk, yielding inconsistent findings (12, 13). However, little is known with regard to the association between dietary TAC intake and risk of stroke. Dietary TAC measures the overall antioxidant capacity of a diet, considering the synergistic effects and interactions of various antioxidants (16). As mentioned above, several epidemiological studies have examined the relationship between dietary TAC and risk of stroke (9, 20–26). But, findings from previously published studies remain inconclusive. For instance, Habibzadeh et al. in a recent nested case–control study, found no significant association between dietary TAC and stroke risk (20). Conversely, a Swedish cohort study of 36, 715 women by Rautiainen et al., showed that dietary TAC was inversely associated with total stroke among cardiovascular disease (CVD)-free women and hemorrhagic stroke among women with CVD history (23). Similarly, our current study found that higher dietary TAC intake was associated with a lower risk of stroke in the current study. The discrepant findings in previous studies May be attributed the following several factors. First, the methods for assessing dietary TAC are different. For example, Yang et al., used vitamin C equivalent antioxidant capacity and ABTS assay to determine the dietary TAC (22), while Del Rio et al., used the TEAC assay in a large Italian cohort (25). Other studies used the FRAP assay (9, 20, 21, 24, 26), which might underestimate the true antioxidant capacity of the whole diet by not considering lipophilic antioxidant (38). Second, discrepancies in dietary data collection methods May contribute to the differing results. Yang et al. used 24-h dietary recalls to evaluate the usual dietary intake (22), whereas the remaining seven studies used a FFQ (9, 20, 21, 23–26). Third, there were the significant differences in dietary habits and lifestyle between different countries. Six of the included studies were conducted in Western countries (9, 22–26), and the remaining two studies in Iran (20, 21). Notably, there are stark differences between Iranian and Western diets. Fourth, the types of foods that contribute the most to dietary TAC vary widely in different countries. In short, discrepancies in the measurement of dietary TAC, types of dietary questionnaire, and differences in dietary habits and lifestyles across countries May contribute to the different results.
Even though evidence on the association between dietary TAC and risk of stroke is inconsistent, several mechanisms have been proposed to explain the observed favorable associations. First, previous studies have indicated that antioxidants abundant in fruits and vegetables can help to prevent oxidative stress (39), which plays a critical role in the pathogenesis of stroke (40). Antioxidants are compounds that scavenge reactive oxygen species (ROS) and protect cells from oxidative damage (41). Second, antioxidants can improve endothelial dysfunction and lower blood pressure, both of which are the known risk factors for stroke (42, 43). A recent systematic review and meta-analysis reported that higher intake of dietary TAC was associated with reduced systolic blood pressure, diastolic blood pressure, and fasting blood sugar, all factors associated with the risk of stroke (4, 30). Third, dietary TAC intake has been inversely associated with plasma concentrations of C-reactive protein, a sensitive biomarker of systemic inflammation (44). Systemic inflammation is a well-known risk factor in the etiology and pathology of stroke (45). All together, these mechanisms discussed above May explain the protective effect of high dietary TAC intake on stroke risk.
There are some strengths and limitations in this meta-analysis. First, this is the first systematic review and dose–response meta-analysis thus far assessing the association between dietary TAC and risk of stroke. Our findings add the evidence for a protective effect of high dietary TAC on stroke, and highlight the importance of increasing dietary TAC intake for the prevention of stroke. Second, stroke diagnosis was confirmed through medical records, minimizing misdiagnosis bias. Third, no signs of publication bias were evident in the funnel plot, and statistical tests for publication bias were also non-significant. Fourth, rigorous article selection was made according to pre-determined inclusion and exclusion criteria. Finally, the dose–response analysis was also conducted to strengthen the association between dietary TAC and stroke risk. Despite the aforementioned strengths, several limitations should also be considered in this study. First, given the observational nature of all included studies, the possibility of residual bias remains. In parallel, two of included studies were case–control studies, which have inherent limitations of recall and selection bias. Thus, we cannot assume the causality of the observed association. Therefore, further prospective cohort studies or randomized controlled trials are needed to confirm the role of dietary TAC in the prevention of stroke. Second, in the present study, dietary TAC measurement was calculated based on self-reported data gathered by 24 h dietary records and FFQs, which might cause misclassification, thereby resulting in the under-or overestimation of dietary TAC. Third, although multiple potential confounding variables have been taken into account, the existence of residual confounders cannot be excluded owing to the undetected or unknown confounders. In addition, adjusted confounding factors were inconsistent across all included studies, leading to some degree of variation in the values of OR, RR or HR. Finally, six of the included studies were performed in Western countries, with only two studies in Asian countries, limiting the generalizability of our findings.
In conclusion, our study demonstrated that higher intake of dietary TAC was significantly associated with a reduced risk of stroke. Our findings contribute additional evidence supporting the favorable effect of high dietary TAC on stroke, and highlight the importance of promoting consumption of dietary TAC for the prevention of stroke. Additionally, it also makes sense to elucidate the potential association between dietary TAC and stroke risk and provides a scientific basis for developing dietary guidelines. Future well-designed prospective studies, particularly in diverse geographic regions and settings, are needed to confirm these findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
YH: Conceptualization, Data curation, Writing – original draft. YN: Data curation, Writing – review & editing. LY: Data curation, Writing – review & editing. LS: Formal analysis, Funding acquisition, Methodology, Writing – review & editing. QZ: Formal analysis, Funding acquisition, Writing – review & editing. XH: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Natural Science Foundation of China (grant number:82004040), Traditional Chinese Medicine Research Project of Zhejiang (Nos. 2020ZB009, 2021ZB010), and Medical and Health research fund project of Zhejiang Province (No. 2022KY006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank the participants from Department of Nutrition, Zhejiang Hospital for their value assistance and support. Besides, we also acknowledge QZ for his important contribution to data collection and analysis in this study. We are grateful to QZ for providing additional data for the current meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1451386/full#supplementary-material
ABTS, 2,2′-azino-bis (3-ethylbenzthiazoline) 6-sulfonic acid; CI, confidence interval; CNKI, China National Knowledge Infrastructure; CVD, cardiovascular disease; FFQ, food frequency questionnaire; FRAP, ferric reducing antioxidant potential; GBD, Global Burden of Disease Study; HR, Hazard ratio; NEAC, non-enzymatic antioxidant capacity; NOS, Newcastle-Ottawa Quality Scale; OR, odds ratio; ORAC, oxygen radical absorbance capacity; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RR, relative risk; TAC, dietary total antioxidant capacity; TEAC, Trolox equivalent antioxidant capacity; TRAP, total radical-trapping antioxidant parameter; VCEAC, vitamin C equivalent antioxidant capacity.
1. Thayabaranathan, T, Kim, J, Cadilhac, DA, Thrift, AG, Donnan, GA, Howard, G, et al. Global stroke statistics 2022. Int J Stroke. (2022) 17:946–56. doi: 10.1177/17474930221123175
2. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
3. Ma, Q, Li, R, Wang, L, Yin, P, Wang, Y, Yan, C, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the global burden of disease study 2019. Lancet Public Health. (2021) 6:e897–906. doi: 10.1016/S2468-2667(21)00228-0
4. Zhang, X, Shu, L, Si, C, Yu, X, Gao, W, Liao, D, et al. Dietary patterns and risk of stroke in adults: a systematic review and Meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. (2015) 24:2173–82. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.035
5. Pandian, JD, Gall, SL, Kate, MP, Silva, GS, Akinyemi, RO, Ovbiagele, BI, et al. Prevention of stroke: a global perspective. Lancet. (2018) 392:1269–78. doi: 10.1016/S0140-6736(18)31269-8
6. Lim, GH, Neelakantan, N, Lee, YQ, Park, SH, Kor, ZH, van Dam, RM, et al. Dietary patterns and cardiovascular diseases in Asia: a systematic review and meta-analysis. Adv Nutr. (2024) 15:100249. doi: 10.1016/j.advnut.2024.100249
7. Morvaridzadeh, M, Sepidarkish, M, Fazelian, S, Rahimlou, M, Omidi, A, Ardehali, SH, et al. Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and meta-analysis. Clin Ther. (2020) 42:e45–63. doi: 10.1016/j.clinthera.2020.01.005
8. He, FJ, Nowson, CA, and GA, MG. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. (2006) 367:320–6. doi: 10.1016/S0140-6736(06)68069-0
9. Colarusso, L, Serafini, M, Lagerros, YT, Nyren, O, la Vecchia, C, Rossi, M, et al. Dietary antioxidant capacity and risk for stroke in a prospective cohort study of Swedish men and women. Nutrition. (2017) 33:234–9. doi: 10.1016/j.nut.2016.07.009
10. Shirley, R, Ord, EN, and Work, LM. Oxidative stress and the use of antioxidants in stroke. Antioxidants. (2014) 3:472–501. doi: 10.3390/antiox3030472
11. Uesugi, S, Ishihara, J, Iso, H, Sawada, N, Takachi, R, Inoue, M, et al. Dietary intake of antioxidant vitamins and risk of stroke: the Japan public health center-based prospective study. Eur J Clin Nutr. (2017) 71:1179–85. doi: 10.1038/ejcn.2017.71
12. Daviglus, ML, Orencia, AJ, Dyer, AR, Liu, K, Morris, DK, Persky, V, et al. Dietary vitamin C, beta-carotene and 30-year risk of stroke: results from the Western electric study. Neuroepidemiology. (2004) 16:69–77. doi: 10.1159/000109673
13. Loh, HC, Lim, R, Lee, KW, Ooi, CY, Chuan, DR, Looi, I, et al. Effects of vitamin E on stroke: a systematic review with meta-analysis and trial sequential analysis. Stroke Vasc Neurol. (2021) 6:109–20. doi: 10.1136/svn-2020-000519
14. Chen, GC, Lu, DB, Pang, Z, and Liu, QF. Vitamin C intake, circulating vitamin C and risk of stroke: a meta-analysis of prospective studies. J Am Heart Assoc. (2013) 2:e000329. doi: 10.1161/JAHA.113.000329
15. Wang, S, and Zhu, F. Dietary antioxidant synergy in chemical and biological systems. Crit Rev Food Sci Nutr. (2017) 57:2343–57. doi: 10.1080/10408398.2015.1046546
16. Zujko, ME, Waśkiewicz, A, Witkowska, AM, Cicha-Mikołajczyk, A, Zujko, K, and Drygas, W. Dietary total antioxidant capacity-a new indicator of healthy diet quality in cardiovascular diseases: a polish cross-sectional study. Nutrients. (2022) 14:3219. doi: 10.3390/nu14153219
17. Huang, D, Zhang, Y, Wang, X, Guo, R, Leng, X, du, Q, et al. Dietary total antioxidant capacity and the risk of developing asthenozoospermia: a hospital-based case-control study in China. Hum Reprod. (2023) 38:537–48. doi: 10.1093/humrep/dead010
18. La Vecchia, C, Decarli, A, Serafini, M, Parpinel, M, Bellocco, R, Galeone, C, et al. Dietary total antioxidant capacity and colorectal cancer: a large case-control study in Italy. Int J Cancer. (2013) 133:1447–51. doi: 10.1002/ijc.28133
19. Henríquez-Sánchez, P, Sánchez-Villegas, A, Ruano-Rodríguez, C, Gea, A, Lamuela-Raventós, RM, Estruch, R, et al. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur J Nutr. (2016) 55:227–36. doi: 10.1007/s00394-015-0840-2
20. Habibzadeh, A, Rahimlou, M, Ravankhah, M, Vahid, F, and Tabrizi, R. Association between dietary total antioxidant capacity and the risk of stroke: a nested case-control study. BMC Nutr. (2024) 10:56. doi: 10.1186/s40795-024-00867-5
21. Milajerdi, A, Shakeri, F, Keshteli, AH, Mousavi, SM, Benisi-Kohansal, S, Saadatnia, M, et al. Dietary total antioxidant capacity in relation to stroke among Iranian adults. Nutr Neurosci. (2020) 23:465–70. doi: 10.1080/1028415X.2018.1520478
22. Yang, C, Jia, X, Wang, Y, Fan, J, Zhao, C, Yang, Y, et al. Association between dietary Total antioxidant capacity of antioxidant vitamins and the risk of stroke among US adults. Antioxidants. (2022) 11:2252. doi: 10.3390/antiox11112252
23. Rautiainen, S, Larsson, S, Virtamo, J, and Wolk, A. Total antioxidant capacity of diet and risk of stroke: a population-based prospective cohort of women. Stroke. (2012) 43:335–40. doi: 10.1161/STROKEAHA.111.635557
24. Devore, EE, Feskens, E, Ikram, MA, den Heijer, T, Vernooij, M, van der Lijn, F, et al. Total antioxidant capacity of the diet and major neurologic outcomes in older adults. Neurology. (2013) 80:904–10. doi: 10.1212/WNL.0b013e3182840c84
25. Del Rio, D, Agnoli, C, Pellegrini, N, Krogh, V, Brighenti, F, Mazzeo, T, et al. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J Nutr. (2011) 141:118–23. doi: 10.3945/jn.110.125120
26. Hantikainen, E, Löf, M, Grotta, A, Trolle Lagerros, Y, Serafini, M, Bellocco, R, et al. Dietary non-enzymatic antioxidant capacity and risk of stroke: the Swedish Women's lifestyle and health cohort. Nutrition. (2020) 73:110723. doi: 10.1016/j.nut.2020.110723
27. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
28. Stang, A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
29. Shu, L, Huang, Y, Si, C, Zhu, Q, Zheng, P, and Zhang, X. Association between ultra-processed food intake and risk of colorectal cancer: a systematic review and meta-analysis. Front Nutr. (2023) 10:1170992. doi: 10.3389/fnut.2023.1170992
30. Farhangi, MA, and Mohammad-Rezaei, A. Higher dietary total antioxidant capacity (TAC) reduces the risk of cardio-metabolic risk factors among adults: an updated systematic review and meta-analysis. Int J Vitam Nutr Res. (2023) 93:178–92. doi: 10.1024/0300-9831/a000708
31. Symons, MJ, and Moore, DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. (2002) 55:893–9. doi: 10.1016/s0895-4356(02)00443-2
32. Grant, RL . Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. (2014) 348:f7450. doi: 10.1136/bmj.f7450
33. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
34. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.7786990. doi: 10.2307/2533446
35. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
36. Greenland, S, and Longnecker, MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
38. Sasanfar, B, Toorang, F, Maleki, F, Esmaillzadeh, A, and Zendehdel, K. Association between dietary total antioxidant capacity and breast cancer: a case-control study in a middle eastern country. Public Health Nutr. (2021) 24:965–72. doi: 10.1017/S1368980019004397
39. Zheng, PF, Shu, L, Zhang, XY, Si, CJ, Yu, XL, Gao, W, et al. Association between dietary patterns and the risk of hypertension among Chinese: a cross-sectional study. Nutrients. (2016) 8:239. doi: 10.3390/nu8040239
40. Jelinek, M, Jurajda, M, and Duris, K. Oxidative stress in the brain: basic concepts and treatment strategies in stroke. Antioxidants. (2021) 10:1886. doi: 10.3390/antiox10121886
41. Slemmer, JE, Shacka, JJ, Sweeney, MI, and Weber, JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. (2008) 15:404–14. doi: 10.2174/092986708783497337
42. Franzini, L, Ardigò, D, Valtueña, S, Pellegrini, N, del Rio, D, Bianchi, MA, et al. Food selection based on high total antioxidant capacity improves endothelial function in a low cardiovascular risk population. Nutr Metab Cardiovasc Dis. (2012) 22:50–7. doi: 10.1016/j.numecd.2010.04.001
43. Grassi, D, Desideri, G, Croce, G, Tiberti, S, Aggio, A, and Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des. (2009) 15:1072–84. doi: 10.2174/138161209787846982
44. Brighenti, F, Valtueña, S, Pellegrini, N, Ardigò, D, del Rio, D, Salvatore, S, et al. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. (2005) 93:619–25. doi: 10.1079/bjn20051400
Keywords: dietary total antioxidant capacity, stroke, systematic review, dose–response meta-analysis, observational studies
Citation: Huang Y, Ni Y, Yu L, Shu L, Zhu Q and He X (2024) Dietary total antioxidant capacity and risk of stroke: a systematic review and dose–response meta-analysis of observational studies. Front. Nutr. 11:1451386. doi: 10.3389/fnut.2024.1451386
Received: 19 June 2024; Accepted: 10 September 2024;
Published: 19 September 2024.
Edited by:
Nafisa M. Jadavji, University of Arizona, United StatesReviewed by:
Mehran Rahimlou, Zanjan University of Medical Sciences, IranCopyright © 2024 Huang, Ni, Yu, Shu, Zhu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingzhen He, MTgyNTcxNTgyMjdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.