- 1Department of Agricultural, Forest and Food Science (DISAFA), University of Torino, Grugliasco, Torino, Italy
- 2Transplant Research Center, Clinical Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran
- 3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 4Department of Immunology, School of Medicine, Jiroft University of Medical Sciences, Jiroft, Iran
- 5Department of Food Science and Technology, Sarvestan Branch, Islamic Azad University, Sarvestan, Iran
- 6Department of Biochemistry and Diet Therapy, School of Nutrition and Food Science, Tabriz University of Medical Sciences, Tabriz, Iran
Background and aim: The regulation of lipid metabolism is crucial for preventing cardiovascular diseases, which are among the leading causes of mortality worldwide. β-hydroxy-β-methylbutyrate (HMB) has garnered attention for its potential role in modulating lipid profiles. However, the magnitude of these effects are unclear due to the heterogeneity of the studies. This study aimed to provide a comprehensive overview of the randomized controlled trials (RCTs) that have examined the effects of HMB on lipid profiles in adults.
Methods: Databases including PubMed, Web of Science, and Scopus, were searched for relevant studies through January 2024. The study protocol was also registered at Prospero (no. CRD42024528549). Based on a random-effects model, we calculated WMDs and 95% confidence intervals (CIs). The outcomes assessed included total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Sensitivity, subgroup and meta-regression analyses were also conducted.
Results: Our analysis included a total of 10 RCTs comprising 421 participants. The pooled data revealed no significant effect of HMB supplementation on TC (WMD: −2.26 mg/dL; 95%CI: −6.11 to 1.58; p = 0.25), TG (WMD: −2.83 mg/dL 95% CI: −12.93 to 7.27; p = 0.58), LDL-C (WMD: 0.13 mg/dL; 95%CI: −3.02 to 3.28; mg; p = 0.94), and HDL-C (WMD: −0.78 mg/dL; 95%CI: −2.04 to 0.48; p = 0.22). The quality of evidence was rated as moderate to low for all outcomes.
Conclusion: The current evidence from RCTs suggests that HMB supplementation does not significantly alter lipid profiles, including TC, TG, LDL-C, and HDL-C. Further research is warranted to confirm these results and explore the potential mechanisms of action of HMB.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=528549, CRD42024528549.
1 Introduction
A metabolite of branched-chain amino acid (BCAA), β-hydroxy-β-methyl butyrate (HMB) is one of the sports supplements widely utilized by athletes for its anticatabolic effects during stressful situations (1). HMB is known to impact various aspects of muscle strength, muscle mass, muscle damage, and exercise recovery (2). Additionally, HMB has a key role in muscle quality by reducing the breakdown of muscle proteins (up to 57%), enhancing muscle protein synthesis (up to 70%), and increasing the stability of muscle membranes (3, 4). Furthermore, this supplement is associated with fat loss and body composition, which has made it one of the most popular supplements (5). As a result of these effects, HMB supplementation could potentially influence the presence and progression of metabolic syndrome, diabetes mellitus (DM), and cardiovascular disease (CVD), suggesting its significance in the broader context of metabolic health and chronic disease prevention (6, 7). In the process of cell growth and membrane repair, 3-hydroxy-3-methylglutarylcoA (HMG-CoA) is metabolized by HMB as the rate-limiting component of cholesterol synthesis (8). Consequently, HMB may contribute to cholesterol synthesis and lipid profiles (9). Assessing the lipid profile and identifying dyslipidemia characterized by lower levels of high-density lipoprotein (HDL), higher levels of low-density lipoprotein (LDL), total cholesterol (TC), and total triglyceride (TG) of serum is very necessary (10). Maintaining optimal blood lipid levels can help prevent chronic kidney disease (CKD), CVD, and DM (10, 11).
Several studies have demonstrated that supplementation with HMB may decrease LDL levels and subcutaneous fat deposition by reducing cholesterol levels (12, 13). It was shown by Nisen et al. that HMB supplementation significantly reduced serum TC (5/8%) and LDL (7/3%). The main point is that decreasing LDL levels among individuals with high cholesterol levels by HMB supplementation is more effective than the other methods (14). In addition, fat utilization, fatty acid oxidation, and the generation of fatty acids in human skeletal muscle cells and adipocytes are facilitated by HMB supplementation, leading to a decrease in TG levels of serum (15, 16). Nevertheless, despite prior studies, a number of studies dispute the efficacy of HMB in improving lipid profiles (17–19). Moreover, further research is needed to explore new methods and supplements designed to influence blood lipid levels (20, 21).
Overall, not only given the limited and conflicting data regarding the impact of HMB on lipid profiles, but also meta-analysis and systematic review studies about this subject are not available. Hence, this study was conducted to investigate the potential effects of HMB supplementation on the lipid profile of adults.
2 Methods
This meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) guidelines (22) and was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42024528549).
2.1 Search strategy
A comprehensive literature search of the PubMed, Scopus, and Web of Science online databases was conducted until January 12, 2024, to identify articles evaluating the effect of HMB supplements on blood lipid parameters. To ensure coverage of the latest publications, email alerts were set to notify us of any new articles listed in these databases. The detailed search strategy is presented in Supplementary Table S1. Two researchers (B. S. and H. J.) separately hand-searched the reference lists of the related review papers, meta-analyses, and randomized clinical trials (RCTs) along with Google Scholar (using search phrases) to find additional RCTs.
2.2 Study selection
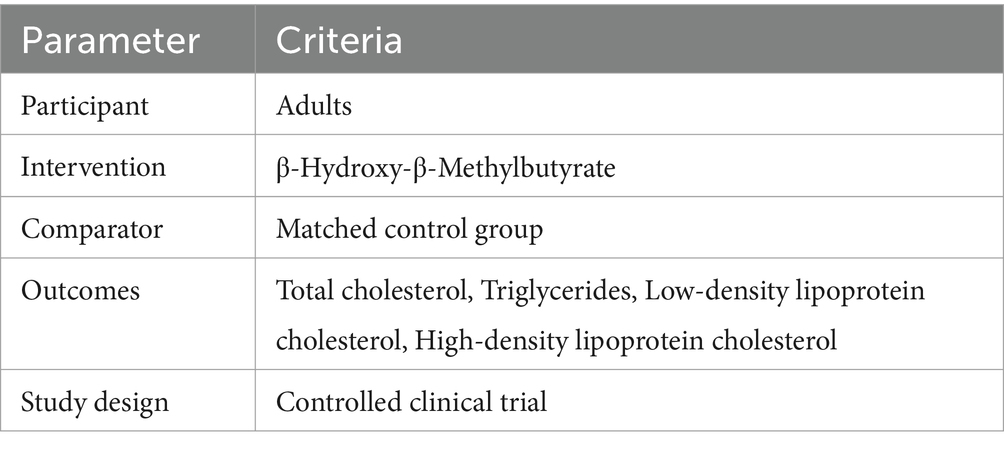
Two authors (B.S and H.J) separately screened the titles and abstracts of all the identified papers in order to determine their eligibility based on our inclusion criteria. Discrepancies were resolved by discussion with MV.B. To ensure a comprehensive and reproducible search strategy, all identified articles were managed using EndNote, a reference management software. This tool was instrumental in organizing the identified articles, removing duplicates, and facilitating the screening process. Furturemore, attempts were made to locate the full text of all related articles through open access databases and university library subscriptions. When these resources were unavailable, the corresponding authors were contacted directly to request a copy for analysis. The following criteria for inclusion were used to select studies for analysis based on the Population, Intervention, Comparison, Outcome (PICO) presented in Table 1: (1) RCTs with a crossover or parallel design; (2) studies involving adults (aged ≥18 years); (3) intervention durations with HMB supplements of at least 2 weeks; (4) included suitable intervention and control groups; (5) had sufficient information on lipid indices with mean or median values (standard errors (SEs), standard deviations (SDs), or 95% confidence intervals (Cls)) for the intervention and control groups at baseline and at the end of the intervention; and (6) full text of the article available in the English language.
We excluded studies based on the following criteria: (1) it was not possible to calculate a net benefit of HMB supplementation; (2) the duration of supplementation was less than 2 weeks; (3) the participants were not adults (18 > years of age); (4) studies with animal, case–control, cross-sectional, cohort designs, or review papers; and (5) the data on the baseline and follow-up lipid profile values were inadequate.
2.3 Data extraction
Two authors (B.S and H.J) separately selected eligible articles based on our inclusion and exclusion criteria. The extracted data were double-checked by (MV.B, H.J, and B.S) and agreed upon by all authors.
An extract of the data was made, including: author names, study location, year of publication, participant characteristics (sex, health status, age, and baseline BMI), intervention and control group sample sizes, dosage and type of supplementation, and co-interventions. A comparison of the baseline and final data for blood lipid indices was made. To ensure consistency, lipid index measurements originally reported in units other than mg/dL were converted and subsequently included in the analysis. In cases where results were reported at multiple measurements, only the measurements at baseline and at the end of the intervention duration were considered. If a study used different doses of HMB, each dose was analyzed separately.
2.4 Assessment of quality and certainty
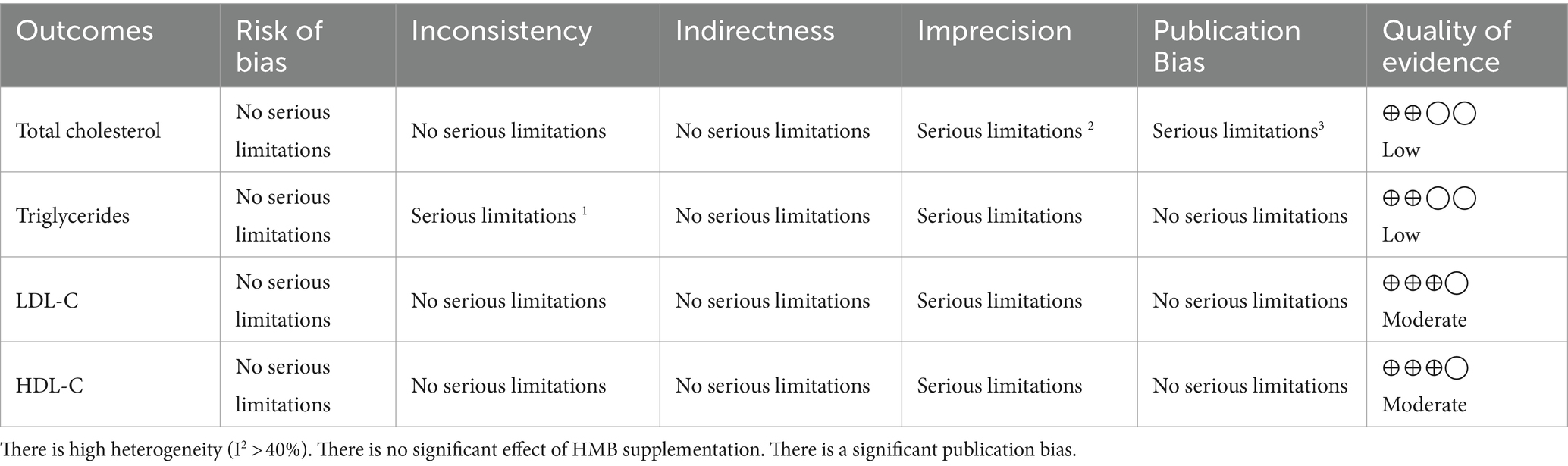
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias 2 (ROB2) tool (23). The following assessment domains were considered when assessing bias in the included studies: (1) adequacy of sequence generation (selection bias), (2) allocation sequence concealment (selection bias), (3) blinding (performance bias), (4) blinding of outcome assessment (detection bias), (5) addressing of dropouts (attrition bias), (6) reporting selective outcomes (reporting bias), and (7) other potential sources of bias. Two authors (B. S. and H. J.) assessed the studies and assigned “Low,” “High,” or “Unclear” for each domain. In the event of any disagreements, a third author (MV. B) was consulted to reach an agreement. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) protocol (24) developed by the GRADE Working Group1 was utilized to assess the certainty of evidence. GRADE introduces a systematic and consistent framework for assessing the quality and validity of evidence derived from trials (25). This assessment resulted in the categorization of certainty into four levels: High, Moderate, Low, or Very Low. The GRADE approach evaluates the certainty of evidence based on five key criteria: (1) risk of bias, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias.
2.5 Statistical analysis
We analyzed the effects of HMB supplementation on the following outcomes: (1) total cholesterol (mg/dL), (2) HDL-C (mg/dL), (3) LDL-C (mg/dL), and (4) triglycerides (mg/dL). Weighted mean differences (WMDs) and 95% Cls were used to express effect sizes. The mean and SD values of these blood lipid indices at baseline and at the end of the intervention were extracted from the papers. If the mean difference was not reported, we used the following formulas to calculate it: (value at the endpoint of the intervention group - value at the baseline of the intervention group) and (value at the endpoint of the control group - value at the baseline of the control group). In the event that SD of mean difference was not reported, we used the following formula to calculate it: in which R (correlation coefficient) of 0.5 was assumed (26). In studies where SE was reported instead of SD, we used the following formula to calculate it: SD = SE × square root (n), where n is the number of subjects. Medians were converted into means, and studies that reported 95% CIs, interquartile ranges (IQRs), and SEs were converted to SDs, all using the methods developed by Hozo et al. (27). Cochran’s Q-test and the test were conducted to calculate between-study heterogeneity, with values of p < 0.1 and an value (≥ 50%) indicating significant heterogeneity. Based on heterogeneity, the results were presented either using the random effects model or using the fixed effects model. For the purpose of analyzing the effect size of each trial, we used a leave-one-out sensitivity analysis, in which one trial was excluded from each analysis. In addition, a subgroup analysis was conducted to evaluate the influence of factors such as different types of HMB supplements, dosage, supplementation duration, baseline blood lipid concentrations, BMI, and health status on study outcomes. Funnel plots, Egger’s weighted regression, and Begg’s rank correlation tests were utilized to identify potential publication bias (28). Statistical analysis was conducted using STATA for Windows version 16.0 (Stata Corporation, College Station, TX, United States). The level of significance was determined as p < 0.05.
3 Results
3.1 Study selection
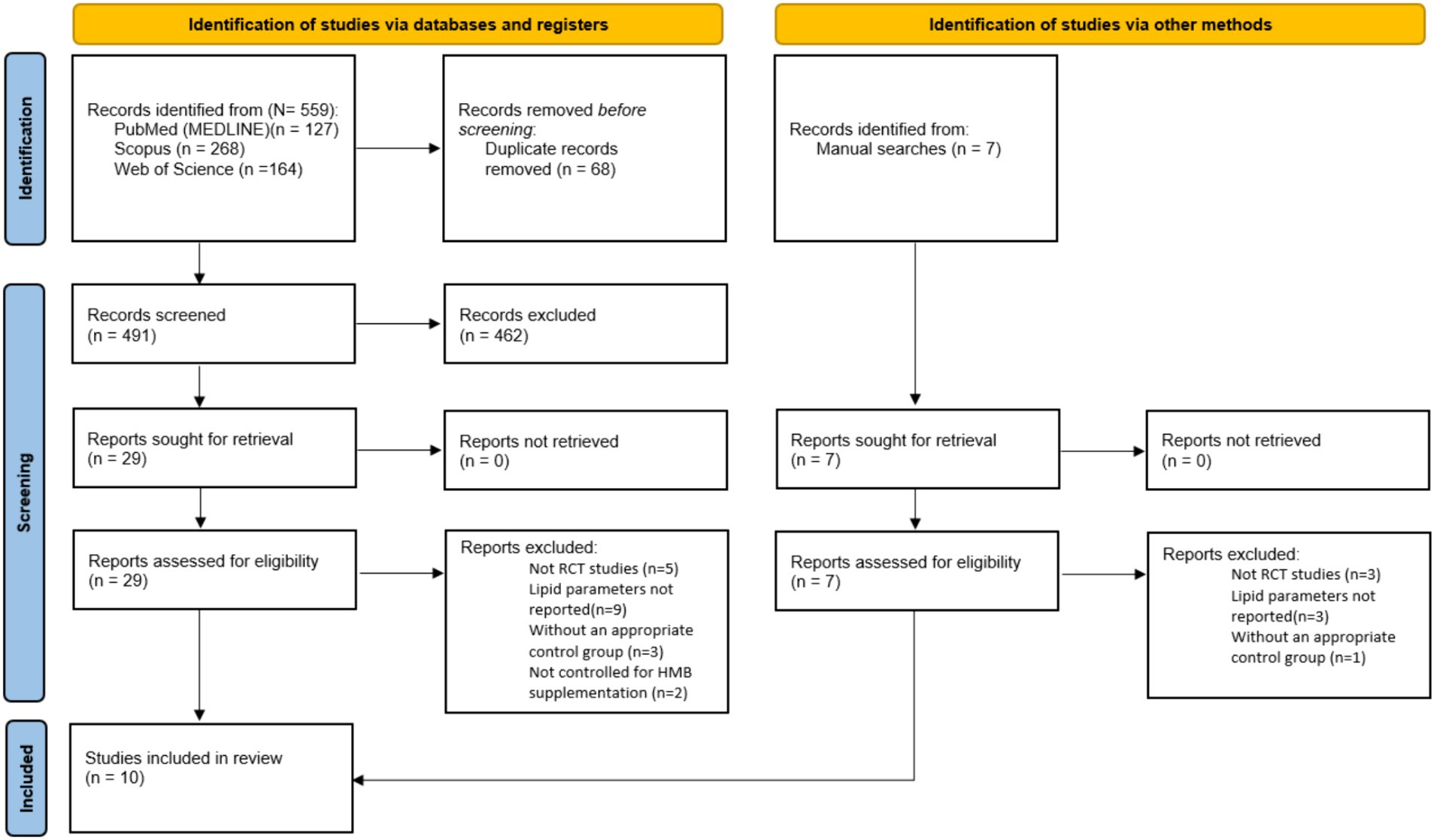
In Figure 1, it can be seen that the search protocol initially yielded a total of 559 studies. Out of these, 68 duplicates were identified and subsequently removed. Following this, an evaluation of the titles and abstracts based on the inclusion criteria resulted in the exclusion of 462 studies that were deemed irrelevant to the subject. A thorough assessment of the full texts of 29 studies led to the removal of 19 studies due to insufficient data reporting. Ultimately, 10 studies with 13 treatment arms were included in this meta-analysis.
3.2 Study characteristics
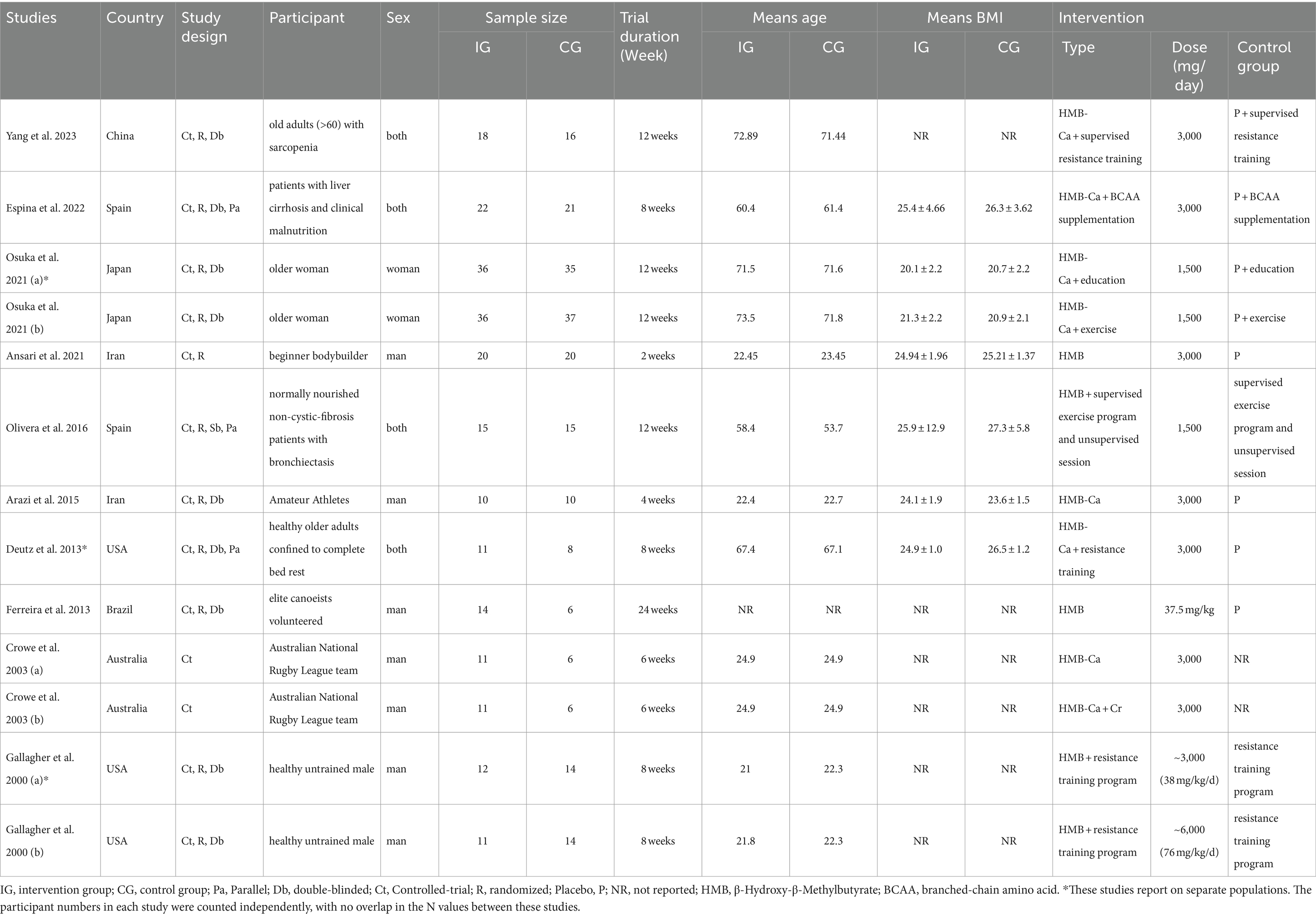
Table 2 provides information on the inclusion of 10 studies in this meta-analysis. All qualified articles included in this analysis were published between 2000 and 2023. The qualified studies were conducted in several countries, including Japan (29), China (30), Iran (31, 32), Brazil (33), Spain (34, 35), Australia (36), and the United States (37, 38). Five studies were conducted on men (31–33, 36, 38), one study was executed on women (29), and the others were conducted on both (30, 34, 35, 37). The intervention periods in the included trials ranged from 2 weeks (32) to 24 weeks (33). The supplement type was HMB in four studies (32–34, 38) and HMB-Ca in the other studies (29–31, 35–37). The dosage of HMB supplements in the included studies ranged from 1500 (29) to ~6000 (38) mg/day. The participants in these trials represented various populations, including healthy individuals (37, 38), patients with liver cirrhosis and clinical malnutrition (35), normally nourished non-cystic-fibrosis patients with bronchiectasis (34), Australian National Rugby League Team (36), elite canoeists volunteered (33), amateur athletes (31), beginner bodybuilders (32), older women with low muscle mass (29), and older adults (>60) with sarcopenia (30).
3.3 Quality assessment
In terms of the general risk of bias in the qualified articles, it was found that eight studies had a low risk of bias (29–33, 35, 37, 38), one study had a moderate risk of bias (34), and one article mentioned a high risk of bias (36). For transparency, traffic light graphs depicting the risk of bias for each outcome, as evaluated using the ROB2 tool, have been added to the Supplementary Figure S4. These graphs offer a clear visual representation of bias across the included studies, highlighting the different levels of risk for each domain.
3.4 Meta-analysis
3.4.1 Effect of HMB supplementation on TC
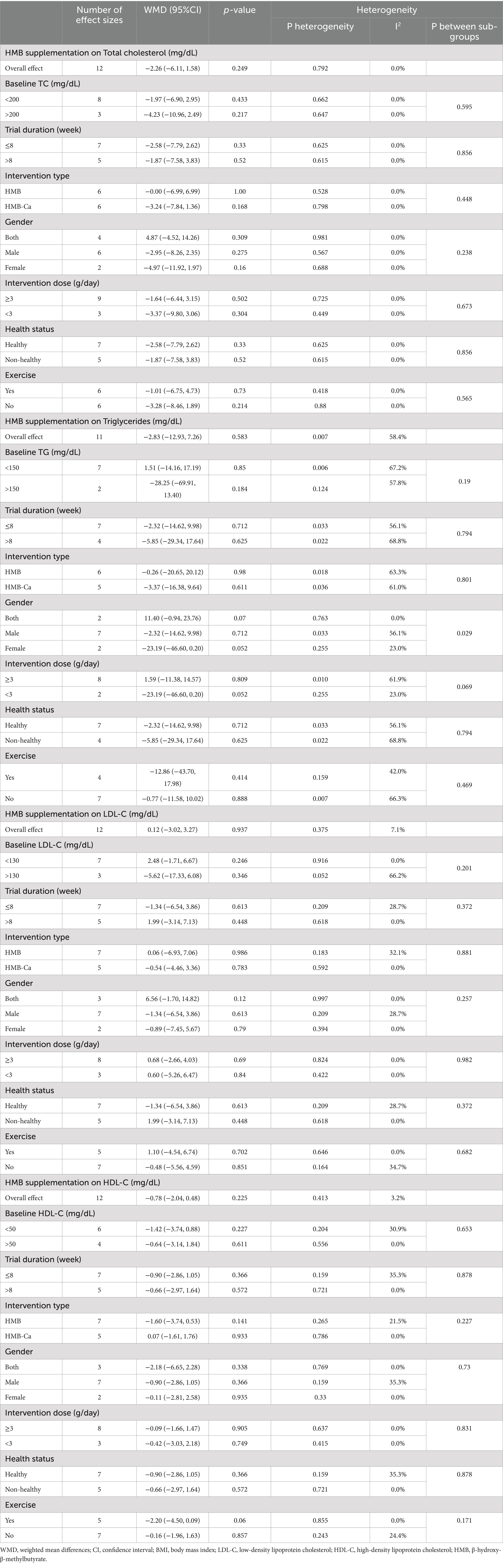
Pooled data from 12 effect sizes revealed no significant impact of HMB supplementation on TC levels compared to those in the control groups (WMD: −2.26 mg/dL; 95%CI: −6.11 to 1.58; p = 0.25; Figure 2A). Additionally, there was no significant heterogeneity among the included trials (I2 = 0.0%, p = 0.79). Subgroup analysis of HMB supplementation on TC is shown in Table 3.
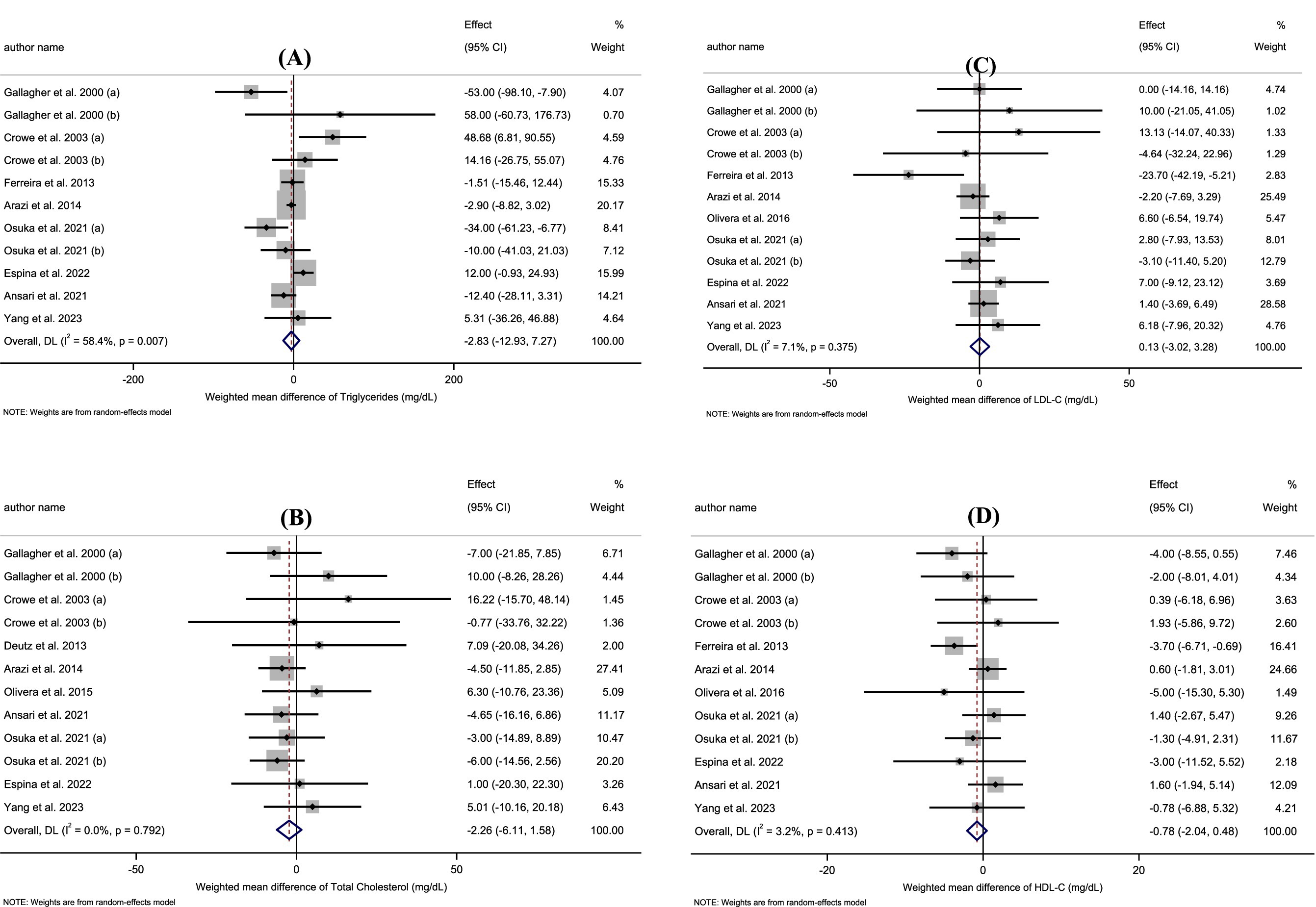
Figure 2. (A–D) Forest plot for the effect of HMB supplementation on A: Triglycerides, B: Total Cholesterol, C: LDL-C, D: HDL-C in adults, expressed as mean differences between intervention and control groups.
3.4.2 Effect of HMB supplementation on TG
According to the 11 effect sizes compared to control groups, HMB supplementation did not significantly reduce TG levels (WMD: −2.83 mg/dL 95% CI: −12.93 to 7.27; p = 0.58; Figure 2B). Additionally, a high degree of heterogeneity was detected between the included trials (I2 = 58.4%, p = 0.01). Subgroup analysis of HMB supplementation on TG is presented in Table 3.
3.4.3 Effect of HMB supplementation on LDL-C
The overall results from evaluating 12 effect sizes indicated that HMB supplementation had no significant effect on LDL-C levels compared to control groups (WMD: 0.13 mg/dL; 95%CI: −3.02 to 3.28; mg; p = 0.94; Figure 2C). Moreover, no significant heterogeneity was observed among the included studies (I2 = 7.1%, p = 0.37). Subgroup analysis of HMB supplementation on LDL-C is shown in Table 3.
3.4.4 Effect of HMB supplementation on HDL-C
After evaluating 12 effect sizes, it was found that HMB supplementation did not change HDL-C levels significantly (WMD: −0.78 mg/dL; 95%CI: −2.04 to 0.48; p = 0.22; Figure 2D). In addition, there was no significant heterogeneity among the included trials (I2 = 3.2%, p = 0.41). Subgroup analysis of HMB supplementation on HDL-C are presented in Table 3.
3.5 Sensitivity analysis and publication bias
The impact of each study on the overall effect size was determined by excluding each study from the analysis, respectively. The overall effect size was not significantly altered when each article was omitted. Upon examination of the funnel plots and conducting Egger’s test, it was observed that there is a notable publication bias in studies evaluating the effect of HMB supplementation on TC levels (p = 0.01). However, among the studies examining other outcomes, no significant publication bias was detected (Supplementary Figure S3).
3.6 Certainty assessment
The GRADE analysis demonstrated that the quality of evidence about the effect of HMB supplementation on LDL-C and HDL-C levels was identified as moderate due to the presence of serious limitations in imprecision. Furthermore, the quality of evidence for TG and TC levels was downgraded to low due to the presence of serious limitations in inconsistency, imprecision, and publication bias (Table 4).
4 Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the effects of HMB supplementation on lipid profiles in adults. The results of our meta-analysis, conducted on 10 RCTs with a total of 421 (intervention group: 227, control group: 194) adult participants, revealed no beneficial effects of HMB supplementation on TC (WMD: −2.26 mg/dL; 95%CI: −6.11 to 1.58; p = 0.25), TG (WMD: −2.83 mg/dL 95% CI: −12.93 to 7.26; p = 0.58), LDL-C (WMD: 0.12 mg/dL; 95%CI: −3.02 to 3.27; mg; p = 0.94) and HDL-C (WMD: −0.78 mg/dL; 95%CI: −2.04 to 0.48; p = 0.22) in adults. Moreover, subgroup analysis revealed that HMB and HMB-Ca supplements had no effects on the lipid profile in all subjects.
In recent years, HMB has been used as one of the nutritional supplements used by athletes to adjust homeostasis and increase lipolysis and fat-free mass. Several studies have shown that supplementation with HMB alters cholesterol synthesis in the liver by converting it to HMG-CoA (9, 38–40). Different studies have been performed on the effects of HBM on weight loss, lipid profile, and muscle strength.
In a recent study, Ansari et al. (2021) investigated the effect of HMB supplementation on lipid profile and some indicators of physical fitness in beginner bodybuilders. According to their findings, compared with the placebo group, the HBM group demonstrated a significant reduction in triglyceride levels, but for other factors such as LDL, HDL, LDL/HDL, and cholesterol, there was no significant difference. An additional study conducted in 2001 by Coelho and Carvalho showed that supplementation with HMB significantly reduced LDL levels in hypercholesterolemic patients (12).
An analysis of nine studies published in 2000 by Nissen et al. examined data from subjects who received 3 grams of HMB per day for 3 to 8 weeks (including men and women, young and older, and athletes and non-athletes). According to the results, supplementation with HMB decreased TC and LDL cholesterol levels. However, HMB has no significant effect on LDL levels in people with normal cholesterol levels (14). This may be interpreted as HMB lowering LDL levels in the presence of high cholesterol levels. Conversely, some studies have found that HMB supplementation does not significantly affect blood cholesterol levels (41).
Arazi et al. studied HMB supplementation and cardiovascular risk factors (hematological parameters, blood pressure, and blood lipid levels) in 2015. Although their results showed the beneficial changes in blood lipids in both groups, no significant differences were found between the two groups concerning blood lipids (31). The results of another study conducted by Gallagher et al. in 2000 indicated that the use of varying doses of HMB (0, 3, or 6 g) did not result in significant changes to the lipid profile levels following 8 weeks of resistance training in untrained men (38). These findings were in line with our findings of the present study, as HMB supplementation had no significant effect on blood lipids. Differences in results between different studies may be due to differences in the type of experimental design, quantity and intensity of exercise, health status of the participants, duration of the experiment, supplemental formulas, methods of evaluating variables and statistical analysis methods.
The background diet of subjects indeed plays a crucial role in the overall effects of HMB supplementation, given that HMB is a metabolite of leucine. It is important to note that most of the included studies managed to control for dietary intake to some extent, often using standardized meal plans during the intervention period. For example, the study by Yang et al. and Espina et al. monitored and controlled participants’ diets to mitigate the confounding effects of dietary variations (30, 35). It was necessary to apply this control in order to reduce the confounding effects of dietary variations, particularly protein and leucine intake, which can have an impact on endogenous HMB concentrations (13). However, it is acknowledged that not all studies could perfectly control for dietary habits, and this represents a limitation of the review. Further, it is suggested that future studies should include more rigorous dietary controls more precisely to account for such variables (40).
One proposed mechanism of action is that HMB may inhibit the activity of enzymes involved in lipolysis, such as hormone-sensitive lipase and adipose tissue triglyceride lipase. This inhibition ultimately leads to reduced breakdown of triglycerides into free fatty acids, thereby reducing the release of triglycerides into the circulation (16). Additionally, HMB may promote fat oxidation by increasing the expression of genes involved in fatty acid metabolism. By stimulating the oxidation of free fatty acids, HMB may contribute to the reduction in triglyceride levels (42, 43). Furthermore, it could indirectly affect lipid metabolism through other pathways. HMB supplementation is known to stimulate muscle protein synthesis. Increased muscle mass is often associated with a higher metabolic rate, potentially leading to favorable changes in lipid profiles (40). Additionally, HMB might influence inflammatory markers, which are linked to cardiovascular health and cholesterol regulation (44). Future research needs to explore these potential indirect effects in greater detail (45).
4.1 Safety and optimal supplementation dosage
The supplementation of HMB has been extensively studied, with evidence suggesting that an optimal dosage of 3 grams per day maximizes strength and lean body mass gains, while higher doses, such as 6 grams per day, do not confer additional benefits (38, 40). Although there is a theoretical basis for skeletal muscles being unable to absorb higher concentrations of HMB, thus explaining the lack of further gains at higher doses (38), the safety of HMB at various dosages has been well-documented. Studies have shown that even at doses up to 50 grams per day in rat models, normalized to human equivalent dosing, no adverse effects were observed, and human studies have corroborated these findings, reporting no negative impacts on cholesterol, blood glucose, liver, or kidney functions at dosages of up to 6 grams per day for extended periods (46). Consequently, HMB is considered a safe supplement within the studied dosage range, potentially enhancing health markers without adverse effects (38, 47).
4.2 Strengths and limitations
This systematic review and meta-analysis has several strengths which include an acceptable number of studies. In addition, the majority of the studies we included were considered to be of high quality according to the Cochrane risk of bias tool. As the studies were conducted in different regions across the world, our findings can be applied to adult populations globally; however, this also highlights the need for future research to explore the impact of ethnicity on HMB supplementation outcomes, since the baseline HMB concentration might differ among various ethnic groups. Furthermore, the search was not limited to a particular time. There are some limitations to this study that need to be acknowledged. One of the primary limitations is the potential heterogeneity among studies included in the systematic reviews and meta-analyses. Different study designs, populations, and intervention protocols may have influenced the observed results, making it difficult to draw definitive conclusions. Additionally, confounding factors, such as exercise and diet, can potentially influence lipid profile changes observed with HMB supplementation. These factors cannot be adequately controlled for all studies included in the systematic reviews and meta-analyses, making it difficult to attribute the observed effects solely to HMB. To ensure the quality and comparability of the included studies, we restricted our analysis to English-language articles. This language limitation may have resulted in the exclusion of relevant studies published in other languages, potentially limiting the generalizability of our findings. In addition, some of the included studies did not clearly report the details of their randomization or blinding of the outcome assessment process. Lastly, most outcomes were moderate to low, according to the GRADE.
5 Conclusion
In conclusion, this systematic review and meta-analysis suggested that HMB supplementation does not significantly alter lipid profiles, including TC, TG, LDL-C, and HDL-C levels. Further research involving larger, more rigorously conducted studies with long-term intervention periods is necessary in order to fully investigate the mechanisms of action and potential clinical significance of HMB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BS: Writing – original draft, Conceptualization, Investigation, Methodology, Writing – review & editing. HB: Writing – original draft, Formal analysis, Writing – review & editing. HJ: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. M-AH: Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. DH: Writing – review & editing, Methodology. MVB: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research protocol was approved and supported by the student research committee, Tabriz University of Medical Sciences (registration code: 75353).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1451282/full#supplementary-material
Footnotes
References
1. Wilson, JM, Fitschen, PJ, Campbell, B, Wilson, GJ, Zanchi, N, Taylor, L, et al. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB). J Int Soc Sports Nutr. (2013) 10:6. doi: 10.1186/1550-2783-10-6
2. Holland, BM, Roberts, BM, Krieger, JW, and Schoenfeld, BJ. Does HMB enhance body composition in athletes? A systematic review and Meta-analysis. J Strength Cond Res. (2022) 36:585–92. doi: 10.1519/jsc.0000000000003461
3. Cheng, W, Phillips, B, and Abumrad, N. Effect of HMB on fuel utilization, membrane stability and creatine kinase content of cultured muscle cells. FASEB J. (1998) 12:A950.
4. Silva, VR, Belozo, FL, Micheletti, TO, Conrado, M, Stout, JR, Pimentel, GD, et al. β-Hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: a systematic review. Nutr Res. (2017) 45:1–9. doi: 10.1016/j.nutres.2017.07.008
5. Holecek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. (2017) 8:529–41. doi: 10.1002/jcsm.12208
6. Wilkinson, DJ, Hossain, T, and Limb, MC. Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism. Clin Nutr. (2018) 37:2068–75. doi: 10.1016/j.clnu.2017.09.024
7. Kerksick, CM, Wilborn, CD, and Roberts, MD. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. (2018) 15:38. doi: 10.1186/s12970-018-0242-y
8. Walker, DK, Thaden, JJ, and Wierzchowska-McNew, A. Determination of β-hydroxy-β-methylbutyrate concentration and enrichment in human plasma using chemical ionization gas chromatography tandem mass spectrometry. J Chromatogr B. (2017) 1040:233–8. doi: 10.1016/j.jchromb.2016.11.010
9. Howatson, G, and van Someren, KA. The prevention and treatment of exercise-induced muscle damage. Sports Med. (2008) 38:483–503. doi: 10.2165/00007256-200838060-00004
10. Jasim, OH, Mahmood, MM, and Ad'hiah, AH. Significance of lipid profile parameters in predicting pre-diabetes. Arch Razi Inst. (2022) 77:277–84. doi: 10.22092/ari.2021.356465.1846
11. Kochan, Z, Szupryczynska, N, Malgorzewicz, S, and Karbowska, J. Dietary lipids and dyslipidemia in chronic kidney disease. Nutrients. (2021) 13:3138. doi: 10.3390/nu13093138
12. Coelho, CW, and Carvalho, T. Effects of hmb supplementation on ldl-cholesterol, strength and body composition of patients with hypercholesterolemia. Med Sci Sports Exerc. (2001) 33:S340. doi: 10.1097/00005768-200105001-01912
13. Nissen, SL, and Abumrad, NN. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J Nutr Biochem. (1997) 8:300–11. doi: 10.1016/S0955-2863(97)00048-X
14. Nissen, S, Sharp, RL, Panton, L, Vukovich, M, and Trappe, S. β-Hydroxy-β-Methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. (2000) 130:1937–45. doi: 10.1093/jn/130.8.1937
15. Standley, RA, Distefano, G, and Pereira, SL. Effects of β-hydroxy-β-methylbutyrate on skeletal muscle mitochondrial content and dynamics, and lipids after 10 days of bed rest in older adults. J Appl Physiol. (2017) 123:1092–100. doi: 10.1152/japplphysiol.00192.2017
16. Bruckbauer, A, Zemel, MB, and Thorpe, T. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutrit Metabolism. (2012) 9:1–13. doi: 10.1186/1743-7075-9-77
17. Hsieh, L-C, Chow, C-J, and Chang, W-C. Effect of Beta-hydroxy-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac J Clin Nutr. (2010) 19:200–8.
18. Tinsley, GM, Moore, ML, Graybeal, AJ, Paoli, A, Kim, Y, Gonzales, JU, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. (2019) 110:628–40. doi: 10.1093/ajcn/nqz126
19. O'connor, D, and Crowe, M. Effects of [beta]-hydroxy-[beta]-methylbutyrate and creatine monohydrate supplementation on the aerobic and anaerobic capacity of highly trained athletes. J Sports Med Phys Fitness. (2003) 43:64–8.
20. Derosa, G, Colletti, A, and Maffioli, P. Lipid-lowering nutraceuticals update on scientific evidence. J Cardiovasc Med. (2020) 21:845–59. doi: 10.2459/jcm.0000000000000970
21. Grant, JK, Dangl, M, Ndumele, CE, Michos, ED, and Martin, SS. A historical, evidence-based, and narrative review on commonly used dietary supplements in lipid-lowering. J Lipid Res. (2024) 65:100493. doi: 10.1016/j.jlr.2023.100493
22. Page, MJ, McKenzie, JE, and Bossuyt, PM. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 10:372. doi: 10.1136/bmj.n71
23. Higgins, JP, Altman, DG, and Gøtzsche, PC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
25. Group GW. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
26. Higgins, JP, and Green, S. Cochrane handbook for systematic reviews of interventions. Wiley (2008).
27. Hozo, SP, Djulbegovic, B, and Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:1–10. doi: 10.1186/1471-2288-5-13
28. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63.
29. Osuka, Y, Kojima, N, and Sasai, H. Effects of exercise and/or β-hydroxy-β-methylbutyrate supplementation on muscle mass, muscle strength, and physical performance in older women with low muscle mass: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2021) 114:1371–85. doi: 10.1093/ajcn/nqab176
30. Yang, C, Song, Y, Li, T, Chen, X, Zhou, J, Pan, Q, et al. Effects of Beta-Hydroxy-Beta-Methylbutyrate supplementation on older adults with sarcopenia: a randomized, double-blind, placebo-controlled study. J Nutrit, Health, Aging. (2023) 27:329–39. doi: 10.1007/s12603-023-1911-1
31. Arazi, H, Rohani, H, and Ghiasi, A. The effect of HMB supplementation on cardiovascular risk factors after four weeks of resistance training in amateur athletes. Int Cardiovasc Res J. (2017) 9:e11400
32. Ansari Kolachahi, S, Gholamrezaei, S, and Moradnia, M. The effect of resistance training with HMB supplementation on lipid profile and some physical fitness indicators of beginner bodybuilders. Medical Sci J Islamic Azad University. (2021) 31:164–72. doi: 10.52547/iau.31.2.164
33. Ferreira, H, Rodacki, ALF, and Gill, P. The effects of supplementation of β-hydroxy-β-methylbutyrate on inflammatory markers in high performance athletes. J Exercise Physiol. (2013) 16:53–63.
34. Olveira, G, Olveira, C, and Doña, E. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (prospective, randomised study). Clin Nutr. (2016) 35:1015–22. doi: 10.1016/j.clnu.2015.10.001
35. Espina, S, Sanz-Paris, A, and Gonzalez-Irazabal, Y. Randomized clinical trial: effects of β-Hydroxy-β-Methylbutyrate (HMB)-enriched vs. HMB-free Oral nutritional supplementation in malnourished cirrhotic patients. Nutrients. (2022) 14:2344. doi: 10.3390/nu14112344
36. Crowe, MJ, O'Connor, DM, and Lukins, JE. The effects of beta-hydroxy-beta-methylbutyrate (HMB) and HMB/creatine supplementation on indices of health in highly trained athletes. Int J Sport Nutr Exerc Metab. (2003) 13:184–97. doi: 10.1123/ijsnem.13.2.184
37. Deutz, NEP, Pereira, SL, and Hays, NP. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. (2013) 32:704–12. doi: 10.1016/j.clnu.2013.02.011
38. Gallagher, PM, Carrithers, JA, Godard, MP, Schulze, KE, and Trappe, SW. Beta-hydroxy-beta-methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Med Sci Sports Exerc. (2000) 32:2116–9. doi: 10.1097/00005768-200012000-00023
39. Beg, Z, and Lupien, P. In vitro and in vivo inhibition of hepatic cholesterol synthesis by 3-hydroxy-3-methylglutaric acid. Biochimica et Biophysica Acta (BBA)-lipids and lipid. Metabolism. (1972) 260:439–48.
40. Wilson, GJ, Wilson, JM, and Manninen, AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab. (2008) 5:1743–7075. doi: 10.1186/1743-7075-5-1
41. Leroux, M, Lemery, T, and Boulet, N. Effects of the amino acid derivatives, β-hydroxy-β-methylbutyrate, taurine, and N-methyltyramine, on triacylglycerol breakdown in fat cells. J Physiol Biochem. (2019) 75:263–73. doi: 10.1007/s13105-019-00677-5
42. Sun, X, and Zemel, MB. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids. (2007) 42:297–305. doi: 10.1007/s11745-007-3029-5
43. Sun, X, and Zemel, MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutrit Metabolism. (2009) 6:26–8. doi: 10.1186/1743-7075-6-26
44. Hsieh, L, Chien, S, Huang, M, Tseng, HF, and Chang, CK. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr. (2006) 15:544–50.
45. Molfino, A, Gioia, G, Rossi Fanelli, F, and Muscaritoli, M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids. (2013) 45:1273–92. doi: 10.1007/s00726-013-1592-z
46. Kaczka, P, Michalczyk, MM, Jastrząb, R, Gawelczyk, M, and Kubicka, K. Mechanism of action and the effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) supplementation on different types of physical performance - a systematic review. J Hum Kinet. (2019) 68:211–22. doi: 10.2478/hukin-2019-0070
Keywords: β-hydroxy-β-methylbutyrate, HMB, lipid profile, dyslipidemia, cardiovascular health, meta-analysis
Citation: Sadeghi B, Bahari H, Jozi H, Hasanzadeh M-A, Hashemi D and Bideshki MV (2024) Effects of β-hydroxy-β-methylbutyrate (HMB) supplementation on lipid profile in adults: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 11:1451282. doi: 10.3389/fnut.2024.1451282
Edited by:
Eric Gumpricht, Isagenix International, LLC, United StatesReviewed by:
Azita Hekmatdoost, National Nutrition and Food Technology Research Institute, IranSiti Wulan, University of Brawijaya, Indonesia
Edilene Maria Queiroz Araujo, Bahia State University, Brazil
Copyright © 2024 Sadeghi, Bahari, Jozi, Hasanzadeh, Hashemi and Bideshki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Vesal Bideshki, QmlkZXNoa2ltQHRiem1lZC5hYy5pcg==; Qm0udmFzYWxAZ21haWwuY29t
 Behrad Sadeghi
Behrad Sadeghi Hossein Bahari
Hossein Bahari Hannane Jozi
Hannane Jozi Mohammad-Ali Hasanzadeh4
Mohammad-Ali Hasanzadeh4 Mohammad Vesal Bideshki
Mohammad Vesal Bideshki