- 1Universal Scientific Education and Research Network (USERN), Tehran, Iran
- 2Student Research Committee, Fasa University of Medical Sciences, Fasa, Iran
- 3Research Center for Immunodeficiencies, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran
- 4Network of Immunity in Infection, Malignancy and Autoimmunity (NIIMA), Universal Scientific Education and Research Network (USERN), Tehran, Iran
While inflammation is a known beneficial mechanism, pro-inflammatory nutrients can lead to chronic inflammation. The energy-adjusted dietary inflammatory index (E-DII) has revealed positive associations with chronic inflammatory diseases. However, more evidence about the demographic risk factors for high E-DII is needed. Therefore, the present study reviewed the high-risk groups of people for high E-DII scores. Men had higher E-DII than women worldwide, which could be explained by the craving for energy induced by stress and higher physical activity. However, in some societies, women had higher consumption of a pro-inflammatory diet, which could be induced by compulsive eating and craving for more sweets and carbohydrates during menstruation and also can be seen among women with premenopausal syndrome. The pro-inflammatory diets were more common among elders in southern America, East Asia, and Arab countries, while some other studies had contradictory results. The proliferation of unhealthy foods, such as fast food and Western dietary patterns enriched with a pro-inflammatory diet, increased youth’s E-DII and decreased the healthy eating index among older people. Also, smokers and alcoholics tended to consume a diet with a higher E-DII, which should be investigated in further studies. Black people consumed the most pro-inflammatory diets compared with White people, especially in pregnant women. Education had a negative association with E-DII, while socioeconomic status was positively associated with a pro-inflammatory diet. Therefore, E-DII consumption had no association with access to healthy foods but is more associated with knowledge and cultural dietary habits. Moreover, further nutritional interventions are required to educate the vulnerable populations and also provide better availability of healthy food enriched with anti-inflammatory nutrients in the future.
1 Introduction
Inflammation is one of the most essential processes in the immune system, which responds to triggers such as injury and infection (1). While acute inflammation contributes to healing injuries and fighting pathogenic microbes, chronic inflammation causes tissue damage and other disorders, such as cancers, diabetes, autoimmune diseases, and cardiovascular diseases (2). The escape of T cells, especially CD8+, from tolerance is the primary mechanism of chronic inflammation initiation (3). Secretion of transforming growth factor-beta and the dysfunction of regulatory T cells trigger further pathways (4–6). Consequently, increased plasma levels of interferons and interleukin-1 (IL-1) and -6 (IL-6) leads to the development of chronic inflammation and result in chronic diseases (4).
Diet has a modest effect on inflammation through the regulation of plasma inflammatory factors, such as IL-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) (7). Recent studies suggested the important role of obesity in the association of diet with chronic inflammation and its related metabolic diseases (8). It has been confirmed that a high-caloric diet and consuming pro-inflammatory nutrients increase fat storage in adipose tissue and result in obesity. The hyperplasia and hypertrophy of adipocytes induced by a high-caloric and -fat diet would increase cellular stress, apoptosis, and oxidative stress (9). Production of reactive oxygen species (ROS) in adipose tissue, induced by a pro-inflammatory diet, increases the IL-6 and CRP (10). Excessive intake of energy and fat causes higher secretion of leptin and other adipokines, which increase pro-inflammatory biomarkers. Also, higher energy intake and consuming fat and carbohydrates directly stimulate adipose tissue to produce IL-6, TNF-α, and other pro-inflammatory biomarkers (11). Intake of carbohydrates increases insulin secretion and stimulates the expression of IL-6 from adipose tissue (12). Therefore, while consuming foods enriched with fruits, fibers, and vegetables had anti-inflammatory properties, higher energy, carbohydrate, and saturated fats increase chronic inflammation (13). Also, high fat intake causes insulin resistance and increases the plasma level of TNF-α (14). Healthy dietary patterns, especially the Mediterranean diet, indicate cardiovascular protective effects by decreasing inflammation and thrombosis (15). Moreover, dietary patterns are associated with the development of different diseases, such as cancer, cardiovascular disease, and obesity, and can influence the outcomes of immunotherapy by regulating gut microbiome composition (16–18).
Following an exhaustive review during the 2000s, a comprehensive index was developed to assess the inflammatory potential of diet patterns for the first time (19). This index, the Dietary Inflammatory Index (DII), was eventually published by Shivappa et al. (20). A higher DII score shows a more pro-inflammatory diet, while a more negative DII represents a more anti-inflammatory diet. Using DII in nutrition studies provides a comprehensive perspective on the inflammatory potential of diet (20). In a few years, DII was rapidly applied in multiple studies worldwide. However, the revision continued, and energy-adjusted DII, also named E-DII, was developed to adjust the proportion of energy intake in assessing the inflammatory potential of diet and reveal the effect of other dietary components compared with DII. E-DII is a more global assessment of dietary inflammatory potential, which fosters a sense of connection and allows us to compare the findings in different studies worldwide (21).
To date, several recent studies showed that E-DII had a tight, significant association with different diseases, such as cardiovascular diseases, metabolic syndrome, and diabetes (22, 23). However, to our knowledge, there is a need for more evidence regarding the risk factors of potent inflammatory diets. For instance, while the proportion of dietary carbohydrates increases with aging, energy intake is significantly decreased in older people (24). Furthermore, social determinants, such as low income, also affect people’s diets; lower-income people are likelier to consume unhealthy diets (25). Therefore, the present study aimed to review different determinants of E-DII, shedding light on the factors that contribute to the inflammatory potential of an individual’s diet and their implications for chronic disease prevention and management.
The present study reviewed the studies that compared the E-DII score among different groups of demographic features to investigate the groups that are possibly susceptible to consumption of pro-inflammatory diet vulnerable to further diseases. The high-quality observational papers that studied the E-DII were included in the present study to investigate the pro- and anti-inflammatory intake compared among different groups of demographic risk factors addressed in their findings.
2 Search strategy
Appropriate related keywords, including pro-inflammatory diet, anti-inflammatory diet, and dietary inflammatory index, energy-adjusted dietary inflammatory index, were searched in electronic databases of Google Scholar, Scopus, Web of Science, and PubMed. We extracted searched articles from inception until October 2023. Medical Subject Headings (MeSH) terms were included. Endnote v.20 was applied to remove duplicated studies and include the full text of relevant articles. Finally, the remaining 34 articles that addressed the comparison of E-DII between demographic risk factor groups were reviewed in our study.
3 Determinants of E-DII
Different risk factors, such as age, sex, marital status, race, education level, socio-economic status, and addiction, have been associated with E-DII (Table 1). Each risk factor has been comprehensively discussed below:
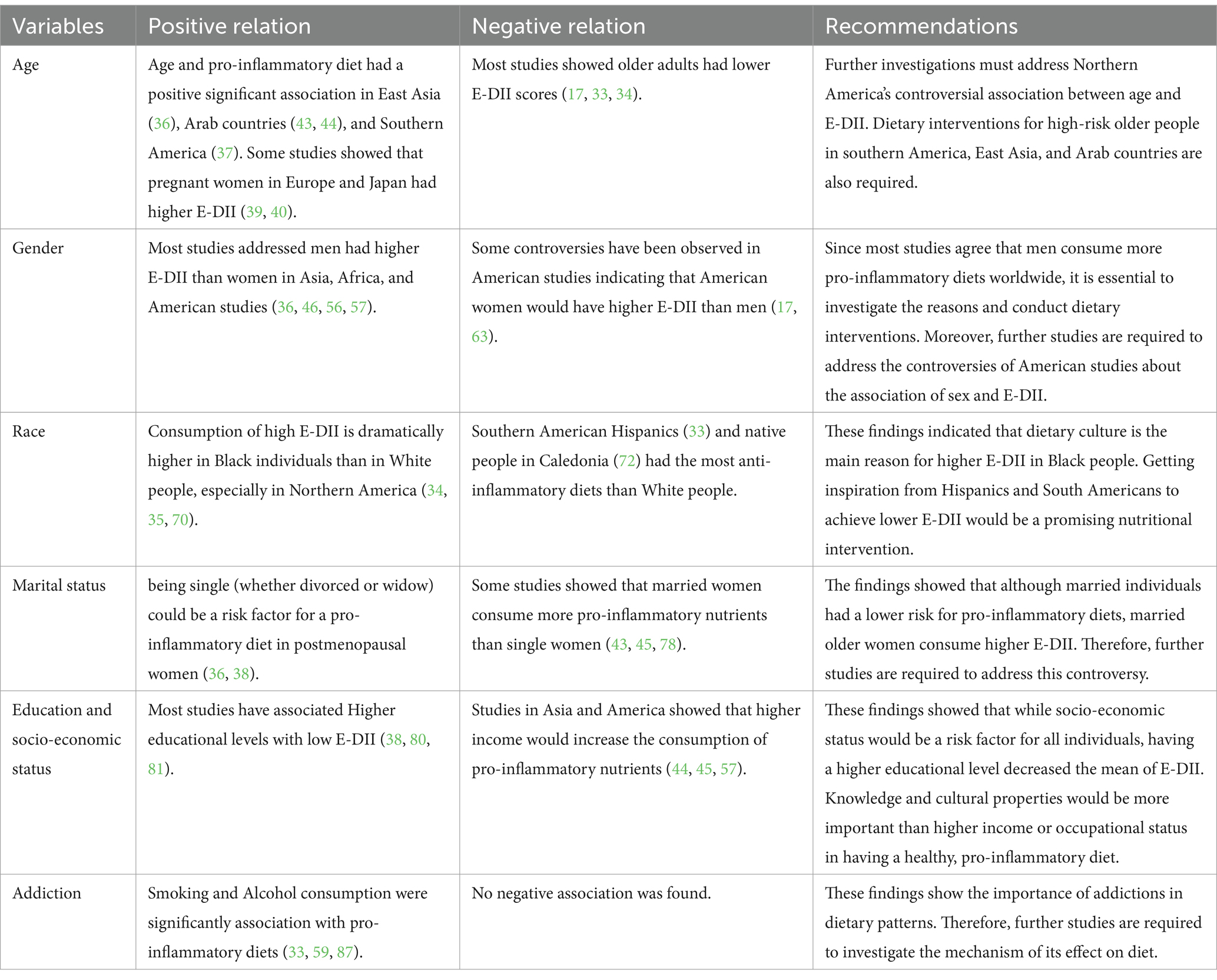
Table 1. Demographic risk factors for high Energy-intake Dietary Inflammatory Index (E-DII), controversies and recommendations.
3.1 Age
Aging is one of the most critical determinants of health and dietary condition (26, 27). The demand for nutrients and energy changes significantly across aging. Decreased physical activity in older people causes physiological anorexia and lower energy intake (28). Also, since older people are vulnerable to morbidities, they require higher amounts of nutrients to achieve the same health condition as their younger ages; however, physiological anorexia associated with aging may prevent proper supplementation (29). Therefore, a healthy diet enriched with anti-inflammatory nutrients for aged people would guarantee their health and prevent further morbidities (30). Consuming anti-inflammatory micronutrients, especially in the elderly, modulates gene expression and inhibits signaling pathways of pro-inflammatory biomarkers such as CRP, IL-6, and TNF-α, which results in lower chronic inflammation and further common diseases of old age (31, 32).
A wide diversity has been observed in the association of age with E-DII, but fortunately, most studies indicated that older people had anti-inflammatory diets (17, 33, 34). However, some others showed a significant increase in E-DII among elders (35). A study on more than 142,000 Korean people showed that the mean age of individuals with a pro-inflammatory diet was dramatically higher (36). Another study on the general population of Brazil, which included 34,000 participants, showed that adults had higher E-DII than seniors (37). Moreover, women also showed varying trends in E-DII across different ages. A post-menopausal women study revealed that the E-DII scores decreased in older participants (38). Also, studies on European and Japanese pregnant women showed the same trend (39, 40); however, McCullough et al. (41) showed that older pregnant women were more likely to consume pro-inflammatory diets. Over the past decades, poor diet quality has been extended among the elderly population because of worsening social, economic, and environmental factors (42).
Interestingly, the findings in Western regions of Asia were controversial. While the studies in Arab countries showed a positive association between age and E-DII (43, 44), the older Persian people had been consuming an anti-inflammatory diet (45, 46). This controversy could be explained by the differences in the geographical and cultural varieties observed in dietary patterns in previous studies (47–49). Eventually, findings of these studies showed that pro-inflammatory diets are more common among elders in southern America, East Asia, and Arab countries. The development of cheap fast foods among different societies, especially among low socioeconomic populations, increased unhealthy eating scores. The trend in eating habits and dietary patterns has changed in the last decades, and Western Dietary patterns have become the most common (50). An unhealthy diet enriched with pro-inflammatory nutrients such as unsaturated fatty acids increases the E-DII score and develops chronic inflammation and further diseases (51). However, further studies are required to address the rationale for these differences and conduct dietary interventions to prevent complications of high E-DII observed in these societies.
3.2 Sex
Sex is a primary risk factor for several pathologic conditions (27). Existing evidence indicates that women and men have differences in lifestyle aspects, such as physical activity, occupation, energy, and nutrient intake (52–54). Therefore, these differences influence the consumed diet and demands for supplementations (55).
The pro-inflammatory diet was more common among men than women in most previous studies, especially in Asian and African regions (36, 46, 56, 57). Consequently, the worldwide trend of dietary patterns has increased the pro-inflammatory properties of men’s diets in different regions (58, 59). Men have higher physical activity and spend most of their time on occupational duties (52). Therefore, it is expected to observe that men ignore healthy nutrition (28). On the other hand, daily stresses increase cortisol secretion and improve people’s appetites for consuming more fat and sweets. Therefore, higher energy and carbohydrate intake increases the amount of pro-inflammatory biomarkers in plasma (60). Since men are more vulnerable to cardiovascular disease and its mortality due to their sex hormone differences than women, extended dietary interventions are required to decrease the E-DII score of men’s diet (61).
The studies in Northern America had the highest controversies. The increasing desire of American men and women of different age groups for unhealthy snacks instead of complete and healthy meals has become a significant concern (62). The increased uncontrolled sugar and energy intake has significant pro-inflammatory effects (62). While the studies by Liang et al. (17), Huang et al. (35), and Li et al. (63) indicated that American women significantly had higher E-DII than men, other studies in Northern America revealed that men were more exposed to high pro-inflammatory potent nutrition. Compulsive eating before menstruation significantly increases the energy intake without enough physical activity. Women with premenopausal syndrome (PMS) have more attraction to sweets due to fluctuations in estrogen and progesterone (64). Moreover, craving for sweets during menstruation increases the intake of energy, carbohydrates, and fats, resulting in higher E-DII (65). Therefore, these conditions could provide an appropriate explanation for the higher E-DII among women than men in the mentioned studies. However, future studies should compare the lifestyle differences between women in America and other countries to explain the reason for this controversy.
3.3 Race
Food culture is unique for each race (66, 67). The races found their way to achieve healthy diets. But in recent decades, the diet has significantly changed worldwide, and consumption of unhealthy diets has been growing due to modern lifestyle (66, 68).
The difference in E-DII between different races has been documented in American studies; however, there is a lack of evidence in other regions. Although existing evidence revealed that White people are more likely to consume anti-inflammatory diets (33–35, 63), some studies showed significant controversial findings (17, 38, 69). Most studies indicated that Black people had a high level of E-DII (34, 35, 70). Also, Chen et al. (38) showed that Black pregnant women dangerously are more likely to consume pro-inflammatory diets associated with poorer delivery outcomes for mothers and neonates. Previous findings showed that Black people were significantly more vulnerable to food insecurity than White people [2021, (71)]. Therefore, further dietary interventions are vital to control Black people’s unhealthy diets and restrict the inflammatory potential of their diet.
The available studies revealed that other racial minorities in North and South America, especially Hispanics, widely use anti-inflammatory diets (33). Moreover, Hispanic pregnant women had healthy anti-inflammatory nutrition during pregnancy (39, 41). The study conducted by Paquet et al. (72) showed that the native people of New Caledonia, Melanesians, are interested in anti-inflammatory foods. This study also showed that Melanesians had a better health status than Europeans (tending to be closer to the Western food pattern) (72). Consequently, it would be concluded that the prevalence of pro-inflammatory diets in Black people is more related to food culture than access to food, literacy level, and inequalities (73, 74).
The study by Masaad et al. (44) showed that Arab people had slightly attended to pro-inflammatory diets than non-Arab people living in the Middle East. On the other hand, Chinese people had significantly lower E-DII scores than the different races, such as Malay and Indian, in East Asia (57). However, there needs to be more evidence addressing the differences between races living around the world comprehensively. The findings of our review on race differences by E-DII suggest that expanding the food culture of races that have an anti-inflammatory diet among races, such as Black and Arab people, would be a promising way to prevent the incidence of diseases related to chronic inflammation in the future.
3.4 Marital status
The role of marital status in dietary intake has been well-known during the last decades (75–77). While Sreeja et al. (36) indicated that married Korean people had lower E-DII scores than single individuals, more prevalence of a pro-inflammatory diet was observed in married American individuals (17). Small sample-sized studies on female participants showed that married women consumed pro-inflammatory diets (43, 45, 78). In contrast, Chen et al. (38) showed that a high E-DII score is more observed in single postmenopausal women than married women. This study also showed that age had a significant positive association with higher E-DII scores in women, as mentioned before (38). Therefore, being single (whether divorced or widow) could be a risk factor for a pro-inflammatory diet in postmenopausal women.
3.5 Education and occupation
Educational and occupational levels are important determinants of healthy eating and lifestyle in all societies (79). In East and West Asia, most evidence showed that individuals with higher educational levels had significantly lower E-DII scores (38, 80, 81). In contrast, the study conducted by Asgari et al. (45) revealed that obese women with higher educational levels had a more pro-inflammatory diet than low-educated people. Interestingly, this study also showed that the participants with higher occupational levels had more E-DII scores than low-income individuals (45). Moreover, other Asian studies showed that higher income and occupational level were tightly associated with a pro-inflammatory diet (44, 57). Healthy eating is an essential aspect of lifestyle for pregnant women and has a vital role in their children’s nutrition (82). Therefore, Asian studies showed that a higher educational level would help decrease E-DII score, while higher occupational level and income provide the conditions for a pro-inflammatory diet.
There is significant controversy in American studies regarding the association of education with E-DII (33, 34, 63, 69). Individuals who were educated in college or higher consumed pro-inflammatory diets, while some studies show that people with low educational levels (less than 9 years of education) obey more anti-inflammatory diets (37). Pereira et al. also suggest that people with higher incomes consume pro-inflammatory nutrients (37). Therefore, while the educational level is closely associated with higher E-DII scores, studies have shown that a higher educational-occupational level increases the pro-inflammatory potential of diet. Also, previous studies showed that both higher education and socioeconomic status develop healthy eating behaviors among different populations, but socioeconomic status is more important than education (83). While educational interventions increased the consumption of whole grains, vegetables, and fruits, recent observations represented that people with inadequate financial resources crave more energy than healthy eating, even if they are well-educated (84, 85). Therefore, although education is a basic factor of healthy eating, financial barriers can influence people to consume more unhealthy, pro-inflammatory nutrients enriched with fat, energy, and carbohydrates.
3.6 Addiction
Although addiction is not a demographic risk factor, it is closely associated with demographic factors such as age, sex, ethnicity, and socioeconomic status (86); thus, we decided to discuss the association of addiction with the E-DII level.
Approximately all existing findings confirmed that alcoholics and smokers are interested in dramatically inflammatory potent nutrients (33, 59, 87). Some studies revealed that a pro-inflammatory diet is more prevalent in smokers up to 3 or 4 times compared with non-smokers (17, 36). Smoking not only decreased the appetite of adults but also had a significant association with unhealthy diet in previous studies (88). Moreover, Chao et al. indicated that dietary intervention and expanding healthy eating habits would be more difficult for smokers (89).
The mechanisms of the association between addiction and unhealthy eating habits, especially higher E-DII, are still unclear. However, the activation of reward centers in the brain could be a responsible mechanism that increases the craving of addicted people for more sweets, carbohydrates, and energy (90). Also, recent investigations showed that addicts experience urge, hunger, and compulsive eating behaviors (91). Consequently, smoking and alcohol are two important neglected risk factors of the inflammatory diet, which the majority of studies have shown can be closely related to unhealthy eating habits. Also, the use of drugs, such as narcotics, cannabis, and modern psychedelics, are other substances whose addiction can be closely related to the desire for inflammatory diets, and there is limited information about them till now. On the other hand, addiction to several materials, such as coffee or tea, would be another risk factor for higher E-DII that would be addressed in further studies.
4 Conclusion
The present study reviewed the papers addressing the demographic risk factors for a pro-inflammatory diet. Although addiction, low educational level, high income, and being single were common risk factors for high E-DII scores, there were some significant controversies regarding sex and race. Several controversies observed in previous studies about age, gender, and race (in some cases) would be required to be investigated in future studies to achieve more precise findings. The high prevalence of pro-inflammatory diets in Arab and Black people could be a hazard to the future of healthy eating worldwide. Most of the existing evidence was analyzed by unadjusted models. Therefore, further studies are required to address the demographic risk factors of high E-DII and provide promising dietary interventions for vulnerable populations. Moreover, nutritional interventions, educating vulnerable populations, and ameliorating their availability of healthy food enriched with anti-inflammatory micronutrients would help to alleviate their dietary habits and prevent further chronic inflammatory diseases.
Author contributions
HP: Methodology, Writing – original draft. SK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Institue, N. C. (2023). Inflammation [online]. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/inflammation (Accessed May 12, 2023)
2. Furman, D, Campisi, J, Verdin, E, Carrera-Bastos, P, Targ, S, Franceschi, C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
3. Mauro, C, and Marelli-Berg, FM. T cell immunity and cardiovascular metabolic disorders: does metabolism fuel inflammation? Front Immunol. (2012) 3:173. doi: 10.3389/fimmu.2012.00173
4. Murakami, M, and Hirano, T. The molecular mechanisms of chronic inflammation development. Front Immunol. (2012) 3:323. doi: 10.3389/fimmu.2012.00323
5. Shinohara, M, Mirakaj, V, and Serhan, CN. Functional metabolomics reveals novel active products in the Dha metabolome. Front Immunol. (2012) 3:81. doi: 10.3389/fimmu.2012.00081
6. Ueha, S, Shand, FH, and Matsushima, K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol. (2012) 3:71. doi: 10.3389/fimmu.2012.00071
7. Galland, L. Diet and inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
8. Sepehrinia, M, and Pourmontaseri, H. Evaluation of the role of body mass index (Bmi) in the association between dietary inflammatory index and non-alcoholic fatty liver diseases: a letter to editor. Scand J Gastroenterol. (2024) 59:761–1. doi: 10.1080/00365521.2024.2323513
9. Van Der Heijden, RA, Sheedfar, F, Morrison, MC, Hommelberg, PP, Kor, D, Kloosterhuis, NJ, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57bl/6j mice. Aging. (2015) 7:256–68. doi: 10.18632/aging.100738
10. Alsaggar, M, Bdour, S, Ababneh, Q, El-Elimat, T, Qinna, N, and Alzoubi, KH. Silibinin attenuates adipose tissue inflammation and reverses obesity and its complications in diet-induced obesity model in mice. BMC Pharmacol Toxicol. (2020) 21:1–8. doi: 10.1186/s40360-020-0385-8
11. Chmurzynska, A, Muzsik, A, KrzyŻanowska-Jankowska, P, Walkowiak, J, and Bajerska, J. The effect of habitual fat intake, Il6 polymorphism, and different diet strategies on inflammation in postmenopausal women with central obesity. Nutrients. (2019) 11:1557. doi: 10.3390/nu11071557
12. Catta-Preta, M, Martins, MA, Brunini, TMC, Mendes-Ribeiro, AC, Mandarim-De-Lacerda, CA, and Aguila, MB. Modulation of cytokines, resistin, and distribution of adipose tissue in C57bl/6 mice by different high-fat diets. Nutrition. (2012) 28:212–9. doi: 10.1016/j.nut.2011.05.011
13. Wu, PY, Chen, KM, and Tsai, WC. The Mediterranean dietary pattern and inflammation in older adults: A systematic review and Meta-analysis. Adv Nutr. (2021) 12:363–73. doi: 10.1093/advances/nmaa116
14. Da Costa, RM, Neves, KB, Mestriner, FL, Louzada-Junior, P, Bruder-Nascimento, T, and Tostes, RC. Tnf-α induces vascular insulin resistance via positive modulation of Pten and decreased Akt/enos/no signaling in high fat diet-fed mice. Cardiovasc Diabetol. (2016) 15:119. doi: 10.1186/s12933-016-0443-0
15. Badimon, L, Chagas, P, and Chiva-Blanch, G. Diet and cardiovascular disease: effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr Med Chem. (2019) 26:3639–51. doi: 10.2174/0929867324666170428103206
16. Bernhart, JA, Turner-Mcgrievy, GM, Wirth, MD, Shivappa, N, and Hebert, JR. The imagine intervention: impacting physical activity, body fat, body mass index, and dietary inflammatory index. Transl J Am Coll Sports Med. (2022) 7:181. doi: 10.1249/TJX.0000000000000181
17. Liang, Z, Feng, Y, Shivappa, N, Hebert, JR, and Xu, X. Dietary inflammatory index and mortality from all causes, cardiovascular disease, and Cancer: A prospective study. Cancers. (2022) 14:4609. doi: 10.3390/cancers14194609
18. Szczyrek, M, Bitkowska, P, Chunowski, P, Czuchryta, P, Krawczyk, P, and Milanowski, J. Diet, microbiome, and Cancer immunotherapy-A comprehensive review. Nutrients. (2021) 13:2217. doi: 10.3390/nu13072217
19. Cavicchia, PP, Steck, SE, Hurley, TG, Hussey, JR, Ma, Y, Ockene, IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
20. Shivappa, N, Steck, SE, Hurley, TG, Hussey, JR, and Hebert, JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
21. Hebert, JR, Shivappa, N, Wirth, MD, Hussey, JR, and Hurley, TG. Perspective: the dietary inflammatory index (dii)—lessons learned, improvements made, and future directions. Adv Nutr. (2019) 10:185–95. doi: 10.1093/advances/nmy071
22. Pourmontaseri, H, Sepehrinia, M, Kuchay, MS, Farjam, M, Vahid, F, Dehghan, A, et al. The association between energy-adjusted dietary inflammatory index and metabolic syndrome and its mediatory role for cardiometabolic diseases: a prospective cohort study. Front Nutr. (2024) 11:1429883. doi: 10.3389/fnut.2024.1429883
23. Sepehrinia, M, Pourmontaseri, H, Naghizadeh, MM, Vahid, F, Hebert, JR, Homayounfar, R, et al. The association between energy-adjusted dietary inflammatory index and 10-year cardiovascular risk: Fasa adult cohort study. Food Sci Nutr. (2024) 12:5530–7. doi: 10.1002/fsn3.4181
24. Giezenaar, C, Chapman, I, Luscombe-Marsh, N, Feinle-Bisset, C, Horowitz, M, and Soenen, S. Ageing is associated with decreases in appetite and energy intake—a meta-analysis in healthy adults. Nutrients. (2016) 8:28. doi: 10.3390/nu8010028
25. Darmon, N, and Drewnowski, A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. (2015) 73:643–60. doi: 10.1093/nutrit/nuv027
26. Bellary, S, Kyrou, I, Brown, JE, and Bailey, CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. (2021) 17:534–48. doi: 10.1038/s41574-021-00512-2
27. Rodgers, JL, Jones, J, Bolleddu, SI, Vanthenapalli, S, Rodgers, LE, Shah, K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. (2019) 6:19. doi: 10.3390/jcdd6020019
28. Closs, VE, Pandolfo Feoli, AM, Gomes, I, and Augustin Schwanke, CH. Healthy eating index of elderly: description and association with energy, macronutrients and micronutrients intake. Arch Latinoam Nutr. (2014) 64:34–41.
29. Malafarina, V, Uriz-Otano, F, Gil-Guerrero, L, and Iniesta, R. The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas. (2013) 74:293–302. doi: 10.1016/j.maturitas.2013.01.016
30. Gielen, E, Beckwee, D, Delaere, A, De Breucker, S, Vandewoude, M, and Bautmans, I. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev. (2021) 79:121–47. doi: 10.1093/nutrit/nuaa011
31. Gholizadeh, M, Khalili, A, Roodi, PB, Saeedy, SAG, Najafi, S, Mohammadian, MK, et al. Selenium supplementation decreases Crp and Il-6 and increases Tnf-alpha: A systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol. (2023) 79:127199. doi: 10.1016/j.jtemb.2023.127199
32. Ramos-Lopez, O, Martinez-Urbistondo, D, Vargas-Nuñez, JA, and Martinez, JA. The role of nutrition on meta-inflammation: insights and potential targets in communicable and chronic disease management. Curr Obes Rep. (2022) 11:305–35. doi: 10.1007/s13679-022-00490-0
33. Mazidi, M, Shivappa, N, Wirth, MD, Hebert, JR, and Kengne, AP. Greater dietary inflammatory index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. (2018) 120:204–9. doi: 10.1017/S0007114518001071
34. Yuan, F, Deng, L, Sun, X, Chen, Z, Shivappa, N, Sheth, AK, et al. Dietary inflammatory index and risk of colorectal adenoma: effect measure modification by race, nonsteroidal anti-inflammatory drugs, cigarette smoking and body mass index? Cancer Causes Control. (2021) 32:837–47. doi: 10.1007/s10552-021-01436-y
35. Huang, Y, Zhang, L, Zeng, M, Liu, F, Sun, L, Liu, Y, et al. Energy-adjusted dietary inflammatory index is associated with 5-year all cause and cardiovascular mortality among chronic kidney disease patients. Front Nutr. (2022) 9:899004. doi: 10.3389/fnut.2022.899004
36. Sreeja, SR, Le, TD, Eom, BW, Oh, SH, Shivappa, N, Hebert, JR, et al. Association between the dietary inflammatory index and gastric disease risk: findings from a Korean population-based cohort study. Nutrients. (2022) 14:2662. doi: 10.3390/nu14132662
37. Pereira, NO, Carvalho, CA, Sperandio, N, Marques, KDS, Viola, P, Shivappa, N, et al. Factors associated with the inflammatory potential of the Brazilian population’s diet. Br J Nutr. (2021) 126:285–94. doi: 10.1017/S0007114520004079
38. Chen, WY, Fu, YP, Zhong, W, and Zhou, M. The association between dietary inflammatory index and sex hormones among postmenopausal women in the us. Front Endocrinol. (2021) 12:771565. doi: 10.3389/fendo.2021.771565
39. Chen, LW, Lyons, B, Navarro, P, Shivappa, N, Mehegan, J, Murrin, CM, et al. Maternal dietary inflammatory potential and quality are associated with offspring asthma risk over 10-year follow-up: the lifeways cross-generation cohort study. Am J Clin Nutr. (2020) 111:440–7. doi: 10.1093/ajcn/nqz297
40. Imai, C, Takimoto, H, Fudono, A, Tarui, I, Aoyama, T, Yago, S, et al. Application of the nutrient-rich food index 9.3 and the dietary inflammatory index for assessing maternal dietary quality in Japan: A single-center birth cohort study. Nutrients. (2021) 13:2854. doi: 10.3390/nu13082854
41. McCullough, LE, Miller, EE, Calderwood, LE, Shivappa, N, Steck, SE, Forman, MR, et al. Maternal inflammatory diet and adverse pregnancy outcomes: circulating cytokines and genomic imprinting as potential regulators? Epigenetics. (2017) 12:688–97. doi: 10.1080/15592294.2017.1347241
42. Long, T, Zhang, K, Chen, Y, and Wu, C. Trends in diet quality among older us adults from 2001 to 2018. JAMA Netw Open. (2022) 5:–e221880. doi: 10.1001/jamanetworkopen.2022.1880
43. Attlee, A, Saravanan, C, Shivappa, N, Wirth, MD, Aljaberi, M, Alkaabi, R, et al. Higher dietary inflammatory index scores are associated with stress and anxiety in dormitory-residing female university students in the United Arab Emirates. Front Nutr. (2022) 9:814409. doi: 10.3389/fnut.2022.814409
44. Masaad, AA, Yusuf, AM, Shakir, AZ, Khan, MS, Khaleel, S, Cheikh Ismail, L, et al. Sleep quality and dietary inflammatory index among university students: a cross-sectional study. Sleep Breath. (2021) 25:2221–9. doi: 10.1007/s11325-020-02169-z
45. Asgari, E, Shiraseb, F, Mirzababaei, A, Tangestani, H, and Mirzaei, K. Positive interaction between cg, cc genotypes of Cryptochrome circadian clocks 1, and energy-adjusted dietary inflammatory index on high sensitivity C-reactive protein level in women with central obesity. Clin Nutr Res. (2023) 12:7–20. doi: 10.7762/cnr.2023.12.1.7
46. Firoozi, D, Masoumi, SJ, Ranjbar, S, Shivappa, N, Hebert, JR, Zare, M, et al. The association between energy-adjusted dietary inflammatory index, body composition, and anthropometric indices in Covid-19-infected patients: A case-control study in Shiraz, Iran. Int J Clin Pract. (2022) 2022:5452488. doi: 10.1155/2022/5452488
47. Mora, N, and Golden, SH. Understanding cultural influences on dietary habits in Asian, middle eastern, and Latino patients with type 2 diabetes: a review of current literature and future directions. Curr Diab Rep. (2017) 17:1–12. doi: 10.1007/s11892-017-0952-6
48. Musaiger, AO. Socio-cultural and economic factors affecting food consumption patterns in the Arab countries. J R Soc Health. (1993) 113:68–74. doi: 10.1177/146642409311300205
49. Roudsari, AH, Vedadhir, A, Amiri, P, Kalantari, N, Omidvar, N, Eini-Zinab, H, et al. Psycho-socio-cultural determinants of food choice: A qualitative study on adults in social and cultural context of Iran. Iran J Psychiatry. (2017) 12:241.
50. Heslin, AM, and Mcnulty, B. Adolescent nutrition and health: characteristics, risk factors and opportunities of an overlooked life stage. Proc Nutr Soc. (2023) 82:142–56. doi: 10.1017/S0029665123002689
51. Kunset, P, Punsawad, C, Petsirasan, R, Suwanbamrung, C, Shohaimi, S, Narkkul, U, et al. Unhealthy dietary patterns and their associations with sociodemographic factors as predictors among underweight and overweight adolescents in southern Thailand. Int J Environ Res Public Health. (2023) 20:6703. doi: 10.3390/ijerph20176703
52. Lee, Y-S. Gender differences in physical activity and walking among older adults. J Women Aging. (2005) 17:55–70. doi: 10.1300/J074v17n01_05
53. Milanović, Z, Pantelić, S, Trajković, N, Sporiš, G, Kostić, R, and James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging. (2013) 8:549–56. doi: 10.2147/CIA.S44112
54. Price, WA, and Nguyen, T. Nutrition and physical degeneration: a comparison of primitive and modern diets and their effects. EnCognitive. com. (2016).
55. Artegoitia, VM, Krishnan, S, Bonnel, EL, Stephensen, CB, Keim, NL, and Newman, JW. Healthy eating index patterns in adults by sex and age predict cardiometabolic risk factors in a cross-sectional study. BMC Nutr. (2021) 7:30. doi: 10.1186/s40795-021-00432-4
56. Ferreira, M, Cronje, HT, Van Zyl, T, Bondonno, N, and Pieters, M. The association between an energy-adjusted dietary inflammatory index and inflammation in rural and urban Black south Africans. Public Health Nutr. (2021) 25:1–13. doi: 10.1017/S136898002100505X
57. Shafiee, NH, Razalli, NH, Shahril, MR, Muhammad Nawawi, KN, Mohd Mokhtar, N, Abd Rashid, AA, et al. Dietary inflammatory index, obesity, and the incidence of colorectal Cancer: findings from a hospital-based case-control study in Malaysia. Nutrients. (2023) 15:982. doi: 10.3390/nu15040982
58. Millar, CL, Dufour, AB, Shivappa, N, Habtemariam, D, Murabito, JM, Benjamin, EJ, et al. A proinflammatory diet is associated with increased odds of frailty after 12-year follow-up in a cohort of adults. Am J Clin Nutr. (2022) 115:334–43. doi: 10.1093/ajcn/nqab317
59. Sharma, I, Zhu, Y, Woodrow, JR, Mulay, S, Parfrey, PS, Mclaughlin, JR, et al. Inflammatory diet and risk for colorectal cancer: A population-based case-control study in Newfoundland, Canada. Nutrition. (2017) 42:69–74. doi: 10.1016/j.nut.2017.05.010
60. Chao, AM, Jastreboff, AM, White, MA, Grilo, CM, and Sinha, R. Stress, cortisol, and other appetite-related hormones: prospective prediction of 6-month changes in food cravings and weight. Obesity. (2017) 25:713–20. doi: 10.1002/oby.21790
61. Ruige, JB, Mahmoud, AM, De Bacquer, D, and Kaufman, J-M. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. (2011) 97:870–5. doi: 10.1136/hrt.2010.210757
62. Nielsen, SJ, Siega-Riz, AM, and Popkin, BM. Trends in energy intake in us between 1977 and 1996: similar shifts seen across age groups. Obes Res. (2002) 10:370–8. doi: 10.1038/oby.2002.51
63. Li, A, Chen, Y, Schuller, AA, Van Der Sluis, LWM, and Tjakkes, GE. Dietary inflammatory potential is associated with poor periodontal health: A population-based study. J Clin Periodontol. (2021) 48:907–18. doi: 10.1111/jcpe.13472
64. Souza, LBD, Martins, KA, Cordeiro, MM, Rodrigues, YDS, Rafacho, BPM, and Bomfim, RA. Do food intake and food cravings change during the menstrual cycle of young women? Rev Bras Ginecol Obstet. (2018) 40:686–92. doi: 10.1055/s-0038-1675831
65. Yukie, M, Aoi, I, Mizuki, K, and Toshiyuki, Y. Change in appetite and food craving during menstrual cycle in young students. Int J Nutr Metabol. (2020) 12:25–30. doi: 10.5897/IJNAM2019.0264
66. Ma, Y, Weng, X, Gao, X, Winkels, R, Cuffee, Y, Gupta, S, et al. Healthy eating index scores differ by race/ethnicity but not hypertension awareness status among us adults with hypertension: findings from the 2011-2018 National Health and nutrition examination survey. J Acad Nutr Diet. (2022) 122:1000–12. doi: 10.1016/j.jand.2021.11.006
67. Vaccaro, JA, and Huffman, FG. Sex and race/ethnic disparities in food security and chronic diseases in us older adults. Gerontol Geriatr Med. (2017) 3:2333721417718344. doi: 10.1177/2333721417718344
68. Simmons, D, Devlieger, R, Van Assche, A, Jans, G, Galjaard, S, Corcoy, R, et al. Effect of physical activity and/or healthy eating on Gdm risk: the Dali lifestyle study. J Clin Endocrinol Metabol. (2016) 102:903–13. doi: 10.1210/jc.2016-3455
69. Wang, L, Sun, M, Guo, Y, Yan, S, Li, X, Wang, X, et al. The role of dietary inflammatory index on the association between sleep quality and Long-term cardiovascular risk: A mediation analysis based on Nhanes (2005-2008). Nat Sci Sleep. (2022) 14:483–92. doi: 10.2147/NSS.S357848
70. Lozano, CP, Wilkens, LR, Shvetsov, YB, Maskarinec, G, Park, SY, Shepherd, JA, et al. Associations of the dietary inflammatory index with total adiposity and ectopic fat through the gut microbiota, Lps, and C-reactive protein in the multiethnic cohort-adiposity phenotype study. Am J Clin Nutr. (2022) 115:1344–56. doi: 10.1093/ajcn/nqab398
71. Allen, AJ, Kuczmarski, MF, Evans, MK, Zonderman, AB, and Waldstein, SR. Race differences in diet quality of urban food-insecure blacks and whites reveals resiliency in blacks. J Racial Ethn Health Disparities. (2016) 3:706–12. doi: 10.1007/s40615-015-0189-5
72. Paquet, M, Shivappa, N, Hebert, JR, Baron-Dubourdieu, D, Boutron-Ruault, MC, Guenel, P, et al. Dietary inflammatory index and differentiated thyroid carcinoma risk: a population-based case-control study in New Caledonia. Am J Epidemiol. (2020) 189:95–107. doi: 10.1093/aje/kwz192
73. Irving, SM, Njai, RS, and Siegel, PZ. Food insecurity and self-reported hypertension among Hispanic, Black, and White adults in 12 states, behavioral risk factor surveillance system, 2009. Prev Chronic Dis. (2014) 11:E161. doi: 10.5888/pcd11.140190
74. Larson, NI, Story, MT, and Nelson, MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. (2009) 36:74–81.e10. doi: 10.1016/j.amepre.2008.09.025
75. Mirmiran, P, Mohammadi, F, Allahverdian, S, and Azizi, F. Association of educational level and marital status with dietary intake and cardiovascular risk factors in Tehranian adults: Tehran lipid and glucose study (Tlgs). Nutr Res. (2002) 22:1365–75. doi: 10.1016/S0271-5317(02)00440-2
76. Wimmer, R, Audetat, A, Binggeli, J, Schuetz, P, and Kaegi-Braun, N. Association of Sociodemographic, socioeconomic and lifestyle characteristics with low protein and energy intake in the healthy Swiss population. Nutrients. (2023) 15:2200. doi: 10.3390/nu15092200
77. Woo, J, Leung, S, Ho, S, Sham, A, Lam, T, and Janus, E. Influence of educational level and marital status on dietary intake, obesity and other cardiovascular risk factors in a Hong Kong Chinese population. Eur J Clin Nutr. (1999) 53:461–7. doi: 10.1038/sj.ejcn.1600777
78. Wang, Y, Armijos, RX, Xun, P, and Weigel, MM. Dietary inflammatory index and Cardiometabolic risk in Ecuadorian women. Nutrients. (2021) 13:2640. doi: 10.3390/nu13082640
79. Daniel, C. Is healthy eating too expensive?: how low-income parents evaluate the cost of food. Soc Sci Med. (2020) 248:112823. doi: 10.1016/j.socscimed.2020.112823
80. Shin, D, Kwon, SC, Kim, MH, Lee, KW, Choi, SY, Shivappa, N, et al. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition. (2018) 55-56:56–62. doi: 10.1016/j.nut.2018.02.026
81. Shiraseb, F, Farazi, M, Rasaei, N, Clark, CCT, Jamili, S, and Mirzaei, K. The interaction between rs 3,807,992 genotypes with the dietary inflammatory index on leptin, leptin resistance, and galectin 3 in obese and overweight women. BMC Endocr Disord. (2022) 22:237. doi: 10.1186/s12902-022-01136-x
82. Van Ansem, WJ, Schrijvers, C, Rodenburg, G, and Van De Mheen, D. Maternal educational level and children’s healthy eating behaviour: role of the home food environment (cross-sectional results from the Inpact study). Int J Behav Nutr Phys Act. (2014) 11:1–12. doi: 10.1186/s12966-014-0113-0
83. Almoraie, NM, Alothmani, NM, Alomari, WD, and Al-Amoudi, AH. Addressing nutritional issues and eating behaviours among university students: a narrative review. Nutr Res Rev. (2024) 15:1–16. doi: 10.1017/S0954422424000088
84. Hamulka, J, Wadolowska, L, Hoffmann, M, Kowalkowska, J, and Gutkowska, K. Effect of an education program on nutrition knowledge, attitudes toward nutrition, diet quality, lifestyle, and body composition in polish teenagers. The Abc of healthy eating project: design, protocol, and methodology. Nutrients. (2018) 10:1439. doi: 10.3390/nu10101439
85. Rippin, H, Hutchinson, J, Greenwood, DC, Jewell, J, Breda, J, Martin, A, et al. Inequalities in education and national income are associated with poorer diet: pooled analysis of individual participant data across 12 European countries. PLoS One. (2020) 15:e0232447. doi: 10.1371/journal.pone.0232447
86. Gordon, K, Kutywayo, A, Frade, S, Naidoo, N, and Mullick, S. Socio-demographic and social support factors related to substance use in south African in-school adolescents: insights from the girls achieve power (gap year) trial in three peri-urban settings. Gates Open Res. (2021) 5:154. doi: 10.12688/gatesopenres.13422.1
87. Vázquez-Salas, RA, Shivappa, N, Galván-Portillo, M, López-Carrillo, L, Hebert, JR, and Torres-Sánchez, L. Dietary inflammatory index and prostate cancer risk in a case-control study in Mexico. Br J Nutr. (2016) 116:1945–53. doi: 10.1017/S0007114516003986
88. Heydari, G, Heidari, F, Yousefifard, M, and Hosseini, M. Smoking and diet in healthy adults: a cross-sectional study in Tehran, Iran, 2010. Iran J Public Health. (2014) 43:485–91.
89. Chao, AM, White, MA, Grilo, CM, and Sinha, R. Examining the effects of cigarette smoking on food cravings and intake, depressive symptoms, and stress. Eat Behav. (2017) 24:61–5. doi: 10.1016/j.eatbeh.2016.12.009
90. Volkow, ND, Michaelides, M, and Baler, R. The neuroscience of drug reward and addiction. Physiol Rev. (2019) 99:2115–40. doi: 10.1152/physrev.00014.2018
91. Moore, CF, Sabino, V, Koob, GF, and Cottone, P. Pathological overeating: emerging evidence for a compulsivity construct. Neuropsychopharmacology. (2017) 42:1375–89. doi: 10.1038/npp.2016.269
Keywords: energy-adjusted dietary inflammatory index, E-DII, prevention, diet, demographic features, pro-inflammatory diet
Citation: Pourmontaseri H and Khanmohammadi S (2024) Demographic risk factors of pro-inflammatory diet: a narrative review. Front. Nutr. 11:1448806. doi: 10.3389/fnut.2024.1448806
Edited by:
Mehran Rahimlou, Zanjan University of Medical Sciences, IranReviewed by:
Dorota Formanowicz, Poznan University of Medical Sciences, PolandCamelia Munteanu, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Jose Luis Fachi, Washington University in St. Louis, United States
Copyright © 2024 Pourmontaseri and Khanmohammadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaghayegh Khanmohammadi, U2hhZ2hheWVnaC5raGFubW9oYW1tYWRpQGdtYWlsLmNvbQ==
 Hossein Pourmontaseri
Hossein Pourmontaseri Shaghayegh Khanmohammadi
Shaghayegh Khanmohammadi