- 1Riddet Institute, Massey University, Palmerston North, New Zealand
- 2INRAE, Institut Agro, STLO, Rennes, France
- 3Inner Mongolia Dairy Technology Research Institute Co. Ltd., Hohhot, China
- 4Oceania Dairy Limited, Glenavy, New Zealand
- 5National Center of Technology Innovation for Dairy—Oceania Innovation Center, Lincoln, New Zealand
- 6National Center of Technology Innovation for Dairy, Hohhot, China
The recommended amino acid requirements of the infant are based on the amino acid composition of mature human breast milk. The amino acid composition of breast milk is usually determined following either acid or alkaline (for tryptophan) hydrolysis. For accuracy, however, the known effect of hydrolysis time on amino acid composition should be accounted for. Also, ideally the amino acid composition of breast milk should be given in units of digested (assumed to be absorbed) amino acids. A review of the literature is presented which gives mean total amino acid concentrations in mature human milk (n = 26 studies), mean hydrolysis correction factors (n = 3 studies) and mean true ileal amino acid digestibility coefficients (n = 3 studies, suckling piglet). There were differences between the estimates of amino acid concentration corrected for hydrolysis time and digestibility, and current FAO (2013) recommendations that were not corrected for these factors. The values based on the published literature up until 2023 (mg/g true protein) corrected for hydrolysis time and digestibility gave higher values (more than 16% higher) for leucine, lysine and threonine, and considerably higher values (greater than 30%) for histidine and tryptophan. Current recommendations may need revision.
1 Introduction
Human breast milk is a complex biological fluid and in nature is the sole source of nutrients for a baby for the first few months of life. The protein composition and consequent amino acid composition of breast milk is the result of millions of years of evolution, and as such, it is generally assumed that the amino acid composition of breast milk from healthy well-nourished women, provides a suitable basis for estimates of the amino acid requirements of the baby postnatally (1, 2). It is of utmost importance to know the amino acid requirements of the infant with accuracy as they provide the building blocks of proteins synthesised during growth and development, and many of the amino acids have important specific physiological roles (3–5).
Breast milk is the preferred source of nutrition for the newborn baby, but for numerous reasons in practice many infants receive infant formula as their sole source of nutrition. It is important, therefore, to have accurate estimates of the amino acid composition of breast milk.
Human milk contains hundreds of different proteins of which the concentrations are variable, and although the amino acid sequences of some of the more common milk proteins are known, not all of the proteins have been sequenced. Moreover, a significant proportion of breast milk amino acids are in the free form. It is for these reasons that the amino acid composition of milk is usually determined by chemical analysis.
Since the development of ion-exchange chromatography and other methods such as HPLC and UHPLC with precolumn derivatization to separate amino acids in complex mixtures, many studies have been reported determining the amino acid composition of human milk. Common to these studies is the need to firstly hydrolyse the breast milk proteins to their constituent free amino acids to allow quantitation. This commonly involves acid hydrolysis (usually 6 M HCl) of the defatted material in an oxygen free environment for 20 to 24 h at 110 degrees Celsius. It is well established, however, that with strong acid hydrolysis, methionine (particularly if oxygen is present), cysteine and tryptophan can be destroyed. Accordingly, methionine and cysteine are usually determined as methionine sulphone and cysteic acid, respectively following performic acid oxidation undertaken before the hydrolysis step, and tryptophan after an alkaline hydrolysis. Also, during hydrolysis tyrosine can become halogenated but this can be prevented by adding phenol to the hydrolysis mixture. What is less widely appreciated, however, is that regardless of the type of hydrolysis, a hydrolysis time longer than 24 h is required for the full release of some amino acids (for example leucine, isoleucine and valine), while others (for example serine, threonine, cysteic acid, tryptophan) can be progressively oxidized (6). The degree of underestimation can be practically important urging some authorities to adopt correction factors (e.g., TNO, the Netherlands: threonine 1.05; serine 1.10; valine 1.07; isoleucine 1.08). For some applications such a degree of underestimation may be acceptable, but it is important that infant formulas mimic the amino acid composition of human milk as accurately as possible.
An approach to determining amino acids that is more accurate than using a set hydrolysis time, is to subject the protein to multiple hydrolyses (different durations of hydrolysis) and then apply a curvilinear mathematical model to allow the prediction of the amounts of amino acids present in the protein, accounting for simultaneous rates of both amino acid release and destruction (7, 8). The Robel and Crane model has been modified (9) to allow for complex mixtures, such as breast milk, that have a free amino acid as well as a bound proteinaceous amino acid component.
Another consideration when equating milk amino acid contents with amino acid requirements for the infant is that not all proteins in human milk have a primary nutrition function, but rather some proteins (e.g., immunoglobulins; lactoferrin; transferrin; lysozyme) may have primary immunological and developmental roles. It appears that these types of proteins are only partially digested between the mouth and end of the small intestine and complete fragments of such proteins can be detected in faeces from breast-fed babies (10–12). Consequently, not all human milk amino acids are absorbed and thus a more refined estimate of amino acid requirements is given by the digestible (assumed absorbed) amino acids in human milk (13). It is the profile of absorbed rather than gross amino acids that needs to be mimicked by the digestible amino acids in infant formulas.
It is well established that the absorption of intact amino acids in humans is essentially complete by the end of the small intestine and that amino acid digestibility should be determined between the mouth and the terminal ileum using a true ileal amino acid digestibility assay (14, 15). Such a measure cannot be readily obtained using human infants, necessitating the need for animal models of digestion. The three-week-old suckled piglet, ingesting milk at an amount per unit stomach volume to mimic the human infant, has been shown to be a suitable candidate model for the three-month-old human baby, from an anatomical and physiological perspective (16–19). A study directly comparing the protein and organic matter digestion of milk in the piglet and human baby, provides empirical evidence for the suitability of the suckled piglet model (20). Other models such as the rat pup have been successfully used to study the digestion of milk proteins (21) but such models rely upon intubation of the milk, and thus exclude suckling, which may affect digestion. The suckled piglet model has been used in several studies to determine the digestibility of amino acids in breast milk.
The objective of this contribution is to review the published literature on the amino acid composition of human milk with an emphasis on the effect of amino acid losses and gains during hydrolysis, and on the absorbability of the breast milk amino acids. A profile of absorbed amino acids in human milk is put forward as the current best estimate of the amino acid needs of the newborn term infant. This amino acid profile is compared with the current FAO recommendations (2). The latter recommended amino acid profile does not consider the effects of hydrolysis time during amino acid analysis, nor the effects of differences in amino acid digestibility.
2 Methods
A systematic review of the literature was conducted to identify publications that reported total amino acid concentrations in human milk, including publications up until the end of 2023. A search was performed using Scopus and Google Scholar. Keywords used were “PubMed”, “amino acid,” “protein composition,” “human milk composition,” “human milk,” “breast milk,” “human milk nutrition,” “characterisation of human milk,” “standardisation of human milk,” and “factors affecting human milk composition.” Reference lists of the selected publications were further searched manually to identify any other relevant articles. A total of 74 articles were identified for potential inclusion.
Within each publication, milk collection methods and methods for amino acid analysis were reviewed. Inclusion criteria included the collection of mature milk from healthy women, defined as collection periods extending between beyond 1 to 10 months post-partum. For publications that presented total amino acid concentrations for multiple lactation periods, those that were within the specified collection period were averaged.
Exclusion criteria included collection from a single donor or from non-healthy women or results from the collection of non-mature milk (defined as less than 1 month post-partum). Studies that conducted amino acid analysis using non-standard methods were also excluded. Publications in which the milk collection methodology or amino acid analysis methods were not well-described were excluded.
When the results from one study were reported in several publications (determined according to the description of methods), these data were only included in the database once. Table 1 lists the studies that were not included in the database and the reason for their exclusion.
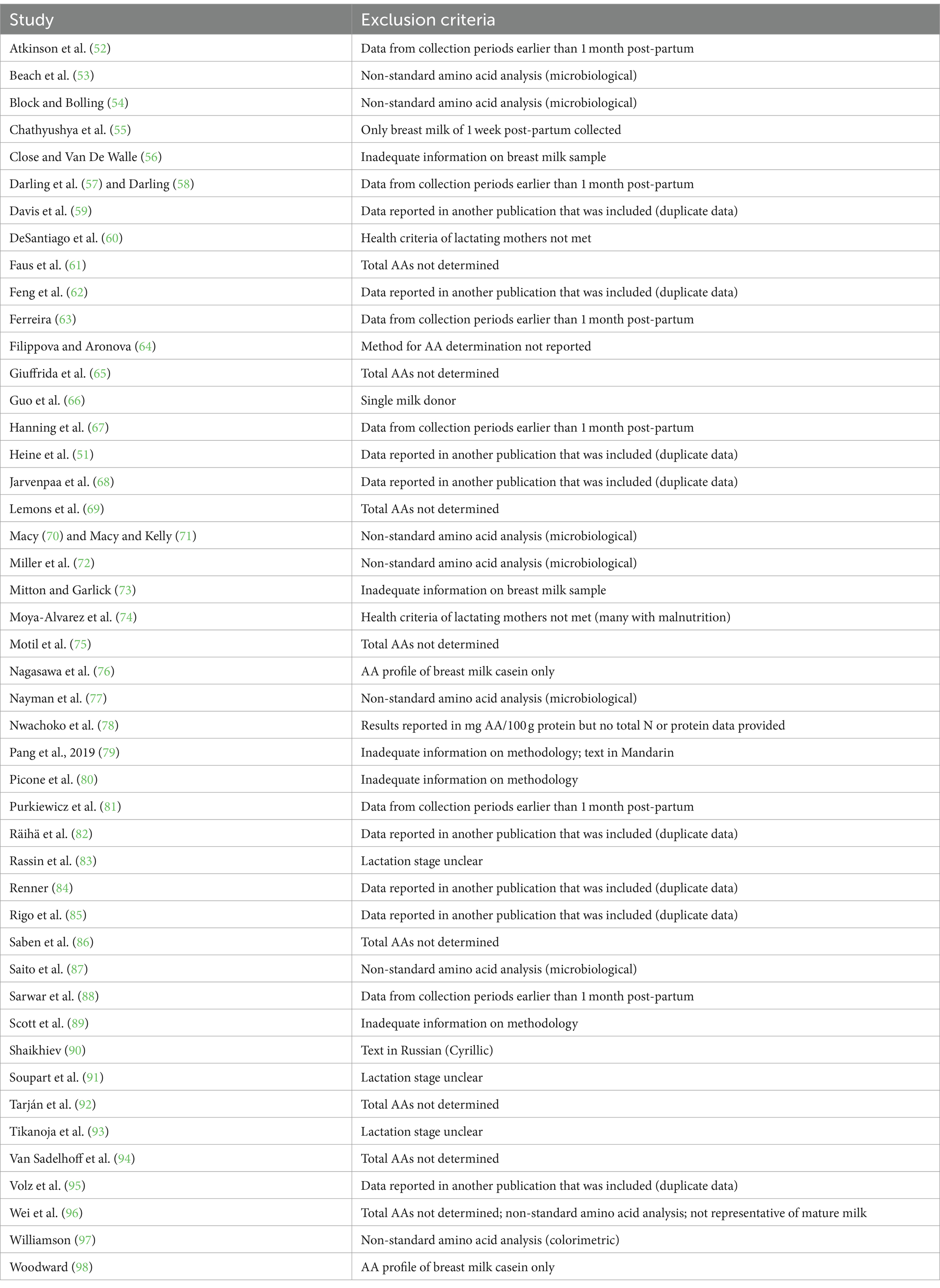
Table 1. Studies that report the amino acid (AA) concentration in mature human milk that were published before 2023 and not included in the present dataset.
A total of 26 studies were included in the database (13, 22–46).
The amino acid composition of human milk was reported using different units in the publications so these were converted when required, to mg amino acid/L milk, mg amino acid/g dry matter (DM) and mg/g true protein (TP). Data that were presented only in moles were first converted to mg of amino acid per L milk by multiplying by the molecular weight of each amino acid (x 10). For each publication, the reported dry matter (DM) content of milk given in that publication was used to convert each amino acid value between mg/L and mg/g DM. When the DM content of the milk was not reported in the publication, the average DM content of milk calculated from publications that reported this value was used (121.2 mg DM/L milk). To convert between mg/g and mg/L, the conversion factor of 1.032 mg milk/L was used (47).
Values were also converted to mg/g true protein (TP) with the TP content of the milk samples calculated as reported by FAO (2) where TP = nitrogen concentration × 6.38 × 0.75. The factor of 0.75 is used as the non-protein content of human milk (comprising mainly urea and free amino acids) is around 25% of the total nitrogen content (2). Where necessary, as different publications used different conversion factors between nitrogen concentration (which is chemically analysed) and crude protein, reported protein concentrations were first converted back to nitrogen concentrations according to the reported conversion factor in each publication.
A review of the literature (until 2023) was also undertaken to identify studies addressing the effect of hydrolysis time on amino acid yield in human breast milk, and studies determining the true ileal digestibility of amino acids in human breast milk.
3 Results and discussion
3.1 Amino acid composition
The overall mean total amino acid compositions of human milk reported in the 26 studies included in the database are given in Table 2. Zhang et al. (48) also conducted a systematic review of the total amino acid concentration in human milk, and their values are included in Table 2.
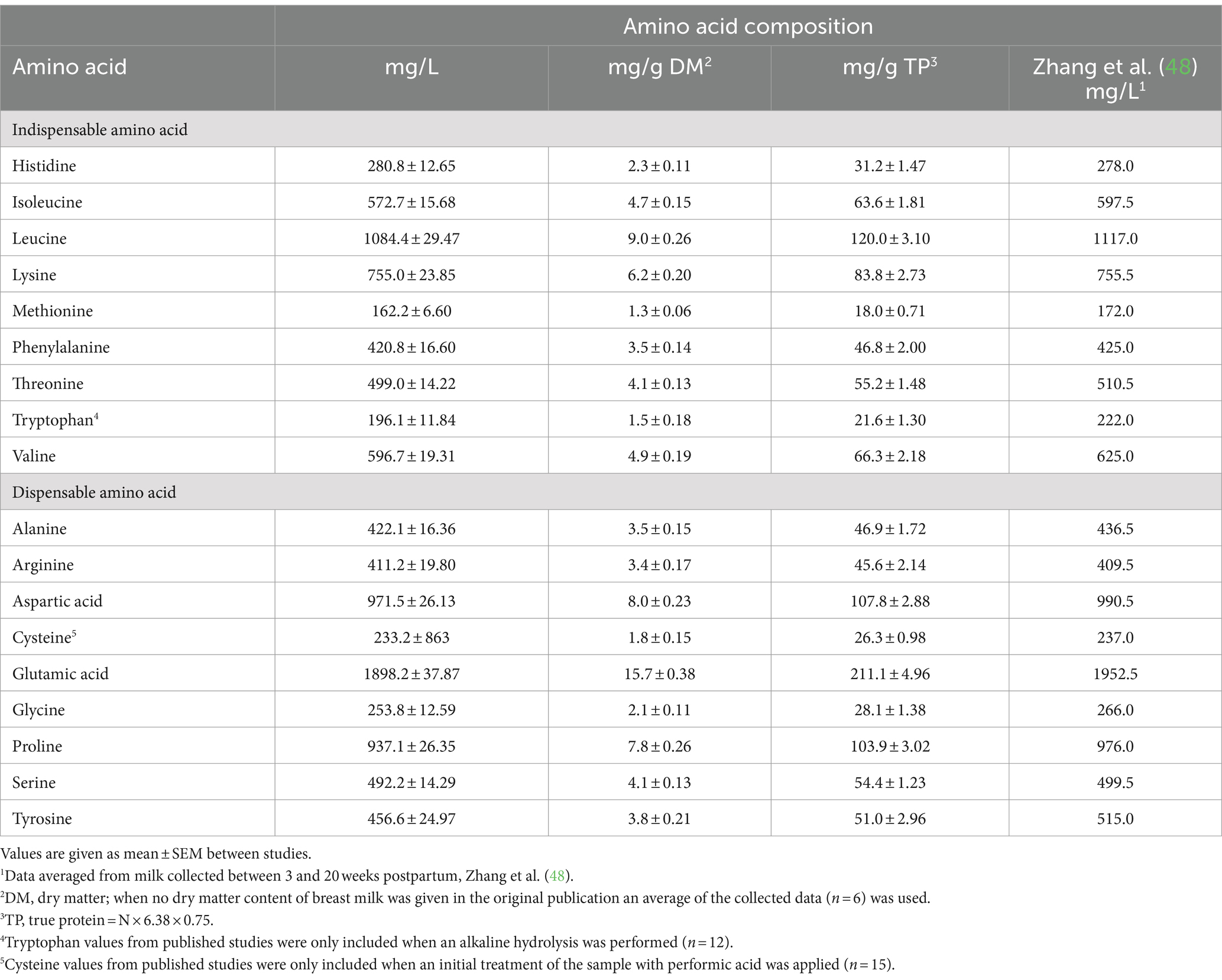
Table 2. Amino acid composition of human milk (based on 20 to 24 h amino acid hydrolysis period) collected from women between 3 and 42 weeks post-partum from data published before 2023 (n = 26 studies) and values reported in the systematic review of Zhang et al. (48).1
The rigorous and detailed review of Zhang et al. (48) covering the literature published up to 2009 provides an important benchmark against which to compare the presently derived data. Data included in the Zhang et al. (48) study related to breast milk samples from complete 24 h collections or at least collections of the entire amount of milk from one or both breasts at a feeding, or pooled or banked milk. The milk was from healthy mothers receiving “free-living” diets and who had delivered healthy mainly term babies. Studies employing microbiological methods of amino acid determination were excluded, and only studies using ion exchange chromatography, HPLC and UHPLC with precolumn derivatization or similar validated methods were used in the analysis. Results largely relate to 20 to 24 h hydrolysis of protein. Attention was paid to ensuring that the studies used appropriate consistent methods for the determination of methionine, cysteine and tryptophan. Overall mean amino acid concentrations in milk were determined for each study and least squares means generated with stage of lactation fitted as an effect in the ANOVA model. Mature milk in the Zhang et al. (48) work was defined as milk from 21 days of lactation up to >136 days of lactation. Lactation stage significantly (p < 0.05) influenced total amino acid composition and data were presented separately for mature milk relating to 21 to 58 days of lactation; 59 to 135 days of lactation and 136 to 540 days of lactation. Most studies used the units of weight of amino acid per 100 ml milk. Where data were given as weight per 100 grams milk, the volume-weight correction, which is quantitatively minor, was not undertaken. The study reviewed human milk composition data from 83 published scientific papers, from 18 countries, with publication dates ranging from 1941 to 2009. For total amino acid content, 26 papers providing 79 mean values from 3,774 subjects were selected by the authors for analysis. The total N concentration of breast milk and the amino acid content of breast milk declined (p < 0.05) moderately for milk from around 2 months of lactation to milk from 5 to 18 months of lactation. This is consistent with the conclusions reached by Lönnerdal et al. (49) and Ren et al. (47) that human milk amino acid content is relatively stable from around 3 to 4 weeks after birth and onwards. For our purposes and to align with the lactation period used in the present work, the mean concentrations calculated over 21 to 135 days of lactation were taken as an estimate of the amino acid composition of mature human breast milk (see Table 2).
There is close agreement between the values for the amino acid composition of human breast milk between Zhang et al. (48) and the present estimates, though differences were found for some of the amino acids. This gives confidence in the presently derived estimates. The presently reported estimates are preferred, as these incorporate the most up-to-date published information (studies published up to 2023, as opposed to 2009).
3.2 Correction for the effect of time of hydrolysis
Three studies were identified that conducted amino acid analysis with multiple hydrolysis intervals (13, 44, 45). The difference between the concentration of each amino acid determined using a 20 to 24 h hydrolysis period and that determined using multiple hydrolyses (estimated amino acid concentration in the milk) for each amino acid in each study was averaged to calculate average correction factors. These correction factors were used to correct the total amino acid concentration in human milk (reported in Table 2; based on 20 to 24 h hydrolysis) to that if multiple hydrolysis had been used for each individual published study, and the mean results are reported in Table 3. In the publication by Charton et al. (44) data corresponding to 24 h hydrolysis were not included, but data were provided (A. Deglaire, personal communication) to allow the correction to be made.

Table 3. Determined correction factors1 for breakdown or incomplete release of amino acids during hydrolysis, and concentration of amino acids in breast milk corrected by these values.
For most of the amino acids the effect of correction was small but for histidine, phenylalanine, threonine, tryptophan, cysteine, serine and tyrosine the differences were considered to be practically important (correction factor ≥ 2%). For leucine, isoleucine and valine the determined correction factors were smaller than expected, though the hydrolysis behaviour is likely to vary with the substrate being analysed. The amino acid affected by hydrolysis to the greatest extent was threonine, which is known to be sensitive to oxidation (6).
3.3 Correction for the true ileal amino acid digestibility
True ileal amino acid digestibility coefficients for human milk determined using the piglet as a model for the human infant were reported in three studies (13, 44, 45). For each amino acid, digestibility coefficients were averaged across the three studies (Table 4) and applied to the data presented in Table 3. The overall mean true ileal amino acid digestibility coefficients and mean amounts of true ileal digestible amino acids are presented in Table 5.
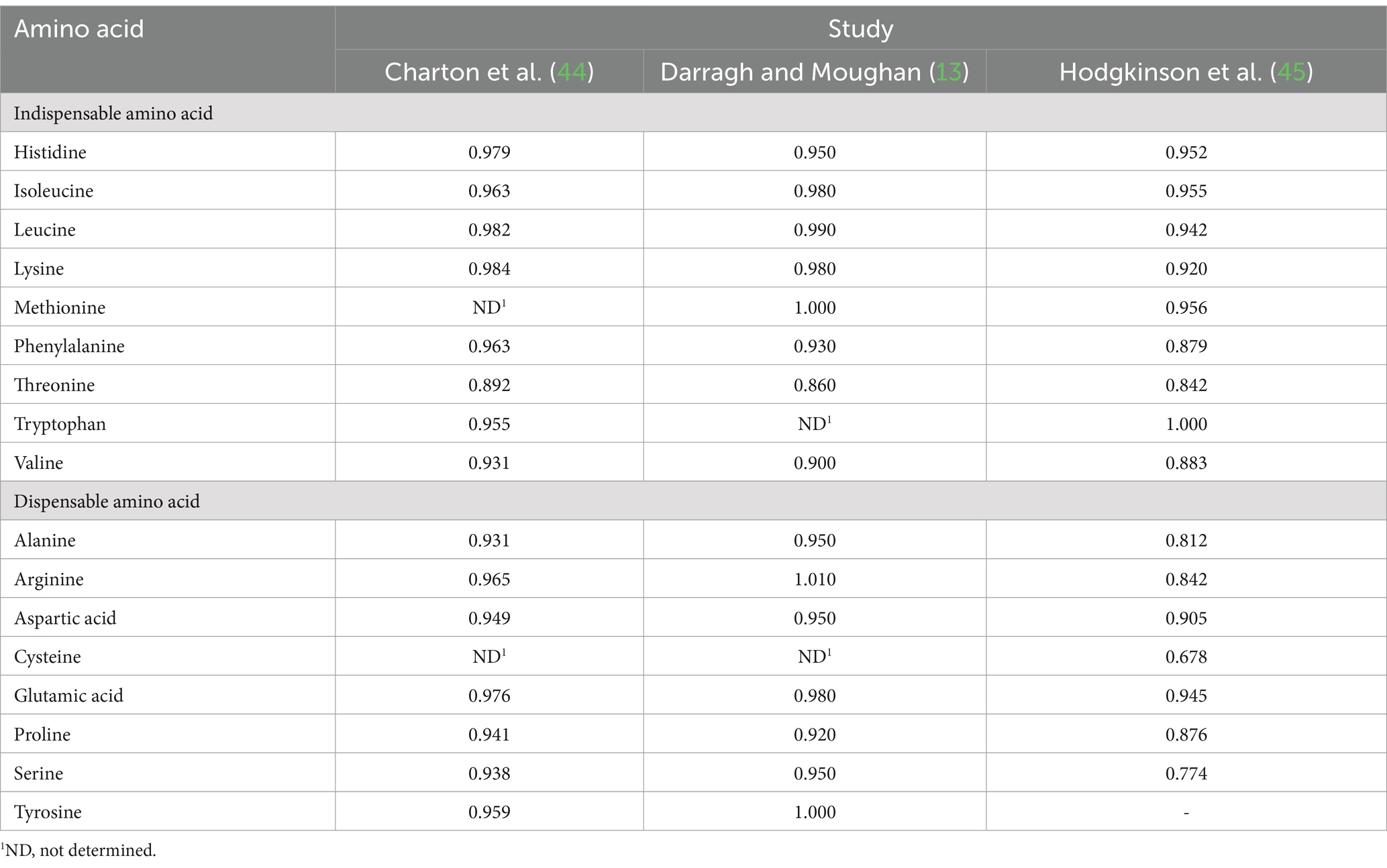
Table 4. Published mean true ileal amino acid digestibility of amino acids in breast milk determined using the piglet as a model for the human infant.
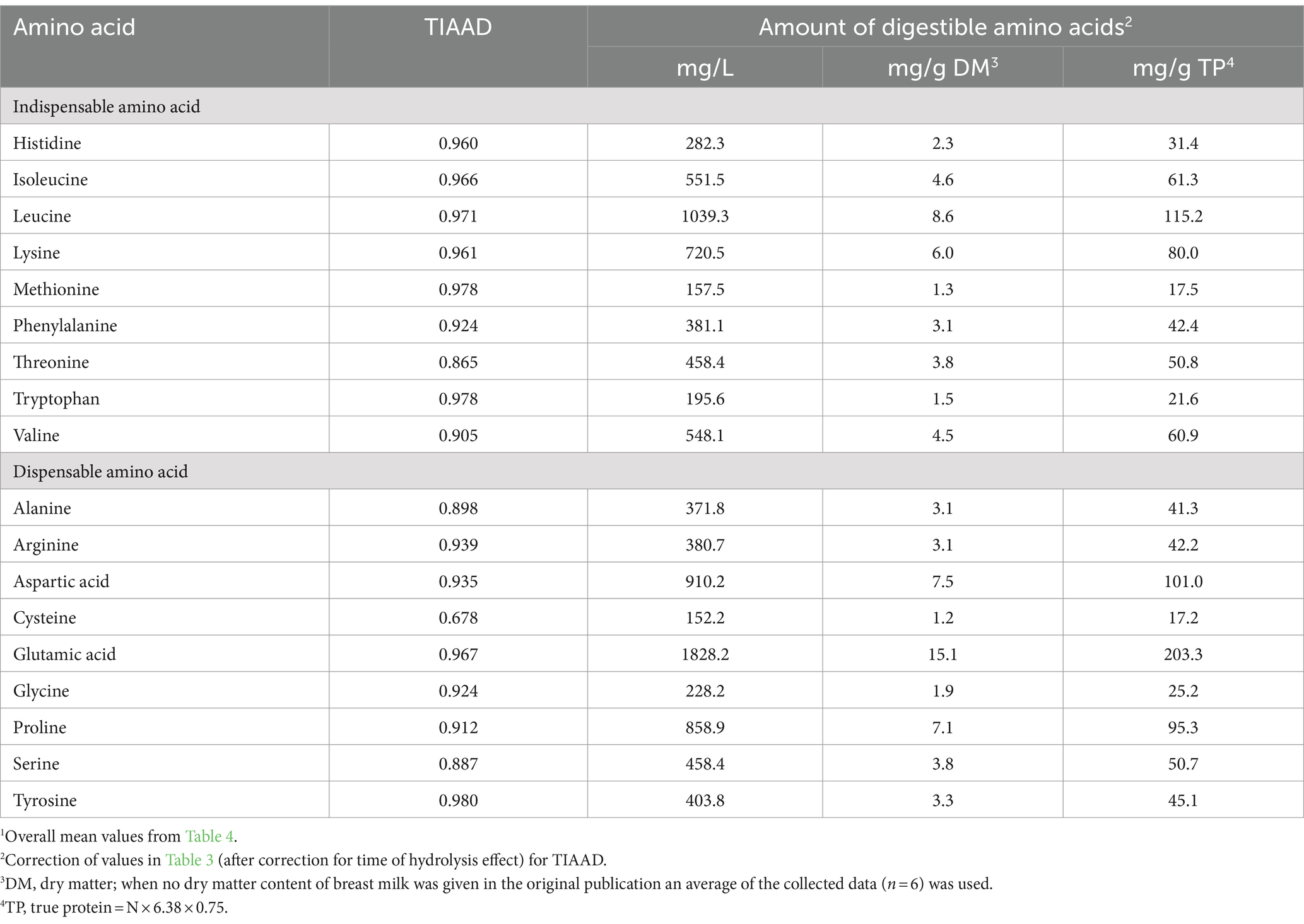
Table 5. True ileal amino acid digestibility coefficients (TIAAD)1 and amounts of true ileal digestible amino acids in human milk presented in different units.
For most of the amino acids, digestibility was high, but for some amino acids, notably cysteine and threonine, true ileal amino acid digestibility was much lower than for the other amino acids. The digestibility of threonine was consistently lower across the three studies, but only one of the studies provided digestibility data for cysteine. Mavromichalis et al. (50) reported high amino acid digestibility for sow’s milk (95–100%), but also found relatively low true ileal digestibility for cysteine and threonine (84%). Although the suckling piglet is a well-accepted animal model for protein digestion in the human infant, there may be differences in digestion (e.g., differences in gut microbial populations), and this should be borne in mind in interpreting the results.
3.4 Comparison with FAO recommendations
The amounts of true ileal digestible amino acids determined from the literature, corrected for multiple hydrolysis time and true ileal digestibility are presented in Table 6 along with the current FAO reference values. The FAO (2) Expert Consultation recommended the amino acid content of breast milk as the current best estimate of amino acid requirements for infants, and gave a recommended amino acid profile for mature human milk based on the deliberations of the FAO/WHO/UNU (1) Expert Consultation. FAO (2) accepted the appropriateness of correcting the total amino acid contents for amino acid digestibility, but did not make the correction at that time as only one published set of values for digestibility was available. The FAO (2) recommended values also relate to only a few older studies on the amino acid composition of human milk (37, 38, 51).

Table 6. Absorbed amino acid composition of human milk based on the present work (Table 5) compared with reference values from FAO (2).1
It is apparent from the values listed in Table 6 that the estimates of amino acid requirements for the human baby as determined after correcting published values for the amino acid content of mature human milk for the effects of hydrolysis time and true ileal amino acid digestibility, are quite different from the most recent FAO recommendations (2). The values presented here and based on the published literature up until 2023, and corrected for the estimated effect of hydrolysis time during amino acid analysis and for true ileal amino acid digestibility, led to higher concentrations (more than 16%) in breast milk for leucine, lysine and threonine and considerably higher values (greater than 30%) for histidine and tryptophan. All of these amino acids play critical roles in infant growth and development (5). A potential limitation of the present data is that although they are based on multiple studies, the hydrolysis correction factors and amino acid digestibility estimates are based on three studies only, and more investigation of these important aspects is required. In addition to the effects of the correction for hydrolysis time and amino acid digestibility, potential differences due to factors such as population (ethnic and nutritional differences), methodology related to milk collection, and advances in amino acid analysis are all undoubtedly important. Nevertheless, the corrected values shown in Table 6 are put forward as the currently most accurate estimates for the absorbed amino acid composition of human breast milk. It would seem appropriate to reassess the FAO international recommendations.
Author contributions
PM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AD: Writing – original draft, Writing – review & editing. YY: Writing – original draft, Writing – review & editing. PW: Writing – original draft, Writing – review & editing. WXJL: Data curation, Resources, Writing – original draft, Writing – review & editing. NS: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SD: Writing - review & editing. IS: Writing - review and editing. SH: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
YY, SD, and IS were employed by Inner Mongolia Dairy Technology Research Institute Co. Ltd. PW was employed by Oceania Dairy Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Joint FAO/WHO/UNU Expert Consultation (2007). Protein and amino acid requirements in human nutrition. Review. Contract No.: 935.
2. Food and Agriculture Organization of the United Nations (FAO). Dietary protein quality evaluation in human nutrition, Report of an FAO Expert Consultation. Rome: FAO (2013).
3. Jonker, R, Engelen, MPKJ, and Deutz, NEP. Role of specific dietary amino acids in clinical conditions. Br J Nutr. (2012) 108:S139–48. doi: 10.1017/S0007114512002358
4. Uauy, R, Kurpad, A, Tano-Debrah, K, Otoo, GE, Aaron, GA, Toride, Y, et al. Role of protein and amino acids in infant and young child nutrition: protein and amino acid needs and relationship with child growth. J Nutr Sci Vitaminol. (2015) 61:S192–4. doi: 10.3177/jnsv.61.S192
6. Rutherfurd, S, and Moughan, P. The chemical analysis of proteins and amino acids In: Feed evaluation science. Wageningen (Netherlands): Wageningen Academic Publishers PJ Moughan and WH Hendriks Editors. (2018). 39–74.
7. Robel, EJ, and Crane, AB. An accurate method for correcting unknown amino acid losses from protein hydrolyzates. Anal Biochem. (1972) 48:233–46. doi: 10.1016/0003-2697(72)90186-8
8. Darragh, AJ, Garrick, DJ, Moughan, PJ, and Hendriks, WH. Correction for amino acid loss during acid hydrolysis of a purified protein. Anal Biochem. (1996) 236:199–207. doi: 10.1006/abio.1996.0157
9. Darragh, AJ, and Moughan, PJ. The effect of hydrolysis time on amino acid analysis. J AOAC Int. (2005) 88:888–93. doi: 10.1093/jaoac/88.3.888
10. Ogra, S, Weintraub, D, and Ogra, PL. Immunologic aspects of human colostrum and milk: III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J Immunol. (1977) 119:245–8. doi: 10.4049/jimmunol.119.1.245
11. Davidson, LA, and Lönnerdal, B. Persistence of human milk proteins in the breast-fed infant. Acta Paediatr. (1987) 76:733–40. doi: 10.1111/j.1651-2227.1987.tb10557.x
12. Prentice, A, MacCarthy, A, Stirling, D, Vasquez-Velasquez, L, and Ceesay, S. Breast-milk IgA and lactoferrin survival in the gastrointestinal tract–a study in rural Gambian children. Acta Paediatr. (1989) 78:505–12. doi: 10.1111/j.1651-2227.1989.tb17928.x
13. Darragh, AJ, and Moughan, PJ. The amino acid composition of human milk corrected for amino acid digestibility. Br J Nutr. (1998) 80:25–34. doi: 10.1017/S0007114598001731
14. Moughan, PJ. Amino acid availability: aspects of chemical analysis and bioassay methodology. Nutr Res Rev. (2003) 16:127–41. doi: 10.1079/NRR200365
15. Moughan, PJ, and Wolfe, RR. Determination of dietary amino acid digestibility in humans. J Nutr. (2019) 149:2101–9. doi: 10.1093/jn/nxz211
16. Moughan, PJ, Birtles, MJ, Cranwell, PD, Smith, WC, and Pedraza, M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev Nutr Diet. (1992) 67:40–113. doi: 10.1159/000419461
17. Puiman, P, and Stoll, B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nut Metabol Care. (2008) 11:601–6. doi: 10.1097/MCO.0b013e32830b5b15
18. Guilloteau, P, Zabielski, R, Hammon, HM, and Metges, CC. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev. (2010) 23:4–22. doi: 10.1017/S0954422410000077
19. Odle, J, Lin, X, Jacobi, SK, Kim, SW, and Stahl, CH. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu Rev Anim Biosci. (2014) 2:419–44. doi: 10.1146/annurev-animal-022513-114158
20. Darragh, AJ, and Moughan, PJ. The three-week-old piglet as a model animal for studying protein digestion in human infants. J Pediatr Gastroenterol Nutr. (1995) 21:387–93. doi: 10.1097/00005176-199511000-00004
21. Wada, Y, Phinney, BS, Weber, D, and Lönnerdal, B. In vivo digestomics of milk proteins in human milk and infant formula using a suckling rat pup model. Peptides. (2017) 88:18–31. doi: 10.1016/j.peptides.2016.11.012
22. Cheung, MW, Pratt, EL, and Fowler, DI. Total amino acid composition in mature human milk; analysis by the ion exchange resin column chromatographic technic. Pediatrics. (1953) 12:353–7.
23. Lonnerdal, B, Forsum, E, and Hambraeus, L. The protein content of human milk. I. A transversal study of Swedish normal material. Nut Rep Int. (1976) 13:125–35.
24. Svanberg, U, Gebre-Medhin, M, Ljungqvist, B, and Olsson, M. Breast milk composition in Ethiopian and Swedish mothers III. Amino acids and other nitrogenous substances. Am J Clin Nutr. (1977) 30:499–507. doi: 10.1093/ajcn/30.4.499
25. Lauber, E, and Reinhardt, M. Studies on the quality of breast milk during 23 months of lactation in a rural community of the Ivory Coast. Am J Clin Nutr. (1979) 32:1159–73. doi: 10.1093/ajcn/32.5.1159
26. Atkinson, SA. (1982). Human milk feeding of premature infants <1.3 kg birthweight: milk analysis and clinical studies during early postnatal life. elibrary.RU.
27. Chavalittamrong, B, Suanpan, S, Boonvisut, S, Chatranon, W, and Gershoff, SN. Protein and amino acids of breast milk from Thai mothers. Am J Clin Nutr. (1981) 34:1126–30. doi: 10.1093/ajcn/34.6.1126
28. Oppe, T. (1977). The composition of mature human milk-report of a working Party of the Committee on medical aspects of food policy. Food and Agriculture Organization of the United Nations.
29. Harzer, G, Haug, M, and Bindels, JG. Biochemistry of maternal milk in early lactation. Hum Nutr Appl Nutr. (1986) 40:11–8.
30. Lonnerdal, B, and Forsum, E. Casein content of human milk. Am J Clin Nutr. (1985) 41:113–20. doi: 10.1093/ajcn/41.1.113
31. Janas, LM, Picciano, MF, and Hatch, TF. Indices of protein metabolism in term infants fed either human milk or formulas with reduced protein concentration and various whey/casein ratios. J Pediatr. (1987) 110:838–48. doi: 10.1016/S0022-3476(87)80394-3
32. Janas, LM, and Picciano, MF. Quantities of amino acids ingested by human milk-fed infants. J Pediatr. (1986) 109:802–7. doi: 10.1016/S0022-3476(86)80697-7
33. Donovan, SM, and Lonnderdal, B. Non-protein nitrogen and true protein in infant formulas. Acta Paediatr Scand. (1989) 78:497–504. doi: 10.1111/j.1651-2227.1989.tb17927.x
34. Yonekubo, A, Onoda, T, Fumikura, M, Fudohta, K, and Yamamoto, Y. Total and free amino acid compositions of human milk in Japan. J Jap Soc Nut Food Sci. (1989) 42:194–7. doi: 10.4327/jsnfs.42.194
35. Zhao, X, Xu, Z, Wang, Y, and Sun, Y. Studies of the relation between the nutritional status of lactating mothers and milk composition as well as the milk intake and growth of their infants in Beijing. Pt. 4. The protein and amino acid content of breast milk. Ying Yang Xue Bao. (1989) 11:227–32.
36. Bellomonte, G, Boniglia, C, Carratù, B, Filesi, C, Giammarioli, S, Mosca, M, et al. Protein and lipid composition of human milk and infant formulas: comparison and nutritional consequences. Ann Ist Super Sanita. (1990) 26:131–9.
37. Davis, TA, Nguyen, HV, Garcia-Bravo, R, Fiorotto, ML, Jackson, EM, and Reeds, PJ. Amino acid composition of the milk of some mammalian species changes with stage of lactation. Br J Nutr. (1994) 72:845–53. doi: 10.1079/BJN19940089
38. Villalpando, S, Butte, NF, Flores-Huerta, S, and Thotathuchery, M. Qualitative analysis of human milk produced by women consuming a maize-predominant diet typical of rural Mexico. Ann Nutr Metab. (1998) 42:23–32. doi: 10.1159/000012714
39. Wu, TC, Chuang, CC, Lau, BH, Hwang, B, Sugawara, M, and Idota, T. Crude protein content and amino acid composition in Taiwanese human milk. J Nutr Sci Vitaminol. (2000) 46:246–51. doi: 10.3177/jnsv.46.246
40. Yamawaki, N, Yamada, M, Kan-no, T, Kojima, T, Kaneko, T, and Yonekubo, A. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med Biol. (2005) 19:171–81. doi: 10.1016/j.jtemb.2005.05.001
41. Feng, P, Gao, M, Holley, T, Zhou, T, Burgher, A, Trabulsi, J, et al. Amino acid composition and protein content of mature human milk from nine countries. FASEB J. (2009) 23:LB448-LB. doi: 10.1096/fasebj.23.1_supplement.LB448
42. Garcia-Rodenas, CL, Affolter, M, Vinyes-Pares, G, De Castro, CA, Karagounis, LG, Zhang, Y, et al. Amino acid composition of breast milk from urban chinese mothers. Nutrients. (2016) 8:606. doi: 10.3390/nu8100606
43. van Sadelhoff, JHJ, van de Heijning, BJM, Stahl, B, Amodio, S, Rings, EHHM, Mearin, ML, et al. Longitudinal variation of amino acid levels in human milk and their associations with infant gender. Nutrients. (2018) 10:1233. doi: 10.3390/nu10091233
44. Charton, E, Henry, G, Cahu, A, Le Gouar, Y, Dahirel, P, Moughan, PJ, et al. Ileal digestibility of nitrogen and amino acids in human milk and an infant formula as determined in neonatal minipiglets. J Nutr. (2023) 153:1063–74. doi: 10.1016/j.tjnut.2023.02.025
45. Hodgkinson, SM, Xiong, X, Yan, Y, Wu, Y, Szeto, IMY, Li, R, et al. An accurate estimate of the amino acid content of human milk collected from Chinese women adjusted for differences in amino acid digestibility. J Nutr. (2023) 153:3439–47. doi: 10.1016/j.tjnut.2023.10.009
46. Close, J, and Van De Walle, A. Composition of the milk of women in the Belgian Congo. III. Total amino acids of transitional milk. Ann Soc Belg Med Trop. (1957) 37:213–24.
47. Ren, Q, Sun, H, Zhao, M, Xu, Y, Xie, Q, Jiang, S, et al. Longitudinal changes in crude protein and amino acids in human milk in Chinese population: a systematic review. J Pediatr Gastroenterol Nutr. (2020) 70:555–61. doi: 10.1097/MPG.0000000000002612
48. Zhang, Z, Adelman, AS, Rai, D, Boettcher, J, and Lőnnerdal, B. Amino acid profiles in term and preterm human milk through lactation: a systematic review. Nutrients. (2013) 5:4800–21. doi: 10.3390/nu5124800
49. Lönnerdal, B, Erdmann, P, Thakkar, SK, Sauser, J, and Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: a developmental perspective. J Nutr Biochem. (2017) 41:1–11. doi: 10.1016/j.jnutbio.2016.06.001
50. Mavromichalis, I, Parr, TM, Gabert, VM, and Baker, DH. True ileal digestibility of amino acids in sow's milk for 17-day-old pigs. J Anim Sci. (2001) 79:707–13. doi: 10.2527/2001.793707x
51. Heine, WE, Klein, PD, and Reeds, PJ. The importance of α-lactalbumin in infant nutrition. J Nutr. (1991) 121:277–83. doi: 10.1093/jn/121.3.277
52. Atkinson, SA, Anderson, GH, and Bryan, MH. Human milk: comparison of the nitrogen composition in milk from mothers of premature and full-term infants. Am J Clin Nutr. (1980) 33:811–5. doi: 10.1093/ajcn/33.4.811
53. Beach, EF, Bernstein, SS, and Macy, IG. Intake of amino acids by breast-milk-fed infants and amino acid composition of cow's milk and human milk. J Pediatr. (1941) 19:190–200. doi: 10.1016/S0022-3476(41)80061-4
54. Block, RJ, and Bolling, D. The amino acid composition of cow and human milk proteins. Arch Biochem. (1946) 10:359–63.
55. Chathyushya, K, Hemalatha, R, Ananthan, R, Devraj, J, Banjara, SK, Alimelu, M, et al. Macronutrient composition of term and preterm human milk of different socio economic groups. Prostaglandins Leukot Essent Fat Acids. (2023) 192:102571. doi: 10.1016/j.plefa.2023.102571
56. Close, J, and Van De Walle, A. Composition of human milk in Belgium Congo. IV. Total amino acids of complete secretion. Ann Soc Belg Med Trop. (1958) 38:907–19.
57. Darling, PB, Dunn, M, Sarwar, G, Brookes, S, Ball, RO, and Pencharz, PB. Threonine kinetics in preterm infants fed their mothers' milk or formula with various ratios of whey to casein. Am J Clin Nutr. (1999) 69:105–14. doi: 10.1093/ajcn/69.1.105
58. Darling, PB. Threonine and phenylalanine metabolism in the human neonate Toronto, Ontario: University of Toronto (1997).
59. Davis, TA, Fiorotto, ML, and Reeds, PJ. Amino acid compositions of body and milk protein change during the suckling period in rats. J Nutr. (1993) 123:947–56. doi: 10.1093/jn/123.5.947
60. DeSantiago, S, Ramírez, I, Tovar, AR, Ortíz, N, Torres, N, and Bourges, H. Amino acid profiles in diet, plasma and human milk in Mexican rural lactating women. Nutr Res. (1999) 19:1133–43. doi: 10.1016/S0271-5317(99)00074-3
61. Faus, O, López Morales, J, Faus, MJ, Periago, JL, Bueno Sánchez, A, Gil, A, et al. Free amino acid content of human milk in Spain. An Esp Pediatr. (1984) 21:557–63.
62. Feng, P, Gao, M, Burgher, A, Hui Zhou, T, and Pramuk, K. A nine-country study of the protein content and amino acid composition of mature human milk. Food Nutr Res. (2016) 60:31042. doi: 10.3402/fnr.v60.31042
63. Ferreira, I. Quantification of non-protein nitrogen components of infant formulae and follow-up milks: comparison with cows' and human milk. Br J Nutr. (2003) 90:127–33. doi: 10.1079/BJN2003882
64. Filippova, G, and Aronova, B. Amino acid composition of human milk at different periods of lactation and effect on it of hedge nettle extract administered in the early puerperal. Zdravookhr Kirg. (1974) 6:24–6.
65. Giuffrida, F, Austin, S, Cuany, D, Sanchez-Bridge, B, Longet, K, Bertschy, E, et al. Comparison of macronutrient content in human milk measured by mid-infrared human milk analyzer and reference methods. J Perinatol. (2019) 39:497–503. doi: 10.1038/s41372-018-0291-8
66. Guo, H, Pang, K, Zhang, X, Zhao, L, Chen, S, Dong, M, et al. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J Dairy Sci. (2007) 90:1635–43. doi: 10.3168/jds.2006-600
67. Hanning, RM, Paes, B, and Atkinson, SA. Protein metabolism and growth of term infants in response to a reduced-protein, 40:60 whey: casein formula with added tryptophan. Am J Clin Nutr. (1992) 56:1004–11. doi: 10.1093/ajcn/56.6.1004
68. Jarvenpaa, A-L, Rassin, DK, Räihä, NC, and Gaull, GE. Milk protein quantity and quality in the term infant II. Effects on acidic and neutral amino acids. Pediatrics. (1982) 70:221–30. doi: 10.1542/peds.70.2.221
69. Lemons, JA, Reyman, D, and Moye, L. Amino acid composition of preterm and term breast milk during early lactation. Early Hum Dev. (1983) 8:323–9.
70. Macy, IG. Composition of human colostrum and milk. Am J Dis Child. (1911) 78:589–603. doi: 10.1001/archpedi.1949.02030050604009
71. Macy, IG, and Kelly, HJ. Human milk and cow's milk in infant nutrition In: Milk: the mammary gland and its secretion : Elsevier (1961). 265–304.
72. Miller, S, Ruttinger, V, Rutledge, MM, Frahm, R, Maurer, S, Moyer, EZ, et al. Human milk studies: XXVII. Essential amino acids in human colostrum and transitional milk. J Nutr. (1950) 40:499–514. doi: 10.1093/jn/40.4.499
73. Mitton, SG, and Garlick, PJ. Changes in protein turnover after the introduction of parenteral nutrition in premature infants: comparison of breast milk and egg protein-based amino acid solutions. Pediatr Res. (1992) 32:447–54. doi: 10.1203/00006450-199210000-00015
74. Moya-Alvarez, V, Eussen, SRBM, Mank, M, Koyembi, JCJ, Nyasenu, YT, Ngaya, G, et al. Human milk nutritional composition across lactational stages in Central Africa. Frontiers. Nutrition. (2022) 9:9. doi: 10.3389/fnut.2022.1033005
75. Motil, KJ, Thotathuchery, M, Bahar, A, and Montandon, CM. Marginal dietary protein restriction reduced nonprotein nitrogen, but not protein nitrogen, components of human milk. J Am Coll Nutr. (1995) 14:184–91. doi: 10.1080/07315724.1995.10718492
76. Nagasawa, T, Kiyosawa, I, and Kuwahara, K. Acrylamide gel electrophoresis and amino acid compositions of human Colostral casein. J Dairy Sci. (1970) 53:92–4. doi: 10.3168/jds.S0022-0302(70)86154-9
77. Nayman, R, Thomson, ME, Scriver, CR, and Clow, CL. Observations on the composition of milk-substitute products for treatment of inborn errors of amino acid metabolism. Comparisons with human milk. A proposal to rationalize nutrient content of treatment products. Am J Clin Nutr. (1979) 32:1279–89. doi: 10.1093/ajcn/32.6.1279
78. Nwachoko, N, Akuru, UB, Odejayi, FM, and Tetam, JG. Amino acids and carbohydrates composition in breast milk of lactating mothers of different age group from Aleto Health Center, Rivers State. Afr J Biochem Res. (2022) 16:19–23. doi: 10.5897/AJBR2021.1132
79. Pang, J, Liu, Z, Jia, N, Li, J, Pei, C, Mi, L, et al. Longitudinal study of protein content and amino acid composition of breast milk at different lactation stages from southern and northern urban Chinese mothers. Food Sci. (2019) 40:167–74.
80. Picone, TA, Benson, JD, Moro, G, Minoli, I, Fulconis, F, Rassin, DK, et al. Growth, serum biochemistries, and amino acids of term infants fed formulas with amino acid and protein concentrations similar to human milk. J Pediatr Gastroenterol Nutr. (1989) 9:351–60. doi: 10.1097/00005176-198910000-00015
81. Purkiewicz, A, Stasiewicz, M, Nowakowski, JJ, and Pietrzak-Fiećko, R. The influence of the lactation period and the type of Milk on the content of amino acids and minerals in human milk and infant formulas. Food Secur. (2023) 12:3674. doi: 10.3390/foods12193674
82. Räihä, NCR, Fazzolari-Nesci, A, Cajozzo, C, Puccio, G, Monestier, A, Moro, G, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr. (2002) 35:275–81. doi: 10.1097/00005176-200209000-00008
83. Rassin, DK, Gaull, GE, Heinonen, K, and Raiha, NCR. Milk protein quantity and quality in low birth weight infants: II. Effects on selected aliphatic amino acids in plasma and urine. Pediatrics. (1977) 59:407–22. doi: 10.1542/peds.59.3.407
84. Renner, E. (1983). Protein quality in relation to estimates of essential amino acid requirements. W-Gmbh Volkswirtschaftlicher, Verlag München. p. 90–130.
85. Rigo, J, Salle, BL, Cavero, E, Richard, P, Putet, G, and Senterre, J. Plasma amino acid and protein concentrations in infants fed human milk or a whey protein hydrolysate formula during the first month of life. Acta Paediat Int J Paediat. (1994) 83:127–31. doi: 10.1111/j.1651-2227.1994.tb13033.x
86. Saben, JL, Sims, CR, Pack, L, Lan, R, Børsheim, E, and Andres, A. Infant intakes of human milk branched chain amino acids are negatively associated with infant growth and influenced by maternal body mass index. Pediatr Obes. (2022) 17:e12876. doi: 10.1111/ijpo.12876
87. Saito, K, Furuichi, E, Kondo, S, Kawanishi, G, Nishikawa, I, Nakazato, H, et al. (1965). Studies on human milk. Snow Brand Products; Tokyo, Japan:1–84.
88. Sarwar, G, Darling, P, Ujiie, M, Botting, HG, and Pencharz, PB. Use of amino acid profiles of pretermand term human milks in evaluating scoring patterns for routine protein quality assessment of infant formulas. J AOAC Int. (1996) 79:498–502. doi: 10.1093/jaoac/79.2.498
89. Scott, P, Sandham, S, Balmer, S, and Wharton, B. Diet-related reference values for plasma amino acids in newborns measured by reversed-phase HPLC. Clin Chem. (1990) 36:1922–7. doi: 10.1093/clinchem/36.11.1922
90. Shaikhiev, A. (1980). Amino acid composition of human milk during the first month of lactation. Moscow: Publishing house “Medicine”.
91. Soupart, P, Moore, S, and Bigwood, EJ. Amino acid composition of human milk. J Biol Chem. (1954) 206:699–704. doi: 10.1016/S0021-9258(19)50839-0
92. Tarján, R, Krámer, M, Szöke, K, Lindner, K, Szarvas, T, and Dworschák, R. The effect of different factors on the composition of human Milk. II. The composition of human milk during lactation: Wirkung verschiedencr Faktoren auf die Zusammensetzung der menschlichen Milch II. Zusammensetzung der menschlichen Milch wahrcnd der Stillperiode: Effet de différents facteurs Sur la composition du Iait humain II. Composition du Iait humain pendant la lactation. Ann Nutr Metab. (1965) 7:136–54. doi: 10.1159/000175095
93. Tikanoja, T, Simell, O, Järvenpää, A-L, and Räihä, NC. Plasma amino acids in preterm infants after a feed of human milk or formula. J Pediatr. (1982) 101:248–52. doi: 10.1016/S0022-3476(82)80134-0
94. Van Sadelhoff, JH, Mastorakou, D, Weenen, H, Stahl, B, Garssen, J, and Hartog, A. Differences in levels of free amino acids and total protein in human foremilk and hindmilk. Nutrients. (2018) 10:1828. doi: 10.3390/nu10121828
95. Volz, VR, Book, LS, and Churella, HR. Growth and plasma amino acid concentrations in term infants fed either whey-predominant formula or human milk. J Pediatr. (1983) 102:27–31. doi: 10.1016/S0022-3476(83)80281-9
96. Wei, M, Deng, Z, Liu, B, Ye, W, Fan, Y, Liu, R, et al. Investigation of amino acids and minerals in Chinese breast milk. J Sci Food Agric. (2020) 100:3920–31. doi: 10.1002/jsfa.10434
97. Williamson, MB. The amino acid composition of human milk proteins. J Biol Chem. (1944) 156:47–52. doi: 10.1016/S0021-9258(17)41673-5
Keywords: breast milk, human milk, human milk protein, indispensable amino acid, infant nutrition, lactation, protein hydrolysis, true ileal amino acid digestibility
Citation: Moughan PJ, Deglaire A, Yan Y, Wescombe P, Lim WXJ, Stroebinger N, Duan S, Szeto IM-Y and Hodgkinson S (2024) Amino acid requirements of the infant: the amino acid composition of human breast milk. Front. Nutr. 11:1446565. doi: 10.3389/fnut.2024.1446565
Edited by:
Hércules Rezende Freitas, Federal University of Rio de Janeiro, BrazilReviewed by:
Cristine Couto Almeida, Oswaldo Cruz Foundation (Fiocruz), BrazilLijun Chen, Beijing Sanyuan Foods Co., Ltd., China
Copyright © 2024 Moughan, Deglaire, Yan, Wescombe, Lim, Stroebinger, Duan, Szeto and Hodgkinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul J. Moughan, cC5qLm1vdWdoYW5AbWFzc2V5LmFjLm56
 Paul J. Moughan
Paul J. Moughan Amelie Deglaire
Amelie Deglaire Yalu Yan
Yalu Yan Philip Wescombe4,5
Philip Wescombe4,5 Wen Xin Janice Lim
Wen Xin Janice Lim Natascha Stroebinger
Natascha Stroebinger Suzanne Hodgkinson
Suzanne Hodgkinson