- 1The First Clinical College, Liaoning University of Traditional Chinese Medicine, Shenyang, China
- 2The Second Clinical College, Liaoning University of Traditional Chinese Medicine, Shenyang, China
- 3Department of Nephropathy, The First Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang, China
Objectives: Observational studies have identified an association between dried fruit intake and kidney function. However, these studies have limitations such as vulnerability to confounders and reverse causality bias. Therefore, this study aimed to explore the potential causal relationship between dried fruit intake and kidney function.
Methods: A two-sample Mendelian randomization (MR) study was conducted using a large-scale genome-wide association study dataset to investigate the causal relationship between dried fruit intake and kidney function markers (blood urea nitrogen (BUN), creatinine (CR), uric acid (UA), cystatin C (CyC), hematuria, microalbuminuria). The main analytical method was inverse variance weighting. In addition, we applied the MR Egger and weighted median to assess the robustness of the results. Finally, Multivariate Mendelian randomization (MVMR) was used to estimate the direct effect of dried fruit intake on kidney function markers.
Results: The univariate MR analysis showed that increased dried fruit intake was associated with lower kidney function markers, including BUN (β: −0.171, 95% confidence interval (CI): −0.239 to −0.102, p = 1.063 × 10−6), CR (β: −0.205, 95% CI: −0.311 to −0.099, p = 1.455 × 10−4), UA (β = −0.317, 95% CI: −0.384 to −0.249, p = 4.439 × 10−20), and CysC (β = −0.323, 95% CI: −0.384 to −0.249, p = 1.074 × 10−11); however, it was unrelated to hematuria and microalbuminuria. Causality persisted after performing MVMR analysis; however, with the addition of alcohol consumption and smoking as exposure factors, the causality for UA (β = −0.296, 95% CI: −0.523 to −0.068, p = 1.094 × 10−2) and CysC (β = −0.238, 95% CI: −0.465 to −0.011, p = 4.024× 10−2) weakened, while the causality for BUN (β = −0.038, 95% CI: −0.215 to 0.138, p = 6.698 × 10−1) and CR (β = −0.038, 95% CI: −0.431 to 0.046, p = 1.347 × 10−1) disappeared.
Conclusion: Increased dried fruit intake was associated with lower kidney function markers (BUN, CR, UA, and CysC) in the absence of smoking and alcohol consumption; however, the causal relationship between dried fruit intake and BUN and CR disappeared in the presence of smoking and alcohol consumption. These results provide a promising avenue for delaying the course of chronic kidney disease.
1 Introduction
Patients with chronic kidney failure are often required to undergo treatments such as dialysis or kidney transplantation, which poses a huge global health challenge (1). According to the 2020 World Health Organization Global Health Estimates, kidney disease is among the top 10 causes of death worldwide (2). In 2024, the Global Kidney Health Atlas by the International Society of Nephrology reported that the global incidence of kidney failure is approximately 146 per million population per year (1). Currently, the main tests for kidney function include creatinine (CR), blood urea nitrogen (BUN), uric acid (UA), cystatin C (CysC), hematuria, microalbuminuria, etc., which represent the metabolic function and filtration function of the kidneys. Kidney disease progression is often associated with infections, inflammatory responses, and oxidative stress due to a weakened immune system throughout the body (3, 4). Furthermore, other acquired factors can also lead to progressive deterioration of kidney function markers, for example, hypertension, hyperlipidemia, and a hypercoagulable state (5). Current nutritional studies of kidney disease have identified an intrinsic relationship between dietary influences and the risk of progression of kidney function markers, which can be reduced by controlling the relevant factors (6).
The Mediterranean diet consists of a diet rich in vegetables, fruits, legumes, nuts, moderate amounts of poultry and seafood, very little red meat, and sweets (7). An article suggested that the Mediterranean dietary pattern improves lipid profiles in kidney transplant recipients and may help slow kidney failure in patients with chronic kidney disease (CKD) (8). Due to its healthy nutrient composition, it can benefit the intestinal microflora in various ways, thus ensuring the normal function of the intestinal barrier, in addition to having a beneficial effect on blood pressure (9–11). There is sufficient evidence to suggest that adherence to the Mediterranean diet is beneficial for patients with CKD, slowing the disease progression and reducing the likelihood of complications (12). Owing to the lack of preservation techniques in ancient times, a series of fruits, such as apricots and grapes, which are prone to decay, were often preserved by drying (13). Various previous studies have demonstrated that dried fruits have anti-inflammatory, antioxidant, and immune-boosting effects (14, 15); however, no cause-and-effect relationships have been documented, and there are no large-scale, multicenter, prospective studies to validate the benefits of dried fruit intake on the kidney function markers. Dried fruits are one dietary option available; however, many other dietary factors may have this effect.
Mendelian randomization (MR) employs genetic variation as an instrumental variable and capitalizes on the attributes of genetic variation to mitigate the impact of confounders and reverse causal bias.MR allows for the assessment of causal inference between exposure and outcome, opening new directions in the design and cost-effectiveness of clinical trials, and laying the groundwork for further research (16). MR of genetic variation as an instrumental variable analysis (IVs) avoids confounding factors and reverse causation on experimental results (17).
In this study, we focused on the causal relationship between dried fruit intake and kidney function markers (including CR, BUN, UA, CysC, hematuria, and microalbuminuria), to provide a scientific basis for delaying the course of CKD.
2 Materials and methods
2.1 Study design
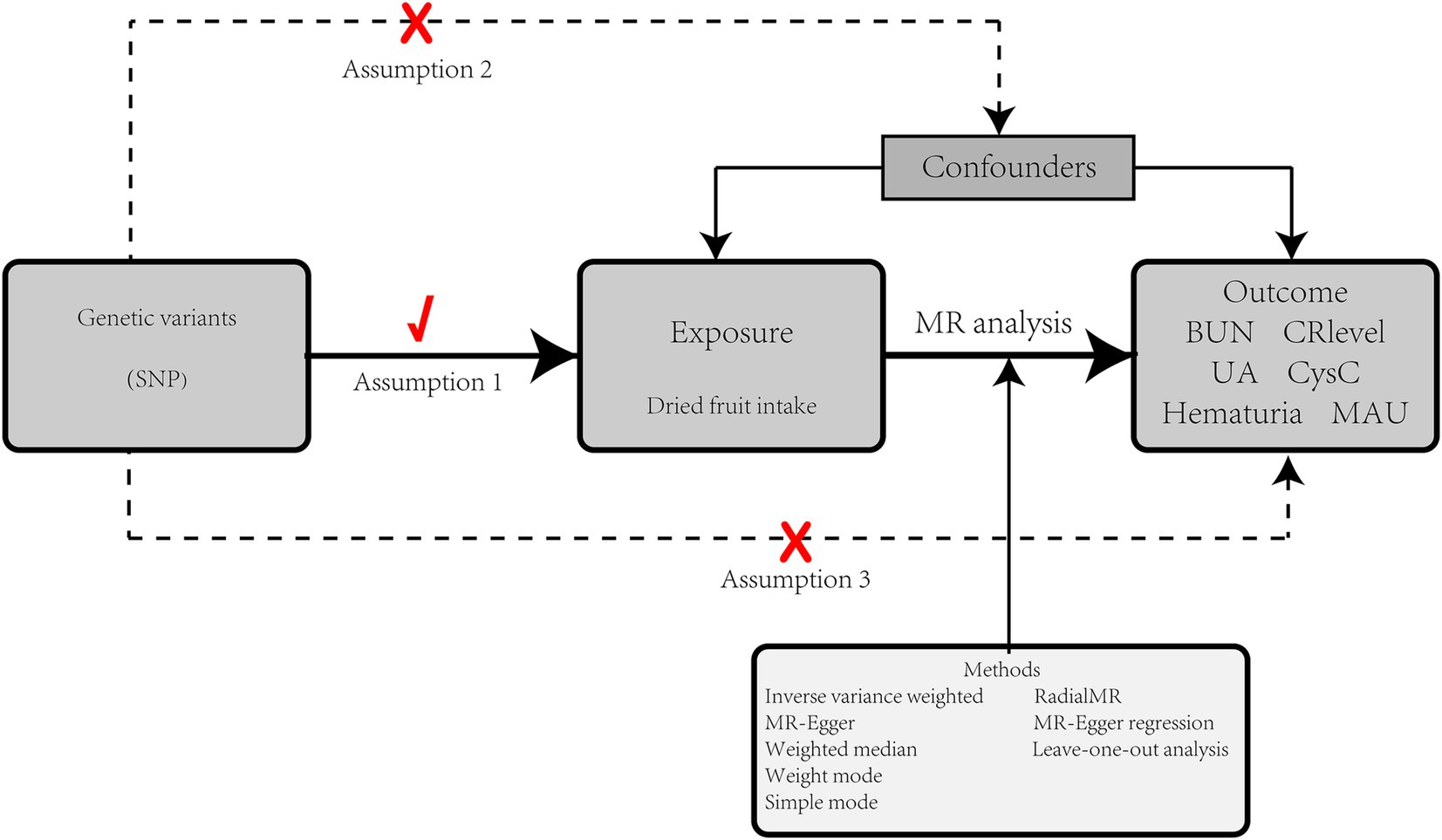
We used two-sample MR analyses to investigate the relationship between dried fruit intake and kidney function markers. Multivariate Mendelian randomization (MVMR) was then used to analyze whether confounders would have an impact on the study results. Correct causal inferences are subject to the following conditions: (1) genetic instrumental variables should be highly correlated with exposure factors; (2) IVs are not associated with any possible confounders; and (3) IVs can influence the results only through exposure factors (16). The GWAS data involved in this study has been ethically approved (18–22). The study design is shown in Figure 1.
2.2 Data sources
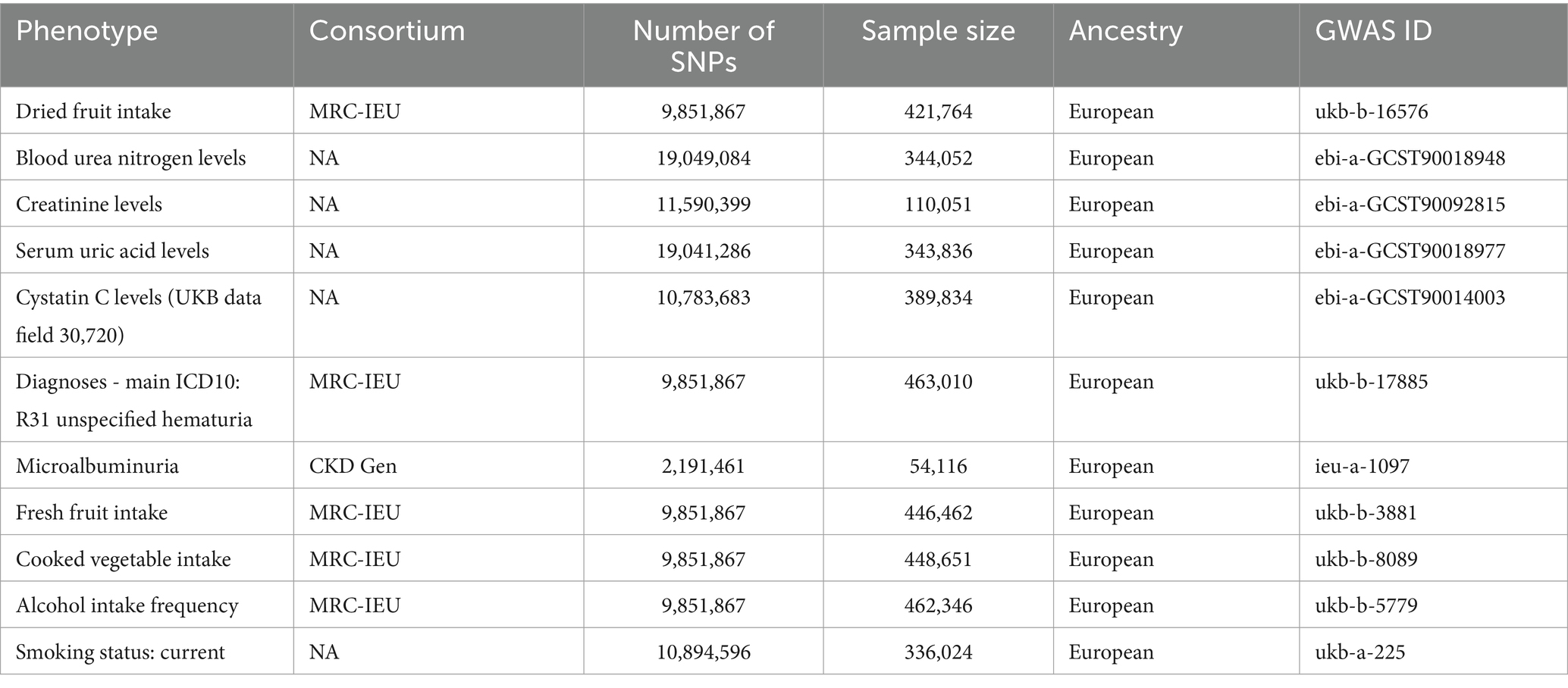
The data involved in this study were obtained from the open GWAS database.1 Dried fruit intake was measured by a questionnaire entitled: “About how many pieces of dried fruit would you eat per day?” (count one prune, one dried apricot, and 10 raisins as one piece; if you do not eat any raisins, write “0”). The following check was performed during the collection process: if the answer was >100, the answer was invalid. Kidney function markers were derived from the following datasets for BUN, CR, UA, and CysC levels (UKB data field 30,720), diagnoses—main ICD10: R31 unspecified hematuria, and microalbuminuria. The MVMR analysis used fresh fruit intake (23), cooked vegetable intake (24), smoking status: current (25), and alcohol intake frequency (26) as confounders to further validate the relationship between dried fruit intake and kidney function markers. The specifics of the dataset are detailed in Table 1.
2.3 IV selection
All genetic IVs involved in this study have a threshold of significance (p < 5 × 10−8) with respect to dry fruit intake; possible linkage disequilibrium was removed (r2 < 0.001, kb = 10,000). In addition, we applied the F-value (F = β2/se2) to assess the strength of each IV (27), with F > 10 indicating no weak instrumental bias. We obtained 43 single nucleotide polymorphisms (SNPs). Data are detailed in Supplementary Table S1.
2.4 Statistical analysis
Our main analytical method was inverse variance weighting (IVW), which is characterized by regressions that do not take into account the presence of an intercept term and are fitted with the inverse of the ending variance as weights (28). The advantage of this method is that it has a strong causality detection capability, which can effectively reduce bias and improve the accuracy of estimation, and the method can summarize the effects of multiple loci when dealing with multiple SNPs, thus providing more comprehensive results. The MR-Egger method is similar to the IVW method, but its regression takes into account the presence of the intercept term (29). When the above analysis is used in the MR analysis, the weighted median (WM) is more accurate in some cases after the results are validated (30). The weighting model weighs the effect of each SNP for further analysis (31).
For multi-effect outliers, we applied the Radial MR test and removed outliers (32). For horizontal pleiotropy, we used the MR-Egger intercept test. Cochran’s Q test was used to identify heterogeneity in the IVW analysis. For directional pleiotropy, we used funnel plot assessment. To determine whether each individual SNP was accountable for causal associations, we applied the leave-one-out method. To completely remove any SNP associated with potential confounders, we utilized the LDlink website (2last accessed on May 21st, 2024) and searched for all rsID after examining the phenotypes to remove any possible confounders. Based on the dietary recommendations mentioned in the Dietary Guidelines for Americans (DGA), 2020–2025, and a Meta-analysis of Lifestyles of Patients with Chronic Kidney Disease (33, 34). We selected several indicators, including fruit intake, cooked vegetable intake, smoking status: current, and frequency of alcohol intake. We selected several indicators, including fruit intake, cooked vegetable intake, smoking status: current, and alcohol intake frequency, and utilized MVMR analyses to further explore the effects of dried fruit intake on kidney function markers. All of the above analyses were statistically analyzed using R software (4.3.1) via “Two Sample MR” (version 0.5.11) and the “Radial MR” (version 1.0) (32) packages.
3 Results
3.1 Univariate MR results
A total of 43 SNPs were analyzed as IVs for MR in this study. We applied the Radial MR test to identify multi-effect outliers, and the results are detailed in Supplementary Figure S1.
After IVW, the results showed that a lower BUN (p = 1.063 × 10−6), CR level (p = 1.455 × 10−4), UA (p = 4.439 × 10−20), and CysC (p = 1.074 × 10−11) had a statistically significant association with dried fruit intake, with no association found for hematuria (p = 1.397 × 10−1) or microalbuminuria (p = 5.128 × 10−1; Supplementary Table S2; Supplementary Figures S2–S7).
After applying LDlink (last accessed on May 21, 2024) to remove SNPs associated with other shapes (Supplementary Table S3), we found that dried fruit intake was associated with lower levels of BUN (β: −0.161, 95% CI: −0.235 to −0.087, p = 2.010 × 10−5), CR level (β: −0.194, 95% CI: −0.310 to −0.077, p = 1.135 × 10−3), UA (β: −0.326, 95% CI: −0.396 to −0.255, p = 1.894 × 10−19), and CysC (β: −0.337, 95% CI: −0.429 to −0.244, p = 8.227 × 10−13) (Table 2). The results were not heterogeneous or horizontally pleiotropic (p > 0.05; Supplementary Table S4). The funnel plot results show that the pattern is essentially symmetrical, proving to be unaffected by potential bias. The leave-one-out plot indicates that the result is not affected by individual variations (Figures 2–5).
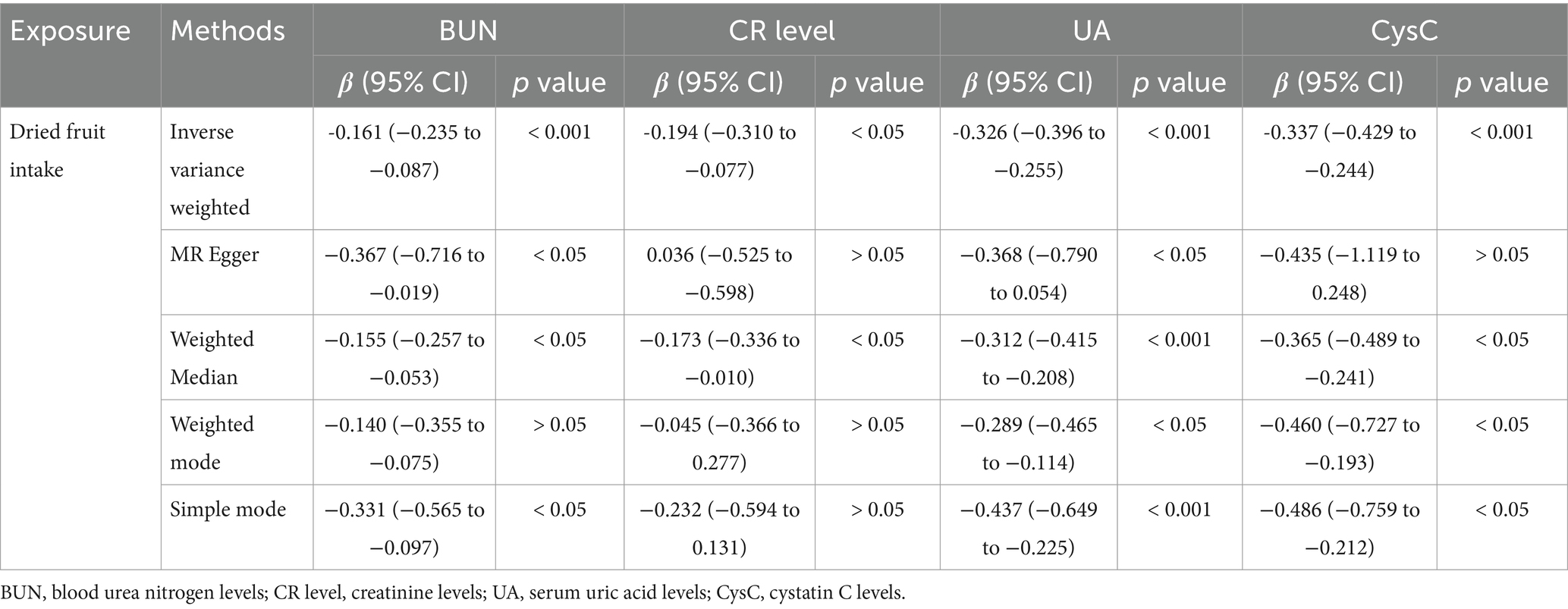
Table 2. MR estimates of the causal association between dried fruit intake on BUN, CR, UA, and CysC level.
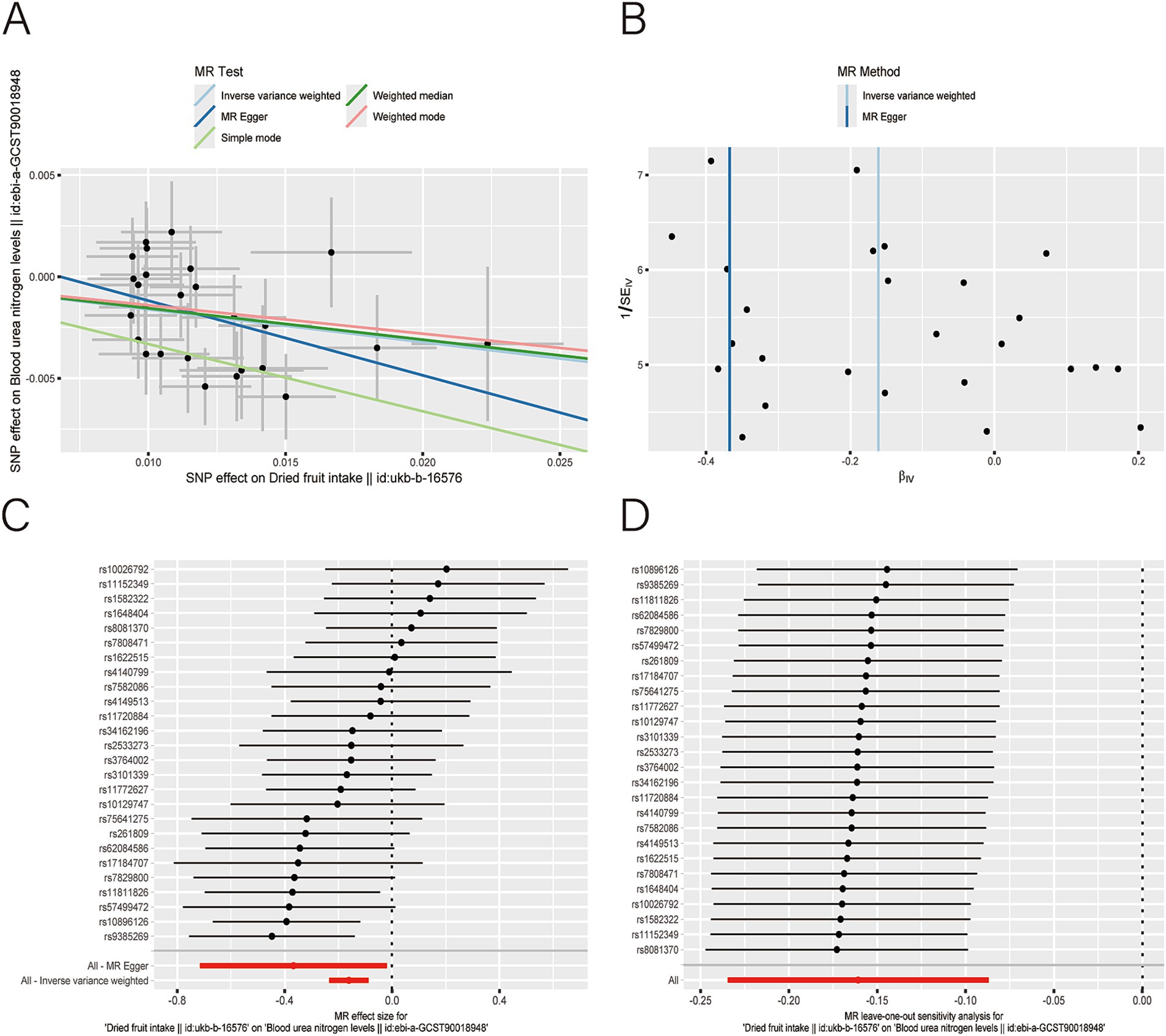
Figure 2. Scatter plot (A), funnel plot (B), forest plot (C), and leave-one-out analysis (D) of single nucleotide polymorphisms (SNPs) associated with dried fruit intake based on blood urea nitrogen (BUN) level.
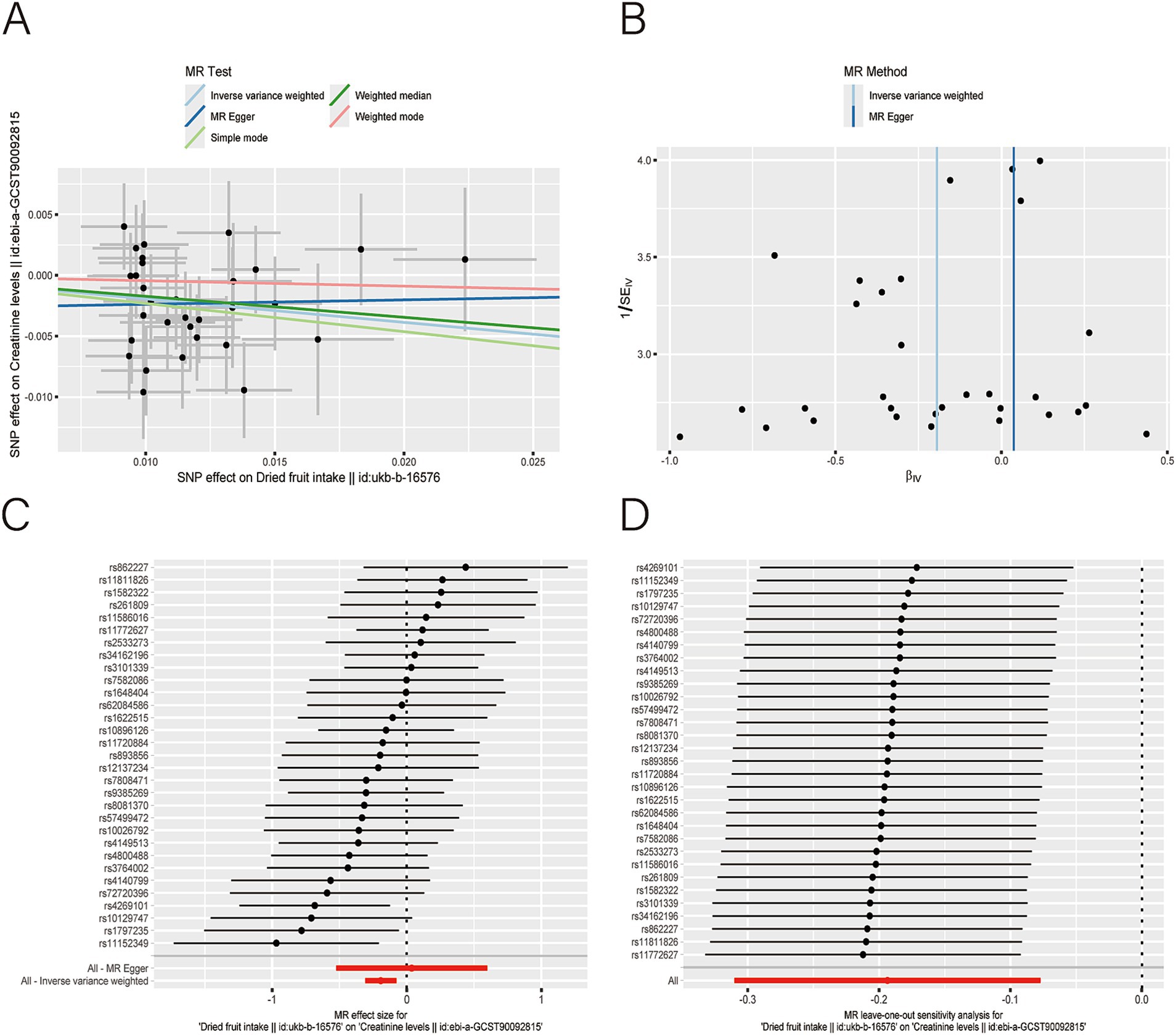
Figure 3. Scatter plot (A), funnel plot (B), forest plot (C), and leave-one-out analysis (D) of single nucleotide polymorphisms (SNPs) associated with dried fruit intake based on serum creatinine (CR) level.
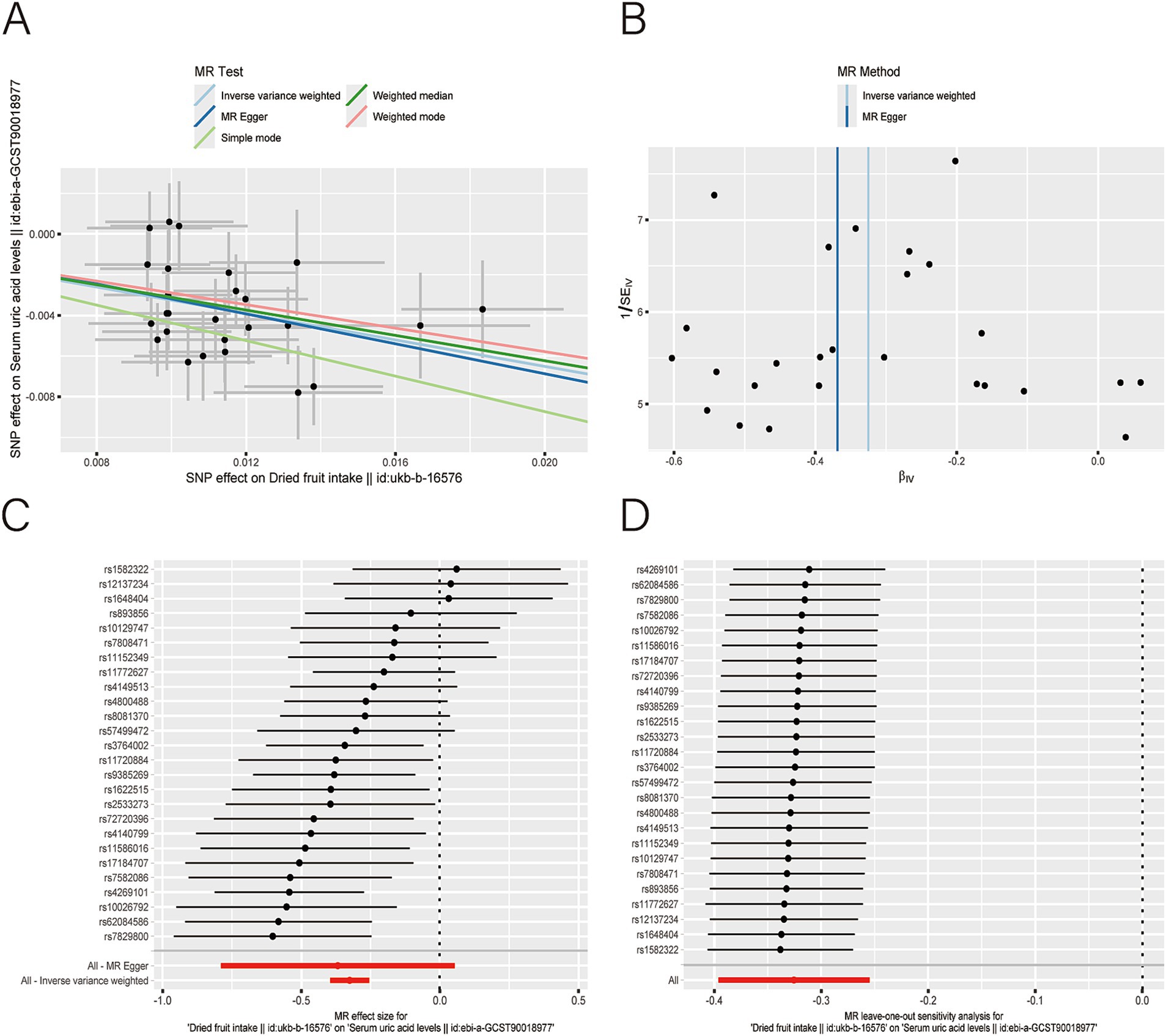
Figure 4. Scatter plot (A), funnel plot (B), forest plot (C), and leave-one-out analysis (D) of single nucleotide polymorphisms (SNPs) associated with dried fruit intake based on serum uric acid (UA) level.
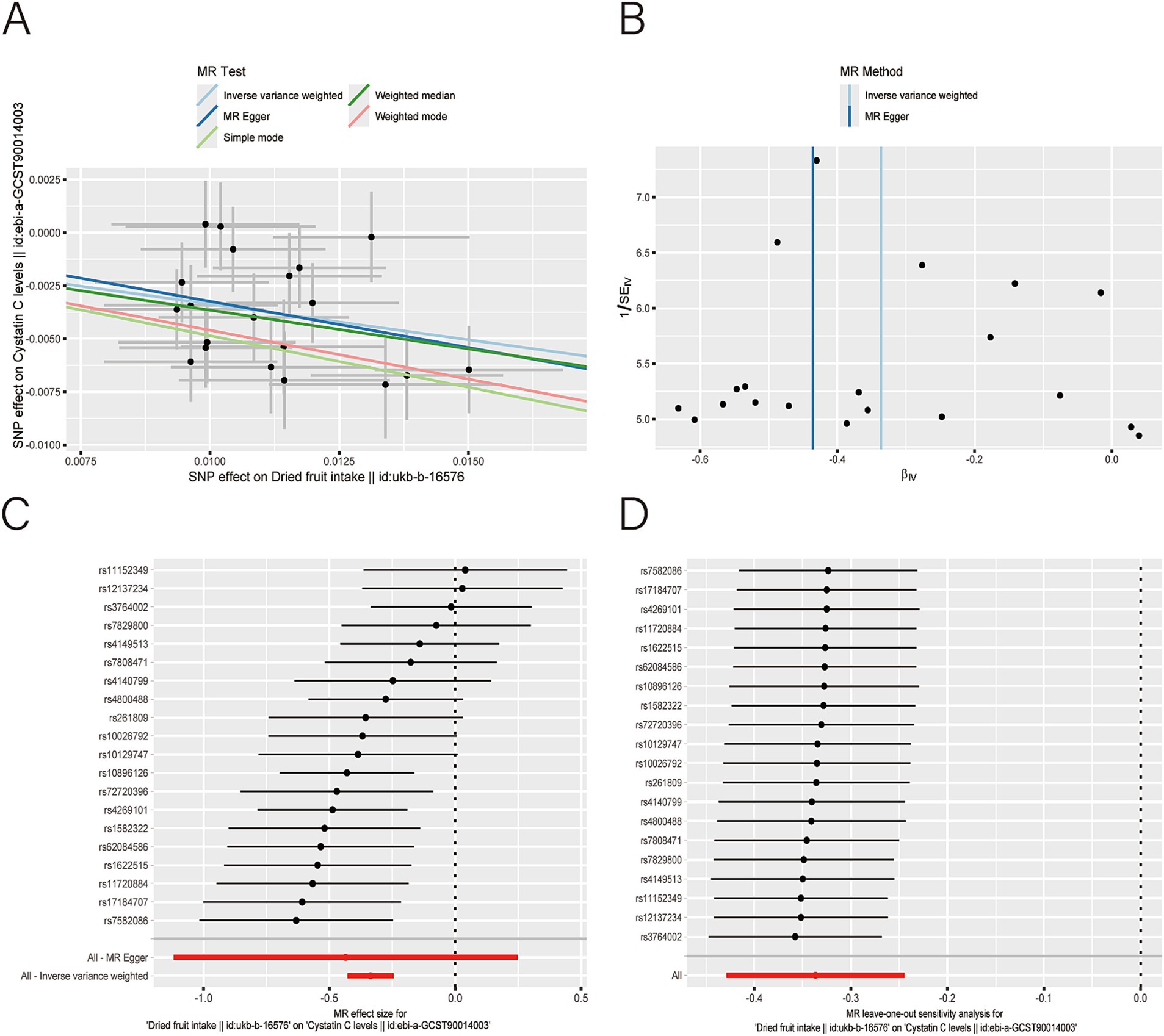
Figure 5. Scatter plot (A), funnel plot (B), forest plot (C), and leave-one-out analysis (D) of single nucleotide polymorphisms (SNPs) associated with dried fruit intake based on cystatin C (CysC) level.
The relationship between dried fruit intake and renal physical and chemical tests was further analyzed using MVMR. When fresh fruit and cooked vegetable intake were used as covariates, there was still evidence of an association between dried fruit intake and lower BUN (β: −0.196, 95% CI: −0.364 to −0.027, p = 2.309 × 10−2), CR level (β: −0.258, 95% CI: −0.437 to −0.080, p = 4.494 × 10−3), UA (β: −0.452, 95% CI: −0.618 to −0.287, p = 8.987 × 10−8), and CysC (β: −0.393, 95% CI: −0.608 to −0.1178, p = 3.405 × 10−4). After adding smoking status: current to the covariates, the MVMR analysis was again performed and found that the MVMR estimates were weakened: BUN (β: −0.173, 95% CI: −0.345 to −0.002, p = 4.787 × 10−2), CR level (β: −0.266, 95% CI: −0.447 to −0.086, p = 3.760 × 10−3), UA (β: −0.421, 95% CI: −0.595 to −0.248, p = 1.961 × 10−6), and CysC (β: −0.347, 95% CI: −0.551 to −0.134, p = 8.400 × 10−4). After adding alcohol intake frequency to the covariates, the MVMR analysis was again performed and found that the MVMR estimates were attenuated or not significant: BUN (β: −0.038, 95% CI: −0.215 to −0.138, p = 6.698 × 10−1), CR level (β: −0.148, 95% CI: −0.341 to 0.046, p = 1.347 × 10−1), UA (β: −0.296, 95% CI: −0.523 to −0.068, p = 1.094 × 10−2), and CysC (β: −0.238, 95% CI: −0.465 to −0.011, p = 4.024 × 10−2; Table 3).
4 Discussion
Our study showed that dried fruit intake has potential preventive value for increased BUN, CR, UA, and CysC levels, and no preventive value for hematuria and microalbuminuria. The results of the MVMR adjusted for fresh fruit intake, cooked vegetable intake, smoking status: current, and alcohol intake frequency found that in the absence of smoking and alcohol consumption as exposure factors, there was still substantial evidence that dried fruit intake was associated with BUN, CR, UA, and CysC levels, whereas when alcohol consumption was added as an exposure factor, the causality of dried fruit intake BUN and CR levels disappeared. The results suggest that this association may help to elucidate the pathophysiological mechanisms underlying the delayed effect of dried fruit intake on the course of CKD, which may be related to the prevention of an increase in kidney function markers. Thus, increased intake of dried fruits may help slow the deterioration of kidney function markers by reducing indicators of kidney function markers, although this effect is diminished or eliminated by smoking and alcohol consumption.
Dried fruit, as a preserved fruit substitute, ensured the supplementation of vitamins and other nutrients in ancient times when the preservation technology was not mature. Although the current development of preservation technology and transportation has ensured the freshness of fruits, their convenience, stability, and other characteristics are still widely popular. Currently, there are few studies on dried fruit intake for kidney disease, and people are more concerned about elements such as protein and energy (6). One of the characteristics of CKD is the increase in oxidative stress, which may be exacerbated by the gradual decrease in kidney excretory function and the accumulation of toxins as CKD worsens. Studies have shown that dried fruits are a rich source of antioxidant polyphenols; a study of common foods in the United States found that raisins (golden seedless) had the highest oxygen radical absorption capacity, followed by dried pears, dried plums, and so on (35). It has also been found that dried fruits always have higher antioxidant activity than the corresponding fresh dried fruits (36). Studies have demonstrated that the primary pathogenic mechanism of kidney injury is the inflammatory response (37). The prospective study “Systemic Inflammation Indicators Predict Survival in a U.S. CKD Population” (38) suggested that dried fruits are known to inhibit the inflammatory response. Furthermore, studies have shown that dried fruits can be used to prevent cancer (39) and reduce mortality (40). The theory of intestinal–kidney syndrome was presented by Ritz at the International Dialysis Congress in 2011; it states that damage to the intestinal barrier function can lead to pathology in several organs, including the heart and kidneys (41). In the same year, Meijers first introduced the concept of the gut–kidney axis and found that microbial levels in the gut can regulate uremic toxins in the body to influence the progression of CKD (42). In 2019 Meijers again interpreted the gut–kidney axis and found that CKD causes dysregulation of the gut microbiota, while ecological dysregulation of gut microbes can lead to the exacerbation of kidney disease (43). Dried fruits inherently contain many bioactive substances that can potentially influence gut microbial composition in ways that have potential health benefits (44).
BUN, CR, UA and CysC often represent the ability of the kidneys to excrete metabolic wastes in a clinical setting. Oxidative stress in kidney cells can lead to kidney damage, including damage to podocytes and the tubular interstitium of the kidney (45). Renal podocytes are an important part of maintaining the glomerular filtration barrier. Damage to podocytes can lead to kidney diseases such as proteinuria or glomerulosclerosis, and renal tubular mesenchyme plays an important role in maintaining renal function, which is closely related to the development of kidney diseases (46). As a portable grape product, Raisins are rich in nutrients, the most abundant of which are resveratrol and other phenols with anti-inflammatory and antioxidant properties (47, 48). Resveratrol has been found to reduce oxidative stress inflammatory cytokine levels, improve renal structure, and have a protective effect on the kidney (49). An in vivo experiment demonstrated that resveratrol inhibits and protects against podocyte apoptosis and improves kidney function (50, 51). A review of phenolic compounds shows that tannins and stilbenes contained in raisins can reduce the burden of oxidative stress on the kidneys and slow disease progression (52). Raisins were reported to reduce the risk of inflammatory injury through their effects on TNF-α (53). Black grapes were reported to improve the altered kidney function in hypercholesterolemic rats, and the effect is better than that of red grape juice (54). A review of dried plums showed that they were effective in promoting the growth of beneficial bacteria in the colon and in improving immune function (55). Furthermore, prunes, as a sub-species of plums, is also rich in phenolic compounds that inhibit the activation of the immune-inflammatory system and oxidative stress (56). Chlorogenic acid in dried plums was found to block inflammatory signaling pathways at multiple levels and reduce inflammatory damage (57). Apricots are a source of numerous vitamins and essential nutrients (58), and are often used as an antipyretic, laxative, emetic, etc. (59); they are rich in phenolic acids and flavanols, which reduce oxidative stress and regulate the balance of intestinal flora (14, 60–62). Japanese apricots (processed into umeboshi from dried and pickled forms) have been found to reduce gastrointestinal mucosal inflammation (63) and to improve the composition of the intestinal flora, with an increase in Mycobacterium spp. and Clostridium spp. cluster IV (64). Studies have found that apigenin, which is found in dried apricots, has a renoprotective effect and can reduce markers of renal function (65). However, there are still few studies on the association between dried fruits and CKD; therefore, animal experiments and large-scale clinical trials are needed to verify the efficacy.
Despite the numerous health benefits of dried fruits, they are often susceptible to contamination by various toxigenic fungi during the entire production process due to the specific way in which they are prepared. The most notable contaminants are aflatoxins (AFs) and ochratoxin A (OTA), with AFs leading to DNA mutations as well as liver and kidney damage, and OTA mainly leading to kidney damage (66). Therefore, care should be taken to purchase dried fruits from regular merchants to prevent damage.
Our MR results showed that there was a statistically significant association between dried fruit intake and lower BUN (p = 1.063 × 10−6), CR level (p = 1.455 × 10−4), UA (p = 4.439 × 10−20), and CysC (p = 1.074 × 10−11). In contrast, there was no statistically significant effect level for hematuria (p = 0.140) and microalbuminuria (p = 0.513). CR, BUN, UA, and CysC levels are the most commonly used indicators for assessing kidney function in patients with CKD, and they are considered to be closely related to the kidney’s ability to excrete metabolic waste from the body; therefore, they are easily influenced by dietary factors. Our results suggest that dried fruit intake has a causal effect on BUN, CR levels, UA, and CysC levels, which are influenced by smoking and drinking. More research is needed to confirm that dried fruit intake reduces CR, BUN, UA, and CysC levels in clinical trials.
There are some limitations to our study. First, MR relies on the validity of the GWAS results and is ethnically homogenous, limiting the ability to generalize the results. Second, possible confounding factors were not completely excluded from this study. Third, the dataset used in this study took the form of a questionnaire; the disadvantage of this method is that it may have incurred bias in the answers, thus affecting the accuracy. In conclusion, further in vivo, in vitro, and clinical trials are needed to provide more conclusive evidence. For example, studying the benefits of substances contained in dried fruits on kidney cells, or whether the addition of dried fruits intake to the daily diet of rats with chronic kidney disease could have a beneficial effect on the rate of kidney function markers such as BUN, CR, UA and CysC.
5 Conclusion
In conclusion, using a large-scale GWAS analysis of a European population, our study demonstrates that dried fruit intake is associated with lower BUN, CR, UA, and CysC levels, and that some of the results are influenced by factors such as alcohol and smoking. However, further studies are required to prove the causal relationship, which includes studies with different ethnic groups, followed by a comprehensive assessment of the impact of experimental participants’ lifestyles, social determinants, environmental exposures, and physicochemical tests that may affect kidney function markers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YG: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XY: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft. WZ: Conceptualization, Data curation, Supervision, Visualization, Writing – original draft, Writing – review & editing. FY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank all those who participated in this paper and also thank the Charlesworth editors for touching up the paper. I also thank the assistance from medical writers, proof-readers and editors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1440896/full#supplementary-material
Footnotes
References
1. Bello, AK, Okpechi, IG, Levin, A, Ye, F, Damster, S, Arruebo, S, et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health. (2024) 12:e382–95. doi: 10.1016/S2214-109X(23)00570-3
2. Organization WH. WHO methods and data sources for country-level causes of death 2000-2019. (2020). Available from: https://iris.who.int/handle/10665/374896 (Accessed on 2024 May 18)
3. Kato, S, Chmielewski, M, Honda, H, Pecoits-Filho, R, Matsuo, S, Yuzawa, Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. (2008) 3:1526–33. doi: 10.2215/CJN.00950208
4. Jdiaa, SS, Mansour, R, El Alayli, A, Gautam, A, Thomas, P, and Mustafa, RA. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. (2022) 35:69–85. doi: 10.1007/s40620-021-01206-8
5. Suh, SH, and Kim, SW. Dyslipidemia in patients with chronic kidney disease: an updated overview. Diabetes Metab J. (2023) 47:612–29. doi: 10.4093/dmj.2023.0067
6. Ikizler, TA, Burrowes, JD, Byham-Gray, LD, Campbell, KL, Carrero, JJ, Chan, W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–S107. doi: 10.1053/j.ajkd.2020.05.006
7. Hidalgo-Mora, JJ, García-Vigara, A, Sánchez-Sánchez, ML, García-Pérez, MÁ, Tarín, J, and Cano, A. The Mediterranean diet: a historical perspective on food for health. Maturitas. (2020) 132:65–9. doi: 10.1016/j.maturitas.2019.12.002
8. MacLaughlin, HL, Friedman, AN, and Ikizler, TA. Nutrition in kidney disease: Core curriculum 2022. Am J Kidney Dis. (2022) 79:437–49. doi: 10.1053/j.ajkd.2021.05.024
9. Zambrano, AK, Cadena-Ullauri, S, Ruiz-Pozo, VA, Tamayo-Trujillo, R, Paz-Cruz, E, Guevara-Ramírez, P, et al. Impact of fundamental components of the Mediterranean diet on the microbiota composition in blood pressure regulation. J Transl Med. (2024) 22:417. doi: 10.1186/s12967-024-05175-x
10. Vázquez-Cuesta, S, Lozano García, N, Rodríguez-Fernández, S, Fernández-Avila, AI, Bermejo, J, Fernández-Avilés, F, et al. Impact of the Mediterranean diet on the gut microbiome of a well-defined cohort of healthy individuals. Nutrients. (2024) 16:793. doi: 10.3390/nu16060793
11. Ticinesi, A, Nouvenne, A, Cerundolo, N, Parise, A, Mena, P, and Meschi, T. The interaction between Mediterranean diet and intestinal microbiome: relevance for preventive strategies against frailty in older individuals. Aging Clin Exp Res. (2024) 36:58. doi: 10.1007/s40520-024-02707-9
12. Bakis, H, Chauveau, P, Combe, C, and Pfirmann, P. Mediterranean diet for cardiovascular risk reduction in chronic kidney disease. Adv Kidney Dis Health. (2023) 30:496–501. doi: 10.1053/j.akdh.2023.07.007
13. Berry, EM, Arnoni, Y, and Aviram, M. The middle eastern and biblical origins of the Mediterranean diet. Public Health Nutr. (2011) 14:2288–95. doi: 10.1017/S1368980011002539
14. Alasalvar, C, Salvadó, JS, and Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. (2020) 314:126192. doi: 10.1016/j.foodchem.2020.126192
15. Carughi, A, Feeney, MJ, Kris-Etherton, P, Fulgoni, V, Kendall, CWC, Bulló, M, et al. Pairing nuts and dried fruit for cardiometabolic health. Nutr J. (2016) 15:23. doi: 10.1186/s12937-016-0142-4
16. Skrivankova, VW, Richmond, RC, Woolf, BAR, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
17. Bowden, J, and Holmes, MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346
18. Sakaue, S, Kanai, M, Tanigawa, Y, Karjalainen, J, Kurki, M, Koshiba, S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
19. Barton, AR, Sherman, MA, Mukamel, RE, and Loh, PR. Whole-exome imputation within UK biobank powers rare coding variant association and fine-mapping analyses. Nat Genet. (2021) 53:1260–9. doi: 10.1038/s41588-021-00892-1
20. Mbatchou, J, Barnard, L, Backman, J, Marcketta, A, Kosmicki, JA, Ziyatdinov, A, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. (2021) 53:1097–103. doi: 10.1038/s41588-021-00870-7
21. Teumer, A, Tin, A, Sorice, R, Gorski, M, and Yeo, NC. Genome-wide association studies identify genetic loci associated with albuminuria in diabetes. Diabetes. (2016) 65:803–17. doi: 10.2337/db15-1313
22. Richardson, TG, Leyden, GM, Wang, Q, Bell, JA, Elsworth, B, Davey Smith, G, et al. Characterising metabolomic signatures of lipid-modifying therapies through drug target mendelian randomisation. PLoS Biol. (2022) 20:e3001547. doi: 10.1371/journal.pbio.3001547
23. Kranz, S, Sharma, B, Pourafshar, S, Mallawaarachchi, I, Ma, JZ, and Scialla, JJ. Fruit and vegetable intake patterns, kidney failure, and mortality in adults with and without chronic kidney disease in the United States. J Nutr. (2024) S0022-3166:00285–2. doi: 10.1016/j.tjnut.2024.05.008
24. Santin, F, Canella, D, Borges, C, Lindholm, B, and Avesani, CM. Dietary patterns of patients with chronic kidney disease: the influence of treatment modality. Nutrients. (2019) 11:1920. doi: 10.3390/nu11081920
25. Liu, WC, Chuang, HC, Chou, CL, Lee, YH, Chiu, YJ, Wang, YL, et al. Cigarette smoke exposure increases Glucose-6-phosphate dehydrogenase, autophagy, fibrosis, and senescence in kidney cells in vitro and in vivo. Oxidative Med Cell Longev. (2022) 2022:1–16. doi: 10.1155/2022/5696686
26. Tian, Y, Liu, J, Zhao, Y, Jiang, N, Liu, X, Zhao, G, et al. Alcohol consumption and all-cause and cause-specific mortality among US adults: prospective cohort study. BMC Med. (2023) 21:208. doi: 10.1186/s12916-023-02907-6
27. Xie, J, Huang, H, Liu, Z, Li, Y, Yu, C, Xu, L, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology. (2023) 77:949–64. doi: 10.1002/hep.32728
28. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
29. Burgess, S, and Thompson, SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
30. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
31. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
32. Bowden, J, Spiller, W, Del Greco, MF, Sheehan, N, Thompson, J, Minelli, C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. (2018) 47:1264–78. doi: 10.1093/ije/dyy101
33. U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025. US: Department of Health and Human Services (2020).
34. Kelly, JT, Su, G, Zhang, L, Qin, X, Marshall, S, González-Ortiz, A, et al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and Meta-analysis. J Am Soc Nephrol. (2021) 32:239–53. doi: 10.1681/ASN.2020030384
35. Wu, X, Beecher, GR, Holden, JM, Haytowitz, DB, Gebhardt, SE, and Prior, RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. (2004) 52:4026–37. doi: 10.1021/jf049696w
36. Omolola, AO, Jideani, AIO, and Kapila, PF. Quality properties of fruits as affected by drying operation. Crit Rev Food Sci Nutr. (2017) 57:95–108. doi: 10.1080/10408398.2013.859563
37. Furman, D, Campisi, J, Verdin, E, Carrera-Bastos, P, Targ, S, Franceschi, C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
38. Chen, Y, Nie, Y, Wu, J, Li, C, Zheng, L, Zhu, B, et al. Association between systemic inflammatory indicators with the survival of chronic kidney disease: a prospective study based on NHANES. Front Immunol. (2024) 15:1365591. doi: 10.3389/fimmu.2024.1365591
39. Alasalvar, C, Salas-Salvado, J, Ros, E, and Sabate, J. (Editors). (2020). Health Benefits of Nuts and Dried Fruits (1st ed.). CRC Press. doi: 10.1201/9781315173337
40. Mossine, VV, Mawhinney, TP, and Giovannucci, EL. Dried fruit intake and Cancer: a systematic review of observational studies. Adv Nutr. (2020) 11:237–50. doi: 10.1093/advances/nmz085
41. Ritz, E. Intestinal-renal syndrome: mirage or reality? Blood Purif. (2011) 31:70–6. doi: 10.1159/000321848
42. Meijers, BKI, and Evenepoel, P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. (2011) 26:759–61. doi: 10.1093/ndt/gfq818
43. Meijers, B, Evenepoel, P, and Anders, HJ. Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol. (2019) 15:531–45. doi: 10.1038/s41581-019-0172-1
44. Alasalvar, C, Chang, SK, Kris-Etherton, PM, Sullivan, VK, Petersen, KS, Guasch-Ferré, M, et al. Dried fruits: bioactives, effects on gut microbiota, and possible health benefits-an update. Nutrients. (2023) 15:1611. doi: 10.3390/nu15071611
45. Ho, HJ, and Shirakawa, H. Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells. (2022) 12:88. doi: 10.3390/cells12010088
46. Yoshimura, Y, and Nishinakamura, R. Podocyte development, disease, and stem cell research. Kidney Int. (2019) 96:1077–82. doi: 10.1016/j.kint.2019.04.044
47. Burin, VM, Ferreira-Lima, NE, Panceri, CP, and Bordignon-Luiz, MT. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: evaluation of different extraction methods. Microchem J. (2014) 114:155–63. doi: 10.1016/j.microc.2013.12.014
48. Painter, JE, and Waters, AR. A review of the health benefits of raisins. J Food Sci. (2013) 78:ii–iii. doi: 10.1111/1750-3841.12139
49. Den Hartogh, DJ, and Tsiani, E. Health benefits of resveratrol in kidney disease: evidence from in vitro and in vivo studies. Nutrients. (2019) 11:1624. doi: 10.3390/nu11071624
50. Wang, F, Li, R, Zhao, L, Ma, S, and Qin, G. Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy. Eur J Pharmacol. (2020) 885:173387. doi: 10.1016/j.ejphar.2020.173387
51. Gong, L, Wang, R, Wang, X, Liu, J, Han, Z, Li, Q, et al. Research progress of natural active compounds on improving podocyte function to reduce proteinuria in diabetic kidney disease. Ren Fail. (2023) 45:2290930. doi: 10.1080/0886022X.2023.2290930
52. Baptista, F, Paié-Ribeiro, J, Almeida, M, and Barros, AN. Exploring the role of phenolic compounds in chronic kidney disease: a systematic review. Molecules. (2024) 29:2576. doi: 10.3390/molecules29112576
53. Puglisi, MJ, Vaishnav, U, Shrestha, S, Torres-Gonzalez, M, Wood, RJ, Volek, JS, et al. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. (2008) 7:14. doi: 10.1186/1476-511X-7-14
54. Ali, S, Alahmadi, A, Hamdy, R, Huwait, EA, Alansari, A, and Ayuob, N. Renoprotective effect of red grape (Vitis vinifera L.) juice and dark raisins against hypercholesterolaemia-induced tubular renal affection in albino rats. Folia Morphol (Warsz). (2019) 78:91–100. doi: 10.5603/FM.a2018.0069
55. Lever, E, Scott, SM, Louis, P, Emery, PW, and Whelan, K. The effect of prunes on stool output, gut transit time and gastrointestinal microbiota: a randomised controlled trial. Clin Nutr. (2019) 38:165–73. doi: 10.1016/j.clnu.2018.01.003
56. Damani, JJ, De Souza, MJ, VanEvery, HL, Strock, NCA, and Rogers, CJ. The role of prunes in modulating inflammatory pathways to improve bone health in postmenopausal women. Adv Nutr. (2022) 13:1476–92. doi: 10.1093/advances/nmab162
57. Nguyen, V, Taine, EG, Meng, D, Cui, T, and Tan, W. Chlorogenic acid: a systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients. (2024) 16:924. doi: 10.3390/nu16070924
58. Leccese, A, Bartolini, S, and Viti, R. Total antioxidant capacity and Phenolics content in apricot fruits. Int J Fruit Sci. (2007) 7:3–16. doi: 10.1300/J492v07n02_02
59. Wani, SM, Masoodi, FA, Ahmad, M, and Mir, SA. Processing and storage of apricots: effect on physicochemical and antioxidant properties. J Food Sci Technol. (2018) 55:4505–14. doi: 10.1007/s13197-018-3381-x
60. Al-Soufi, MH, Alshwyeh, HA, Alqahtani, H, Al-Zuwaid, SK, Al-Ahmed, FO, Al-Abdulaziz, FT, et al. A review with updated perspectives on nutritional and therapeutic benefits of apricot and the industrial application of its underutilized parts. Molecules. (2022) 27:5016. doi: 10.3390/molecules27155016
61. Lu, Y, and Han, X. Therapeutic implications of phenolic acids for ameliorating inflammatory bowel disease. Nutrients. (2024) 16:1347. doi: 10.3390/nu16091347
62. Średnicka-Tober, D, Kazimierczak, R, Ponder, A, and Hallmann, E. Biologically active compounds in selected organic and conventionally produced dried fruits. Food Secur. (2020) 9:1005. doi: 10.3390/foods9081005
63. Enomoto, S, Yanaoka, K, Utsunomiya, H, Niwa, T, Inada, K, Deguchi, H, et al. Inhibitory effects of Japanese apricot (Prunus mume Siebold et Zucc.; Ume) on Helicobacter pylori-related chronic gastritis. Eur J Clin Nutr. (2010) 64:714–9. doi: 10.1038/ejcn.2010.70
64. Tamura, M, Ohnishi, Y, Kotani, T, and Gato, N. Effects of new dietary fiber from Japanese apricot (Prunus mume Sieb. Et Zucc.) on gut function and intestinal microflora in adult mice. Int J Mol Sci. (2011) 12:2088–99. doi: 10.3390/ijms12042088
65. Allemailem, KS, Almatroudi, A, Alharbi, HOA, AlSuhaymi, N, Alsugoor, MH, Aldakheel, FM, et al. Apigenin: a bioflavonoid with a promising role in disease prevention and treatment. Biomedicine. (2024) 12:1353. doi: 10.3390/biomedicines12061353
Keywords: Mendelian randomization, dried fruit intake, kidney function, genome-wide association study, causal relationship
Citation: Gao Y, Yue X, Zhao W and Yuan F (2024) Association between dried fruit intake and kidney function: research from univariate and multivariate Mendelian randomized studies. Front. Nutr. 11:1440896. doi: 10.3389/fnut.2024.1440896
Edited by:
Lanfranco D’Elia, University of Naples Federico II, ItalyReviewed by:
Salvatore Vaccaro, IRCCS Local Health Authority of Reggio Emilia, ItalyMohammad Irshad Reza, North Dakota State University, United States
Copyright © 2024 Gao, Yue, Zhao and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Yuan, MTc3MDI0MzM4OTJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuhang Gao
Yuhang Gao Xinghai Yue2
†
Xinghai Yue2
†