- 1Chinese-German Joint Institute for Natural Product Research, Shaanxi International Cooperation Demonstration Base, Shaanxi University of Technology, Hanzhong, China
- 2Department of Biological Sciences, Faculty of Science, National University of Singapore, Singapore, Singapore
- 3Department of Biochemistry, Faculty of Natural and Applied Sciences, Umaru Musa Yar’adua University Katsina, Katsina, Nigeria
- 4Biomedical Research and Training Centre, Yobe State University, Damaturu, Nigeria
- 5Centre of Molecular and Environmental Biology, Department of Biology, University of Minho, Braga, Portugal
- 6Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
Background: 3-caffeoylquinic acid (3-CQA), a member of the chlorogenic acid family, possesses diverse pharmacological properties, such as scavenging, antioxidant, and antiapoptotic activity, rendering substantial value to alimentary consumables and therapeutic substances. However, the pervasiveness of non-standard practices, notably the misuse and abuse of indigenous botanicals, coupled with the inherent susceptibility of 3-CQA to degradation under light and heat exposure, engenders discernible disparateness in the quality profiles of the same kinds of herbs. Consequently, precise quantification of 3-CQA becomes imperative.
Methods: In this context, an artificial antigen was synthesized as a specific conjugate of 3-CQA and bovine serum albumin (3-CQA-BSA), followed by the generation of a monoclonal antibody (mAb) against the conjugate. Through optimization, a mAb-based indirect competitive chemiluminescence enzyme immunoassay (ic-CLEIA) was developed.
Results: It demonstrated an IC50 and the calibration range of 2.97 ng/mL and 0.64–13.75 ng/mL, respectively, outperforming the conventional enzyme-linked immunosorbent assay (ELISA). Notably, the ic-CLEIA displayed 10.71% cross-reactivity with 3,5-dicaffeoylquinic acid, alongside minimal cross-reactivity toward other isomeric counterparts and analogs. Validation experiments on herbs and Chinese patent medicines using ic-CLEIA, confirmed by high-performance liquid chromatography (HPLC) analysis, revealed a robust correlation coefficient of 0.9667 between the two modalities.
Conclusion: These findings unequivocally demonstrated that the proposed ic-CLEIA represents a viable and reliable analytical method for 3-CQA determination. This method holds significant potential for ensuring the quality control and therapeutic efficacy germane to herbs and patent medicines, spanning diverse therapeutic milieus and applications.
1 Introduction
Phenolic compounds, as secondary metabolites of plants, have garnered significant attention in recent years due to their remarkable bioactivity (1–3). Among these compounds, chlorogenic acids (CGAs), a class of phenolic acids, have particularly attracted interest owing to their antioxidative and free radical-scavenging properties (4, 5). CGAs comprise a family of esters derived from quinic acid and specific trans-cinnamic acids, predominantly caffeic, p-coumaric, and ferulic acid. Within the CGA family, although the quantification of 5-caffeoylquinic acid (5-CQA) has been extensively reported (6, 7), limited research exists on its structural isomers like 3-caffeoylquinic acid (3-CQA) and 4-caffeoylquinic acid (4-CQA).
Considering this, this study focuses on 3-CQA, an ester of caffeic acid and (−)-quinic acid. On the one hand, 3-CQA displays intriguing biological effects, low toxicity, and promising commercial value. Notably, it acts as the primary active ingredient in Honeysuckle and Euphorbia, demonstrating antibacterial, antiviral, antioxidant, anti-inflammatory, hypolipidemic, and neuroprotective effects (Figure 1) (8–11). Recently, it has been reported to alleviate and prevent diabetes mellitus and associated complications such as diabetic nephropathy and diabetic retinopathy (12). In addition, 3-CQA has shown promise in countering high-fat diet (HFD)-induced activation of the TLR4 signaling pathway and the expression of TNF-α and IL-6 in the liver, thus ameliorating non-alcoholic fatty liver disease (NAFLD) (13). On the other hand, beyond its presence in Honeysuckle and Euphorbia, 3-CQA is widely distributed in other plants of the Euphorbiaceae, Lonicerae, and Soniaceae families, as well as in various vegetables and fruits (Figure 1) (14–17). However, the prevalence of non-standard practices, along with the vulnerability of the compound per se to degradation from light and heat, often leads to considerable variations and challenges in quality control.
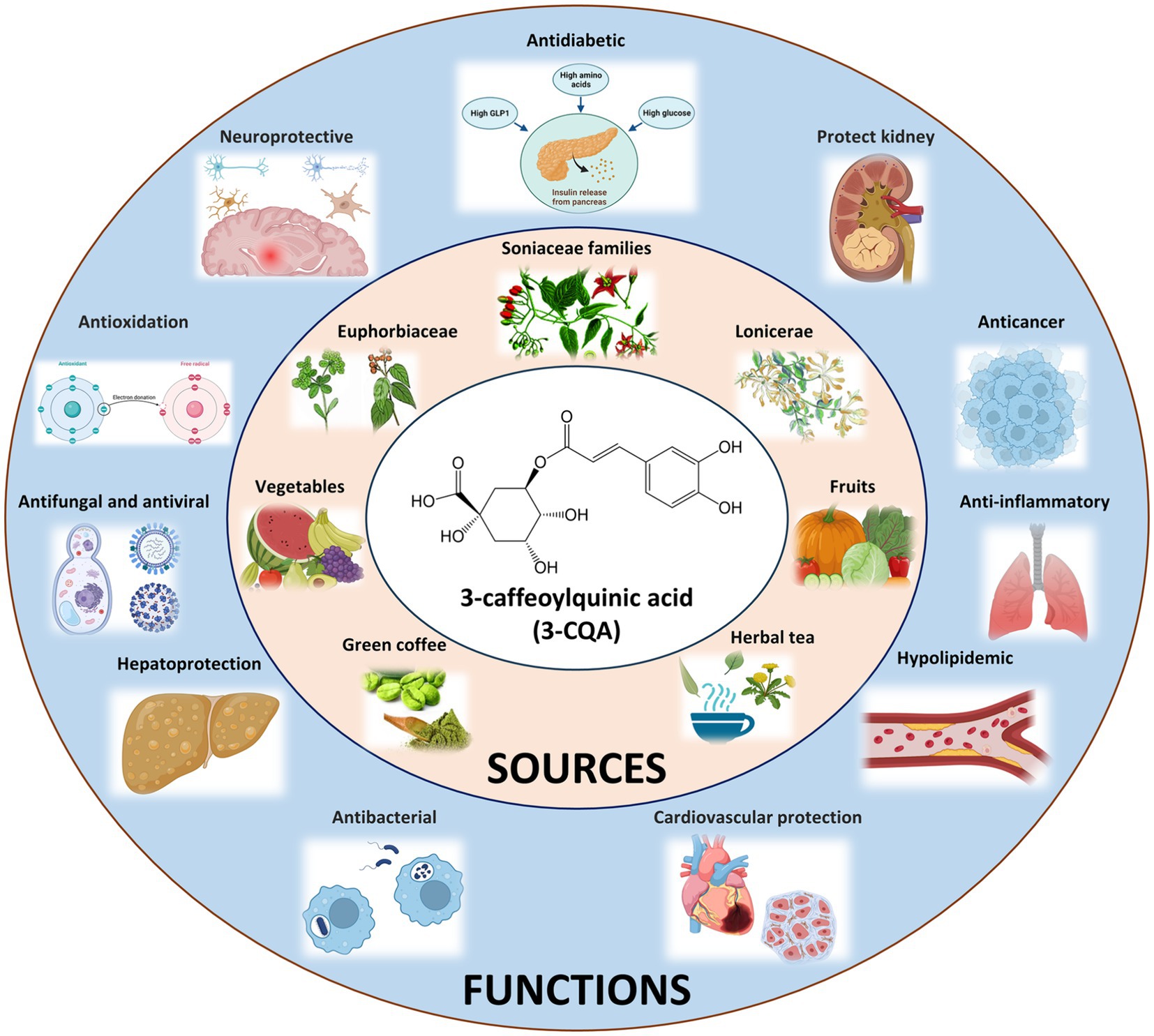
Figure 1. Origins and Bioactive Impacts of 3-CQA. The chemical structure of 3-CQA is represented within the white circle, sources of 3-CQA are delineated within the pink circle, and the bioactive effects of 3-CQA are elucidated within the blue circle.
Given the impressive pharmacological activities and the scarcity of quantification studies, there is an imperative demand for the development of analytical methods for 3-CQA-containing herbs and associated products. In this context, we capitalized on the intrinsic advantages of canonical chemiluminescence enzyme-linked immunosorbent assay (CLEIA) over other instrumental techniques and traditional immunoassays, particularly in terms of high sensitivity, time-saving, ease of operation, and high-throughput evaluation. Specifically, we generated an artificial antigen by chemically conjugating 3-CQA with bovine serum albumin (BSA) and produced a monoclonal antibody (mAb). Following the optimization of the reaction system, a mAb-based indirect competitive CLEIA (ic-CLEIA) was developed, with a half-maximum inhibitory concentration (IC50 value) of 2.97 ng/mL and a calibration range of 0.64–13.75 ng/mL, surpassing those of the conventional enzyme-linked immunosorbent assay (ELISA). Further cross-reactivity (CR) tests revealed that the established ic-CLEIA offered favorable specificity to 3-CQA. Moreover, we determined 3-CQA content in six herbs and seven patient medicine samples, with the results showing a strong correlation coefficient of 0.9667 between the ic-CLEIA and high-performance liquid chromatography (HPLC).
These findings indicate that the proposed ic-CLEIA enables rapid, simple, and environmentally friendly detection of 3-CQA. More importantly, to our knowledge, this study represents the first incorporation of ic-CLEIA into the quantification of 3-CQA, holding significant potential for ensuring the quality control of herbal products and patent medicines across various applications. Our study thus serves as an instructive example for 3-CQA determination and opens avenues for extending the application of this method to the quantification of other molecules within the CGA family.
2 Materials and methods
2.1 Preparation of artificial antigens
The 3-CQA artificial antigen was synthesized through the carbodiimide method (Figure 2) (18). All chemicals utilized were of analytical grade and purchased from Sigma (St Louis, MO, United States). Initially, 30 mg of either BSA or ovalbumin (OVA), 6 mg of 6-aminohexanoic acid, and 25 mg of 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide (EDAC) were precisely weighted and dissolved in an 8 mL solution of 2-(N-morpholino) ethanesulfonic acid. The mixture was stirred at room temperature for 12 h. Upon reaction completion, the mixture was transferred to a dialysis bag and subjected to dialysis in phosphate-buffered saline (PBS) at 4°C for 72 h, yielding a solution of aminated carrier protein. Subsequently, 7.09 mg of 3-CQA, along with 4.38 mg of N-hydroxysuccinimide (NHS) and 3.3 mg of EDAC were weighed and dissolved in 500 μL N, N-dimethylformamide (DMF). The reaction was conducted at 17°C, 130 rpm for 16 h to generate the activated intermediate of 3-CQA. This activated intermediate was then added dropwise to the aminated carrier protein solution. Followed by stirring overnight, the mixture was dialyzed against 10 mM PBS, (pH 7.4) at 4°C for 2 days, with the dialysis solution changed four times, ensuring the removal of any unreacted 3-CQA. The resulting 3-CQA-BSA and 3-CQA-OVA bio-conjugates were finally stored at −20°C until use. Spectroscopic analysis was conducted using a Specord 50 UV–VIS spectrophotometer (Analytik AG, Jena, Germany) to confirm the successful coupling of 3-CQA to the carrier protein.
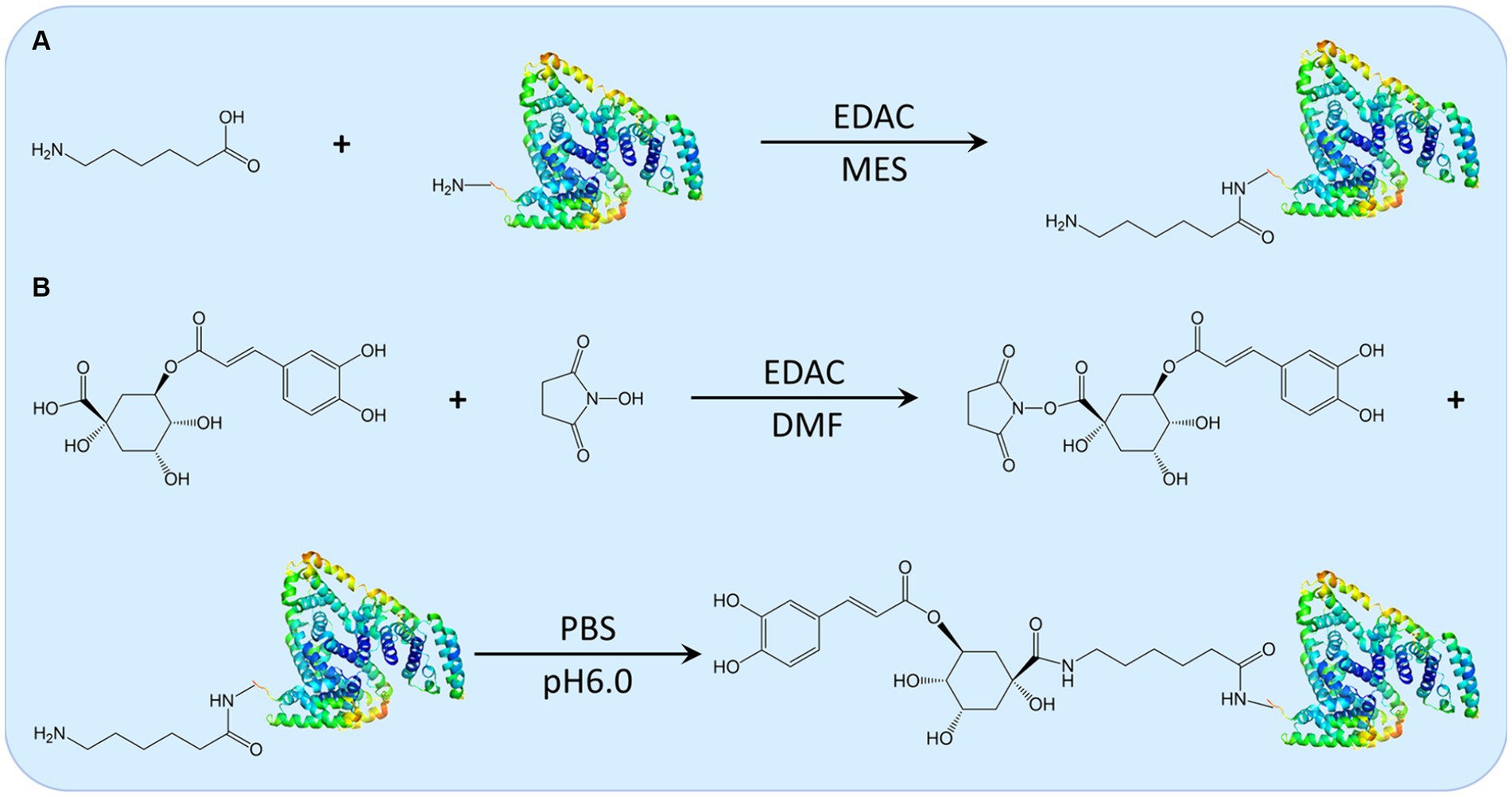
Figure 2. The schematic illustration of the synthesis route for 3-CQA-BSA Conjugate. Depiction of the synthesis routes for the aminated carrier protein (A) and the activated intermediate 3-CQA, along with the 3-CQA-BSA bio-conjugate (B).
2.2 Generation of monoclonal antibody
The hybridoma technique was employed to produce the monoclonal antibody as follows. Six female BALB/c mice (6 to 10 weeks old) were obtained from Beijing HFK Bio-technology (Beijing, China). Following 2 weeks of adaptive feeding, the mice were immunized with equal amounts of 3-CQA-BSA (1 mg/mL, dissolved in PBS) and Freund’s complete adjuvant (Sigma, St Louis, MO, United States). Subsequently, four booster injections were administered at two-week intervals, each containing 3-CQA-BSA solution emulsified with Freund’s incomplete adjuvant (Sigma, St Louis, MO, United States). After the fifth injection, blood samples were collected from each mouse and centrifuged. The specificity of the obtained serum was determined using an indirect competitive ELISA (ic-ELISA). The positive mice were then identified, and splenocytes from these mice were fused with sp2/0 mouse myeloma cells (ATCC, Manassas, VA, United States) using PEG4000 at 37°C. Hybridomas were sequentially cultivated in HAT medium (containing hypoxanthine, aminopterin, and thymidine) and HT medium (lacking amino purine) at 37°C in a CO2 incubator (Thermo, Franklin, MA, United States) after fusion. After 10 days, subclone culture of surviving hybridoma cells was performed using a limited dilution method, and positive clones were detected by ic-ELISA. The identified clones were further amplified to produce ascites, which were then purified using the ammonium sulfate precipitation method (19). Following dialysis against H2O for 48 h, the mAb was lyophilized. The purity and concentration of purified mAb were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and a BCA Protein Assay Kit (Sangon biotech, Shanghai, China), respectively. The isotype of mAb was identified using a mouse monoclonal antibody isotyping kit (Sino Biological, Beijing, China).
All the procedures described above complied with the Chinese Regulations of Laboratory Animals and were approved by the institutional experimental animal ethics committee (Chinese-German Joint Institute for Natural Product Research, Shaanxi University of Technology, and Approval Number 2021–01).
2.3 Optimization of working buffer and establishment of the standard curve for ic-CLEIA
To establish the optimal conditions for the ic-CLEIA, a checkerboard titration was employed. The protocols of the ic-CLEIA were performed as previously (20). A gradient concentration of 3-CQA-OVA (0.125, 0.5, 2.0, and 8.0 μg/mL; 100 μL/well) was diluted in PBS and added to a 96-well white microliter plate (Costar Inc., Cambridge, MA, United States), followed by incubation at 4°C overnight. After washing each well three times with PBST (PBS solution containing 0.05% Tween-20, pH 7.4), the plates were blocked with 5% skimmed milk powder (SMP) (Sigma, St Louis, MO, United States) in PBS (200 μL/well) at 37°C for 2 h. Subsequently, different concentrations of mAb (1:2000, 1:4000, 1:8000, 1:16000, 1:32000, 1:64000, and 1:128000; 100 μL/well) were added to different wells, followed by incubation at 4°C for 50 min. For ic-CLEIA standard curve, 3-CQA standard solutions in PBS (0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, and 100 ng/mL; 50 μL) and the diluted antibody in PBS (50 μL) were added to each well and incubated at 4°C for 50 min. After a washing step, goat anti-mouse IgG-horse radish peroxidase (HRP) (SinoBiological, Beijing, China) dissolved in PBS supplemented with 2% SMP was added at a 1:5000 dilution (100 μL/well), and the plates were incubated at 37°C for 50 min. Afterward, 100 μL of chemiluminescent substrate solution (Heliosense Biotechnology, Xiamen, China) was pipetted into each well, and the emitted photons were immediately read at 595 nm using a Tecan Infinite F200 reader (Mannedorf, Switzerland). To investigate the effects of different factors on the overall reaction system, different pH (6.5, 7.0, 7.4, and 8.0), methanol aqueous solution (5, 10, 30, and 50%; v/v), Tween-20 (0.01, 0.02, 0.04, 0.06, and 0.08%, v/v), sodium chloride solution (0.1 M, 0.2 M, and 0.4 M) were prepared and tested.
All incubation steps were performed in the dark throughout the procedure. The competitive inhibition curves for 3-CQA were plotted using GraphPad Prism 8.0 (GraphPad Inc., San Diego, CA).
2.4 Establishment of the standard curve for ic-ELISA
The protocols of ic-ELISA are similar to the ic-CLEIA, 3-CQA-OVA antigen, anti-3-CQA mAb, and goat anti-mouse IgG-HRP were successively incubated under optimized conditions. 3-CQA standard solutions in PBS (1, 2, 4, 8, 16, 32, 64, 128, 256, and 512 ng/mL; 50 μL) and the diluted antibody in PBS (50 μL) were added to each well and incubated at 4°C for 50 min. Subsequent to four washes, 3, 3′, 5, 5′-tetramethylbenzidine (TMB, 100 μL/well) solution was added and incubated at 37°C for 10 min. The reaction was then terminated by adding 2 M H2SO4 (50 μL/well), and the absorbance was measured at the wavelength of 450 nm (OD450). The competitive inhibition curve was plotted using Lg[10*C3-CQA (ng/mL)] as the abscissa and B/B0 (B0 is the OD450 value without standard, and B is the OD450 value with standard present) as the ordinate.
2.5 Cross-reactivity assay
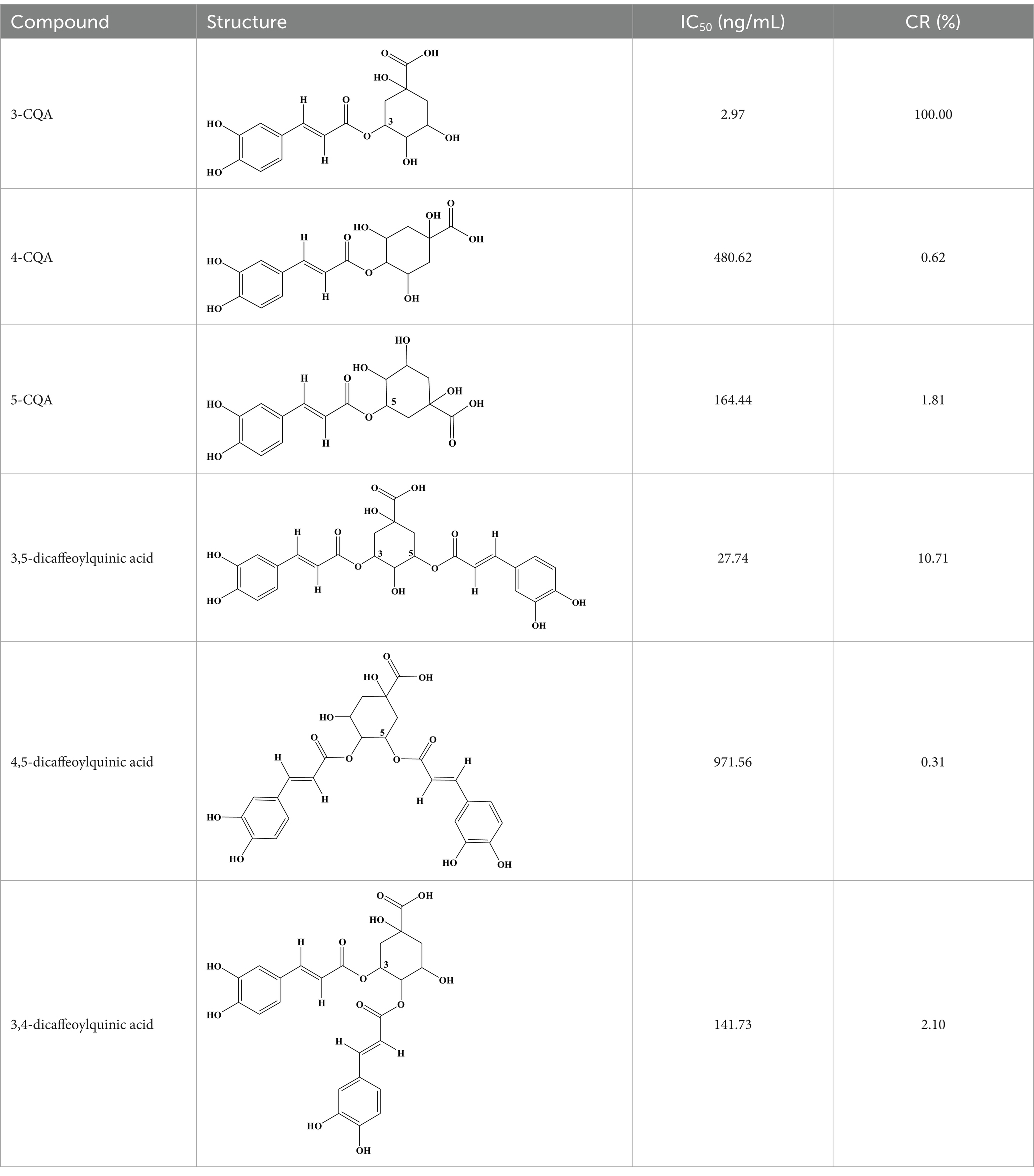
To evaluate CR, the structural analogs of 3-CQA including 4-CQA, 5-CQA, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, and tea polyphenols (DESITE Biotech, Chengdu, China) were used in place of 3-CQA within the ic-CLEIA assay. The respective CR values of these analogs were determined using the following equation:
2.6 HPLC analysis
HPLC determination of 3-CQA was performed using a Dionex Ultimate 3,000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, United States). The method was established with reference to the Chinese Pharmacopeia (21). Briefly, 3-CQA solutions were prepared by dissolving in 50% methanol at different concentrations (25, 50, 100, 200, 400, and 800 μg/mL). Subsequently, the 3-CQA solutions were separated using a Shimadzu Intersil ODS3-C18 column (reversed-phase, 150 × 4.6 mm, 4.0 μm) with a mobile phase system consisting of acetonitrile and 0.4% (v/v) phosphoric acid aqueous. The remaining parameters were set as follows: column temperature = 30°C, injection volume = 10 μL, and detection wavelength = 327 nm (Supplementary Table S1), and the retention time of 3-CQA was 11.2 min. The standard curve of 3-CQA-HPLC was plotted based on the retention time of the 3-CQA reference. It was then used to extrapolate the amount of 3-CQA in the herbs and patent medicines.
2.7 Sample preparation and extraction of 3-CQA
Samples of six herbs (Honeysuckle, Eucommia, Dandelion, Houttuynia, Virgate wormwood, and Lonicera stem), seven Chinese patent medicines (Shuang-Huang-Lian capsule, Vitamin C Yin-Qiao tablet, Yin-Zhi-Huang granule, Yin-Huang granule, Kou-Yan-Qing granule, compound Jin-Yin-Hua granule, and Jin-Sang-Zi lozenge) were purchased from local pharmacies and supermarkets in Hanzhong, Shaanxi, China. The extraction of 3-CQA was conducted following the procedure prescribed in the Chinese Pharmacopeia (21). Each herb (0.1 g) and patent medicine (0.5 g) were ground into powder and dissolved in 50% methanol (5 mL). The resulting solutions were then subjected to sonication for 30 min at 250 W. After sonication, the extracts were weighed, and the lost weight was replenished with 50% methanol, followed by centrifugation at 4000 rpm for 5 min. The whole process was carried out under protected conditions to avoid exposure to light. The obtained supernatant was directly employed for immunoassay analysis after dilution. For HPLC analysis, the supernatant was further filtered through a 0.22 μm cellulose membrane prior to analysis.
2.8 Recovery analysis
A total of six samples, comprising three herbs and three patent medicines, were prepared in triplicate. For each sample, 0.5 mg and 1.0 mg of 3-CQA were added, leaving one sample remaining without the addition of 3-CQA as a control. The 3-CQA in the resulting mixture was extracted, followed by appropriate dilution for subsequent HPLC and ic-CLEIA analyses. Each spiked sample was measured four times in parallel. The spiked recoveries were calculated using the following formula:
3 Results
3.1 Characterization of the artificial antigen and antibody
The characteristic peaks of BSA and 3-CQA were observed at 280 nm and 326 nm, respectively. In contrast, the characteristic absorption peaks of the 3-CQA-BSA bio-conjugate were identified between 280 and 326 nm (Figure 3A), demonstrating the successful synthesis of the complete artificial antigen. Likewise, the absorption peak of 3-CQA-OVA was shifted compared to that of 3-CQA and OVA (data not shown), indicating that the conjugated antigen 3-CQA-OVA was also successfully synthesized.
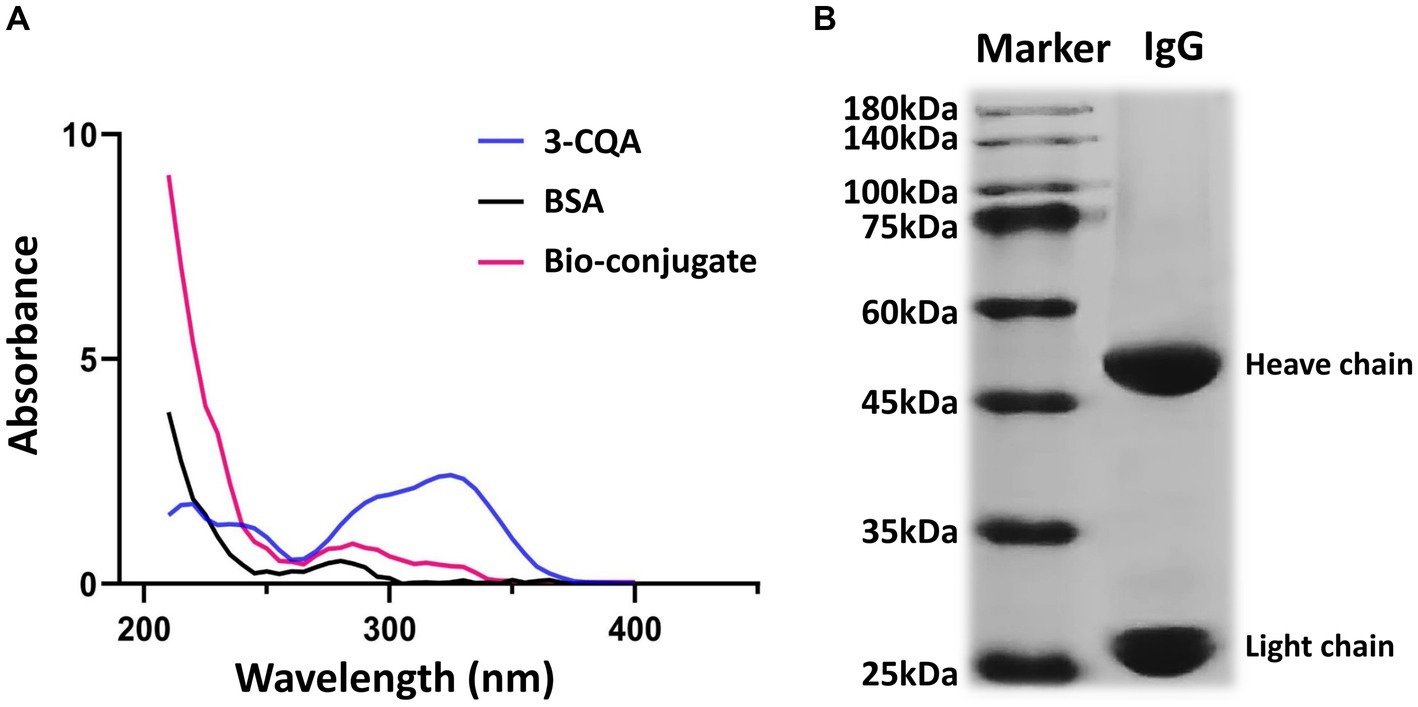
Figure 3. Characterization of the hapten and anti-3-CQA mAb. (A) Ultraviolet spectrometry analysis of 3-CQA, BSA, and 3-CQA-BSA bio-conjugate. (B) SDS-PAGE analysis of the mAb.
Regarding the antibody against the 3-CQA-BSA, the antiserum titer of the immunized mice was determined to be 1: 160000 using the ic-ELISA (Supplementary Figure S1). Subsequent purification of mouse ascites yielded two distinct bands corresponding to the heavy (50 kDa) and light chains (25 kDa) on SDS-PAGE (Figure 3B), affirming the high purity of the obtained antibodies. The purified mAb was then concentrated to 3.12 mg/mL and was classified as an IgG1 isotype with a kappa light chain.
3.2 Optimization of ic-CLEIA condition
The relative light unit (RLU) value decreased sharply with the antibody titer from 1:2000 to 1:8000, and the trend gradually flattened as the titer reached 1:16000 (Supplementary Figure S2). Additionally, when the coating concentration of 3-CQA-OVA was below 2 μg/mL, the RLU exhibited a sharp decline (Supplementary Figure S2). Consequently, an antibody dilution of 1:8000 (0.39 μg/mL) and a coating concentration of 2 μg/mL were deemed appropriate conditions for ic-CLEIA. Regarding the pH conditions, both slightly acidic and slightly alkaline environments resulted in an increase in the IC50. Conversely, a neutral pH led to minimum IC50 while the RLU0/IC50 ratio reached the maximum (Figure 4A). Therefore, pH = 7.0 was selected as the optimal pH value. The impact of surfactants (Tween-20) and organic solvents (methanol) on the reaction system was also investigated. The IC50 values displayed a continuous increasing trend with higher concentration of methanol and Tween-20 added (Figures 4B,C). As such, deionized water in the absence of surfactants and organic reagents was chosen as the optimal reaction condition. Moreover, the effect of salt concentration on ic-CLEIA was explored. As shown in Figure 4D, the IC50 value progressively decreases with the increase of salt ion concentration in the range of 0.1–0.4 M, and reached the minimum value at 0.4 M. In case of the absence of salt ions, the IC50 value was distinctly lower than the case of the added salt ions, with a maximum value of RLU0/IC50 value. With comprehensive consideration, deionized water without Na+, surfactant, and organic reagent was selected as the working buffer, and the pH value was adjusted to 7.0.
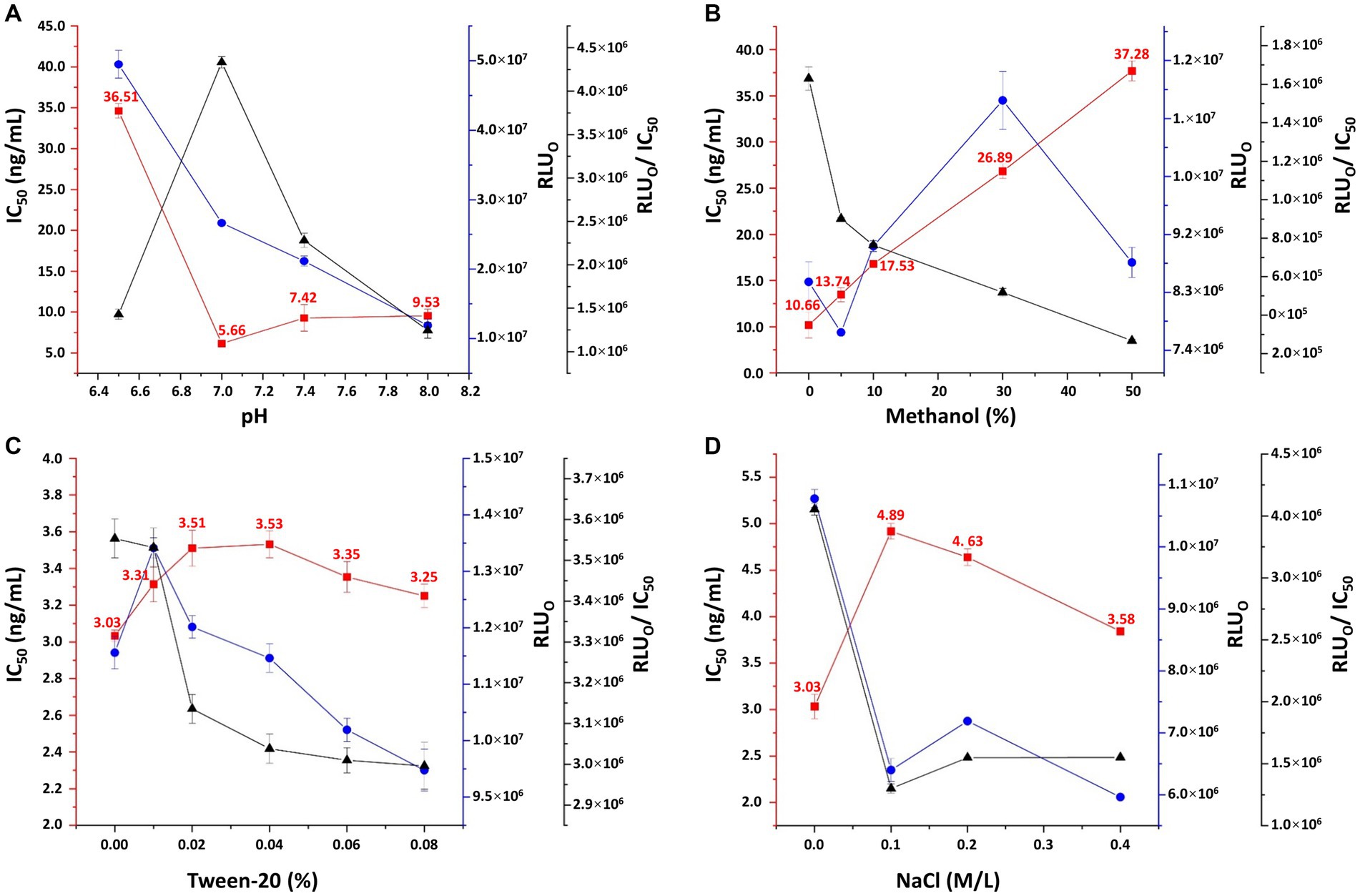
Figure 4. Optimization of pH (A), methanol concentration (B), Tween-20 concentration (C), and sodium chloride concentration (D) in working buffer. RLU0: relative light unit without competing reagents. Each value represents the average of four replicates.
3.3 Establishment of enzyme-linked immunoassay method
Under the optimized conditions, representative competitive inhibition curve revealed an IC50 value of 2.97 ng/mL and the limit of detection (LOD, IC10) value of 0.39 ng/mL (Figure 5A). The linear range was from 0.64 ng/mL (IC20) to 13.75 ng/mL (IC80). An ic-ELISA method for detecting 3-CQA was established under the same conditions as ic-CLEIA, with a linear range of 10.21–127.36 ng/mL, an IC50 value of 36.06 ng/mL, and a LOD value of 6.71 ng/mL (Figure 5B).
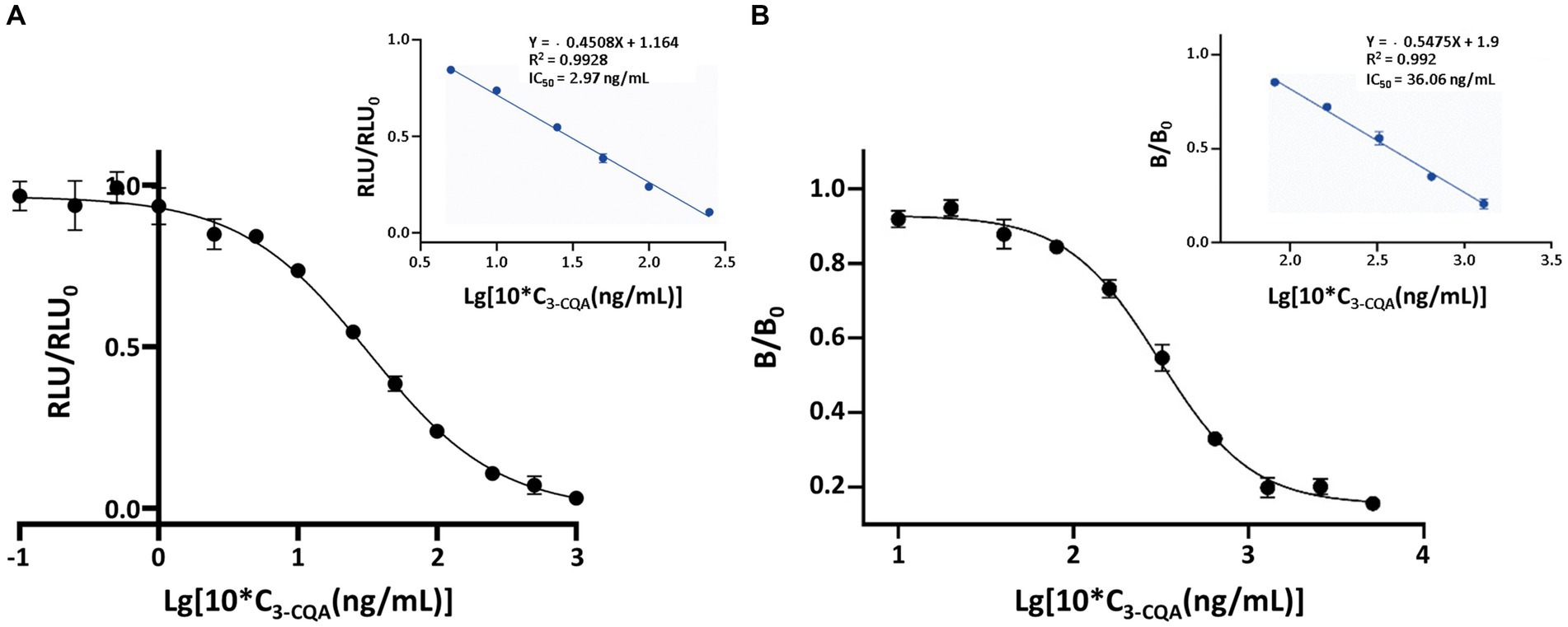
Figure 5. Standard curves of the ic-CLEIA (A) and ic-ELISA (B) for 3-CQA. RLU and RLU0 mean the relative light unit with and without competing reagents, respectively. B and B0 mean the absorbance value with and without competing reagents, respectively. Each value represents the average of four replicates.
3.4 Cross-reactivity
4-CQA, 5-CQA, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, and 3,4-dicaffeoylquinic acid were subjected to ic-CLEIA and their IC50 values were determined sequentially. The CR was 0.62, 1.81, 10.71, 0.31, and 2.10%, respectively (Table 1).
3.5 High-performance liquid chromatography analysis of 3-CQA
A reversed-phase HPLC was developed for 3-CQA determination, and the retention time was 11.2 min (Supplementary Figure S3). The regression curve equation was Y = 0.5494X + 4.744 (R2 = 0.9999, n = 4; Supplementary Figure S4).
3.6 Real and spiked samples detection
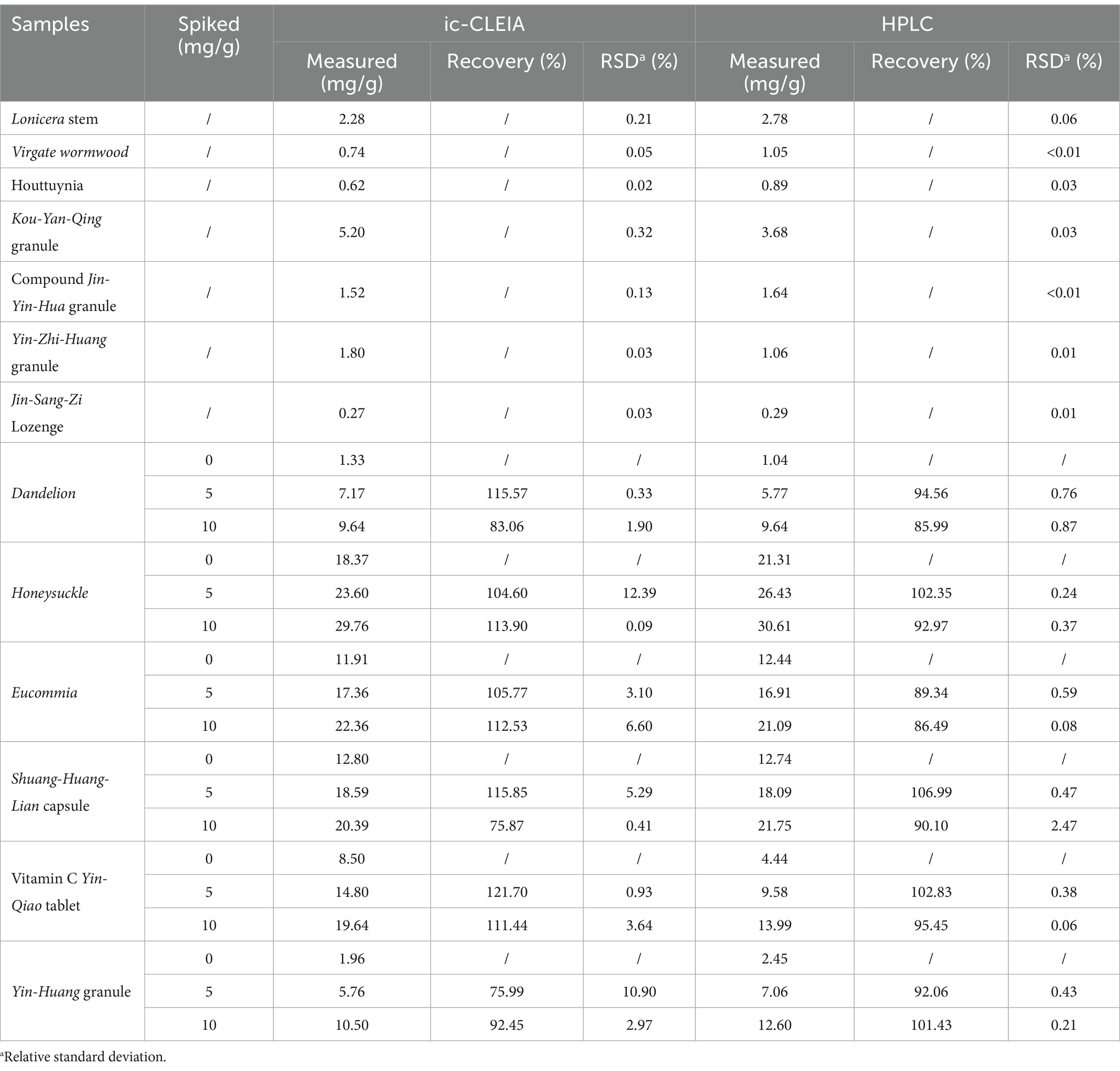
3-CQA was extracted from herbs, and patent medicines samples, and the 3-CQA content was determined using the established ic-CLEIA method. The 3-CQA contents of Honeysuckle and Eucommia were significantly higher than that of other herbs. Shuang-huang-lian capsules contain about 1.5% (w/w) 3-CQA, the highest content among the patent medicines (Table 2).
3-CQA standard was added to herb and patent medicines to obtain specific concentration (5 or 10 mg/g). The recovery of 3-CQA after extraction was determined by the ic-CLEIA or HPLC. The ic-CLEIA showed recoveries ranging from 75.87 to 121.70%, and the relative standard deviation was 0.09 to 12.39% (Table 2).
3.7 Comparison of ic-CLEIA and HPLC for detection of 3-CQA
The results of the ic-CLEIA method showed good correlation with those of HPLC (R2 = 0.9667), indicating that the method developed could achieve reliable and accurate determination of 3-CQA in samples (Supplementary Figure S5).
4 Discussion
3-CQA, a prominent member of the CGA family, exhibits a broad spectrum of bioactive and therapeutic functions (8, 22, 23). Its widespread distribution in edible foods and herbs (24, 25), coupled with the prevalence of non-standard practices in its determination, underscores the pressing need for the development of a robust quantification method. In this pursuit, we sought to establish a highly sensitive and specific ic-CLEIA method for 3-CQA analysis, as reports have yet to explore the application of ic-CLEIA for determining 3-CQA levels prior to this study.
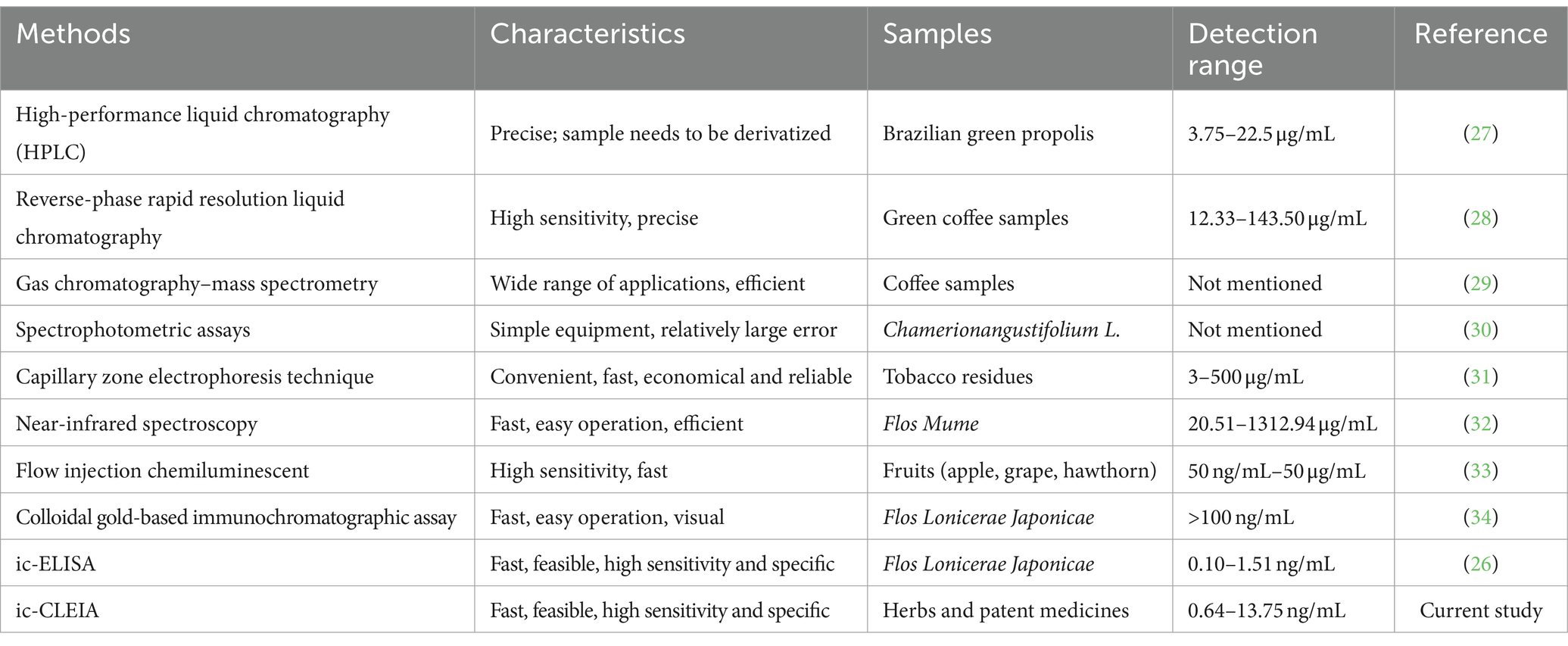
The inherent small molecule nature of 3-CQA renders it devoid of immunogenicity. We devised a method wherein 3-CQA was chemically conjugated with BSA or OVA to generate an artificial antigen capable of eliciting an immune response in mice. Leveraging the mAb specifically targeting the conjugate, we developed an ic-CLEIA with remarkable efficacy. It demonstrated an IC50 of 2.97 ng/mL, displaying linear absorbance values across a concentration range of 0.64–13.75 ng/mL. Impressively, the LOD of the ic-CLEIA was 0.39 ng/mL, comparable to the reported LOD (0.1 ng/mL) using ELISA (26), while significantly outperforming the ic-ELISA (6.71 ng/mL) in our parallel investigations. In addition, in comparison to previously reported instrumental methods for 3-CQA analysis (Table 3), including HPLC, reverse-phase rapid resolution liquid chromatography, capillary zone electrophoresis technique, and near-infrared spectroscopy, our ic-CLEIA emerged as a superior alternative reflected in the aspects such as high sensitivity and avoidance of expensive equipment and specialized operations. While Zhang’s ic-ELISA demonstrated a detection range of 0.10–1.51 ng/mL, which is narrower than our ic-CLEIA range of 0.64–13.75 ng/mL, our ic-CLEIA method provides a broader detection range to accommodate a wider variety of sample concentrations.
Moreover, in terms of specificity, the established ic-CLEIA revealed relatively low cross-reactivity (<5%) with most 3-CQA structural analogs, with only 3, 5-dicaffeoylquinic acid exhibiting slightly higher cross-reactivity (10.71%) (Table 1). This occurrence implies the active role of the substitution of hydroxyl moiety in position 3 of the quinic acid in influencing cross-reactivity patterns. These results suggest the relatively high specificity of the ic-CLEIA method in determining 3-CQA. Moreover, the spiked recoveries, a critical index for evaluating the reliability of immunoassays (35), ranged from 75.87 to 121.70%, with relative standard deviations varying from 0.09 to 12.39%, thereby affirming the capability of the ic-CLEIA for detecting 3-CQA in herbs and patent medicines.
One thing worth highlighting is the eco-friendliness and user-friendly attributes of our detection process. Through optimization, we uncovered that the deionized water in the absence of ions, surfactants, and organic reagents serves as an optimal buffer for ic-CLEIA. The exclusion of organic solvents and the elimination of complex sample pre-treatment present clear advantages over HPLC and other instrumental techniques. Meanwhile, in this study, we set the interaction time at 50 min; considering the vulnerability of 3-CQA and other CGA family compounds, further optimization of reaction time may enable even greater efficiency in simultaneous detection of 3-CQA, realizing the rapid high-throughput quantification.
Furthermore, the established ic-CLEIA was applied for the analysis of 3-CQA contents in 13 herb or patent medicine samples, revealing varying levels of 3-CQA across different samples (Supplementary Table S2), with higher contents observed in Honeysuckle and Eucommia. According to the Chinese Pharmacopeia, the minimum effective contents of 3-CQA in Honeysuckle, Eucommia, and Lonicera stem are 1.5% (15 mg/g), 0.08% (0.8 mg/g), and 0.1% (1 mg/g), respectively (21). Consequently, the herbs in this study met the criteria for qualified 3-CQA content. In addition, these herbs with therapeutic effects as individual components are essential raw materials in patient medicines, such as Lianhua Qingwen capsule, renowned for their antipyretic, antibacterial, and anti-inflammatory effects (36). We tested a total of 7 patent medicines, five of which adhere to clear 3-CQA content regulations in pharmacopeia: Shuang-huang-lian capsules (≥ 3 mg/capsule), Yin-huang granules (≥ 5 mg/bag), Yin-Zhi-huang granules (≥ 1.8 mg/bag), Kou-yan-qing granules (≥ 4 mg/bag), and Vitamin C Yin-qiao tablets (≥ 1.5 mg/tablet) (21). Detailed information on these medicines (Supplementary Table S2) confirms their compliance with the minimum 3-CQA content requirements. Additionally, good linearity was observed between the ic-CLEIA and HPLC analysis in detecting 3-CQA (R2 = 0.9667, Supplementary Figure S5), further attesting to the reliability and efficiency of our developed ic-CLEIA for 3-CQA analysis.
Of course, although herbal medicine quality control mandates active ingredient detection, it is equally essential to achieve real-time and rapid detection of toxic and harmful substances generated or retained during herbal growth and processing. Our study serves as a compelling paradigm for 3-CQA determination, opening avenues for extending the application of this method to quantify other members within the CGA family, like 4-CQA, contributing to advancements in herbal medicine analysis and quality assurance.
5 Conclusion
Collectively, we established an ic-CLEIA with a lower IC50 value (2.97 ng/mL) and a wider detection range (0.64–13.75 ng/mL) compared to the ic-ELISA (IC50: 36.06 ng/mL; detection range: 10.21–127.36 ng/mL). Without complex sample pre-treatment steps, this novel approach was validated under different sample conditions, including herbs and patent medicines. The recoveries were satisfactory (75.87–121.70%) with RSD of 0.09 to 12.39%. The results were strongly correlated with HPLC analysis (R2 = 0.9667). In conclusion, the established method was simple, time-saving, sensitive and efficient for rapid detection of 3-CQA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was approved by Chinese-German Joint Institute for Natural Product Research, Shaanxi University of Technology, and approval no. 2021–01. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LW: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft. MD: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. RW: Data curation, Validation, Writing – review & editing. MI: Writing – review & editing. XZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was finically supported by Expert Workstation Program of Hanzhong Municipality (2022–27); the Incubation Project of State Key Laboratory of Biological Resources and Ecological Environment of Qinba Areas (SLGPT2019KF04–04), and the Graduate Innovation Fund Project of Shaanxi University of Technology (SLGYCX2318).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1439287/full#supplementary-material
References
1. Soto-Hernandez, M, Palma Tenango, M, and García-Mateos, M. Phenolic compounds-biological activity. (2017).
2. Zhang, Y, Cai, P, Cheng, G, and Zhang, Y. A brief review of phenolic compounds identified from plants: their extraction, analysis, and biological activity. Nat Prod Commun. (2022) 17:10697. doi: 10.1177/1934578X211069721
3. Lou, Z, Zheng, X, Bede, D, Dai, W, Wan, C, Wang, H, et al. New perspectives for mechanisms, ingredients, and their preparation for promoting the formation of beneficial bacterial biofilm. J Food Meas Charact. (2023) 17:2386–403. doi: 10.1007/s11694-022-01777-5
4. Liang, N, and Kitts, D. Role of Chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. (2015) 8:16. doi: 10.3390/nu8010016
5. Liang, N, Dupuis, J, Yada, R, and Kitts, D. Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol Cell Biochem. (2019) 457:105–18. doi: 10.1007/s11010-019-03516-9
6. Nevena, GL, Branislava, R, Emilia, S, Dusica, R, Ivan, N, Nebojsa, K, et al. Determination of 5-Caffeoylquinic acid (5-Cqa) as one of the major classes of Chlorogenic acid in commercial tea and coffee samples. Vojnosanit Pregl. (2015) 72:1018–23. doi: 10.2298/VSP130915096G
7. Wianowska, D, and Gil, M. Recent advances in extraction and analysis procedures of natural Chlorogenic acids. Phytochem Rev. (2019) 18:273–302. doi: 10.1007/s11101-018-9592-y
8. Liu, W, Li, J, Zhang, X, Zu, Y, Yang, Y, Liu, W, et al. Current advances in naturally occurring Caffeoylquinic acids: structure, bioactivity, and synthesis. J Agric Food Chem. (2020) 68:10489–516. doi: 10.1021/acs.jafc.0c03804
9. Anggreani, E, and Lee, CY. Neuroprotective effect of Chlorogenic acids against Alzheimer’s disease. International journal of food science. Nutr Diet. (2017) 6:330–7. doi: 10.19070/2326-3350-1700059
10. Adeosun, W, More, G, Steenkamp, P, Prinsloo, G, Moco, S, Levanova, A, et al. Influence of seasonal and geographic variation on the anti-Hsv-1 properties and Chlorogenic acids content of Helichrysum Aureonitens Sch. Bip. Front Mol Biosci. (2022) 9:961859. doi: 10.3389/fmolb.2022.961859
11. Singdam, P, Naowaboot, J, Senggunprai, L, Boonloh, K, Hipkaeo, W, and Pannangpetch, P. The mechanisms of Neochlorogenic acid (3-Caffeoylquinic acid) in improving glucose and lipid metabolism in rats with insulin resistance induced by a high fat-high fructose diet. Trends Sci. (2023) 20:6455–5. doi: 10.48048/tis.2023.6455
12. Yan, Y, Zhou, X, Guo, K, Zhou, F, and Yang, H. Use of Chlorogenic acid against diabetes mellitus and its complications. J Immunol Res. (2020) 2020:1–6. doi: 10.1155/2020/9680508
13. Shi, A, Li, T, Zheng, Y, Song, Y, Wang, H, Wang, N, et al. Chlorogenic acid improves Nafld by regulating gut microbiota and Glp-1. Front Pharmacol. (2021) 12:693048. doi: 10.3389/fphar.2021.693048
14. Lu, H, Tian, Z, Cui, Y, Liu, Z, and Ma, X. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr Rev Food Sci Food Saf. (2020) 19:3130–58. doi: 10.1111/1541-4337.12620
15. Meinhart, AD, Damin, FM, Caldeirão, L, de Jesus, FM, da Silva, LC, da Silva, CL, et al. Study of new sources of six Chlorogenic acids and Caffeic acid. J Food Compos Anal. (2019) 82:103244. doi: 10.1016/j.jfca.2019.103244
16. Gamiotea-Turro, D, Camaforte, NAP, Valerino-Diaz, AB, Ortiz Nuñez, Y, Rinaldo, D, Dokkedal, AL, et al. Qualitative and quantitative analysis of Ethanolic extract and phenolic fraction of Jatropha Aethiopica (Euphorbiaceae) leaves and their hypoglycemic potential. J Agric Food Chem. (2018) 66:1419–27. doi: 10.1021/acs.jafc.7b05648
17. Yu, HC, Huang, SM, Lin, WM, Kuo, CH, and Shieh, CJ. Comparison of artificial neural networks and response surface methodology towards an efficient ultrasound-assisted extraction of Chlorogenic acid from Lonicera Japonica. Molecules (Basel, Switzerland). (2019) 24:2304. doi: 10.3390/molecules24122304
18. Song, J, Wang, R-M, Wang, Y-Q, Tang, Y-R, and Deng, A-P. Hapten design, modification and preparation of artificial antigens. Chin J Anal Chem. (2010) 38:1211–8. doi: 10.1016/S1872-2040(09)60063-3
19. Liu, J, Zhang, H, Zhang, D, Gao, F, and Wang, J. Production of the monoclonal antibody against Sudan 2 for immunoassay of Sudan dyes in egg. Anal Biochem. (2012) 423:246–52. Epub 2012/02/14. doi: 10.1016/j.ab.2012.02.001
20. Xu, L, Suo, XY, Zhang, Q, Li, XP, Chen, C, and Zhang, XY. Elisa and Chemiluminescent enzyme immunoassay for sensitive and specific determination of Lead (ii) in water, food and feed samples. Foods (Basel, Switzerland). (2020) 9:305. doi: 10.3390/foods9030305
21. Commission CP . The pharmacopoeia of the People’srepublic of China. China Medical Science Press Beijing (2020).
22. Ud Din, SR, Saeed, S, Khan, SU, Kiani, FA, Alsuhaibani, AM, and Zhong, M. Bioactive compounds (bacs): a novel approach to treat and prevent cardiovascular diseases. Curr Probl Cardiol. (2023) 48:101664. doi: 10.1016/j.cpcardiol.2023.101664
23. Gupta, A, Atanasov, AG, Li, Y, Kumar, N, and Bishayee, A. Chlorogenic acid for Cancer prevention and therapy: current status on efficacy and mechanisms of action. Pharmacol Res. (2022) 186:106505. doi: 10.1016/j.phrs.2022.106505
24. Li, Z, Zhang, J, Meng, Q, Yang, L, Qiu, M, Li, Y, et al. The content and distribution of 18 phenolic compounds in 462 batches of edible flowers from 73 species commercially available in China. Food Res Int. (2023) 166:112590. doi: 10.1016/j.foodres.2023.112590
25. Woźniak, D, Nawrot-Hadzik, I, Kozłowska, W, Ślusarczyk, S, and Matkowski, A. Handbook of Dietary Phytochemicals. Berlin: Springer, pp. 1065–1104. (2021).
26. Zhang, B, Nan, TG, Zhan, ZL, Kang, LP, Yang, J, Lai, CJ, et al. A monoclonal antibody-based enzyme-linked immunosorbent assay for the determination of Chlorogenic acid in honeysuckle. J Pharm Biomed Anal. (2018) 148:1–5. doi: 10.1016/j.jpba.2017.09.023
27. Sun, S, Liu, M, He, J, Li, K, Zhang, X, and Yin, G. Identification and determination of seven phenolic acids in Brazilian green Propolis by Uplc-Esi-Qtof-Ms and Hplc. Molecules (Basel, Switzerland). (2019) 24:1791. doi: 10.3390/molecules24091791
28. Velkoska-Markovska, L, Jankulovska, MS, Petanovska-Ilievska, B, and Hristovski, K. Development and validation of Rrlc–Uv method for determination of Chlorogenic acid in green coffee. Acta Chromatogr. (2020) 32:34–8. doi: 10.1556/1326.2019.00547
29. Heo, J, Adhikari, K, Choi, KS, and Lee, J. Analysis of caffeine, Chlorogenic acid, Trigonelline, and volatile compounds in cold brew coffee using high-performance liquid chromatography and solid-phase microextraction-gas chromatography-mass spectrometry. Foods (Basel, Switzerland). (2020) 9:1746. doi: 10.3390/foods9121746
30. Kaškonienė, V, Stankevičius, M, Drevinskas, T, Akuneca, I, Kaškonas, P, Bimbiraitė-Survilienė, K, et al. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion Angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry. (2015) 115:184–93. doi: 10.1016/j.phytochem.2015.02.005
31. Li, Z, Huang, D, Tang, Z, Deng, C, and Zhang, X. Fast determination of Chlorogenic acid in tobacco residues using microwave-assisted extraction and capillary zone electrophoresis technique. Talanta. (2010) 82:1181–5. doi: 10.1016/j.talanta.2010.06.037
32. Yan, H, Pu, Z, Wang, Y, Guo, S, Wang, T, Li, S, et al. Rapid qualitative identification and quantitative analysis of Flos Mume based on Fourier transform near infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. (2021) 249:119344. doi: 10.1016/j.saa.2020.119344
33. Wang, X, Wang, J, and Yang, N. Chemiluminescent determination of Chlorogenic acid in fruits. Food Chem. (2007) 102:422–6. doi: 10.1016/j.foodchem.2006.03.002
34. Zhang, B, Nan, TG, Xin, J, Zhan, ZL, Kang, LP, Yuan, Y, et al. Development of a colloidal gold-based lateral flow dipstick immunoassay for rapid detection of Chlorogenic acid and Luteoloside in Flos Lonicerae Japonicae. J Pharm Biomed Anal. (2019) 170:83–8. doi: 10.1016/j.jpba.2019.03.035
35. Xu, J, Zhu, LY, Shen, H, Zhang, HM, Jia, XB, Yan, R, et al. A critical view on spike recovery for accuracy evaluation of analytical method for medicinal herbs. J Pharm Biomed Anal. (2012) 62:210–5. doi: 10.1016/j.jpba.2011.12.034
Keywords: 3-caffeoylquinic acid (3-CQA), indirect competitive chemiluminescence enzyme immunoassay (ic-CLEIA), monoclonal antibody (mAb), enzyme-linked immunosorbent assay (ELISA), quality control
Citation: Wu L, Dang M, Wu R, Isah MB and Zhang X (2024) Development of an ic-CLEIA for precise detection of 3-CQA in herbs and patent medicines: ensuring quality control and therapeutic efficacy. Front. Nutr. 11:1439287. doi: 10.3389/fnut.2024.1439287
Edited by:
Geraldine M. Dowling, Atlantic Technological University, IrelandReviewed by:
Lingguang Yang, Yichun University, ChinaZaixiang Lou, Jiangnan University, China
Kai Zhou, Jiujiang University, China
Copyright © 2024 Wu, Dang, Wu, Isah and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoying Zhang, eHpoYW5nNjdAdW9ndWVscGguY2E=
 Longjiang Wu1
Longjiang Wu1 Mei Dang
Mei Dang Rao Wu
Rao Wu Murtala Bindawa Isah
Murtala Bindawa Isah Xiaoying Zhang
Xiaoying Zhang