- 1Institute of Biomedical Science, National Sun Yat-sen University, Kaohsiung, Taiwan
- 2Division of Urology, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan
- 3Department of Medical Research, Chi Mei Medical Center, Yongkang, Tainan, Taiwan
- 4Department of Information Management, Southern Taiwan University of Science and Technology, Tainan, Taiwan
- 5Department of Urology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
- 6Department of Pathology, Chi Mei Medical Center, Tainan, Taiwan
- 7Institute of Precision Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
Purpose: This study investigates the complex relationship between body mass index (BMI) and bladder cancer outcomes, utilizing Taiwan’s national database. Bladder cancer remains a significant health concern, especially in Taiwan, prompting a comprehensive retrospective analysis to explore the impact of obesity on survival outcomes.
Materials and methods: A meticulous exclusion process, based on Taiwan National Health Insurance System Database, refined the initial dataset of 15,086 bladder cancer patients to 10,352. Categorizing patients into BMI groups (underweight, normal weight, and obesity), the study examined baseline characteristics, comorbidities, and survival outcomes. The analysis involved Cox regression and subgroup assessments stratified by clinical stage.
Results: Among our patients, 71.5% are male, 78.5% are over 60 years of age, and 18.8% are between 45 and 60 years old. Despite a higher prevalence of comorbidities, obesity patients exhibited a more favorable prognosis, supporting the obesity paradox. The overall and specific mortality ratio of obesity patients were 0.76 fold and 0.82-fold compared with normal-weight patients (overall: 95% confidence interval [CI], 0.71–0.82, p < 0.0001; specific: 95% CI, 0.75–0.90, p < 0.0001). Conversely, underweight patients displayed an increased risk of both overall and cancer-specific mortality compared to normal-weight patients (p < 0.0001).
Conclusion: This study highlights the potential protective role of higher BMI in bladder cancer survival, revealing a more favorable prognosis among obesity patients, highlighting the need for cautious interpretation and suggesting avenues for future research. These insights could guide BMI-targeted intervention strategies, allowing clinicians to consider BMI as a factor in personalized treatment planning for bladder cancer patients.
Introduction
Cancer treatment has evolved significantly from surgery and radiotherapy to targeted therapies and immunotherapies, achieving increased efficacy, reduced toxicity, and improved patient outcomes (1). Bladder cancer ranks among the prevalent malignancies affecting the urinary system globally, with an annual Age Standardized Incidence Rate (ASR) of 9.6 per 100,000 for males and 2.4 per 100,000 for females worldwide (2). Non-muscle-invasive bladder cancer is associated with an overall survival rate of approximately 90% (3). In patients with muscle-invasive bladder cancer undergoing bladder-preserving combined-modality therapy, the 5- and 10-year overall survival rates are 57 and 36%, respectively (4). In contrast, survival for metastatic bladder cancer remains poor, with a median survival of 3–6 months without treatment and extending to 12–15 months with treatment (5).
Data from the Taiwan Cancer Registry (TCR) shows that the projected age-standardized incidence rates per 100,000 people for men are 13.0 in 2020 and 10.4 in 2025, while for women, the rates are 4.7 in 2020 and 3.7 in 2025 (6). The accelerated aging of the population in Taiwan underscores the continued significance of bladder cancer as a serious health concern. Therefore, a comprehensive examination of potential risk factors and factors influencing progression remains imperative.
Overweight and obesity may exhibit an escalated risk of bladder cancer, demonstrating a Dose–Response correlation (7). This association is likely attributed to inflammatory processes, alterations in sex hormone metabolism, abnormal insulin and insulin-like growth factor levels, adipokine pathways, and microenvironment perturbations that contribute to tumor cell growth and proliferation (8). The implications for survival outcomes are intricate, marked by contradictory findings in the literature. Some studies report a negative correlation between higher body mass index (BMI) and prognosis; for instance, a large multi-institutional series showed that obesity is associated with worse cancer-specific outcomes in patients treated with radical cystectomy for urothelial carcinoma of the bladder, with higher risks of disease recurrence and cancer-specific mortality (9). However, other studies identify better survival outcomes. Data from the PROspective MulticEnTer RadIcal Cystectomy Series (2011) indicated that the overall survival rate of obese patients was superior to that of normal-weight patients (10). Additionally, for patients with non-muscle invasive bladder cancer treated with transurethral resection of bladder tumor and adjuvant intravesical BCG, Huang et al. (11) found that obese patients had better overall survival than non-obese patients. This phenomenon, known as the “obesity paradox,” refers to the observation that higher BMI is associated with decreased mortality risk in certain cancers, contradicting expectations (12). The obesity paradox is well recognized in the cardio-metabolic literature but is less commonly discussed in oncology (13). However, it has been reported in various cancers, including lymphoma, leukemia, colorectal, gastric, renal, and lung cancers (14).
Given that Asian populations typically exhibit higher body fat accumulation and elevated levels of adipocytokines at equivalent BMI levels compared to their Western counterparts (15), assessing this correlation within the Taiwanese group is paramount. The primary objective of this study is to rigorously analyze the association between BMI and bladder cancer, utilizing nationally representative data from the Taiwanese population and stratifying the analysis by cancer stages. This approach aims to contribute valuable insights into the complex interplay between adiposity and bladder cancer risk and prognosis.
Materials and methods
Data source
Datasets from the TCR, the Taiwan’s National Health Insurance Research Database (NHIRD), and the Taiwan’s Cause-of-Death Database were used in this study. These claims datasets were all from the Health and Welfare Data Science Center (HWDC), an integrated health-related database center. The TCR, established in 1979, monitors cancer incidence and mortality rates across Taiwan. It collects data on individual demographics, cancer stages, primary tumor sites, histology, and treatment types. Bladder cancer incidence data from 1997 to 2016 were obtained from this publicly available, nationwide, population-based registry. Since reaching maturity in 2003, the registry has maintained high data quality, with timeliness under 14 months, completeness exceeding 98%, a morphological verification rate of approximately 93%, and fewer than 1% of cases registered solely by death certificate (6). NHIRD is based on Taiwan’s national health insurance program, which includes detailed healthcare information of more than 99% of Taiwan’s population from 1996 to 2018. For research purposes, the HWDC released the de-identified claims to the public in an anonymous format. This study was conducted in compliance with the Declaration of Helsinki and has been approved by the Research Ethics Committee of Chi Mei Hospital (IRB no. 11301–017). In addition, patient informed consent was waived by the Research Ethics Committee of Chi Mei Hospital.
Definitions of study subjects
Patients with bladder cancer between 2011 and 2018 were selected from the TCR using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3): C67. The patients with incomplete information in the TCR were excluded from this study. Exclusion criteria were applied to ensure a homogeneous sample and reduce bias. Patients with clinical stage 0 or missing data were excluded due to the impact of unclear staging on prognosis and treatment analysis. Those with missing height or weight data were excluded to maintain accurate BMI calculations, a key factor in the study. Patients without data on smoking, drinking, or betel nut chewing were excluded as these lifestyle factors can influence cancer outcomes, and missing data would introduce confounding. Finally, patients under 20 years old were excluded due to the extreme rarity of cancer in children and adolescents (16), with available data primarily limited to case reports, ensuring a more clinically relevant and homogenous study population.
The World Health Organization (WHO) defines overweight as a BMI ≥25 kg/m2 and obesity as a BMI ≥30 kg/m2, regardless of race or sex (17). However, Asian populations have an increased risk of diabetes and cardiovascular diseases even at a BMI ≤25 kg/m2. Consequently, the WHO Asia-Pacific region defines overweight as BMI ≥23 kg/m2 and obesity as BMI ≥25 kg/m2 (18). Therefore, in our study, we used a BMI cutoff of 25 kg/m2 to define obesity. A flowchart summarizing the selection of study subjects is presented in Figure 1.
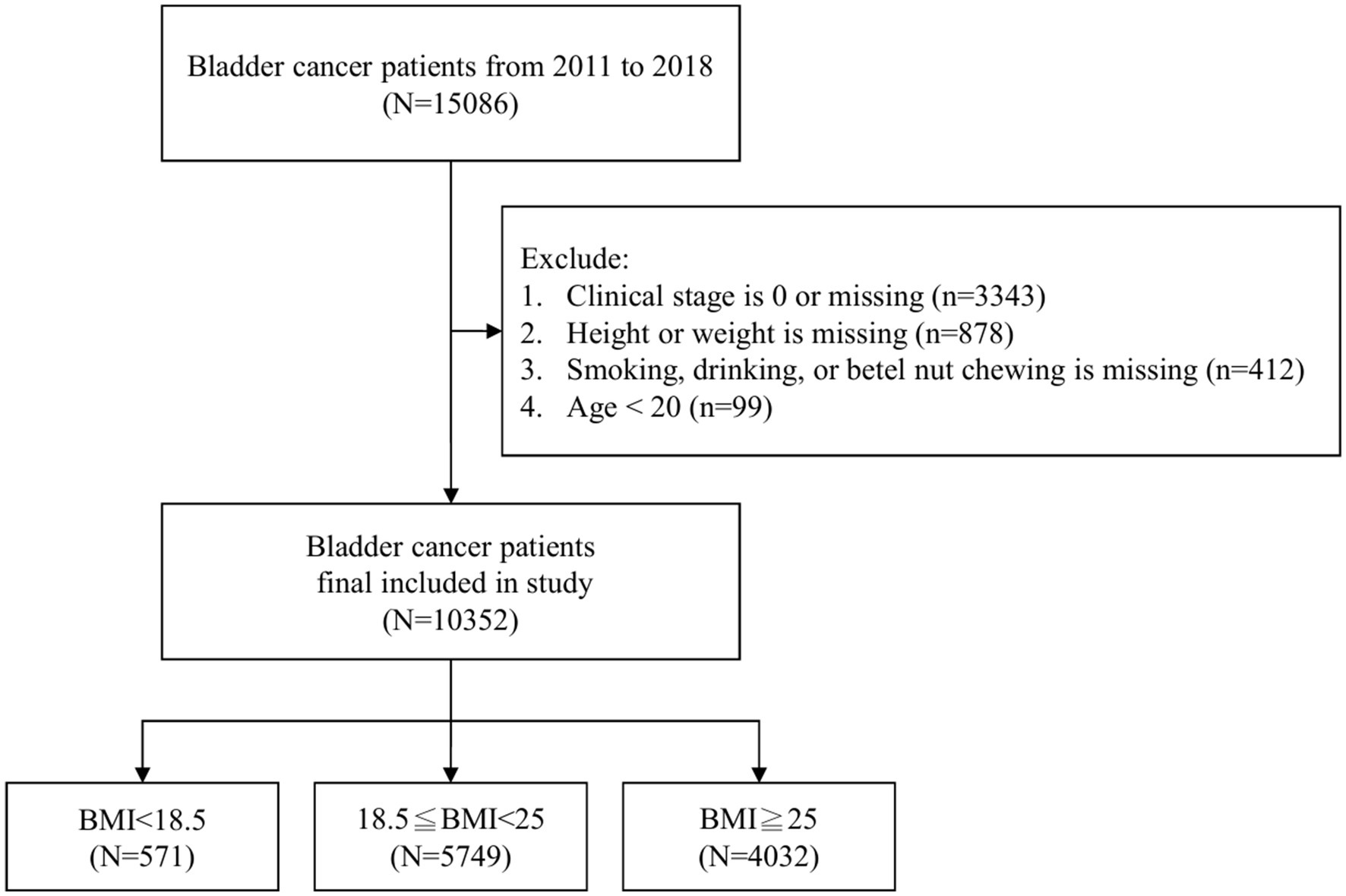
Figure 1. Exclusion process in the initial dataset of 15,086 bladder cancer patients, resulting in 10,352 individuals categorized into underweight (BMI < 18.5, n = 571), normal weight (18.5 ≤ BMI < 25, n = 5,749), and overweight (BMI ≥ 25, n = 4,032).
Measurement
The primary outcome of this study was mortality. Mortality was determined from the Cause-of-Death Dataset. The mortality risk was estimated using two endpoints: overall and cancer-specific mortalities. The time for the overall mortality was set from the date of bladder cancer diagnosis to the date of death, regardless of the cause. Cancer-specific mortality was defined as the cause of death due to bladder cancer.
Patients with comorbidities were defined as patients with the diseases included below at least 1 year before the diagnosis of bladder cancer. This definition was based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes in Appendix 1. Comorbidities included the following: myocardial infarction; congestive heart failure, peripheral vascular disease, cardiovascular disease, dementia, chronic obstructive pulmonary disease, renal disease, diabetes mellitus, hypertension, and liver disease.
In the stratification, patients with bladder cancer were categorized by gender, age group, and clinical stage to assess the risk of overall and cancer-specific mortality across different BMI categories. This approach allowed for a more nuanced understanding of how these factors influence mortality risks. Gender plays a significant role in bladder cancer outcomes, as men have a fourfold higher incidence rate than women, while female patients often present with higher-grade disease and tend to experience worse outcomes (19). Age was divided into three categories: 20–45 years, 45–60 years, and 60 years or older. Lastly, patients were stratified by clinical stage, which was divided into early-stage (Stage I and II) and advanced-stage (Stage III and IV) bladder cancer. Clinical stage is a critical determinant of survival, with higher stages indicating more aggressive disease. Stratifying by clinical stage helps control for disease severity, ensuring accurate and meaningful comparisons between groups.
Statistical analysis
Continuous variables are presented as means ± standard deviations, while categorical variables are presented as frequencies with percentages. The Cox proportional regression model was chosen for its ability to assess the relationship between predictors and the time to an event, and was used to estimate the hazard ratios (HRs) of the overall and cancer-specific mortalities. This model is ideal for survival analysis, as it does not assume a specific distribution for the time variable. Confounders like age, sex, smoking status, and comorbidities were adjusted for to minimize bias and clarify the relationship between the main exposure and outcome. Adjusted HRs were calculated from the multivariable Cox regression with adjustment of age at diagnosis, sex, body mass index (BMI), Charlson Comorbidity Index (CCI) score, drinking, chewing betel nut, clinical stage, treatment type, and comorbidities. The stratified analysis was also presented to estimate the risk ratios of the overall and cancer-specific mortalities between bladder cancer patients with underweight, normal-weight and overweight patients. To avoid violation of the proportional hazards assumption, the estimated HRs were checked using the Schoenfeld residuals test. In addition, considering the differences in BMI classification between Asian populations and WHO standards, a sensitivity analysis was conducted using Taiwan’s BMI classification (underweight: <18.5 kg/m2; normal weight: 18.5–24 kg/m2; overweight: ≥24 kg/m2) to compared with the findings from WHO classification. The SAS 9.4 (SAS Institute, Inc., Cary, NC, United States) was used to perform all statistical analyses. A p-value <0.05 was set for statistical significance.
Results
In the initial dataset of 15,086 patients, a meticulous exclusion process led to the removal of 4,732 individuals from the analysis, following criteria such as clinical stage 0 or missing data (n = 3,343), absence of information on weight or height (n = 878), unrecorded data regarding smoking, drinking, or betel nut chewing (n = 412), and an age less than 20 (n = 99). The refined sample of 10,352 bladder cancer patients was then categorized into three distinct BMI groups: underweight (BMI < 18.5, n = 571), normal weight (18.5 ≤ BMI < 25, n = 5,749), and overweight (BMI ≥ 25, n = 4,032) as illustrated in Figure 1.
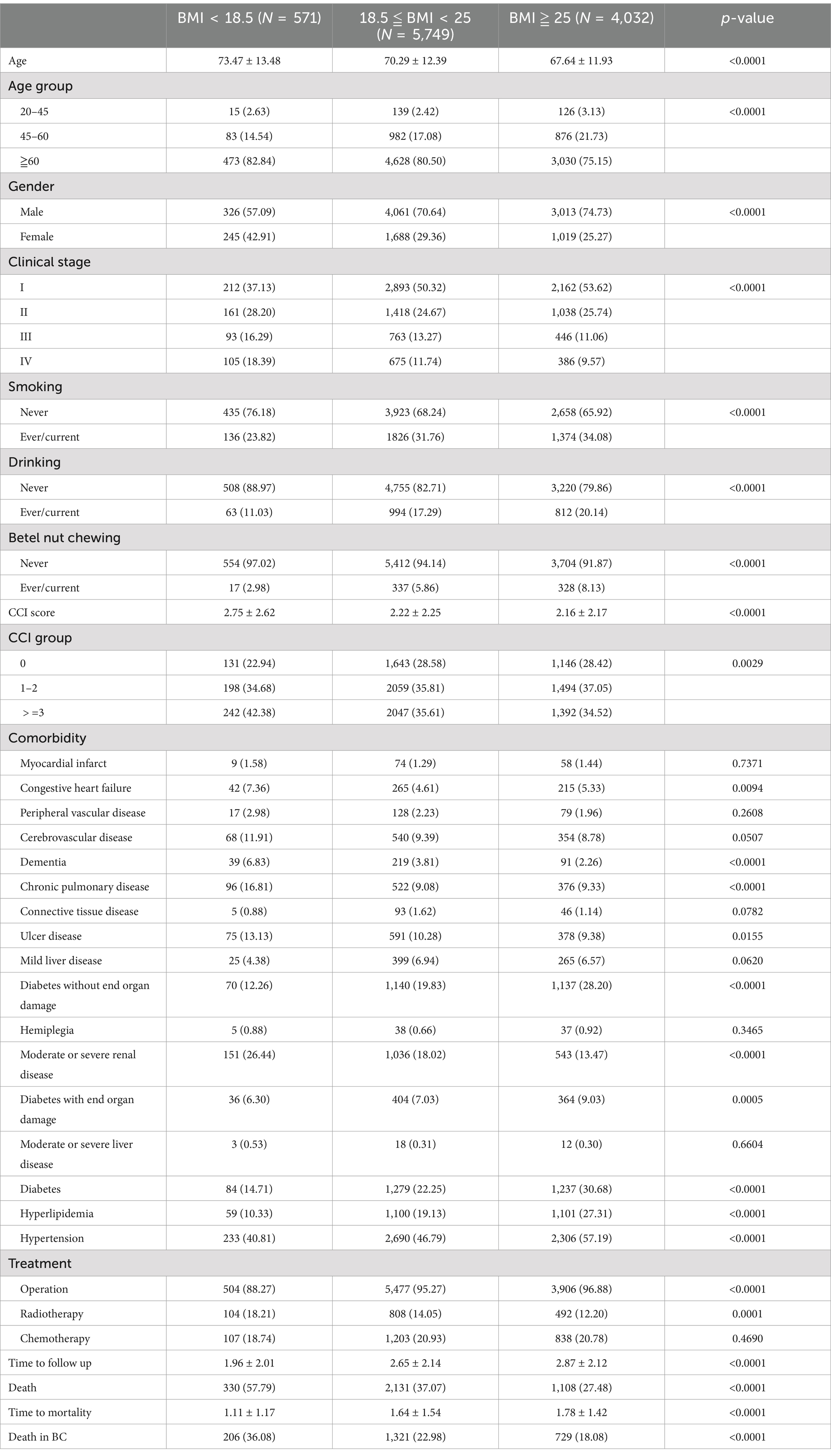
The baseline demographic and disease characteristics of bladder cancer patients, stratified by BMI category, revealed significant differences. Patients in the higher BMI cohort were younger, predominantly male (p < 0.0001) compared to those in lower BMI categories. Overweight patients had a higher prevalence of comorbidities, including diabetes (30.68%), hyperlipidemia (27.31%), and hypertension (57.19%), surpassing their normal or underweight counterparts (p < 0.0001). Overweight patients also exhibited a more favorable prognosis, marked by a lower overall mortality (27.48%), reduced bladder cancer-specific mortality (18.08%), and an extended survival period (1.78 ± 1.42 years) (p < 0.0001) (Table 1).
In terms of mortality risk, adjusted Cox regression analyses showed that the overall mortality rate in overweight patients was 0.76-fold (95% confidence interval [CI], 0.71–0.82, p < 0.0001) adjusted HR compared with normal-weight patients. In addition, the specific mortality rate of overweight patients was 0.82-fold (95% CI, 0.75–0.90, p < 0.0001) adjusted HR compared with normal-weight patients. The HRs with confidence intervals provide an estimate of the relative risk of overall and cancer-specific mortality. The crude HRs reflect the unadjusted risk, while the adjusted HRs (AHRs) account for factors such as age, sex, comorbidities, and treatment modalities, offering a more accurate assessment of the impact of each factor on survival outcomes. Conversely, the overall and specific mortality ratio of underweight patients were 1.58 fold and 1.50-fold compared with normal-weight patients (overall: 95% confidence interval [CI], 1.41–1.78, p < 0.0001; specific: 95% CI, 1.29–1.74, p < 0.0001) (Table 2).
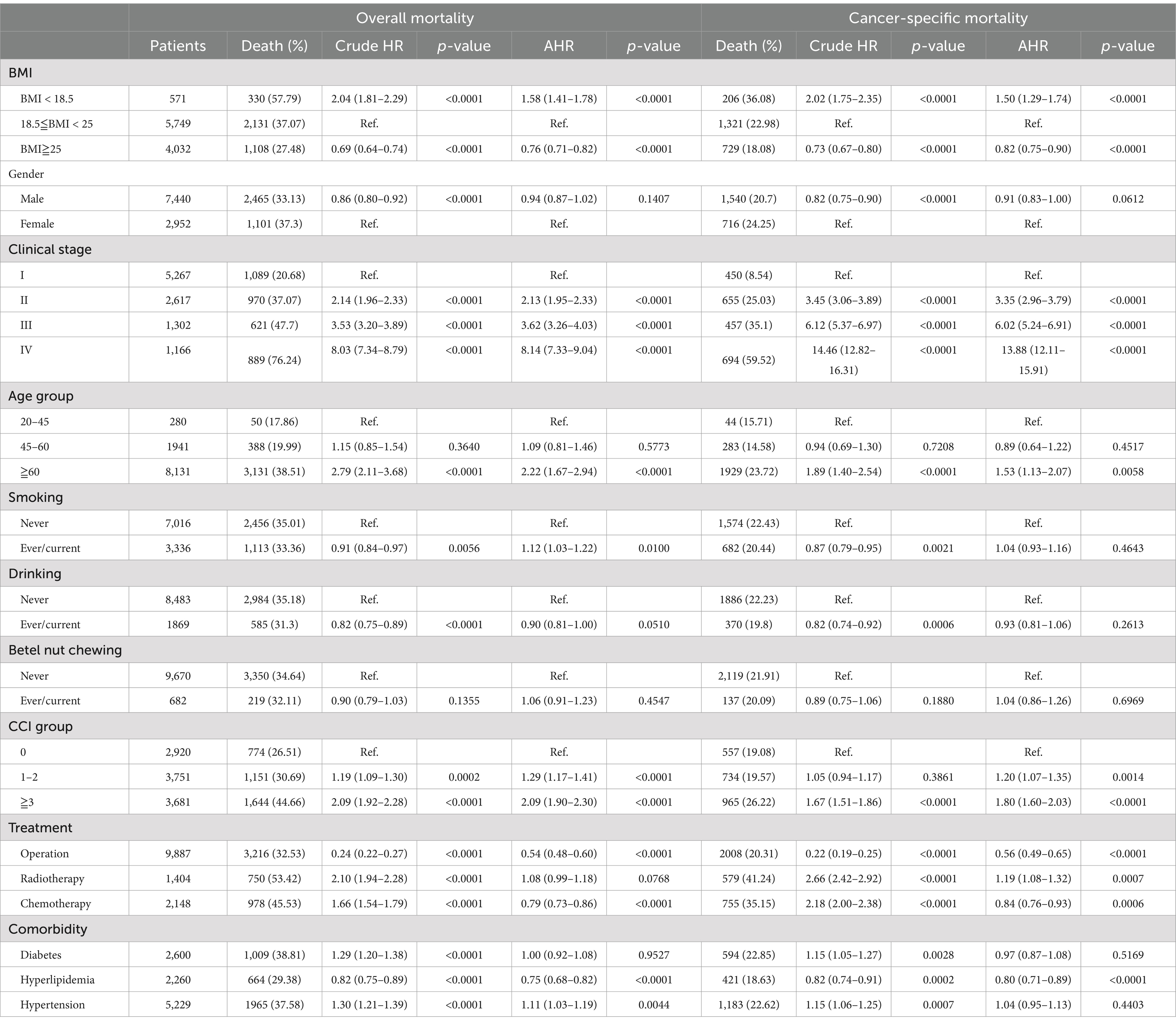
Table 2. The risk of overall and cancer-specific mortality among bladder cancer patients and stratified by gender, age group, and clinical stage.
In the subgroup analysis, overweight patients consistently exhibited a lower mortality rate, a trend that was significant across both genders and in overall and cancer-specific mortality (p < 0.01). When stratified by clinical stage, underweight patients in stages I, II, and III showed an increased risk of both overall and cancer-specific mortality (p < 0.01). Although a higher mortality rate was observed in stage IV underweight patients, this did not reach statistical significance (p > 0.05). Overweight patients exhibited a significantly lower overall and cancer-specific mortality rate across all stages, with the exception of cancer-specific mortality in stage I patients (AHR: 0.92, 95% CI: 0.75–1.12, p = 0.4104) (Table 3).
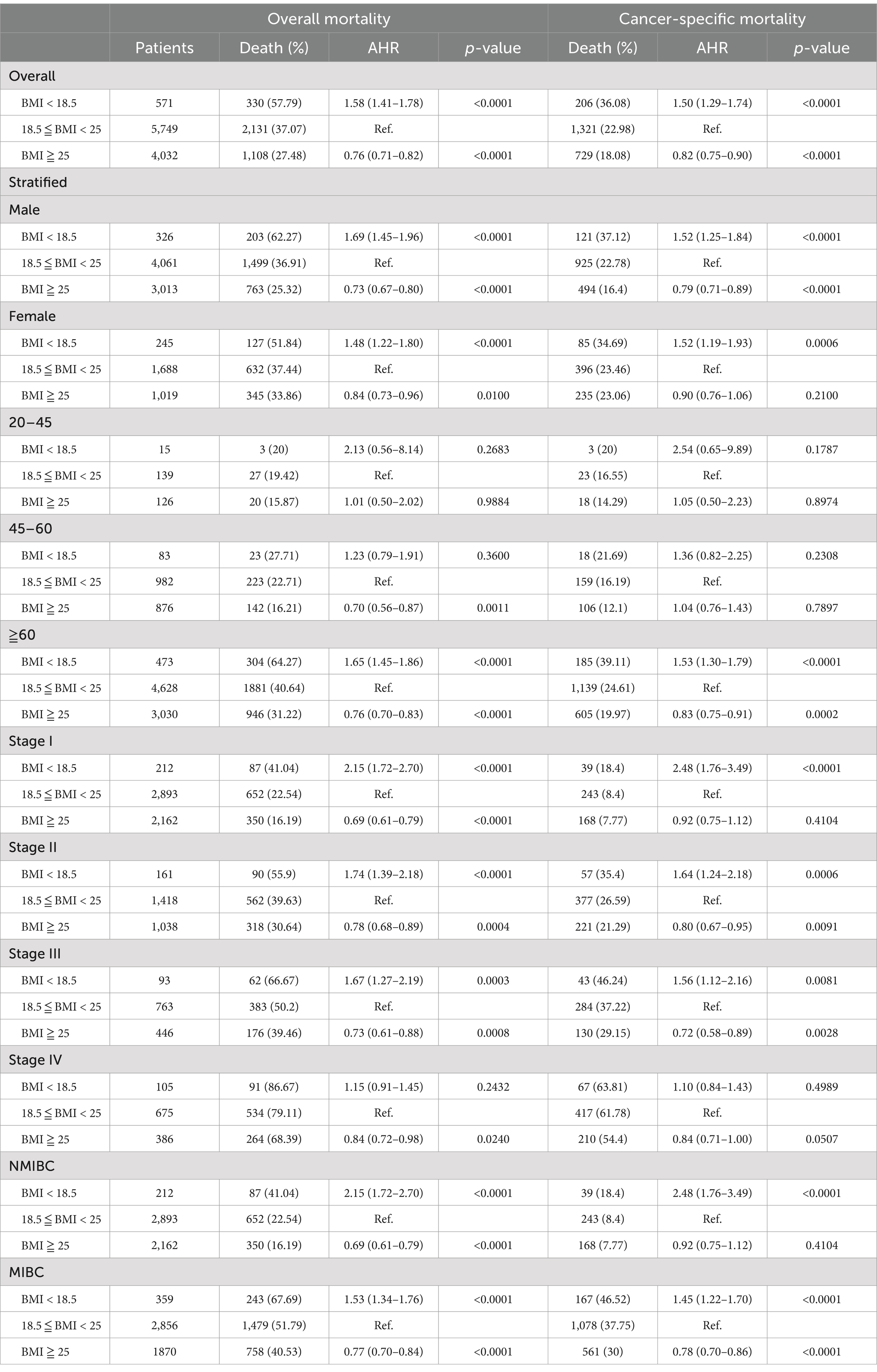
Table 3. The risk of overall and cancer-specific mortality among bladder cancer patients in different BMI groups stratified by gender, age group, and clinical stage.
Kaplan–Meier survival curves also demonstrated significantly trends of OS and PFS between different BMI categories with log-rank p < 0.0001 (Figures 2, 3). Additionally, the sensitivity analyses using Taiwan’s BMI classification also presented the consistent results with the above primary analysis (Supplementary Table S1).

Figure 2. Kaplan–Meier survival curves for OS stratified by BMI categories. A significant difference in survival was observed between the groups (Log-rank p < 0.0001). The number of patients at risk is indicated at the bottom of the figure for each time point.
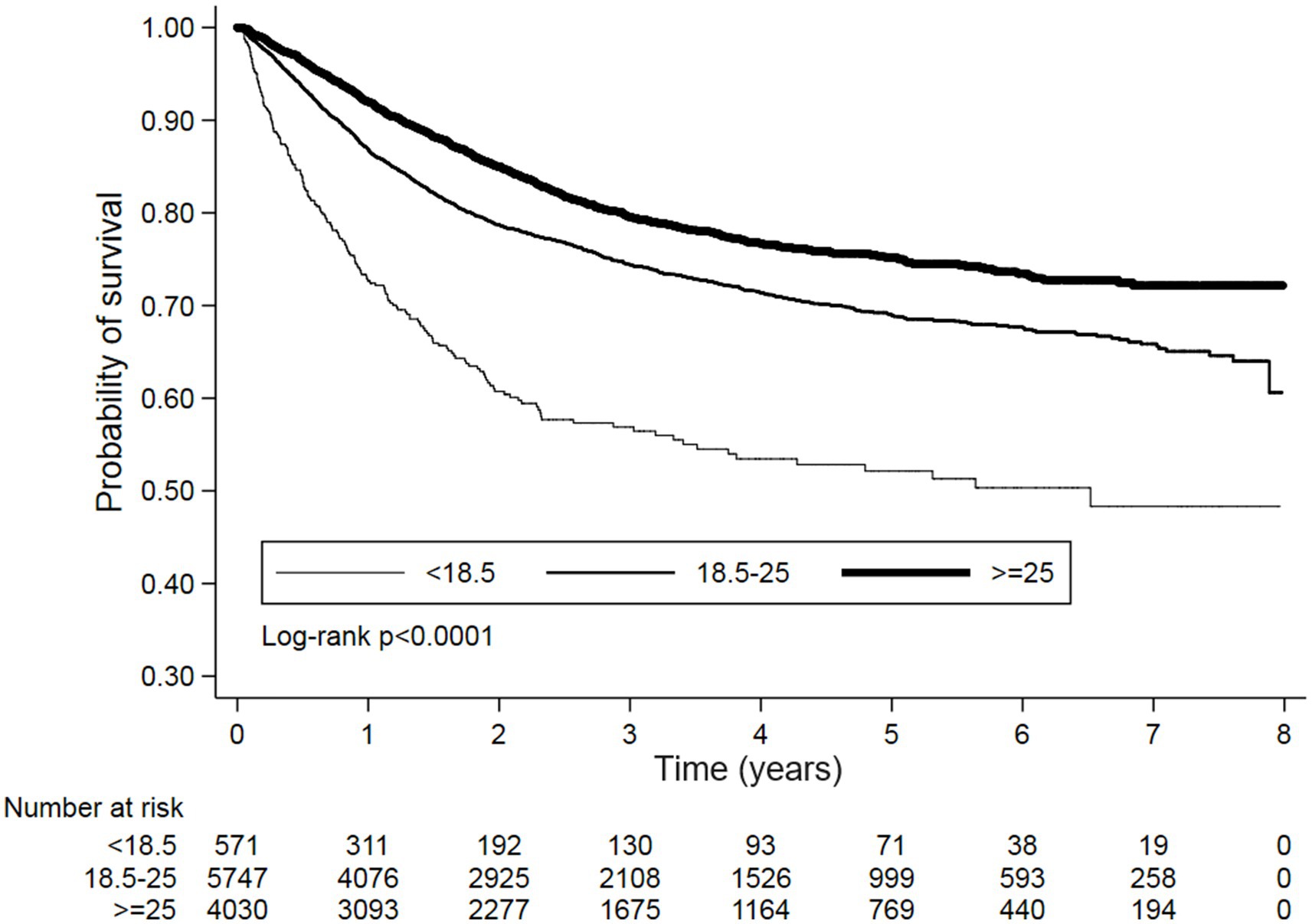
Figure 3. Kaplan–Meier survival curves for PFS stratified by BMI categories. A significant difference in survival was observed between the groups (Log-rank p < 0.0001). The number of patients at risk is indicated at the bottom of the figure for each time point.
Discussion
This study represents the inaugural comprehensive retrospective analysis within Taiwan’s national database, exploring the survival outcomes of bladder cancer among patients categorized by different BMI groups. Within the overweight group, despite a higher prevalence of comorbidities, a noteworthy decrease in both overall and cancer-specific mortality was observed, accompanied by an extended survival period. Our data support the obesity paradox hypothesis for greater precision, suggesting that the higher prevalence of comorbidities does not appear to significantly impact overall mortality in the subsequent 2–3 years.
The relationship between obesity and bladder cancer is complex, with ongoing debate in the scientific community. Systematic reviews suggest a modest association between obesity and bladder cancer incidence (RR = 1.1, 95% CI 1.07–1.13) and a similar trend for overweight individuals (RR = 1.07, 95% CI 1.03–1.1). A dose–response analysis indicated a BMI increase of 5 kg/m2 raised bladder cancer risk by 3% (RR = 1.03, 95% CI 1.02–1.05) (20). Data from the Korean National Health Insurance database found the highest bladder cancer risk in those with BMI ≥ 30 (HR = 1.17) (21). Additionally, a meta-analysis revealed a significantly higher recurrence rate in obese patients (HR = 1.76, 95% CI: 1.36–2.28) and a linear relationship between BMI and recurrence risk (HR = 1.01, 95% CI: 1.01–1.02), though obesity was not significantly associated with overall survival (HR = 1.21, 95% CI: 0.97–1.52) (22).
To elucidate the complex relationship between obesity and bladder cancer, several mechanisms have been proposed. A study by Zhao et al. (23) demonstrated that elevated levels of IGF-1 were associated with increased bladder cancer risk, while higher levels of IGF-binding protein-3 were protective, with a dose–response relationship between these factors and cancer risk. In obesity, hyperinsulinemia caused by insulin resistance leads to reduced growth hormone secretion, but IGF-I levels remain stable due to increased hepatic GH sensitivity, with suppressed IGFBP-1 levels in response to elevated insulin (24), which may decreasing the protection toward bladder cancer. Another study in mice fed a high-fat diet revealed greater bladder inflammation and premalignant alterations, including increased dysplasia, proliferation, apoptosis, and activation of inflammatory pathways (NFκB, IKKβ, JNK, and c-JUN). The tumor microenvironment plays a key role in cancer development, growth, and progression (25).
The role of BMI in predicting survival outcomes for bladder cancer patients exhibits substantial variability across studies. A meta-analysis focusing on urothelial cancer patients undergoing radical surgery demonstrated that overweight individuals had improved Cancer-Specific Survival (CSS) and Recurrence-Free Survival (RFS), while obesity and underweight were associated with unfavorable survival outcomes (26). The significant heterogeneity observed in this review was partially attributed to the inclusion of studies from both Asian and Western populations, utilizing varying BMI category definitions. In our study, we addressed this heterogeneity by employing WHO-defined BMI categories tailored to the Asian population, aiming to rectify these discrepancies and reinforcing the observed findings associated with the obesity paradox. However, our database has limitations, lacking long-term follow-up data, which may be relevant to the study by Arthuso et al. (27), indicating diminishing differences observed in longer-term survival up to 5 years.
In our study, a higher proportion of obese patients were found to have diabetes, hypertension, and hyperlipidemia. Studies including meta-analyses have shown a positive correlation between these comorbidities and bladder cancer risk, as well as metabolic syndrome (28–30). A systematic review indicated that diabetic patients had a significantly higher risk for all-cause mortality (HR 1.24, 95% CI: 1.07–1.44) and cancer-specific mortality (HR 1.67, 95% CI: 1.29–2.16) compared to non-diabetics (31). Teleka et al. (32) found that systolic blood pressure was positively associated with bladder cancer-specific mortality (HR 1.10 [1.01–1.20]) among never-smokers, although weaker and non-significant associations were observed for others. Regarding hyperlipidemia, Tu et al. (33) reported that elevated cholesterol (CHOL), low HDL, and elevated triglycerides (TG) were linked to worse OS. Elevated CHOL, LDL, and TG, as well as lower HDL, significantly affected PFS. Furthermore, studies have shown that diet-induced and LDLR deficiency-induced hypercholesterolemia can enhance both bladder cancer stemness and progression (34). However, in our study, diabetes and hypertension did not show decreased OS or PFS, while hyperlipidemia was associated with significant OS and PFS benefits. This suggests that specific research on bladder cancer mortality in Taiwanese populations, and the impact of lipid profiles, warrants further investigation. Given the evidence of increased mortality in bladder cancer patients with comorbidities, and the fact that among 28 cancer types, bladder cancer patients have the highest risk of dying from cardiovascular disease (35), the persistence of the “obesity paradox” highlights its important impact on outcomes.
Although our study supports the “obesity paradox” hypothesis in bladder cancer, with observed reductions in both overall and cancer-specific mortality for overweight patients, these findings warrant cautious interpretation. The data contribute to the notion of the obesity paradox but highlight the need for greater precision in understanding how BMI influences bladder cancer outcomes. The obesity paradox has been observed in lung, renal, and metastatic melanoma cancers (36), which typically experience higher rates of surgical complications and early cancer-related deaths. Variations in BMI at pre-, peri-, and post-diagnosis may influence results (13), as evidenced by sarcopenia independently predicting Overall Survival (OS) and Cancer-Specific Survival (CSS) in a multicenter study of patients undergoing radical cystectomy for bladder cancer (37). Given that BMI is an inadequate measure of adiposity, other measurements, such as adipose compartment areas, may offer a more accurate assessment of the paradox. Studies adjusting for lean muscle wasting found no significant associations between obesity or adiposity measurements and all-cause mortality in patients treated with radical cystectomy (38). Confounding factors such as smoking, socioeconomic status, physical activity, diet, and ethnicity could impact our assumptions; for instance, exercise decreases and current smoking increases the risk of bladder cancer-specific mortality (39).
Additional biases, such as collider stratification bias, must be considered. Smoking, a well-established risk factor for bladder cancer, acts as a collider variable. Consequently, among non-obese cancer patients, the likelihood of other risk factors, such as smoking, increases, potentially generating an artificial inverse association (13). However, in our study, the obesity paradox persisted even after stratifying for smoking status, with underweight patients showing significantly increased overall mortality and overweight patients demonstrating significantly decreased overall mortality (Table 3).
Regarding detection bias, evidence indicates that diabetes and excess body weight can negatively impact bladder cancer prognosis and outcomes (31, 40). The phenomenon of reverse causality, where the prevalence of sarcopenia significantly increases during cancer treatment in patients with bladder cancer (41), further underscores the intricacies involved. These considerations emphasize the necessity for a cautious interpretation of our findings, acknowledging the potential influence of these biases on our understanding of the relationship between BMI and bladder cancer outcomes.
In the subgroup analysis of our study, while the obesity paradox persisted across stages, some exceptions were noted. Stage IV underweight patients displayed an insignificant mortality rate, and overweight stage I patients exhibited higher cancer-specific mortality. These findings align with previous studies, suggesting that underweight patients may experience increased postoperative complications after radical cystectomy, although no direct link between malnutrition and complications was identified (42). Additionally, evidence indicates that patients diagnosed with clinical T1 bladder cancer, treated with Bacillus Calmette-Guérin immunotherapy or transurethral resection and categorized as obese, have worse cancer-specific outcomes compared to their non-obese counterparts (43, 44). The observation that the obesity paradox diminishes in early-stage cancer hints at the potential root of this phenomenon in bladder cancer, suggesting that the better compliance and fitness of obese patients in withstanding intense treatment and surgical complications may contribute, and these advantages may diminish as treatment becomes less demanding.
The potential underlying mechanism of the obesity paradox, amidst its confounding factors, involves various speculations. The fat mass and obesity-associated protein (FTO), known for its association with body mass and obesity, influences the energy metabolism of cancer cells. However, studies have indicated a significant decrease in FTO mRNA expression in bladder urothelial carcinoma compared to controls, suggesting an oncogenic role in bladder cancer (45). Molecular pathways like PI3K/AKT/mTOR, activated in obese patients due to high circulating levels of IGF1, could be a potential therapeutic target in bladder cancer patients with high BMI (46). The transcription factor Nuclear factor erythroid 2–related factor2 (Nrf2), linked to detoxification and antioxidant response, may correlate with obesity and insulin resistance (47), impacting resistance to cisplatin and overall bladder cancer-specific survival (48). Adipokines released by adipose tissue may also play a role. Kashiwagi et al. (49) demonstrated that the downregulation of adiponectin expression and the upregulation of leptin expression were independent predictors for the recurrence of non-muscle-invasive bladder tumors and the progression of muscle-invasive bladder tumors, respectively. These findings suggest that synthetic adiponectin may exhibit antitumor activity against bladder cancer (49). Furthermore, in bladder cancer patients receiving neoadjuvant platinum-based therapies, low/intermediate mRNA levels of BRCA1 were associated with increased tumor pathological response and overall survival (50), while DNA damage in normal breast epithelia of women with a BRCA mutation positively correlated with BMI and biomarkers of metabolic dysfunction (51).
This study is notable for its comprehensive, population-based approach, including both non-muscle invasive and muscle-invasive bladder cancer in the context of the obesity paradox. However, it has several limitations. Firstly, the method of collecting anthropometric data, such as BMI, is not specified; if self-reported, this may introduce inaccuracies due to recall bias. Direct measurements by healthcare professionals are preferable for accuracy. Secondly, reliance solely on BMI may not fully capture the impact of obesity on bladder cancer outcomes, as it does not account for body fat distribution or other indicators like waist-to-hip ratio (WHR).
In Taiwan, Traditional Chinese Medicine (TCM) is frequently integrated with conventional treatments to support cancer patients. The National Health Insurance program covers TCM, making it accessible to the majority of the population. Many cancer patients use TCM to alleviate the side effects of conventional therapies, manage cancer cachexia (52), enhance quality of life, and potentially improve survival rates through mechanisms such as the regulation of ion channels in cancer cells (53). Consequently, TCM usage could introduce bias if not adequately controlled in studies. For example, TCM use might be more common among individuals with anorexia and cachexia, who may experience better outcomes due to this additional support. This could lead to collider bias and reverse causation, where the association between TCM usage and improved outcomes may reflect the selective use of TCM among patients with specific conditions rather than a direct therapeutic effect.
Additionally, confounding variables such as smoking, socioeconomic status, and diet were not extensively controlled, potentially affecting the observed relationship between BMI and cancer outcomes. Collider stratification bias could also be a concern, as smoking, a risk factor for bladder cancer, may create an artificial inverse association with BMI in non-obese patients. Finally, the follow-up duration may be too short to capture long-term survival outcomes, and the findings may not be generalizable beyond the Taiwanese population to other demographic groups with different obesity profiles and cancer outcomes.
Conclusion
The complexity of the relationship between BMI and bladder cancer outcomes stems from the varying impacts of BMI across different cancer stages and the observed “obesity paradox.” Previous research has added to this complexity by demonstrating conflicting results that both support and challenge the existence of the obesity paradox. Our study reveals an obesity paradox in which overweight patients exhibit a 0.76-fold reduction in overall mortality and a 0.82-fold reduction in specific mortality compared to normal-weight patients. Conversely, underweight patients show a 1.58-fold increase in overall mortality and a 1.50-fold increase in specific mortality. This study advances existing knowledge by providing a comprehensive analysis of both non-muscle invasive bladder cancer and muscle-invasive bladder cancer within the context of the obesity paradox.
The clinical implications underscore the importance of considering BMI as a prognostic factor in bladder cancer management, with potential benefits of integrating BMI-based assessments into clinical guidelines. Incorporating BMI-specific follow-up strategies—particularly using the WHO Asia-Pacific BMI definitions—may help tailor risk assessments and interventions to regional population characteristics. Further research is warranted to elucidate the mechanisms underlying the obesity paradox in bladder cancer, including the influence of metabolic and inflammatory pathways. Additionally, given the limitations of BMI as an indicator of adiposity, alternative measurements like body composition analysis may provide more precise insights into the impact of adiposity on cancer outcomes.
Data availability statement
The datasets presented in this article are not readily available because the data sources are the Taiwan Nation Health Insurance Database and Taiwan Cancer Registry. The data are available with permission from the Taiwan Health and Welfare Data Science Center (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html, accessed on May 02, 2024). Restrictions apply to the availability of these data, which were used under license for this study. Requests to access the datasets should be directed to https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Chi Mei Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
W-HT: Writing – original draft, Writing – review & editing, Conceptualization, Validation. T-YC: Writing – original draft, Conceptualization. C-HH: Formal analysis, Writing – original draft, Methodology, Visualization. SH: Writing – original draft, Project administration. AC: Supervision, Writing – original draft. C-FL: Writing – review & editing, Methodology, Validation. Y-LS: Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1433632/full#supplementary-material
References
1. Sonkin, D, Thomas, A, and Teicher, BA. Cancer treatments: past, present, and future. Cancer Genet. (2024) 286-287:18–24. doi: 10.1016/j.cancergen.2024.06.002
2. Richters, A, Aben, KKH, and Kiemeney, L. The global burden of urinary bladder cancer: an update. World J Urol. (2020) 38:1895–904. doi: 10.1007/s00345-019-02984-4
3. Lopez-Beltran, A, Cookson, MS, Guercio, BJ, and Cheng, L. Advances in diagnosis and treatment of bladder cancer. BMJ. (2024) 384:e076743. doi: 10.1136/bmj-2023-076743
4. Holzbeierlein, J, Bixler, BR, Buckley, DI, Chang, SS, Holmes, RS, James, AC, et al. Treatment of non-metastatic muscle-invasive bladder Cancer: AUA/ASCO/SUO guideline (2017, amended 2020, 2024). J Urol. (2024) 212:3–10. doi: 10.1097/JU.0000000000003981
5. Pfail, JL, Small, AC, Cumarasamy, S, and Galsky, MD. Real world outcomes of patients with bladder Cancer: effectiveness versus efficacy of modern treatment paradigms. Hematol Oncol Clin North Am. (2021) 35:597–612. doi: 10.1016/j.hoc.2021.01.005
6. Hsiao, BY, Su, SY, Jhuang, JR, Chiang, CJ, Yang, YW, and Lee, WC. Ensemble forecasting of a continuously decreasing trend in bladder cancer incidence in Taiwan. Sci Rep. (2021) 11:8373. doi: 10.1038/s41598-021-87770-2
7. Sun, JW, Zhao, LG, Yang, Y, Ma, X, Wang, YY, and Xiang, YB. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One. (2015) 10:e0119313. doi: 10.1371/journal.pone.0119313
8. Avgerinos, KI, Spyrou, N, Mantzoros, CS, and Dalamaga, M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
9. Chromecki, TF, Cha, EK, Fajkovic, H, Rink, M, Ehdaie, B, Svatek, RS, et al. Obesity is associated with worse oncological outcomes in patients treated with radical cystectomy. BJU Int. (2013) 111:249–55. doi: 10.1111/j.1464-410X.2012.11322.x
10. Gierth, M, Zeman, F, Denzinger, S, Vetterlein, MW, Fisch, M, Bastian, PJ, et al. Influence of body mass index on clinical outcome parameters, complication rate and survival after radical cystectomy: evidence from a Prospective European multicentre study. Urol Int. (2018) 101:16–24. doi: 10.1159/000488466
11. Huang, L-K, Lin, YC, Chuang, HH, Chuang, CK, Pang, ST, Wu, CT, et al. Body composition as a predictor of oncological outcome in patients with non-muscle-invasive bladder cancer receiving intravesical instillation after transurethral resection of bladder tumor. Front Oncol. (2023) 13:13. doi: 10.3389/fonc.2023.1180888
12. Park, Y, Peterson, LL, and Colditz, GA. The plausibility of obesity paradox in Cancer-point. Cancer Res. (2018) 78:1898–903. doi: 10.1158/0008-5472.CAN-17-3043
13. Lennon, H, Sperrin, M, Badrick, E, and Renehan, AG. The obesity paradox in Cancer: a review. Curr Oncol Rep. (2016) 18:56. doi: 10.1007/s11912-016-0539-4
14. Cespedes Feliciano, EM, Kroenke, CH, and Caan, BJ. The obesity paradox in Cancer: how important is muscle? Annu Rev Nutr. (2018) 38:357–79. doi: 10.1146/annurev-nutr-082117-051723
15. Tan, KCB. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
16. Polat, H, Utangac, MM, Gulpinar, MT, Cift, A, Erdogdu, IH, and Turkcu, G. Urothelial neoplasm of the bladder in childhood and adolescence: a rare disease. Int Braz J Urol. (2016) 42:242–6. doi: 10.1590/S1677-5538.IBJU.2015.0200
17. WHO Consultation. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253. doi: 10.26717/BJSTR.2020.30.004979
18. World Health Organization. Regional Office for the Western, P., the Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia (2000).
19. Doshi, B, Athans, SR, and Woloszynska, A. Biological differences underlying sex and gender disparities in bladder cancer: current synopsis and future directions. Oncogenesis. (2023) 12:44. doi: 10.1038/s41389-023-00489-9
20. Shi, J, Zhao, L, Gao, Y, Niu, M, Yan, M, Chen, Y, et al. Associating the risk of three urinary cancers with obesity and overweight: an overview with evidence mapping of systematic reviews. Syst Rev. (2021) 10:58. doi: 10.1186/s13643-021-01606-8
21. Choi, JB, Lee, EJ, Han, KD, Hong, SH, and Ha, US. Estimating the impact of body mass index on bladder cancer risk: stratification by smoking status. Sci Rep. (2018) 8:947. doi: 10.1038/s41598-018-19531-7
22. Lin, Y, Wang, Y, Wu, Q, Jin, H, Ma, G, Liu, H, et al. Association between obesity and bladder cancer recurrence: a meta-analysis. Clin Chim Acta. (2018) 480:41–6. doi: 10.1016/j.cca.2018.01.039
23. Zhao, H, Grossman, HB, Spitz, MR, Lerner, SP, Zhang, K, and Wu, X. Plasma levels of insulin-like growth Factor-1 and binding Protein-3, and their association with bladder Cancer risk. J Urol. (2003) 169:714–7. doi: 10.1016/S0022-5347(05)63999-7
24. Lewitt, MS, Dent, MS, and Hall, K. The insulin-like growth factor system in obesity, insulin resistance and type 2 diabetes mellitus. J Clin Med. (2014) 3:1561–74. doi: 10.3390/jcm3041561
25. de Andrade, CT, Rocha, GZ, Zamuner, M, Dos Reis, RB, and Reis, LO. Obesity influence on bladder inflammation and cancer: a cystitis model. Int J Clin Exp Pathol. (2022) 15:373–9.
26. Yang, Z, Bai, Y, Hu, X, Wang, X, and Han, P. The prognostic value of body mass index in patients with urothelial carcinoma after surgery: a systematic review and Meta-analysis. Dose Response. (2020) 18:1559325820979247. doi: 10.1177/1559325820979247
27. Arthuso, FZ, Fairey, AS, Boulé, NG, and Courneya, KS. Associations between body mass index and bladder cancer survival: is the obesity paradox short-lived? Can Urol Assoc J. (2022) 16:E261–e267. doi: 10.5489/cuaj.7546
28. Kok, VC, Zhang, HW, Lin, CT, Huang, SC, and Wu, MF. Positive association between hypertension and urinary bladder cancer: epidemiologic evidence involving 79,236 propensity score-matched individuals. Ups J Med Sci. (2018) 123:109–15. doi: 10.1080/03009734.2018.1473534
29. Shih, HJ, Lin, KH, Wen, YC, Fan, YC, Tsai, PS, and Huang, CJ. Increased risk of bladder cancer in young adult men with hyperlipidemia: a population-based cohort study. Medicine (Baltimore). (2021) 100:e28125. doi: 10.1097/MD.0000000000028125
30. Ahmadinezhad, M, Arshadi, M, Hesari, E, Sharafoddin, M, Azizi, H, and Khodamoradi, F. The relationship between metabolic syndrome and its components with bladder cancer: a systematic review and meta-analysis of cohort studies. Epidemiol Health. (2022) 44:e2022050. doi: 10.4178/epih.e2022050
31. Lu, Y, and Tao, J. Diabetes mellitus and obesity as risk factors for bladder Cancer prognosis: a systematic review and meta-analysis. Front Endocrinol. (2021) 12:699732. doi: 10.3389/fendo.2021.699732
32. Teleka, S, Jochems, SHJ, Häggström, C, Wood, AM, Järvholm, B, Orho-Melander, M, et al. Association between blood pressure and BMI with bladder cancer risk and mortality in 340,000 men in three Swedish cohorts. Cancer Med. (2021) 10:1431–8. doi: 10.1002/cam4.3721
33. Tu, KY, Li, CC, Li, WM, Yeh, HC, Ke, HL, Wu, WJ, et al. Lipid profiles impact on the oncologic outcome of upper tract urothelial carcinoma. World J Oncol. (2024) 15:287–97. doi: 10.14740/wjon1800
34. Yang, L, Sun, J, Li, M, Long, Y, Zhang, D, Guo, H, et al. Oxidized low-density lipoprotein links hypercholesterolemia and bladder Cancer aggressiveness by promoting Cancer Stemness. Cancer Res. (2021) 81:5720–32. doi: 10.1158/0008-5472.CAN-21-0646
35. Liao, J, and Zhou, Z. Long-term cardiovascular mortality risk in patients with bladder cancer: a real-world retrospective study of 129,765 cases based on the SEER database. Front Cardiovasc Med. (2023) 10:10. doi: 10.3389/fcvm.2023.1142417
36. Petrelli, F, Cortellini, A, Indini, A, Tomasello, G, Ghidini, M, Nigro, O, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. (2021) 4:e213520. doi: 10.1001/jamanetworkopen.2021.3520
37. Mayr, R, Gierth, M, Zeman, F, Reiffen, M, Seeger, P, Wezel, F, et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. (2018) 9:505–13. doi: 10.1002/jcsm.12279
38. Psutka, SP, Boorjian, SA, Moynagh, MR, Schmit, GD, Frank, I, Carrasco, A, et al. Mortality after radical cystectomy: impact of obesity versus adiposity after adjusting for skeletal muscle wasting. J Urol. (2015) 193:1507–13. doi: 10.1016/j.juro.2014.11.088
39. Liss, MA, White, M, Natarajan, L, and Parsons, JK. Exercise decreases and smoking increases bladder cancer mortality. Clin Genitourin Cancer. (2017) 15:391–5. doi: 10.1016/j.clgc.2016.11.006
40. Gill, E, Sandhu, G, Ward, DG, Perks, CM, and Bryan, RT. The Sirenic links between diabetes, obesity, and bladder Cancer. Int J Mol Sci. (2021) 22:11150. doi: 10.3390/ijms222011150
41. Hansen, TTD, Omland, LH, von Heymann, A, Johansen, C, Clausen, MB, Suetta, C, et al. Development of sarcopenia in patients with bladder Cancer: a systematic review. Semin Oncol Nurs. (2021) 37:151108. doi: 10.1016/j.soncn.2020.151108
42. Swalarz, M, Swalarz, G, Juszczak, K, Maciukiewicz, P, Czurak, K, Matuszewski, M, et al. Correlation between malnutrition, body mass index and complications in patients with urinary bladder cancer who underwent radical cystectomy. Adv Clin Exp Med. (2018) 27:1141–7. doi: 10.17219/acem/89863
43. Ferro, M, Vartolomei, MD, Russo, GI, Cantiello, F, Farhan, ARA, Terracciano, D, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. (2019) 37:507–14. doi: 10.1007/s00345-018-2397-1
44. Kluth, LA, Xylinas, E, Crivelli, JJ, Passoni, N, Comploj, E, Pycha, A, et al. Obesity is associated with worse outcomes in patients with T1 high grade urothelial carcinoma of the bladder. J Urol. (2013) 190:480–6. doi: 10.1016/j.juro.2013.01.089
45. Wen, L, Pan, X, Yu, Y, and Yang, B. Down-regulation of FTO promotes proliferation and migration, and protects bladder cancer cells from cisplatin-induced cytotoxicity. BMC Urol. (2020) 20:39. doi: 10.1186/s12894-020-00612-7
46. Santoni, M, Cimadamore, A, Massari, F, Piva, F, Aurilio, G, Martignetti, A, et al. Key role of obesity in genitourinary tumors with emphasis on urothelial and prostate cancers. Cancers. (2019) 11:1225. doi: 10.3390/cancers11091225
47. Li, S, Eguchi, N, Lau, H, and Ichii, H. The role of the Nrf2 signaling in obesity and insulin resistance. Int J Mol Sci. (2020) 21:6973. doi: 10.3390/ijms21186973
48. Hayden, A, Douglas, J, Sommerlad, M, Andrews, L, Gould, K, Hussain, S, et al. The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol Oncol. (2014) 32:806–14. doi: 10.1016/j.urolonc.2014.02.006
49. Kashiwagi, E, Abe, T, Kinoshita, F, Ushijima, M, Masaoka, H, Shiota, M, et al. The role of adipocytokines and their receptors in bladder cancer: expression of adiponectin or leptin is an independent prognosticator. Am J Transl Res. (2020) 12:3033–45. doi: 10.1016/S2666-1683(20)32677-X
50. Font, A, Taron, M, Gago, JL, Costa, C, Sánchez, JJ, Carrato, C, et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer. Ann Oncol. (2011) 22:139–44. doi: 10.1093/annonc/mdq333
51. Bhardwaj, P, Iyengar, NM, Zahid, H, Carter, KM, Byun, DJ, Choi, MH, et al. Obesity promotes breast epithelium DNA damage in women carrying a germline mutation in BRCA1 or BRCA2. Sci Transl Med. (2023) 15:eade1857. doi: 10.1126/scitranslmed.ade1857
52. Liu, H. Effect of traditional medicine on clinical Cancer. Biomed J Sci Techn Res. (2020) 30:23548–51.
Keywords: bladder cancer, obesity paradox, Taiwan Cancer Registry, mortality, body mass index
Citation: Tseng W-H, Chiang T-Y, Ho C-H, Huang SK, Chiu AW, Li C-F and Shiue Y-L (2024) Navigating the obesity paradox in bladder cancer prognosis—insights from the Taiwan National Health Insurance System Database. Front. Nutr. 11:1433632. doi: 10.3389/fnut.2024.1433632
Edited by:
Dechao Feng, University College London, United KingdomReviewed by:
Ewelina Książek, Wroclaw University of Economics, PolandHengrui Liu, University of Cambridge, United Kingdom
Copyright © 2024 Tseng, Chiang, Ho, Huang, Chiu, Li and Shiue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-Yi Chiang, YjEwMTA5NjEwOEB0bXUuZWR1LnR3;Yow-Ling Shiue, c2hpcmxleUBpbXN0Lm5zeXN1LmVkdS50dw==
 Wen-Hsin Tseng
Wen-Hsin Tseng Ting-Yi Chiang1,2*
Ting-Yi Chiang1,2* Chung-Han Ho
Chung-Han Ho Yow-Ling Shiue
Yow-Ling Shiue