- Department of Nephrology, The First Hospital of Jilin University, Changchun, China
Background: Immunoglobulin A nephropathy (IgAN) is the most prevalent form of chronic kidney disease (CKD), marked by diverse pathological patterns and variable prognostic outcomes. Nutritional indexes are crucial for disease assessment and prognosis prediction. This study investigates associations between nutritional indexes and renal function in patients with IgAN.
Methods: A cohort of 736 adults diagnosed with IgAN, who underwent renal biopsy at the First Hospital of Jilin University between January 2010 and October 2022, was examined. Clinical and laboratory data were reviewed, and four nutritional indexes were calculated: controlling nutritional status (CONUT) score, geriatric nutritional risk index (GNRI), body mass index (BMI), and prognostic nutritional index (PNI). Cox-proportional hazard analysis evaluated factors associated with end-stage renal disease (ESRD).
Results: Patients with ESRD showed significantly lower GNRI (91.84 vs. 98.94, p < 0.001) and median PNI (41.90 vs. 46.30, p < 0.001), with higher median CONUT score (2.00 vs. 1.00, p = 0.001) compared to those without ESRD. PNI, GNRI, and CONUT scores correlated significantly with C2 in MEST-C classification. Kaplan–Meier analysis indicated increased ESRD probability in individuals with specific thresholds of PNI, GNRI, or CONUT scores. Additionally, GNRI emerged as an independent predictor of ESRD (hazard ratio: 0.963, 95% CI: 0.940–0.979, p < 0.001), along with platelet count, serum creatinine, eGFR (CKD-EPI), and triglyceride levels.
Conclusion: GNRI, PNI, and CONUT scores hold potential in reflecting IgAN severity and predicting ESRD risk. GNRI especially may serve as a valuable tool for identifying high-risk individuals for ESRD in IgAN.
1 Introduction
IgA nephropathy (IgAN) is the most prevalent form of primary glomerulonephritis globally, with approximately 40% of patients progressing to end-stage renal disease (ESRD) within 10–20 years (1). The hallmark pathology involves the deposition of IgA and complement 3 in the glomerular mesangial region, with increased production and circulation of galactose-deficient IgA1 (gd-IgA1) considered as the initiating factor (2). Furthermore, studies have implicated IgAN in mucosal immune dysfunction and chronic inflammation (3). Managing nutrient intake, particularly protein, is crucial for patients with chronic kidney disease (CKD) to safeguard renal function. However, inadequate dietary intake can compromise immune system function and mucosal integrity (4).
Assessing nutritional status has become a focal point in enhancing disease management, particularly in renal diseases. Despite this, a unanimous standard for nutritional evaluation in renal conditions is lacking. Conventionally, nutritional assessment relies on indicators like body mass index (BMI) or laboratory parameters such as total cholesterol, prealbumin, and albumin levels. However, emerging tools like the controlling nutritional status (CONUT) score, geriatric nutritional risk index (GNRI), and prognostic nutritional index (PNI) have gained traction across various patient demographics, ranging from surgical candidates to individuals with cancer or chronic ailments (5–7). Despite this progress, their potential for predicting the prognosis of IgAN remains largely unexplored. Therefore, this study aims to investigate the correlation between four distinct nutritional indexes and disease severity, along with the likelihood of developing ESRD in patients with IgAN.
2 Materials and methods
2.1 Study population
We conducted a retrospective review of adults who had undergone renal biopsy at the First Hospital of Jilin University between January 2010 and October 2022 who were diagnosed with IgAN. Out of the initial pool of 1,077 patients, 341 were excluded based on specific criteria: (1) eGFR (CKD-EPI) <15 mL/min/1.73 m2 (n = 31); (2) presence of autoimmune diseases (n = 14); (3) secondary IgAN conditions (n = 96), such as hepatitis virus-related glomerulonephritis and Henoch-Schonlein purpura nephritis; (4) acute infectious diseases (n = 7) or cancer (n = 7); (5) incomplete or absent follow-up data (n = 26 and n = 160, respectively). Ultimately, 736 eligible patients with IgAN were enrolled in the study. The research protocol underwent thorough scrutiny, was strictly reviewed, and received approval from the ethical committees of the First Hospital of Jilin University (No. 2024-442).
2.2 Clinical and laboratory data collection
Following the confirmation of a diagnosis of IgAN through pathology, comprehensive clinical and laboratory data were systematically collected. Clinical parameters included age, gender, mean arterial pressure (MAP), smoking status, alcohol consumption habits, and history of diabetes and/or hematuria. Laboratory measurements included glomerular filtration rate (eGFR), serum albumin levels, blood urea nitrogen (BUN), serum creatinine (Cr), uric acid (UA), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum immunoglobulin levels (IgG, IgA, and IgM), complement 3 and 4 levels, C-reactive protein (CRP) levels, anti-streptolysin O (ASO) titers, proteinuria, lymphocyte and leukocyte levels, and platelet counts. eGFR was determined using the CKD Epidemiology Collaboration (CKD-EPI) formula (8). Smoking history was assessed based on the Brinkman Index, calculated as the product of years of smoking and cigarettes smoked per day, with a threshold exceeding 200 indicating significant smoking exposure. Similarly, alcohol history was determined by an index calculated as the product of years of drinking and grams of alcohol consumed per day, with a threshold surpassing 2000 denoting notable alcohol consumption.
2.3 Renal pathology evaluation
The Oxford Classification Scoring System (MEST-C) was employed to assess the pathological status of each case. This system classifies cellular/fibrocellular crescents (C), interstitial fibrosis/tubular atrophy (T), segmental glomerulosclerosis (S), endocapillary hypercellularity (E), and mesangial hypercellularity (M) (9).
2.4 Selection and calculation of nutritional indexes
Four distinct nutritional indexes were incorporated into our study: PNI, BMI, GNRI, and CONUT score. PNI is calculated as serum albumin (g/L) + 0.005 × blood lymphocyte count (/mm3) (10). BMI is determined by weight (kg) divided by height (m) squared. GNRI is computed as (1.519 × serum albumin (g/dL)) + (41.7 × weight (kg)/ideal body weight (kg)), where the ideal weight is calculated as [height (m)]2 multiplied by 22 (11). The CONUT score was derived using a formula (Supplementary Table S1) that includes total cholesterol score, lymphocyte count score, and serum albumin score.
2.5 Statistical analysis
The statistical analyses were carried out using IBM SPSS Statistics version 24.0. In the analysis results, continuous variables are presented as median with interquartile ranges, while categorical variables are expressed as frequencies and percentages. To compare continuous and categorical variables, appropriate tests, including chi-square or Fisher’s exact test, Kruskal–Wallis test, and the Mann–Whitney U test, were utilized. Spearman’s correlation analysis was also employed to assess the relationships between nutritional indexes and variables.
Receiver operating characteristics (ROC) curve analysis was used to define low and high PNI (or GNRI, CONUT score) with cutoff values set at 42.78, 92.03, or 1.50, respectively. Furthermore, Kaplan–Meier analysis was conducted to compare the renal survival rates of patients with low and high PNI, GNRI, or CONUT score. Additionally, univariable and multivariable Cox-proportional hazard analyses were performed to identify factors associated with ESRD. Prior to conducting multivariable Cox-proportional hazard analysis, variables that had less than a 20% impact on hazard ratios (HRs) of other factors were removed to adjust for confounding variables. A significance level of p < 0.05 (two-tailed) was considered statistically significant for all analyses.
3 Results
3.1 Features of the study population with or without ESRD
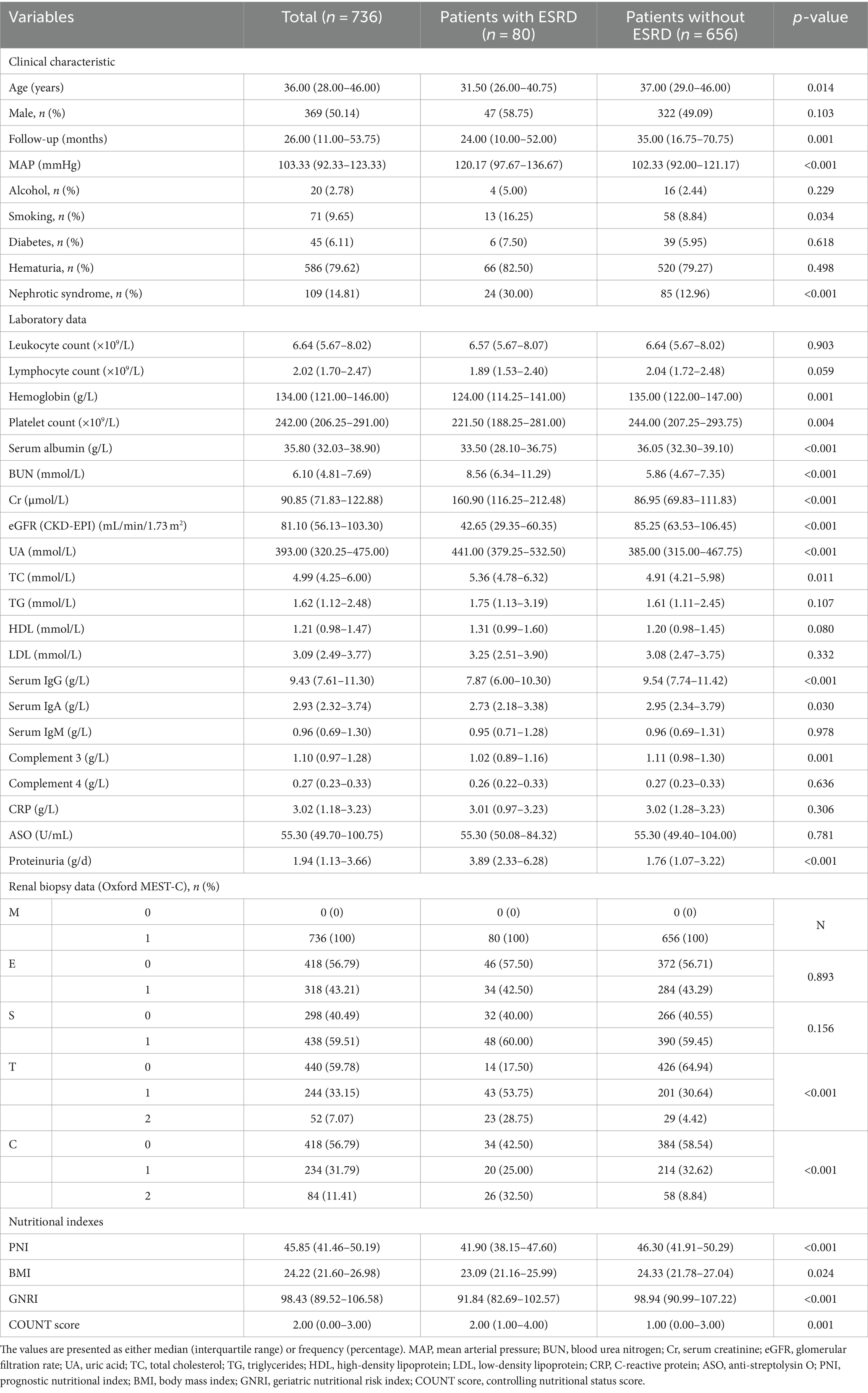
This research encompassed 736 patients diagnosed with IgAN. Table 1 illustrates the baseline characteristics of individuals with or without ESRD. The cohort had a median age of 36.00 years, with males comprising 50.14% of the sample. Over a median follow-up period of 26.00 months, 80 patients (10.87%) were identified as having ESRD. Those with ESRD tended to be younger and had higher mean arterial pressure (MAP), along with a notable prevalence of smoking and nephrotic syndrome. Laboratory findings indicated lower hemoglobin levels, platelet count, serum albumin, eGFR, serum IgG, and complement 3 among patients with ESRD, juxtaposed with elevated BUN, Cr, UA, total cholesterol (TC), and proteinuria levels. Renal biopsy data revealed a higher incidence of T1, T2, and C2 lesions in individuals without ESRD. Furthermore, significant disparities in nutritional indexes were evident between the groups, particularly reflected in markedly lower values of prognostic nutritional index (PNI) (median 41.90 vs. 46.30, p < 0.001) and geriatric nutritional risk index (GNRI) (median 91.84 vs. 98.94, p < 0.001) among patients with ESRD.
3.2 Correlation between variables and nutritional indexes
An analysis of the correlation between variables and nutritional indexes was conducted. Notably, nephrotic syndrome, serum albumin, and serum IgG demonstrated significant correlations with PNI, GNRI, and CONUT score, while hemoglobin exhibited a moderate correlation with PNI and GNRI. Proteinuria exhibited the most robust correlation with PNI, with a correlation coefficient of −0.465, indicating a strong negative association. The CONUT score showed a notable correlation with proteinuria, albeit slightly weaker, with a correlation coefficient of 0.379. Importantly, all correlations were statistically significant, with p-values less than 0.001. Complement 3 and HDL were moderately correlated with BMI and GNRI. Conversely, while some factors, such as BUN, Cr, eGFR, and UA showed correlations with PNI, BMI, GNRI, or CONUT score, these associations were weak (refer to Table 2).
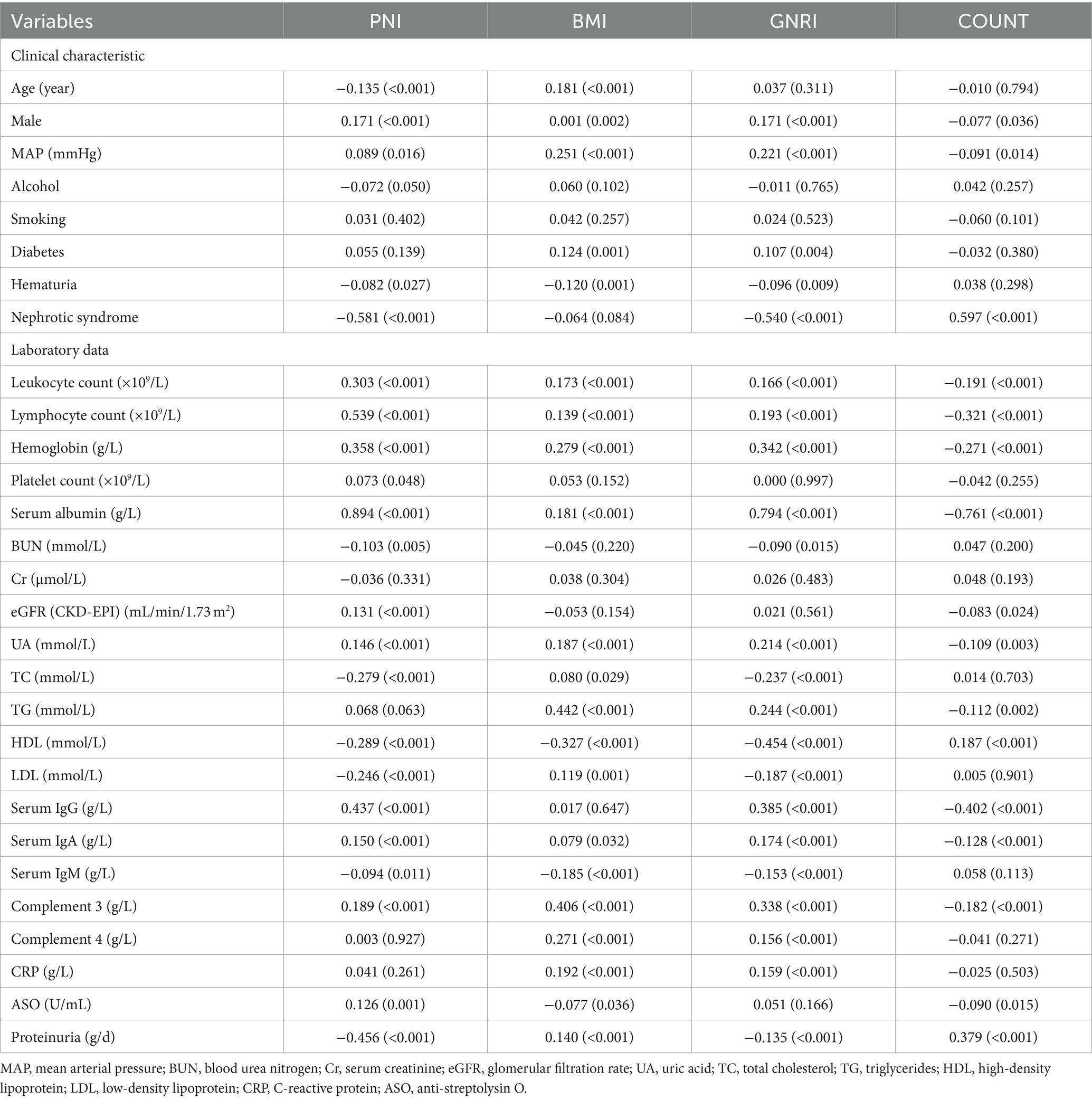
Table 2. The correlation analysis results depicting the relationships between variables and nutritional indexes in IgAN.
Moreover, the study compared four nutritional indexes across the MEST-C classification. PNI, GNRI, and CONUT score demonstrated similar trends concerning cellular/fibrocellular crescents (C) classification. Specifically, patients classified as C2 exhibited lower PNI and GNRI scores compared to those in C0 and C1, whereas the CONUT score showed the opposite trend (refer to Figure 1). No significant differences were observed in other groups.
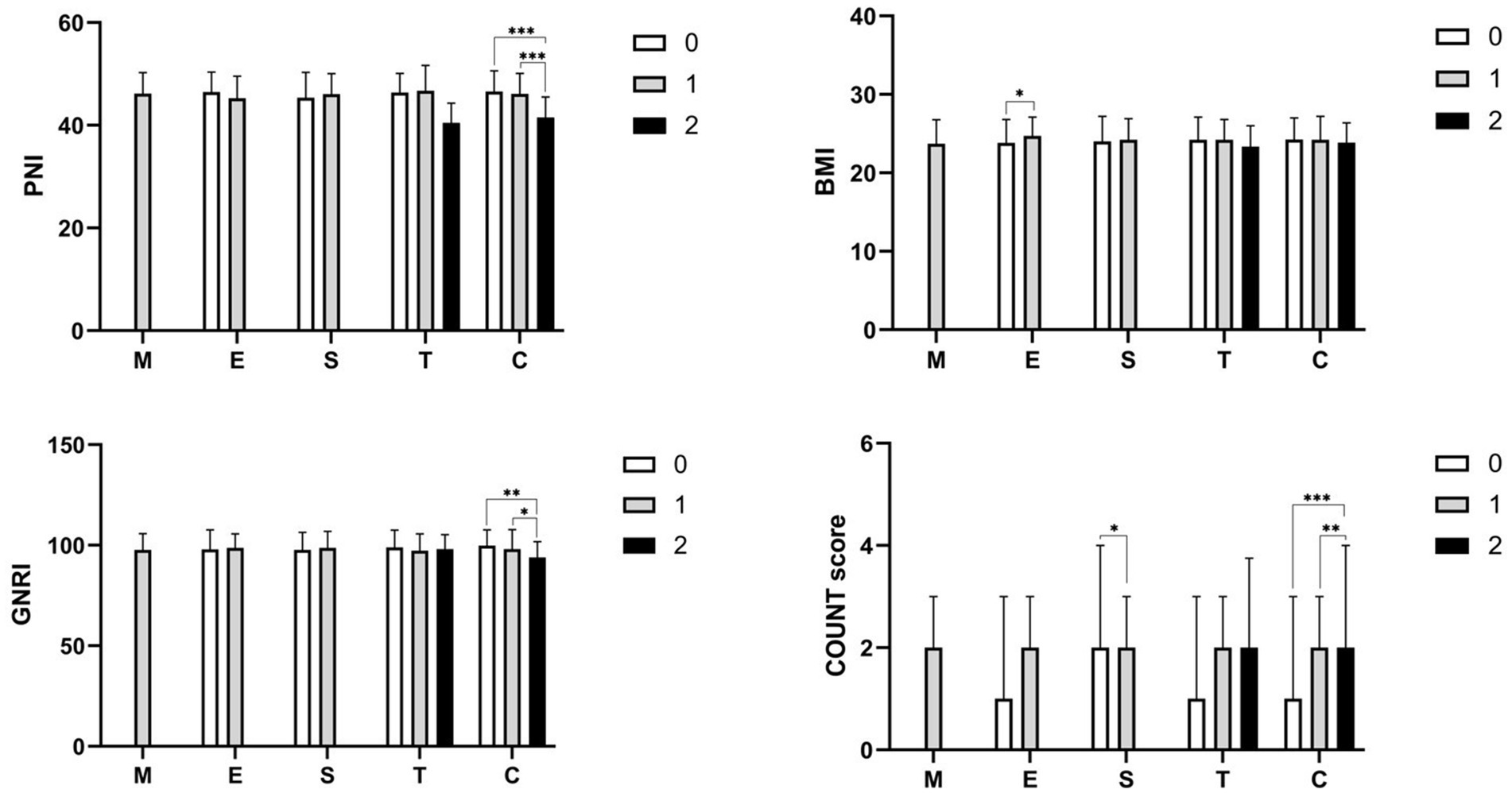
Figure 1. The comparison of various nutritional indexes categorized by MEST-C classification. Statistical significance levels are denoted as *p < 0.05, **p < 0.01, and ***p < 0.001 between the different groups.
3.3 Factors associated with ESRD in patients with IgAN
Among the four nutritional indexes analyzed, PNI (AUC = 0.627, sensitivity 71.6%, specificity 57.5, 95% CI 0.560–0.693, p < 0.001), GNRI (AUC = 0.629, sensitivity 73.2%, specificity 52.5, 95% CI 0.563–0.694, p < 0.001), and CONUT (AUC = 0.614, sensitivity 73.8%, specificity 50.6, 95% CI 0.320–0.452, p < 0.001) demonstrated potential in predicting ESRD. Figure 2 depicts optimal cutoff values for predicting ESRD in the analysis provided, which were determined to be PNI ≤ 42.78, GNRI ≤ 92.03, and CONUT ≥ 1.50. These values were identified as key thresholds indicating heightened risk of ESRD development.
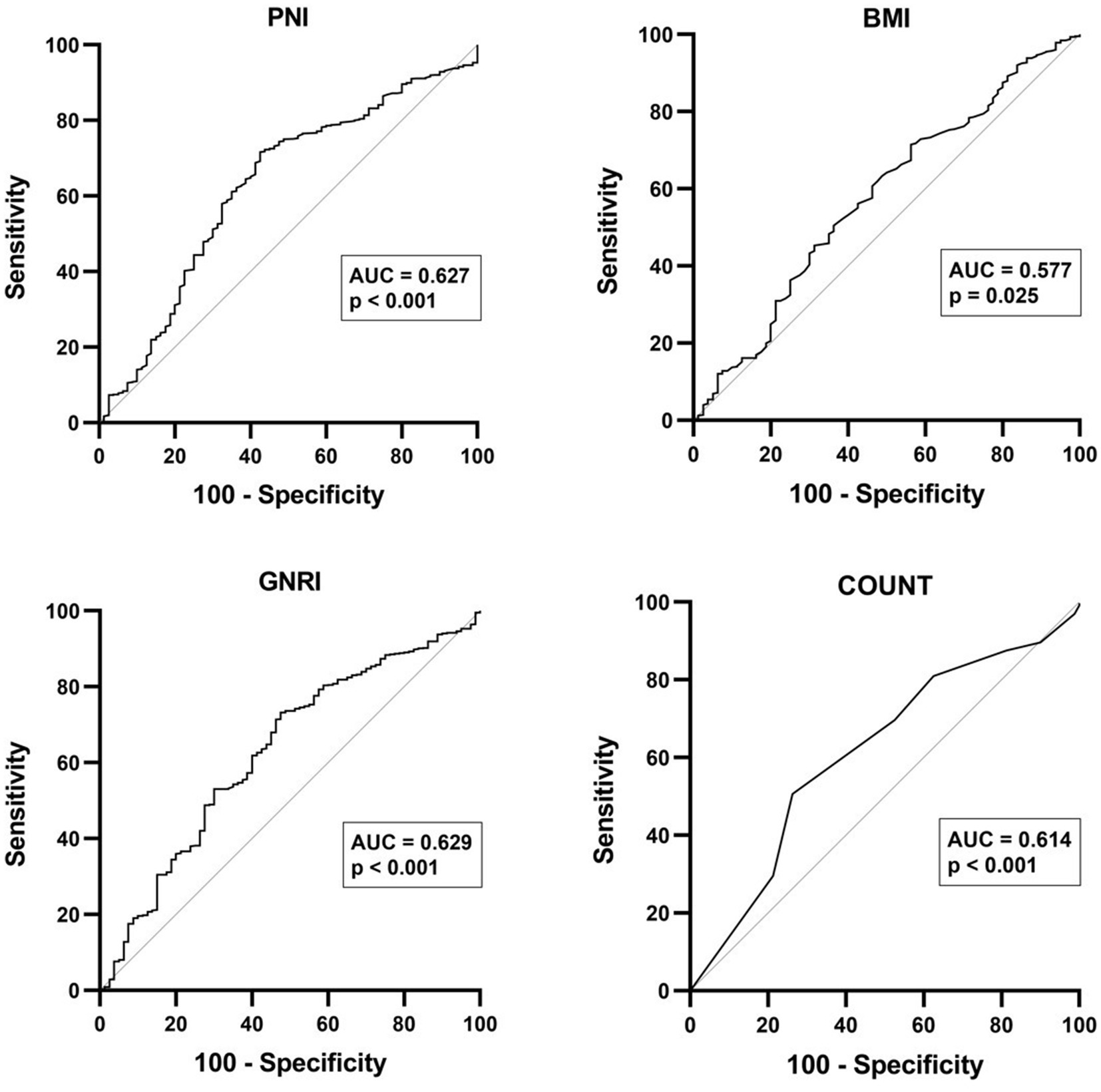
Figure 2. The receiver operating characteristic (ROC) curve analysis for PNI, BMI, GNRI, and CONUT score in predicting ESRD among patients with IgA nephritis.
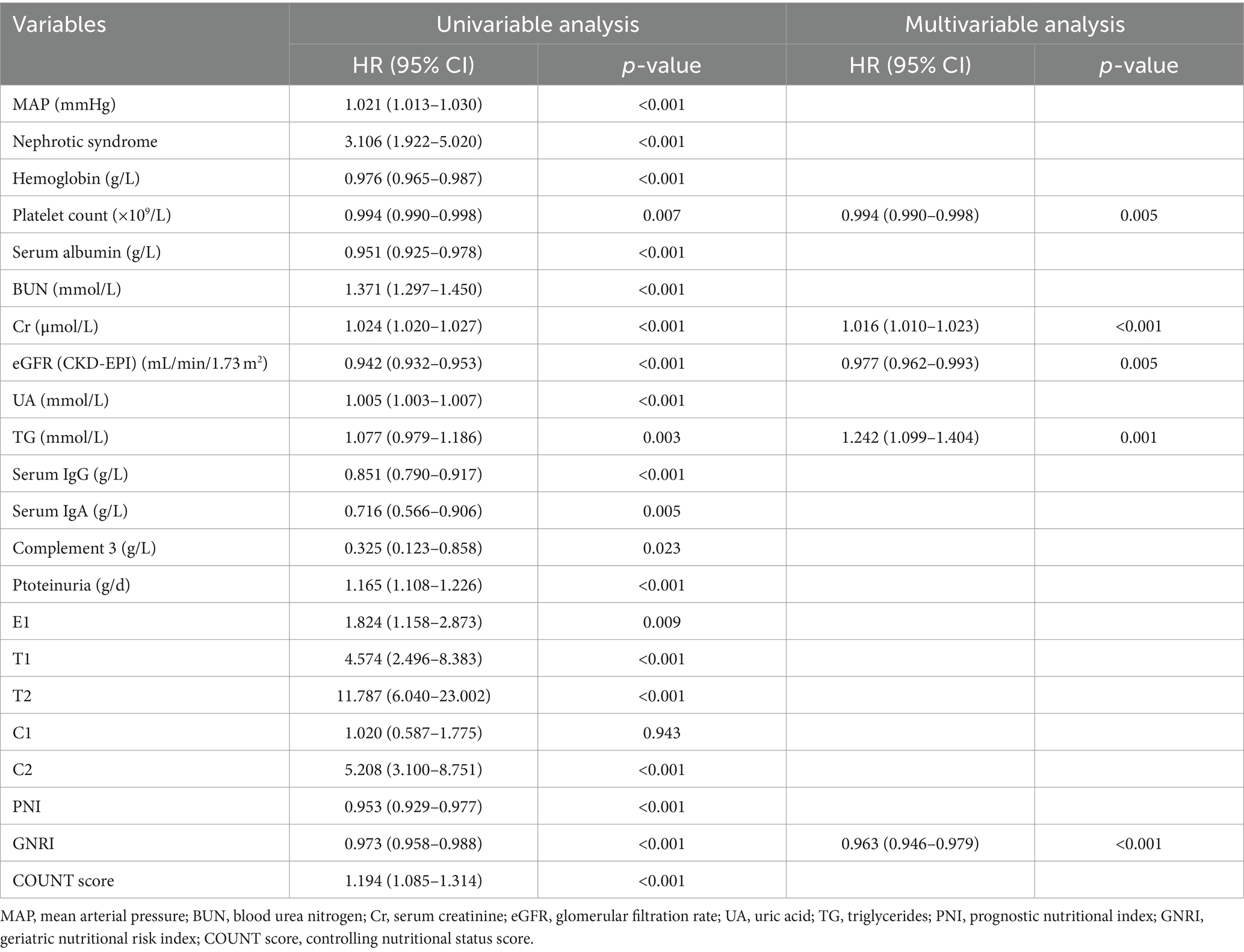
Subsequently, a Cox-proportional hazard analysis was conducted to identify factors associated with ESRD. Various factors used in the univariable analysis, including nephrotic syndrome, mean arterial pressure (MAP), hemoglobin, platelet count, serum albumin, BUN, Cr, eGFR, UA, TG, serum IgG, serum IgA, complement 3, proteinuria, E1, T1, T2, C1, C2, PNI, GNRI, and CONUT were significantly associated with ESRD. The Kaplan–Meier analysis showed that individuals with PNI ≤ 42.78, GNRI ≤ 92.03, or CONUT ≥ 1.50 exhibited a substantially elevated probability of progressing to ESRD, as illustrated in Figure 3. These specific cutoff values served as important indicators of increased risk for ESRD onset. However, in multivariable analysis, only GNRI, platelet count, Cr, eGFR, and TG emerged as independent factors for ESRD (refer to Table 3). In the multivariable analysis includes GNRI and clinical parameters (total cholesterol, platelet count and glomerular filtration rate), the concordance index (C-index) (0.924, 95%CI 0.899–0.949) shows a good aggregate concordance, which is the highest among the multivariable analysis of four nutritional indexes with the same clinical parameters (Supplementary Table S2).
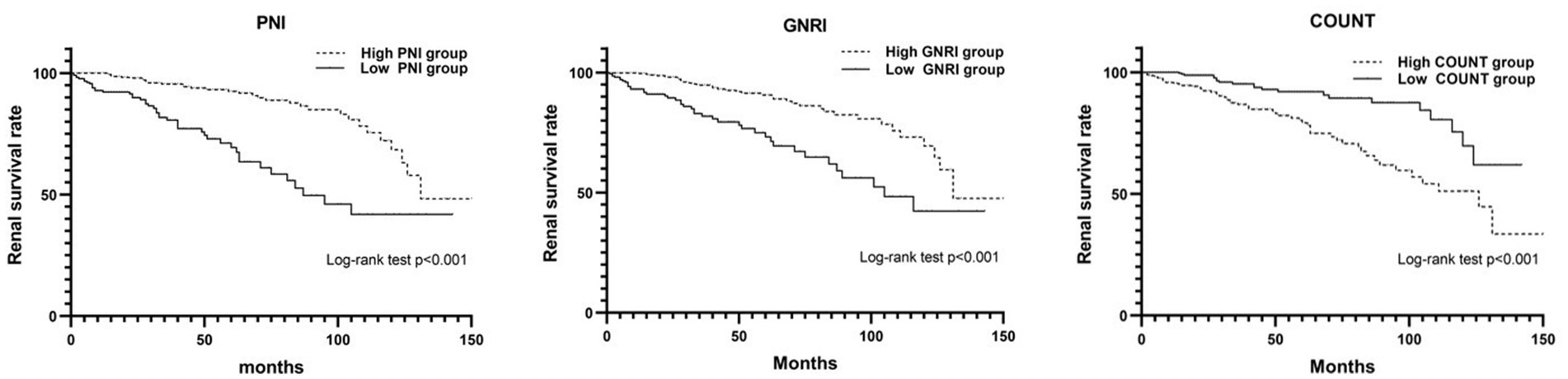
Figure 3. The Kaplan–Meier curve analysis for depicting renal survival rate categorized by PNI, GNRI, and COUNT score in IgAN.
4 Discussion
This study represents the inaugural assessment of the clinical utility of four distinct nutritional indexes in patients diagnosed with IgAN. Among these indexes, the PNI, GNRI, and CONUT score demonstrated superior correlations with the severity of crescents in patients with IgAN and were also linked with the risk of progressing to ESRD. Notably, both the PNI and GNRI were significantly lower in patients with IgAN who developed ESRD. However, in the multivariable Cox-proportional hazard analysis, only GNRI emerged as a predictor of ESRD, alongside platelet count, serum creatinine, and eGFR calculated using the CKD-EPI equation, and triglyceride levels.
The presence of cellular or fibrocellular crescents in patients with IgAN has been associated with adverse renal outcomes (12). Specifically, crescents present in over 25% of glomeruli (classified as C2 in MEST-C) independently predicted the development of ESRD, even in patients undergoing corticosteroid or immunosuppressive therapy (12–14). Recent investigations have indicated that GNRI serves as an independent marker reflecting the extent of crescentic lesions, suggesting its potential utility in monitoring crescent severity (15). Our study further revealed that not only GNRI but also PNI and CONUT score exhibited strong correlations with crescent proportions.
Furthermore, both PNI and CONUT score exhibited significant associations with proteinuria levels, serum IgG, and serum albumin. Serum albumin, serving as a gauge of nutritional status and an acute-phase protein, is reduced during systemic inflammatory responses and has been recognized as an independent risk factor for ESRD in IgAN (15). Elevated serum IgG levels have been correlated with heightened crescentic lesions and improved cumulative renal survival rates (16, 17). Proteinuria, a hallmark characteristic of IgAN, not only signifies disease activity (where increased proteinuria can precipitate hypoalbuminemia) but also mirrors systemic inflammation and endothelial dysfunction (18).
Furthermore, decreased levels of serum complement 3, indicative of systemic inflammation, were noted in patients with IgAN and ESRD. A lower median complement 3 (1.02 vs. 1.11, p = 0.001) demonstrated a notable correlation with BMI and Geriatric Nutritional Risk Index (GNRI) (r = 0.406 and r = 0.338, respectively; all p < 0.001). Intense mesangial complement 3 deposition was linked to more severe pathological lesions, underscoring the pivotal role of complement 3 in the progression of IgAN (19). Furthermore, low serum complement 3 levels and mesangial complement 3 deposition were identified as independent risk factors for renal progression in IgAN (20).
GNRI transcends its conventional role as a mere nutritional assessment tool. Recent research suggests its potential association with inflammation indicators in elderly patients and systemic inflammatory response in cancer cases (21, 22). Within the context of IgAN, our study delves into the correlation between GNRI and serum markers such as albumin, IgG, and complement 3, illuminating the intricate interplay between nutrition and inflammation (23). As evidence mounts regarding their intertwined nature, the pressing question arises: how do we tailor nutritional interventions for patients with IgAN?
Nephrologists have long recognized the pivotal role of dietary adjustments in slowing CKD progression, offering clinicians standardized guidelines. While moderating protein intake remains central to alleviating renal strain, studies underscore the peril of insufficient nutrition, culminating in immune system dysregulation and gut mucosal impairment (24).
Recent insights spotlight the gut’s pivotal role in IgAN pathogenesis (25), underscoring the intricate interplay between gut-associated lymphoid tissue and renal health, dubbed the gut-kidney axis. The advent of targeted interventions like Nefecon, designed to modulate gut mucosal function, underscores this nexus. Hence, there is a palpable need for updated dietary guidelines tailored to patients with IgAN, particularly those grappling with weight management or stringent dietary restrictions.
Recent strides in IgAN therapeutics, exemplified by Nefecon, an oral formulation of budesonide tailored to target the gut mucosa, has emerged as a groundbreaking therapy for IgAN. Through its efficacy demonstrated in the first phase 3 trial, marked improvements in key indicators such as the 24 h urine protein-to-creatinine ratio (UPCR) and estimated glomerular filtration rate have been observed (26). This underscores the pivotal role of the gut in IgAN pathogenesis, reaffirming the concept of the gut-kidney axis.
Given these advancements, there is a compelling case for revisiting dietary guidelines for patients with IgAN to better manage renal burden and disease progression. This is especially pertinent for individuals grappling with weight management or facing stringent dietary constraints. For instance, adopting a gluten-free diet presents a promising approach. Studies have highlighted heightened gut permeability in IgAN patients (27), with gluten potentially exacerbating this phenomenon by triggering the production of IgA anti-alimentary antigens (28).
Compelling evidence further links celiac disease with an increased likelihood of IgAN development (29), underscoring the potential impact of dietary interventions. Animal studies have provided insights into the mechanisms at play, with findings suggesting a correlation between gluten-rich diets and heightened renal IgA deposition, indicative of experimental IgAN induction (30). However, the absence of randomized controlled trials exploring the efficacy of gluten-free diets in IgAN necessitates further research in this domain.
4.1 Study limitations and future avenues
This retrospective study has limitations, including inherent biases stemming from selection biases and missing data. Additionally, the scarcity of patients with IgAN and M0 lesions in our biopsy cohort poses a challenge, precluding their inclusion in the study.
Moving forward, two avenues merit further exploration: firstly, stratifying GNRI to identify optimal thresholds, which is crucial given the prevalence of malnutrition coexisting with obesity in patients with CKD (31), thereby necessitating nuanced nutritional assessments. Among a cohort exceeding 3 million US veterans, an intriguing pattern emerged in the association between BMI and kidney function decline, revealing a U-shaped relationship. This trend was particularly pronounced with advancing age (32), suggesting a nuanced interplay between BMI and renal health among the elderly. Meanwhile, GNRI, comprising parameters like albumin, height, and weight, warrants careful consideration. Notably, excessive weight can skew GNRI readings, potentially yielding misleading results. Hence, ensuring accurate interpretation of GNRI necessitates accounting for factors such as body composition and underlying health conditions to avoid erroneous assessments.
Secondly, integrating GNRI with lymphocyte count, which is common in PNI and COUNT score, given its previously established association with crescentic lesions in IgAN, holds promise for refining disease severity indexes.
5 Conclusion
Our findings underscore the utility of GNRI, PNI, and COUNT score as markers reflecting crescentic proportions in patients with IgAN, with implications for ESRD risk stratification. Notably, GNRI emerges as a potentially valuable tool for identifying high-risk patients with IgAN warranting closer monitoring for ESRD progression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data being analyzed in our retrospective research is completely anonymized. There is no direct involvement or impact on the individuals whose data is being studied. Obtaining informed consent from each participant would be impractical and unnecessary, as the research does not pose any risks or potential harm to the subjects.
Author contributions
CQ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. HL: Data curation, Methodology, Software, Writing – review & editing. YH: Formal analysis, Validation, Writing – review & editing. WW: Supervision, Validation, Writing – review & editing. MS: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Special Project for Medical and Sanitary Talent of Jilin Province, grant number JLSWSRCZX2023-33.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1431910/full#supplementary-material
References
1. Prapa, P, Carmen, A, and Heather, NR. IgA nephropathy: Core curriculum 2021. Am J Kidney Dis. (2023) 78:429–41. doi: 10.1053/j.ajkd.2021.01.024
2. Kar, NL, Sydney, CWT, Francesco, PS, Jan, N, Yasuhiko, T, Agnes, BF, et al. IgA nephropathy. Nat Rev Dis Primers. (2016) 11:16001. doi: 10.1038/nrdp.2016.1
3. Jan, N, Zina, M, Bruce, AJ, Milan, R, Robert, JW, Yusuke, S, et al. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv Otorhinolaryngol. (2011) 72:60–3. doi: 10.1159/000324607
4. Philipp, S, Martina, B, Zeno, S, and Ulrich, K. Loss of appetite in acutely ill medical inpatients: physiological response or therapeutic target? Swiss Med Wkly. (2014) 29:w13957. doi: 10.4414/smw.2014.13957
5. Liu, CC, Liu, PH, Chen, HT, Chen, JY, Lee, CW, Cheng, WJ, et al. Association of preoperative prognostic nutritional index with risk of postoperative acute kidney injury: a meta-analysis of observational studies. Nutrients. (2023) 15:2929. doi: 10.3390/nu15132929
6. Jinyu, S, Tong, L, Yizhong, G, Chenan, L, Qi, Z, Hailun, X, et al. Cholesterol-modified prognostic nutritional index (CPNI) as an effective tool for assessing the nutrition status and predicting survival in patients with breast cancer. BMC Med. (2023) 21:512. doi: 10.1186/s12916-023-03225-7
7. Kimiaki, T, Hiroshi, T, Tomomi, M, Kasumi, Y, Kota, K, Yuka, MM, et al. Prognostic value of the controlling nutritional status (CONUT) score in patients at dialysis initiation. Nutrients. (2022) 14:2317. doi: 10.3390/nu14112317
8. Andrew, SL, Lesley, AS, Christopher, HS, Yaping, LZ, Alejandro, FC, Harold, IF, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
9. Hernán, T, Jonathan, B, Daniel, CC, Cook, HT, Rosanna, C, Mark, H, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. (2017) 91:1014–21. doi: 10.1016/j.kint.2017.02.003
10. Onodera, T, Goseki, N, and Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
11. Olivier, B, Gilles, M, Claire, D, Isabelle, C, Vincent, JP, Ioannis, N, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderlymedical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
12. Mark, H, Jacobien, CV, Zhi-Hong, L, Charles, EA, Jonathan, B, Jan, UB, et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J Am Soc Nephrol. (2017) 28:691–701. doi: 10.1681/ASN.2016040433
13. Zaoqiang, L, Lichang, L, Rongling, Z, Xuefei, L, Fuhua, L, Kun, B, et al. Volume of crescents affects prognosis of IgA nephropathy in patients without obvious chronic renal pathology. Am J Nephrol. (2021) 52:507–18. doi: 10.1159/000516187
14. Sehoon, P, Chung Hee, B, Su-Kil, P, Hee, GK, Hye, SH, Eujin, P, et al. Clinical significance of crescent formation in IgA nephropathy - a multicenter validation study. Kidney Blood Press Res. (2019) 44:22–32. doi: 10.1159/000497808
15. Yasuhiro, K, Kosuke, M, Kumiko, T, Ritsuko, K, Shigeru, T, and Akihiro, T. Association between serum albumin level and incidence of end-stage renal disease in patients with immunoglobulin a nephropathy: a possible role of albumin as an antioxidant agent. PLoS One. (2018) 13:e0196655. doi: 10.1371/journal.pone.0196655
16. Yangang, G, Jiajia, L, Jianping, W, Rui, Z, Qianqian, H, Zizhen, L, et al. Association between geriatric nutritional risk index and pathological phenotypes of IgA nephropathy. PeerJ. (2023) 11:e14791. doi: 10.7717/peerj.14791
17. Di, L, Jing, Y, Yexin, L, Xiaofang, T, Xia, T, Ming, X, et al. Serum immunoglobulin G provides early risk prediction in immunoglobulin a nephropathy. Int Immunopharmacol. (2019) 66:13–8. doi: 10.1016/j.intimp.2018.10.044
18. Karen, EP, Martin, B, John, ET, Vidya, MA, Gordon, DOL, and Angela, CS. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. (2003) 63:624–33. doi: 10.1046/j.1523-1755.2003.00768.x
19. Xiaoyue, H, Yanan, L, Weiwei, Z, and Rong, L. The clinical and pathological effects of serum C3 level and mesangial C3 intensity in patients with IgA nephropathy. Anal Cell Pathol (Amst). (2024) 2024:8889306. doi: 10.1155/2024/8889306
20. Seung, JK, Hyang, MK, Beom, JL, Hyung, JO, Dong, EY, Dong, HS, et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One. (2012) 7:e40495. doi: 10.1371/journal.pone.0040495
21. Simone, G, Matthias, K, Janine, K, Lena, JV, Michael, F, Julia, M, et al. Geriatric nutritional risk index correlates with length of hospital stay and inflammatory markers in older inpatients. Clin Nutr. (2016) 35:1522–9. doi: 10.1016/j.clnu.2016.04.010
22. Guo-Tian, R, Qi, Z, Xi, Z, Meng, T, Meng-Meng, S, Xiao-Wei, Z, et al. Geriatric nutrition risk index: prognostic factor related to inflammation in elderly patients with cancer cachexia. J Cachexia Sarcopenia Muscle. (2021) 12:1969–82. doi: 10.1002/jcsm.12800
23. Franziska, S, Bettina, K, Carla, G, and Philipp, S. Inflammation and nutrition: friend or foe? Nutrients. (2023) 15:1159. doi: 10.3390/nu15051159
24. Katona, P, and Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Diseases Off. Publ. Infect. Diseases Soc. Am. (2008) 46:1582–8. doi: 10.1086/587658
25. Loreto, G, Vincenzo, DL, and Rosanna, C. The mucosal immune system and IgA nephropathy. Semin Immunopathol. (2021) 43:657–68. doi: 10.1007/s00281-021-00871-y
26. Richard, L, Jens, K, Andrew, S, Jürgen, F, Vladimir, T, Hernán, T, et al. Lancet. (2023) 402:859–70. doi: 10.1016/S0140-6736(23)01554-4
27. Rostoker, G, Wirquin, V, Terzidis, H, Petit-Phar, M, Chaumette, MT, Delchier, JC, et al. Mucosal immunity in primary glomerulonephritis III study of intestinal permeability. Nephron. (1993) 63:286–90. doi: 10.1159/000187211
28. Coppo, R, Amore, A, Roccatello, D, Gianoglio, B, Molino, A, Piccoli, G, et al. IgA antibodies to dietary antigens and lectin-binding IgA in sera from Italian, Australian, and Japanese IgA nephropathy patients. Am J Kidney Dis. (1991) 17:480–7. doi: 10.1016/s0272-6386(12)80644-5
29. Welander, A, Sundelin, B, Fored, M, and Ludvigsson, JF. Increased risk of IgA nephropathy among individuals with celiac disease. J Clin Gastroenterol. (2013) 47:678–83. doi: 10.1097/MCG.0b013e318284792e
30. Coppo, R, Mazzucco, G, Martina, G, Roccatello, D, Amore, A, Novara, R, et al. Gluten-induced experimental IgA glomerulopathy. Lab Investig. (1989) 60:499–506.
31. Tsuda, S, Nakayama, M, Tanaka, S, Haruyama, N, Yoshitomi, R, Fukui, A, et al. The association of controlling nutritional status score and prognostic nutritional index with cardiovascular diseases: the Fukuoka kidney disease registry study. J Atheroscler Thromb. (2023) 30:390–407. doi: 10.5551/jat.63501
Keywords: IgA nephropathy (IgAN), nutrition, index, end-stage renal disease, renal pathology
Citation: Qian C, Li H, Hou Y, Wang W and Sun M (2024) Clinical implications of four different nutritional indexes in patients with IgA nephropathy. Front. Nutr. 11:1431910. doi: 10.3389/fnut.2024.1431910
Edited by:
Diego Augusto Santos Silva, Federal University of Santa Catarina, BrazilReviewed by:
Mariia Ivanova, European Institute of Oncology IRCCS, ItalyLaureline Berthelot, INSERM U1064 Centre de Recherche en Transplantation et Immunologie, France
Copyright © 2024 Qian, Li, Hou, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mindan Sun, c3VubWRAamx1LmVkdS5jbg==
 Chuyue Qian
Chuyue Qian Huimin Li
Huimin Li Wanning Wang
Wanning Wang Mindan Sun
Mindan Sun