- 1Student Research Committee, Nutrition and Food Security Research Center and Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
- 3Nutrition and Food Security Research Center and Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
- 4Isfahan Kidney Diseases Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 5St Boniface Hospital, University of Manitoba, Winnipeg, MB, Canada
Background: Hemodialysis (HD) patients have a low quality of life (QOL), and dietary intakes may impact both somatic and psychosocial aspects of QOL. Nevertheless, the relationship between QOL and different dietary fats has not yet been evaluated.
Objective: The purpose of this study was to assess the association between QOL and the types/quantities of dietary fats intake in HD patients.
Methods: In this multi-center cross-sectional study, 251 adult patients under dialysis for at least 3 months were included. Participants’ dietary intakes were collected using a validated 168-item semi-quantitative FFQ during the past year. Moreover, to assess QOL, Kidney Disease Quality of Life Short Form (KDQOL-SF 1/3) was used. The linear regression between QOL and different types of dietary fats was conducted. p < 0.05 was statistically significant.
Results: Overall, 66 women and 185 men participated in our study. Regression analysis adjusted for total calorie intake showed that there was a negative association between QOL and total fat (95% CI: −0.187, −0.043), SFA (95% CI: −0.688, −0.143), MUFA (95% CI: −0.389, −0.065) and PUFA (95% CI: −0.401, −0.056) when types of dietary fats were individually included to the regression analysis. When all types of dietary fats were simultaneously entered into the analysis, the association between QOL and MUFA (95% CI: −0.243, 1.031) and PUFA (95% CI: −1.159, 0.084) were attenuated. The regression coefficient for SFA remained significant (95% CI: −0.968, −0.138). Also, there was a marginally significant association between SFA and the risk of low QOL was observed when all types of dietary fats were simultaneously entered into the analysis (OR = 1.051, 95% CI: 0.998–1.104).
Conclusion: Our investigation found a negative association between SFA consumption and QOL among different types of dietary fats. Furthermore, SFA mediated the relationship between QOL, MUFA, PUFA, and total fat. So, modification of dietary fat intake could enhance QOL in HD patients.
Introduction
Most patients with end-stage renal disease (ESRD) should be under renal replacement therapy such as hemodialysis (HD) (1). Although HD is necessary for survival in these patients, it cannot mimic all kidney functions (2). Therefore, patients suffer from several metabolic dysfunctions that affect their quality of life (QOL). Evidence showed that the physical and psychosocial aspects of QOL in HD patients were not as good as healthy subjects (3, 4). Assessment of QOL can provide a comprehensive medical judgement and promote patient-physician relationships (5). Limited physical activity, emotional distress, increased financial burden, HD’s time-consuming nature, and negative social pressure are the main reasons for poor QOL in HD patients (6). Nevertheless, the importance of malnutrition to QOL should not be neglected (7–9).
HD patients are susceptible to protein-energy malnutrition (PEM) because of poor appetite, decreased dietary intake and loss of nutrients through dialysis membranes (10, 11). More significant morbidity, functional impairment, more prolonged hospitalizations, and decreased QOL are strongly related to PEM (12, 13). Poor quality of life may contribute to eating disorders and consequently lead to malnutrition (14). Alternatively, previous findings indicated that malnutrition negatively affects the QOL of the patients by reducing their muscle strength (15) and affecting psychological and neurological complications (16). As malnourished patients have a worse quality of life, early diagnosis and treatment of malnutrition are crucial (12, 13). Research have shown that PEM is often caused by insufficient dietary energy intake in HD patients (17, 18). Therefore, the intake of sources of dietary energy should be sufficient. Dietary fat is the most energy-dense macronutrient (19). Moreover, fat is considered the main store of energy in the body (20). Therefore, adequate dietary fat intake may prevent PEM and positively affect QOL.
Besides the importance of dietary fat as a source of energy, the profile of dietary fats has a significant role in physiological functions (21). Furthermore, since CKD patients are at high risk of cardiovascular disease, types and amounts of dietary fats should be noticed (22). Meanwhile, nutritional recommendations regarding fat intake in CKD patients have been given less attention. For instance, Kidney Disease Improving Global Outcomes guidelines (KDIGO) did not recommend dietary fat intake in HD patients (23). Moreover, as a specific kidney disease dietary recommendation, the 2020 KDOQI update, only recommended supplementation with long-chain ω-3 PUFAs for managing dyslipidemia in kidney disease (24). However, they made no recommendations regarding the dietary intake of these fatty acids. According to KDOQI, 1.3–4 g/d supplementation of long-chain n-3 PUFA is recommended for adults on MHD to lower triglycerides and LDL cholesterol and raise HDL levels (24). In general, Mono-unsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) are considered healthy dietary fats (25). Conversely, saturated fatty acid (SFA) intake is a major cardiovascular risk factor (26). PUFAs, particularly docosahexaenoic acid (DHA), are concentrated in the neuronal cell membrane (27). These fatty acids are crucial for the functions and development of the nervous system (28). Furthermore, fatty acids contribute to membrane fluidity and function, synaptic transmission and metabolism of neurotransmitters (29–31). A study reported that SFA intake was directly, and consumption of MUFAs and alpha-linolenic acid (ALA) inversely related to anxiety risk (32). Moreover, the beneficial effects of supplementing with n-3 PUFA on improving depressive symptoms and QOL among HD patients were reported (33, 34). In contrast, a meta-analysis showed that n-3 PUFA supplementation had no significant impact on anxiety (35). Therefore, results regarding the association between dietary fat and QOL are inconsistency. Also, there were no studies regarding the relationship between QOL and dietary fats in HD patients. The present study aimed to investigate the association between QOL and the profile of dietary fat intake (i.e., total fat, SFA, MUFA, PUFA, and cholesterol) in HD patients.
Materials and methods
Study population and protocol
This multi-center cross-sectional study was conducted on 251 maintenance HD patients from September 2021 to March 2022.Participants were recruited from the five governmental and charity hemodialysis centers in Isfahan, Iran. We included patients if they met the following criteria: undergoing maintenance HD for at least the previous 90 days, being at least 18 years old, and having the ability and willingness to participate in our study. Individuals were excluded if their daily energy intake was above 4,200 kcal/d (17,573 kJ) or less than 800 kcal/d (3,347 kJ) (36). A brief description of the study’s significance, methods, goals, and timeline was provided to all participants. All volunteers filled out the informed consent forms before starting the study. This study was approved by The Research Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.RESEARCH.REC.1399.605).
Dietary assessment
A semi-quantitative food frequency questionnaire (FFQ) containing 168 food items was used to assess dietary intakes during the past year. The reliability and validity of this questionnaire were previously evaluated and found to be acceptable in the Iranian population (37). FFQ was completed via face-to-face interviews with a trained dietitian only once at the baseline. For each food item, two parameters were asked: 1) the frequency of consumption (never or < 1 times/month, 1–3 times/month, 1 times/week, 2–4 times/week, 5–6 times/week, 1 times/day, 2–3 times/day, 4–5 times/day and ≥6 times/day) in the previous year, and 2) The usual amounts of food consumed every time were based on a standard portion size. Then mean daily intake was calculated for each food item using the following formula: (frequency of consumption × amount of food item intake (g) / duration of reported frequency (day). For example, in the case of consuming one potato (90 grams) two times per week, the mean daily intake of potato was 2 × 90/7 = 25.7 g/day. Then macro/micronutrients intake were assessed based on the mean daily intake by Nutritionist IV software (First Databank, Hearst Corp).
Assessment of quality of life
We used the Kidney Disease Quality of Life Short Form (KDQOL-SF 1/3) to assess QOL in HD patients. Combination of SF-36 generic instrument with the kidney disease-specific instrument which forms the KDQOL-SF TM version 1.3. This questionnaire is composed of 80 items arranged into 19 categories. There are 43 items focused on kidney disease in KDQOL-SFTM 1.3. This questionnaire contained 11 domains including a list of symptoms and problems (12 items), the impact of renal disease on daily life (8 items), the burden of renal disease (4 items), occupational status (2 items), cognitive function (3 items), social contacts quality (3 items), sexual function (2 items), sleep quality (4 items), social support (2 items), dialysis staff encouragement (2items), and patient satisfaction (1 items).
SF-36 consists of 8 domains (36 items) that measure functioning and well-being. These domains covered physical functions (10 items), physical roles (4 items), pain (2 items), general health (5 items), emotional health (5 items), psychological roles (3 items), social activities (2 items), and energy/fatigue (4 items). Finally, a 0–10 scale is used for rating respondents’ overall health. A physical component summary (PCS) and a mental component summary (MCS) are further summarized by the SF-36 instrument. Subjects scored from 0 to 100, with higher values indicating better QOL (38). The reliability and validity of this questionnaire have been previously assessed and found to be acceptable in Iranian HD patients (39).
Anthropometric measurements
Height was measured with 0.1 cm precision using non-stretchable tape. It was measured in a standing position while shoulders and barefoot touching the wall. Dry weight was defined as the minimum tolerable weight achieved after a dialysis session by means of gradual change in post-dialysis weight at which there are no signs or symptoms of either hypovolemia or hypervolemia (40). Dry weight was measured to the nearest 0.1 kg using a calibrated digital floor scale after dialysis session when no signs or symptoms of either hypovolemia or hypervolemia were observed. Also, individuals were asked to wear light clothing without shoes (41). The body mass index (BMI) was determined by dividing dry weight to squared height. Waist circumference (WC) was also measured to the nearest 0.1 cm using a stable tension tape (42). It was measured at the midpoint between the lowest rib and iliac crest in a standing position. Hip circumference (HC) was measured at the maximum circumference over the buttocks to the nearest 0.1 cm (43). By dividing the WC by the HC, the waist-to-hip ratio (WHR) was determined. A flexible, non-stretchable tape was used to measure the mid-upper arm circumference (MUAC). It was measured on the bare left arm between the inferior border of the acromion process (shoulder bone) and the tip of the olecranon process (elbow) to the nearest 0.1 cm (44).
Assessment of quality of HD
To assess the quality of HD, we used the Urea reduction ratio (URR) and Kt/V. URR was calculated by following (45):
the dialyzer urea clearance (K) is multiplied by dialysis time to calculate the Kt/V (t) divided by the subject’s urea distribution volume (V) (45).
Assessment of other variables
Age, place of habitation, marital and occupational status, education, family income, smoking history, menopausal status and alcohol intake were collected by oral questions. Medical records were used to gather data, including medications, dialysis vintage, dialysis frequency, dialysis duration and cause of renal failure.
Statistical analysis
The normal distribution of dependent variables was assessed by Kolmogorov–Smirnov test and Q-Q plot. Qualitative variables were expressed as numbers (percentages). Quantitative variables were reported as mean ± standard deviation (SD). Comparisons between groups were performed using one-way analysis of variance (ANOVA) and also the Chi-square test, as appropriate. Linear regression was used to investigate the relationship between dietary fat intake and QOL. Logistic regression anlaysis was applied to assess risk of low QOL (median cut) per one-unit increase in different dietary fat intake. The regression coefficient and 95% confidence intervals were reported. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS version 21.
Results
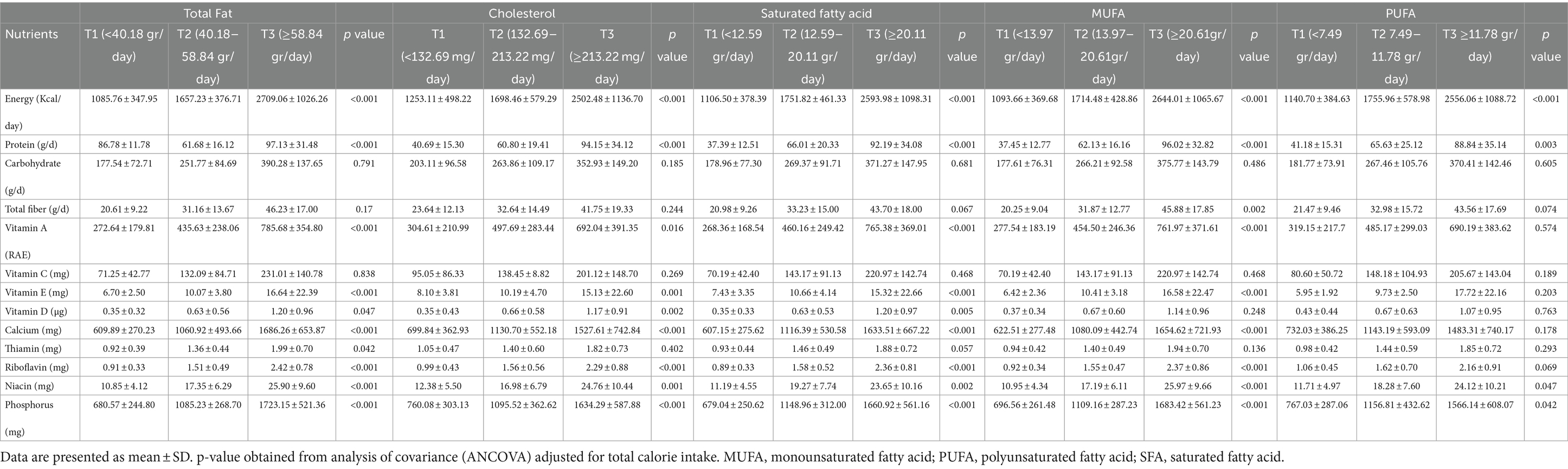
Overall, 66 women and 185 men were included in this study. Mean intake of total fat, cholesterol, SFA, MUFA and PUFA was 58.5 g/d, 201.57 mg/d, 18.86 g/d, 20.18 g/d and 11.85 g/d, respectively. The general characteristics of participants across tertiles of specific types of fat intake are summarized in Table 1. The percentage of men was higher in the last tertile of all types of dietary fats compared with the lower tertiles (p < 0.001 for all types of dietary fats). Similarly, subjects in the top tertile of all types of dietary fats had higher weight (p = 0.002 for total fat, p = 0.02 for cholesterol, p = 0.019 for SFA, p = 0.001 for MUFA and p = 0.023 for PUFA) and height (p < 0.001for total fat, cholesterol, SFA and MUFA, and p = 0.008 for PUFA). Individuals in higher tertiles of PUFA were younger than those in the lowest tertile (p = 0.038). URR decreased across tertiles of total fat (p = 0.005), MUFA (p = 0.022), and PUFA (p = 0.026). Additionally, subjects in the highest tertile of SFA had less dialysis duration per session than the lowest tertile (p = 0.016). Moreover, there were mostly retired patients in the top tertile of all types of dietary fats, whereas most unemployed individuals were in the lowest tertile (p < 0.001 for total fat, cholesterol, SFA, MUFA and p = 0.01 for PUFA). No significant differences were observed regarding other characteristics throughout the tertiles of different types of dietary fat. Energy-adjusted nutrient intake across tertiles of specific types of dietary fatsis shown in Table 2. Participants with the highest adherence to total fat intake had a higher consumption of energy (p < 0.001), protein (p < 0.001), vitamin A (p < 0.001), vitamin E (p < 0.001), calcium (p < 0.001), thiamin (p = 0.042), riboflavin (p < 0.001), niacin (p < 0.001), and phosphorus (p < 0.001). Moreover, individuals with the highest consumption of cholesterol had a higher intake of energy (p < 0.001), protein (p < 0.001), vitamin A (p = 0.016), vitamin E (p < 0.001), calcium (p < 0.001), riboflavin (p < 0.001), niacin (p < 0.001), as well as phosphorus (p < 0.001).Patients with higher consumption of SFA consume more energy (p < 0.001), protein (p < 0.001), vitamin A (p < 0.001), vitamin E (p < 0.001), calcium (p < 0.001), riboflavin (p < 0.001), niacin (p < 0.001), and phosphorus (p < 0.001). Subjects in the top tertile of MUFA had the highest consumption of energy (p < 0.001), protein (p < 0.001), vitamin A (p < 0.001), vitamin E (p < 0.001), calcium (p < 0.001), riboflavin (p < 0.001), niacin (p = 0.002), and phosphorus (p < 0.001). Individuals in the last tertile of PUFA had the highest amounts of energy (p < 0.001), protein(p = 0.003), niacin (p = 0.047), and phosphorus (p = 0.042). Other nutrients were not significantly different across tertiles of different dietary fats.
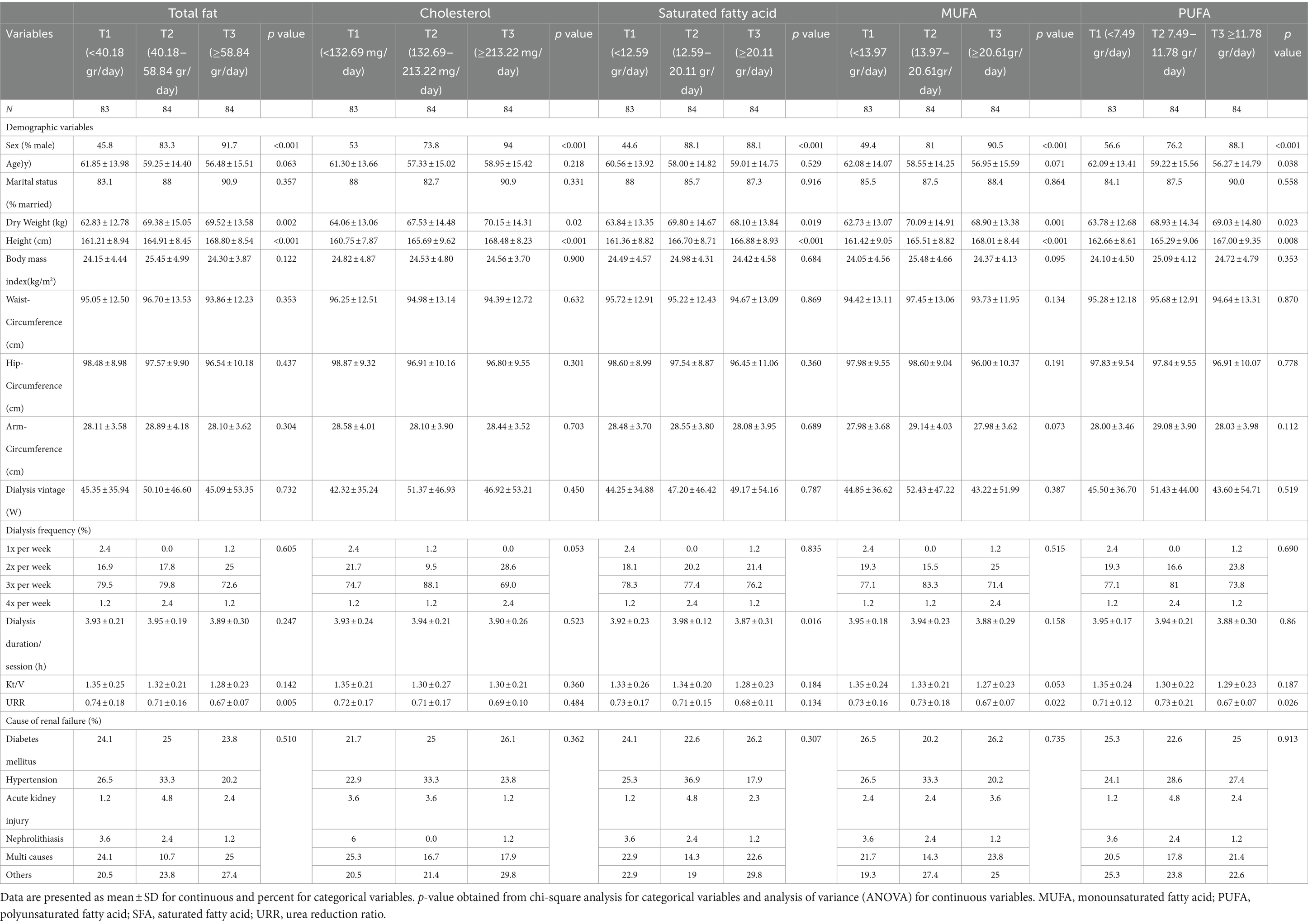
Table 1. General characteristics of hemodialysis patients across tertiles of different types of dietary fats.
Results of the calorie adjusted linear regression between QOL and different types of dietary fats among HD patients are presented in Table 3. There was a negative association between QOL and total fat (B = −0.115; 95% CI: −0.187, −0.043), SFA (B = −0.416; 95% CI: −0.688, −0.143), MUFA (B = −0.227; 95% CI: −0.389, −0.065) and PUFA (B = −0.228; 95% CI: −0.401, −0.056) when types of dietary fats were individually included to the regression analysis. We did not observe any relation between cholesterol intake and the score of QOL. When all dietary fats were simultaneously entered into the analysis, the association between QOL and MUFA (B = 0.394; 95% CI: −0.243, 1.031) and PUFA (B = −0.537; 95% CI: −1.159, 0.084) were attenuated. Inversely, regression coefficient for SFA remained significant (B = −0.553; 95% CI: −0.968, −0.138).
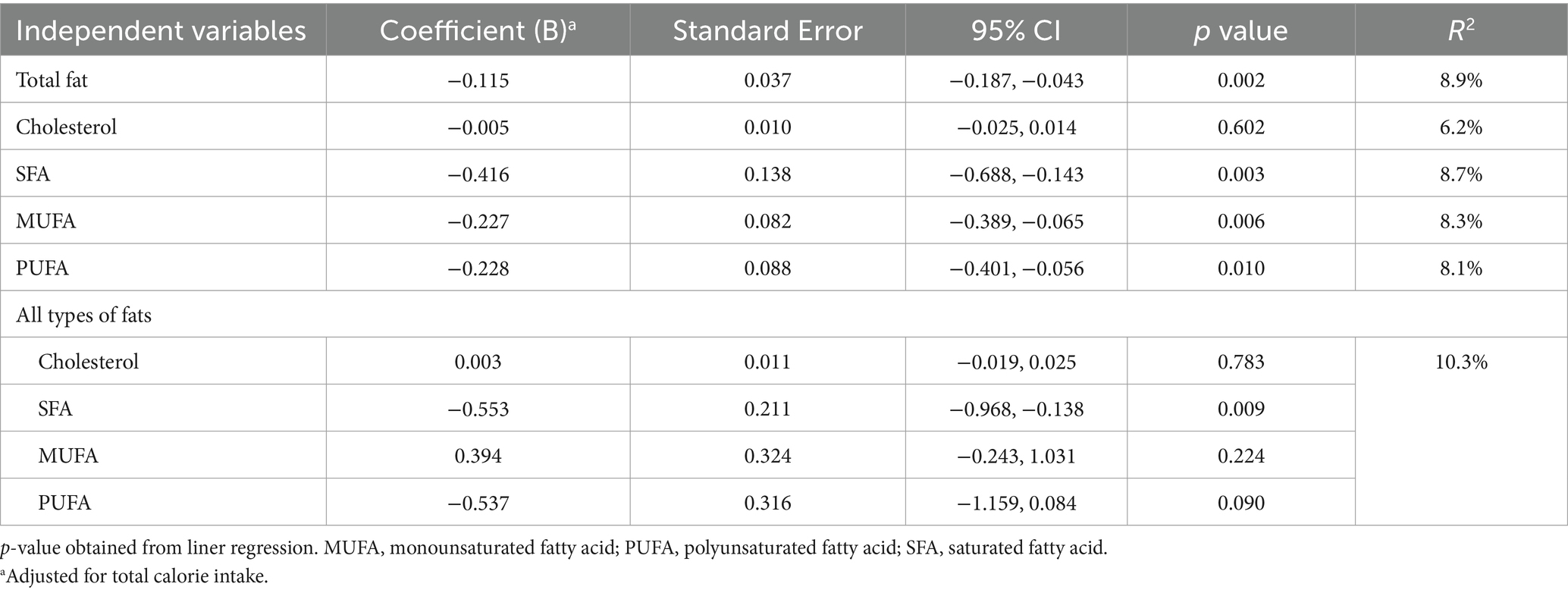
Table 3. Linear regression between quality of life and different types of dietary fats among hemodialysis patients.
The risk of low QOL per one-unit increase in intake of different dietary fats is presented in Table 4. There was no significant relationship between risk of low QOL and all types of dietary fat except for cholesterol in crude model (OR = 0.998, 95% CI: 0.996–1.000; p = 0.018). After adjusting for energy intake, we observed a significant association between the risk of low QOL and one-unit increase in intake of total fat (OR = 1.013, 95% CI: 1.004–1.022; p = 0.004), SFA (OR = 1.040, 95% CI: 1.006–1.076; p = 0.021), MUFA (OR = 1.028, 95% CI: 1.008–1.049; p = 0.005), and PUFA (OR = 1.029, 95% CI: 1.008–1.051; p = 0.007). Also, when all dietary fats were simultaneously entered into the analysis, we could not detect any significant relation between risk of low QOL and intake of dietary fats. In fully adjusted model (Model 2), there was a significant association between the risk of low QOL and on-unit increase in intake of total fat (OR = 1.011, 95% CI: 1.002–1.020), SFA (OR = 1.038, 95% CI: 1.004–1.074), MUFA (OR = 1.024, 95% CI: 1.004–1.045), and PUFA (OR = 1.024, 95% CI: 1.003–1.046). In Model 2 and after including all dietary fats simultaneously, the relation between risk of low QOL and MUFA and PUFA was attenuated and only a marginally significant association between SFA and the risk of low QOL was observed (OR = 1.051, 95% CI: 0.998–1.104).
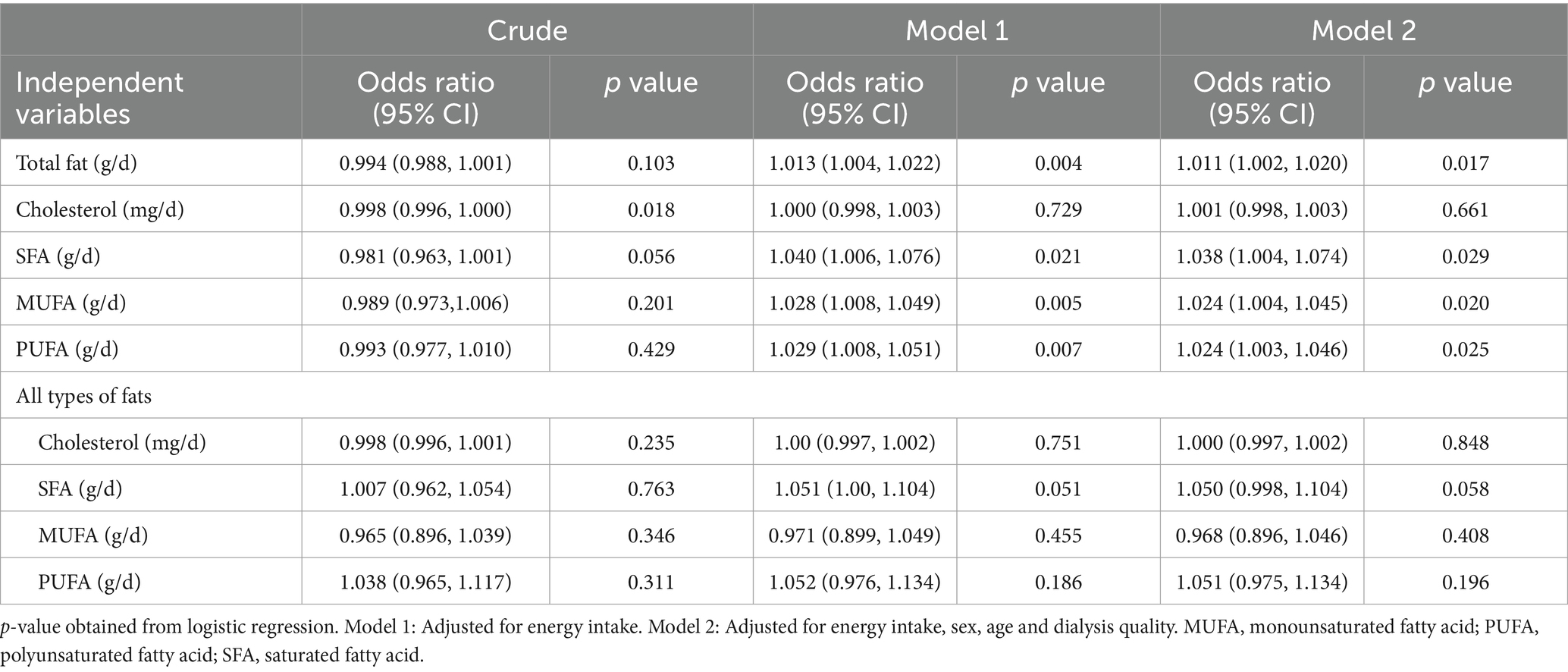
Table 4. The Risk of low quality of life per one-unit increase of different dietary fats among hemodialysis patients.
Discussion
We found that dietary intake of SFA, MUFA, PUFA and total fat was inversely associated with QOL. Nevertheless, the relation between QOL and MUFA and PUFA was attenuated when SFA was simultaneously entered into the regression analysis. These findings revealed that the relation between QOL and MUFA, PUFA and total fat was mediated by SFA.
Over the past decades, sources of dietary fat in the general population have changed significantly. The main change is substituting PUFA and MUFA for SFA (21). There is a consensus that PUFA and MUFA is healthy lipids due to their ability to decrease the risk of cardiovascular diseases (25). However, SFA is recognized as a major CVD risk factor (26). Moreover, higher intake of SFA has been linked to declined cognitive function (46) and increased risk of depression (47), anxiety (32) and dementia (48).These pieces of evidence support our findings that SFA intake was negatively associated with QOL. It is suggested that the impact of changes in dietary fat on QOL in different diseases should be assessed in future studies.
We found that the negative association between QOL and MUFA or PUFA was mediated by SFA and results were insignificant when SFA was simultaneously entered into the regression analysis. Foods containing fats typically contain a wide range of fatty acids, including SFA, MUFA and PUFA (49). SFAs are found in both plant and animal food sources. Animal-source foods such as red meat, bacon, lard, and high-fat dairy products are condensed sources of SFA (50). On the other hand, oils extracted from palm kernel, coconut, olive, soybean and sunflower are considered plant sources of SFA (51). For instance, the concentration of SFA in olive oil may be up to 25% (52). Therefore, there is a co-consumption of MUFA/PUFA and SFA; subjects who consumed more vegetable oil may intake large amounts of SFA.
Consequently, assessment of dietary intake by questionnaires cannot determine the exact intake of different types of dietary fats, and more reliable methods such as dietary biomarkers (e.g., plasma phosphor acid fatty acid composition and adipose tissue) should be used (53), Evidence showed that using biomarkers may alter the conclusion of a study. A meta-analysis reported that blood concentration of PUFA was inversely associated with the risk of cancer. However, there was no significant association between dietary intake of PUFA and the risk of cancer (54).
We found an indirect association between SFA intake and QOL. Metabolic changes that occurred after consuming various types of fatty acids may explain our finding. High dietary SFA intake elevates inflammation by stimulating the secretion of proinflammatory compounds (e.g., TNF -α and IL-6) and reducing anti-inflammatory cytokines such as interleukin-10 and Arginase-1 (55).
Previous findings indicated that chronic inflammation could negatively affect the emotional and social domains of QOL (56). Therefore, the association between high dietary intake of SFA and decreased QOL is reasonable. In addition, a high intake of dietary SFAs promotes insulin resistance by releasing inflammatory cytokines and endotoxins (57). Insulin resistance and impaired glucose regulation can result in neuronal dysfunctions and lead to cognitive deficits (58–60).
The limitations of this study should also be addressed. First, we could not evaluate the relationship between trans fatty acid and QOL because we had no data regarding the trans fatty acid content of the foods. Second, we did not assess dietary intake by a biomarker. Third, the cross-sectional design of our study had several limitations regarding a causal relationship between dietary SFA intake and QOL.
Conclusion
In conclusion, among different types of dietary fats, we found an inverse relationship between dietary intake of SFA and QOL. Also, the relation between QOL and MUFA, PUFA and total fat was mediated by SFA.
In future studies, a specific focus should be placed on the type of fat intake as a key factor affecting the patient’s quality of life. Furthermore, to evaluate the quantity and type of consumed fat in such studies, a suitable biomarker is recommended. It is hoped that the association between types of fat intake and symptoms and complications of this disease will be evaluated comprehensively to improve the guidelines and recommendations for HD patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was ethically approved by the Research Council and Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran, (Code: IR.MUI.RESEARCH.REC.1399.605). All participants provided written informed consent to participate in the study.
Author contributions
FN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SF: Writing – review & editing. MV: Writing – review & editing. GA: Writing – review & editing. FM: Writing – review & editing. HH: Writing – review & editing. SM: Writing – review & editing. MR: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Isfahan University of Medical science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth, A . End-stage renal disease and renal replacement therapy in older patients. Nephro-urol Monthly. (2012) 4:425–30. doi: 10.5812/numonthly.1825
2. Lee, K-Y . A unified pathogenesis for kidney diseases, including genetic diseases and cancers, by the protein-homeostasis-system hypothesis. Kidney Res Clin Pract. (2017) 36:132–44. doi: 10.23876/j.krcp.2017.36.2.132
3. Majkowicz, M, Afeltowicz, Z, Dębska-Slizień, A, Lichodziejewska-Niemierko, M, and Rutkowski, B. Jakość życia chorych hemodializowanych, dializowanych otrzewnowo oraz pacjentów onkologicznych. Psychoonkologia. (1999) 4:53–63.
4. Sapilak, BJ, Kurpas, D, Steciwko, A, and Melon, M. Czy jakość życia jest istotna dla chorych dializowanych? Na podstawie 3-letniej obserwacji pacjentów. Problemy lekarskie. (2006) 45:89–93.
5. Klim, M. (2014). The nurse’s role in relieving pain in patients on dialysis. Renal Disease and Transplantation Forum.
6. Mittal, SK, Ahern, L, Flaster, E, Maesaka, JK, and Fishbane, S. Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant. (2001) 16:1387–94. doi: 10.1093/ndt/16.7.1387
7. Chen, M-F, Chang, R-E, Tsai, H-B, and Hou, Y-H. Effects of perceived autonomy support and basic need satisfaction on quality of life in hemodialysis patients. Qual Life Res. (2018) 27:765–73. doi: 10.1007/s11136-017-1714-2
8. Giannaki, CD, Hadjigavriel, M, Lazarou, A, Michael, A, Damianou, L, Atmatzidis, E, et al. Restless legs syndrome is contributing to fatigue and low quality of life levels in hemodialysis patients. World J Nephrol. (2017) 6:236–42. doi: 10.5527/wjn.v6.i5.236
9. Rhee, CM, Chen, Y, You, AS, Brunelli, SM, Kovesdy, CP, Budoff, MJ, et al. Thyroid status, quality of life, and mental health in patients on hemodialysis. Clin J Am Soc Nephrol. (2017) 12:1274–83. doi: 10.2215/CJN.13211216
10. Kuhlmann, MK, Kribben, A, Wittwer, M, and Hörl, WH. OPTA—malnutrition in chronic renal failure. Nephrol Dial Transplant. (2007) 22:iii13–9. doi: 10.1093/ndt/gfm016
11. Nagy, E, Mahmoud, M, El-Kannishy, G, and Sayed-Ahmed, N. Impact of malnutrition on health-related quality of life in patients on maintenance hemodialysis. Ther Apher Dial. (2021) 25:467–74. doi: 10.1111/1744-9987.13588
12. Sohrabi, Z, Eftekhari, MH, Eskandari, MH, Rezaeianzadeh, A, and Sagheb, MM. Malnutrition-inflammation score and quality of life in hemodialysis patients: is there any correlation? Nephro-urol Monthly. (2015) 7:e27445. doi: 10.5812/numonthly.7(3)2015.27445
13. Campbell, KL, Ash, S, and Bauer, JD. The impact of nutrition intervention on quality of life in pre-dialysis chronic kidney disease patients. Clin Nutr. (2008) 27:537–44. doi: 10.1016/j.clnu.2008.05.002
14. Mitchison, D, Morin, A, Mond, J, Slewa-Younan, S, and Hay, P. The bidirectional relationship between quality of life and eating disorder symptoms: a 9-year community-based study of Australian women. PLoS One. (2015) 10:e0120591. doi: 10.1371/journal.pone.0120591
15. Alfonso-Rosa, RM, Del Pozo-Cruz, B, Del Pozo-Cruz, J, Del Pozo-Cruz, JT, and Sañudo, B. The relationship between nutritional status, functional capacity, and health-related quality of life in older adults with type 2 diabetes: a pilot explanatory study. J Nutr Health Aging. (2013) 17:315–21. doi: 10.1007/s12603-013-0028-5
16. Başoğlu, M, Yetimalar, Y, Gürgör, N, Büyükçatalbaş, S, Kurt, T, Seçil, Y, et al. Neurological complications of prolonged hunger strike. Eur J Neurol. (2006) 13:1089–97. doi: 10.1111/j.1468-1331.2006.01531.x
17. Carrero, JJ . Mechanisms of altered regulation of food intake in chronic kidney disease. J Ren Nutr. (2011) 21:7–11. doi: 10.1053/j.jrn.2010.10.004
18. Fouque, D, Kalantar-Zadeh, K, Kopple, J, Cano, N, Chauveau, P, Cuppari, L, et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. (2008) 73:391–8. doi: 10.1038/sj.ki.5002585
19. Atwater, WO . Experiments on the metabolism of matter and energy in the human body, 1898–1900. Washington, DC: US Government Printing Office (1902).
20. Yu, Y-H, and Ginsberg, HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. (2005) 96:1042–52. doi: 10.1161/01.RES.0000165803.47776.38
21. Collins, AJ . Cardiovascular mortality in end-stage renal disease. Am J Med Sci. (2003) 325:163–7. doi: 10.1097/00000441-200304000-00002
22. Kochan, Z, Szupryczynska, N, Malgorzewicz, S, and Karbowska, J. Dietary lipids and dyslipidemia in chronic kidney disease. Nutrients. (2021) 13:3138. doi: 10.3390/nu13093138
23. KDIGO . 2024 clinical practice guideline for the evaluation and Management of Chronic Kidney Disease. Kidney Int. (2024) 105:S117–s314. doi: 10.1016/j.kint.2023.10.018
24. Ikizler, TA, Burrowes, JD, Byham-Gray, LD, Campbell, KL, Carrero, JJ, Chan, W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–s107. doi: 10.1053/j.ajkd.2020.05.006
25. Yuzbashian, E, Asghari, G, Mirmiran, P, Hosseini, F-S, and Azizi, F. Associations of dietary macronutrients with glomerular filtration rate and kidney dysfunction: Tehran lipid and glucose study. J Nephrol. (2015) 28:173–80. doi: 10.1007/s40620-014-0095-7
26. Hu, FB . WC Willett optimal diets for prevention of coronary heart disease. JAMA. (2002) 88:2569–78.
27. Valenzuela, A . Docosahexaenoic acid (DHA), an essential fatty acid for the proper functioning of neuronal cells: their role in mood disorders. Grasas Aceites. (2009) 60:203–12. doi: 10.3989/gya.085208
28. Czyż, K, Bodkowski, R, Herbinger, G, and Librowski, T. Omega-3 fatty acids and their role in central nervous system-a review. Curr Med Chem. (2016) 23:816–31. doi: 10.2174/0929867323666160122114439
29. Gupta, A, Jadhav, A, Petkar, S, and Dubey, V. (2013). Study of lipid derangement in Pyschiatric disorder.
30. Chen, H-F, and Su, H-M. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic–pituitary–adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J Nutr Biochem. (2013) 24:70–80. doi: 10.1016/j.jnutbio.2012.02.006
31. Bazan, NG . Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol. (2005) 32:089–104. doi: 10.1385/MN:32:1:089
32. Fatemi, F, Siassi, F, Qorbani, M, and Sotoudeh, G. Higher dietary fat quality is associated with lower anxiety score in women: a cross-sectional study. Ann General Psychiatry. (2020) 19:1–9. doi: 10.1186/s12991-020-00264-9
33. Moeinzadeh, F, Shahidi, S, Mortazavi, M, Dolatkhah, S, Kajbaf, M, Javanmard, SH, et al. Effects of omega-3 fatty acid supplementation on serum biomarkers, inflammatory agents, and quality of life of patients on hemodialysis. Iran J Kidney Dis. (2016) 10:381–7.
34. Gharekhani, A, Khatami, M-R, Dashti-Khavidaki, S, Razeghi, E, Noorbala, A-A, Hashemi-Nazari, S-S, et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol. (2014) 70:655–65. doi: 10.1007/s00228-014-1666-1
35. Deane, KH, Jimoh, OF, Biswas, P, O'Brien, A, Hanson, S, Abdelhamid, AS, et al. Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: systematic review and meta-analysis of randomised trials. Br J Psychiatry. (2021) 218:135–42. doi: 10.1192/bjp.2019.234
36. Azadbakht, L, Mirmiran, P, Hosseini, F, and Azizi, F. Diet quality status of most Tehranian adults needs improvement. Asia Pac J Clin Nutr. (2005) 14:163–8.
37. Mirmiran, P, Esfahani, FH, Mehrabi, Y, Hedayati, M, and Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
38. Hays, RD, Kallich, J, Mapes, D, Coons, S, Amin, N, Carter, W, et al. Kidney disease quality of life short form (KDQOL-SF), version 1.3: a manual for use and scoring. Santa Monica, CA: Rand Corp (1997).
39. Pakpour, AH, Yekaninejad, M, Molsted, S, Harrison, AP, Hashemi, F, and Saffari, M. Translation, cultural adaptation assessment, and both validity and reliability testing of the kidney disease quality of life–short form version 1.3 for use with Iranian patients. Nephrology. (2011) 16:106–12. doi: 10.1111/j.1440-1797.2010.01389.x
40. Sinha, AD, and Agarwal, R. (2017). Setting the dry weight and its cardiovascular implications. Seminars in dialysis. Wiley Online Library.
41. Lohman, TG, Roche, AF, and Martorell, R. (1988). Anthropometric standardization reference manual. Chicago: Human kinetics book.
42. Esmaili, H, Bahreynian, M, Qorbani, M, Motlagh, ME, Ardalan, G, Heshmat, R, et al. Prevalence of general and abdominal obesity in a nationally representative sample of Iranian children and adolescents: the CASPIAN-IV study. Iran J Pediatr. (2015) 25:401. doi: 10.5812/ijp.25(3)2015.401
43. Organization WH . (2008). Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva. 8–11 December. 2011.
44. Chen, ST, Ngoh, HJ, and Harith, S. Prevalence of malnutrition among institutionalized elderly people in northern peninsular Malaysia: gender, ethnicity and age-specific. Sains Malays. (2012) 41:141–8.
45. Maduell, F, Garcia-Valdecasas, J, Garcia, H, Hdez-Jaras, J, Sigüenza, F, Del Pozo, C, et al. Urea reduction ratio considering urea rebound. Nephron. (1998) 78:143–7. doi: 10.1159/000044902
46. Cao, G-Y, Li, M, Han, L, Tayie, F, Yao, S-S, Huang, Z, et al. Dietary fat intake and cognitive function among older populations: a systematic review and meta-analysis. J Prev Alzheimers Dis. (2019) 6:204–11. doi: 10.14283/jpad.2019.9
47. Li, D, Liang, H, Tong, Y, Zheng, H, and Li, Y. Association between saturated fatty acid intake and depressive symptoms in midlife women: a prospective study. J Affect Disord. (2020) 267:17–22. doi: 10.1016/j.jad.2020.01.173
48. Lee, Y, Back, JH, Kim, J, Kim, S-H, Na, DL, Cheong, H-K, et al. Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr. (2010) 22:174–87. doi: 10.1017/S1041610209991189
49. Lunn, J, and Theobald, H. The health effects of dietary unsaturated fatty acids. Nutr Bull. (2006) 31:178–224. doi: 10.1111/j.1467-3010.2006.00571.x
50. Knight, EL, Stampfer, MJ, Hankinson, SE, Spiegelman, D, and Curhan, GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. (2003) 138:460–7. doi: 10.7326/0003-4819-138-6-200303180-00009
51. Micha, R, and Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. (2010) 45:893–905. doi: 10.1007/s11745-010-3393-4
52. Kelebek, H, Kesen, S, and Selli, S. Comparative study of bioactive constituents in Turkish olive oils by LC-ESI/MS/MS. Int J Food Prop. (2015) 18:2231–45. doi: 10.1080/10942912.2014.968788
53. Parry, SA, Rosqvist, F, Peters, S, Young, RK, Cornfield, T, Dyson, P, et al. The influence of nutritional state on the fatty acid composition of circulating lipid fractions: implications for their use as biomarkers of dietary fat intake. Ups J Med Sci. (2021) 126:7649. doi: 10.48101/ujms.v126.7649
54. Kim, Y, and Kim, J. N-6 polyunsaturated fatty acids and risk of cancer: accumulating evidence from prospective studies. Nutrients. (2020) 12:2523. doi: 10.3390/nu12092523
55. Chan, KL, Pillon, NJ, Sivaloganathan, DM, Costford, SR, Liu, Z, Théret, M, et al. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J Biol Chem. (2015) 290:16979–88. doi: 10.1074/jbc.M115.646992
56. Nowakowski, AC . Chronic inflammation and quality of life in older adults: a cross-sectional study using biomarkers to predict emotional and relational outcomes. Health Qual Life Outcomes. (2014) 12:1–12. doi: 10.1186/s12955-014-0141-0
57. Glass, CK, and Olefsky, JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. (2012) 15:635–45. doi: 10.1016/j.cmet.2012.04.001
58. Greenwood, CE, and Winocur, G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. (2005) 26:42–5. doi: 10.1016/j.neurobiolaging.2005.08.017
59. Steen, E, Terry, BM, Rivera, EJ, Cannon, JL, Neely, TR, Tavares, R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease–is this type 3 diabetes? J Alzheimers Dis. (2005) 7:63–80. doi: 10.3233/JAD-2005-7107
Keywords: dietary fat, fatty acids, quality of life, hemodialysis, cross-sectional study
Citation: Navab F, Foshati S, Vajdi M, Askari G, Moeinzadeh F, Heshamtipour H, Mirzaeian S and Rouhani MH (2024) Is there any association between type of dietary fat and quality of life in hemodialysis patients? A cross-sectional study. Front. Nutr. 11:1430595. doi: 10.3389/fnut.2024.1430595
Edited by:
Atefeh Ashabi, Semnan University of Medical Sciences, IranReviewed by:
Kenji Nagao, Ajinomoto, JapanJeanette Mary Andrade, University of Florida, United States
Copyright © 2024 Navab, Foshati, Vajdi, Askari, Moeinzadeh, Heshamtipour, Mirzaeian and Rouhani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Hossein Rouhani, c21fcm91aGFuaUBudXRyLm11aS5hYy5pcg==
 Fatemeh Navab1
Fatemeh Navab1 Sahar Foshati
Sahar Foshati Mahdi Vajdi
Mahdi Vajdi Gholamreza Askari
Gholamreza Askari Mohammad Hossein Rouhani
Mohammad Hossein Rouhani