- 1State Key Laboratory of Food Science and Resources, China-Canada Joint Lab of Food Science and Technology (Nanchang), Key Laboratory of Bioactive Polysaccharides of Jiangxi Province, Nanchang University, Nanchang, China
- 2Department of Medical Imaging, Ganzhou People's Hospital, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, China
Background: Fruits and vegetables (FVs) are widely believed to mitigate the risk of atherosclerosis (AS). However, the causal relationships between specific FVs and AS risk factors remain unclear.
Methods: This study performed two-sample Mendelian Randomization (MR) analysis to infer the causality of the intake of 28 kinds of FVs with AS, as well as its risk factors including blood low-density lipoprotein cholesterol (LDL-C), triglycerides (TG) and C-reactive protein (CRP). GWAS genetic data for these exposures and outcomes were extracted from the IEU open GWAS project. Heterogeneity was evaluated using both Inverse Variance Weighted (IVW) and MR-Egger methods. MR-Egger regression was specifically deployed to detect potential pleiotropy. Furthermore, a “leave-one-out” sensitivity analysis was conducted to determine the impact of each individual single nucleotide polymorphism (SNP) on the combined outcome.
Results: The analysis confirms a causal relationship between total fruit consumption and reduced levels of LDL-C (OR = 0.911, p = 0.007) and CRP (OR = 0.868, p = 0.008). Similarly, total vegetable intake is also causally associated with a reduction in CRP levels (OR = 0.858, p = 0.018). Specifically, garlic intake exhibits the most significant causal relationship with reduced risk of AS (OR = 0.985, p = 0.036) and also causally associated with lower levels of LDL-C and TG. Berry (OR = 0.929, p = 0.010) and potato (OR = 0.957, p = 0.020) intake both display a significant causal negative association with TG levels, while peach/nectarine consumption is significantly associated with reduced CRP levels (OR = 0.913, p = 0.010).
Conclusion: This is the first MR study that systemically examined the causality between commonly consumed FVs and AS. Our findings highlight the atheroprotective effects of various FVs, particularly garlic, on cardiovascular health and the importance of tailored nutritional recommendations to prevent AS.
Introduction
Atherosclerosis (AS) is a pathological state characterized by the localized accumulation of cholesterol and other lipids primarily within the intimal layer of medium and large arteries (1). In 2019, it was estimated that cardiovascular diseases (CVD) were responsible for the deaths of ~17.9 million people, representing 32% of all global fatalities (2). The majority of these deaths stemmed from myocardial infarction and stroke, which are often consequences of AS (3). AS usually progresses insidiously over decades, and it might not produce noticeable symptoms until there is considerable narrowing of the arteries or an abrupt plaque rupture (4). Being a disease influenced by multiple factors, including genetic predisposition, lifestyle choices, and metabolic disturbances, AS presents challenges in devising a one-size-fits-all treatment strategy. Managing AS typically involves significant lifestyle modifications, particularly with respect to diet and physical activity (5). Diets rich in fruits and vegetables (FVs) have been linked to a lower risk of developing atherosclerotic conditions (6). Epidemiological research has consistently demonstrated an inverse association between such dietary patterns and subclinical markers of AS, including carotid intima-media thickness (IMT) and the presence of atherosclerotic plaques (7). For instance, Ellingsen et al. calculated the intake of total fruits and berries in elderly men using a food frequency questionnaire (FFQ) and measured their carotid IMT (8). They found that carotid IMT was significantly lower in the highest quartile of fruit and berry intake compared to the lowest quartile. This inverse association held even after adjustments for age, smoking, and other dietary components. Similarly, Blekkenhorst et al. quantified the total vegetable intake of older adult women with subclinical AS using FFQ and discovered that participants consuming ≥3 servings of vegetables had significantly 4.6–5.0% lower carotid IMT compared with those consuming <2 servings (7).
Fruits and vegetables are typically rich in dietary fiber and various phytochemicals, such as plant sterols and flavonoids. Dietary fiber can slow gastric emptying and the transit of food through the intestines, reducing the absorption of fats and cholesterol, which assists in lowering blood lipid levels (9). Furthermore, dietary fiber can regulate the composition of gut commensals, which plays a pivotal role in the regulation of metabolism and immune responses across multiple organs and tissues (10, 11). Plant sterols, which structurally resemble cholesterol, can compete with it for intestinal absorption (12), while flavonoids can enhance vascular function and exert anti-inflammatory effects (13, 14). Additionally, Vitamins C and E, along with β-carotene and various other antioxidants, are essential in mitigating oxidative stress, which is a significant contributing factor in the development of AS. Oxidative stress promotes the oxidation of low-density lipoprotein (LDL), a critical step that leads to inflammatory responses and the eventual buildup of atherosclerotic plaques within the arteries (15). Moreover, FVs are rich sources of potassium and magnesium, minerals that are essential for maintaining normal blood pressure, a significant risk factor for AS (16). In fact, specific fruits such as apples, bananas, berries, cherries, grapes, grapefruits, melons, and, plums (17–24), as well as vegetables like cabbage, carrots, celery, garlic, onions, and tomatoes (25–30) have been associated, to varying degrees, with a protective effect against AS. However, findings remain inconsistent, and a definitive causal link between the consumption of these FVs and AS has yet to be established. Furthermore, since each type of fruit and vegetable has a unique phytochemical profile, it is still unclear which are the most effective at reducing the risk of AS. The systematic evaluation and comparison of the causal relationships between the consumption of various specific FVs and the development of AS require further investigation. The impact of dietary components on health may become evident only over a prolonged timeframe, complicating the assessment of long-term effects. Therefore, conducting randomized double-blind clinical trials to explore these potential causal links poses significant challenges.
Mendelian Randomization (MR) is a statistical method that uses genetic variants as instrumental variables (IVs) to infer causal relationships between risk factors and health outcomes. This approach assumes that the genetic variants are associated with the exposure but not with any confounders, enabling estimates of direct causal effects. In the present study, we utilized a two-sample MR method to examine and compare the potential causal relationships between the consumption of various FVs and the risk of developing AS. We also assessed the impact of these dietary elements on AS risk factors, including blood lipid levels and chronic inflammation. The fruits analyzed in this study were apples, bananas, berries, cherries, grapes, grapefruits, mangoes, melons, oranges, peaches/nectarines, pears, pineapples, and plums. The vegetables included broccoli, cabbage/kale, carrots, cauliflower, celery, courgettes, cucumbers, garlic, green beans, lettuce, onions, potatoes, spinach, sweet peppers, and tomatoes. This study underlines the potential for more tailored dietary recommendations in the prevention and management of AS.
Materials and methods
Study design
As depicted in Figure 1, this study performed to two-sample MR analysis to infer the causality between the consumption of various fruits and vegetables (FVs) and AS, as well as its risk factors including the levels of LDL-C, TG and CRP. FV consumption was used as exposure and AS, LDL-C, TG, and CRP were used as outcome. MR analysis followed three core assumptions: genetic variation is strongly associated with exposure; the genetic variation is not associated with confounding factors; the genetic variation influence outcome only through the exposure.
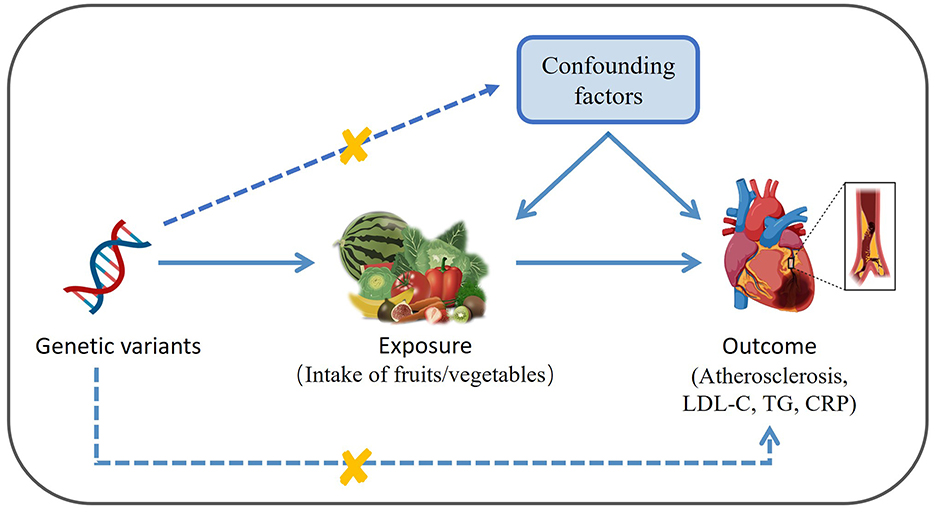
Figure 1. Diagram depicting the model of two-sample Mendelian Randomization analysis of the causal links between the intake of various fruits/vegetables and atherosclerosis as well as its risk factors (created with BioRender.com). LDL-C, low density lipoprotein cholesterol; TG, triglyceride; CRP, C-reactive protein.
Data source
GWAS datasets for FV intake, AS, LDL-C, and C-reactive protein (CRP) were obtained from publicly available database, the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/), with detailed information provided in Supplementary Table S1. Genetic datasets for the intake of various FVs were extracted from the IEU analysis of UK Biobank phenotypes. The datasets for the intake of various FVs included data from 64,949 participants, while those for total fruit and total vegetable intake involved 446,462 and 435,435 participants, respectively. In addition, genetic data for coronary atherosclerosis (ukb-d-I9_CORATHER) included 346,860 participants, comprising 14,334 cases and 332,526 controls. Genetic data for LDL-C (ieu-b-110), triglycerides (TG) (ieu-b-111), and CRP (ebi-a-GCST90029070) were collected from 440,546, 441,016, and 427,367 individuals, respectively. Participants included both males and females, all of whom were of European descent. The original genetic datasets for FV intake utilized ordered categorical phenotypes, while for LDL-C, TG, and CRP, continuous phenotypes were used. The dataset for AS employed binary phenotypes. For LDL-C and TG, age, sex, and genotype chip were used as covariates.
Selection of instrumental variables
In order to identify SNPs that can act as IVs for the consumption of specific FVs, the following criteria were established: Association with FV intake: The SNP must show a statistically significant association with the intake of a specific FV (p < 5 × 10−6). For SNPs associated with the consumption of total fruits or vegetables, a more stringent significance level of p < 5 × 10−7 is required. Association with outcome: the SNP must also be associated with the outcome of interest (AS and risk factors) with a p-value > 10−4. This ensures that the SNP is not too strongly linked with the outcome, which could confound the causal inference. Linkage disequilibrium (LD) minimization: to ensure that identified SNPs are independent from one another, an LD threshold of R2 <0.01 is set within a 5,000-kilobase (kb) window. This minimizes the possibility that the SNP associations are due to LD rather than a direct relationship with FV intake. The 5 super-populations in the 1,000 genomes project were used as a reference panel. SNPs that are missing from the reference panel were excluded from the analysis. Instrument strength: the strength of the IVs is assessed using the F-statistic. An F-value of <10 is indicative of a weak instrument, which could introduce significant bias into the causal estimates. Such weak IVs should be excluded from the analysis to avoid misleading conclusions (31). Confounding factors: to mitigate the influence of potential confounders, we conducted a Phenome-wide association analysis using tools provided by the IEU Open GWAS project. This analysis involved searching for the effects of specific genetic variants across all publicly available datasets. Any SNPs that showed significant (p < 5 × 10−6) associations with potential confounders like meat, dairy, tobacco, and alcohol were excluded.
MR analysis approach
For the performance of two-sample MR analyses, R software (version 4.3.1) along with the TwoSampleMR package (version 0.5.7) were utilized. The effects of selected IVs were harmonized to ensure the effect of a SNP on the exposure and the effect of that SNP on the outcome must each correspond to the same allele. The Inverse Variance Weighted (IVW) method was adopted as the main analytical approach, under the assumption that each SNP acts as a valid IV. Complementary methods, including MR Egger and the weighted median, were also implemented. The choice between fixed and mixed random effects models was dependent on the presence of heterogeneity; the mixed random effects model was adopted in the case of detected heterogeneity, while the fixed effect model was employed otherwise. The outcomes of these analyses were expressed as β (effect size) and odds ratios (OR) with corresponding 95% confidence intervals (CI), thereby quantifying the strength and precision of the inferred causal relationships. After the analysis, the multiple test results were also adjusted for false discovery rate (FDR) using the Benjamini-Hochberg (BH) procedure. Statistical significance was defined as a p-value that was lower than 0.05.
Heterogeneity, pleiotropy, and sensitivity test
To ensure the reliability of our results, multiple tests were carried out to evaluate heterogeneity, pleiotropy, and sensitivity. Heterogeneity was evaluated using both Inverse Variance Weighted (IVW) and MR-Egger methods. To detect any potential horizontal pleiotropy, MR-Egger regression was specifically employed. If the intercept does not significantly differ from 0 (p > 0.05), it is considered that there is no sign of pleiotropy. Furthermore, a “leave-one-out” sensitivity analysis was conducted to determine the impact of each individual SNP on the combined outcome. This analysis entailed recalculating the effect estimates while systematically excluding one SNP at a time.
Results
Association between various FVs and AS
Initially, we investigated the potential causal relationship between the total consumption of fruits or vegetables and the risk of AS. No significant associations were observed for either category (fruit OR = 0.990, p = 0.183; vegetable OR = 0.991, p = 0.333, Figure 2). Subsequently, we examined the associations between specific FVs and AS. All the results of MR analyses for various FV on AS and its risk factors, including heterogeneity and pleiotropy tests are provided in Supplementary Table S2. After applying predetermined screening criteria, 745 SNPs were identified as IVs for analyzing the consumption of 28 different FVs. The F-statistics for each of these selected IVs exceeded the threshold of 10, indicating a minimal risk of weak instrument bias. Detailed information about these IVs is provided in Supplementary Table S3. When evaluating 13 distinct types of fruit, we found no significant association with AS (Figure 2; Supplementary Table S2). Among the 15 tested vegetable varieties, the intake of garlic showed the most significant causal association with a decreased risk of AS (OR = 0.985, p = 0.036, Figures 2, 6A; Supplementary Table S2). Despite previous reports suggesting an antiatherogenic effect of onions and tomatoes (30, 32), our analysis did not detect any significant association between their consumption and the risk of AS. A sensitivity test was conducted using the leave-one-out method, and it was found that no single IV significantly impacted the results (Supplementary Table S4).
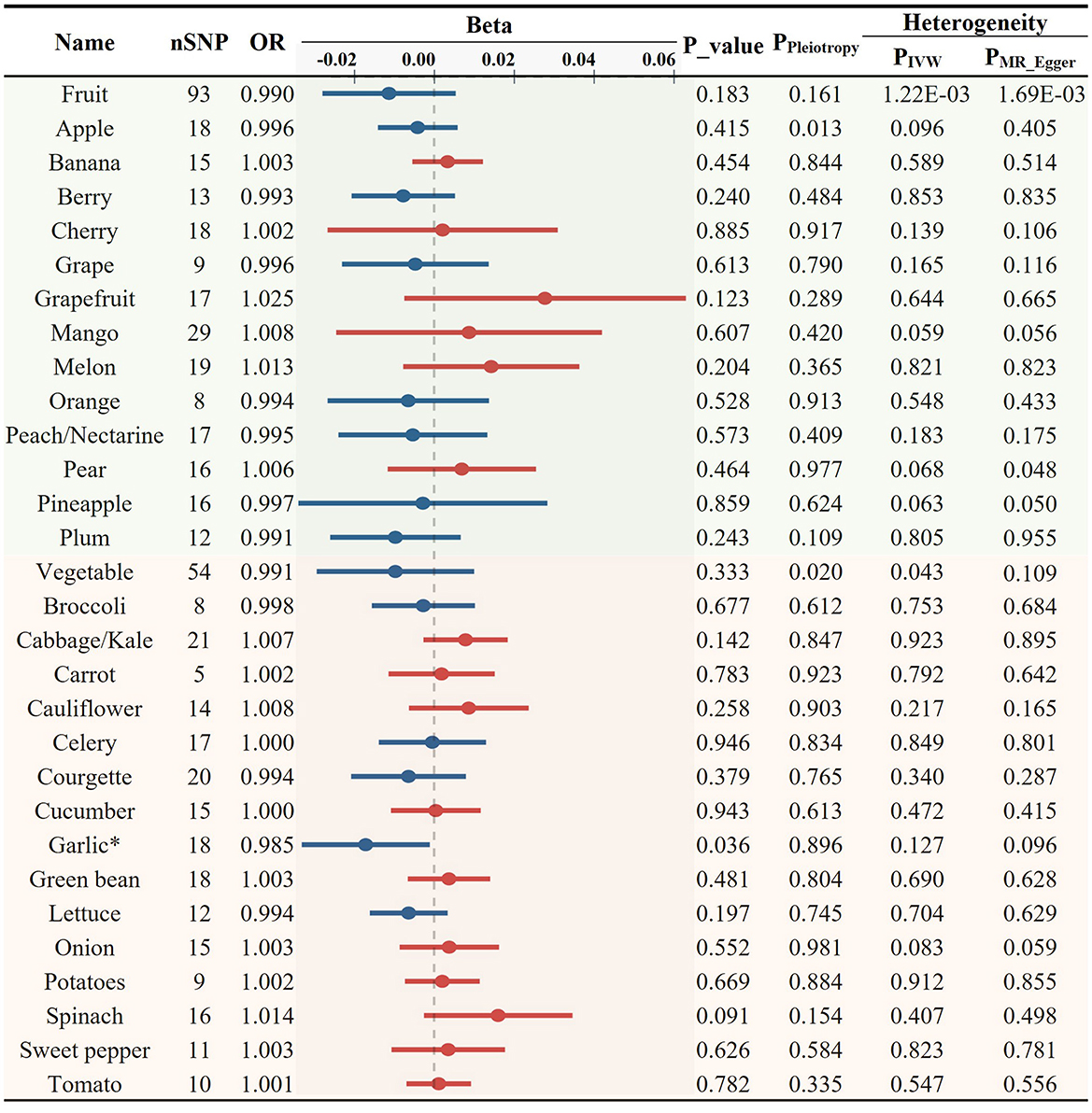
Figure 2. Causal relationships between fruit and vegetable consumption and atherosclerosis risk (*p < 0.05).
Association between various FVs and blood lipids
As elevated levels of LDL-C and TG are recognized risk factors for AS, we further examined the causal relationships between the total intake of fruits/vegetables and blood lipid profiles. Fruit consumption was significantly associated with reduced levels of LDL-C (OR = 0.911, p = 0.007, Figures 3, 6B) while no significant association was found with total vegetable consumption (Figure 3).
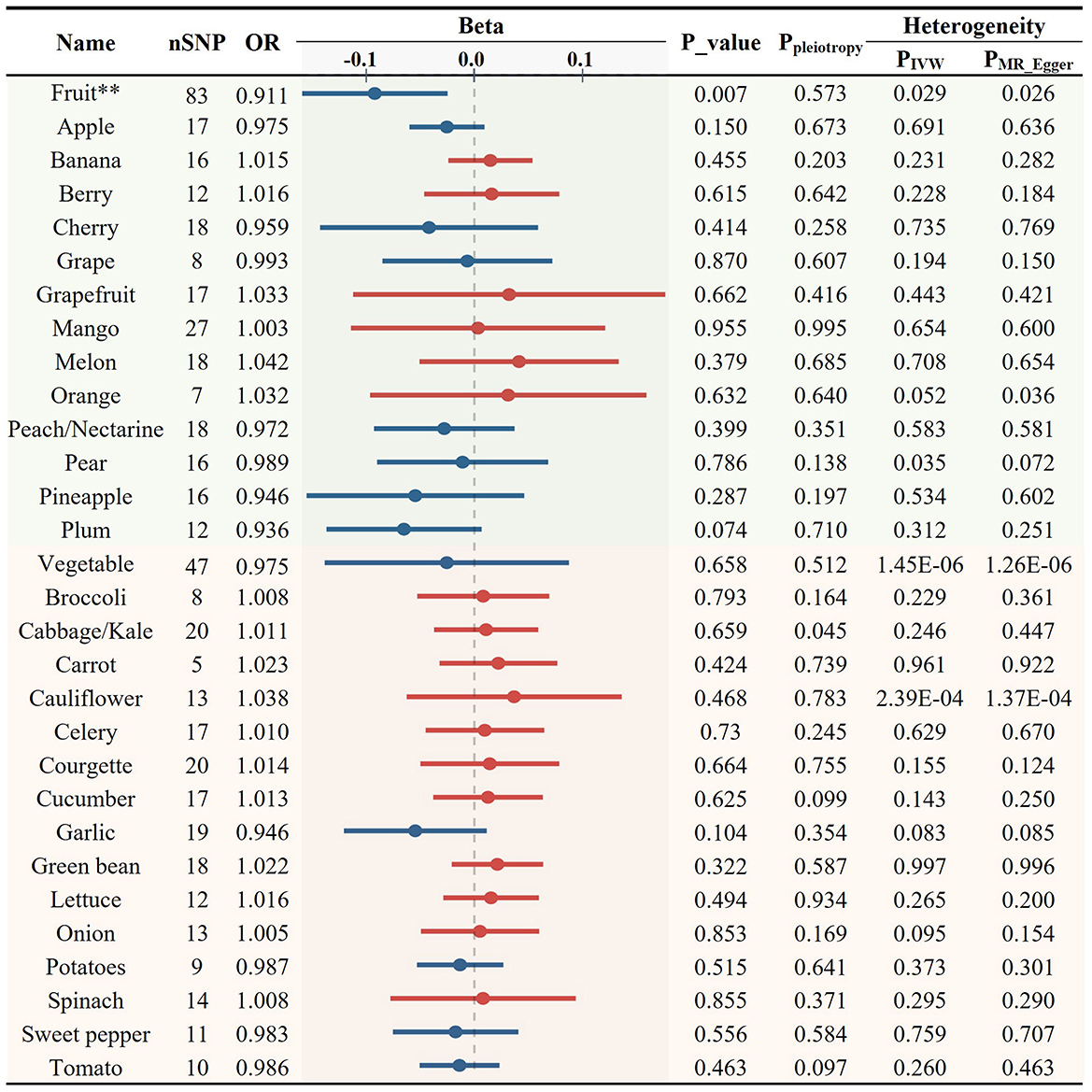
Figure 3. Causal relationships between fruit and vegetable consumption and the levels of circulating low-density lipoprotein cholesterol (**p < 0.01).
Next, we used 408 SNPs as IVs for LDL-C and 401 SNPs for TG to investigate the impact of 28 specific FVs on lipid levels (Supplementary Tables S5, S6). As shown in Figures 3, 4 and Supplementary Table S2, garlic intake displayed a negative association with the levels of LDL-C (OR = 0.946, p = 0.104) and TG (OR = 0.933, p = 0.078), which is consistent with its negative association with AS. Additionally, plum consumption had a marginally negative association with LDL-C (OR = 0.936, p = 0.074, Figure 3). Moreover, berry consumption was significantly and inversely associated with TG levels (OR = 0.929, p = 0.010, Figures 4, 6C). The intake of potatoes also showed a significant negative association with TG levels (OR = 0.957, p = 0.020, Figure 4). Sensitivity assessment revealed that the exclusion of any single IV did not substantially alter the findings (Supplementary Tables S7, S8). These outcomes suggest that certain FVs may have cholesterol and TG-lowering effects.
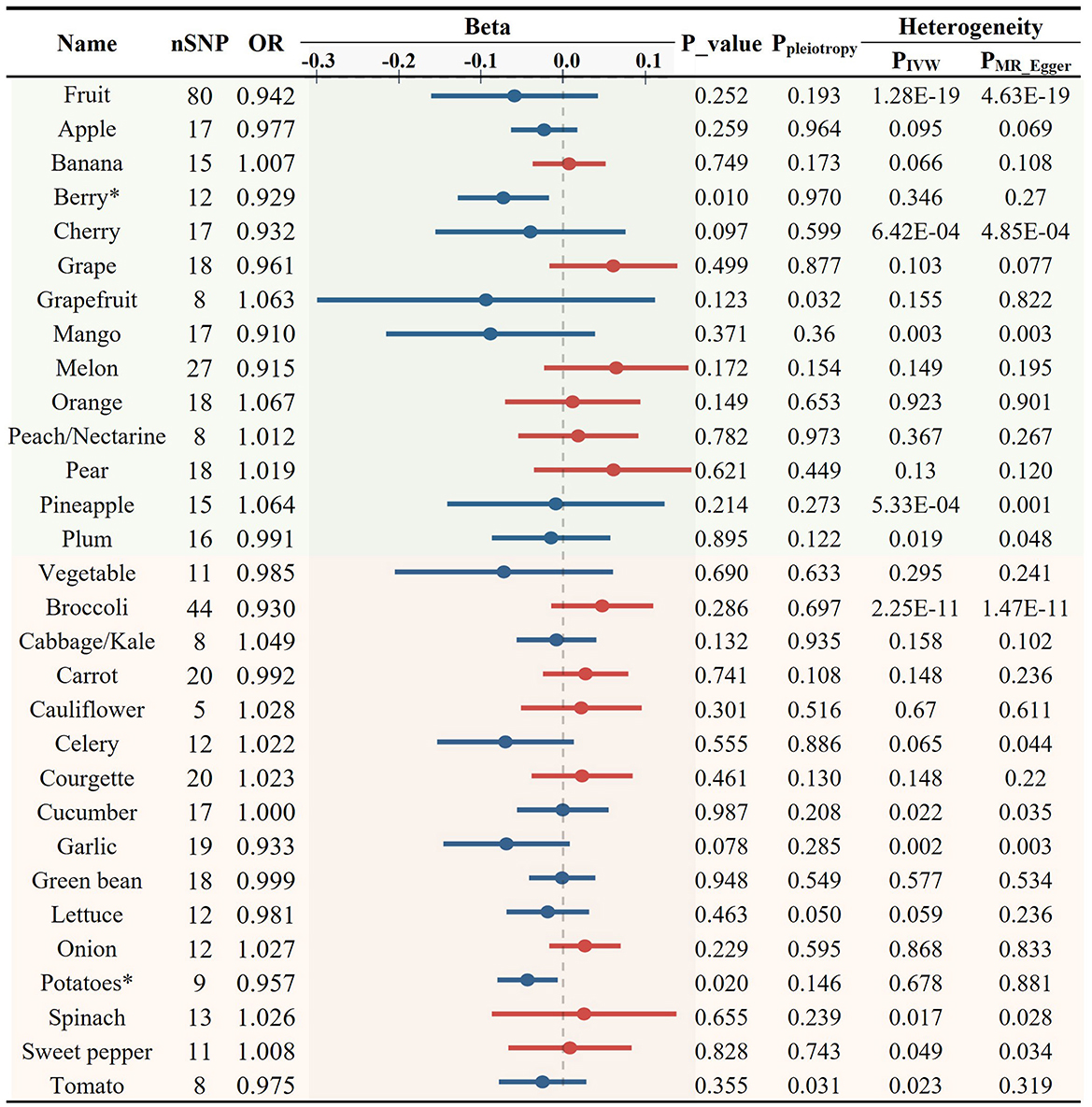
Figure 4. Causal relationships between fruit and vegetable consumption and the levels of blood triglyceride (*p < 0.05).
Association between various FVs and inflammation
Inflammation plays an important role in the development and progression of AS. CRP serves as a marker of chronic inflammation. High-sensitivity CRP test is particularly useful for the assessment of CVD risk. We found both consumption of total fruits (OR = 0.868, p = 0.008) and vegetables (OR = 0.858, p = 0.018) showed a significant negative causal association with CRP levels (Figures 5, 6D, E). Upon the application of predetermined screening criteria, a total of 392 SNPs with F-statistics > 10 were identified as IVs for the analysis of 28 FVs. Detailed information regarding these IVs is presented in Supplementary Table S9. Out of the 28 FVs, peach/nectarine consumption showed the most significant negatively causal association with the levels of CRP (OR = 0.913, p = 0.010, Figures 5, 6F; Supplementary Table S2). Consistent with its negative association with AS and the levels of LDL-C and TG, garlic also showed a trend to be negatively associated with CRP (OR = 0.974, p = 0.361, Figure 5). Sensitivity test demonstrated that omitting each IV in turn did not notably influence the overall results (Supplementary Table S10).
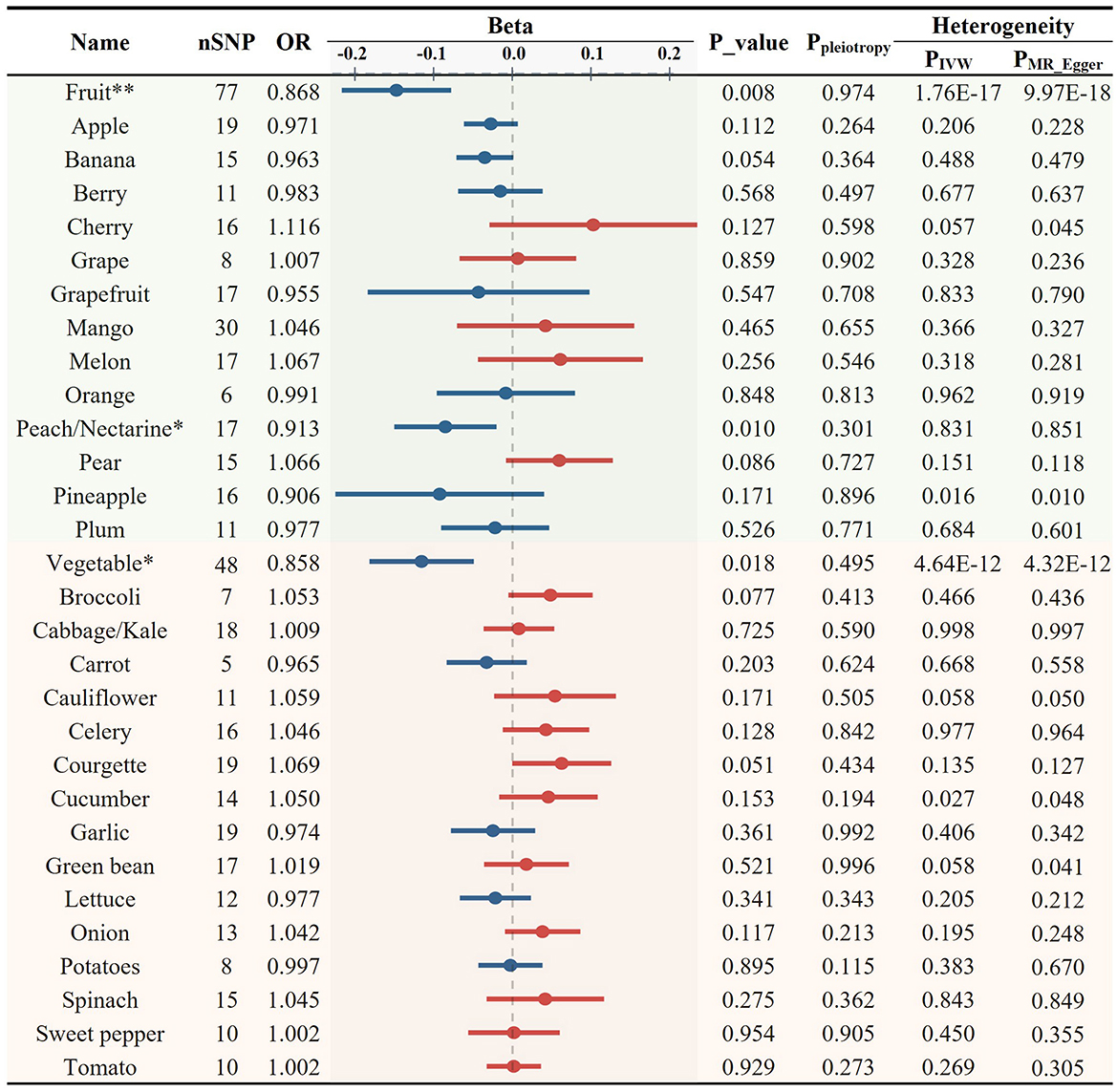
Figure 5. Causal relationships between fruit and vegetable consumption and the levels of C-reactive protein (*p < 0.05, **p < 0.01).
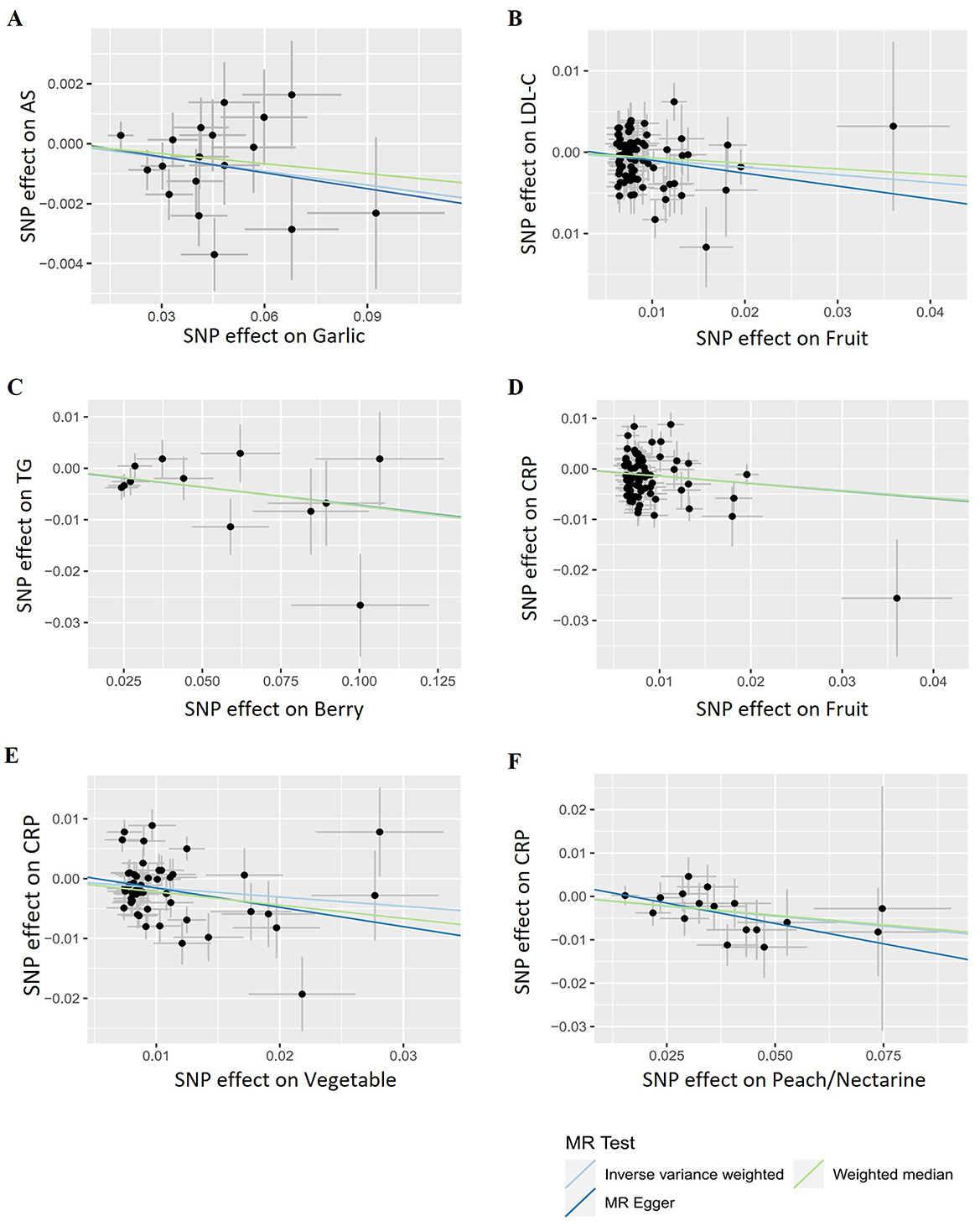
Figure 6. Representative scatter plots of the causal relationships between fruits/vegetables and atherosclerosis as well as its risk factors. (A) Garlic and atherosclerosis. (B) Fruit and LDL-C. (C) Berry and TG. (D) Fruit and C-reactive protein (CRP). (E) Vegetable and CRP. (F) Peach/nectarine and CRP.
Discussion
CVDs remain the leading cause of diet-associated mortality and morbidity worldwide (33). An insufficient intake of fruits and vegetables is a major contributor to cardiometabolic deaths (34). Fruits and vegetables are abundant sources of dietary fiber, essential micronutrients, and a range of bioactive compounds, including vitamins, minerals, polyphenols, carotenoids, and organosulfur compounds (35). These elements may play a critical role in preventing the development of CVD and in modulating intermediary risk factors such as blood lipid levels, blood pressure, and body mass (36). Chronic inflammation is closely linked to the development of AS. LDL-C in the bloodstream can become oxidized through inflammatory processes, leading to its adherence to arterial walls and increasing its atherogenic potential. Monocytes that enter the arterial wall can transform into macrophages, ingest oxidized LDL, and become foam cells, which are a hallmark of early atherosclerotic plaques (37).
In this work, we utilized two-sample MR analysis approach to investigate the causality between the intake of various FVs and AS, as well as its risk factors, including blood lipids, and chronic inflammation. Although not statistically significant, the overall intake of fruits and vegetables appeared to exhibit negative causal associations with the risk of AS. Among the 13 types of fruit and 15 types of vegetables studied, garlic exhibited the most significant negative causal association with AS. Moreover, our results also suggest a marginally significant inverse causal relationship between garlic intake and levels of circulating LDL-C and TG in humans.
Prior research has shown that garlic compounds can reduce intracellular oxidative stress and inhibit NF-κB activation (38, 39). A clinical trial reported that long-term supplementation with aged garlic extract improved peripheral tissue perfusion in patients with AS (40). Moreover, a meta-analysis suggested that garlic supplementation may lower the levels of serum TC and LDL-C (41). According to literature review, garlic is one of the vegetables with the strongest protective effects against AS. Consistently, garlic showed the strongest atheroprotective effect among the FVs examined in this study. It is always valuable to validate biological findings using different approaches.
Mechanisms have been reported on how garlic may improve AS. Garlic contains bioactive compounds such as allicin, diallyl disulfide (DADS), and S-allyl cysteine (SAC), etc. (42). Allicin can inhibit the activation of JNK and p38 MAPK and decrease SR-A and CD36 expression, thereby preventing macrophage foam formation (43). It also activates PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells, thereby reducing lipid accumulation. Additionally, allicin promotes cholesterol efflux through upregulation of ATP Binding Cassette Subfamily A Member 1 (ABCA1) (44). It has also been suggested that alliin could inhibit platelet aggregation and prevent the progression of AS (42). DADS inhibits hepatic lipid synthesis by downregulating SREBP-1c expression and promote lipolysis and fatty acid oxidative metabolism by upregulating the PPARα in hepatocytes. SAC can also inhibit SREBP-1 mediated lipogenesis by activating AMPK through calcium/calmodulin-dependent kinase and silent information regulator T1 (45). In addition, the molecular mechanisms involved in the antioxidant, anti-inflammatory, antithrombotic, and endothelium-protective effects of garlic compounds have also been demonstrated (42).
While some clinical studies suggest that tomato supplementation can lower serum TC, TG, and blood pressure, there are still discrepancies in the results (30). Additionally, these human trials typically involve small sample sizes and do not accurately model AS. Smith et al. employed ApoE−/− mouse model to investigate the anti-atherosclerotic properties of tomatoes and observed no significant effects (46). Although animal studies indicate that onion extract might decrease arterial lesions and lipid levels and potentially improve AS by affecting various pathways (47), clinical trials to confirm these effects are lacking (46). In our study, neither tomatoes nor onions demonstrated a clear relationship with AS, blood lipid levels, or CRP levels. They contain functional compounds such as lycopene and quercetin, which are theoretically atheroprotective (48). The amount consumed may be a critical factor in determining their effectiveness.
Berries are rich in anthocyanins, such as cyanidin, pelargonidin, delphinidin, peonidin, malvidin, and petunidin, which are known for their antioxidative activity (49). They have been extensively reviewed for their protective effects against CVD. A meta-analysis involving 41 studies and 2,788 participants showed that anthocyanin supplementation significantly reduced TG and LDL-C levels (50). Consistent with these findings, our study detected a significant negative causal association between berry consumption and TG levels. Animal studies have demonstrated mechanistic insights into the role of berries in lipid metabolism. It has been revealed that polyphenolic flavonoids and phenolic acids found in berries enhance the activity of paraoxonase, which is linked to the antioxidant properties of HDL cholesterol, and also boost the liver's production of apolipoprotein A-I (51). Commonly consumed berries, such as blueberries and strawberries, have been demonstrated to suppress fatty acid synthesis, reduce aortic lesions, and alleviate both inflammation and oxidative damage. The polyphenol content and profile are important factors in determining the hypolipidemic effect of berries (52).
Potatoes, being starchy vegetables, have been the subject of conflicting reports regarding their impact on TG levels. Although a cohort study reported only weak associations with high TG levels following boiled potato consumption (53), another study indicated that a steamed potato-enriched diet significantly decreased cholesterol and TG levels in rats, compared to a wheat starch-enriched diet (54). Our findings support a causal link between the consumption of potatoes and lowered TG levels.
A diet rich in fruits and vegetables is beneficial for reducing inflammation, which is attributed to the high content of antioxidants and biologically active substances present in these foods. Our analysis revealed that the consumption of both fruits and vegetables can potentially reduce inflammation. Peaches, in particular, contain a broad spectrum of phytochemicals, including phenolic compounds and carotenoids, as well as vitamins, volatile aroma compounds, and organic acids (55). Research has shown that both fresh and preserved peach supplements can reduce oxidative stress, inflammation, and tissue damage caused by CCl4. They inhibit the activation of inflammatory mediators such as TNF-α, IL-1β, RAGE, and NF-κB (56). Another study demonstrated that consumption of polyphenol-rich peach and plum juice may prevent risk factors for obesity-related metabolic disorders and cardiovascular disease in Zucker rats (57). Consistent with these studies, our research found a significant causal association between peach/nectarine consumption and reduced CRP levels in humans.
We primarily used two-sample MR analysis to explore the direct relationships between individual FV intakes and AS risks. This approach could not capture the potential interactions among these dietary components. Therefore, we further performed multivariable MR (MVMR) analysis on the significant FVs to assess the independent effect of each FV, considering the influence of other FVs on the outcomes. As shown in Supplementary Table S11, garlic intake still shows a negative causal association with AS (OR = 0.985, p = 0.009) and TG (OR = 0.940, p = 0.049). Berry intake continues to be negatively associated with TG (OR = 0.934, p = 0.024). Similarly, peach intake remains negatively associated with CRP levels (OR = 0.900, p = 0.012). Overall, the effect size and significance levels remained similar to those observed in previous analyses.
Some limitations in this study should also be noted. Firstly, it is challenging to fully consider the complex interactions given the complexity of dietary compositions. Future studies may benefit from exploring these interactions more comprehensively using advanced statistical models and larger datasets. Second, population stratification can impact the results of MR analyses, as it may introduce bias where the association between genetic variants and environmental factors is mistakenly interpreted as causal relationships. Additionally, Heterogeneity was detected in some MR tests in this study. We adopted a random effects model to address this heterogeneity, which accounts for the potential differences in effect sizes among the various genetic instrumental variables. However, when interpreting these results, it is still necessary to carefully consider the potential impacts of heterogeneity. Our sample primarily consists of individuals of European descent, which may limit the generalizability of our findings. Future studies are needed to validate the results in different populations. Furthermore, intake amount and intake forms are key factors affecting the health benefits of FVs. However, due to the lack of relevant genetic datasets, this study could not provide a detailed relationship between these factors and the protective benefits. Therefore, the causal relationships revealed in this study and their underlying mechanisms require further in-depth investigation.
Conclusion
This is the first study that systemically assesses and compares the atheroprotective effects of a diverse range of FVs. The findings indicate that, under MR assumptions, particular FVs may confer a protective effect against AS and its risk factors. Among the 28 FVs evaluated, garlic emerged as the one with the most pronounced negative causal relationship with the condition. Additionally, the consumption of berries and potatoes was notably associated with reduced TG levels. Regarding inflammation, a general intake of fruits and vegetables was linked with potential anti-inflammatory effects, with the consumption of peaches/nectarines specifically showing a significant and causal association with decreased CRP levels. Although the deeper mechanisms behind these causal relationships require further investigation, these discoveries shed light on the nuanced contributions of FVs to cardiovascular health and underscore the significance of consuming certain types of produce for their health advantages.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SY: Data curation, Formal analysis, Investigation, Writing – original draft, Validation. ZC: Methodology, Software, Writing – review & editing. HL: Writing – review & editing, Conceptualization. ZL: Conceptualization, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft. SN: Funding acquisition, Project administration, Supervision, Writing – review & editing. MX: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Key Program of the National Natural Science Foundation of China (32130082), Central Government Guide Local Special Fund Project for Scientific and Technological Development of Jiangxi Province (20221ZDD02001), National Natural Science Foundation of China (32402112), and Jiangxi Provincial Natural Science Foundation (20242BAB20323).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1426763/full#supplementary-material
References
1. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. (2014) 5:927.
2. Zerihun M, Qvit N. Selective inhibitors targeting Fis1/Mid51 protein-protein interactions protect against hypoxia-induced damage in cardiomyocytes. Front Pharmacol. (2023) 14:1275370. doi: 10.3389/fphar.2023.1275370
3. Kim HC. Epidemiology of Cardiovascular disease and its risk factors in Korea. Glob Health Med. (2021) 3:134–41. doi: 10.35772/ghm.2021.01008
5. Belardo D, Michos ED, Blankstein R, Blumenthal RS, Ferdinand KC, Hall K, et al. Practical, evidence-based approaches to nutritional modifications to reduce atherosclerotic cardiovascular disease: An American Society for Preventive Cardiology Clinical Practice Statement. American Journal of Preventive Cardiology. (2022) 10:100323. doi: 10.1016/j.ajpc.2022.100323
6. He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. (2007) 21:717–28. doi: 10.1038/sj.jhh.1002212
7. Blekkenhorst LC, Bondonno CP, Lewis JR, Woodman RJ, Devine A, Bondonno NP, et al. Cruciferous and total vegetable intakes are inversely associated with subclinical atherosclerosis in older adult women. J Am Heart Assoc. (2018) 7:e008391. doi: 10.1161/JAHA.117.008391
8. Ellingsen I, Hjerkinn E, Seljeflot I, Arnesen H, Tonstad S. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. Br J Nutr. (2008) 99:674–81. doi: 10.1017/S0007114507832521
9. Bazzano LA. Effects of soluble dietary fiber on low-density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep. (2008) 10:473–7. doi: 10.1007/s11883-008-0074-3
10. Li Z, Nie Q, Nie S-P. Comprehensive insights: unraveling the mechanisms of gut commensals in glucose metabolism regulation. Sci China Life Sci. (2024) 67:414–7. doi: 10.1007/s11427-023-2455-y
11. Li Z, Chen L, Sepulveda M, Wang P, Rasic M, Tullius SG, et al. Microbiota-dependent and-independent effects of obesity on transplant rejection and hyperglycemia. Am J Transplant. (2023) 23:1526–35. doi: 10.1016/j.ajt.2023.06.011
12. Trautwein EA, Duchateau GS, Lin Y, Mel'nikov SM, Molhuizen HO, Ntanios FY. Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur J Lipid Sci Technol. (2003) 105:171–85. doi: 10.1002/ejlt.200390033
13. Ciumǎrnean L, Milaciu MV, Runcan O, Vesa S´C, Rǎchis ´ an AL, Negrean V, et al. The effects of flavonoids in cardiovascular diseases. Molecules. (2020) 25:4320. doi: 10.3390/molecules25184320
14. Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. (2003) 169:203–14. doi: 10.1016/S0021-9150(03)00012-1
15. Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci. (2011) 342:135–42. doi: 10.1097/MAJ.0b013e318224a147
16. Vaskonen T. Dietary minerals and modification of cardiovascular risk factors. J Nutr Biochem. (2003) 14:492–506. doi: 10.1016/S0955-2863(03)00074-3
17. Xu Z-R, Li J-Y, Dong X-W, Tan Z-J, Wu W-Z, Xie Q-M, et al. Apple polyphenols decrease atherosclerosis and hepatic steatosis in Apoe–/– mice through the Ros/Mapk/Nf-?b pathway. Nutrients. (2015) 7:7085–105. doi: 10.3390/nu7085324
18. Bucalen FCK. Antioxidant and anti-atherosclerotic potential of banana (Musa Spp): a review of biological mechanisms for prevention and protection against atherosclerosis. Avicenna Jo Phytomed. (2023) 13:240. doi: 10.22038/AJP.2022.20616
19. Wu X, Wang TT, Prior RL, Pehrsson PR. Prevention of atherosclerosis by berries: the case of blueberries. J Agric Food Chem. (2018) 66:9172–88. doi: 10.1021/acs.jafc.8b03201
20. Lietava J, Beerova N, Klymenko SV, Panghyova E, Varga I, Pechanova O. Effects of cornelian cherry on atherosclerosis and its risk factors. Oxid Med Cell Longevity. (2019) 2019:2515270. doi: 10.1155/2019/2515270
21. Fernandez ML, Barona J. Grapes and atherosclerosis. Grapes Health 53–76. doi: 10.1007/978-3-319-28995-3_4
22. Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, et al. Red Grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J Agric Food Chem. (2006) 54:1887–92. doi: 10.1021/jf058171g
23. Zeng Y, Guan M, Li C, Xu L, Zheng Z, Li J, et al. Bitter Melon (Momordica Charantia) attenuates atherosclerosis in Apo-E knock-out mice possibly through reducing triglyceride and anti-inflammation. Lipids Health Dis. (2018) 17:1–7. doi: 10.1186/s12944-018-0896-0
24. Gallaher CM, Gallaher DD. Dried plums (Prunes) reduce atherosclerosis lesion area in apolipoprotein E-deficient mice. Br J Nutr. (2008) 101:233–9. doi: 10.1017/S0007114508995684
25. Joo HK, Choi S, Lee YR, Lee EO, Park MS, Park KB, et al. Anthocyanin-rich extract from Red Chinese cabbage alleviates vascular inflammation in endothelial cells and Apo E–/– mice. Int J Mol Sci. (2018) 19:816. doi: 10.3390/ijms19030816
26. Soleti R, Coué M, Trenteseaux C, Hilairet G, Fizanne L, Kasbi-Chadli F, et al. Carrot supplementation improves blood pressure and reduces aortic root lesions in an atherosclerosis-prone genetic mouse model. Nutrients. (2021) 13:1181. doi: 10.3390/nu13041181
27. Budiarto A, Nasihun T, Hussaana A. Effect of celery extract administration on 8-Ohdg and number of foam cell in wistar strain rats with a high-fat diet. Sains Med. (2017) 8:2421. doi: 10.30659/sainsmed.v8i2.2421
28. Orekhov AN, Grünwald J. Effects of garlic on atherosclerosis. Nutrition. (1997) 13:656–63. doi: 10.1016/S0899-9007(97)83010-9
29. He B, Hao J, Sheng W, Xiang Y, Zhang J, Zhu H, et al. Fistular onion stalk extract exhibits anti-atherosclerotic effects in rats. Exp Ther Med. (2014) 8:785–92. doi: 10.3892/etm.2014.1790
30. Yanai H. Anti-atherosclerotic effects of tomatoes. Funct Foods Health Dis. (2017) 7:411–28. doi: 10.31989/ffhd.v7i6.351
31. Nie Q, Luo Q, Yan W, Zhang T, Wang H, Wu J. Rheumatoid arthritis and coronary atherosclerosis: a two-sample mendelian randomization study. Front Cardiovasc Med. (2023) 10:1033644. doi: 10.3389/fcvm.2023.1033644
32. Li W, Tang C, Jin H, Du J. Effects of onion extract on endogenous vascular H2s and adrenomedulin in rat atherosclerosis. Curr Pharm Biotechnol. (2011) 12:1427–39. doi: 10.2174/138920111798281135
33. Wang J, Liu F, Li J, Huang K, Yang X, Chen J, et al. Fruit and vegetable consumption, cardiovascular disease, and all-cause mortality in China. Sci China Life Sci. (2022) 65:119–28. doi: 10.1007/s11427-020-1896-x
34. He Y, Li Y, Yang X, Hemler EC, Fang Y, Zhao L, et al. The Dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982-2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. (2019) 7:540–8. doi: 10.1016/S2213-8587(19)30152-4
35. Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. (2016) 374:1332–43. doi: 10.1056/NEJMoa1501451
36. Tang GY, Meng X, Li Y, Zhao CN, Liu Q, Li HB. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. (2017) 9:857. doi: 10.3390/nu9080857
37. Mehu M, Narasimhulu CA, Singla DK. Inflammatory cells in atherosclerosis. Antioxidants. (2022) 11:233. doi: 10.3390/antiox11020233
38. Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa B activation. J Nutr. (2001) 131:1020S−6S. doi: 10.1093/jn/131.3.1020S
39. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. (1994) 60:417–20. doi: 10.1055/s-2006-959522
40. Lindstedt S, Wlosinska M, Nilsson AC, Hlebowicz J, Fakhro M, Sheikh R. Successful improved peripheral tissue perfusion was seen in patients with atherosclerosis after 12 months of treatment with aged garlic extract. Int Wound J. (2021) 18:681–91. doi: 10.1111/iwj.13570
41. Ried K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J Nutr. (2016) 146:389S−96S. doi: 10.3945/jn.114.202192
42. Li M, Yun W, Wang G, Li A, Gao J, He Q. Roles and mechanisms of garlic and its extracts on atherosclerosis: a review. Front Pharmacol. (2022) 13:954938. doi: 10.3389/fphar.2022.954938
43. Wang X-H, Zhang J-H, Hu Y-N, Zhang H. Effects and mechanisms of allicin on atherosclerosis in high-fat diet fed mice. Edit Off Chin J Arterioscler. (2017) 25:140–4.
44. Lin X-L, Hu H-J, Liu Y-B, Hu X-M, Fan X-J, Zou W-W, et al. Allicin induces the upregulation of abca1 expression Via Pparγ/Lxrα signaling in Thp-1 macrophage-derived foam cells. Int J Mol Med. (2017) 39:1452–60. doi: 10.3892/ijmm.2017.2949
45. Hwang YP, Kim HG, Choi JH, Do MT, Chung YC, Jeong TC, et al. S-Allyl cysteine attenuates free fatty acid-induced lipogenesis in human Hepg2 cells through activation of the Amp-activated protein kinase-dependent pathway. J Nutr Biochem. (2013) 24:1469–78. doi: 10.1016/j.jnutbio.2012.12.006
46. Smith BW, Miller RJ, Wilund KR, O'Brien WD Jr, Erdman JW Jr. Effects of tomato and soy germ on lipid bioaccumulation and atherosclerosis in Apoe-/- mice. J Food Sci. (2015) 80:H1918–25. doi: 10.1111/1750-3841.12968
47. Vatsala TM, Singh M, Murugesan RG. Effects of onion in induced atherosclerosis in rabbits: I. Reduction of arterial lesions and lipid levels. Artery. (1980) 7:519–30.
48. Zheng G, Zhao J, Ru J, Guo H (editors). The anti-atherosclerosis protein regulation network delivered by onion quercetin. In: 2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (Basel: MDPI) (2021).
49. Ponder A, Hallmann E, Kwolek M, Srednicka-Tober D, Kazimierczak R. Genetic differentiation in anthocyanin content among berry fruits. Curr Issues Mol Biol. (2021) 43:36–51. doi: 10.3390/cimb43010004
50. Jang HH, Hwang IG, Lee YM. Effects of anthocyanin supplementation on blood lipid levels: a systematic review and meta-analysis. Front Nutr. (2023) 10:1207751. doi: 10.3389/fnut.2023.1207751
51. Basu A. Role of berry bioactive compounds on lipids and lipoproteins in diabetes and metabolic syndrome. Nutrients. (2019) 11:1983. doi: 10.3390/nu11091983
52. Jaroslawska J, Juskiewicz J, Wroblewska M, Jurgonski A, Krol B, Zdunczyk Z. Polyphenol-rich strawberry pomace reduces serum and liver lipids and alters gastrointestinal metabolite formation in fructose-fed rats. J Nutr. (2011) 141:1777–83. doi: 10.3945/jn.111.143677
53. Moholdt T, Devlin BL, Nilsen TIL. Intake of boiled potato in relation to cardiovascular disease risk factors in a large Norwegian Cohort: The Hunt Study. Nutrients. (2019) 12:73. doi: 10.3390/nu12010073
54. Robert L, Narcy A, Rock E, Demigne C, Mazur A, Rémésy C. Entire potato consumption improves lipid metabolism and antioxidant status in cholesterol-fed rat. Eur J Nutr. (2006) 45:267–74. doi: 10.1007/s00394-006-0594-y
55. Bento C, Goncalves AC, Silva B, Silva LR. Peach (Prunus Persica): phytochemicals and health benefits. Food Rev Int. (2022) 38:1703–34. doi: 10.1080/87559129.2020.1837861
56. Gasparotto J, Somensi N, Bortolin RC, Girardi CS, Kunzler A, Rabelo TK, et al. Preventive supplementation with fresh and preserved peach attenuates Ccl4-induced oxidative stress, inflammation and tissue damage. J Nutr Biochem. (2014) 25:1282–95. doi: 10.1016/j.jnutbio.2014.07.004
Keywords: fruits and vegetables, atherosclerosis, Mendelian Randomization, LDL-C and TG, C-reactive protein
Citation: Yang S, Cao Z, Liu H, Li Z, Nie S and Xie M (2024) Identifying atheroprotective fruits and vegetables by Mendelian Randomization analysis. Front. Nutr. 11:1426763. doi: 10.3389/fnut.2024.1426763
Received: 08 May 2024; Accepted: 27 September 2024;
Published: 14 October 2024.
Edited by:
Mauro Serafini, University of Teramo, ItalyReviewed by:
Han Feng, Tulane University, United StatesLuo Qiang, Children's Hospital of Chongqing Medical University, China
Copyright © 2024 Yang, Cao, Liu, Li, Nie and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhipeng Li, emhpcGVuZ2xpQG5jdS5lZHUuY24=
 Shenji Yang
Shenji Yang Zhikang Cao
Zhikang Cao Huidong Liu2
Huidong Liu2 Zhipeng Li
Zhipeng Li Shaoping Nie
Shaoping Nie