
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 18 June 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1422084
This article is part of the Research Topic Vitamin D: From Pathophysiology to Clinical Impact, volume II View all 24 articles
Objective: This study aimed to investigate the association between serum 25-hydroxyvitamin D (25(OH)D) concentrations and mortality in long-term prescription opioid users.
Methods: The study included 1856 long-term prescription opioid users from the National Health and Nutrition Examination Survey (NHANES, 2001–2018). Mortality status were determined by matching with the National Death Index (NDI) records until December 31, 2019. Multivariable Cox proportional hazard models were constructed to assess the association.
Results: Over a median follow-up period of 7.75 years, there were 443 cases of all-cause mortality, including 135 cardiovascular disease (CVD) deaths and 94 cancer deaths. After multivariable adjustment, participants with serum 25(OH)D concentrations within 50.00 to <75.00 nmol/L and ≥ 75 nmol/L had a lower risk of all-cause mortality, with hazard ratios (HRs) of 0.50 (95% confidence interval [CI] 0.29, 0.86) and 0.54 (95% CI 0.32, 0.90), respectively. Nevertheless, no significant association was found between serum 25(OH)D concentrations and the risk of CVD or cancer mortality. The RCS analysis revealed a non-linear association of serum 25(OH)D concentration with all-cause mortality (p for non-linear = 0.01). Per 1-unit increment in those with serum 25(OH)D concentrations <62.17 nmol/L corresponded to a 2% reduction in the risk of all-cause mortality (95% CI 0.97, 1.00), but not changed significantly when 25(OH)D concentrations ≥62.17 nmol/L.
Conclusion: In conclusion, a non-linear association existed between serum 25(OH)D concentrations and all-cause mortality in long-term prescription opioid users. Maintaining serum 25(OH)D concentrations ≥62.17 nmol/L may be beneficial in preventing all-cause mortality in this population.
Prescription opioids are widely recognized as the most commonly used and effective analgesics for managing moderate to severe pain, and the rates of prescription opioid use are increasing in many countries (1). Despite their significant pain-relieving properties, long-term use of prescription opioids increases the risk of overdose, misuse, addiction, and other associated risks (2), with 16,706 fatalities attributed to prescription opioid overdose in 2021 (3). Increasing evidence suggests long-term prescription opioid use is associated with all-cause mortality (4–6). However, the results regarding the association between long-term prescription opioid use and cardiovascular or cancer mortality were contradictory. Ekholm et al. (6) reported no association was observed for long-term prescription opioid use with cardiovascular and cancer mortality. In contrast, Nalini et al. (7) found long-term opiate use was associated with an increased risk of cardiovascular mortality, and Song et al. (4) also observed a higher mortality rate due to cancer or circulatory system diseases in long-term opioid users.
Vitamin D is a steroid hormone, and 25-hydroxyvitamin D (25(OH)D) serves as the predominant circulating form of vitamin D in the bloodstream. Vitamin D in the body is primarily synthesized through the skin upon exposure to ultraviolet radiation, and it can also be obtained in small quantities from the diet (8). Apart from its role in maintaining bone health, vitamin D plays a crucial role in immune regulation, cell growth, and cellular differentiation (9). Several epidemiological studies have identified a link between low 25(OH)D concentrations and an increased risk of mortality in the general population (10, 11). Nevertheless, recent intervention studies have failed to demonstrate the benefit of vitamin D supplementation on mortality (12–14). Prior study have reported that chronic pain patients with vitamin D deficiency faced an increased risk of receiving higher opioid doses and using them for extended periods (15). Recent study have indicated vitamin D deficiency was associated with an increased risk of prescription opioid use and the exacerbation of opioid addiction (16). The absence of vitamin D signaling can increase sensitivity to morphine reward, leading to greater exogenous opioid consumption (16). Furthermore, vitamin D may exert analgesic effects through stimulating the body’s anti-inflammatory response, scavenging reactive oxygen species, and regulating the endogenous opioid pathway, thereby affecting opioid use (17–19). Despite the presence of evidence indicates a connection between vitamin D deficiency and the use of prescription opioids, no reports have addressed the association of vitamin D deficiency with mortality in long-term prescription opioid users. Due to the variation in the impact of long-term prescription opioid use on mortality outcomes, further research is needed to elucidate the relationship between them.
To address this research gap, we conducted a prospective investigation to examine the associations of serum 25(OH)D concentrations with all-cause and cause-specific mortality in long-term prescription opioid users. We hypothesized that low 25(OH)D concentrations would be associated with an increased risk of all-cause and cause-specific mortality in this population.
National Health and Nutrition Examination Survey (NHANES) is a nationally representative cross-sectional survey that utilizes a stratified multistage sampling design to evaluate the health and nutritional status of the non-institutionalized population in the United States (20). The research protocol was approved by the Institutional Review Board of the National Center for Health Statistics (NCHS), and written informed consent was obtained from all participants. Since the de-identified data analyzed during the current study were publicly available from NHANES, the study did not require any review board approval again.
This study included a total of 50,201 participants aged ≥20 years from 2001 to 2018. We excluded non-long-term prescription opioid users (n = 47,426), individuals receiving medications for opioid dependence or withdrawal (including buprenorphine; naloxone, buprenorphine; methadone, n = 79), those with missing vitamin D data (n = 331), those with missing follow-up data (n = 1), and those with missing covariate data (n = 508). Ultimately, the analysis included 1856 participants. The participant inclusion process was detailed in Supplementary Figure S1.
Before 2005–2006, serum 25(OH)D concentrations were measured using the DiaSorin RIA kit (Stillwater, MN, USA). Since 2005–2006, a standardized liquid chromatography–tandem mass spectrometry (LC–MS/MS) method had been used to measure serum 25(OH)D concentrations. Following the analytical guidelines of Centers for Disease Control and Prevention (CDC), serum 25(OH)D concentrations from 2005 to 2006 and earlier were converted to equivalent concentrations from LC–MS/MS using regression, and LC–MS/MS equivalent data were utilized in all analyses.
During the household interview survey, participants were asked if they had taken a medication in the past month for which they needed a prescription. Participants who answered “yes” were asked to report the names of the medications through medication containers, verbal reports, or pharmacy receipts. Additionally, participants were also asked how long they had been taking the medication. All recorded medications were classified using the Multum Lexicon therapeutic classification system. Participants who reported the use of narcotic analgesics or narcotic analgesic combinations were considered as prescription opioid users, and the duration of prescription opioid use ≥90 days was considered as long-term use (21). Methadone, naloxone, and buprenorphine were often used to treat opioid dependence or withdrawal, we exclude participants taking these drugs from prescription opioid users.
Mortality status and follow-up time were determined by matching with the National Death Index (NDI) records available until December 31, 2019. According to the International Classification of Diseases, 10th Revision (ICD-10), cardiovascular disease-specific mortality was defined as deaths due to heart disease (I00-I09, I11, I13, I20-I51) or cerebrovascular disease (I60-I6), while cancer-specific mortality was defined as deaths due to malignant neoplasms (C00-C97).
Sociodemographic information [age, sex, race/ethnicity, education, poverty-income ratio (PIR)], living habits (physical activity, cotinine, alcohol consumption), comorbidity (history of hypertension, cardiovascular disease (CVD), diabetes, and cancer), as well as body mass index (BMI), were obtained from NHANES. Race/ethnicity was categorized as Non-Hispanic White, Non-Hispanic Black, Mexican American, and Other. Education was divided into less than high school, high school, and more than high school. PIR was classified as ≤130, 130–300%, and > 300% (22). Physical activity was calculated as weekly minutes of moderate and vigorous activity multiplied by metabolic equivalent (MET) levels. Alcohol consumption was categorized as never (<12 drinks in a lifetime), former (≥12 drinks in a year or ≥ 12 drinks in a lifetime but none in the past year), mild (female: <2 drinks/d, male: <3 drinks/d), moderate (female: 2 to <3 drinks/d, male: 3 to <4 drinks/d, or 2–4 binges/month), and heavy (female: ≥3 drinks/d, male: ≥4 drinks/d, or ≥ 5 binges/month) (23). BMI was calculated as weight (kilograms) divided by the square of height (meters) and classified as <25, 25–30, and ≥ 30 (24). Hypertension was assessed by systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 80 mmHg, or taking antihypertensive drugs (25). Diabetes was determined by self-reported diagnosis by doctor or glycosylated hemoglobin (HbA1c) level ≥ 6.5% or taking anti-diabetic drugs. CVD was confirmed through self-reported diseases, including coronary heart disease, congestive heart failure, heart attack, stroke, and angina.
All analyses were conducted using appropriate sample weights to account for the complex sampling design of NHANES. Serum 25(OH)D concentrations were classified into four groups: severe deficiency (<25.00 nmoL/L), deficiency (25.00 to <50.00 nmoL/L), insufficiency (50.00 to <75.00 nmoL/L), and sufficiency (≥75.00 nmoL/L) (26). The reference group was defined as those with serum 25(OH)D concentrations <25.00 nmoL/L. Continuous variables and categorical variables were presented as means (standard errors, SE) and frequencies (weighted percentages), respectively. Differences between the four 25(OH)D groups were assessed using ANOVA for continuous variables and chi-square test for categorical variables. Multivariable Cox proportional hazards regression models were created to investigate the association between serum 25(OH)D concentrations and risks of all-cause or cause-specific mortality. Model 1 made no adjustments; model 2 was adjusted for age, sex, race/ethnicity; model 3 was further adjusted for education, alcohol consumption, cotinine, physical activity, BMI, PIR, hypertension, diabetes, cancer, and CVD. Restricted cubic spline analysis (RCS) with 5 knots (5th, 28th, 50th, 73th, and 95th) was applied to examine the non-linear relationship between serum 25(OH)D concentrations and all-cause mortality. Stratified analyses were further performed by age (<60 or ≥ 60 years), sex (male or female), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, and Other), BMI (< 25, 25–30, and ≥ 30), and cancer (yes or no). We also conducted several sensitivity analyses: (1) Participants who died within 2 years of follow-up were excluded to minimize reverse causality; (2) Participants with a history of cardiovascular disease or cancer were further excluded; (3) Additionally, adjustments were made for the blood drawing season to account for the influence of seasonal variations on vitamin D status.
All analyses were conducted using R 4.2.3 (Boston, MA, USA), and a two-tailed p-value <0.05 was considered statistically significant.
Of the 1856 participants with long-term prescription opioid use (mean [SE] age, 54.12 (0.5) years; 808 male [weighted, 40.7%]; 1,060 [weighted, 78.5%] non-Hispanic White; mean [SE] serum 25(OH)D concentrations, 70.19 (1.2) nmol/l), 32.4% were with deficient vitamin D (<50 nmoL/L), and 67.2% were with insufficient vitamin D (<75.00 nmoL/L). Table 1 shows the baseline characteristics of the study participants stratified by serum 25(OH)D concentrations. Compared to sufficient vitamin D (≥75.00 nmoL/L), the age of participants with severe deficient vitamin D (<25.00 nmoL/L) was younger. Severe deficient vitamin D (<25.00 nmoL/L) was more likely to occur in participants with of female, Non-Hispanic Black and Mexican American, with less physical activity, with obesity or history of CVD and diabetes. In addition, participants with severe deficient vitamin D were less likely to have higher PIR (>300%), have history of cancer, and have mild alcohol consumption.
During a median follow-up of 7.75 years, we identified 443 all-cause deaths (weighted, 18.7%), including 135 CVD deaths (weighted, 4.9%), and 94 cancer deaths (weighted, 3.8%).
Table 2 displays the results of Cox proportional hazards regression analyses between serum 25(OH)D concentrations and mortality. After multivariable adjustment (model3), compared to participants with serum 25(OH)D concentrations <25.00 nmol/L, the hazard ratio (HR) for all-cause mortality for those with serum 25(OH)D concentrations ranging from 50.00 to <75.00 nmol/L was 0.50 (95% confidence interval [CI] 0.29, 0.86), for those with serum 25(OH)D concentrations ≥75 nmol/L was 0.54 (95% CI 0.32, 0.90). Nevertheless, no significant inverse associations were observed for serum 25(OH)D concentrations with CVD-specific mortality and cancer-specific mortality after multivariable adjustment. It is important to note that there was no linear association between serum 25(OH)D concentrations and all-cause mortality (p for trend = 0.19).
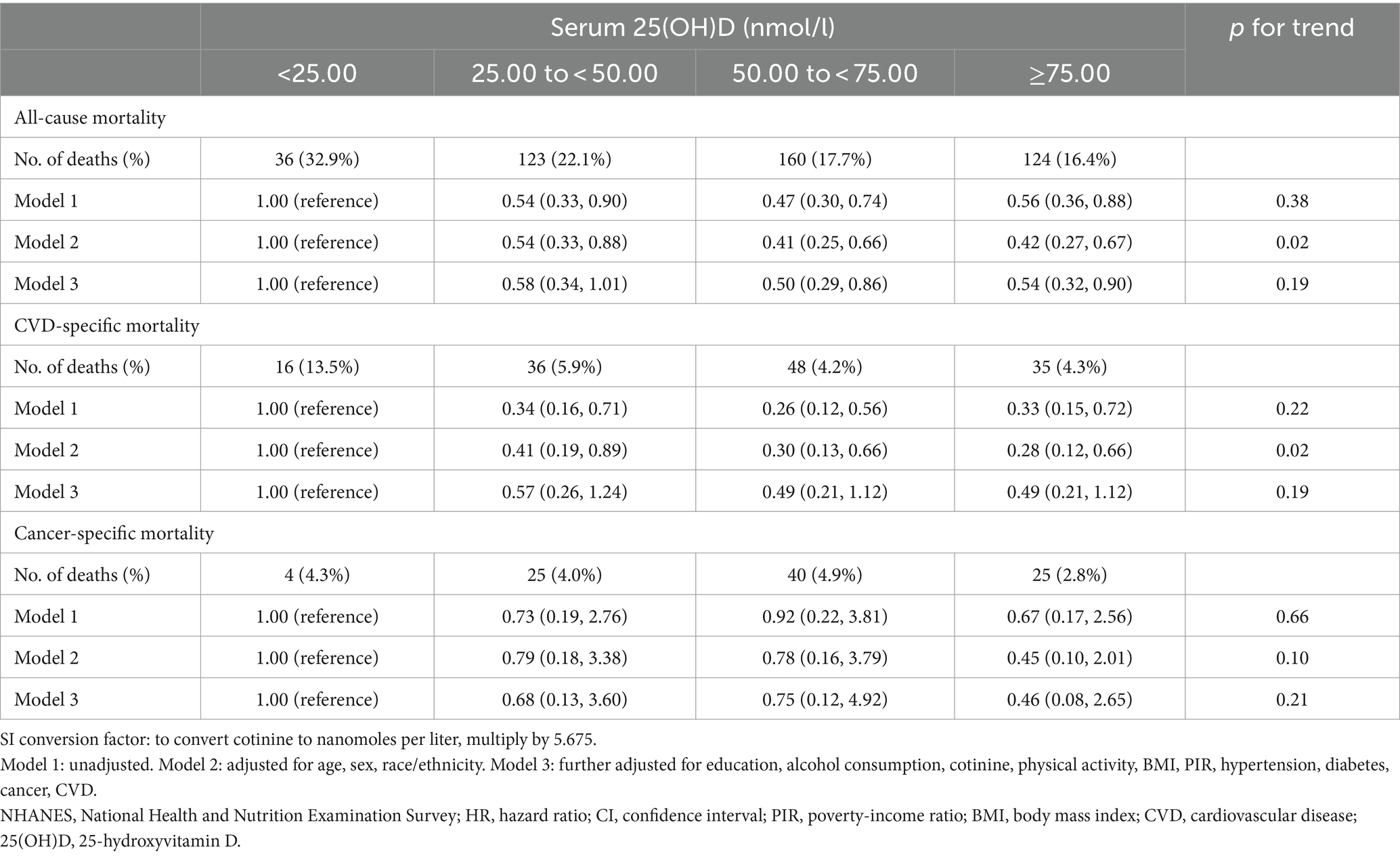
Table 2. HRs (95% CI) for mortality risk according to serum 25(OH)D concentrations in long-term prescription opioid users in NHANES, 2001–2018.
We used the RCS curve to estimate the dose–response relationship between serum 25(OH)D concentrations and all-cause mortality, and a non-linear association was observed after adjusting all confounders (p for non-linear = 0.01) (Figure 1). The inflection points for serum 25(OH)D concentrations were 62.17 nmoL/L and 94.52 nmol/L. Subsequently, piecewise COX proportional hazards regression was performed to analyze a threshold effect of serum 25(OH)D concentrations on all-cause mortality (Table 3). When serum 25(OH)D concentrations were < 62.17 nmoL/L, per 1-unit increment in serum 25(OH)D concentrations, there was a 2% lower risk of all-cause mortality (HR 0.98, 95%CI [0.97, 1.00]). However, there was no benefit effect for lower risk of all-cause mortality when elevate serum 25(OH)D concentrations for participants with serum 25(OH)D concentrations were 62.17- <94.52 nmol/L (HR 1.01, 95%CI [0.98, 1.03]) or ≥ 94.52 nmol/L (HR 0.97, 95%CI [0.93, 1.02]).
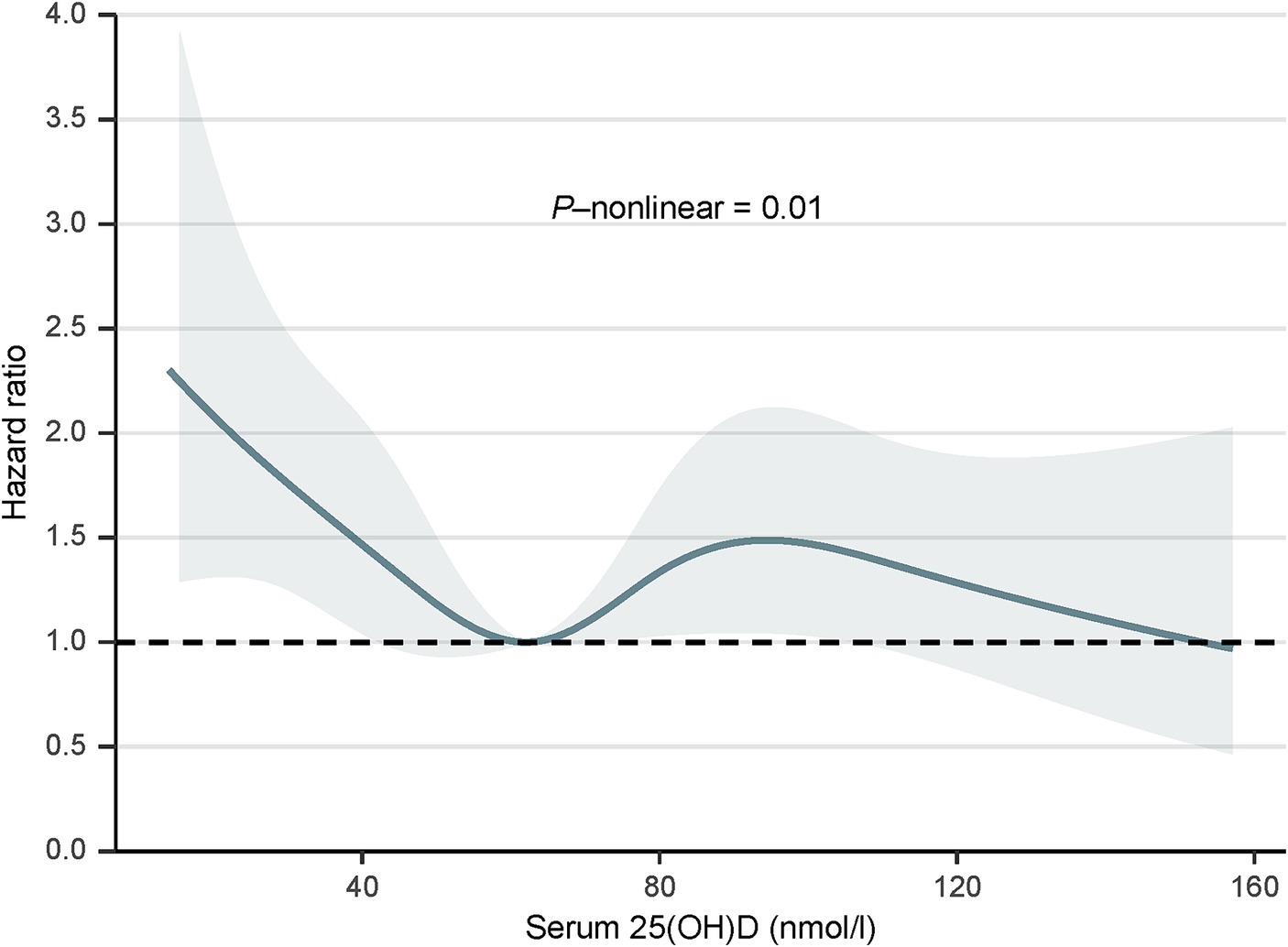
Figure 1. Dose–response relationship between serum 25(OH)D concentrations and all-cause mortality, 2001 to 2018. The model was adjusted for age, sex, race/ethnicity, education, alcohol consumption, cotinine, physical activity, BMI, PIR, hypertension, diabetes, cancer, and CVD. SI conversion factor: to convert cotinine to nanomoles per liter, multiply by 5.675. PIR, poverty-income ratio; BMI, body mass index; CVD, cardiovascular disease; 25(OH)D, 25-hydroxyvitamin D.
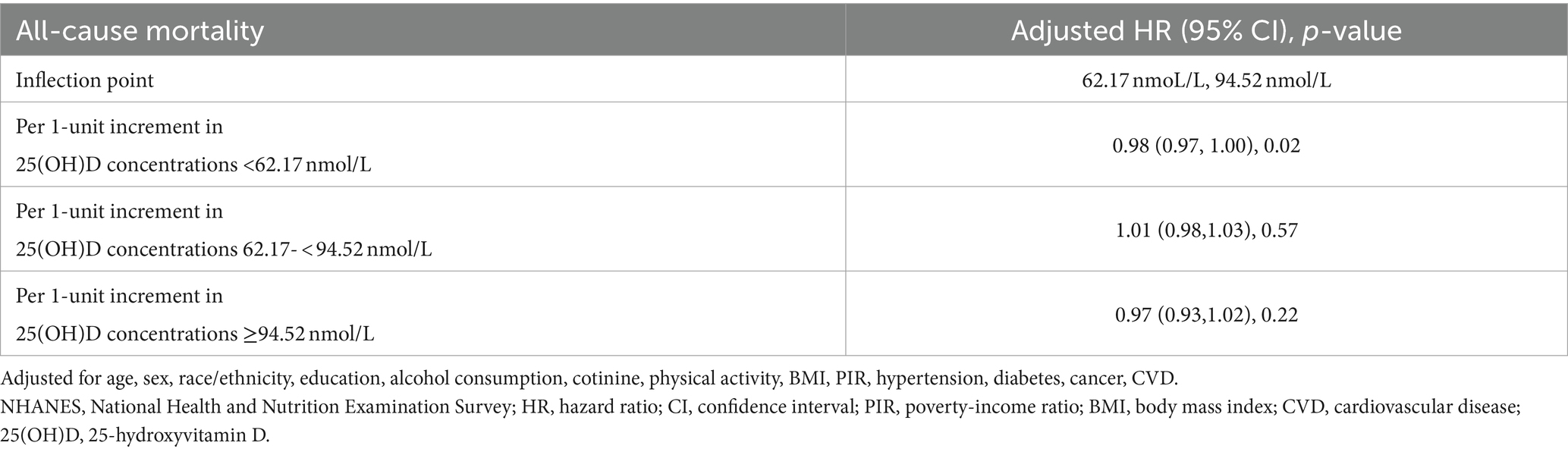
Table 3. Threshold effect analysis of serum 25(OH)D concentrations on all-cause mortality in long-term prescription opioid users in NHANES, 2001–2018.
In stratified analyses, the benefit of high serum 25(OH)D concentrations (≥ 62.17 nmol/L) and low serum 25(OH)D concentrations (< 62.17 nmol/L) for the survival rate of participants with long-term prescription opioid use stratified by age, sex, race/ethnicity, BMI, and cancer was similar. No modification factors were found between serum 25(OH)D concentrations and these stratified variables (all p for interaction >0.05) (Figure 2). In sensitivity analyses, the results were also typically robust when excluding deaths that occurred within 2 years (Supplementary Table S1); excluding participants with history of CVD or cancer (Supplementary Table S2); further adjustment of blood drawing season (Supplementary Table S3).
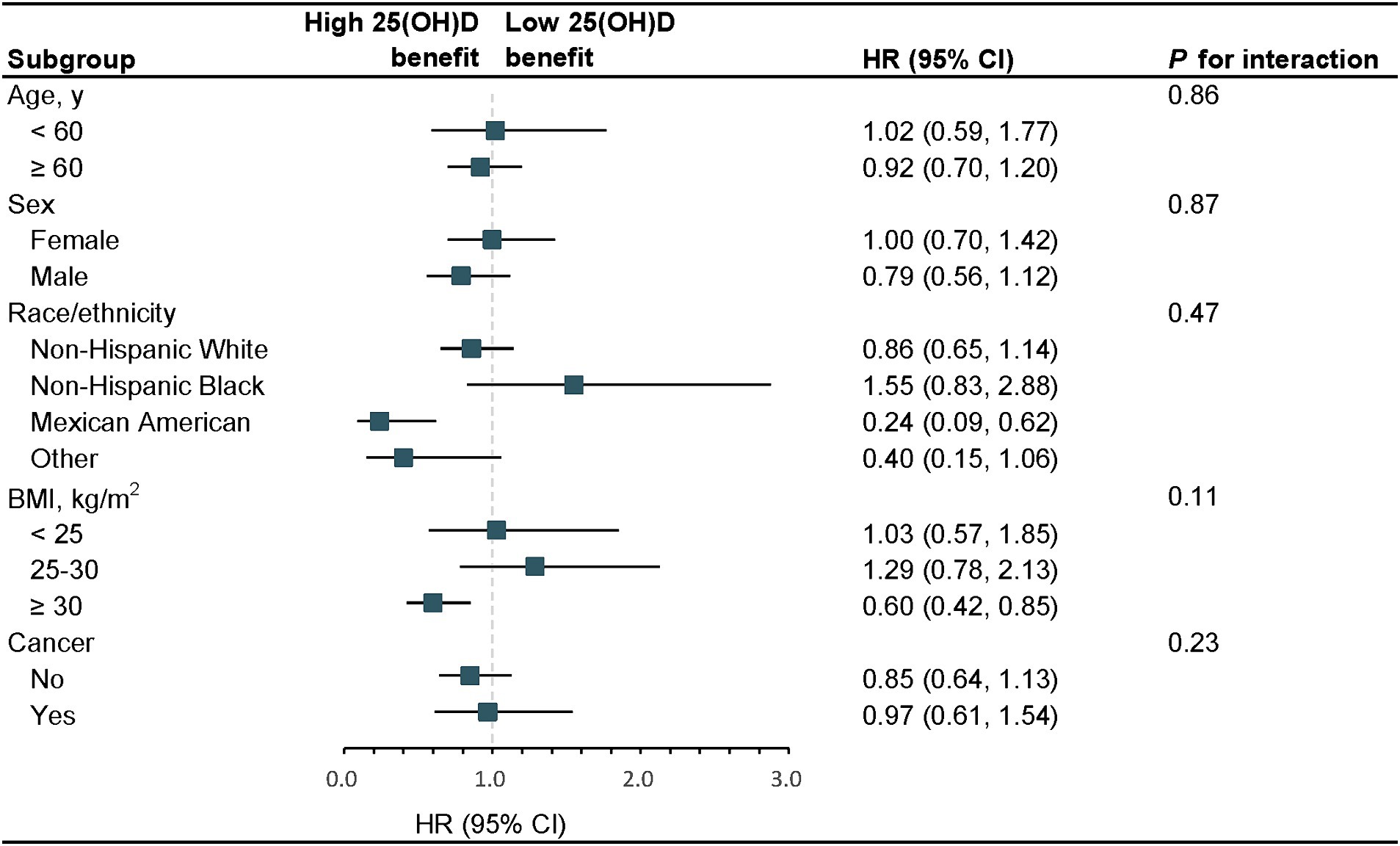
Figure 2. Stratified analyses of serum 25(OH)D concentrations with the risk of all-cause mortality, 2001–2018. The model was adjusted for age, sex, race/ethnicity, education, alcohol consumption, cotinine, physical activity, BMI, PIR, hypertension, diabetes, cancer, and CVD. The strata variable was not included in the adjustment when stratifying by itself. SI conversion factor: to convert cotinine to nanomoles per liter, multiply by 5.675. HR, hazard ratio; CI, confidence interval; PIR, poverty-income ratio; BMI, body mass index; CVD, cardiovascular disease; 25(OH)D, 25-hydroxyvitamin D.
In this prospective cohort study of participants with long-term prescription opioid use, we discovered significantly non-linear inverse association between serum 25(OH)D concentrations and all-cause mortality, nevertheless, no inverse associations were observed for serum 25(OH)D concentrations with CVD-specific mortality and cancer-specific mortality. Lower vitamin D in participants with serum 25(OH)D concentrations < 62.17 nmol/L were associated with an increased risk of all-cause mortality. Stratified and sensitivity analyses supported the stability of our conclusions.
Our research revealed that a significant proportion (67.2%) of long-term prescription opioid users had insufficient serum 25(OH)D concentrations, highlighting a prevalent vitamin D deficiency within this population. Preclinical study have demonstrated that vitamin D deficiency accelerated the development of opioid tolerance and exacerbated opioid dependence, resulting in elevated opioid consumption (16). Turner et al. (15) have reported that chronic pain patients with vitamin D deficiency exhibited significantly greater prescription opioid doses and prolonged duration of use. Excessive or long-term use of prescription opioids undoubtedly increases the risk of mortality. Consequently, there is a basis to hypothesize that vitamin D deficiency heightens the mortality risk in long-term prescription opioid users. Prior epidemiological studies have indicated the association between low serum 25(OH)D concentrations and increased all-cause mortality risk within the general population or specific subgroups (27–31), aligning with our findings. The majority of studies have demonstrated that low serum 25(OH)D concentrations are associated with increased incidence of cardiovascular events and cardiovascular mortality (32–34). Nevertheless, our study did not observe a relationship between low serum 25(OH)D concentrations and the risk of cardiovascular mortality, potentially attributable to the focus on specific high-risk population, a smaller sample size, and differences in ethnicity. Likewise, we did not detect low serum 25(OH)D concentrations linked to an increased risk of cancer mortality. The association between vitamin D status and cancer mortality was controversial (35). A meta-analysis conducted by Chowdhury et al. (36) proposed that elevated baseline serum 25(OH)D concentrations were linked to a decreased risk of cancer mortality. Conversely, several other extensive prospective studies failed to identify an association between serum 25(OH)D concentrations and the risk of cancer mortality (37–39).
In long-term prescription opioid users, we observed a non-linear relationship between serum 25(OH)D concentrations and the risk of all-cause mortality (27, 40, 41), aligning with earlier findings in diverse populations. In comparison to the optimal serum 25(OH)D concentrations reported in other populations, our study revealed that sustaining serum 25(OH)D concentrations above 62.17 nmol/L corresponded to a decreased risk of all-cause mortality in long-term prescription opioid users. Collectively, these studies indicate the presence of a ceiling effect for serum 25(OH)D concentrations concerning health outcomes, including mortality, wherein surpassing a specific threshold may not yield additional advantages. Although consensus regarding the ideal serum 25(OH)D concentrations is lacking, the National Institutes of Health advised maintaining levels above 50.00 ng/mL to mitigate the health hazards linked to vitamin D insufficiency (42). Despite observational studies suggest that low serum 25(OH)D concentrations contribute to mortality risk, recent intervention studies have failed to demonstrate the advantages of vitamin D supplementation (12–14). Likewise, Neale et al. (43) observed that monthly vitamin D supplementation in the elderly did not result in lower all-cause mortality. A meta-analysis conducted by Zhang et al. (44) indicated that vitamin D supplementation alone only reduced cancer mortality in the general population. Wu et al. (45) discovered that monthly vitamin D supplementation did not lead to a reduction in prescription opioid use. However, these experiments included participants with high baseline serum 25(OH)D concentrations, such as 115 nmol/L in Neale RE et al.’s study and 66.4 nmol/L in Wu et al.’s study (exceeding 50 nmol/L), thereby constraining the efficacy of supplementation tests within the low serum 25(OH)D concentration subgroups. Subsequent studies exploring the potential of vitamin D supplements to mitigate all-cause mortality in long-term prescription opioid users should consider enrolling participants with lower baseline serum 25(OH)D concentrations.
This study possesses several strengths. Firstly, to the best of our knowledge, this was the first study to investigate the relationship between vitamin D deficiency and mortality in long-term prescription opioid users. Secondly, this study relied on a nationally representative sample and exhibited excellent follow-up rates. Lastly, several sensitivity analyses were performed to affirm the model’s stability, thereby bolstering the reliability of our study’s findings. Nonetheless, this study possesses certain limitations. Firstly, owing to its nature of cross-sectional design, it was unable to establish a causal relationship between vitamin D deficiency and mortality. Then, despite numerous potential confounding factors had been adjusted for, residual confounding factors might persist. Additionally, the absence of repeated measurements of serum 25(OH)D concentrations precluded the evaluation of the influence of dynamic fluctuations in vitamin D status on mortality.
In conclusion, a non-linear association was observed between serum 25(OH)D concentrations and all-cause mortality in long-term prescription opioid users. Lower vitamin D in participants with serum 25(OH)D concentrations < 62.17 nmol/L were associated with an increased risk of all-cause mortality.
Publicly available datasets were analyzed in this study. This data can be found at: http://www.cdc.gov/nchs/nhanes.htm.
The studies involving humans were approved by NCHS Ethics Review Board, National Center for Health Services. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SD: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JW: Data curation, Investigation, Writing – review & editing. PW: Data curation, Validation, Visualization, Writing – review & editing. ZH: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1422084/full#supplementary-material
25(OH)D, 25-hydroxyvitamin D; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center of Health Statistics; CDC, Centers for Disease Control and Prevention; ICD-10, International Classification of Diseases, 10th; NDI, National Death Index; PIR, poverty-income ratio; BMI, body mass index; MET, metabolic equivalent; CVD, cardiovascular disease; HbA1c, glycosylated hemoglobin; SE, standard errors; HR, hazard ratio; CI, confidence interval; RCS, restricted cubic spline
1. Dowell, D, Ragan, KR, Jones, CM, Baldwin, GT, and Chou, R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. (2022) 71:1–95. doi: 10.15585/mmwr.rr7103a1
2. Chou, R, Deyo, R, Devine, B, Hansen, R, Sullivan, S, Jarvik, JG, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evid Rep Technol Assess. (2014) 218:1–219. doi: 10.23970/AHRQEPCERTA218
3. Drug Overdose Death Rates: National Institute on Drug Abuse. (2024). Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates/.
4. Song, I-A, and Choi H-r, OTK. Long-term opioid use and mortality in patients with chronic non-cancer pain: ten-year follow-up study in South Korea from 2010 through 2019. eClinicalMedicine. (2022) 51:101558. doi: 10.1016/j.eclinm.2022.101558
5. Häuser, W, Schubert, T, Vogelmann, T, Maier, C, Fitzcharles, M-A, and Tölle, T. All-cause mortality in patients with long-term opioid therapy compared with non-opioid analgesics for chronic non-cancer pain: a database study. BMC Med. (2020) 18:162. doi: 10.1186/s12916-020-01644-4
6. Ekholm, O, Kurita, GP, Hjsted, J, Juel, K, and Sjgren, P. Chronic pain, opioid prescriptions, and mortality in Denmark: a population-based cohort study. Pain. (2014) 155:2486–90. doi: 10.1016/j.pain.2014.07.006
7. Nalini, M, Shakeri, R, Poustchi, H, Pourshams, A, Etemadi, A, Islami, F, et al. Long-term opiate use and risk of cardiovascular mortality: results from the Golestan cohort study. Eur J Prev Cardiol. (2021) 28:98–106. doi: 10.1093/eurjpc/zwaa006
9. DeLuca, HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. (2004) 80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S
10. Heath, A, Kim, I, Hodge, A, English, D, and Muller, D. Vitamin D status and mortality: a systematic review of observational studies. Int J Environ Res Public Health. (2019) 16:383. doi: 10.3390/ijerph16030383
11. Zittermann, A, Iodice, S, Pilz, S, Grant, WB, Bagnardi, V, and Gandini, S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. (2012) 95:91–100. doi: 10.3945/ajcn.111.014779
12. Manson, JE, Cook, NR, Lee, IM, Christen, W, Bassuk, SS, Mora, S, et al. Vitamin D supplements and prevention of Cancer and cardiovascular disease. N Engl J Med. (2019) 380:33–44. doi: 10.1056/NEJMoa1809944
13. Zittermann, A, Ernst, JB, Prokop, S, Fuchs, U, Dreier, J, Kuhn, J, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. (2017) 38:2279–86. doi: 10.1093/eurheartj/ehx235
14. Scragg, R, Stewart, AW, Waayer, D, Lawes, CMM, Toop, L, Sluyter, J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study. JAMA. Cardiology. (2017) 2:608. doi: 10.1001/jamacardio.2017.0175
15. Turner, MK, Hooten, WM, Schmidt, JE, Kerkvliet, JL, Townsend, CO, and Bruce, BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. (2008) 9:979–84. doi: 10.1111/j.1526-4637.2008.00415.x
16. Kemény, LV, Robinson, KC, Hermann, AL, Walker, DM, Regan, S, Yew, YW, et al. Vitamin D deficiency exacerbates UV/endorphin and opioid addiction. Sci Adv. (2021) 7:eabe4577. doi: 10.1126/sciadv.abe4577
17. Santos, MCQ, da Silva, T, da Silva, F, Siebert, C, Kroth, A, Silveira, EMS, et al. Effects of vitamin D administration on nociception and spinal cord pro-oxidant and antioxidant markers in a rat model of neuropathic pain. Braz J Med Biol Res. (2021) 54:e11207. doi: 10.1590/1414-431x2021e11207
18. Poisbeau, P, Aouad, M, Gazzo, G, Lacaud, A, Kemmel, V, Landel, V, et al. Cholecalciferol (vitamin D 3) reduces rat neuropathic pain by modulating opioid signaling. Mol Neurobiol. (2019) 56:7208–21. doi: 10.1007/s12035-019-1582-6
19. Leal, LKAM, Lima, LA, de Aquino, PEA, de Sousa, JAC, Gadelha, CVJ, Calou, IBF, et al. Vitamin D (VD3) antioxidative and anti-inflammatory activities: peripheral and central effects. Eur J Pharmacol. (2020) 879:173099. doi: 10.1016/j.ejphar.2020.173099
20. Akinbami, LJ, Chen, T-C, Davy, O, Ogden, CL, Fink, S, Clark, J, et al. National Health and Nutrition Examination Survey, 2020 Prepandemic file: sample design, Estimation, and Analytic Guidelines. Vital Health Stat 1. (2022) 190:1–36. doi: 10.15620/cdc:115434
21. Stokes, A, Berry, KM, Collins, JM, Hsiao, CW, Waggoner, JR, Johnston, SS, et al. The contribution of obesity to prescription opioid use in the United States. Pain. (2019) 160:2255–62. doi: 10.1097/j.pain.0000000000001612
22. Beydoun, MA, Beydoun, HA, Banerjee, S, Weiss, J, Evans, MK, and Zonderman, AB. Pathways explaining racial/ethnic and socio-economic disparities in incident all-cause dementia among older US adults across income groups. Transl Psychiatry. (2022) 12:478. doi: 10.1038/s41398-022-02243-y
23. Wu, J, Yang, P, Wu, X, Yu, X, Zeng, F, and Wang, H. Association between secondhand smoke exposure and severe headaches or migraine in never-smoking adults. Headache: the journal of head and face. Pain. (2023) 63:1341–50. doi: 10.1111/head.14640
24. Zhang, M, Wang, J, Li, X, Zhang, L, Zhang, Y, Wen, Z, et al. Association between dietary supplement use and mortality in cancer survivors with different body mass index and frailty status: a cohort study. Front Nutr. (2024) 11:1395362. doi: 10.3389/fnut.2024.1395362
25. Whelton, PK, Carey, RM, Aronow, WS, Casey, DE, Collins, KJ, Dennison Himmelfarb, C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2018) 71:e127–248. doi: 10.1016/j.jacc.2017.11.006
26. Holick, MF, Binkley, NC, Bischoff-Ferrari, HA, Gordon, CM, Hanley, DA, Heaney, RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
27. Xiao, Q, Cai, B, Yin, A, Huo, H, Lan, K, Zhou, G, et al. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the NHANES database prospective cohort study. BMC Med. (2022) 20:308. doi: 10.1186/s12916-022-02510-1
28. Wan, Z, Guo, J, Pan, A, Chen, C, Liu, L, and Liu, G. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care. (2021) 44:350–7. doi: 10.2337/dc20-1485
29. Jayedi, A, Soltani, S, and Shab-Bidar, S. Vitamin D status and all-cause mortality in patients with chronic kidney disease: a systematic review and dose-response Meta-analysis. J Clin Endocrinol Metabol. (2017) 102:2136–45. doi: 10.1210/jc.2017-00105
30. Garland, CF, Kim, JJ, Mohr, SB, Gorham, ED, Grant, WB, Giovannucci, EL, et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. (2014) 104:e43–50. doi: 10.2105/AJPH.2014.302034
31. Ford, ES, Zhao, G, Tsai, J, and Li, C. Vitamin D and all-cause mortality among adults in USA: findings from the National Health and nutrition examination Survey linked mortality study. Int J Epidemiol. (2011) 40:998–1005. doi: 10.1093/ije/dyq264
32. Sutherland, JP, Zhou, A, and Hyppönen, E. Vitamin D deficiency increases mortality risk in the UK biobank: a nonlinear Mendelian randomization study. Ann Intern Med. (2022) 175:1552–9. doi: 10.7326/M21-3324
33. Schottker, B, Jorde, R, Peasey, A, Thorand, B, Jansen, EHJM, Groot, L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. (2014) 348:g3656. doi: 10.1136/bmj.g3656
34. Brøndum-Jacobsen, P, Benn, M, Jensen, GB, and Nordestgaard, BG. 25-Hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death. Arterioscler Thromb Vasc Biol. (2012) 32:2794–802. doi: 10.1161/ATVBAHA.112.248039
35. Mondul, AM, Weinstein, SJ, Layne, TM, and Albanes, D. Vitamin D and Cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. (2017) 39:28–48. doi: 10.1093/epirev/mxx0005
36. Chowdhury, R, Kunutsor, S, Vitezova, A, Oliver-Williams, C, Chowdhury, S, Kiefte-de-Jong, JC, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. (2014) 348:g1903. doi: 10.1136/bmj.g1903
37. Ong, J-S, Gharahkhani, P, An, J, Law, MH, Whiteman, DC, Neale, RE, et al. Vitamin D and overall cancer risk and cancer mortality: a Mendelian randomization study. Hum Mol Genet. (2018) 27:4315–22. doi: 10.1093/hmg/ddy307
38. Zmijewski, M, Gaksch, M, Jorde, R, Grimnes, G, Joakimsen, R, Schirmer, H, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. (2017) 12:e0170791. doi: 10.1371/journal.pone.0170791
39. Khaw, K-T, Luben, R, and Wareham, N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. Am J Clin Nutr. (2014) 100:1361–70. doi: 10.3945/ajcn.114.086413
40. Shi, J-W, Wu, J-N, Zhu, X-Y, Zhou, W-H, Yang, J-Y, and Li, M-Q. Association of serum 25-hydroxyvitamin D levels with all-cause and cause-specific mortality among postmenopausal females: results from NHANES. J Transl Med. (2023) 21:629. doi: 10.1186/s12967-023-04413-y
41. Fan, X, Wang, J, Song, M, Giovannucci, EL, Ma, H, Jin, G, et al. Vitamin D status and risk of all-cause and cause-specific mortality in a large cohort: results from the UK biobank. J Clin Endocrinol Metabol. (2020) 105:e3606–19. doi: 10.1210/clinem/dgaa432
42. Ross, AC, Manson, JE, Abrams, SA, Aloia, JF, Brannon, PM, Clinton, SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metabol. (2011) 96:53–8. doi: 10.1210/jc.2010-2704
43. Neale, RE, Baxter, C, Romero, BD, McLeod, DSA, English, DR, Armstrong, BK, et al. The D-Health trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. (2022) 10:120–8. doi: 10.1016/S2213-8587(21)00345-4
44. Zhang, Y, Fang, F, Tang, J, Jia, L, Feng, Y, Xu, P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. (2019) 366:l4673. doi: 10.1136/bmj.l4673
45. Wu, Z, Camargo, CA, Malihi, Z, Bartley, J, Waayer, D, Lawes, CMM, et al. Monthly vitamin D supplementation, pain, and pattern of analgesic prescription: secondary analysis from the randomized, double-blind, placebo-controlled vitamin D assessment study. Pain. (2018) 159:1074–82. doi: 10.1097/j.pain.0000000000001189
Keywords: prescription opioids, pain, mortality, NHANES, 25-hydroxyvitamin D
Citation: Dai S, Wu J, Wang P and Hu Z (2024) Associations of vitamin D status with all-cause and cause-specific mortality in long-term prescription opioid users. Front. Nutr. 11:1422084. doi: 10.3389/fnut.2024.1422084
Received: 23 April 2024; Accepted: 07 June 2024;
Published: 18 June 2024.
Edited by:
Francesca Gorini, National Research Council (CNR), ItalyReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaCopyright © 2024 Dai, Wu, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Hu, SHV6aDM3MUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.