- 1Department of Critical Care Medicine and Neurosurgery of Huashan Hospital, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Institutes of Brain Science, Fudan University, Shanghai, China
- 2Department of General Practice of Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Infectious Diseases of Huashan Hospital, Fudan University, Shanghai, China
- 4Department of Neurosurgery, Liaocheng People's Hospital, Liaocheng, China
- 5Department of Radiology of Huashan Hospital, Fudan University, Shanghai, China
- 6Department of Clinical Nutrition, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
- 7Department of Geriatric of Huashan Hospital, National Clinical Research Center for Aging and Medicine, Fudan University, Shanghai, China
Background: The Geriatric Nutritional Risk Index (GNRI) is a straightforward and objective tool for nutritional screening in older patients and has been demonstrated to possess prognostic predictive value in several diseases. Nonetheless, there is a lack of research on the nutritional risk associated with brain abscess in the older. This study aimed to evaluate the prevalence of nutritional risk among these patients by GNRI and to investigate its potential prognostic value for clinical outcomes.
Materials and methods: From August 2019 to April 2023, 100 older patients diagnosed with brain abscess were enrolled in this single-center prospective cohort study, which evaluated the prognostic value of the Geriatric Nutritional Risk Index (GNRI) in elderly brain abscess patients. Data collected included demographic, and clinical characteristics at admission and calculated the GNRI, and the Glasgow Outcome Scale (GOS) score 6 months post-discharge. A GOS score of 5 was considered indicative of a good recovery, whereas scores ranging from 1 to 4 were classified as poor recovery.
Results: The results revealed that 48% of older brain abscess patients were at risk of malnutrition according to the GNRI. These patients had significantly higher post-admission C-reactive protein (CRP) levels (p = 0.017), more comorbidities (p < 0.001), and higher age-adjusted Charlson Comorbidity Index (aCCI) scores (p < 0.001) compared to those without nutritional risk. Spearman correlation analysis showed that GNRI scores were negatively correlated with CRP levels, comorbidities, and aCCI scores, and positively correlated with Glasgow Outcome Scale (GOS) scores (Spearman’s ρ = 0.624, p < 0.001). Multivariate logistic regression revealed that lower GNRI values were linked to reduced GOS levels (OR = 0.826, 95% CI: 0.775–0.880). ROC analysis determined a GNRI threshold of 97.50 for predicting poor recovery, with 90.57% sensitivity and 87.23% specificity.
Conclusion: The older brain abscess patients exhibited a high malnutrition risk. GNRI showed an important predictive value for recovery in older patients, which could be helpful in clinical intervention and rehabilitation.
Introduction
Brain abscess, a serious intracranial infection, manifests as a pus collection within the brain tissue (1). Its complex pathogenesis involves a variety of infectious agents, such as bacteria (2), fungi (3), and parasites (4). Risk factors for the formation of a brain abscess include skull injuries, peripheral infections, a compromised immune system, malnutrition, and factors associated with advanced age. Brain abscesses can lead to severe consequences such as increased intracranial pressure, consciousness disturbances, brain herniation, abscess rupture causing meningitis or ventriculitis, focal neurological deficits, and seizures (5). The mortality rate for brain abscesses is around 10%, with 45% of affected patients experiencing neurological deficits post-treatment (4, 6, 7). In older patients, this mortality rate increases, with studies indicating a range of 20 to 40% (4, 7). The Glasgow outcome scale (GOS) is a scale used to systematically evaluate post-brain injury recovery, categorizing outcomes into five levels: death, vegetative state, severe disability, moderate disability, and good recovery (8). The GOS has been employed widely as a metric to assess the effectiveness of brain injury treatment and rehabilitation strategies (9, 10).
Nutritional status significantly influences the progression of brain abscesses and the efficacy of treatments (11). Inadequate nutrition weakens immune defenses, increasing infection risks (12). Furthermore, nutritional health is vital during treatment and recovery, with nutritional deficits potentially delaying wound healing, impeding neurological recovery, and prolonging the recovery process (13). Such deficiencies can negatively impact patient lifespan, and quality of life, and impose significant economic costs (14). The incidence of malnutrition and risk of nutritional deficits in elderly populations within hospital and nursing facility settings is nearly 50%. This condition is detrimental, with a profound link to clinical outcomes, and it significantly amplifies the risk of geriatric syndromes including dependency in activities of daily living, myasthenia gravis, and frailty (15). Given the heightened risk of malnutrition among the older due to aging, conducting a simple and objective assessment of malnutrition risk is imperative to develop effective nutritional interventions. The geriatric nutritional risk index (GNRI) serves as an objective screen tool to evaluate the nutritional status of older individuals and its association with health risks (16). The GNRI has emerged as a powerful prognostic indicator for the older, particularly regarding long-term postoperative outcomes (17–19). Numerous studies have demonstrated the utility of the GNRI in predicting outcomes among a wide range of clinical conditions in the older, including chronic kidney disease (20), heart failure (21), chronic obstructive pulmonary disease (22), and sepsis (23). Additionally, GNRIs have relevant applications in brain-related injuries. Su et al.’s study revealed that the GNRI is a prognostic factor for death in older patients with moderate to severe traumatic brain injuries (24). Furthermore, evidence from another study highlighted that a lower GNRI is an independent predictor of brain infarction and hemorrhage in patients on maintenance hemodialysis (25). Similarly, the study by Dai et al. demonstrated that GNRI was related to the risk of stroke-associated pneumonia (26).
To our knowledge, no study has examined the nutritional status of brain abscess patients and its correlation with clinical outcomes. Therefore, this study employed the GNRI to assess the risk of malnutrition among older brain abscess patients, exploring the relationship between nutritional status and clinical features. Additionally, we employed the GOS to evaluate recovery post-discharge in these patients, further exploring GNRI’s predictive value for GOS scores in older brain abscess patients’ post-discharge.
Materials and methods
Study design and participants
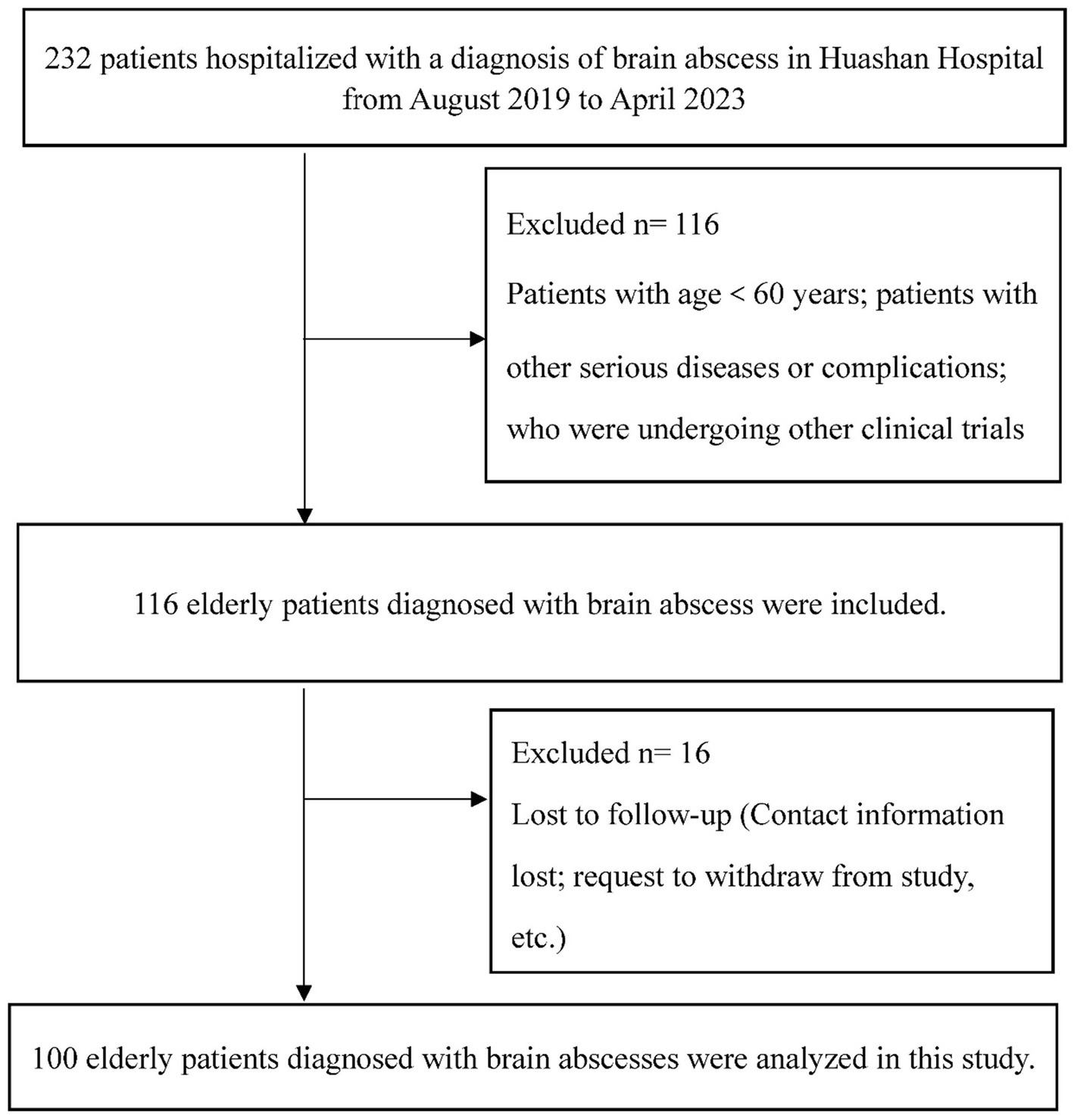
This single-center prospective cohort study was executed at Huashan Hospital, Fudan University. The study population comprised individuals admitted with a diagnosis of brain abscess between August 2019 and April 2023. Diagnosis of brain abscess in surgical patients was confirmed through pathology and pus culture, while conservatively managed patients were diagnosed through cerebrospinal fluid culture or in conjunction with magnetic resonance imaging and clinical manifestations (27). Patients were subjected to a follow-up period of 6 months post-discharge. The inclusion criteria for this study were as follows: (a) patients diagnosed with brain abscess confirmed by imaging and/or cerebrospinal fluid culture; (b) participants must be aged 60 or older; (c) voluntary participation and signed informed consent. The exclusion criteria for this study encompass the following: (a) individuals under the age of 60; (b) patients who are lost to follow-up; (c) those with severe health conditions such as heart or lung failure, advanced cancer, or other critical illnesses that may influence nutritional status and the assessment of prognosis; (d) participants who are unconscious or have a mental illness that prevents them from cooperating with the assessment process (Figure 1). Ethical endorsement for this study’s protocol was granted by the Research Ethics Committee of Huashan Hospital, Fudan University. This approval was secured to ascertain adherence to the ethical guidelines outlined in the Declaration of Helsinki (1975), as well as its later amendments, thereby ensuring the protection of participants’ rights and well-being throughout the research.
Data collection
Demographic and clinical characteristics
Demographic and clinical data were systematically gathered, encompassing age, gender, and histories of tobacco use and alcohol consumption, which were obtained through patient history collection. Serum albumin levels and C-reactive protein (CRP) concentrations were documented for all subjects upon their initial evaluation, conducted within the first 48 h post-admission. These blood indicators were collected through routine blood tests (Sysmex XN-9000 analyzer) and biochemical analyses (VITROS 5600 Integrated System) performed by the laboratory department, ensuring accurate and standardized measurements. Additional details regarding therapeutic interventions employed, comorbidities were also collected. Comorbid conditions encompass a spectrum of diseases and disorders, including chronic illnesses (e.g., hypertension, diabetes mellitus, cardiovascular disorders, pulmonary pathologies, renal dysfunctions), psychiatric disorders (e.g., depressive disorders, anxiety disorders, bipolar affective disorders), autoimmune diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus), neurological pathologies (e.g., Alzheimer’s disease, Parkinson’s disease), metabolic disorders (e.g., obesity, dyslipidemia), infectious diseases (e.g., chronic hepatitis, HIV/AIDS), and gastroenterological conditions (e.g., chronic gastritis, inflammatory bowel disease). The age-adjusted Charlson Comorbidity Index (aCCI) serves as a pivotal tool for assessing mortality risks linked to a spectrum of prognostic clinical indicators. We utilized this index to quantify the cumulative risk profile of patient populations (28).
Nutritional assessment
Anthropometric parameters were ascertained by trained medical personnel employing a calibrated scale equipped with a stadiometer (RGZ 120, China). Body mass index (BMI) was calculated utilizing the formula: weight (kg) /height2 (m2). The classification of BMI adhered to the guidelines established by the Chinese Obesity Working Group, delineating the categories as follows: underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), and overweight (>23.9 kg/m2).
The GNRI is derived through the following equation (16):
The ideal weight within this equation is ascertained via the Lorenz formula, which is contingent upon the individual’s sex and stature. Should the ratio of the current to ideal weight exceed 1, it is subsequently adjusted to 1.
Lorenz formula:
Outcomes
The length of stay (LOS) of each patient was recorded. LOS was defined as the interval, measured in days, extending from the initial date of admission to the terminal date of discharge. GOS was employed for the assessment of overall functional recuperation in overall. The scale is stratified into quintuple gradations as follows: GOS = 1, death; GOS = 2, vegetative state; GOS = 3, severe disability; GOS = 4, moderate disability; and GOS = 5, good recovery.
Statistical analysis
Participant characteristics in this study were summarized as mean ± standard deviation (SD) for normally distributed continuous variables and median (interquartile range, IQR) for non-normally distributed continuous variables. Categorical variables were reported as frequencies and percentages. The t-test and Wilcoxon rank-sum test assessed differences in key indicators between groups with and without nutritional risk. Pearson correlation analysis explored factors associated with GNRI scores. Univariate logistic regression on GOS included variables with p < 0.20 in a subsequent multivariate analysis. Odds ratios (OR) and 95% confidence intervals (CI) were reported. A stratified histogram depicted GOS score distributions across nutritional risk categories in brain abscess patients. The prognostic value of GNRI for the older brain abscess patients was determined using receiver operating characteristic curve (ROC) analysis, a GOS score of 5 was classified as indicative of good recovery, whereas scores ranging from 1 to 4 were considered to reflect poor recovery. Statistical analyses were two-sided, with an alpha level of 0.05, performed using SAS V.9.4 (SAS Institute, Cary, NC, United States). R version 4.3.2, with ggplot2 and pROC packages, was used for histogram and ROC curve visualizations.
Results
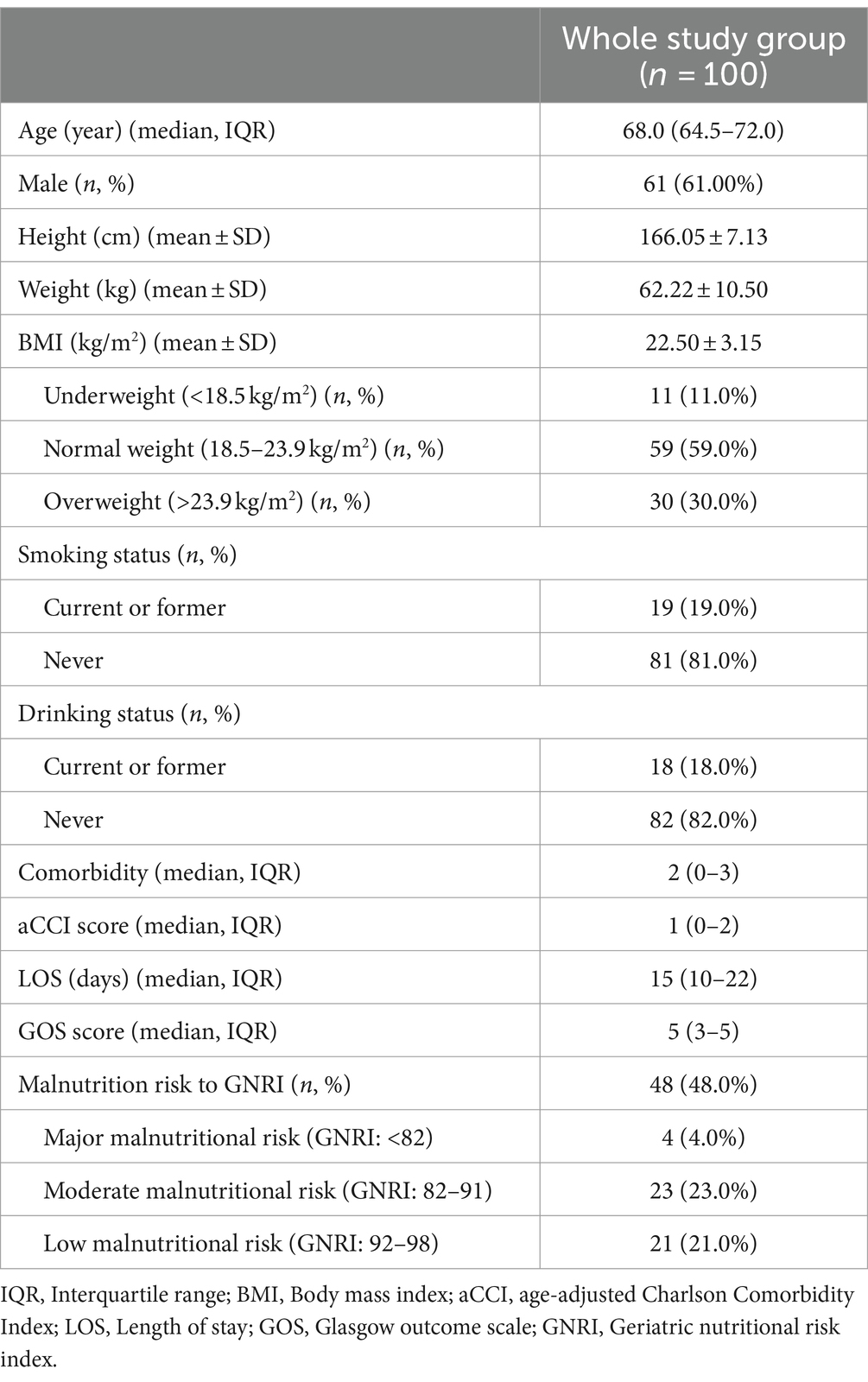
This study’s final analysis included a cohort of 100 older patients diagnosed with brain abscess. Table 1 revealed the median age to be 68 years (IQR 64.5–72), with females constituting 61% (61/100) of the sample. In this cohort, 11% (11/100) were underweight, and 30% (30/100) were overweight. The proportion of alcohol consumption and smoking was noted in 19% (19/100) and 18% (18/100) of the patients, respectively. Comorbidities were prevalent in older brain abscess patients, with the median number of comorbidities being 2 (IQR 0–3). The most common comorbidity was hypertension (36%, n = 36), followed by diabetes (31%, n = 31). GNRI assessments conducted within 48 h of admission indicated malnutrition risks in 48% of patients, with 4% facing major malnutrition risk, 23% moderate risk, and 21% low risk. The median hospital stay duration was 15 days (IQR 10–22), and the median GOS score at 6 months post-discharge was 5 (IQR 3–5), reflecting generally favorable outcomes.
As shown in Table 2, individuals at risk for malnutrition had significantly lower BMI compared to those not at risk (p < 0.001). This group also had a higher median comorbidity count of 3 (IQR 1–4) (p < 0.001), suggesting a higher burden of comorbid conditions. Furthermore, aCCI scores were significantly higher in the malnutrition risk group (p < 0.001). Admission CRP levels were also higher in the malnutrition risk group than in the non-risk group (p = 0.017), indicating more severe inflammation. Treatment strategies varied, with a larger fraction of the malnutrition risk group receiving conservative medical treatment (66.7%) compared to the non-risk group (38.5%) (p = 0.003). Additionally, the malnutrition risk group had lower median GOS scores of 3 (IQR 3–4) (p < 0.001), indicating a worse prognosis.
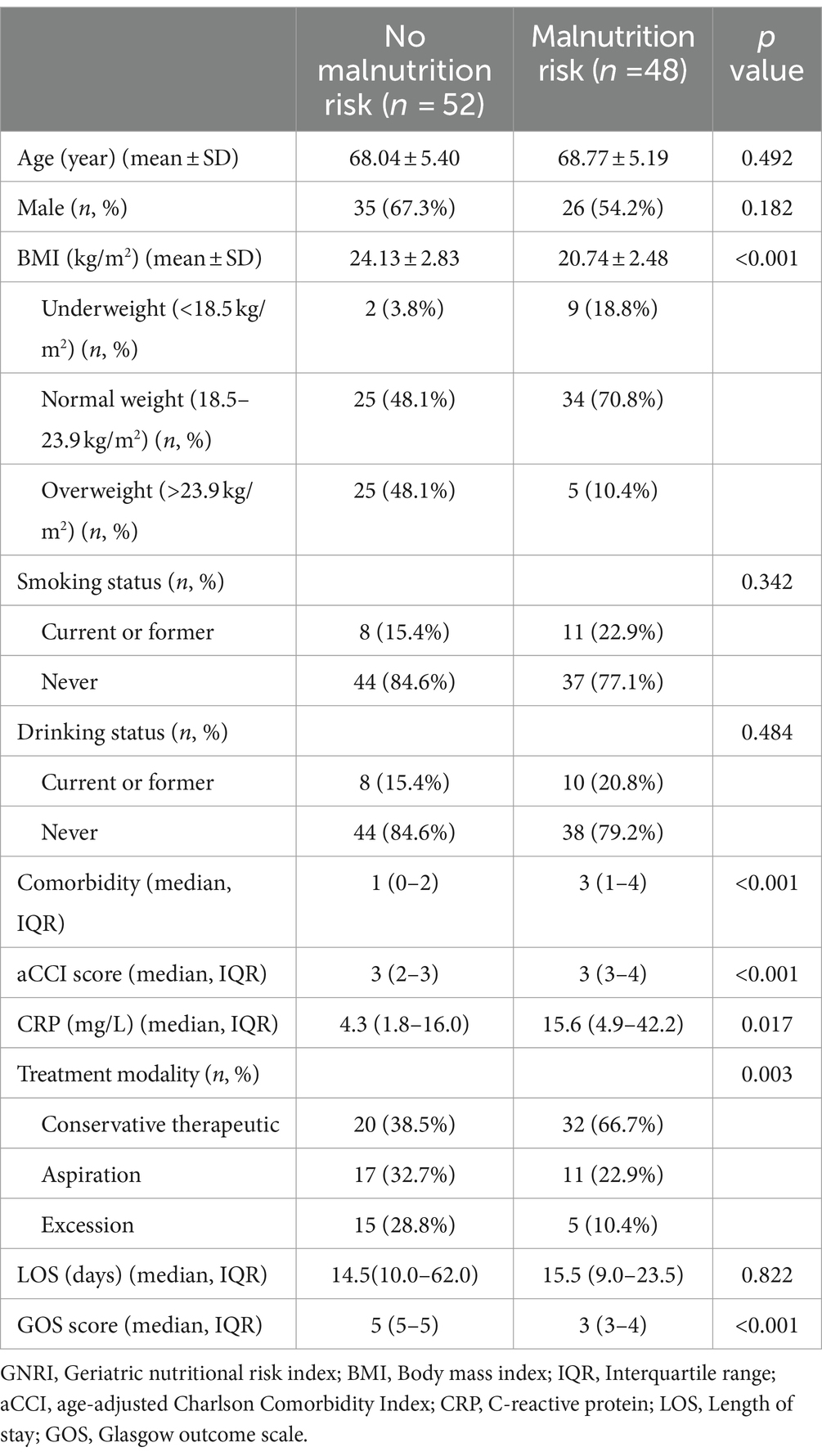
Table 2. A comparative analysis of demographic and clinical characteristics between patients with and without malnutrition risk according to GNRI.
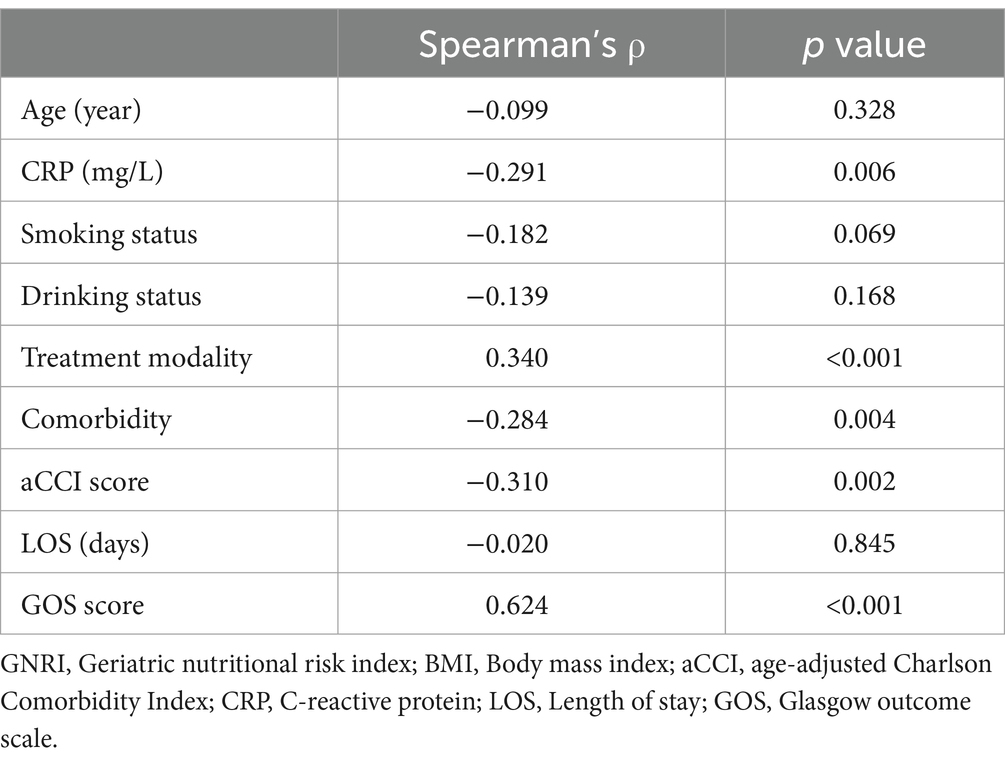
Table 3 demonstrated the relationships between GNRI scores and various clinical parameters. Specifically, CRP levels (Spearman’s ρ = −0.291, p = 0.006), the total number of comorbidities (Spearman’s ρ = −0.284, p = 0.004), and aCCI scores (Spearman’s ρ = −0.310, p = 0.002) are inversely correlated with GNRI scores. In contrast, GOS scores show a strong positive correlation with GNRI scores (Spearman’s ρ = 0.624, p < 0.001), indicating that higher nutritional status is associated with better neurological outcomes. Figure 2 delineates the distribution of GOS scores across varying malnutrition risk categories, with the precise value distribution elaborated in Supplementary Table S1 (p < 0.001). Furthermore, by assigning numerical values to surgical methods—1 for conservative therapeutic, 2 for aspiration, and 3 for excision—a positive correlation is observed between these values and GNRI scores (Spearman’s ρ = 0.340, p < 0.001). This suggests a preference for conservative treatment in patients with poorer nutritional status, consistent with the trends shown in Table 2.
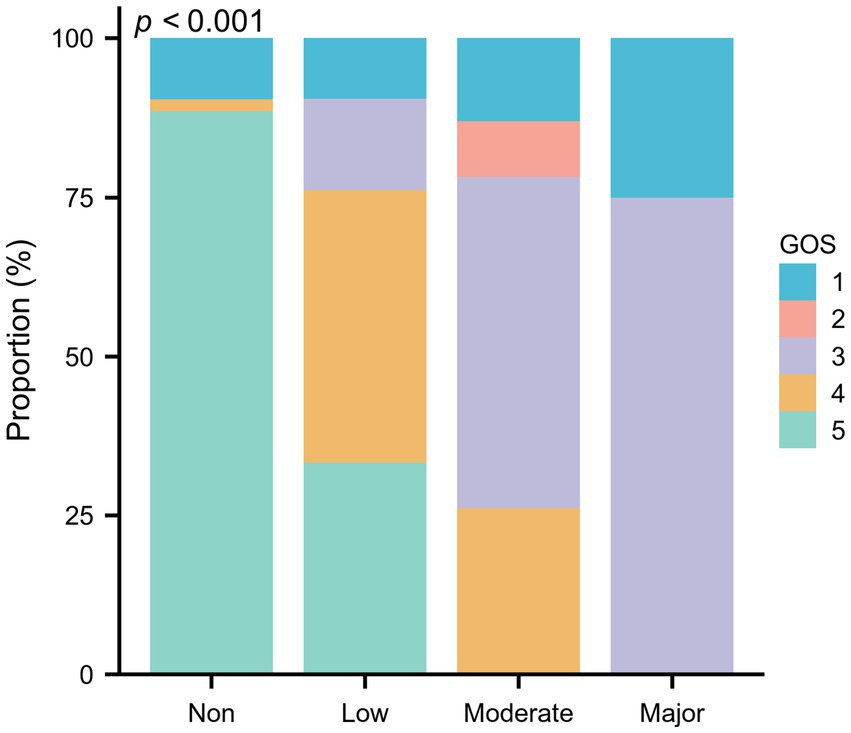
Figure 2. The proportion of GOS scores in different nutritional categories assessed by GNRI among 100 elderly patients with brain abscess.
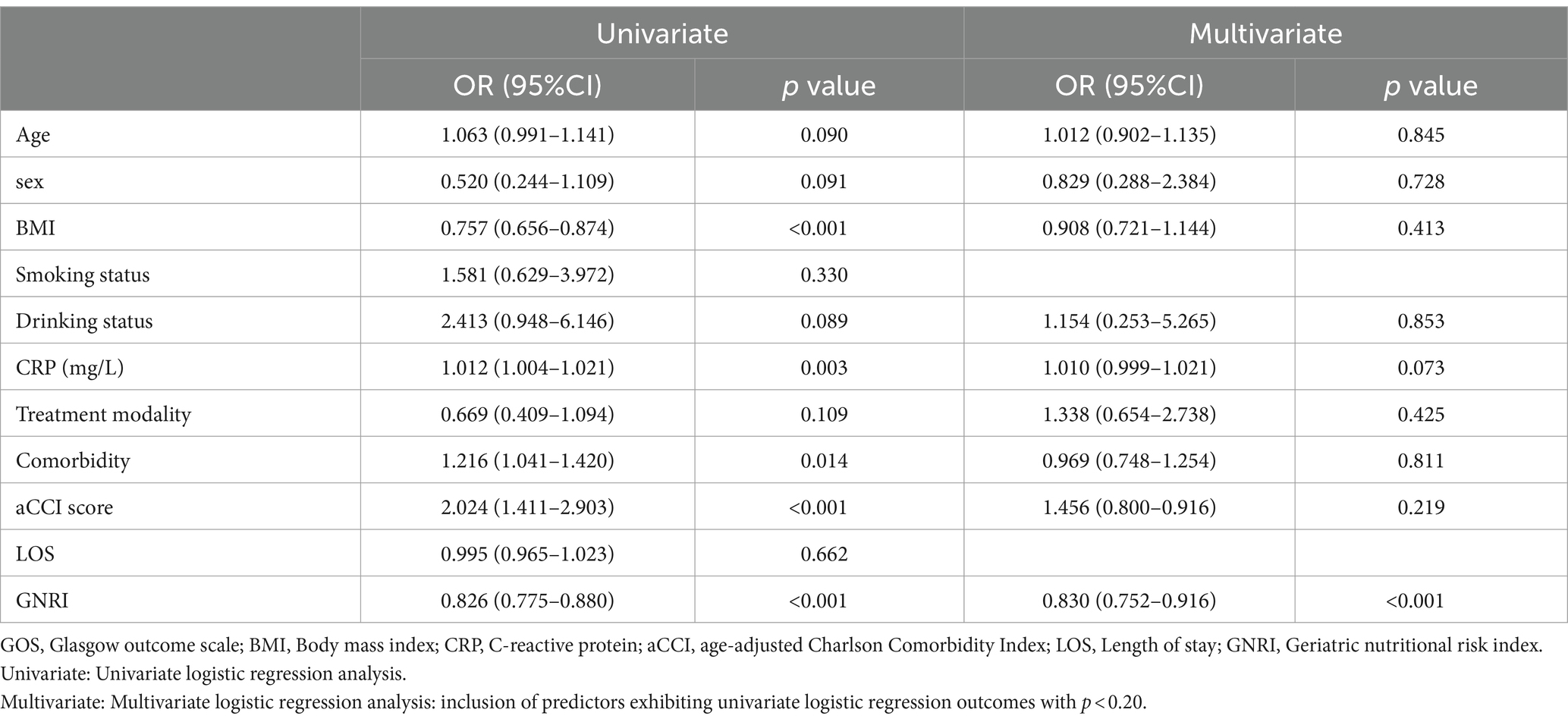
In the univariate logistic regression analyses, the association of each predictor variable with the GOS score was assessed. The results revealed no significant correlation for age or gender with the GOS score. BMI and GNRI score were significantly negatively correlated with the GOS score, whereas CRP, aCCI score, and number of comorbidities showed a positive correlation with the GOS score. In the subsequent multivariable logistic regression analysis, which incorporated variables with a p-value of less than 0.2 from the univariate analysis, GNRI was found to have a significant negative correlation with GOS score (Table 4). This highlights GNRI’s potential as a predictive factor for outcomes. Additionally, Figure 3 showed the ROC curve for GNRI in predicting poor recovery. The analysis yielded an Area Under the Curve (AUC) of 0.903, with the 95% Confidence Interval (CI) extending from 0.832 to 0.975. At the optimal threshold of 97.50, with a sensitivity of 90.57% and a specificity of 87.23% in predicting recovery outcomes.

Figure 3. ROC curve analysis of GNRI for poor recovery in the elderly hospitalized with brain abscess.
Discussion
This study represents the first study into the association between malnutrition risk, as determined by the GNRI, and clinical outcomes in older patients with brain abscess. Findings from this prospective cohort study revealed that nearly half of the older brain abscess patients exhibited malnutritional risk, which is related to their treatment modality as well. Concurrently, the GNRI displayed associations with both the quantum of comorbidities and the aCCI score, underscoring the intricate interplay between nutritional status and the aggregate health burden in this demographic. Notably, older brain abscess patients presenting with lower GNRI scores were observed to have diminished GOS scores, indicating that GNRI has a predictive impact on their clinical outcomes.
Malnutrition, often underreported and underdiagnosed, is widespread among older hospitalized patients, contributing to adverse clinical outcomes such as prolonged hospital stays and elevated mortality rates (29). The GNRI provides an efficient, objective, and age-specific approach to nutritional screening in the older (16). It assesses the nutritional risks of the older in a timely and accurate manner by utilizing current weight metrics and serum albumin levels, thereby minimizing recall bias associated with past weight changes (16). In this study, the GNRI was employed to assess the malnutrition risk in older patients with brain abscesses, revealing a significant prevalence of 48%. Similarly, Bao et al.’s study revealed that among older patients experiencing early neurological deterioration following acute ischemic stroke, 48.3% were at risk of malnutrition according to GNRI (30). In another study, the GNRI assessment revealed a malnutrition risk of approximately 42.5% (97/228) among older patients with mild traumatic brain injury (31). These results above suggest that brain injury in older patients is often accompanied by malnutrition. This high incidence of malnutrition in the older adults may be attributed to various factors. With advancing age, oral issues such as missing teeth impair chewing ability, impacting food intake and digestion (32). Moreover, older individuals frequently contend with chronic diseases like diabetes and heart disease, potentially increasing metabolic demands and subsequently heightening the risk of malnutrition (33). This study’s findings revealed a significant negative correlation between GNRI and the level of comorbidity, further substantiated that the heightened burden of chronic diseases contributes to the increased risk of malnutrition.
As an infectious disease affecting the nervous system, brain abscesses induce inflammation that escalates the body’s metabolic demands, thereby heightening nutritional requirements (1, 34). This, combined with the adverse effects of infection and pharmacological treatments on appetite, can precipitate a further decline in the nutritional status of patients with brain abscesses (34). In the correlation analysis conducted in this study, the GNRI and CRp values upon admission in older patients with brain abscesses were found to be significantly negatively correlated (p = 0.006), thereby further indicating the relation between the level of inflammation and the nutritional risk. Furthermore, we observed that patients at nutritional risk underwent more conservative treatment during hospitalization compared to their counterparts without nutritional risk (38.5% vs. 66.7%). This finding implies that the nutritional status of older patients with brain abscess may influence their treatment approach.
In clinical practice, the typical focus on infection control and acute neurological symptom management in brain abscess cases often overshadows the scrutiny of patients’ nutritional status. The findings of this study indicated that older brain abscess patients at higher malnutrition risk exhibit poorer clinical prognoses (p < 0.001). A positive correlation emerged between GNRI scores and GOS scores (p < 0.001), indicating a nutritional status may be significantly associated with recovery and long-term outcomes in brain abscess patients. Several studies also have demonstrated a correlation between elevated malnutrition risks and adverse clinical outcomes in geriatric patients (31, 35). It can be seen that in the process of diagnosis and treatment of geriatric diseases, the assessment of nutritional status cannot be ignored. However, there is no significant correlation was observed between smoking, alcohol consumption, and either the nutritional status or the GOS in elderly patients with brain abscess in this study. This lack of association is likely due to the standardized, individualized treatment these patients received, which effectively minimized the impact of lifestyle factors on their outcomes (36). The findings emphasize the importance of tailored clinical management in mitigating the potential negative effects of smoking and alcohol use in this patient population.
GNRI has been employed as an independent predictive factor for morbidity and mortality in older hospitalized patients with cancer (37). Research on older colorectal cancer patients revealed that the preoperative GNRI served as an independent prognostic factor for those who underwent curative colorectal resection (38). In another study, the GNRI has been identified as an independent predictor of prognosis and postoperative complications after radical nephroureterectomy for upper urinary tract urothelial carcinoma (39). In addition to its extensive application in forecasting postoperative complications and the long-term prognosis of tumors, the GNRI was also employed in other diseases for its prognostic significance for recuperation and clinical outcomes. A study focused on older patients with mild traumatic brain injury revealed that a higher GNRI was associated with a decreased risk of incomplete recovery at the 6-month mark. Furthermore, the ROC analysis established a GNRI threshold of 97.85 (31). And GNRI was considered a promising tool for predicting mortality outcomes in older patients with moderate to severe traumatic brain injuries (24). These findings indicated that GNRI may have applicability in diseases related to brain injury. In our multivariate regression analysis, we found that lower GNRI values were positively associated with poorer prognosis (i.e., lower GOS score). Moreover, the results of the ROC analysis, which yielded an AUC of 0.903, underscore the significant potential of GNRI in predicting the long-term outcomes for older patients with brain abscess.
Our study offered several strengths. Firstly, as a prospective cohort study, the high standardization of our data collection protocols ensures data quality, while simultaneously reducing selection and recall biases. Secondly, this is the inaugural investigation into the prevalence of nutritional risk among older patients with brain abscesses, providing a novel insight into the clinical implications of GNRI scores. Furthermore, our use of logistic regression analysis has elucidated the relationship between GNRI and the prognostic score for geriatric brain abscesses (GOS), affirming GNRI’s utility in predicting outcomes for older hospitalized patients with brain abscesses. Meanwhile, our study still has certain limitations that should be considered. The study was conducted at a single center, which may limit the generalizability of the findings to other healthcare settings or diverse patient populations. The potential for selection bias and the lack of variability in patient demographics could affect the broader applicability of our results. Although the study utilized a standard nutritional assessment, it did not include specific evaluations for macro or micronutrient deficiencies. This omission may have restricted our understanding of the detailed nutritional profiles of elderly patients with brain abscesses and their impact on treatment efficacy and recovery outcomes. Additionally, reliance on a single prognostic measure, the GOS, may not fully capture the nuanced aspects of patient recovery, suggesting a need for more diverse and sensitive outcome assessment tools in future studies. Therefore, future research should be conducted across multiple centers, incorporate larger patient samples, and integrate additional nutritional assessment methods, such as Dual-energy X-ray Absorptiometry (DXA) or Bioelectrical Impedance Analysis (BIA). To deepen our understanding of the nutritional status of older patients with brain abscess and further explore the impact of nutritional status on treatment and clinical outcomes.
This study on the nutritional status of older patients with brain abscesses revealed that nearly half are at risk of malnutrition according to the GNRI. The GNRI is correlated with the number of comorbidities, aCCI scores, and treatment modalities, underscoring the intricate relationship between nutritional status and the overall health burden in this demographic. Moreover, the study observed that lower GNRI scores in these patients were associated with decreased GOS scores, and found that GNRI is a prognostic indicator of clinical outcomes. Clinically, heightened attention is warranted for patients with GNRI scores below the critical threshold of 97.5, and prompt nutritional interventions should be administered. Consequently, we advocate regular nutritional evaluations for all older patients with brain abscesses, ensuring early intervention with nutritional therapy to preserve their nutritional health and mitigate the risk of adverse outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Huashan Hospital affiliated with Fudan University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XP: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. YZ: Data curation, Formal analysis, Writing – original draft. DJ: Data curation, Methodology, Writing – review & editing. MZ: Data curation, Methodology, Writing – review & editing. JF: Data curation, Writing – review & editing. YN: Supervision, Validation, Writing – review & editing. MT: Supervision, Visualization, Writing – review & editing. SH: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from Huashan Hospital Affiliated to Fudan University (No. Y845) and China Primary Health Care Foundation (No. GWJJ2021100301).
Acknowledgments
The authors express their gratitude to the staff responsible for data collection and patient follow-up, as well as to the participants and their families, for their invaluable cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1410483/full#supplementary-material
References
1. Brouwer, MC, Tunkel, AR, McKhann, GM 2nd, and van de Beek, D. Brain abscess. N Engl J Med. (2014) 371:447–56. doi: 10.1056/NEJMra1301635
2. Sonneville, R, Ruimy, R, Benzonana, N, Riffaud, L, Carsin, A, Tadié, JM, et al. An update on bacterial brain abscess in immunocompetent patients. Clin Microbiol Infect. (2017) 23:614–20. doi: 10.1016/j.cmi.2017.05.004
3. Kurokawa, M, Kurokawa, R, Baba, A, Kim, J, Tournade, C, McHugh, J, et al. Deadly Fungi: invasive fungal Rhinosinusitis in the head and neck. Radiographics. (2022) 42:2075–94. doi: 10.1148/rg.220059
4. Bodilsen, J, Duerlund, LS, Mariager, T, Brandt, CT, Petersen, PT, Larsen, L, et al. Clinical features and prognostic factors in adults with brain abscess. Brain. (2023) 146:1637–47. doi: 10.1093/brain/awac312
5. Bodilsen, J, Duerlund, LS, Mariager, T, Brandt, CT, Wiese, L, Petersen, PT, et al. Risk factors and prognosis of epilepsy following brain abscess: a nationwide population-based cohort study. Neurology. (2023) 100:e1611–20. doi: 10.1212/WNL.0000000000206866
6. Bodilsen, J, Dalager-Pedersen, M, van de Beek, D, Brouwer, MC, and Nielsen, H. Incidence and mortality of brain abscess in Denmark: a nationwide population-based study. Clin Microbiol Infect. (2020) 26:95–100. doi: 10.1016/j.cmi.2019.05.016
7. Bodilsen, J, Dalager-Pedersen, M, van de Beek, D, Brouwer, MC, and Nielsen, H. Long-term mortality and epilepsy in patients after brain abscess: a nationwide population-based matched cohort study. Clin Infect Dis. (2020) 71:2825–32. doi: 10.1093/cid/ciz1153
8. McMillan, T, Wilson, L, Ponsford, J, Levin, H, Teasdale, G, and Bond, M. The Glasgow outcome scale −40 years of application and refinement. Nat Rev Neurol. (2016) 12:477–85. doi: 10.1038/nrneurol.2016.89
9. Bilgin, S, Guclu-Gunduz, A, Oruckaptan, H, Kose, N, and Celik, B. Gait and Glasgow coma scale scores can predict functional recovery in patients with traumatic brain injury. Neural Regen Res. (2012) 7:1978–84. doi: 10.3969/j.issn.1673-5374.2012.25.009
10. Cooper, DJ, Rosenfeld, JV, Murray, L, Arabi, YM, Davies, AR, D'Urso, P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. (2011) 364:1493–502. doi: 10.1056/NEJMoa1102077
11. Gaviani, P, Silvani, A, Lamperti, E, Botturi, A, Simonetti, G, Milanesi, I, et al. Infections in neuro-oncology. Neurol Sci. (2011) 32:233–6. doi: 10.1007/s10072-011-0803-1
12. Muzumdar, D, Jhawar, S, and Goel, A. Brain abscess: an overview. Int J Surg. (2011) 9:136–44. doi: 10.1016/j.ijsu.2010.11.005
13. Zhang, J, Chen, Z, Xie, L, Zhao, C, Zhao, H, Fu, C, et al. Treatment of a subdural empyema complicated by intracerebral abscess due to Brucella infection. Braz J Med Biol Res. (2017) 50:e5712. doi: 10.1590/1414-431x20165712
14. Ngarka, L, Siewe Fodjo, JN, Aly, E, Masocha, W, and Njamnshi, AK. The interplay between neuroinfections, the immune system and neurological disorders: a focus on Africa. Front Immunol. (2021) 12:803475. doi: 10.3389/fimmu.2021.803475
15. Donini, LM, Poggiogalle, E, Molfino, A, Rosano, A, Lenzi, A, Fanelli, FR, et al. Mini-nutritional assessment, malnutrition universal screening tool, and nutrition risk screening tool for the nutritional evaluation of older nursing home residents. J Am Med Dir Assoc. (2016) 17:e11–8. doi: 10.1016/j.jamda.2016.06.028
16. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
17. Mochizuka, Y, Suzuki, Y, Kono, M, Hasegawa, H, Hashimoto, D, Yokomura, K, et al. Geriatric nutritional risk index is a predictor of tolerability of antifibrotic therapy and mortality risk in patients with idiopathic pulmonary fibrosis. Respirology. (2023) 28:775–83. doi: 10.1111/resp.14523
18. Song, F, Ma, H, Wang, S, Qin, T, Xu, Q, Yuan, H, et al. Nutritional screening based on objective indices at admission predicts in-hospital mortality in patients with COVID-19. Nutr J. (2021) 20:46. doi: 10.1186/s12937-021-00702-8
19. Chang, LW, Hung, SC, Li, JR, Chiu, KY, Yang, CK, Chen, CS, et al. Geriatric nutritional risk index as a prognostic marker for patients with metastatic castration-resistant prostate Cancer receiving docetaxel. Front Pharmacol. (2020) 11:601513. doi: 10.3389/fphar.2020.601513
20. Lin, TY, and Hung, SC. Geriatric nutritional risk index is associated with unique health conditions and clinical outcomes in chronic kidney disease patients. Nutrients. (2019) 11:2769. doi: 10.3390/nu11112769
21. Sun, T, Ma, M, Huang, X, Zhang, B, Chen, Z, Zhao, Z, et al. Prognostic impacts of geriatric nutritional risk index in patients with ischemic heart failure after percutaneous coronary intervention. Clin Nutr. (2023) 42:1260–7. doi: 10.1016/j.clnu.2023.05.023
22. Chai, X, Chen, Y, Li, Y, Chi, J, and Guo, S. Lower geriatric nutritional risk index is associated with a higher risk of all-cause mortality in patients with chronic obstructive pulmonary disease: a cohort study from the National Health and nutrition examination survey 2013-2018. BMJ Open Respir Res. (2023) 10:e001518. doi: 10.1136/bmjresp-2022-001518
23. Lee, JS, Choi, HS, Ko, YG, and Yun, DH. Performance of the geriatric nutritional risk index in predicting 28-day hospital mortality in older adult patients with sepsis. Clin Nutr. (2013) 32:843–8. doi: 10.1016/j.clnu.2013.01.007
24. Su, WT, Tsai, CH, Huang, CY, Chou, SE, Li, C, Hsu, SY, et al. Geriatric nutritional risk index as a prognostic factor for mortality in elderly patients with moderate to severe traumatic brain injuries. Risk Manag Healthc Policy. (2021) 14:2465–74. doi: 10.2147/RMHP.S314487
25. Tsuneyoshi, S, Matsukuma, Y, Kawai, Y, Hiyamuta, H, Yamada, S, Kitamura, H, et al. Association between geriatric nutritional risk index and stroke risk in hemodialysis patients: 10-years outcome of the Q-cohort study. Atherosclerosis. (2021) 323:30–6. doi: 10.1016/j.atherosclerosis.2021.03.006
26. Dai, C, Yan, D, Xu, M, Huang, Q, and Ren, W. Geriatric nutritional risk index is related to the risk of stroke-associated pneumonia. Brain Behav. (2022) 12:e2718. doi: 10.1002/brb3.2718
27. Bodilsen, J, D'Alessandris, QG, Humphreys, H, Iro, MA, Klein, M, Last, K, et al. European society of clinical microbiology and infectious diseases guidelines on diagnosis and treatment of brain abscess in children and adults. Clin Microbiol Infect. (2024) 30:66–89. doi: 10.1016/j.cmi.2023.08.016
28. Charlson, M, Szatrowski, TP, Peterson, J, and Gold, J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47:1245–51. doi: 10.1016/0895-4356(94)90129-5
29. Kaegi-Braun, N, Mueller, M, Schuetz, P, Mueller, B, and Kutz, A. Evaluation of nutritional support and in-hospital mortality in patients with malnutrition. JAMA Netw Open. (2021) 4:e2033433. doi: 10.1001/jamanetworkopen.2020.33433
30. Bao, Y, Zhang, Y, Du, C, Ji, Y, Dai, Y, and Jiang, W. Malnutrition and the risk of early neurological deterioration in elderly patients with acute ischemic stroke. Neuropsychiatr Dis Treat. (2022) 18:1779–87. doi: 10.2147/NDT.S366851
31. Zhu, B, Ou, Y, Guo, X, Liu, W, and Wu, L. Poor nutritional status is associated with incomplete functional recovery in elderly patients with mild traumatic brain injury. Front Neurol. (2023) 14:1131085. doi: 10.3389/fneur.2023.1131085
32. Corcoran, C, Murphy, C, Culligan, EP, Walton, J, and Sleator, RD. Malnutrition in the elderly. Sci Prog. (2019) 102:171–80. doi: 10.1177/0036850419854290
33. Mathew, AC, Das, D, Sampath, S, Vijayakumar, M, Ramakrishnan, N, and Ravishankar, SL. Prevalence and correlates of malnutrition among elderly in an urban area in Coimbatore. Indian J Public Health. (2016) 60:112–7. doi: 10.4103/0019-557X.184542
34. Katona, P, and Katona-Apte, J. The interaction between nutrition and infection. Clin Infect Dis. (2008) 46:1582–8. doi: 10.1086/587658
35. Fang, P, Yang, Q, Zhou, J, Yang, Y, Luan, S, Xiao, X, et al. The impact of geriatric nutritional risk index on esophageal squamous cell carcinoma patients with neoadjuvant therapy followed by esophagectomy. Front Nutr. (2022) 9:983038. doi: 10.3389/fnut.2022.983038
36. Jindal, A, Pandey, A, Sharma, MK, Mukund, A, Vijayaraghavan, R, Arora, V, et al. Management practices and predictors of outcome of liver abscess in adults: a series of 1630 patients from a liver unit. J Clin Exp Hepatol. (2021) 11:312–20. doi: 10.1016/j.jceh.2020.10.002
37. Hao, X, Li, D, and Zhang, N. Geriatric nutritional risk index as a predictor for mortality: a meta-analysis of observational studies. Nutr Res. (2019) 71:8–20. doi: 10.1016/j.nutres.2019.07.005
38. Hayama, T, Hashiguchi, Y, Ozawa, T, Watanabe, M, Fukushima, Y, Shimada, R, et al. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep. (2022) 12:3682. doi: 10.1038/s41598-022-07540-6
39. Wu, P, Liu, J, Wang, X, Lai, S, Wang, J, Wang, J, et al. Development and validation of a nomogram based on geriatric nutritional risk index for predicting prognosis and postoperative complications in surgical patients with upper urinary tract urothelial carcinoma. J Cancer Res Clin Oncol. (2023) 149:18185–200. doi: 10.1007/s00432-023-05462-y
Keywords: geriatric nutritional risk index, malnutrition, brain abscess, recovery outcomes, prognosis
Citation: Pei X, Zhang Y, Jiang D, Zhang M, Fu J, Niu Y, Tian M and Huang S (2024) Geriatric nutritional risk index has a prognostic value for recovery outcomes in elderly patients with brain abscess. Front. Nutr. 11:1410483. doi: 10.3389/fnut.2024.1410483
Edited by:
Miquel Martorell, Universidad de Concepción, ChileReviewed by:
Jiahe Tan, First Affiliated Hospital of Chongqing Medical University, ChinaAntoni Sureda, University of the Balearic Islands, Spain
Weiming Liu, Capital Medical University, China
Copyright © 2024 Pei, Zhang, Jiang, Zhang, Fu, Niu, Tian and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanshan Huang, cGF0cmlja3N0YXI3NkBhbHVtbmkuc2p0dS5lZHUuY24=; Mi Tian, bXRpYW4xOUBmdWRhbi5lZHUuY24=; Yang Niu, MTMwMDMxMDkwNzZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xu Pei
Xu Pei Yutu Zhang2†
Yutu Zhang2† Junyan Fu
Junyan Fu Mi Tian
Mi Tian Shanshan Huang
Shanshan Huang