
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 12 July 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1408898
 Shuai Shi1
Shuai Shi1 Qiang Fang2*
Qiang Fang2*Aim: Antioxidants diet is beneficial for the prognosis of chronic kidney disease (CKD). However, the relationship between the Dietary Antioxidant Quality Score (DAQS), a measure of overall quality on antioxidant diet, and hyperuricemia related mortality is unclear. This study aimed to investigate the relationship between the DAQS and hyperuricemia mortality in CKD patients.
Methods: In this cohort study, data were collected in the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2018. The DAQS was calculated based on the six dietary antioxidants. Mortality status were determined by NHANES-linked National Death Index public access files through December 31, 2019. Weighted Cox proportional hazard models were used to investigate the association between the DAQS and hyperuricemia related mortality.
Results: A total of 3,684 participants were included. During the median follow-up of 63.83 months, 820 deaths were recorded. The results showed that higher dietary antioxidants intake associated with lower hyperuricemia related mortality risk among CKD patients (HR = 1.28, 95%CI: 1.07 to 1.54). In subgroup analyses, the association of antioxidants intake and hyperuricemia related mortality risk remained exist in groups of aged ≥65 years (HR = 1.23, 95%CI: 1.01 to 1.52), with hypertension (HR = 1.26, 95%CI: 1.02 to 1.55), with dyslipidemia (HR = 1.30, 95%CI: 1.07 to 1.58), with CVD (HR = 1.31, 95%CI: 1.03 to 1.67), and diabetes (HR = 1.62, 95%CI: 1.24 to 2.12).
Conclusion: Higher antioxidants intake associated with lower odds of hyperuricemia related mortality in CKD patients. Future interventional studies are needed to elucidate the beneficial effect of antioxidants diets.
Chronic kidney disease (CKD), characterized by the progressive renal function decline, is a global health problem affecting millions of individuals worldwide (1). In the United States, CKD affects 37 million adults (2). CKD has continued to rise in rank among leading cause of mortality with 1.2 million global deaths attributed to CKD in 2017 (1). The globally all-age CKD mortality rate has increased by 41.5% from 1999 to 2017 (1). Therefore, accurately identifying factors affecting the prognosis of CKD is crucial for implementing reasonable intervention and reducing the disease burden.
Uric acid (UA), as an end product of purine metabolism in humans, has emerged as a potential risk factor for adverse outcomes in CKD (3). Elevated UA levels are associated with increased oxidative stress (OS) and inflammation, both of which plays a crucial role in CKD progression (4). Increased serum UA levels are associated with higher risk of all-cause and cardiovascular disease (CVD) mortality among CKD patients (5, 6). A review reported that the primary benefit of lowering serum urate is by reducing the incidence of cardiovascular events and mortality in CKD (7). Therefore, identifying strategies to mitigate the detrimental effects of UA is of paramount importance.
Medical nutrition therapy is essential for CKD patients as it can slow disease progression (2). Dietary antioxidants, which can neutralize harmful reactive oxygen species (ROS) and protect against cellular damage, have gained considerable attention for their ability to counteract OS and mitigate inflammation (8–10). An antioxidant-rich diet may confer protective effects against CKD development and progression, while moderate dietary antioxidants intake is linked to reduced mortality risk in early-stage CKD patients (11–13). The Dietary Antioxidant Quality Score (DAQS) is a comprehensive measure that assesses the overall quality of antioxidant intake from dietary sources. The DAQS considers various antioxidants including vitamin A, vitamin C, vitamin E, zinc, magnesium, and selenium (14), providing a quantitative assessment of antioxidant intake. The DAQS has been used to evaluate the association between antioxidant intake and various health outcomes, such as diabetes (14), metabolic syndrome (15), and systemic lupus erythematosus (16). However, the relationship between dietary antioxidant intake and hyperuricemia-related mortality in CKD remains unknown. Therefore, this study aims to investigate the association of dietary antioxidant intake, hyperuricemia, with mortality in CKD patients and further explore the ameliorative effect of antioxidant intake on the relationship between hyperuricemia and all-cause mortality.
The study population of this cohort study were extracted from the National Health and Nutrition Examination Surveys (NHANES) (2009–2018). NHANES, major program of the National Center for Health Statistics (NCHS), is designed to assess the health and nutritional status of adults and children in the United States, with combined interviews and physical examinations.
Participants with CKD were included from the database. CKD was defined as urinary albumin to creatinine ratio (UACR) >30 mg/g and/or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 according to the “KDIGO 2021 Guidelines” (17). Urinary albumin was measured by solid-phase fluorescent immunoassay. And eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for standardized creatinine (18). The equation is eGFR (mL/min/1.73m2) = 141 × min (Scr/κ, 1)α × max (Scr/κ, 1)-1.209 × 0.933age × 1.108 (if female) × 1.159 (if black). κ is 0.7 for females and 0.9 for males, α is −0.329 for females and − 0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1. Exclusion criteria were as follows: (1) <18 years old, (2) missing data on uric acid, (3) missing data on energy intake, (4) with implausible energy intake (<500 kcal or > 8,000 kcal in male or < 500 kcal or > 5,000 kcal in female), and (5) missing survival information. The NHANES protocol was approved by the NCHS Research Ethics Review Board and all participants signed an informed consent.
UA in serum was measured by using a timed endpoint method based on Beckman Coulter UniCel® DxC800 (19). Hyperuricemia was defined as serum UA level > 7.0 mg/dL in males and > 6.0 mg/dL in females (20).
The DAQS were calculated based on six antioxidant vitamins and minerals, including vitamin A, C, E, zinc, magnesium, and selenium. A 24-h dietary interview was conducted by trained interviewers to collect data on dietary intake of six dietary antioxidant micronutrients. The daily intakes for each antioxidant were calculated as the sum of dietary and supplement intake. For the DAQS, daily nutrient intake of each of six nutrients/minerals were compared with their respective daily recommended intake (RDI) as determined by the Dietary Guidelines for Americans 2015–2020 (14). Then, each antioxidant vitamin/mineral was assigned a value of either 0 or 1, that 0 defined as intake of <2/3 of the RDI and 1 defined as intake ≥2/3 of the RDI. The summed DAQS ranged from 0 (very poor quality) to 6 (high quality). Then, the DAQS was classified into two groups: 1–4 (low quality) and 5–6 (high quality).
Potential covariates were considered in this study. Included covariates were as follows: age, gender, race, marital status, poverty income ratio (PIR), smoking, CVD, diabetes, hemoglobin A1c (HbA1c), alkaline phosphatase (ALP), and asparate aminotransferase (AST). Information on age, gender, race, marital status, PIR, smoking, disease status and medication use was collected from household interviews using standardized questionnaires. Smoking was defined as participants who had a positive answer to the question “Smoked at least 100 cigarettes in life” (21). CVD was determined by a combination of self-reported physician diagnoses and cardiovascular medication usage. Diabetes was defined as meeting any of the following criteria: self-report of a diagnosis by a doctor or other health care professional, HbA1c ≥6.5% or fasting plasma glucose ≥7.0 mmol/L, and taking hypoglycemic medications and/or insulin (22). In addition, HbA1c, ALT and AST were measured when participants provided their blood samples. Details about procedure of blood collection and analysis were described in the NHANES Laboratory/Medical Technologists Procedures Manual (23).
The outcome of our research was all-cause mortality, defined as death from any cause. All-cause mortality was extracted from the National Death Index (NDI) database of the Centers for Disease Control through December 31, 2019. All data in this study were available.1 Follow-up time was defined from the data of participation to the data of death on December 31, 2019, whichever came first.
Data were analyzed based on the prescribed guidelines for analysis of complex NHANES data set, taking into account the masked variance and utilizing the proposed weighting methodology (24). Continuous variables were presented as mean and standard error (S.E), while categorical variables were presented as frequency and percentage (%). Groups different among continuous and categorical variables were compared using the weighted t tests and chi-square tests, respectively. Confounders were selected for variables with statistical differences using a weighted univariate Cox proportional hazard model. The association between DAQS and UA related mortality was analyzed by weighted univariate and multivariable Cox proportional hazard models. Covariates were adjusted for age, gender, race, marital status, PIR, smoking, CVD, diabetes, HbA1c, ALP, and AST. Subgroup analyses were performed to further investigate the association between DAQS and UA related mortality in groups among age, hypertension, dyslipidemia, CVD, diabetes and CKD stage. Furthermore, imputations were performed for missing variables. p < 0.05 was considered statistically significant. All statistical analyses were conducted by using SAS 9.4 (SAS Institute Inc., Cary, NC, United States) and R software (version 4.2.2), while missing variates were performed by Python (version 3.9.12).
In total, 4,624 participants in database from 2009 to 2018 were CKD. First, individuals were excluded with aged younger than 18 years (n = 527) and without uric acid information (n = 2), and total number of people was 4,095. In addition, individuals without energy intake information (n = 357) and with implausible energy intake (n = 49). Then, individuals missing survival information were excluded (n = 5). Finally, 3,684 participants were enrolled in the final analysis. Figure 1 shows the flow diagram of participants selection. After imputation, significant difference was not observed among missing values (Supplementary Table S1). A total of 820 deaths were identified during a follow-up period of 63.83 months. And 21 deaths due to renal disease. As shown in Table 1, the mean age was 58.94 years in this population. Among the group of alive, participants had higher education level, more physical exercises, less drinkers, and less comorbidities including CVD, diabetes and cancer. Participants dead were composed of more people who were older, who had high UA level, who were severe or end stage of CKD, and who were dyslipidemia and hypertension comorbidities.
The relation between UA and all-cause mortality was observed in Table 2. The risk of all-cause mortality was increased in the population with hyperuricemia (HR = 1.20, 95%CI: 1.01 to 1.41). Table 3 shows the association of overall antioxidants intake with UA related mortality. After adjusting age, gender, race, marital status, PIR, smoking, CVD, diabetes, hemoglobin, ALP, and AST, the lower DAQS was associated with increased risk of all-cause mortality in hyperuricemia population (HR = 1.28, 95%CI: 1.07 to 1.54). In participants with higher DAQS, the association of hyperuricemia with all-cause mortality was not found (HR = 1.07, 95%CI: 0.76 to 1.50). Higher antioxidants intake may ameliorate the risk of hyperuricemia related mortality.
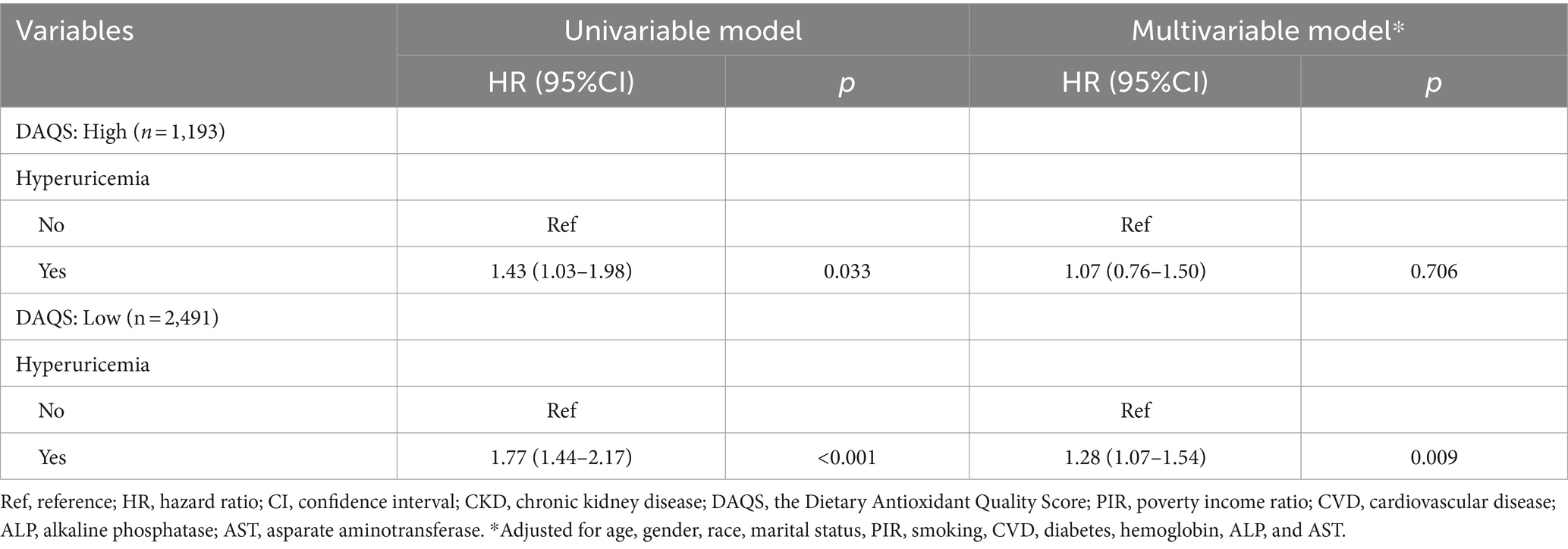
Table 3. Associations between dietary antioxidants intake and hyperuricemia related mortality in CKD patients.
In order to investigate whether patient characteristics and comorbidities could influence the association between DAQS and hyperuricemia related mortality, subgroup analyses were performed. Patients with lower DAQS were associated with hyperuricemia related mortality in subgroups. The association was observed among participants aged ≥65 years (HR = 1.23, 95%CI: 1.01 to 1.52), with hypertension (HR = 1.26, 95%CI: 1.02 to 1.55), with dyslipidemia (HR = 1.30, 95%CI: 1.07 to 1.58), with CVD (HR = 1.31, 95%CI: 1.03 to 1.67), and diabetes (HR = 1.62, 95%CI: 1.24 to 2.12). Notably, this association was observed in all stage of CKD (see Table 4).
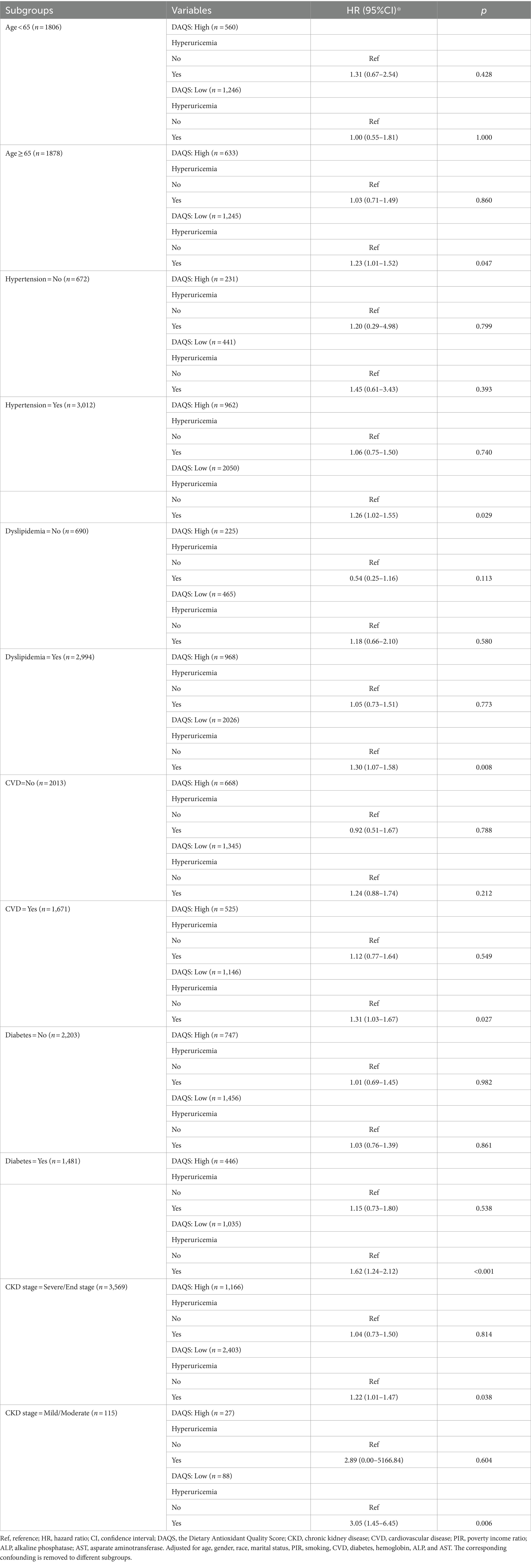
Table 4. Association between DAQS and hyperuricemia related mortality in subgroups of age, hypertension, dyslipidemia, CVD, diabetes, and CKD stage.
In present study, the relationship was investigated between antioxidants intake and odds of hyperuricemia related mortality. After adjusted covariates, who found higher DAQS was associated with lower hyperuricemia related mortality in CKD patients. And subgroup analysis showed that this association was consistent across various subgroups, including individuals aged ≥65 years, individuals with hypertension, dyslipidemia, CVD and diabetes. These results suggested that higher antioxidants intake may facilitate the prognosis among CKD, and reduce odds of hyperuricemia related mortality in CKD patients (see Figure 2).

Figure 2. Association between dietary antioxidants intake and hyperuricemia related mortality by age, hypertension, dyslipidemia, CVD, diabetes and CKD stage among CKD patients. CVD, cardiovascular; CKD, chronic kidney disease.
Moderate dietary antioxidants intake has shown potential benefit for CKD patients (13). UA was associated with adverse outcomes in patients with CKD (3, 6, 25). Excess UA could active OS, and antioxidants intake has a positive effect on hyperuricemia (26). By higher antioxidants intake, the deleterious synergistic effects of hyperuricemia and OS were counteracted, ultimately improving the prognosis of patients with CKD. In subgroup analysis, we also observed the association between low DAQS and hyperuricemia related mortality among elderly patients and patients with comorbidities. These results are consistent with previous studies (13, 14). It may be because these subgroups of individuals have higher levels of OS, and are more sensitive to the effects of exogenous dietary antioxidant intake. And our study shows a potential ameliorative effect of antioxidants intake on the odds of hyperuricemia related mortality among different CKD stages. OS was present in the early stages of CKD and progressed with worsening renal function, and was more severe in end-stage renal disease patients with hemodialysis (25).
The mechanisms underlying the ameliorative effect of dietary antioxidants intake on the risk of hyperuricemia related mortality in CKD patients could be explained through several pathways. Firstly, dietary antioxidants play a crucial role in counteracting OS by neutralizing ROS and protecting against cellular damage (25, 27). Antioxidants, such as vitamin A, C, and E, as well as minerals like zinc, selenium, and magnesium, scavenge free radicals and inhibit oxidative damage (13). Inadequate antioxidants intake may lead to a diminished antioxidant capacity, rendering CKD patients more susceptible to OS-induced damage. This imbalance between ROS production and antioxidant defense mechanisms can further exacerbate the pro-inflammatory state and endothelial dysfunction commonly observed in CKD (28). As a consequence, the increased OS may contribute to the progression of renal dysfunction, cardiovascular complications, and ultimately, mortality in CKD patients. Secondly, it is worth considering the interplay between UA and inflammation in CKD patients. Hyperuricemia has been associated with increased levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (29, 30). These inflammatory mediators can further stimulate UA production, creating a vicious cycle of inflammation and hyperuricemia (4). Inflammatory plays a pivotal role in the pathogenesis of CKD, promoting renal fibrosis, endothelial dysfunction, and CVD (31–33). Inflammatory milieu, combined with hyperuricemia, may have synergistic effects on CKD progression and mortality. Thirdly, it is important to consider the potential impact of dietary antioxidants on UA metabolism. Antioxidants, particularly vitamin C, have been shown to enhance UA excretion by stimulating renal urate transporters (34). Inadequate antioxidants intake may impair this excretion process, leading to UA accumulation and subsequently hyperuricemia. Additionally, antioxidants can inhibit xanthine oxidase (XO), the enzyme responsible for UA production, thereby reducing UA levels (35). Insufficient antioxidants intake may result in increased XO activity, promoting UA synthesis and exacerbating hyperuricemia. These mechanisms further support the association between lower antioxidants intake and hyperuricemia-related mortality in CKD patients.
The clinical importance of our findings lies in the potential for dietary interventions to modulate the risk of hyperuricemia related mortality in CKD patients. Encouraging patients to consume a diet rich in antioxidants, including fruits, vegetables, whole grains, and legumes, may offer a practical and cost-effective approach to improve prognosis in patients with high risk. Moreover, it highlights the importance of considering individual patient characteristics, such as age, comorbidities, and CKD stage, when tailoring dietary recommendations to optimize antioxidant intake.
There are several advantages of our study. The nationally representative sample and long-term follow-up afforded substantial power to detect association between dietary antioxidants intake and hyperuricemia related mortality risk. However, the study still has several limitations. First, dietary information was collected using a 24-h dietary recall, which may introduce recall bias and may not accurately represent usual dietary intake. Second, several potential factors may contribute to variability in serum UA levels, including dietary factors, medication use, renal function, genetic factors, and lifestyle factors such as alcohol consumption and physical activity. Additionally, laboratory methods for UA measurement can also contribute to variability. These suggested that potential factors should be considered in the interpretation of the study findings and their impact on the reliability of serum UA levels. Third, only all-cause mortality was investigated in the current study. As lower incidence of specific mortality, which made it’s impossible to investigate the association between dietary antioxidants intake and specific mortality.
Antioxidants intake may have an ameliorative effect on the risk of hyperuricemia related mortality in CKD. Higher antioxidants intake reduced the risk of hyperuricemia related mortality in CKD patients. The findings highlight the clinical importance of promoting antioxidant-rich diets as part of the comprehensive management of CKD patients. Potential factors may contribute to variability in serum UA level, these should be considered for the explaining of the study. Future longitudinal and causal studies are required to validate our findings and explore the optimal strategies for implementing antioxidants interventions in CKD patients.
Publicly available datasets were analyzed in this study. This data can be found here: NHANES, https://www.cdc.gov/nchs/nhanes/.
The requirement of ethical approval was waived by the Affiliated Taizhou People's Hospital of Nanjing Medical University for the studies involving humans because the Affiliated Taizhou People's Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SS: Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. QF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1408898/full#supplementary-material
1. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Lond Engl. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Naber, T, and Purohit, S. Chronic kidney disease: role of diet for a reduction in the severity of the disease. Nutrients. (2021) 13:3277. doi: 10.3390/nu13093277
3. Hisatome, I, Li, P, Miake, J, Taufiq, F, Mahati, E, Maharani, N, et al. Uric acid as a risk factor for chronic kidney disease and cardiovascular disease - Japanese guideline on the Management of Asymptomatic Hyperuricemia. Circ J Off J Jpn Circ Soc. (2021) 85:130–8. doi: 10.1253/circj.CJ-20-0406
4. Gherghina, M-E, Peride, I, Tiglis, M, Neagu, TP, Niculae, A, and Checherita, IA. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci. (2022) 23:3188. doi: 10.3390/ijms23063188
5. Luo, Q, Xia, X, Li, B, Lin, Z, Yu, X, and Huang, F. Serum uric acid and cardiovascular mortality in chronic kidney disease: a meta-analysis. BMC Nephrol. (2019) 20:18. doi: 10.1186/s12882-018-1143-7
6. Xia, X, Luo, Q, Li, B, Lin, Z, Yu, X, and Huang, F. Serum uric acid and mortality in chronic kidney disease: a systematic review and meta-analysis. Metabolism. (2016) 65:1326–41. doi: 10.1016/j.metabol.2016.05.009
7. Ejaz, AA, Nakagawa, T, Kanbay, M, Kuwabara, M, Kumar, A, Garcia Arroyo, FE, et al. Hyperuricemia in kidney disease: a major risk factor for cardiovascular events, vascular calcification, and renal damage. Semin Nephrol. (2020) 40:574–85. doi: 10.1016/j.semnephrol.2020.12.004
8. Deledda, A, Annunziata, G, Tenore, GC, Palmas, V, Manzin, A, and Velluzzi, F. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxid Basel Switz. (2021) 10:708. doi: 10.3390/antiox10050708
9. Serafini, M, and Peluso, I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr Pharm Des. (2016) 22:6701–15. doi: 10.2174/1381612823666161123094235
10. Thomas, MS, Calle, M, and Fernandez, ML. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv Nutr Bethesda Md. (2023) 14:44–54. doi: 10.1016/j.advnut.2022.10.002
11. Tsubota-Utsugi, M, Satoh, M, Watanabe, J, Takebayashi, J, Oki, T, Tatsumi, Y, et al. Association between an antioxidant-rich Japanese diet and chronic kidney disease: the Ohasama study. J Atheroscler Thromb. (2024) 31:64423:461–77. doi: 10.5551/jat.64423
12. Wang, M, Huang, Z-H, Zhu, Y-H, He, P, and Fan, Q-L. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011-2018. Food Funct. (2023) 14:9279–86. doi: 10.1039/d3fo01157g
13. Li, Y, Ling, G-C, Ni, R-B, Ni, S-H, Sun, S-N, Liu, X, et al. Association of dietary total antioxidant capacity with all-cause and cardiovascular mortality in patients with chronic kidney disease: based on two retrospective cohort studies of NHANES. Ren Fail. 45:2205950. doi: 10.1080/0886022X.2023.2205950
14. Wang, W, Wang, X, Cao, S, Duan, Y, Xu, C, Gan, D, et al. Dietary antioxidant indices in relation to all-cause and cause-specific mortality among adults with diabetes: a prospective cohort study. Front Nutr. (2022) 9:849727. doi: 10.3389/fnut.2022.849727
15. Shahavandi, M, Shahinfar, H, Payande, N, Sheikhhossein, F, Djafarian, K, and Shab-Bidar, S. The association between dietary antioxidant quality score with metabolic syndrome and its components in Iranian adults: a cross-sectional study. Food Sci Nutr. (2021) 9:994–1002. doi: 10.1002/fsn3.2067
16. Pocovi-Gerardino, G, Correa-Rodríguez, M, Rubio, J-LC, Fernández, RR, Ortego-Centeno, N, and Rueda-Medina, B. Diet quality and high-sensitivity C-reactive protein in patients with systemic lupus erythematosus. Biol Res Nurs. (2019) 21:107–13. doi: 10.1177/1099800418803176
17. Rovin, BH, Adler, SG, Barratt, J, Bridoux, F, Burdge, KA, Chan, TM, et al. KDIGO 2021 clinical practice guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
18. Deng, Y, Zhao, Q, and Gong, R. Association between metabolic associated fatty liver disease and chronic kidney disease: a cross-sectional study from NHANES 2017–2018. Diabetes Metab Syndr Obes Targets Ther. (2021) 14:1751–61. doi: 10.2147/DMSO.S292926
19. NHANES (2009-2010) Standard biochemistry profile data documentation, codebook, and frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/BIOPRO_F.htm (Accessed October 31, 2023).
20. Tan, Y, Fu, Y, Yao, H, Wu, X, Yang, Z, Zeng, H, et al. Relationship between phthalates exposures and hyperuricemia in U.S. general population, a multi-cycle study of NHANES 2007-2016. Sci Total Environ. (2023) 859:160208. doi: 10.1016/j.scitotenv.2022.160208
21. Wang, Y, Zhu, Y, Chen, Z, Chen, S, Fu, G, and Fu, J. Association between electronic cigarettes use and whole blood cell among adults in the USA-a cross-sectional study of National Health and nutrition examination survey analysis. Environ Sci Pollut Res Int. (2022) 29:88531–9. doi: 10.1007/s11356-022-21973-6
22. Xu, F, Earp, JE, Adami, A, Weidauer, L, and Greene, GW. The relationship of physical activity and dietary quality and diabetes prevalence in US adults: findings from NHANES 2011–2018. Nutrients. (2022) 14:3324. doi: 10.3390/nu14163324
23. Wan, Z, Guo, J, Pan, A, Chen, C, Liu, L, and Liu, G. Association of Serum 25-Hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care. (2021) 44:350–7. doi: 10.2337/dc20-1485
24. NAMCS/NHAMCS - Survey Methods and Analytic Guidelines. (2019) https://www.cdc.gov/nchs/ahcd/survey_methods.htm (Accessed November 3, 2023).
25. Roumeliotis, S, Roumeliotis, A, Dounousi, E, Eleftheriadis, T, and Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: a review. Nutrients. (2019) 11:1911. doi: 10.3390/nu11081911
26. Lin, Z, Chen, H, Lan, Q, Chen, Y, Liao, W, and Guo, X. Composite dietary antioxidant index is negatively associated with hyperuricemia in US adults: an analysis of NHANES 2007–2018. Int J Endocrinol. (2023) 2023:6680229–12. doi: 10.1155/2023/6680229
27. Roumeliotis, S, Roumeliotis, A, Gorny, X, and Mertens, PR. Could antioxidant supplementation delay progression of cardiovascular disease in end-stage renal disease patients? Curr Vasc Pharmacol. (2021) 19:41–54. doi: 10.2174/1570161118666200317151553
28. Andrade-Sierra, J, Pazarín-Villaseñor, L, Yanowsky-Escatell, FG, Díaz-de la Cruz, EN, García-Sánchez, A, Cardona-Muñoz, EG, et al. The influence of the severity of early chronic kidney disease on oxidative stress in patients with and without type 2 diabetes mellitus. Int J Mol Sci. (2022) 23:11196. doi: 10.3390/ijms231911196
29. Ren, Q, Tao, S, Guo, F, Wang, B, Yang, L, Ma, L, et al. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomed Int J Phytother Phytopharm. (2021) 87:153552. doi: 10.1016/j.phymed.2021.153552
30. Lv, Z, Cui, J, and Zhang, J. Associations between serum urate and telomere length and inflammation markers: Evidence from UK biobank cohort. Front Immunol. (2022) 13:1065739. doi: 10.3389/fimmu.2022.1065739
31. Kalantar-Zadeh, K, Jafar, TH, Nitsch, D, Neuen, BL, and Perkovic, V. Chronic kidney disease. Lancet Lond Engl. (2021) 398:786–802. doi: 10.1016/S0140-6736(21)00519-5
32. Zhu, Q, Chen, Y, Cai, X, Cai, L, Hong, J, Luo, Q, et al. The non-linear relationship between triglyceride-glucose index and risk of chronic kidney disease in hypertensive patients with abnormal glucose metabolism: a cohort study. Front Med. (2022) 9. doi: 10.3389/fmed.2022.1018083
33. Wang, J, Liu, X, Pan, D, Cai, X, Xue, Y, and Huang, J. Chronic kidney disease in the shadow of COVID-19: insights from the bibliometric analysis. Int Urol Nephrol. (2024) 56:683–97. doi: 10.1007/s11255-023-03706-x
34. Torralba, KD, De Jesus, E, and Rachabattula, S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: current and future directions. Int J Rheum Dis. (2012) 15:499–506. doi: 10.1111/1756-185X.12010
Keywords: chronic kidney disease, antioxidant dietary, hyperuricemia, mortality, NHANES
Citation: Shi S and Fang Q (2024) The association between dietary antioxidant quality score and uric acid related mortality in patients with chronic kidney disease. Front. Nutr. 11:1408898. doi: 10.3389/fnut.2024.1408898
Received: 29 March 2024; Accepted: 01 July 2024;
Published: 12 July 2024.
Edited by:
Akio Shimizu, The University of Nagano, JapanReviewed by:
Alessandro De Oliveira, Universidade Federal de São João del-Rei, BrazilCopyright © 2024 Shi and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Fang, cWlhbmdmYW5nXzA2MDVAb3V0bG9vay5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.