
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 01 May 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1395362
This article is part of the Research Topic Nutrition and Metabolism in Cancer: Role in Prevention and Prognosis View all 30 articles
 Mengqi Zhang1†
Mengqi Zhang1† Jia Wang2†
Jia Wang2† Xiaoxuan Li1
Xiaoxuan Li1 Lihua Zhang3
Lihua Zhang3 Yixuan Zhang3
Yixuan Zhang3 Zengjin Wen3
Zengjin Wen3 Jiaqi Zhang3
Jiaqi Zhang3 Yuchen Fan3
Yuchen Fan3 Zhenkang Qiu4*
Zhenkang Qiu4*Background: The association between Body Mass Index (BMI), frailty index (FI), and dietary supplement in cancer survivors has been a subject of growing interest. This study investigates the relationship of BMI and FI with mortality in American cancer survivors and explores the impact of dietary supplement usage on different BMI and FI groups.
Methods: Three thousand nine hundred and thirty-two cancer patients from the National Health and Nutrition Examination Survey (NHANES) database were included in the analyses. BMI, FI, and supplement usage were obtained through the NHANES structured survey and the 49-item FI tool. Weighted logistic and Cox proportional hazards models, Kaplan–Meier survival analyses, and propensity score matching (PSM) were used to elucidate the relationships between BMI, FI, dietary supplement, and mortality outcomes.
Results: The study found significant associations between higher BMI and increased frailty (Odds ratio [OR] = 1.04, 95% confidence interval [95% CI], 1.02–1.06). BMI < 25 kg/m2 and FI > 0.2 are associated with an increased mortality rate. Dietary supplement use can reduce all-cause and cancer mortality in cancer patients with BMI < 25 kg/m2 (Hazard ratio [HR] = 0.63, 95% CI, 0.47–0.84; HR = 0.48, 95% CI, 0.29–0.80) or FI ≤ 0.2 (HR = 0.77, 95% CI, 0.60–0.99; HR = 0.59, 95% CI, 0.39–0.89). In cancer patients with BMI < 25 kg/m2 and FI ≤ 0.2, dietary supplement users had lower all-cause and cancer mortality (HR = 0.49, 95% CI, 0.30–0.79; HR = 0.25, 95% CI, 0.10–0.60).
Conclusion: The study revealed a negative correlation between BMI and the FI among the cancer patient cohort as well as their complex impact on mortality and highlighted the role of dietary supplement in cancer prognosis, indicating benefits for non-frail patients with BMI < 25 kg/m2.
In 2022, there were over 20.0 million new cancer cases and nearly 9.7 million cancer-related deaths all over the world, making it one of the leading causes of death globally (1). The burden of cancer is substantial and continuously evolving, with prevalence and mortality rates influenced by a myriad of factors including genetic susceptibility, infections, tobacco, alcohol, radiation, dietary habits, lifestyles, and other environmental exposures (2–4). Central to improving cancer outcomes is the identification and understanding of prognostic factors (5).
Body Mass Index (BMI) is widely recognized in clinical settings for its utility in evaluating the general health and nutritional status of patients (6–16). Studies have demonstrated mixed impacts of BMI on cancer patient survival, making it a controversial component in the holistic management of cancer patients (10–16). Beyond BMI, frailty is increasingly recognized in guiding healthcare and predicting clinical outcomes of patients, particularly among the elderly. It represents an individual’s health status and capacity to withstand stressors such as illness or treatment (17). The frailty index (FI) has been identified as a significant predictor of prognosis in many diseases, such as cardiovascular diseases, respiratory illnesses, infections, and cerebrovascular diseases (18–20). Additionally, the role of dietary supplement in the management and prognosis of cancer patients is increasingly being recognized (21–23).
The primary objective of this study is to investigate the associations between BMI, FI, and mortality in a cohort of American cancer patients from the National Health and Nutrition Examination Survey (NHANES) and explore the impact of dietary supplement usage on survival outcomes within different contexts of BMI and FI.
This research encompasses the analysis of the NHANES database from 1999 to 2018, which is a comprehensive data collection representing the non-institutionalized, civilian population of the United States through a national, multistage, stratified, clustered probability sampling approach. The National Center for Health Statistics Ethics Review Board sanctioned the survey, and all participants provided their written consent.
The analysis incorporated data from ten NHANES cycles spanning the years 1999 to 2018, initially involving 101,316 individuals. Exclusions were made for 96,150 individuals due to a lack of self-reported cancer history, 304 individuals due to absent follow-up information, three individuals missing data on dietary supplement, and 927 individuals with incomplete data on other variables. Finally, the study included 3,932 participants for the final analysis. The selection process is depicted in Figure 1.
BMI was determined by the ratio of weight in kilograms (kg) to the square of height in meters (m2). Based on BMI, participants were classified into three categories: normal weight (BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obese (BMI ≥ 30 kg/m2). The FI comprised 49 items spanning multiple systems, including cognition, dependence, depression, comorbidities, hospital utilization and general health, physical performance, and anthropometry and laboratory values. The FI score is the ratio of observed deficits to the total possible deficits (24). Details of the FI components and their scoring are available in Supplementary Table S1. Individuals who answered “yes” to the question “Have you used or taken any vitamins, minerals, or other dietary supplement in the past month” in the NHANES survey were categorized as dietary supplement users.
The National Center for Health Statistics (NCHS) provided public use linked mortality files. Mortality status was ascertained by linking the unique study identifier with the National Death Index (last followed up on 31 December 2019, updated in 2022). Causes of death were determined according to the International Statistical Classification of Diseases and Related Health Problems (ICD), tenth revision. This classification system was used to classify cases based on the information on the major cause of death (ICD-10). The main findings of this study were mortality from all-cause, and cancer (codes C00–C97).
Detailed information on covariates includes age, gender, race/ethnicity, education level (grades 0–12, high school graduate/GED, some college or above), marital status, smoking, alcohol consumption, Healthy Eating Index-2015 (HEI-2015), physical activity, hypertension, hyperlipidemia and diabetes history, and Charlson Comorbidity Index (CCI). Hypertension was defined as mean systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, a self-reported diagnosis of hypertension, and/or the use of antihypertensive medication. Hyperlipidemia was defined as triglyceride ≥150 mg/dL, and/or total cholesterol ≥200 mg/dL, and/or low-density lipoprotein ≥130 mg/dL, and/or high-density lipoprotein <40 mg/dL in males or < 50 mg/dL in females, and/or the use of lipid-lowering drug. Diabetes was defined as HbA1c ≥ 6.5%, a self-reported diagnosis of diabetes, and/or the use of anti-diabetic medication. CCI was calculated according to questionnaire survey and examination (25).
All statistical analyses were conducted by NHANES analysis and reporting criteria. The variance inflation factor (VIF) was applied to assess multicollinearity, with a VIF value above 10 indicating significant multicollinearity (26). The analysis revealed no substantial multicollinearity within this study (Supplementary Table S2).
The accumulation of person-years started from the date of enrollment until the date of either death or censoring. Restricted cubic splines, incorporating three knots located at the 5th, 50th, and 95th percentiles, were employed to model non-linear relationships within the data. Additionally, a likelihood ratio test was conducted to compare the model that includes both linear and cubic spline terms against a model featuring only a linear term. Three weighted logistics regression models were utilized to explore the association between BMI and FI. Three weighted Cox proportional hazard models were constructed to study the relationships between dietary supplement and mortality. Kaplan–Meier survival analyses were utilized to investigate the survival differences in different BMI and FI groups. To further ensure the robustness of the findings, four sensitivity analyses were conducted. Firstly, a propensity score matching (PSM) analysis at a 1:1 ratio was implemented to equate differences between users and non-users of dietary supplement. To conduct sensitivity analyses to access the stability of the results, individuals who were under 65 years old, over 80 years old, had a BMI less than 18.5 kg/m2, or died within 2 years of follow-up were excluded separately. Additionally, the relationship between dietary supplement usage and mortality was reevaluated without considering the complexity of the sampling design.
All statistical assessments were performed using a two-sided approach, with a p-value <0.05 denoting statistical significance. The analyses were conducted using R 4.3.1 software.
Table 1 presents the study population’s baseline characteristics according to the use of dietary supplement in the past 30 days. The weighted mean age of the study population was 61.77 years (confidence interval [95% CI], 61.40–62.14 years), and 2,016 participants were females (weighted percentage [WP], 54.92%). Dietary supplement users were more likely to be younger, male, non-White, married, and current smokers, and have a lower level of educational attainment, HEI-2015, and physical activity (all p-value <0.05). There were 1,101 (WP, 29.29%), 1,410 (WP, 35.55%), and 1,421 (WP, 35.16%) participants in the BMI < 25 kg/m2, 25 ≤ BMI < 30 kg/m2, and BMI ≥ 30 kg/m2, respectively, and 2,145 (WP, 62.75%) and 1,787 (WP, 37.25%) in the non-frail and frail groups, respectively, all of which were not statistically significant between the dietary supplement users and non-users groups.
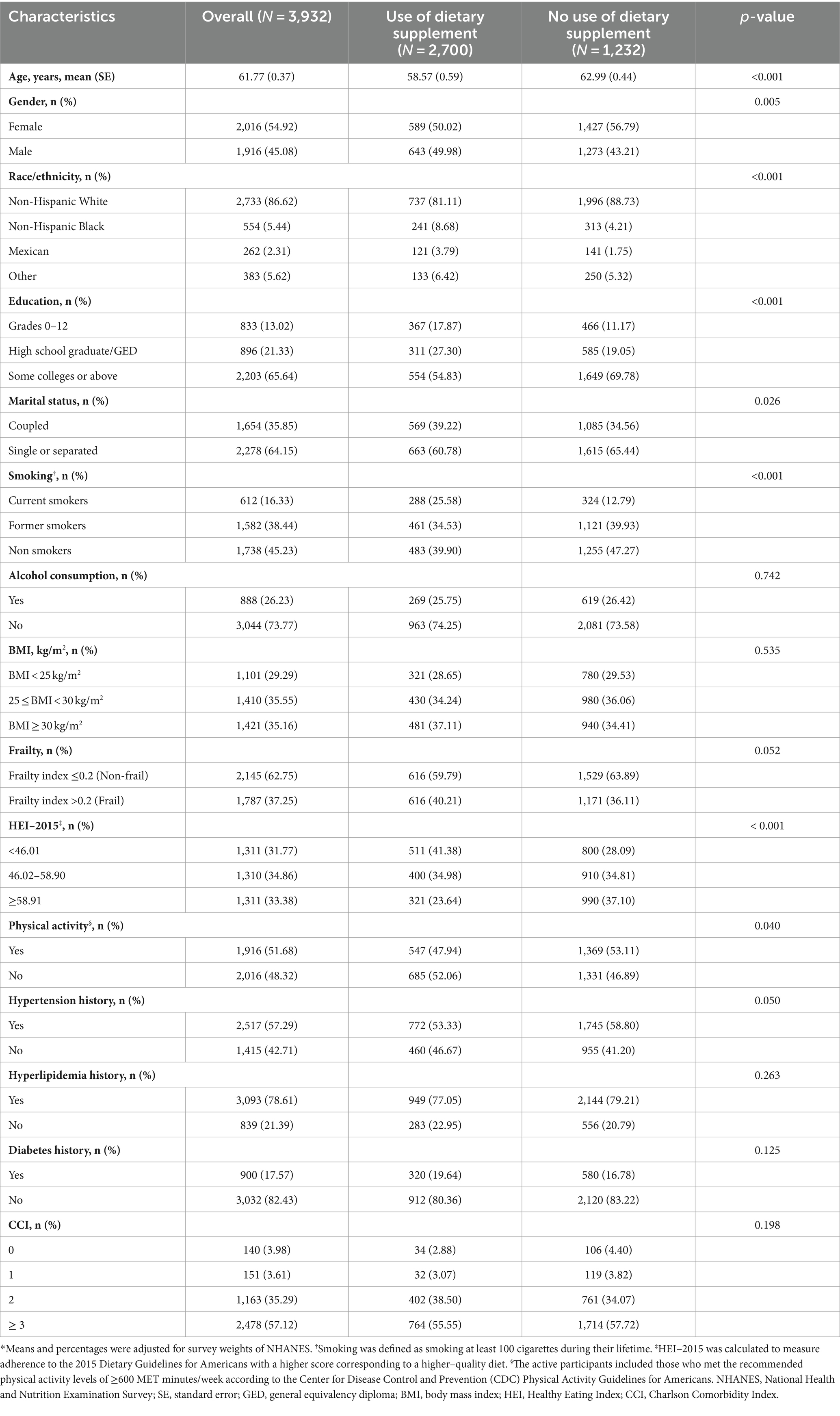
Table 1. Characteristics of US adults according to the use of dietary supplement in the past 30 days, NHANES 1999–2018*.
Table 2 displays the associations between BMI and FI by the survey-weighted logistics regression models. The univariate and multivariate analyses adjusted for confounding factors indicate a significantly higher frailty risk in groups with 25 ≤ BMI < 30 kg/m2 and BMI ≥ 30 kg/m2 compared to those with BMI < 25 kg/m2 (p-value <0.05). In the fully adjusted model (model 2), compared to the normal weight group, the overweight (Odds ratio [OR] = 1.28; 95% CI, 1.01–1.64) and obese group (OR = 1.61; 95% CI, 1.24–2.09, p for trend <0.001) were associated with higher FI. The multivariate-adjusted ORs for every 1 kg/m2 in BMI in the association with FI was 1.04 (95% CI, 1.02–1.06). Furthermore, restricted cubic spline curves further visualize the relationships between BMI and FI (Figure 2A). After adjusting for confounders, a significant positive linear relationship was observed between BMI and FI (p value for overall <0.001, p value for non-linearity = 0.188).
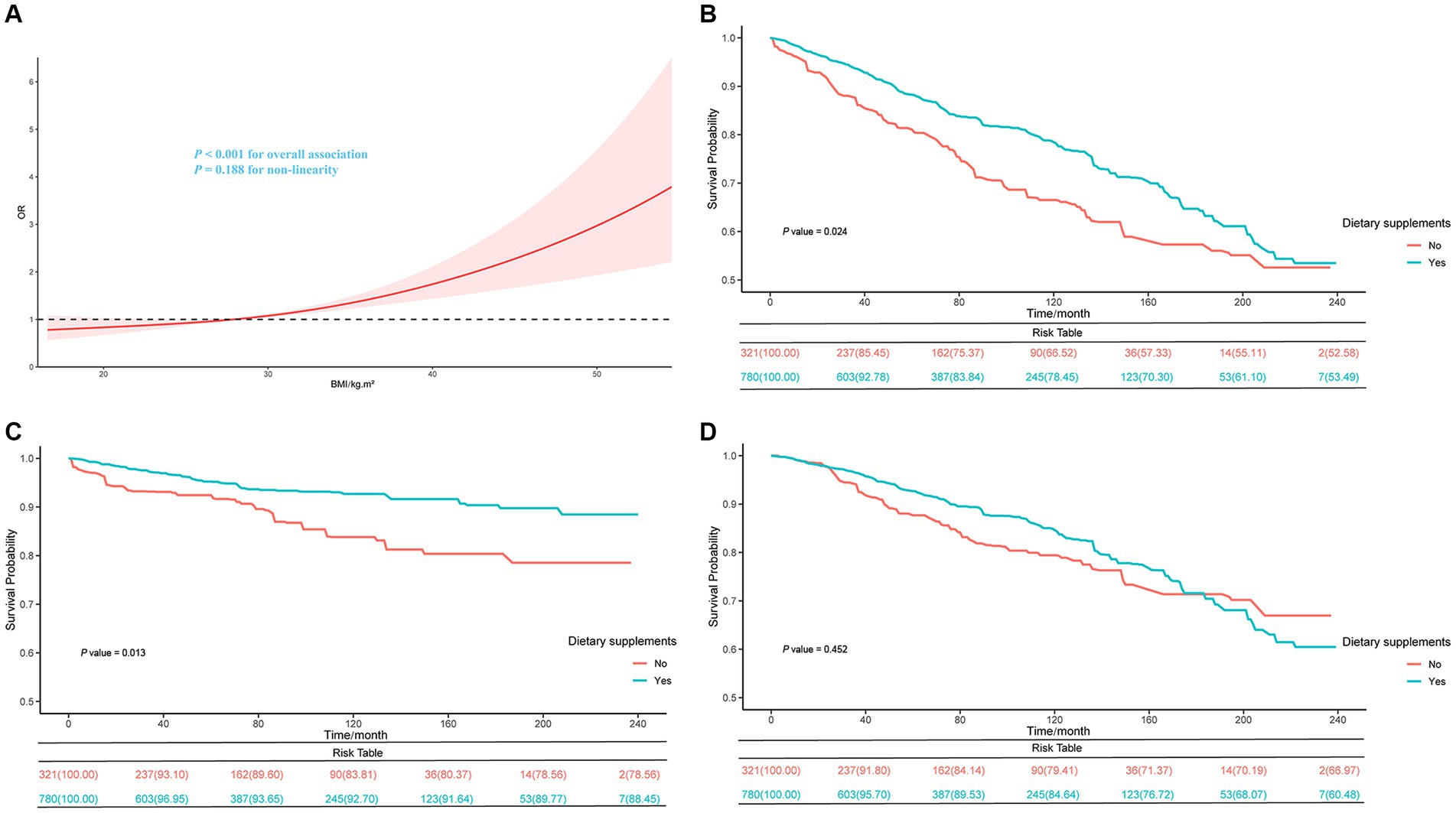
Figure 2. Restricted cubic spline curve of the relationships between BMI and FI in cancer patients (A) and Kaplan–Meier survival curves on all-cause (B), cancer (C), and non-cancer (D) mortality between dietary supplement users and non-users in BMI < 25 kg/m2 group.
The research observed significant associations between BMI, FI, and mortality in cancer patients (Table 3). Patients in the 25 ≤ BMI < 30 kg/m2 group exhibited a reduced risk of all-cause mortality (Hazard ratio [HR] = 0.78, 95% CI, 0.65–0.94, p-value = 0.008) and cancer-specific mortality (HR = 0.73, 95% CI, 0.58–0.92, p-value = 0.007) compared to the reference group (BMI < 25 kg/m2). Notably, patients with a BMI ≥ 30 kg/m2 also showed a lower risk of all-cause (HR = 0.71, 95% CI, 0.55–0.91, p-value = 0.006) and cancer mortality (HR = 0.67, 95% CI, 0.50–0.89, p-value = 0.005), although the association with non-cancer mortality was not statistically significant (HR = 0.81, 95% CI, 0.56–1.19, p-value = 0.285). Regarding the FI, patients with a FI > 0.2 were associated with substantially increased risks of all-cause (HR = 2.22, 95% CI, 1.86–2.66, p-value<0.001), cancer (HR = 2.46, 95% CI, 1.93–3.13, p-value<0.001), and non-cancer mortality (HR = 1.81, 95% CI, 1.42–2.31, p-value<0.001) compared to those with a FI ≤ 0.2.
During a median of 7.63 years of follow-up, 1,211 deaths (WP, 23.96%; 95% CI, 21.78–26.14%) were documented. Among these, there were 795 deaths attributed to cancer events (WP of 8.05%; 95% CI, 7.01–9.08%).
After adjusting for all covariates, in the BMI < 25 kg/m2 group, dietary supplement users had lower risks of all-cause (HR = 0.63; 95% CI, 0.47–0.84) and cancer mortality (HR = 0.48; 95% CI, 0.29–0.80) compared to dietary supplement non-users. However, we found no significant association between dietary supplement use and lower mortality in the overweight and obese group (Supplementary Table S3). Moreover, in the group with BMI < 25 kg/m2, the Kaplan–Meier survival curves related to all-cause mortality and cancer mortality showed that dietary supplement users had a higher survival rate compared to non-users of dietary supplement (Figures 2B,C). No difference in non-cancer mortality was observed between the two groups(Figure 2D). In the non-frail group, dietary supplement users had lower risks of all-cause (HR = 0.77; 95% CI, 0.60–0.90) and cancer mortality (HR = 0.59; 95% CI, 0.39–0.89) compared to dietary supplement non-users. No significant difference in mortality was observed within the frail group (Supplementary Table S4). Additionally, by combining the three BMI groups with the two frailty groups into six combined categories, it was observed that within the BMI < 25 kg/m2 and non-frail group, dietary supplement users had a lower risk of all-cause mortality (HR = 0.49; 95% CI, 0.30–0.79) and cancer mortality (HR = 0.25; 95% CI, 0.10–0.60) compared to non-users. No significant association between dietary supplement use and lower mortality rates was found in the other five groups (Table 4).
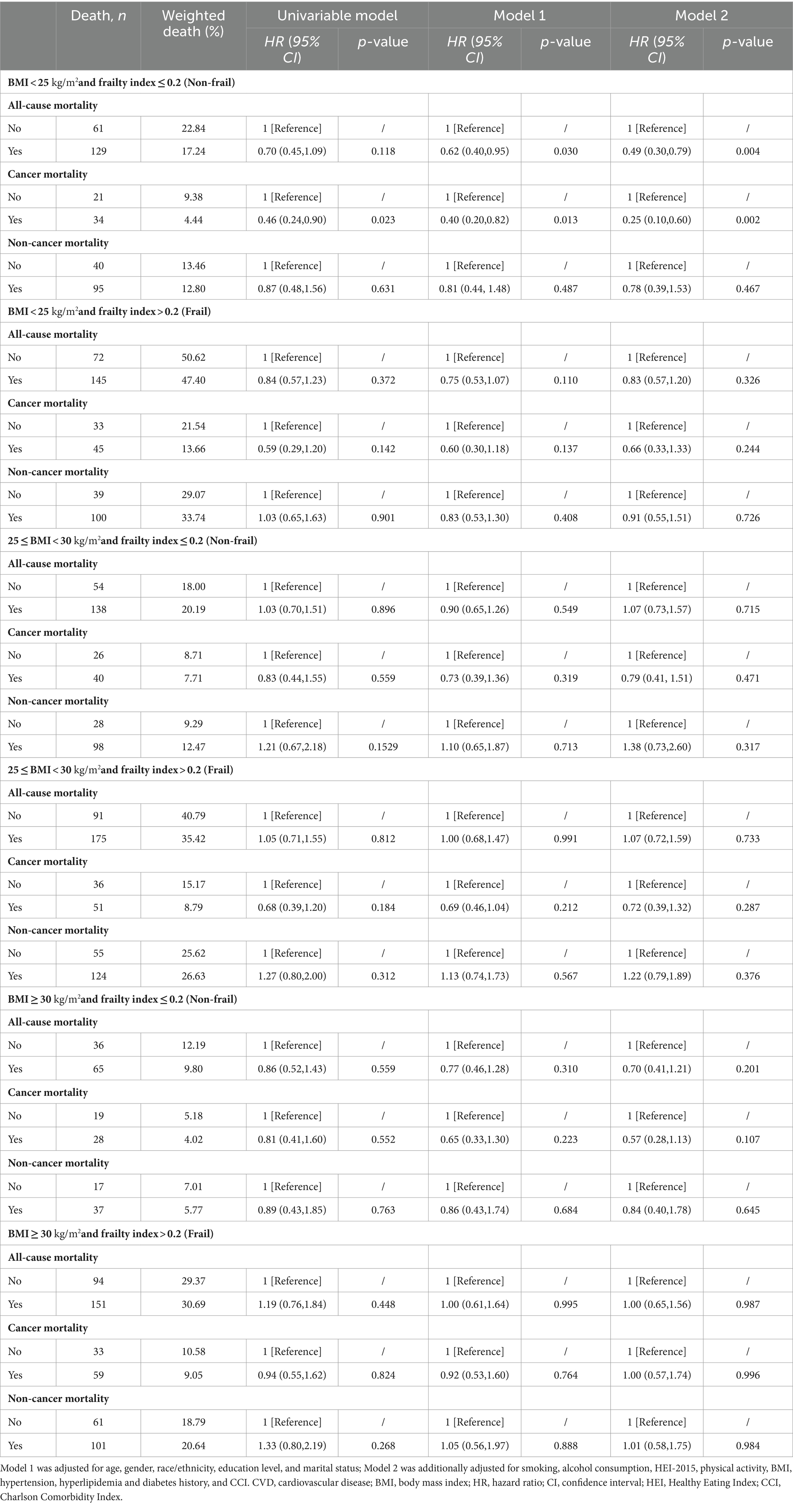
Table 4. Survey-weighted associations between dietary supplement and all-cause, cancer, and non-cancer mortality among cancer survivors in different BMI (kg/m2) and frailty groups.
The results remained robust after PSM analysis (Supplementary Table S5), excluding participants less than 65 years old (Table 5), excluding participants with BMI < 18.5 kg/m2 (Table 6), excluding deaths with a follow-up period of fewer than 2 years (Supplementary Table S6), excluding participants over 80 years old (Supplementary Table S7), and repeating the main analyses without consideration of complex sampling designs (Supplementary Table S8).
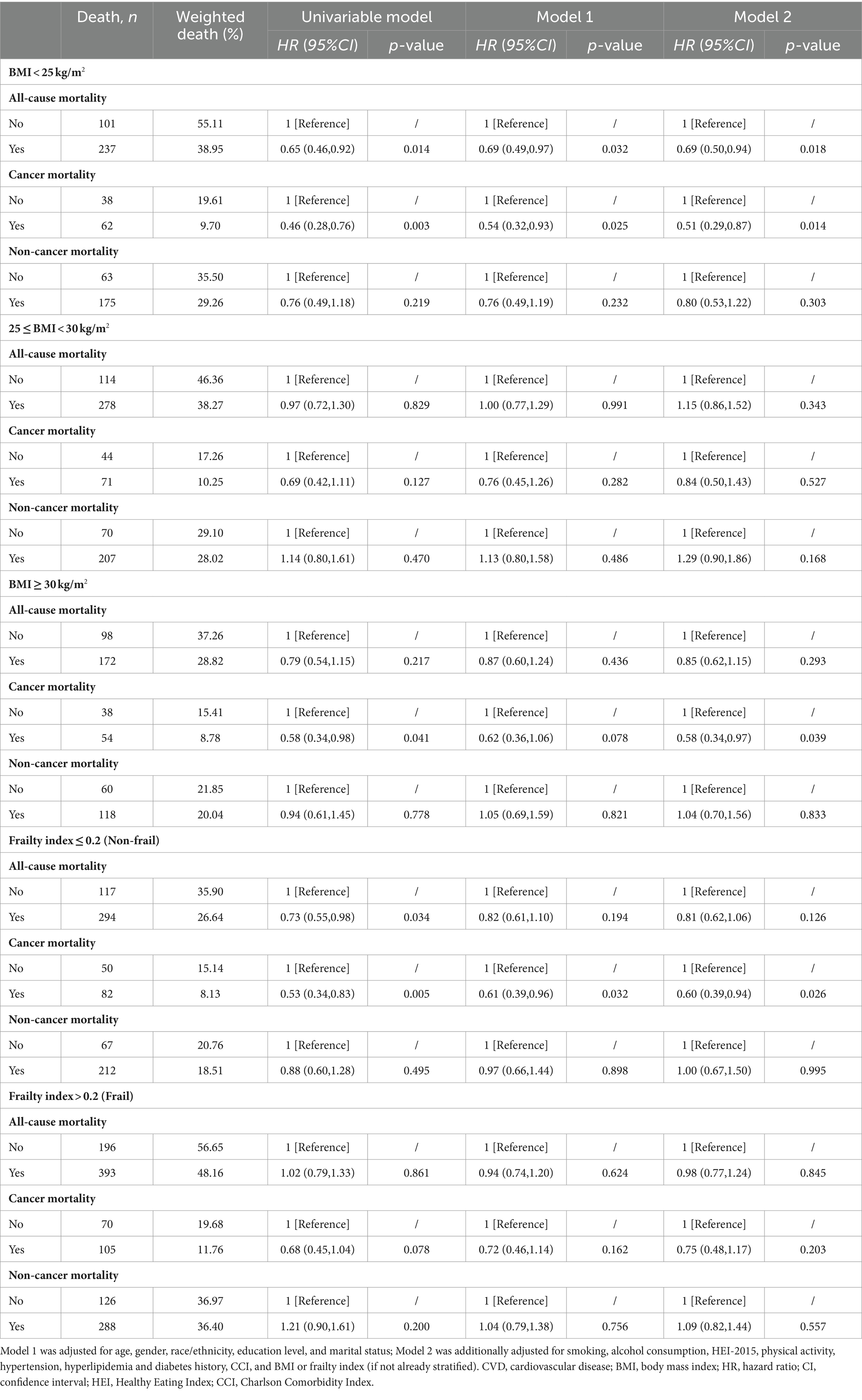
Table 5. Sensitivity analysis of the associations between dietary supplements and all-cause, cancer and non-cancer mortality among cancer survivors in different BMI (kg/m2) or frailty groups after exclusion of participants less than 65 years old.
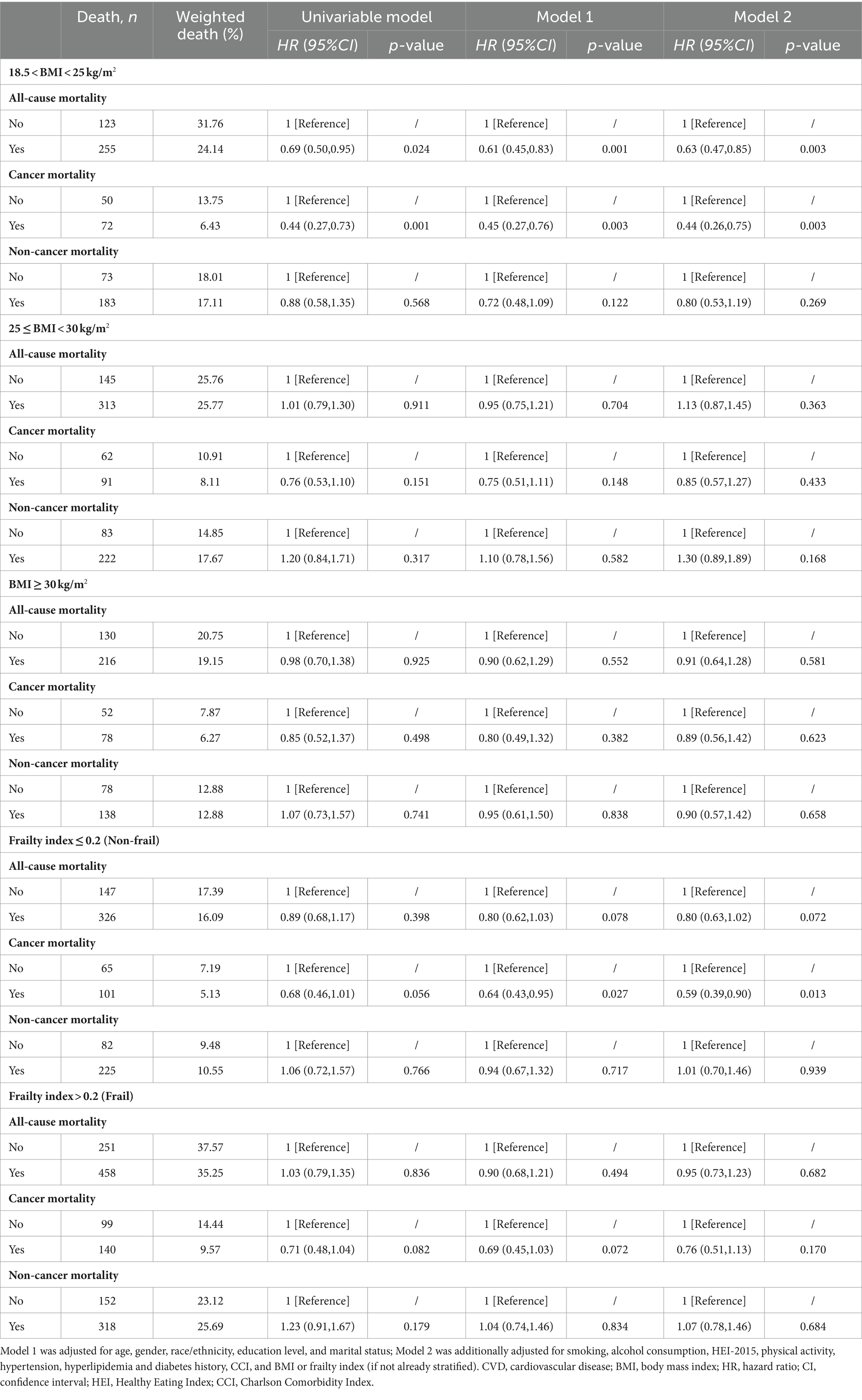
Table 6. Sensitivity analysis of the associations between dietary supplements and all-cause, cancer and non-cancer mortality among cancer survivors in different BMI (kg/m2) or frailty groups after exclusion of participants with BMI < 18.5 kg/m2.
This cohort study discovered that a high BMI is linked to an increased risk of frailty in 3,932 US cancer survivors. BMI < 25 kg/m2 and FI > 0.2 were associated with an increased risk of death. Additionally, the use of dietary supplement can reduce the risk of death in cancer survivors with BMI < 25 kg/m2 and/or FI ≤ 0.2.
BMI has long been a crucial indicator for assessing the nutritional status and prognosis of cancer patients (2, 10, 27–31). In the US, approximately 7.8% of incident cancers (123,300/1,570,975 cases) and 6.5% of cancer-related deaths (38,230/587,521 deaths) were caused by effects of excess body weight, alcohol consumption, physical inactivity, and unhealthy diet (32). Excess weight has become one of the leading preventable causes of cancers similar to tobacco use (30). However, recent studies have indicated that overweight or obese cancer patients often have better outcomes, a phenomenon known as the “obesity paradox.” A clinical study involving 250 cancer patients undergoing αPD-(L)1 checkpoint blockade found that obese patients had significantly improved progression-free survival (PFS) (median: 237 versus 141 days, p = 0.0034) and overall survival (OS) (median: 523 versus 361 days, p = 0.0492) compared to non-obese patients (33). Similar survival advantages for obese patients were also found in studies by Naik GS (34) and Cortellini A (35). Obese patients have more energy reserves when facing cancer treatment, while malnutrition, underweight, and cachexia may impair immune function and surveillance, facilitating infections, treatment-related toxicity, recurrence, and distant metastasis (36). In this study, for the group with BMI ≥ 25 kg/m2, despite the presence of some individuals who may be frail due to obesity or resistant to treatment, greater nutritional reserves and tolerance to therapy might be one of the reasons for the overall better prognosis compared to the group with BMI < 25 kg/m2. This is consistent with the “obesity paradox.”
Considering the limited reliability of BMI in predicting clinical outcomes, frailty emerges as a more comprehensive measure linking patient health status with prognosis. Frailty is described as a complex, multidimensional, and recurring state of decreased physiologic reserve that leads to reduced resilience and adaptability, and heightened susceptibility to stressors (17). The numerous indicators used for calculating the FI are closely associated with the risk of mortality. Research indicates that cognitive decline can affect patients’ treatment decisions and adherence, thereby impacting the management and prognosis of diseases (37). Difficulties in activities of daily living reflect a decline in physical function, which may lead to a reduced quality of life and limited treatment options (38). The presence of depressive symptoms, such as low mood, fatigue, or loss of appetite, not only directly affects the patient’s mental health but also results in decreased treatment compliance (39). Comorbid conditions, such as heart disease, diabetes, arthritis, etc., may interact with cancer and its treatment, potentially increasing the risk of mortality (25). Changes in physical performance and anthropometric measurements may be signs of declining bodily functions and malnutrition (40). Laboratory values can reflect systemic health issues that can directly affect the survival rates of cancer patients (41). Finally, higher rates of hospitalization and healthcare utilization often indicate more severe health issues, which can lead to higher mortality rates (42). As a result, cancer patients with a high FI face a higher risk of mortality due to cumulative deficits in these multiple areas.
This study has revealed a correlation between higher BMI values and an increased risk of frailty in cancer patients. The correlation remains significant even after accounting for various health and socioeconomic factors. Obesity is associated with decreased muscle mass and strength, which can adversely affect overall bodily functions and resilience against external stressors (43). Obesity’s link with mental health issues, particularly depression, is well-documented and may further compound the effects of frailty (44, 45). Additionally, the limited physical activity and heightened risk of chronic diseases associated with obesity also contribute to reduced quality of life and self-care capabilities (46).
Cancer is commonly regarded as a catabolic disease. Tumors can modify a patient’s metabolism, resulting in increased energy and protein consumption, which can lead to malnutrition. Chemotherapy, radiotherapy, and other treatments can cause adverse effects such as appetite loss, taste changes, nausea, and vomiting, which can further exacerbate malnutrition. Given these circumstances, many cancer patients choose to take additional dietary supplements (such as vitamins, minerals, amino acids, herbs, and other similar components) (47–49). Studies suggest that appropriate nutritional interventions can improve the overall nutritional status of cancer patients, reduce complications, and potentially enhance quality of life (50–54). A randomized clinical trial involved 100 colorectal cancer patients undergoing adjuvant chemotherapy after curative surgery, where they were randomly assigned to receive either probiotics or a placebo postoperatively. The results showed that probiotics significantly reduced gastrointestinal reactions and helped balance intestinal flora (51). A study involving 128 gastrointestinal cancer patients receiving chemotherapy confirmed that fish oil-enriched nutrition could increase skeletal muscle and lean body mass, prevent the rise in serum CRP levels, and thereby improve chemotherapy tolerance (52). Additionally, a prospective cohort study of 247 survivors of colorectal cancer identified longitudinal associations between the consumption of macronutrients and micronutrients and the metabolic products of the tryptophan-kynurenine pathway. These associations may have potential implications for improving the health-related quality of life (HRQoL) of survivors (53). Another cohort study conducted on 30,239 individuals from the UK Biobank showed that cancer patients who regularly used dietary supplement (including vitamins, minerals, or non-vitamin non-mineral supplement) after diagnosis had a slightly lower risk of all-cause and cancer-specific mortality. This effect was particularly significant for non-vitamin non-mineral supplement, which showed a significant reduction in the risk of all-cause mortality (54).
However, research indicates that nearly 30% of patients do not inform their healthcare providers about their use of dietary supplements or other alternative treatments (55), leading to ambiguity in the clinical management and scientific research of dietary supplement application. This research, based on data from standardized questionnaires in the NHANES database, confirmed that the addition of dietary supplement is beneficial for non-obese and/or non-frail patient prognosis. However, in the overweight, obese, or frail population, dietary supplement cannot improve prognosis. The study further confirms that the impact of dietary supplements on cancer survivors is affected by BMI and FI through PSM and sensitivity analyses. This suggests that the efficacy of dietary supplement is not constant but is influenced by individual health conditions and other factors. A meta-analysis investigating the efficacy of probiotics, prebiotics, and synbiotics in treating anxiety revealed that the intervention group exhibited a significant reduction in anxiety scores compared to the placebo group within subgroups characterized by mental issues. However, no significant difference in anxiety scores was found between the two groups within subgroups characterized by physical problems or perfectly healthy. Similar disparities in treatment outcomes were also identified in subgroup analyses that utilized gastrointestinal symptoms or region as grouping variables (56). Another meta-analysis, based on individual participant data, synthesized the impact of small-quantity lipid-based nutrient supplements (SQ-LNSs) on child growth. The results revealed that the SQ-LNSs intervention had a more pronounced effect on child development within subgroups characterized by a greater prevalence of stunting, lower socioeconomic status, higher incidence of acute malnutrition, or elevated rates of anemia (57). These findings advocate for tailored recommendations regarding dietary supplement usage based on individual characteristics.
However, this study has several limitations. The analyses did not consider the type, dosage, and duration of dietary supplement use, which could have an impact on the results. Additionally, the study did not address more clinical details such as the types of cancer, anti-tumor medications, comorbid medications, and their duration, due to the limited types of data available in the NHANES database. Finally, it is important to note that the 49 items used to calculate the FI in this study are just one of many. This highlights the need for the development of more FI survey forms that are tailored to different patient groups and clinical scenarios. Before recommending dietary supplement to cancer survivors, it is important to take into account the overall health status, nutritional needs, and potential risks and benefits. For frail cancer patients, it is crucial to develop personalized treatment plans. This should include adjusting medication dosages, providing appropriate nutritional support, and implementing comprehensive rehabilitation programs.
Utilizing data from the NHANES and the NCHS, along with the 49-items, this study observed that among cancer survivors, a positive correlation was noted between BMI and FI. BMI < 25 kg/m2 and FI > 0.2 were associated with heightened risks of all-cause mortality and cancer-related mortality. The administration of dietary supplements appears to confer benefits to patients with a BMI < 25 kg/m2 and FI ≤ 0.2. However, more investigations are warranted to determine the optimal type, dosage form, and duration of usage of dietary supplement, as well as the characteristics of the intended population in the future.
Publicly available datasets were analyzed in this study. This data can be found here: National Health and Nutrition Examination Survey https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving humans were approved by the National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MZ: Methodology, Software, Writing – original draft. JW: Methodology, Software, Writing – original draft. XL: Visualization, Writing – original draft. LZ: Resources, Writing – original draft. YZ: Formal analysis, Writing – original draft. ZW: Data curation, Writing – original draft. JZ: Validation, Writing – original draft. YF: Conceptualization, Writing – original draft. ZQ: Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Shandong Province (ZR2023QH190).
We express our gratitude to the staff at the National Center for Health Statistics at the CDC, whose efforts in designing, collecting, administering, and subsequently releasing the NHANES data for public use. We also extend our sincere thanks to all study participants. Additionally, we thank Yuren 930 for allowing the authors to collaborate on this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1395362/full#supplementary-material
BMI, Body Mass Index; FI, Frailty index; NHANES, National Health and Nutrition Examination Survey; PSM, propensity score matching; OR, odds ratio; 95% CI, 95% confidence interval; HR, Hazard ratio; HEI-2015, Healthy Eating Index-2015; CCI, Charlson Comorbidity Index; VIF, variance inflation factor; WP, weighted percentage
1. Cancer today. (2022). Available at: https://gco.iarc.who.int/today/ (Accessed February 14, 2024)
2. Molina-Montes, E, Ubago-Guisado, E, Petrova, D, Amiano, P, Chirlaque, M-D, Agudo, A, et al. The role of diet, alcohol, BMI, and physical activity in cancer mortality: summary findings of the EPIC study. Nutrients. (2021) 13:4293. doi: 10.3390/nu13124293
3. Lu, L, Mullins, CS, Schafmayer, C, Zeißig, S, and Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. (2021) 41:1137–51. doi: 10.1002/cac2.12220
4. Zhang, Y-B, Pan, X-F, Chen, J, Cao, A, Zhang, Y-G, Xia, L, et al. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. (2020) 122:1085–93. doi: 10.1038/s41416-020-0741-x
5. Mao, JJ, Pillai, GG, Andrade, CJ, Ligibel, JA, Basu, P, Cohen, L, et al. Integrative oncology: addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin. (2022) 72:144–64. doi: 10.3322/caac.21706
6. Keys, A, Fidanza, F, Karvonen, MJ, Kimura, N, and Taylor, HL. Indices of relative weight and obesity. Int J Epidemiol. (2014) 43:655–65. doi: 10.1093/ije/dyu058
7. Elmaleh-Sachs, A, Schwartz, JL, Bramante, CT, Nicklas, JM, Gudzune, KA, and Jay, M. Obesity management in adults: a review. JAMA. (2023) 330:2000–15. doi: 10.1001/jama.2023.19897
8. Min, J, Kim, JS, Kim, HW, Ko, Y, Oh, JY, Jeong, Y-J, et al. Effects of underweight and overweight on mortality in patients with pulmonary tuberculosis. Front Public Health. (2023) 11:1236099. doi: 10.3389/fpubh.2023.1236099
9. Yoo, TK, Han, K-D, Rhee, E-J, and Lee, W-Y. Association between underweight and risk of heart failure in diabetes patients. J Cachexia Sarcopenia Muscle. (2024) 15:671–80. doi: 10.1002/jcsm.13417
10. Ligibel, JA, Alfano, CM, Courneya, KS, Demark-Wahnefried, W, Burger, RA, Chlebowski, RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. (2014) 32:3568–74. doi: 10.1200/JCO.2014.58.4680
11. Martin, L, Senesse, P, Gioulbasanis, I, Antoun, S, Bozzetti, F, Deans, C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. (2015) 33:90–9. doi: 10.1200/JCO.2014.56.1894
12. Kroenke, CH, Neugebauer, R, Meyerhardt, J, Prado, CM, Weltzien, E, Kwan, ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. (2016) 2:1137–45. doi: 10.1001/jamaoncol.2016.0732
13. Oswalt, C, Liu, Y, Pang, H, Le-Rademacher, J, Wang, X, and Crawford, J. Associations between body mass index, weight loss and overall survival in patients with advanced lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:2650–60. doi: 10.1002/jcsm.13095
14. Antoun, S, Lanoy, E, Ammari, S, Farhane, S, Martin, L, Robert, C, et al. Protective effect of obesity on survival in cancers treated with immunotherapy vanishes when controlling for type of cancer, weight loss and reduced skeletal muscle. Eur J Cancer. (2023) 178:49–59. doi: 10.1016/j.ejca.2022.10.013
15. Chan, DSM, Vieira, R, Abar, L, Aune, D, Balducci, K, Cariolou, M, et al. Postdiagnosis body fatness, weight change and breast cancer prognosis: global Cancer update program (CUP global) systematic literature review and meta-analysis. Int J Cancer. (2023) 152:572–99. doi: 10.1002/ijc.34322
16. Wen, H, Deng, G, Shi, X, Liu, Z, Lin, A, Cheng, Q, et al. Body mass index, weight change, and cancer prognosis: a meta-analysis and systematic review of 73 cohort studies. ESMO Open. (2024) 9:102241. doi: 10.1016/j.esmoop.2024.102241
17. Ethun, CG, Bilen, MA, Jani, AB, Maithel, SK, Ogan, K, and Master, VA. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. (2017) 67:362–77. doi: 10.3322/caac.21406
18. Fan, J, Yu, C, Guo, Y, Bian, Z, Sun, Z, Yang, L, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. (2020) 5:e650–60. doi: 10.1016/S2468-2667(20)30113-4
19. Sapp, DG, Cormier, BM, Rockwood, K, Howlett, SE, and Heinze, SS. The frailty index based on laboratory test data as a tool to investigate the impact of frailty on health outcomes: a systematic review and meta-analysis. Age Ageing. (2023) 52:afac309. doi: 10.1093/ageing/afac309
20. Lai, H-Y, Huang, S-T, Anker, SD, von Haehling, S, Akishita, M, Arai, H, et al. The burden of frailty in heart failure: prevalence, impacts on clinical outcomes and the role of heart failure medications. J Cachexia Sarcopenia Muscle. (2024) 15:660–70. doi: 10.1002/jcsm.13412
21. Smith-Ryan, AE, Hirsch, KR, Saylor, HE, Gould, LM, and Blue, MNM. Nutritional considerations and strategies to facilitate injury recovery and rehabilitation. J Athl Train. (2020) 55:918–30. doi: 10.4085/1062-6050-550-19
22. Mao, X-Y, Yin, X-X, Guan, Q-W, Xia, Q-X, Yang, N, Zhou, H-H, et al. Dietary nutrition for neurological disease therapy: current status and future directions. Pharmacol Ther. (2021) 226:107861. doi: 10.1016/j.pharmthera.2021.107861
23. Singh, B, Eshaghian, E, Chuang, J, and Covasa, M. Do diet and dietary supplements mitigate clinical outcomes in COVID-19? Nutrients. (2022) 14:1909. doi: 10.3390/nu14091909
24. Hakeem, FF, Bernabé, E, and Sabbah, W. Association between Oral health and frailty among American older adults. J Am Med Dir Assoc. (2021) 22:559–563.e2. doi: 10.1016/j.jamda.2020.07.023
25. Charlson, ME, Carrozzino, D, Guidi, J, and Patierno, C. Charlson comorbidity index: a critical review of Clinimetric properties. Psychother Psychosom. (2022) 91:8–35. doi: 10.1159/000521288
26. Liu, M, Zhang, Z, Zhou, C, Ye, Z, He, P, Zhang, Y, et al. Predicted fat mass and lean mass in relation to all-cause and cause-specific mortality. J Cachexia Sarcopenia Muscle. (2022) 13:1064–75. doi: 10.1002/jcsm.12921
27. McGee, DLDiverse Populations Collaboration. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. (2005) 15:87–97. doi: 10.1016/j.annepidem.2004.05.012
28. Avgerinos, KI, Spyrou, N, Mantzoros, CS, and Dalamaga, M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
29. Cancer Facts & Figures. (2024). Available at: (https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html)
30. Risk Factors: Obesity – NCI. (2015) Available at: (https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity)
31. Lauby-Secretan, B, Scoccianti, C, Loomis, D, Grosse, Y, Bianchini, F, and Straif, K. Body fatness and Cancer — viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
32. Islami, F, Goding Sauer, A, Miller, KD, Siegel, RL, Fedewa, SA, Jacobs, EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. (2018) 68:31–54. doi: 10.3322/caac.21440
33. Wang, Z, Aguilar, EG, Luna, JI, Dunai, C, Khuat, LT, Le, CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. (2019) 25:141–51. doi: 10.1038/s41591-018-0221-5
34. Naik, GS, Waikar, SS, Johnson, AEW, Buchbinder, EI, Haq, R, Hodi, FS, et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer. (2019) 7:89. doi: 10.1186/s40425-019-0512-5
35. Cortellini, A, Bersanelli, M, Buti, S, Cannita, K, Santini, D, Perrone, F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. (2019) 7:57. doi: 10.1186/s40425-019-0527-y
36. Gallo, M, Adinolfi, V, Barucca, V, Prinzi, N, Renzelli, V, Barrea, L, et al. Expected and paradoxical effects of obesity on cancer treatment response. Rev Endocr Metab Disord. (2021) 22:681–702. doi: 10.1007/s11154-020-09597-y
37. Ranzini, L, Schiavi, M, Pierobon, A, Granata, N, and Giardini, A. From mild cognitive impairment (MCI) to dementia in chronic obstructive pulmonary disease. Implications for clinical practice and disease management: a mini-review. Front Psychol. (2020) 11:337. doi: 10.3389/fpsyg.2020.00337
38. Wildiers, H, Heeren, P, Puts, M, Topinkova, E, Janssen-Heijnen, MLG, Extermann, M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. (2014) 32:2595–603. doi: 10.1200/JCO.2013.54.8347
39. Lima, MP, Machado, WL, and Irigaray, TQ. Predictive factors of treatment adherence in cancer outpatients. Psychooncology. (2018) 27:2823–8. doi: 10.1002/pon.4897
40. Couderc, A-L, Liuu, E, Boudou-Rouquette, P, Poisson, J, Frelaut, M, Montégut, C, et al. Pre-therapeutic sarcopenia among Cancer patients: an up-to-date Meta-analysis of prevalence and predictive value during Cancer treatment. Nutrients. (2023) 15:1193. doi: 10.3390/nu15051193
41. Blohmer, J-U, Dunst, J, Harrison, L, Johnston, P, Khayat, D, Ludwig, H, et al. Cancer-related anemia: biological findings, clinical implications and impact on quality of life. Oncology. (2005) 68:12–21. doi: 10.1159/000083129
42. Shin, J, Shin, DW, Lee, J, Hwang, JH, Yoo, JE, Ko, H, et al. Association between perception of care coordination and health outcomes in Korean cancer survivors. Health Qual Life Outcomes. (2020) 18:21. doi: 10.1186/s12955-020-1279-6
43. Cruz-Jentoft, AJ, Baeyens, JP, Bauer, JM, Boirie, Y, Cederholm, T, Landi, F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
44. Luppino, FS, de Wit, LM, Bouvy, PF, Stijnen, T, Cuijpers, P, Penninx, BWJH, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. (2010) 67:220–9. doi: 10.1001/archgenpsychiatry.2010.2
45. Payne, ME, Porter Starr, KN, Orenduff, M, Mulder, HS, McDonald, SR, Spira, AP, et al. Quality of life and mental health in older adults with obesity and frailty: associations with a weight loss intervention. J Nutr Health Aging. (2018) 22:1259–65. doi: 10.1007/s12603-018-1127-0
46. Demark-Wahnefried, W, Schmitz, KH, Alfano, CM, Bail, JR, Goodwin, PJ, Thomson, CA, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin. (2018) 68:64–89. doi: 10.3322/caac.21441
47. Kaur, H, Hoenemeyer, T, Parrish, KB, and Demark-Wahnefried, W. Dietary supplement use among older Cancer survivors: socio-demographic associations, supplement types, reasons for use, and cost. Nutrients. (2022) 14:3402. doi: 10.3390/nu14163402
48. Yoon, SL, and Grundmann, O. Relevance of dietary supplement use in gastrointestinal-Cancer-associated Cachexia. Nutrients. (2023) 15:3391. doi: 10.3390/nu15153391
49. Zhang, FF, Hudson, MM, Chen, F, Li, Z, Huang, I-C, Bhakta, N, et al. Dietary supplement use among adult survivors of childhood cancer: a report from the St Jude lifetime cohort study. Cancer. (2023) 129:1602–13. doi: 10.1002/cncr.34700
50. Arends, J, Bachmann, P, Baracos, V, Barthelemy, N, Bertz, H, Bozzetti, F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. (2017) 36:11–48. doi: 10.1016/j.clnu.2016.07.015
51. Huang, F, Li, S, Chen, W, Han, Y, Yao, Y, Yang, L, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota Dysbiosis caused by chemotherapy in colorectal Cancer patients. Nutrients. (2023) 15:356. doi: 10.3390/nu15020356
52. Shirai, Y, Okugawa, Y, Hishida, A, Ogawa, A, Okamoto, K, Shintani, M, et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci Rep. (2017) 7:4826. doi: 10.1038/s41598-017-05278-0
53. Holthuijsen, DDB, van Roekel, EH, Bours, MJL, Ueland, PM, Breukink, SO, Janssen-Heijnen, MLG, et al. Longitudinal associations of macronutrient and micronutrient intake with plasma kynurenines in colorectal cancer survivors up to 12 months posttreatment. Am J Clin Nutr. (2023) 118:865–80. doi: 10.1016/j.ajcnut.2023.08.003
54. Lam, CS, Koon, HK, Loong, HH-F, Chung, VC-H, and Cheung, YT. Associations of dietary supplement use with all-cause and cause-specific mortality in patients diagnosed with cancer: a large prospective cohort study in the UK biobank. Eur J Nutr. (2023) 62:879–89. doi: 10.1007/s00394-022-03044-1
55. Sanford, NN, Sher, DJ, Ahn, C, Aizer, AA, and Mahal, BA. Prevalence and nondisclosure of complementary and alternative medicine use in patients with Cancer and Cancer survivors in the United States. JAMA Oncol. (2019) 5:735–7. doi: 10.1001/jamaoncol.2019.0349
56. Zhao, Z, Xiao, G, Xia, J, Guo, H, Yang, X, Jiang, Q, et al. Effectiveness of probiotic/prebiotic/synbiotic treatments on anxiety: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord. (2023) 343:9–21. doi: 10.1016/j.jad.2023.09.018
57. Dewey, KG, Stewart, CP, Wessells, KR, Prado, EL, and Arnold, CD. Small-quantity lipid-based nutrient supplements for the prevention of child malnutrition and promotion of healthy development: overview of individual participant data meta-analysis and programmatic implications. Am J Clin Nutr. (2021) 114:3S–14S. doi: 10.1093/ajcn/nqab279
Keywords: body mass index, frailty index, dietary supplement, cancer, mortality, National Health and Nutrition Examination Survey
Citation: Zhang M, Wang J, Li X, Zhang L, Zhang Y, Wen Z, Zhang J, Fan Y and Qiu Z (2024) Association between dietary supplement use and mortality in cancer survivors with different body mass index and frailty status: a cohort study. Front. Nutr. 11:1395362. doi: 10.3389/fnut.2024.1395362
Received: 03 March 2024; Accepted: 16 April 2024;
Published: 01 May 2024.
Edited by:
Antonio Moschetta, University of Bari Aldo Moro, ItalyReviewed by:
Salvatore Vaccaro, IRCCS Local Health Authority of Reggio Emilia, ItalyCopyright © 2024 Zhang, Wang, Li, Zhang, Zhang, Wen, Zhang, Fan and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenkang Qiu, ZHJfcWl1emtAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.