
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 24 June 2024
Sec. Nutritional Epidemiology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1395016
Worldwide, as well as in Mexico, the leading cause of death is cardiovascular disease (CVD). Hypertension is the main risk factor for CVD; about 50% of the adult population suffers from this condition. High sodium (Na) intake combined with low potassium (K) intake can trigger cardiovascular disorders such as high blood pressure (BP). The aim of this study was to estimate the mean excretion of Na and K in Mexican adults using a spot urine sample, and its association with cardiovascular disorders. Information on 2,778 adults, 20–59 years of age, who participated in ENSANUT-2016 was analyzed. Na and K were estimated using Tanaka formulae. Biomarkers such as glucose, total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol, and anthropometry were measured. Mean Na was 3,354 mg/day (95%CI: 3,278, 3,429), 1,440 mg/day of K (95%CI: 1,412, 1,469), and the Na-K ratio was 2.4. The excretion of Na was greater in adults with high BP (3,542 mg/day) compared to those with normal BP (3,296 mg/day). In adults with hypertension, excretion of K was 10% greater (1,534 mg/day) than in adults with normal BP (1,357 mg/day). In adults with moderate reduction of renal function, Na excretion was 22% less (2,772 mg/day) than in adults with normal kidney function (3,382 mg/day). The results of this study show that the cardiovascular health of Mexican adults is at risk, as they showed high Na excretion and low K excretion.
Sodium (Na) and potassium (K) are essential to human homeostasis because they help in the maintenance of osmotic balance, the transmission of nerve impulses, as well as in muscle contraction or relaxation (1). In addition, these ions move against concentration gradients by the constant pumping of Na K ATP-ase in the plasma membrane of the cells. This allows three Na + ions to be expelled into the extracellular matrix and two K+ ions to enter the cytoplasm, which regulates cell volume and maintains the homeostasis of organs such as the kidney (2).
High Na intake combined with low K intake can increase vascular volume (3) and trigger vascular disorders, such as high blood pressure. Over time, this interaction between Na and K can raise blood pressure that is sensitive to salt and increase cardiovascular risk (4). It has been estimated that hypertension affects 49.4% of Mexican adults, of which 70.0% are unaware of their condition (5).
Therefore, the World Health Organization (WHO) suggests that Na consumption should be less than 2 g/day (6), while K consumption should be higher than 3.5 g/day (7). However, daily consumption of Na worldwide is 4.3 g/day (8), while K consumption is around 2.25 g/day (9). In Mexico, the national dietary intake is 3.1 g/day and 3.4 g/day of Na and K, respectively (10). In addition, a cohort study conducted by a Mexican entity, using a 24-h urine collection, estimated that 89.4% of adults have excessive Na intake, as well as inadequate K intake and Na-K ratio (11). Since the main determinant of urinary Na and K excretion is its intake, it can be determined through urine analysis.
High salt intake increases protein excretion in the urine, which causes damage to target organs: reduced kidney function, brain damage and damage to the heart; this, in turn, increases the risk of developing cardiovascular disorders (12). In addition, this excess consumption is favoring the development of stomach cancer, osteoporosis, and obesity (12). Although the gold standard calls for urine collected within 24 h, it has been shown that a spot urine sample obtained after fasting overnight can indicate daily excretion of Na and K for population studies, when using validated equations (13).
To date, there is no information that has described the average of Na and K excretion in a representative sample of adults in Mexico. Therefore, the objective of this study is to estimate mean Na and K excretion in Mexican adults participating in the National Health and Nutrition Survey 2016 (ENSANUT-2016, by its acronym in Spanish), using spot urine samples. As the Mexican population has diverse sociodemographic backgrounds and experiences a relevant burden of cardiovascular disorders influenced by dietary choices, we also aimed to obtain Na and K excretion estimates across the categories of these characteristics.
The National Health and Nutrition Survey 2016 in Mexico (ENSANUT-2016) was designed to quantify the frequency and distribution of health and nutrition conditions in the Mexican population, as well as associated risk factors at the national, regional, and urban vs. rural level. ENSANUT-2016 had a transversal, probabilistic design, with regional representation (North, Center, Mexico City and South), as well as representation by area of residence, including urban (population ≥ 2,500 inhabitants) and rural (population < 2,500 inhabitants) (14). An adult ≥20 years of age was selected in each home that was visited, with a 91% response rate in 8,262 participants. Subsequently, a sub-sample of 70% of adults –aged 20 to 59 years– was selected and urine samples were collected (14).
Previously trained personnel applied validated questionnaires to collect sociodemographic information, personal pathological history, and lifestyle variables from participants.
Weight and height measurements were obtained by trained personnel using international protocols (15). The body mass index (BMI) was classified according to the WHO criteria: normal BMI (18.5 to 24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obesity (≥ 30.0 kg/m2) (15).
An 11 mL spot urine sample was collected in a conical plastic tube. The first morning urine was discarded before collection. The sample was collected at the participant’s home and was subsequently refrigerated (2–8°C) for a maximum of 7 days. Afterwards, samples were frozen at −70°C until they were processed in the National Institute of Public Health laboratory. Microalbuminuria was measured by immunoturbidimetry technique with reaction type EP -2. Creatinine was measured by the Jaffé method, and Na and K were measured through indirect potentiometry with specific electrodes for the analyte and a Na glass electrode. Na and K were estimated from the Tanaka formula (16):
The creatinine level was measured to evaluate alterations in kidney function; it was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, based on the Glomerular filtration rate (GFR, ml/min/1.73m2) (17), and categorized as follows: normal (≥90); mildly reduced (60–89); moderately reduced (30–59); severely reduced (15–29) (18).
A blood sample was collected after ≥8 h of fasting. Serum biomarkers were analyzed using the Syncron® Clinical UniCel DxC 600 system. Glucose level to classify diabetes (prediabetes: fasting glucose ≥100 mg/dL and ≤ 125 mg/dL, or HbA1c ≥5.7 and < 6.5%; diagnosed diabetes: if they answered “yes” to the question: “Has a doctor ever told you that you have diabetes or high blood sugar?”; undiagnosed diabetes: if they answered “no” to the previous question and had fasting blood glucose ≥126 mg/dL or HbA1c ≥6.5% at the time of the survey), total cholesterol (high cholesterol levels: ≥200 mg/dL), triglycerides (high triglyceride levels: ≥150 mg/dL), hypoalphalipoproteinemia-cholesterol (HDL-c [Low HDL-c levels: women <50 mg/dL or men <40 mg/dL]), and Low-density lipoprotein-cholesterol (LDL-c [High LDL-c levels: ≥100 mg/dL]), were analyzed by endpoint coupled methods.
Blood pressure (BP) was measured using an Omron HEM-907 XL digital sphygmomanometer following the protocol recommended by the American Heart Association (19). Adults were classified as with normal BP (systolic BP <120 mmHg and diastolic BP <80 mmHg). To identify an adult with high blood pressure, two variables were constructed: (1) diagnosed hypertension: if the adult answered “yes” to the question: “Has a doctor told you that you have high blood pressure?”; (2) undiagnosed hypertension: if the adult answered “no” to the previous question and they had elevated BP (systolic BP 120–129 mmHg and diastolic BP < 80 mmHg); hypertension stage 1 (systolic BP 130–139 mmHg or diastolic BP 80–89 mmHg); hypertension stage 2 (systolic BP >140 mmHg or diastolic BP >90 mmHg).
All participants signed the informed consent approved by the Institutional Review Board of the Mexican National Institute of Public Health (MNIPH). This study was a secondary data analysis; the Ethics and Research Commissions of the MNIPH with Commission number 1401, Bioethics registration 17 CEI00120130424, and COFEPRIS registration CEI 17007 36 approved the original protocol on March 16, 2015.
The mean, [95% confidence intervals (95%CI)] and the median [interquartile range (IQR)] of urinary excretion of Na, K, creatinine, and eGFR were computed for the overall sample and stratified by sex. Sex-based mean comparisons were performed using two-tailed t-tests, while medians were contrasted employing quantile regression models, including a binary sex indicator as the primary predictor. Subsequently, mean values (95%CIs) for Na and K excretion, as well as for the Na-K ratio, were tabulated across sociodemographic characteristics (e.g., sex, age groups, education) and cardiovascular disorders (e.g., diabetes status, dyslipidemia, renal function). Two-tailed t-tests (for binary variables) or analysis of variance (Bonferroni-adjusted ANOVA; for categorical variables with more than two groups) were utilized to compare Na excretion, K excretion, and the Na-K ratio mean values across strata of these variables. Additionally, the mean values (95%CIs) of anthropometric variables (e.g., BMI, waist circumference), blood pressure, fasting glucose, eGFR, cholesterol, HDL-c, LDL-c, and triglycerides were tabulated across urinary Na/K excretion quartiles and compared through ANOVA tests. The prevalence of diabetes status categories, hypertension strata, cardiovascular disease binary indicators, and smoking status groups was also tabulated across Na/K excretion quartiles, and comparisons were made using χ2 tests or Fisher exact tests. Finally, the prevalence of high sodium intake, insufficient potassium intake, and sodium–potassium ratio was calculated in the overall sample and stratified by sex. All calculations were adjusted for the complex survey design of ENSANUT using the SVY module in STATA version 14 (College Station, TX, USA).
Information on 2,778 participants between 20 and 59 years of age (49.9% women and 50.1% men) was analyzed, indicating that ≈76% were overweight or obese, 46% had high blood pressure, 32% had impaired fasting glucose or diabetes, and 2% had coronary artery disease (Supplementary Table S1).
Table 1 shows that the mean urinary Na concentration was 133.6 mmol/L (95%CI: 128.7, 138.6) and that of K was 46.5 mmol/L (95%CI: 44.1, 48.9). The distribution of Na and K was not normal, so the median values for Na (129.2 mmol/L) and K (38.8 mmol/L) were estimated. When categorizing by sex, no differences were found in the average concentration of Na and K in the urine. Only a higher concentration of creatinine was observed in men (171.8 mmol/L; 95%CI: 162.9, 180.7) compared to women (142.7 mmol/L; 95%CI: 133.1, 152.3). Likewise, the mean urinary Na excretion was 3,354 mg/day (95%CI: 3,278, 3,429), mean K excretion was 1,440 mg/day (95%CI: 1,412, 1,469), and the Na-K ratio was 2.4 (95%CI: 2.3, 2.5) (Supplementary Table S2).

Table 1. Urinary sodium, potassium, and creatinine concentrations and eGFR of Mexican adults 20–59 years old by sex.
In obese adults, urinary Na and K excretion was ≈14% greater (Na 3,553.9 mg/day; and K 1,538.6 mg/day) than in adults with normal BMI (Na 3,113.7 mg/day; and K 1,331.1 mg/day). Excretion of Na was greater in urban and metropolitan areas –as compared to rural areas–, as well as in the northern region of the country. Concerning blood pressure, urinary excretion of Na was greater in individuals with high blood pressure stages 1 and 2 and compared to individuals with normal blood pressure or who had a previous diagnosis of hypertension. Furthermore, excretion of Na was greater in individuals with prediabetes and those who were found to have type 2 diabetes, compared to those with normal fasting glucose levels and those previously diagnosed with type 2 diabetes (Supplementary Table S2).
Na excretion was lower in individuals with a hypercholesterolemia diagnosis and elevated LDL-c, compared to individuals with normal cholesterol and LDL-c. However, it was higher in those with hypoalphalipoproteinemia (low HDL-c) compared to individuals who had normal HDL-c. Na excretion was positively associated (higher) in individuals diagnosed with cerebrovascular disease and coronary artery disease (Supplementary Table S2).
Urinary K excretion was positively associated with older age (50–59 years), SES, the northern region of the country, and BMI. Likewise, a positive trend was observed in relation to K excretion and blood pressure, which was higher in individuals who had a previous diagnosis of hypertension and type 2 diabetes, as well as those who were found to have type 2 diabetes and prediabetes through the survey, as compared to those with normal fasting glucose levels (Supplementary Table S2).
Greater K excretion was observed in individuals with a diagnosis of cerebrovascular disease, compared to adults without this condition. In adults diagnosed with hypertension, K excretion was 10% higher (1,534 mg/day; 95%CI: 1,470, 1,599) than in adults with normal blood pressure (1,357 mg/day; 95%CI: 1,357, 1,435). In relation to adults with moderate reduction of renal function (MRFR), Na excretion was 22% lower (2,772 mg/day; 95%CI: 2,300, 3,244) than in adults with normal renal function (3,382 mg/day; 95%CI: 3,301, 3,462). Likewise, the Na-K ratio was 2.1 in adults with MRFR and 2.4 in adults with normal kidney function (Supplementary Table S2).
When categorizing urinary Na excretion in quartiles (Table 2), it was observed that the mean waist circumference was greater in women (97.8 cm) and men (99.5 cm) in the fourth quartile (4,436 mg/day) than in the first quartile of Na (2,291 mg/day), being 90.7 cm in women and 90.5 cm in men. In the fourth quartile of K (1,948 mg/day), the mean BMI was higher (29.6 kg/m2) than in the first quartile of K (1,030 mg/day and BMI of 26.6 kg/m2) (Table 3). The values for mean waist circumference in men and women, as well as for hypertension diagnosis, were positively associated with a higher urinary excretion of K (first quartile to fourth quartile) (Table 3).
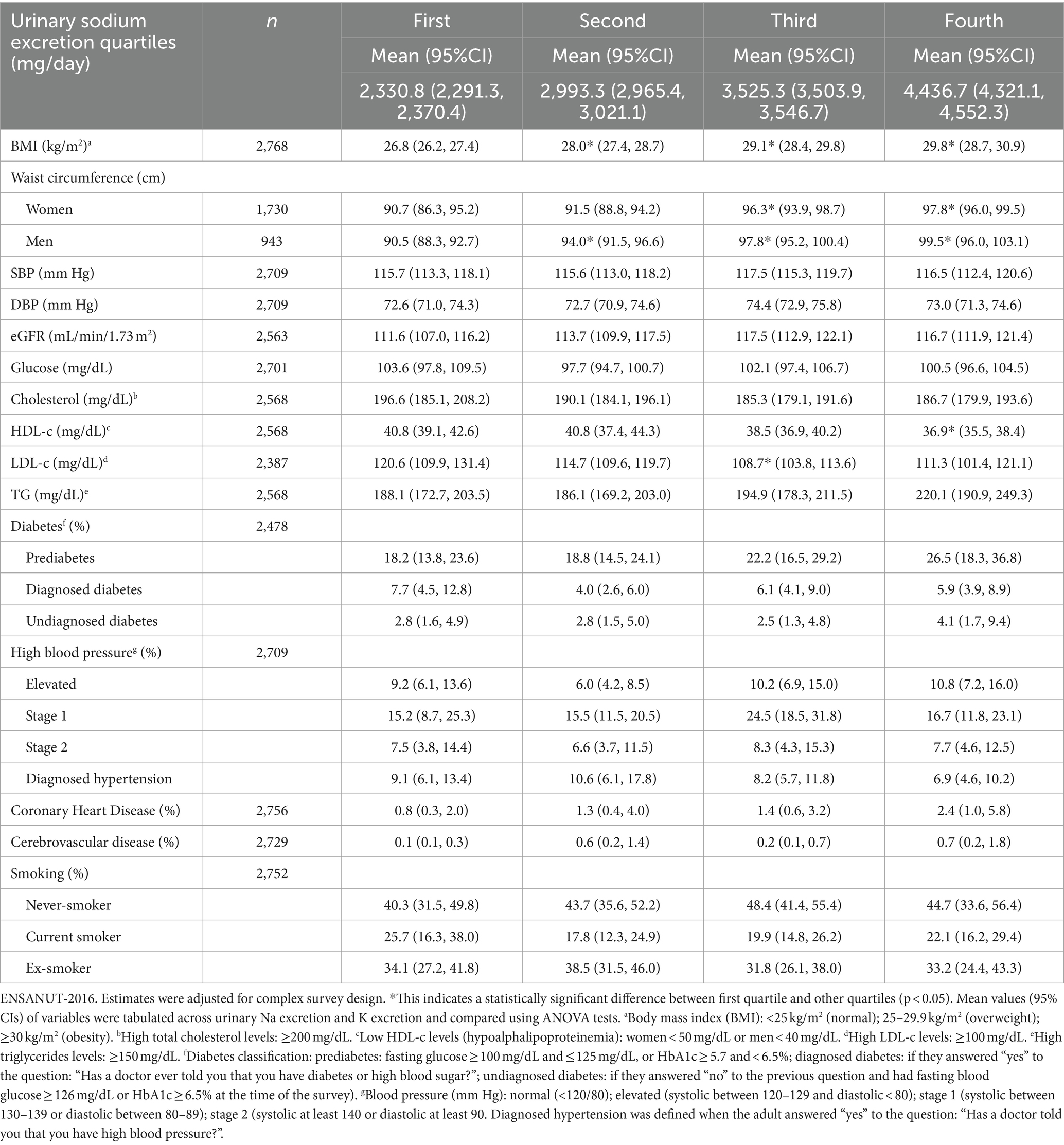
Table 2. Nutritional status and clinical characteristics according to quartiles of urinary sodium excretion in the Mexican adults’ population.
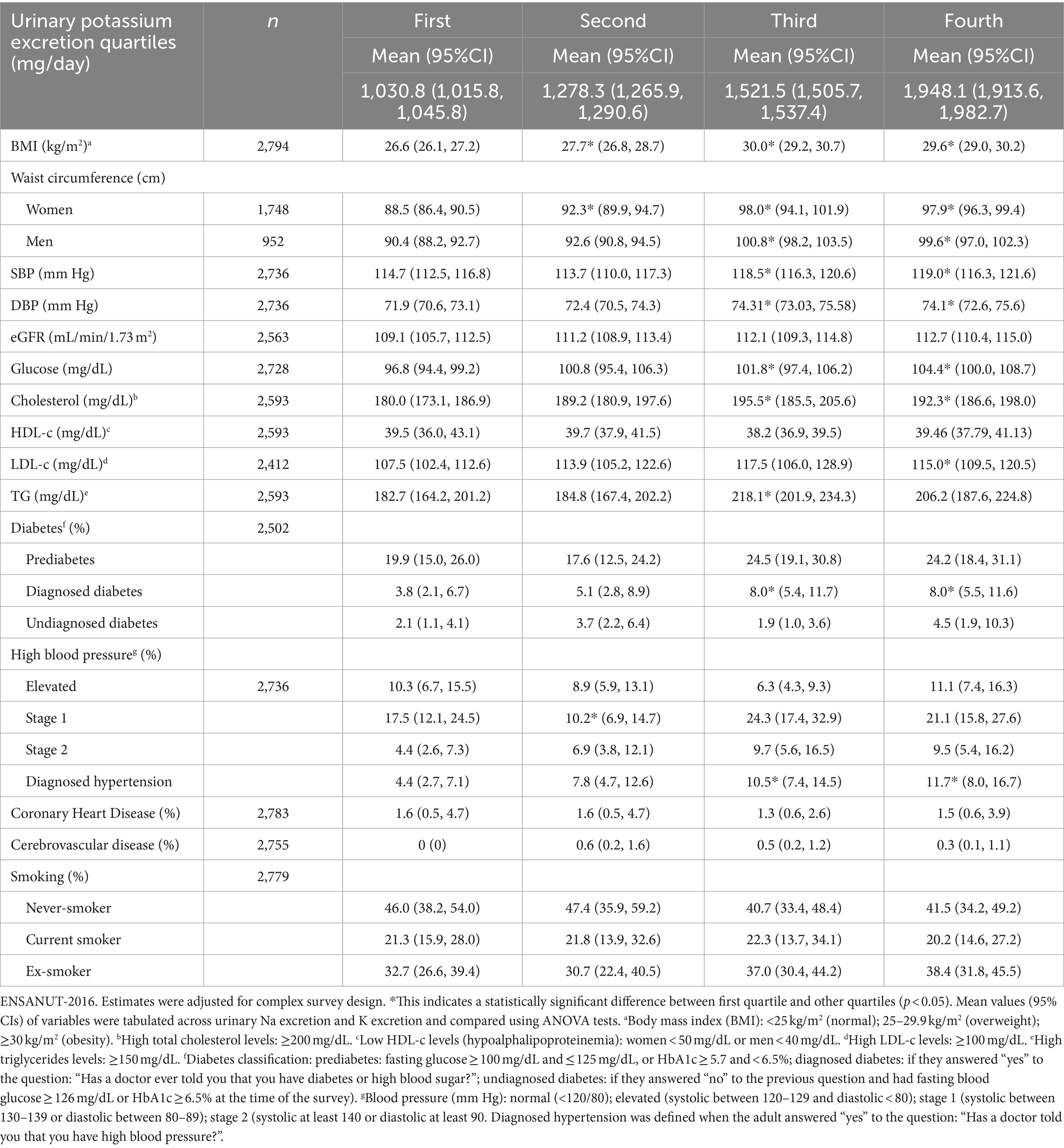
Table 3. Nutritional status and clinical characteristics according to quartiles of urinary potassium excretion in the Mexican adults’ population.
Figure 1 shows the proportion of participants with high Na and K intake, as well as a Na-K ratio greater than 1.0. About 97% of the adult population intakes Na above the WHO recommendations. However, the proportion of the adult population that exceeds the Na intake recommended by the AHA is 89%. Nevertheless, the Mexican adult population has insufficient K intake in accordance with WHO recommendations. Likewise, the entire Mexican adult population has a Na-K ratio > 1. No differences by sex were found.
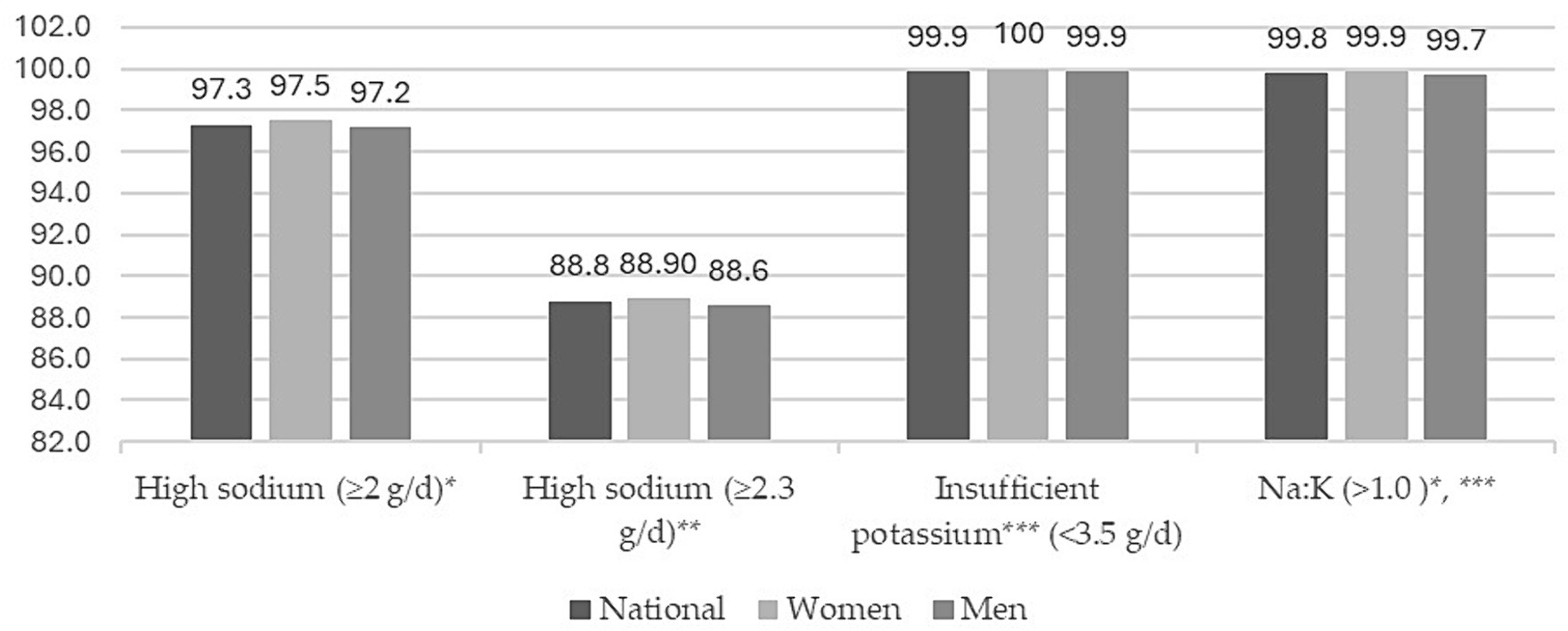
Figure 1. Proportions (%) of high sodium, insufficient potassium intake, and sodium–potassium ratio by sex in Mexican population. ENSANUT-2016.
In this nationwide representative sample of 2,778 Mexican adults aged 20 to 59 years, a mean urinary Na excretion of 133.6 mmol/L and K excretion of 46.5 mmol/L was observed, with a Na-K ratio of 2.4. In addition, urinary Na excretion was higher in individuals with stage 1 and stage 2 hypertension, in individuals with prediabetes, and in those found to have type 2 diabetes, whereas it was positively associated in individuals diagnosed with cerebrovascular disease and coronary heart disease; whereas Na excretion was lower in individuals diagnosed with hypercholesterolemia and elevated LDL-c compared to individuals with normal cholesterol and LDL-c. Likewise, higher K excretion was observed in individuals with a previous diagnosis of hypertension and type 2 diabetes, as well as in those with type 2 diabetes and prediabetes detected through the survey, cerebrovascular disease, and adults with a diagnosis of hypertension.
In this study, Na and K intakes were slightly lower according to what was found in a systematic review in America, where they found mean excretion of 157.3 mmol/24 h for Na and 57.7 mmol/24 h for K (20). Likewise, in Latin America and the Caribbean, it has been identified that the mean Na intake is close to 149.13 mmol/L, which is like that identified in this study, but lower than the estimates for Colombia, Brazil, and Chile (204.4 mmol/L) (21). In Mexico, estimates from diet are very similar to those obtained in this study where a urine spot sample was obtained (3.1 g/day vs. 3.4 g/day, respectively) (10).
These Na and K excretion values were lower than those observed in a study conducted in more than 100,000 individuals from 17 low-and middle-income countries (4,930 and 2,120 mg/day, respectively) (22). However, the median Na excretion in the present study (3,315 mg/day) would reflect a Na consumption that far exceeds the American College of Cardiology/American Heart Association recommendations of 1,500–2,400 mg/day. Thus, more than 50% of Mexican adults would have an excessive Na intake according to these recommendations (23).
Nonetheless, the WHO and the Pan American Health Organization (PAHO) have established a goal to reduce Na consumption in adults to less than 2 grams (<87 mmol) per day by 2025 (6, 24). On the other hand, various organisms recommend K consumption to be above 3.5 grams per day (≥90 mmol). The data from our study indicate that more than 90% of Mexican adults exceed the Na recommendation and fail to meet the K recommendation (7).
Urinary Na excretion was higher in individuals with elevated BP and hypertension stages 1 and 2, compared to individuals with normal BP or who had a previous diagnosis of hypertension. Likewise, Na excretion was higher in individuals with prediabetes and those found to have diabetes type 2 through the survey, compared to individuals with normal fasting glucose and those previously diagnosed with diabetes type 2. Na excretion was lower in individuals with a hypercholesterolemia diagnosis and elevated LDL-c, compared to individuals with normal cholesterol and LDL-c. These findings possibly reflect a phenomenon of “reverse causality” and point to a possible modification of dietary habits in individuals who know their clinical condition (25). However, Na excretion was higher in individuals diagnosed with cerebrovascular disease and heart disease, which possibly translates to poor dietary control in these patients. Likewise, a review study showed that decreased sodium intake increases serum cholesterol, as well as HDL-c and LDL-c concentrations (26).
The positive association of Na excretion with male sex, BMI, residence in urban and metropolitan areas, socioeconomic level, and being in the northern region of the country possibly reflects a higher intake of high-sodium foods (for example, highly processed foods) (27) and low fruit and vegetable intake (28). In turn, these are associated with unhealthy dietary patterns that are conducive to chronic degenerative diseases (29).
When a person is diagnosed with high blood pressure, the first strategy for treatment and prevention of complications is to restrict sodium intake to less than 2,300 mg/day (30). This can coexist with the loss of K and consequently develop muscle weakness, irregular heart rhythm and increased sensitivity to salt (31). However, in our study, individuals with a previous diagnosis of hypertension (HT) had higher K excretion than individuals with normal blood pressure.
In the past three decades, elevated BP has been the main risk factor for preventable deaths (32). In Mexico, mortality due to high BP has increased by 29.9% in the past six years and in 2016 it was responsible for 18.1% of all deaths (33). It has been shown that daily excretion of Na and K can be used to estimate intake; nonetheless, measuring these electrolytes in urine samples collected within 24 h poses practical limitations for use in population studies. For this reason, different formulae have been developed to estimate the daily intake of Na and K from spot urine samples. Among the most used are the INTERSALT, Kawasaki, and Tanaka formulae, since these have achieved high precision when compared with the reference method (13). This last method, which we applied in our study, has a correlation of 0.72 for Na and 0.78 for K, and has shown to be potentially useful in studies with many individuals (34).
Diverse studies show a direct association between reduced consumption of Na and lower BP. In this sense, the lower sodium consumption is, the lower the cardiovascular risk (35). On the other hand, there is strong evidence of the association between an increase in K consumption and a linear reduction in BP and CVD (36). Since these minerals are closely related to BP and CVD, calculating the Na-K ratio is recommended. A ratio of osmolality of Na-K ratio < 1 in urine can be a useful indicator to meet levels of Na and K recommended by the WHO, as well as to reduce hypertension and CVD risk (3, 36, 37). It has been shown that a Na-K ratio of 1.13 (95%CI: 1.04, 1.22) for coronary heart disease and 1.20 (95%CI: 1.01, 1.42) for heart failure can cause a risk of 1.11 (95%CI: 1.04, 1.19) for a combined outcome of cardiovascular disease (37–39).
In the last decades, eating patterns in Mexico have shifted toward increased consumption of ultra-processed foods, accompanied by a reduction in fruit and vegetable intake (40). Therefore, the modern diet is high in Na, sugar, and fat content, but low in fiber (41). In addition, processed and ultra-processed products contribute, on average, 46% of the total Na in the diet, whereas minimally processed foods contribute more than 50% of the intake of K (10). In parallel, about 49% of Mexican adults have hypertension and the main cause of death is cardiovascular disease (5). If this trend continues, the Na-K ratio could increase, and this would have an impact on increased cardiovascular risk (42). Simulation studies have estimated that reducing Na consumption in Mexico as recommended by the WHO could reduce around 27,700 deaths from cardiovascular diseases, mainly ischemic heart disease, hypertensive disease, and stroke (43).
Among the strengths of the study are its representativeness, sample size, and the laboratory methods used. On the other hand, concerning the limitations of the present study, we cannot fail to mention the inherent difficulty of establishing causal association—for example, when evaluating the association between Na excretion and metabolic alterations, given that individuals with a previous diagnosis had lower levels compared to individuals who were assumed to be healthy or were unaware of their condition. This problem of reverse causality is common to several types of studies, especially cross-sectional ones (44). Another limitation, which is inherent to the use of the urine spots technique for population-level research because of its low risk of error and lower collection burden, and because it is more affordable than the collection of 24-h urine, is that these equations have been shown to be an inaccurate estimate of Na intake of 24-h dietary recall (45) and can overestimate low intake and underestimate high intake (45, 46). Additionally, the measurement of sodium in a single urine sample is affected by the salt content of recently ingested foods and diurnal excretion patterns. Therefore, we recognize that the association between sodium intake and blood pressure would likely have been improved if multiple urine samples had been measured rather than just the one (47) that we obtained in our study.
On the other hand, the excretion of K measured in urine is less precise than that of Na and represents approximately 80% of the intake. Underestimating the intake of K will lead to an overestimation of the proportion of individuals with low intake (47).
Until now, Mexico had not had representative population data on urinary Na and K excretion. The results of this study will provide an estimate of Na and K intake in the Mexican adult population.
The present study, performed in a representative sample of Mexican adults, shows high Na excretion and relatively low K excretion, in accordance with international recommendations aimed at reducing cardiovascular risk. However, concrete actions are required at the public policy level to reduce sodium intake in the Mexican population. Nevertheless, this information can serve as a reference for public policies aimed at controlling arterial hypertension and reducing CVD.
The data that support the findings of this study are available from the corresponding author upon reasonable request. The National Health and Nutrition Survey (ENSANUT, by its Spanish acronym) database used in this study is publicly available and can be accessed at https://ensanut.insp.mx/.
The studies involving humans were approved by the Institutional Review Board of the National Institute of Public Health in Mexico. This study was a secondary data analysis; Ethics and Research Commissions of the MNIPH with the Commission number 1401, Bioethics registration 17 CEI00120130424, and COFEPRIS registration CEI 17007 36 approved the original protocol. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
IC: Conceptualization, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. JV: Formal analysis, Methodology, Validation, Visualization, Writing – review & editing. MF: Writing – original draft, Writing – review & editing, Conceptualization, Investigation. SB: Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1395016/full#supplementary-material
1. Pirahanchi, Y, and Aeddula, NR. Sodium potassium pump (Na+ K+ pump). St. Petersburg, FL: StatPearls publishing (2023).
2. Pivovarov, AS, Calahorro, F, and Walker, RJ. Na+/K+-pump and neurotransmitter membrane receptors. Invertebr Neurosci. (2019) 19:1. doi: 10.1007/s10158-018-0221-7
3. Adrogué, HJ, and Madias, NE. The impact of sodium and potassium on hypertension risk. Semin Nephrol. (2014) 34:257–72. doi: 10.1016/j.semnephrol.2014.04.003
4. Welsh, CE, Welsh, P, Jhund, P, Delles, C, Celis-Morales, C, Lewsey, JD, et al. Urinary sodium excretion, blood pressure, and risk of future cardiovascular disease and mortality in subjects without prior cardiovascular disease. Hypertension. (2019) 73:1202–9. doi: 10.1161/HYPERTENSIONAHA.119.12726
5. Campos-Nonato, I, Hernández-Barrera, L, Oviedo-Solís, C, Ramírez-Villalobos, D, Hernández, B, and Barquera, S. Epidemiología de la hipertensión arterial en adultos mexicanos: diagnóstico, control y tendencias. Ensanut 2020. Salud Publica Mex. (2021) 63:12851. doi: 10.21149/12851
6. WHO . Guideline: Sodium intake for adults and children. Geneva: World Health Organization (2012).
7. World Health Organization (WHO) . Guideline: Potassium intake for adults and children. Geneva: World Health Organization (2012).
8. World Health Organization . WHO global report on sodium intake reduction. Geneva: World Health Organization (2023).
9. Reddin, C, Ferguson, J, Murphy, R, Clarke, A, Judge, C, Griffith, V, et al. Global mean potassium intake: a systematic review and Bayesian meta-analysis. Eur J Nutr. (2023) 62:2027–37. doi: 10.1007/s00394-023-03128-6
10. Vargas-Meza, J, Cervantes-Armenta, MA, Campos-Nonato, I, Nieto, C, Marrón-Ponce, JA, Barquera, S, et al. Dietary sodium and potassium intakes: data from the Mexican National Health and nutrition survey 2016. Nutrients. (2022) 14:281. doi: 10.3390/nu14020281
11. Vallejo, M, Col, E, and Madero, M. Assessment of sodium and potassium intake by 24 h urinary excretion in a healthy Mexican cohort. Arch Med Res. (2017) 48:195–202. doi: 10.1016/j.arcmed.2017.03.012
12. He, FJ, Tan, M, Ma, Y, and MacGregor, GA. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:632–47. doi: 10.1016/j.jacc.2019.11.055
13. Vidal-Petiot, E, Joseph, A, Resche-Rigon, M, Boutten, A, Mullaert, J, Ortho, MPD, et al. External validation and comparison of formulae estimating 24-h sodium intake from a fasting morning urine sample. J Hypertens. (2018) 36:785–92. doi: 10.1097/HJH.0000000000001609
14. Instituto Nacional de Salud Pública . Encuesta Nacional de Salud y Nutrición de Medio Camino 2016. México: Instituto Nacional de Salud Pública (2016).
15. World Health Organization . Physical status: The use of and interpretation of anthropometry, report of a WHO expert committee. Geneva: World Health Organization (1995).
16. Tanaka, T, Okamura, T, Miura, K, Kadowaki, T, Ueshima, H, Nakagawa, H, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. (2002) 16:97–103. doi: 10.1038/sj.jhh.1001307
17. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
18. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). (2013) 3:19–62. doi: 10.1038/kisup.2012.64
19. Muntner, P, Shimbo, D, Carey, RM, Charleston, JB, Gaillard, T, Misra, S, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. (2019):35–66. doi: 10.1161/HYP.0000000000000087
20. Valero-Morales, I, Tan, M, Pei, Y, He, FJ, and MacGregor, GA. 24-hour sodium and potassium excretion in the Americas: a systematic review and meta-analysis. Rev Panam Salud Publica. (2022) 46:1. doi: 10.26633/RPSP.2022.199
21. Carrillo-Larco, RM, and Bernabe-Ortiz, A. Sodium and salt consumption in Latin America and the Caribbean: a systematic-review and meta-analysis of population-based studies and surveys. Nutrients. (2020) 12:556. doi: 10.3390/nu12020556
22. O’Donnell, M, Mente, A, Rangarajan, S, McQueen, MJ, Wang, X, Liu, L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. (2014) 371:612–23. doi: 10.1056/NEJMoa1311889
23. Eckel, RH, Jakicic, JM, Ard, JD, de Jesus, JM, Miller, NH, Hubbard, VS, et al. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. Circulation. (2013) 129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1
24. World Health Assembly 66 . (2013). Follow-up to the political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. Available at: https://apps.who.int/iris/handle/10665/150161 (Accessed May 28, 2023)
25. Hernán, MA. A definition of causal effect for epidemiological research. J Epidemiol Community Health (1978). (2004) 58:265–71. doi: 10.1136/jech.2002.006361
26. Graudal, NA, Hubeck-Graudal, T, and Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. (2017) 4:CD004022. doi: 10.1002/14651858.CD004022.pub4
27. Nieto, C, Tolentino-Mayo, L, Medina, C, Monterrubio-Flores, E, Denova-Gutiérrez, E, and Barquera, S. Sodium content of processed foods available in the Mexican market. Nutrients. (2018) 10:2008. doi: 10.3390/nu10122008
28. García-chávez, CG, Monterrubio-flores, E, Ramírez-silva, I, Aburto, TC, Pedraza, LS, and Rivera-dommarco, J. Contribución de los alimentos a la ingesta total de energía en la dieta de los mexicanos mayores de cinco años. Salud Publica Mex. (2020) 62:166. doi: 10.21149/10636
29. Marrón-Ponce, JA, Flores, M, Cediel, G, Monteiro, CA, and Batis, C. Associations between consumption of ultra-processed foods and intake of nutrients related to chronic non-communicable diseases in Mexico. J Acad Nutr Diet. (2019) 119:1852–65. doi: 10.1016/j.jand.2019.04.020
30. American Heart Association . (2021). How much sodium should I eat per day? Available at: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/sodium/how-much-sodium-should-i-eat-per-day (Accessed May 28, 2023)
31. Gumz, ML, Rabinowitz, L, and Wingo, CS. An integrated view of potassium homeostasis. N Engl J Med. (2015) 373:60–72. doi: 10.1056/NEJMra1313341
32. Forouzanfar, MH, Afshin, A, Alexander, LT, Anderson, HR, Bhutta, ZA, Biryukov, S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1659–724. doi: 10.1016/S0140-6736(16)31679-8
33. Campos-Nonato, I, Vargas Meza, J, Nieto, C, Ariza, AC, and Barquera, S. Reducing sodium consumption in Mexico: a strategy to decrease the morbidity and mortality of cardiovascular diseases. Front Public Health. (2022) 10:857818. doi: 10.3389/fpubh.2022.857818
34. Ji, C, Sykes, L, Paul, C, Dary, O, Legetic, B, Campbell, NRC, et al. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica. (2012) 32:307–15. doi: 10.1590/S1020-49892012001000010
35. Mozaffarian, D, Fahimi, S, Singh, GM, Micha, R, Khatibzadeh, S, Engell, RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. (2014) 371:624–34. doi: 10.1056/NEJMoa1304127
36. Kapoor, K, Fashanu, O, Post, WS, Lutsey, PL, Michos, ED, deFilippi, CR, et al. Relation of dietary sodium intake with subclinical markers of cardiovascular disease (from MESA). Am J Cardiol. (2019) 124:636–43. doi: 10.1016/j.amjcard.2019.05.014
37. O’Donnell, M, Mente, A, Rangarajan, S, McQueen, MJ, O’Leary, N, Yin, L, et al. Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: prospective cohort study. BMJ. (2019) 364:1–14. doi: 10.1136/bmj.l772
38. Iwahori, T, Miura, K, Ueshima, H, Tanaka-Mizuno, S, Chan, Q, Arima, H, et al. Urinary sodium-to-potassium ratio and intake of sodium and potassium among men and women from multiethnic general populations: the INTERSALT study. Hypertens Res. (2019) 42:1590–8. doi: 10.1038/s41440-019-0263-1
39. Prentice, RL, Huang, Y, Neuhouser, ML, Manson, JE, Mossavar-Rahmani, Y, Thomas, F, et al. Associations of biomarker-calibrated sodium and potassium intakes with cardiovascular disease risk among postmenopausal women. Am J Epidemiol. (2017) 186:1035–43. doi: 10.1093/aje/kwx238
40. Marrón-Ponce, JA, Tolentino-Mayo, L, Hernández-F, M, and Batis, C. Trends in ultra-processed food purchases from 1984 to 2016 in Mexican households. Nutrients. (2019) 11:1–15. doi: 10.3390/nu11010045
41. Rivera Dommarco, MA, Fuentes, ML, González de Cosío Martínez, T, Aguilar Salinas, CA, Hernández Licona, G, and Barquera, S. La obesidad en México. Estado de la política pública y recomendaciones para su prevención y control. Cuernavaca: Salud Pública de México (2018). 270 p.
42. Schnabel, L, Kesse-Guyot, E, Allès, B, Touvier, M, Srour, B, Hercberg, S, et al. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med. (2019) 179:490–8. doi: 10.1001/jamainternmed.2018.7289
43. Vargas-Meza, J, Nilson, EAF, Nieto, C, Khandpur, N, Denova-Gutiérrez, E, Valero-Morales, I, et al. Modelling the impact of sodium intake on cardiovascular disease mortality in Mexico. BMC Public Health. (2023) 23:983. doi: 10.1186/s12889-023-15827-0
44. Rothman, KJ, and Greenland, S. Causation and causal inference in epidemiology. Am J Public Health. (2005) 95:S144–50. doi: 10.2105/AJPH.2004.059204
45. Polonia, J, Lobo, MF, Martins, L, Pinto, F, and Nazare, J. Estimation of populational 24-h urinary sodium and potassium excretion from spot urine samples. J Hypertens. (2017) 35:477–86. doi: 10.1097/HJH.0000000000001180
46. Ji, C, Miller, MA, Venezia, A, Strazzullo, P, and Cappuccio, FP. Comparisons of spot vs 24-h urine samples for estimating population salt intake: validation study in two independent samples of adults in Britain and Italy. Nutr Metab Cardiovasc Dis. (2014) 24:140–7. doi: 10.1016/j.numecd.2013.06.011
Keywords: sodium, potassium, urinary excretion, hypertension, cardiovascular disease, Mexico
Citation: Campos Nonato I, Mendoza K, Vargas Meza J, Flores Aldana M and Barquera S (2024) Sodium and potassium excretion and its association with cardiovascular disorders in Mexican adults. Front. Nutr. 11:1395016. doi: 10.3389/fnut.2024.1395016
Received: 02 March 2024; Accepted: 06 June 2024;
Published: 24 June 2024.
Edited by:
AslıUçar, Ankara University, TürkiyeReviewed by:
Gül Kızıltan, Başkent University, TürkiyeCopyright © 2024 Campos Nonato, Mendoza, Vargas Meza, Flores Aldana and Barquera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorge Vargas Meza, am9yZ2UudmFyZ2FzQGluc3AubXg=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.