- 1Department of Psychology, University of South Florida, Tampa, FL, United States
- 2Orthosports, Concord, NSW, Australia
- 3Department of Cell Biology and Physiology, Brigham Young University, Provo, UT, United States
Introduction
Ketogenic (very low carbohydrate) diets have well-established, as well as potential, benefits in the treatment of neurological disorders. Over a century ago the ketogenic diet was adopted as an effective treatment for epilepsy (1). More recently, ketogenic diets have demonstrated promising therapeutic potential in a broad range of neurological disorders, including Alzheimer's disease, Parkinson's disease, multiple sclerosis, ischemic stroke, migraine, major depressive disorder, bipolar disorder and psychotic illness (2–5), as well as a potential treatment for traumatic brain injury (6). This research has identified great promise in the use of the ketogenic diet to improve brain functioning, particularly in response to psychiatric disorders and injury.
The ketogenic diet, however, is not without its detractors. A concern with the ketogenic diet is that in some individuals very low carbohydrate consumption can lead to dramatic increases in the level of low-density lipoprotein cholesterol (LDL-C) (7, 8), which is considered a primary cause of cardiovascular disease (CVD) (9). Whereas the ketogenic diet is beneficial for mental health and in the treatment of neurological disorders, but for some individuals with elevated LDL-C, is that benefit obtained at the cost of increasing their risk of developing CVD? We have addressed this issue with an analysis of the benefits vs. potential harms of a ketogenic diet-induced increase in LDL-C.
Is elevated LDL-C inherently atherogenic?
An elevated level of LDL-C has been described as “unequivocally recognized as the principal driving force in the development of (atherosclerotic cardiovascular disease)” (9) and that “the key initiating event in atherogenesis is the retention of low-density lipoprotein (LDL) cholesterol (LDL-C) … within the arterial wall” (10). The view that high LDL-C is atherogenic provides the basis for why an LCD-induced increase in LDL-C has been seen as increasing the risk for developing CVD (8, 11–18). In one example, a ketogenic diet-induced increase in LDL-C was the topic of an editorial that stated these individuals should “work closely with their doctor to implement lifestyle changes and/or medical therapy directed toward lipid lowering with the aim of reducing cardiovascular risk” (18).
Although LDL-C as a cause of CVD is the consensus of key opinion leaders, there are findings that are not supportive of this perspective. An inconsistent, and largely ignored, finding is that cardiovascular and all-cause mortality in people with familial hypercholesterolemia (FH), who have extremely high levels of LDL-C from birth, declines with advanced age, resulting in an overall normal lifespan (19–23). Moreover, people with FH exhibit an equivalent degree of aspects of cardiovascular morbidity, such as ischemic stroke (24), as the general population. These findings challenge the consensus that high LDL-C is inherently atherogenic.
What has been largely ignored in the consensus opinion of FH is that only a subset of individuals with FH die prematurely of CVD. A close assessment of this research reveals that this subset of FH individuals develop coagulopathy, independent of their LDL-C levels (25–29). In one representative study, Jansen et al. (28) reported that FH patients that developed CVD had a polymorphism for the prothrombin gene, which is also associated with premature CVD in the non-FH population (30). Sugrue et al. (31), as well, reported that FH individuals with coronary heart disease (CHD) had higher levels of clotting factors (plasma fibrinogen and factor VIII), and conversely, Sebestjen et al. (32) found reduced markers of fibrinolysis in FH individuals that experienced a myocardial infarction, both of which were independent of their LDL-C.
In complementary research, high LDL-C appears to protect against bacterial infection, which is a risk factor for CVD (33–39). The protection of individuals with high LDL-C from infection and its sequalae is manifested, in one example, by the significantly lower rate of sepsis, and sepsis-induced organ damage, in people with high LDL-C, compared to those with low LDL-C (40).
With regard to the critical factors leading to CVD susceptibility, it has long been recognized that coronary artery calcium (CAC) scoring is superior to LDL-C as the single best predictor of fatal and non-fatal coronary events (41–44). For example, approximately half of FH individuals assessed showed zero CAC, which would indicate they have a low risk for developing CVD, despite their high LDL-C levels (45). Moreover, this study demonstrated that a high CAC score and elevated fasting glucose, unlike LDL-C, were both associated with coronary events (Figure 1). Similar findings were reported by Mortensen et al. (46) in a study of non-FH individuals. These findings led Bittencourt et al. (47), to conclude that “treatment of individuals with very high LDL-C (>190 mg/dl) irrespective of their clinical risk … might not be the most prudent approach.”
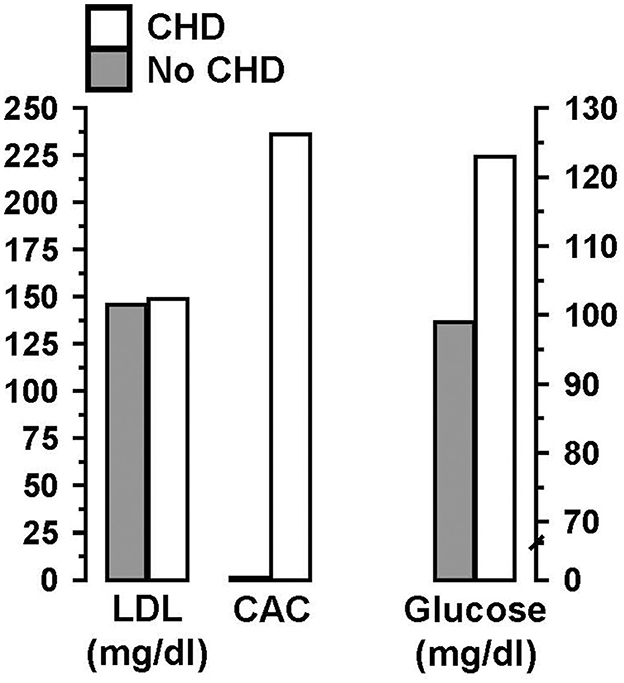
Figure 1. In individuals with familial hypercholesterolemia (FH), a high coronary artery calcium (CAC) score and elevated fasting glucose, unlike high low-density lipoprotein cholesterol (LDL-C), were associated with coronary heart disease (CHD). Data from Miname et al. (45).
At a mechanistic level, concerns with a ketogenic diet-induced increase in LDL-C have not taken into account that the “total LDL-C” measure reported in a conventional lipid panel represents a heterogeneous population of different LDL particle types (48, 49), one of which is referred to as lipoprotein (a) [Lp(a)]. An elevation of Lp(a) is an independent risk factor for the development of CVD (50–54). The association of Lp(a) to CVD may be driven, in part, by its strong atherogenic effects at multiple metabolism levels, particularly in promoting thrombosis (55, 56). For example, Yang et al. (57) demonstrated that the combination of high Lp(a) and fibrinogen levels were correlated with the highest incidence of ischemic stroke in statin-treated patients, while LDL-C levels were unrelated to stroke incidence. Finally, Willeit et al. (58) showed that Lp(a) is a critical component of the association of LDL-C with CVD; without the Lp(a)component, LDL-C, alone, was not associated with CVD.
Insulin resistance and cardiovascular disease
Hyperinsulinemia and hyperglycemia, collectively referred to as insulin resistance (IR), are strong and independent risk factors for CVD (59–63). IR may develop into type 2 diabetes, which typically is not accompanied by an elevation of LDL-C (64), and yet it has the greatest risk for CVD (65). There are multiple mechanisms by which IR exerts an adverse effect on blood vessel structure and functioning leading to CVD (60, 61, 66–71). For example, Yu et al. (72) reported that elevated fasting plasma glucose, hemoglobin A1c and triglycerides (TG), unlike, LDL-C, were all positively correlated with the severity of coronary stenosis. Thus, IR is superior to LDL-C as a marker for CVD risk.
An important but often ignored influence on LDL-C structure and function is referred to as atherogenic dyslipidemia, in which elevated LDL-C is accompanied by elevated triglycerides and low HDL, which is a common metabolic state in people with Type 2 diabetes and obesity (73–75). Under atherogenic dyslipidemia conditions, the composition of the LDL particles (LDL-P) exhibits a shift toward a greater density of small, dense LDL-P (sdLDL) and a reduced density of large, buoyant LDL-P (lbLDL). This shift in the dominance of sdLDL over lbLDL is characteristic of a pro-atherogenic state, originally described as “phenotype B” (76). Phenotype B, in contrast to those with low triglycerides, high lbLDL and high HDL (phenotype A), is strongly associated with an increased incidence of CVD (48, 56, 77–90). One example of this finding is that an elevated level of sdLDL, but not LDL-C or lbLDL, was an independent risk factor for ischemic stroke (87) (Figure 2). Numerous observational studies, as well, have shown that lbLDL is not associated with CVD (91–94).
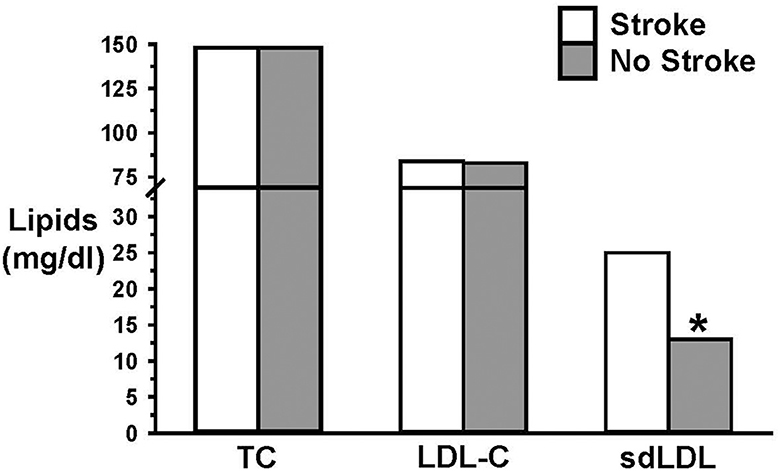
Figure 2. Elevated small dense LDL (sdLDL), but not LDL-C, was an independent risk factor for ischemic stroke. Data from Zhou et al. (87). * = p < 0.05.
It is therefore important to recognize that the primary reason why LDL-C is a poor marker for CVD risk is because it is a hybrid measure, composed of different sizes of LDL particles (sdLDL and lbLDL), as well as Lp(a) (discussed previously), each with a different association to metabolic health and CVD risk (90, 95) [see also Gjuladin-Hellon et al. (96) and Diamond et al. (97) for related review and discussion].
Effects of low carbohydrate diets on cardiovascular disease risk factors
Carbohydrate restriction has been shown to improve a broad range of CVD risk factors (49, 98–122). It is notable that along with the improvement in metabolic measures, LCD reduces the need for hypoglycemic and antihypertensive medications (111, 123–132). Moreover, LCDs attenuate the atherogenic dyslipidemia risk triad (reducing TGs, sdLDL, increasing lbLDL and HDL) (49, 96, 105, 133–136). Long-term trials and case reports have demonstrated the benefits of LCD (49, 100, 102, 137–144) and in documenting improvements in numerous CVD risk biomarkers (133, 144–146).
Despite the improvements in CVD risk factors with LCD, there remain concerns about LCD because of the absence of research on individuals with diet-induced high LDL-C and coronary events. A case study on a father and son diagnosed with FH may be of value in appreciating how atherogenic dyslipidemia is expressed as CVD risk, indirectly in relation to LCD. In this study, a father and son shared the same LDL mutation which resulted in both being diagnosed with FH. Despite their equivalently high levels of total cholesterol (344 vs. 352 mg/dl; father vs. son) and LDL-C (267 vs. 271 mg/dl; father vs. son), only the son (54 years old), but not the father (84 years old), had coronary heart disease (CHD). Although dietary assessments were not provided, the authors suggested that differences in their lifestyles and diets may have been a contributing factor to their differential incidence of CHD, independent of their LDL-C. Specifically, the father's triglycerides at 124.0 mg/dl were almost half of the 230.0 mg/dl measured in his son, and the father's HDL at 54.0 mg/dl was far greater than his son's HDL at 34.8. Thus, the high triglycerides and low HDL of the son provided the basis of the authors' perspective that the son exhibited LDL subclass pattern B, which is associated with a high risk of CVD and a high carbohydrate diet (75, 76). Overall, these findings are consistent with the work of Sijbrands et al. (22), who concluded that cardiovascular outcomes in people with FH are not determined solely by high LDL-C, and instead are the result of the interactions among lipids, genetics and dietary factors.
Discussion
We have addressed concerns regarding high LDL-C that can develop in a subset of individuals on a ketogenic diet. Our commentary has evaluated whether these concerns are justified. We have briefly summarized research which has demonstrated that LDL-C is a faulty marker of CVD risk because it is a hybrid measure composed of multiple components, each with a different association to CVD. Specifically, LDL-C includes lbLDL, sdLDL, and Lp(a), each of which can be influenced by proximal influences on CVD, such as insulin resistance, hypertension, hyperglycemia and more generally, metabolic syndrome. Thus, sdLDL and Lp(a) are not intrinsically atherogenic; each becomes an atherogenic component of the maelstrom of metabolic dysfunction that occurs in response to metabolic syndrome.
The component of LDL-C that dominates in metabolically healthy people is the lbLDL particle, which is not associated with CVD events. Observational trials and RCTs have demonstrated that individuals with high LDL-C and a dominance of lbLDL (phenotype pattern A) and an LCD-like lipid profile (low TGs and high HDL-C), have a lower rate of coronary events than those with pattern B (high LDL-C, high TGs, and low HDL-C) (147, 148).
In summary, our review of the literature provides support for the conclusion that elevated LDL-C occurring in an individual on a ketogenic diet does not place a person at an elevated risk for CVD. Indeed, a person on a ketogenic diet would exhibit a dominance of beneficial lipid markers (low triglycerides, high HDL, high lbLDL), as well as beneficial non-lipid markers (low inflammation, blood glucose, and blood pressure). These findings support the conclusion that pharmacological or dietary interventions to reduce LDL-C in an individual on LCD are not warranted. Indeed, this favorable cluster of LCD-induced changes in biomarkers should not only result in a reduced risk of CVD, it should promote beneficial health outcomes based on the important role of LDL in optimizing immune functioning.
Author contributions
DD: Writing – original draft, Writing – review & editing. PM: Writing – review & editing. BB: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hohn S, Dozieres-Puyravel B, Auvin S. History of dietary treatment from Wilder's hypothesis to the first open studies in the 1920s. Epilepsy Behav. (2019) 101:106588. doi: 10.1016/j.yebeh.2019.106588
2. Grigolon RB, Gerchman F, Schoffel AC, Hawken ER, Gill H, Vazquez GH, et al. Mental, emotional, and behavioral effects of ketogenic diet for non-epileptic neuropsychiatric conditions. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 102:109947. doi: 10.1016/j.pnpbp.2020.109947
3. Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A, et al. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci Biobehav R. (2018) 94:11–6. doi: 10.1016/j.neubiorev.2018.07.020
4. Myette-Côté É, Soto-Mota A, Cunnane SC. Ketones: potential to achieve brain energy rescue and sustain cognitive health during ageing. Brit J Nutr. (2022) 128:407–23. doi: 10.1017/S0007114521003883
5. Newport MT. Alzheimer's Disease: What If There Was a Cure (The Story of Ketones). 3 ed: Nashville: Turner Publishing Company. (2023).
6. McDougall A, Bayley M, Munce SEP. The ketogenic diet as a treatment for traumatic brain injury: a scoping review. Brain Injury. (2018) 32:416–22. doi: 10.1080/02699052.2018.1429025
7. Norwitz NG, Soto-Mota A, Feldman D, Parpos S, Budoff M. Case Report: Hypercholesterolemia “lean mass hyper-responder” phenotype presents in the context of a low saturated fat carbohydrate-restricted diet. Front Endocrinol. (2022) 13:830325. doi: 10.3389/fendo.2022.830325
8. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. (2022) 6:nzab144. doi: 10.1093/cdn/nzab144
9. Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
10. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz826
11. Mindrum MR. Let's be clear about expected cardiovascular risk: a commentary on the massive rise in ldl cholesterol induced by carbohydrate restriction in the proposed “lean mass hyper-responder” phenotype. Current Developments in Nutr. (2022) 6:nzac042. doi: 10.1093/cdn/nzac042
12. Buren J, Ericsson M, Damasceno NRT, Sjodin A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. (2021) 13:814. doi: 10.3390/nu13030814
13. Mansoor N, Vinknes KJ, Veierod MB, Retterstol K. Effects of low-carbohydrate diets vs. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. (2016) 115:466–79. doi: 10.1017/S0007114515004699
14. Moore JM, Diefenbach D, Nadendla M, Hiebert N. Evidence for a lean mass hyperresponder phenotype is lacking with increases in LDL cholesterol of clinical significance in all categories of response to a carbohydrate-restricted diet. Curr Dev Nutr. (2022) 6:nzac043. doi: 10.1093/cdn/nzac043
15. Gardner CD, Landry MJ, Perelman D, Petlura C, Durand LR, Aronica L, et al. Effect of a ketogenic diet versus mediterranean diet on HbA1c in individuals with prediabetes and type 2 diabetes mellitus: the interventional keto-med randomized crossover trial. Am J Clin Nutr. (2022) 116:640–652. doi: 10.1093/ajcn/nqac154
16. Naveh N, Avidan Y, Zafrir B. Extreme hypercholesterolemia following a ketogenic diet: exaggerated response to an increasingly popular diet. Cureus J Med Sci. (2023) 15:e43683. doi: 10.7759/cureus.43683
17. Houttu V, Grefhorst A, Cohn DM, Levels JHM, van Lennep JR, Stroes ESG, et al. Severe dyslipidemia mimicking familial hypercholesterolemia induced by high-fat, low-carbohydrate diets: a critical review. Nutrients. (2023) 15:962. doi: 10.3390/nu15040962
18. Norwitz NG, Mindrum MR, Giral P, Kontush A, Soto-Mota A, Wood TR, et al. Elevated LDL-cholesterol levels among lean mass hyper-responders on low-carbohydrate ketogenic diets deserve urgent clinical attention and further research. J Clin Lipidol. (2022) 16:765–8. doi: 10.1016/j.jacl.2022.10.010
19. Mundal L, Sarancic M, Ose L, Iversen PO, Borgan JK, Veierod MB, et al. Mortality among patients with familial hypercholesterolemia: a registry-based study in Norway, 1992-2010. J Am Heart Assoc. (2014) 3:e001236. doi: 10.1161/JAHA.114.001236
20. Harlan WR, Graham JB, Estes EH. Familial hypercholesterolemia - a genetic and metabolic study. Medicine. (1966) 45:77. doi: 10.1097/00005792-196603000-00001
21. Williams RR, Hasstedt SJ, Wilson DE, Ash KO, Yanowitz FF, Reiber GE, et al. Evidence that men with familial hypercholesterolemia can avoid early coronary death - an analysis of 77 gene carriers in 4 utah pedigrees. JAMA. (1986) 255:219–24. doi: 10.1001/jama.255.2.219
22. Sijbrands EJ, Westendorp RG, Defesche JC, de Meier PH, Smelt AH, Kastelein JJ. Mortality over two centuries in large pedigree with familial hypercholesterolaemia: family tree mortality study. BMJ. (2001) 322:1019–23. doi: 10.1136/bmj.322.7293.1019
23. Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. (2008) 29:2625–33. doi: 10.1093/eurheartj/ehn422
24. Hovland A, Mundal LJ, Igland J, Veierod MB, Holven KB, Bogsrud MP, et al. Risk of ischemic stroke and total cerebrovascular disease in familial hypercholesterolemia: a register study from Norway. Stroke. (2019) 50:172–4. doi: 10.1161/STROKEAHA.118.023456
25. Diamond DM, Alabdulgader AA, de Lorgeril M, Harcombe Z, Kendrick M, Malhotra A, et al. Dietary recommendations for familial hypercholesterolaemia: an evidence-free zone. BMJ Evid Based Med. (2021) 26:295–301. doi: 10.1136/bmjebm-2020-111412
26. Ravnskov U, de Lorgeril M, Kendrick M, Diamond DM. Inborn coagulation factors are more important cardiovascular risk factors than high LDL-cholesterol in familial hypercholesterolemia. Med Hypotheses. (2018) 121:60–3. doi: 10.1016/j.mehy.2018.09.019
27. Huijgen R, Kastelein JJ, Meijers JC. Increased coagulation factor VIII activity in patients with familial hypercholesterolemia. Blood. (2011) 118:6990–1. doi: 10.1182/blood-2011-10-386227
28. Jansen ACM, van Aalst-Cohen ES, Tanck MWT, Cheng S, Fontecha MR, Li J, et al. Genetic determinants of cardiovascular disease risk in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. (2005) 25:1475–81. doi: 10.1161/01.ATV.0000168909.44877.a7
29. Ravnskov U, de Lorgeril M, Kendrick M, Diamond DM. Importance of coagulation factors as critical components of premature cardiovascular disease in familial hypercholesterolemia. Int J Molec Sci. (2022) 23:9146. doi: 10.3390/ijms23169146
30. Burzotta F, Paciaroni K, De Stefano V, Crea F, Maseri A, Leone G, et al. G20210A prothrombin gene polymorphism and coronary ischaemic syndromes: a phenotype-specific meta-analysis of 12 034 subjects. Heart. (2004) 90:82–6. doi: 10.1136/heart.90.1.82
31. Sugrue DD, Trayner I, Thompson GR, Vere VJ, Dimeson J, Stirling Y, et al. Coronary artery disease and haemostatic variables in heterozygous familial hypercholesterolaemia. Br Heart J. (1985) 53:265–8. doi: 10.1136/hrt.53.3.265
32. Sebestjen M, Zegura B, Guzic-Salobir B, Keber I. Fibrinolytic parameters and insulin resistance in young survivors myocardial infarction with heterozygous familial hypercholesterolemia. Wien Klin Wochenschr. (2001) 113:113–8.
33. Karbasi-Afshar R, Khedmat H, Izadi M. Helicobacter pylori Infection and atherosclerosis: a systematic review. Acta Med Iran. (2015) 53:78–88.
34. Khoshbayan A, Taheri F, Moghadam MT, Chegini Z, Shariati A. The association of Chlamydia pneumoniae infection with atherosclerosis: review and update of in vitro and animal studies. Microb Pathog. (2021) 154:104803. doi: 10.1016/j.micpath.2021.104803
35. Ravnskov U. High cholesterol may protect against infections and atherosclerosis. Qjm-Int J Med. (2003) 96:927–34. doi: 10.1093/qjmed/hcg150
36. Ravnskov U, McCully KS. Infections may be causal in the pathogenesis of atherosclerosis. Am J Med Sci. (2012) 344:391–4. doi: 10.1097/MAJ.0b013e31824ba6e0
37. Shi H, Li Y, Dong C, Si G, Xu Y, Peng M, et al. Helicobacter pylori infection and the progression of atherosclerosis: a systematic review and meta-analysis. Helicobacter. (2022) 27:e12865. doi: 10.1111/hel.12865
38. Wang X, He Q, Jin D, Ma B, Yao K, Zou X. Association between helicobacter pylori infection and subclinical atherosclerosis: a systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e27840. doi: 10.1097/MD.0000000000027840
39. Khan S, Rahman HN, Okamoto T, Matsunaga T, Fujiwara Y, Sawa T, et al. Promotion of atherosclerosis by Helicobacter cinaedi infection that involves macrophage-driven proinflammatory responses. Sci Rep. (2014) 4:4680. doi: 10.1038/srep04680
40. Guirgis FW, Donnelly JP, Dodani S, Howard G, Safford MM, Levitan EB, et al. Cholesterol levels and long-term rates of community-acquired sepsis. Crit Care. (2016) 20:408. doi: 10.1186/s13054-016-1579-8
41. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. (2010) 303:1610–6. doi: 10.1001/jama.2010.461
42. Mohlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, et al. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. (2011) 57:E886-E. doi: 10.1016/S0735-1097(11)60886-3
43. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. (2016) 67:139–47. doi: 10.1016/j.jacc.2015.10.058
44. Kavousi M, Elias-Smale S, Rutten JH, Leening MJ, Vliegenthart R, Verwoert GC, et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. (2012) 156:438–44. doi: 10.7326/0003-4819-156-6-201203200-00006
45. Miname MH, Bittencourt MS, Moraes SR, Alves RIM, Silva PRS, Jannes CE, et al. Coronary artery calcium and cardiovascular events in patients with familial hypercholesterolemia receiving standard lipid-lowering therapy. JACC Cardiovasc Imaging. (2019) 12:1797–804. doi: 10.1016/j.jcmg.2018.09.019
46. Mortensen MB, Cainzos-Achirica M, Steffensen FH, Botker HE, Jensen JM, Sand NPR, et al. Association of coronary plaque with low-density lipoprotein cholesterol levels and rates of cardiovascular disease events among symptomatic adults. Jama Netw Open. (2022) 5:e2148139. doi: 10.1001/jamanetworkopen.2021.48139
47. Bittencourt MS, Nasir K, Santos RD, Al-Mallah MH. Very high LDL cholesterol: the power of zero passes another test. Atherosclerosis. (2020) 292:207–8. doi: 10.1016/j.atherosclerosis.2019.11.019
48. Steffen BT, Guan WH, Remaley AT, Stein JH, Tattersall MC, Kaufman J, et al. Apolipoprotein B is associated with carotid atherosclerosis progression independent of individual cholesterol measures in a 9-year prospective study of Multi-Ethnic Study of Atherosclerosis participants. J Clin Lipidol. (2017) 11:1181–91. doi: 10.1016/j.jacl.2017.07.001
49. Norwitz NG, Loh V. A standard lipid panel is insufficient for the care of a patient on a high-fat, low-carbohydrate ketogenic diet. Front Med. (2020) 7:97. doi: 10.3389/fmed.2020.00097
50. Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat. Rev Cardiol. (2019) 16:305–18. doi: 10.1038/s41569-018-0153-2
51. Alonso R, Argueso R, Alvarez-Banos P, Muniz-Grijalvo O, Diaz-Diaz JL, Mata P. Familial hypercholesterolemia and lipoprotein(a): two partners in crime? Curr Atheroscler Rep. (2022) 24:427–34. doi: 10.1007/s11883-022-01019-5
52. Vuorio A, Watts GF, Kovanen PT. Lipoprotein(a) as a risk factor for calcific aortic valvulopathy in heterozygous familial hypercholesterolemia. Atherosclerosis. (2019) 281:25–30. doi: 10.1016/j.atherosclerosis.2018.11.040
53. Jansen AC, van Aalst-Cohen ES, Tanck MW, Trip MD, Lansberg PJ, Liem AH, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med. (2004) 256:482–90. doi: 10.1111/j.1365-2796.2004.01405.x
54. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. (2019) 13:374–92. doi: 10.1016/j.jacl.2019.04.010
55. Reyes-Soffer G, Westerterp M. Beyond Lipoprotein(a) plasma measurements: Lipoprotein(a) and inflammation. Pharmacol Res. (2021) 169:105689. doi: 10.1016/j.phrs.2021.105689
56. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
57. Yang C, Zhu CG, Sui YG, Guo YL, Wu NQ, Dong Q, et al. Synergetic impact of lipoprotein(a) and fibrinogen on stroke in coronary artery disease patients. Eur J Clin Invest. (2024) 2024:e14179. doi: 10.1111/eci.14179
58. Willeit P, Yeang C, Moriarty PM, Tschiderer L, Varvel SA, McConnell JP, et al. Low-density lipoprotein cholesterol corrected for lipoprotein(a) cholesterol, risk thresholds, and cardiovascular events. J Am Heart Assoc. (2020) 9:e016318. doi: 10.1161/JAHA.119.016318
59. Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. (1990) 263:2893–8. doi: 10.1001/jama.263.21.2893
60. Lu MC, Fang WC, Li WC, Yeh WC, Shieh YH, Chen JY. The association between insulin resistance and cardiovascular disease risk: a community-based cross-sectional study among taiwanese people aged over 50 years. Int J Environ Res Public Health. (2020) 17:7195. doi: 10.3390/ijerph17197195
61. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
62. Adeva-Andany MM, Fernandez-Fernandez C, Carneiro-Freire N, Castro-Quintela E, Pedre-Pineiro A, Seco-Filgueira M. Insulin resistance underlies the elevated cardiovascular risk associated with kidney disease and glomerular hyperfiltration. Rev Cardiovasc Med. (2020) 21:41–56. doi: 10.31083/j.rcm.2020.01.5102
63. Pyorala M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia predicts coronary heart disease risk in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Circulation. (1998) 98:398–404. doi: 10.1161/01.CIR.98.5.398
64. Razi F, Forouzanfar K, Bandarian F, Nasli-Esfahani E. LDL-cholesterol measurement in diabetic type 2 patients: a comparison between direct assay and popular equations. J Diabetes Metab Disord. (2017) 16:43. doi: 10.1186/s40200-017-0326-2
65. Wang Y, Wan EYF, Mak IL, Ho MK, Chin WY, Yu EYT, et al. The association between trajectories of risk factors and risk of cardiovascular disease or mortality among patients with diabetes or hypertension: A systematic review. PLoS ONE. (2022) 17:e0262885. doi: 10.1371/journal.pone.0262885
66. Slivnick J, Lampert BC. Hypertension and heart failure. Heart Fail Clin. (2019) 15:531–41. doi: 10.1016/j.hfc.2019.06.007
67. Nieuwdorp M, van Haeften TW, Gouverneur MCLG, Mooij HL, van Lieshout MHP, Levi M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. (2006) 55:480–6. doi: 10.2337/diabetes.55.02.06.db05-1103
68. Ghosh K. Diabetes as a prothrombotic state. In: Mechanisms of Vascular Defects in Diabetes Mellitus. (2017). p. 361–76. doi: 10.1007/978-3-319-60324-7_16
69. Tan KCB, Chow WS Ai VHG, Metz C, Bucala R, Lam KSL. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. (2002) 25:1055–9. doi: 10.2337/diacare.25.6.1055
70. Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. (2010) 59:2152–9. doi: 10.2337/db09-1772
71. Domingues N. Insulin resistance as a predictor of cardiovascular diseases. Rev. Portuguesa De Cardiol. (2021) 40:545–6. doi: 10.1016/j.repc.2021.06.004
72. Yu Y, Zhou ZW, Su K, Xi LL, Zhang L, Yu LW, et al. Association between coronary artery atherosclerosis and plasma glucose levels assessed by dual-source computed tomography. J Thor Dis. (2018) 10:6050. doi: 10.21037/jtd.2018.10.62
73. Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. (2010) 45:907–14. doi: 10.1007/s11745-010-3408-1
74. Toth PP. Insulin resistance, small LDL particles, and risk for atherosclerotic disease. Curr Vasc Pharmacol. (2014) 12:653–7. doi: 10.2174/15701611113119990125
75. Siri-Tarino PW, Krauss RM. Diet, lipids, and cardiovascular disease. Curr Opin Lipidol. (2016) 27:323–8. doi: 10.1097/MOL.0000000000000310
76. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. (1990) 82:495–506. doi: 10.1161/01.CIR.82.2.495
77. Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev. (2017) 2017:1273042. doi: 10.1155/2017/1273042
78. Dev K, Sharma SB, Garg S, Aggarwal A, Madhu SV. Glycated apolipoprotein B-A surrogate marker of subclinical atherosclerosis. Diabetes Metab Synd. (2016) 10:78–81. doi: 10.1016/j.dsx.2015.09.012
79. Soran H, Durrington PN. Susceptibility of LDL and its subfractions to glycation. Curr Opin Lipidol. (2011) 22:254–61. doi: 10.1097/MOL.0b013e328348a43f
80. Younis NN, Soran H, Pemberton P, Charlton-Menys V, Elseweidy MM, Durrington PN. Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clin Sci. (2013) 124:343–9. doi: 10.1042/CS20120304
81. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. (2007) 115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793
82. Zhang B, Menzin J, Friedman M, Korn JR, Burge RT. Predicted coronary risk for adults with coronary heart disease and low HDL-C: an analysis from the US National Health and Nutrition Examination Survey. Curr Med Res Opin. (2008) 24:2711–7. doi: 10.1185/03007990802363198
83. Haffner SM, Mykkanen L, Robbins D, Valdez R, Miettinen H, Howard BV, et al. A preponderance of small dense LDL is associated with specific insulin, proinsulin and the components of the insulin resistance syndrome in non-diabetic subjects. Diabetologia. (1995) 38:1328–36. doi: 10.1007/BF00401766
84. Austin MA, Mykkanen L, Kuusisto J, Edwards KL, Nelson C, Haffner SM, et al. Prospective study of small LDLs as a risk factor for non-insulin dependent diabetes mellitus in elderly men and women. Circulation. (1995) 92:1770–8. doi: 10.1161/01.CIR.92.7.1770
85. Shi HL, Guo JW, Xu K, Zhang FJ, Zhou YL. Study on the value of small dense low-density lipoprotein in predicting cardiovascular and cerebrovascular events in the high-risk stroke population. J Clin Lab Anal. (2022) 36:e24278. doi: 10.1002/jcla.24278
86. Zhao CX, Cui YH, Fan QA, Wang PH, Hui RT, Cianflone K, et al. Small dense low-density lipoproteins and associated risk factors in patients with stroke. Cerebrov Dis. (2009) 27:99–104. doi: 10.1159/000175768
87. Zhou PY, Liu JC, Wang LY, Feng WM, Cao ZH, Wang P, et al. Association of small dense low-density lipoprotein cholesterol with stroke risk, severity and prognosis. J Atheroscler Thromb. (2020) 27:1310–24. doi: 10.5551/jat.53132
88. Gerber PA, Thalhammer C, Schmied C, Spring S, Amann-Vesti B, Spinas GA, et al. Small, dense LDL particles predict changes in intima media thickness and insulin resistance in men with type 2 diabetes and prediabetes–a prospective cohort study. PLoS ONE. (2013) 8:e72763. doi: 10.1371/journal.pone.0072763
89. Bokemark L, Wikstrand J, Attvall S, Hulthe J, Wedel H, Fagerberg B. Insulin resistance and intima-media thickness in the carotid and femoral arteries of clinically healthy 58-year-old men. The Atherosclerosis and Insulin Resistance Study (AZR). J Internal Medicine. (2001) 249:59–67. doi: 10.1046/j.1365-2796.2001.00735.x
90. Lee CK, Liao CW, Meng SW, Wu WK, Chiang JY, Wu MS. Lipids and lipoproteins in health and disease: focus on targeting atherosclerosis. Biomedicines. (2021) 9:985. doi: 10.3390/biomedicines9080985
91. Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. (2014) 34:1069–77. doi: 10.1161/ATVBAHA.114.303284
92. St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. (2005) 25:553–9. doi: 10.1161/01.ATV.0000154144.73236.f4
93. Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. (2014) 34:196–201. doi: 10.1161/ATVBAHA.113.302401
94. Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, et al. Small dense LDL cholesterol and coronary heart disease: results from the framingham offspring study. Clin Chem. (2010) 56:967–76. doi: 10.1373/clinchem.2009.137489
95. Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. (2002) 43:1363–79. doi: 10.1194/jlr.R200004-JLR200
96. Gjuladin-Hellon T, Davies IG, Penson P, Amiri Baghbadorani R. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: a systematic review and meta-analysis. Nutr Rev. (2019) 77:161–80. doi: 10.1093/nutrit/nuy049
97. Diamond DM, Bikman BT, Mason P. Statin therapy is not warranted for a person with high LDL-cholesterol on a low-carbohydrate diet. Curr Opin Endocrinol Diabetes Obes. (2022) 29:497–511. doi: 10.1097/MED.0000000000000764
98. Volek JS, Feinman RD. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr Metab. (2005) 2:31. doi: 10.1186/1743-7075-2-31
99. Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res. (2008) 47:307–18. doi: 10.1016/j.plipres.2008.02.003
100. Dashti HM, Mathew TC, Khadada M, Al-Mousawi M, Talib H, Asfar SK, et al. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol Cell Biochem. (2007) 302:249–56. doi: 10.1007/s11010-007-9448-z
101. Karam JG, McFarlane SI, Feinman RD. Carbohydrate restriction and cardiovascular risk. Curr Cardiovasc Risk Rep. (2008) 2:88–94. doi: 10.1007/s12170-008-0018-z
102. Kelly T, Unwin D, Finucane F. Low-carbohydrate diets in the management of obesity and type 2 diabetes: a review from clinicians using the approach in practice. Int J Environ Res Public Health. (2020) 17:2557. doi: 10.3390/ijerph17072557
103. Barrea L, Caprio M, Watanabe M, Cammarata G, Feraco A, Muscogiuri G, et al. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit Rev Food Sci. (2022) 63:8320–36. doi: 10.1080/10408398.2022.2054935
104. Gram-Kampmann EM, Hansen CD, Hugger MB, Jensen JM, Brond JC, Hermann AP, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: an open-label randomized controlled trial. Diab Obes Metab. (2022) 24:693–703. doi: 10.1111/dom.14633
105. Volek JS, Phinney SD, Krauss RM, Johnson RJ, Saslow LR, Gower B, et al. Alternative dietary patterns for americans: low-carbohydrate diets. Nutrients. (2021) 13:3299. doi: 10.3390/nu13103299
106. Bailey WA, Westman EC, Marquart ML, Guyton JR. Low glycemic diet for weight loss in hypertriglyceridemic patients attending a lipid clinic. J Clin Lipidol. (2010) 4:508–14. doi: 10.1016/j.jacl.2010.08.019
107. Foley PJ. Effect of low carbohydrate diets on insulin resistance and the metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. (2021) 28:463–8. doi: 10.1097/MED.0000000000000659
108. Harvey C, Schofield GM, Zinn C, Thornley SJ, Crofts C, Merien FLR. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: a randomised clinical trial. PeerJ. (2019) 7:e6273. doi: 10.7717/peerj.6273
109. Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. (2015) 31:1–13. doi: 10.1016/j.nut.2014.06.011
110. Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. (2005) 142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006
111. Danan A, Westman EC, Saslow LR Ede G. The ketogenic diet for refractory mental illness: a retrospective analysis of 31 inpatients. Front Psychiatry. (2022) 13:951376. doi: 10.3389/fpsyt.2022.951376
112. Das S, McCreary J, Shamim S, Kalayjian T. Reversal of severe hypertriglyceridemia with intermittent fasting and a very-low-carbohydrate ketogenic diet: a case series. Curr Opin Endocrinol Diabetes Obes. (2020) 27:308–11. doi: 10.1097/MED.0000000000000566
113. O'Neill BJ. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. (2020) 27:301–7. doi: 10.1097/MED.0000000000000569
114. Cipryan L, Litschmannova M, Maffetone PB, Plews DJ, Dostal T, Hofmann P, et al. Very low-carbohydrate high-fat diet improves risk markers for cardiometabolic health more than exercise in men and women with overfat constitution: secondary analysis of a randomized controlled clinical trial. Front Nutr. (2022) 9:867690. doi: 10.3389/fnut.2022.867690
115. Stoica RA, Diaconu CC, Rizzo M, Toth PP, Stefan SD, Serafinceanu C, et al. Weight loss programmes using low carbohydrate diets to control the cardiovascular risk in adolescents (Review). Exp Ther Med. (2021) 21:90. doi: 10.3892/etm.2020.9522
116. Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in alzheimer's disease. Antioxidants. (2018) 7:83. doi: 10.3390/antiox7050063
117. Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. (2015) 56:e95–e8. doi: 10.1111/epi.13038
118. Wood RJ, Volek JS, Davis SR, Dell'Ova C, Fernandez ML. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr Metab. (2006) 3:19. doi: 10.1186/1743-7075-3-19
119. Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. (2010) 51:3324–30. doi: 10.1194/jlr.M005769
120. Westman EC, Yancy WS, Olsen MK, Dudley T, Guyton JR. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int J Cardiol. (2006) 110:212–6. doi: 10.1016/j.ijcard.2005.08.034
121. Fernandez ML, Wood RJ, Dell'Ova C, Davis S, Volek J. Weight loss induced by a carbohydrate restricted diet favorably affects markers of inflammation and heart disease without increasing plasma homocysteine concentrations. Faseb J. (2006) 20:A426-A. doi: 10.1096/fasebj.20.4.A426-b
122. Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. (2009) 44:297–309. doi: 10.1007/s11745-008-3274-2
123. Krebs JD, Bell D, Hall R, Parry-Strong A, Docherty PD, Clarke K, et al. Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with type 2 diabetes. J Am Coll Nutr. (2013) 32:11–7. doi: 10.1080/07315724.2013.767630
124. Ahmed SR, Bellamkonda S, Zilbermint M, Wang J, Kalyani RR. Effects of the low carbohydrate, high fat diet on glycemic control and body weight in patients with type 2 diabetes: experience from a community-based cohort. BMJ Open Diabetes Res Care. (2020) 8:e000980. doi: 10.1136/bmjdrc-2019-000980
125. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrino. (2018) 13:263–72. doi: 10.1080/17446651.2018.1523713
126. Westman EC, Yancy WS. Using a low-carbohydrate diet to treat obesity and type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. (2020) 27:255–60. doi: 10.1097/MED.0000000000000565
127. Moriconi E, Camajani E, Fabbri A, Lenzi A, Caprio M. Very-low-calorie ketogenic diet as a safe and valuable tool for long-term glycemic management in patients with obesity and type 2 diabetes. Nutrients. (2021) 13:758. doi: 10.3390/nu13030758
128. Yancy WS, Mitchell NS, Westman EC. Ketogenic diet for obesity and diabetes. Jama Intern Med. (2019) 179:1734–5. doi: 10.1001/jamainternmed.2019.5148
129. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. (2021) 8:688540. doi: 10.3389/fnut.2021.688540
130. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. (2019) 69:360–1. doi: 10.3399/bjgp19X704525
131. Bouillet B, Rouland A, Petit JM, Verges B, A. low-carbohydrate high-fat diet initiated promptly after diagnosis provides clinical remission in three patients with type 1 diabetes. Diabetes Metab. (2020) 46:511–3. doi: 10.1016/j.diabet.2019.06.004
132. Gavidia K, Kalayjian T. Treating diabetes utilizing a low carbohydrate ketogenic diet and intermittent fasting without significant weight loss: a case report. Front Nutr. (2021) 8:687081. doi: 10.3389/fnut.2021.687081
133. Athinarayanan SJ, Hallberg SJ, McKenzie AL, Lechner K, King S, McCarter JP, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol. (2020) 19:208. doi: 10.1186/s12933-020-01178-2
134. Sharman MJ, Kraemer WJ, Love DM, Avery NG, Gomez AL, Scheett TP, et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. (2002) 132:1879–85. doi: 10.1093/jn/132.7.1879
135. Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. (2014) 161:309–18. doi: 10.7326/M14-0180
136. Wakabayashi I, Daimon T. Comparison of discrimination for cardio-metabolic risk by different cut-off values of the ratio of triglycerides to HDL cholesterol. Lipids Health Dis. (2019) 18:156. doi: 10.1186/s12944-019-1098-0
137. Dashti HM, Mathew TC. Prevention of obesity using low carbohydrate ketogenic diet. Kuwait Med J. (2009) 41:3–12.
138. Brown A, McArdle P, Taplin J, Unwin D, Unwin J, Deakin T, et al. Dietary strategies for remission of type 2 diabetes: a narrative review. J Hum Nutr Diet. (2022) 35:165–78. doi: 10.1111/jhn.12938
139. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women. JAMA. (2007) 297:969–77. doi: 10.1001/jama.297.9.969
140. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiov Diabetol. (2018) 17:1–16. doi: 10.1186/s12933-018-0698-8
141. Hallberg SJ, McKenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diab Ther. (2018) 9:583–612. doi: 10.1007/s13300-018-0373-9
142. Unwin D, Khalid AA, Unwin J, Crocombe D, Delon C, Martyn K, et al. Insights from a general practice service evaluation supporting a lower carbohydrate diet in patients with type 2 diabetes mellitus and prediabetes: a secondary analysis of routine clinic data including HbA1c, weight and prescribing over 6 years. BMJ Nutr Prev Health. (2020) 3:285–94. doi: 10.1136/bmjnph-2020-000072
143. Unwin D, Unwin J, Crocombe D, Delon C, Guess N, Wong C. Renal function in patients following a low carbohydrate diet for type 2 diabetes: a review of the literature and analysis of routine clinical data from a primary care service over 7 years. Curr Opin Endocrinol Diabetes Obes. (2021) 28:469–79. doi: 10.1097/MED.0000000000000658
144. Unwin DJ, Tobin SD, Murray SW, Delon C, Brady AJ. Substantial and sustained improvements in blood pressure, weight and lipid profiles from a carbohydrate restricted diet: an observational study of insulin resistant patients in primary care. Int J Environ Res Public Health. (2019) 16:2680. doi: 10.3390/ijerph16152680
145. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
146. Phelan S, Wyatt H, Nassery S, Dibello J, Fava JL, Hill JO, et al. Three-year weight change in successful weight losers who lost weight on a low-carbohydrate diet. Obesity (Silver Spring). (2007) 15:2470–7. doi: 10.1038/oby.2007.293
147. Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Low high-density lipoprotein cholesterol and response to simvastatin therapy in scandinavian simvastatin survival study (4s) - response. Circulation. (2002) 106:E8-E. doi: 10.1161/01.CIR.0000019970.99823.B2
Keywords: ketogenic diet, low carbohydrate diet, cardiovascular disease, neurological disorder, cholesterol, low-density lipoprotein (LDL), risk factor
Citation: Diamond DM, Mason P and Bikman BT (2024) Opinion: Are mental health benefits of the ketogenic diet accompanied by an increased risk of cardiovascular disease? Front. Nutr. 11:1394610. doi: 10.3389/fnut.2024.1394610
Received: 01 March 2024; Accepted: 16 April 2024;
Published: 01 May 2024.
Edited by:
Beth Ann Zupec-Kania, Ketogenic Therapies, LLC, United StatesReviewed by:
Eric Westman, Duke University, United StatesCopyright © 2024 Diamond, Mason and Bikman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David M. Diamond, ZGRpYW1vbmRAdXNmLmVkdQ==
 David M. Diamond
David M. Diamond Paul Mason2
Paul Mason2