- 1Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 3Jiangxi Provincial Geriatric Hospital, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 4Department of Cardiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
Objective: Nutritional status is closely associated with the prognosis of heart failure. This study aims to assess the relationship between the Controlling Nutritional Status (CONUT) score and in-hospital mortality among patients with acute decompensated heart failure (ADHF) in Jiangxi, China.
Methods: A retrospective cohort study was conducted. Multivariable Cox regression models and restricted cubic spline regression were employed to evaluate the relationship between the CONUT score and in-hospital mortality in ADHF patients from Jiangxi, China. The predictive value of the CONUT score for in-hospital mortality in ADHF patients was analyzed using receiver operating characteristic curves. Subgroup analyses were performed to identify risk dependencies of the CONUT score in specific populations.
Results: The study included 1,230 ADHF patients, among whom 44 (3.58%) mortality events were recorded. After adjusting for confounding factors, a positive correlation was found between the CONUT score and the risk of in-hospital mortality in ADHF patients. Restricted cubic spline regression analysis indicated a non-linear relationship between the CONUT score and the risk of in-hospital mortality in ADHF patients, estimating a rapid increase in mortality risk when the CONUT score exceeded 5. Receiver operating characteristic analysis demonstrated a good predictive value of the CONUT score for all-cause mortality events in ADHF patients [area under the curve = 0.7625, optimal threshold = 5.5]. Additionally, a relatively higher risk associated with the CONUT score was observed in male patients and those with concomitant cerebral infarction.
Conclusion: This study reveals a positive correlation between the CONUT score and the risk of in-hospital mortality in ADHF patients. Based on the findings of this study, we recommend maintaining a CONUT score below 5 for patients with ADHF in Jiangxi, China, as it may significantly contribute to reducing the risk of in-hospital all-cause mortality.
Introduction
Heart failure (HF) is an increasingly grave global public health challenge, with an estimated prevalence exceeding 37.7 million individuals (1). Despite advancements in the management and treatment of HF due to rapid developments in medical technology in recent years, the annual mortality rate remains high (2–5). Most hospital admissions for HF patients are associated with acute decompensated heart failure (ADHF) (6, 7), which refers to a sudden exacerbation of HF symptoms and signs caused by various factors (8, 9), manifesting as hemodynamic abnormalities and end-organ damage (10, 11). The in-hospital mortality rate for ADHF varies between 4% and 15.8%, imposing significant physical and emotional burdens on patients and their families (12–15). Early identification of risk factors for in-hospital mortality in ADHF patients is crucial for improving their short-term prognosis.
Malnutrition is notably common among ADHF patients, likely related to fluid retention causing intestinal edema, decreased secretion of growth peptides, leading to anorexia, malabsorption, and inflammatory responses (16, 17). Evidence suggests a link between nutritional status and the prognosis of ADHF (18–21). Consequently, incorporating nutritional status into the overall assessment of patients to mitigate malnutrition-related risks is essential. Various nutritional assessment scales have been developed by clinicians and researchers for more accurate assessment, such as the Mini Nutritional Assessment, handgrip strength, Subjective Global Assessment, and the Controlling Nutritional Status (CONUT) score (22–25). Unlike other nutritional scores, the CONUT score relies mainly on objective values of serum albumin (ALB), total lymphocyte count (TL), and total cholesterol (TC), and is primarily used to evaluate the prognosis of cancer, coronary artery disease, and patients undergoing dialysis (26–30). A recent retrospective cohort study from Japan demonstrated that the CONUT score could assess the in-hospital all-cause mortality risk in Japanese ADHF patients (31). However, the predictive value of the CONUT score for in-hospital mortality in ADHF patients remains unclear, particularly in the Chinese population. Given the high mortality rate associated with ADHF, early clarification of the predictive value of the CONUT score for all-cause mortality during hospitalization in ADHF patients could be highly beneficial. To address this, the current study aims to assess the predictive value of the CONUT score for in-hospital all-cause mortality among ADHF patients in a cohort from Jiangxi, China.
Methods
Study design and data source
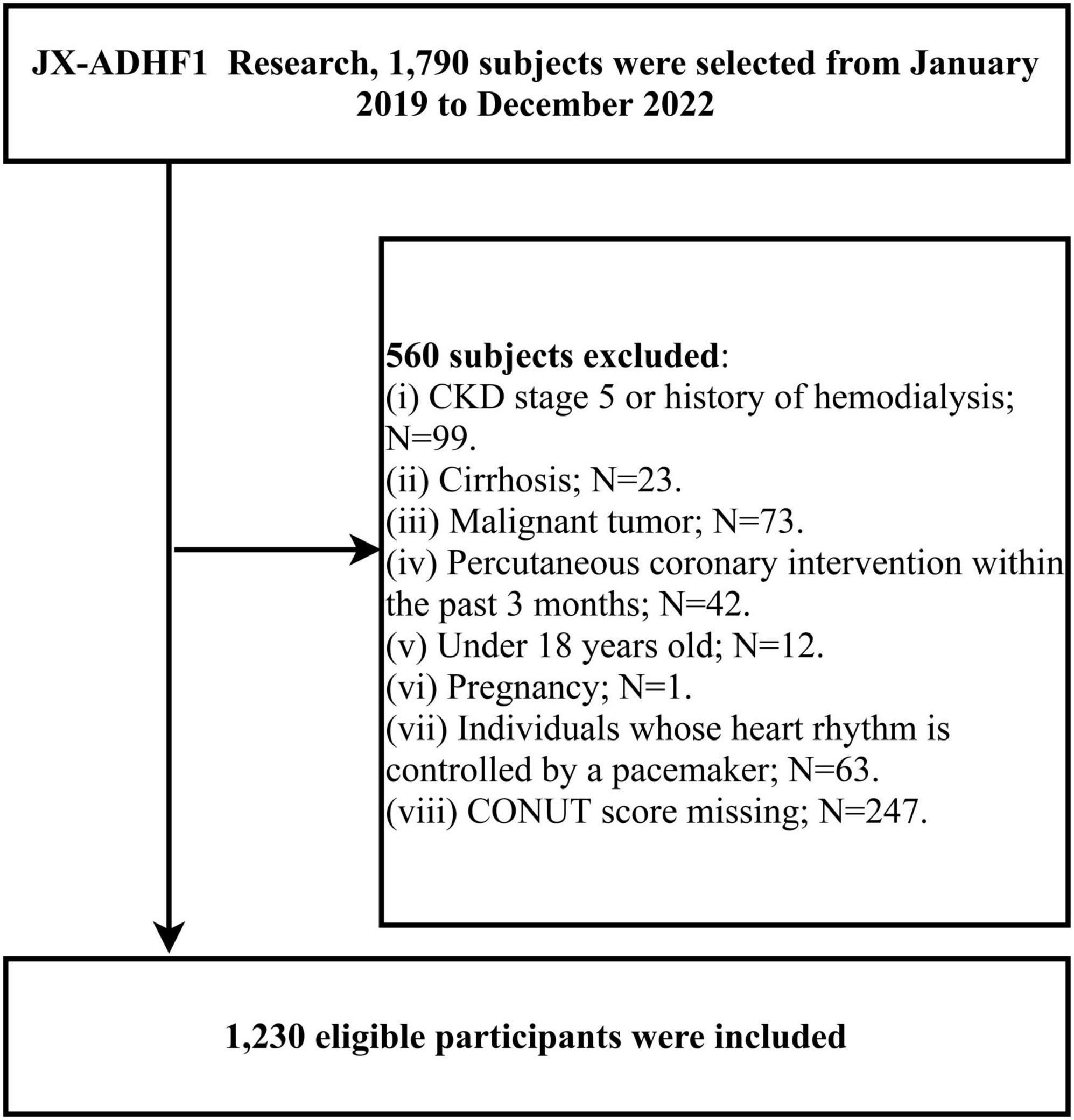
This research utilized data from the JX-ADHF1 (Jiangxi-Acute Decompensated Heart Failure 1) study, a retrospective observational study that consecutively included 1,790 ADHF patients admitted to the Jiangxi Provincial People’s Hospital between January 2019 and December 2022. The definition of ADHF was based on the latest European Society of Cardiology (ESC) guidelines for acute and chronic heart failure available at the time. In this study, we excluded participants with the following characteristics: (1) those with stage 5 chronic kidney disease or a history of hemodialysis (n = 99), and those with cirrhosis (n = 23); (2) those with malignancies (n = 73); (3) those who underwent percutaneous coronary intervention within the past three months (n = 42); (4) those under 18 years of age (n = 12); (5) pregnant participants (n = 1); (6) those with pacemakers (n = 63); and (7) those with missing CONUT score information (n = 247): missing TL (n = 25), ALB (n = 25), TC (n = 197). Ultimately, the study included 1,230 participants. The detailed screening process for the study population was shown in Figure 1.
Ethical approval
The JX-ADHF1 study adhered to the ethical principles of the Declaration of Helsinki. The use of study data was approved with informed consent from the participants, and the research protocol was authorized by the Ethics Review Committee of Jiangxi Provincial People’s Hospital (IRB: 2024-01).
Baseline information measurement and assessment
Participants were received by medical staff upon admission and their gender, age, body mass index (BMI), smoking and drinking status, New York Heart Association (NYHA) functional classification at admission, comorbidities [hypertension, diabetes, cerebral infarction, coronary heart disease, pulmonary infection, atrial fibrillation (PASP)], as well as blood pressure, and most recent echocardiogram results were recorded in the medical record system (including left ventricular ejection fraction (LVEF), mitral regurgitation, tricuspid regurgitation and PASP data). In addition, we recorded information on the medications administered to the subjects during their hospitalization, including furosemide, spirolactone, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor inhibitors (ARB)/angiotensin receptor neprilysin inhibitors (ARNI), beta-blockers, digitalis, sodium-dependent glucose transporters 2 (SGTL-2), antiplatelet agent and lipid-lowering treatment information.
Laboratory parameters were measured within 24 hours after admission in a standard laboratory using an automatic analyzer, including N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP), white blood cell count (WBC), hemoglobin (Hb), neutrophil count (NEUT), TL, ALB, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), TC, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). It should be noted that liver enzyme-related parameters and lipid parameters were determined by venous blood drawn on an empty stomach upon admission or the next morning.
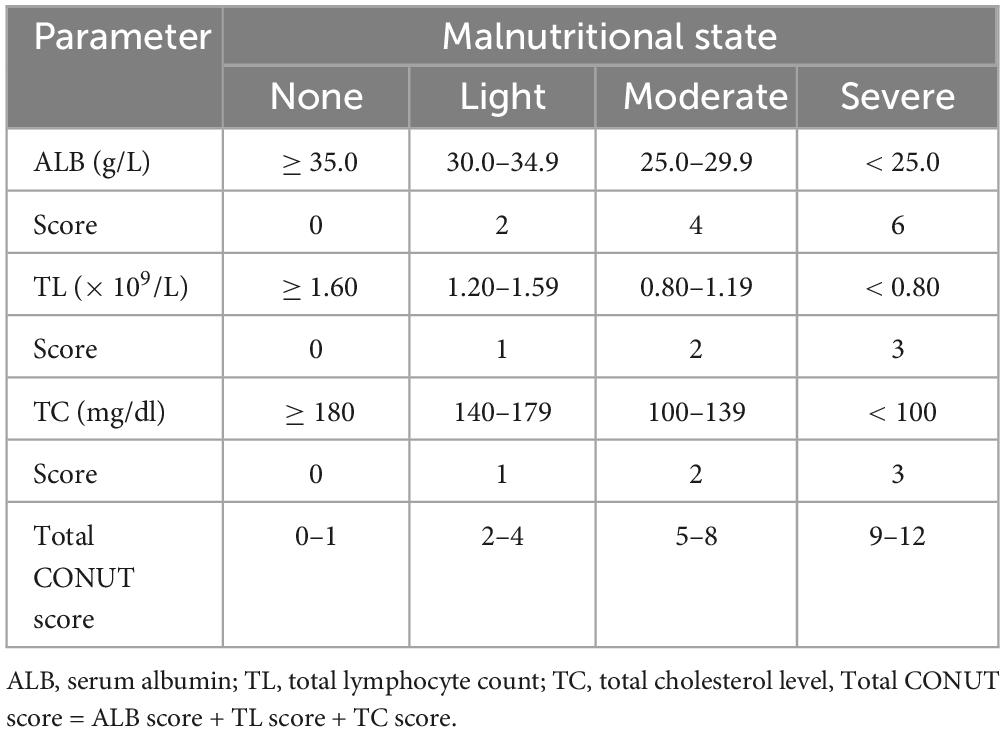
The CONUT score was defined based on ALB, TC, and TL, categorized as no malnutrition (0–1), mild malnutrition (2–4), moderate malnutrition (5–8), and severe malnutrition (9–12); detailed information was provided in Table 1.
Outcome definition
The start time of follow-up was defined as the admission time of patients with ADHF, and the study primary end point was death from any cause during hospitalization, with the secondary endpoint event being death from cardiovascular causes.
Statistical analysis
The baseline information of the study population was described using continuous variables as means [standard deviations] or medians (interquartile range: 25th–75th percentiles) and categorical variables as frequencies (%). Variance analysis or the Kruskal-Wallis H test was used for intergroup comparisons of continuous data, while the chi-square test was employed for intergroup comparisons of categorical data. Kaplan-Meier analysis was utilized to plot cumulative survival curves for different CONUT score groups.
To estimate the risk of high CONUT scores relative to low scores for the outcome indicator, multivariable Cox regression models were used to examine the relationship between CONUT scores and all-cause/cardiovascular mortality during hospitalization in ADHF patients, reporting adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). Model 1 adjusted for gender, age, hypertension, diabetes, cerebral infarction, and coronary heart disease; Model 2 further adjusted for NYHA classification, LVEF, systolic blood pressure (SBP), diastolic blood pressure (DBP), drinking, and smoking status on the basis of Model 1; Model 3 further included WBC, AST, TG, HDL-C, LDL-C, NT-proBNP, and Cr adjustments based on Model 2. Model 4 further adjusted for etiology of ADHF, AF, Pulmonary infection, Hb, BMI and anti-heart failure treatment. Restricted cubic spline regression was employed to assess the dose-response relationship between CONUT scores and all-cause/cardiovascular mortality during hospitalization in ADHF patients.
Receiver operating characteristic (ROC) curves were used to evaluate the predictive accuracy of the CONUT score and its components for all-cause mortality during hospitalization in ADHF patients, identifying the best threshold, sensitivity, and specificity. The DeLong test was used to compare whether there was a difference in the area under the curve (AUC) values between the CONUT score and its components. Finally, stratified analysis was conducted based on age, gender, NYHA classification, LVEF, and the presence of hypertension, diabetes, cerebral infarction, and coronary heart disease. Likelihood ratio tests were used to compare differences between subgroups and to assess the presence of interaction effects.
For all tests, statistical significance was set at P < 0.05. All analyses were conducted using R language version 3.4.3 and Empower(R) version 2.0.
Results
Baseline characteristics of participants
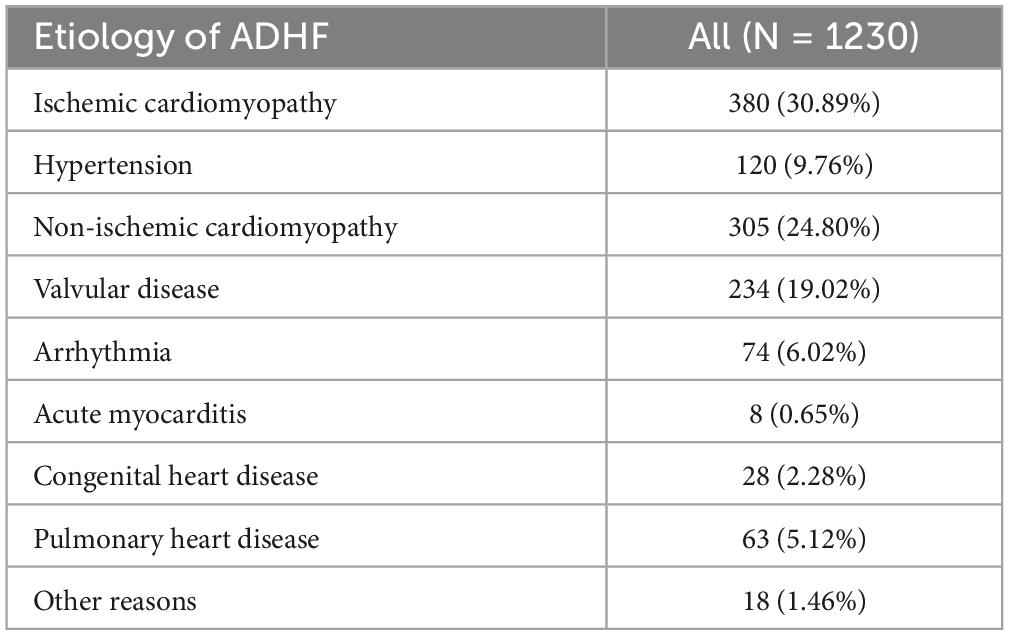
Among the 1,230 eligible adult participants, there were 724 males and 506 females, with an average age of 68 years. Based on the subjects’ disease onset characteristics and chronic heart failure history, we summarized the causes of these patients into the following nine categories: ischemic cardiomyopathy, hypertension, non-ischemic cardiomyopathy (including dilated cardiomyopathy, restrictive cardiomyopathy, hypertrophic cardiomyopathy, stress cardiomyopathy, systemic lupus erythematosus cardiomyopathy, diabetic cardiomyopathy and alcoholic cardiomyopathy), valvular disease, arrhythmia, acute myocarditis, congenital heart disease, pulmonary heart disease and other reasons (includes pericardial disease, hypothyroidism, anemic heart disease, severe infections, rapid progression of other systemic diseases, and unknown etiologies). As can be seen in Table 2, the main etiologies of ADHF patients in the current cohort were ischemic cardiomyopathy, non-ischemic cardiomyopathy and valvular disease.
Table 3 presents the baseline characteristics of the participants according to the four categories of CONUT scores. The results indicated significantly statistical differences in gender, age, BMI, LVEF category, NYHA classification, tricuspid regurgitation, PASP, DBP, WBC, NEUT, TG, TL, ALB, TC, HDL-C, LDL-C, AST, Cr, NT-proBNP, HF treatment with furosemide, spironolactone, ACEI/ARB/ARNI, digitalis and beta-blockers, complicated with pulmonary inflammation, and all-cause mortality rate across different CONUT score groups. Participants with higher CONUT scores generally had lower levels of TL, ALB, TC, Hb, HDL-C, LDL-C, but higher levels of PASP, AST, Cr, NT-proBNP (all P < 0.05). In addition, patients with high CONUT scores had a higher probability of having combined pulmonary inflammation, HF with preserved ejection fraction and tricuspid regurgitation, and a lower probability of having used furosemide, spironolactone, ACEI/ARB/ARNI, and beta-blockers.

Table 3. The malnutrition status defined by CONUT score shows the baseline characteristics of the subjects.
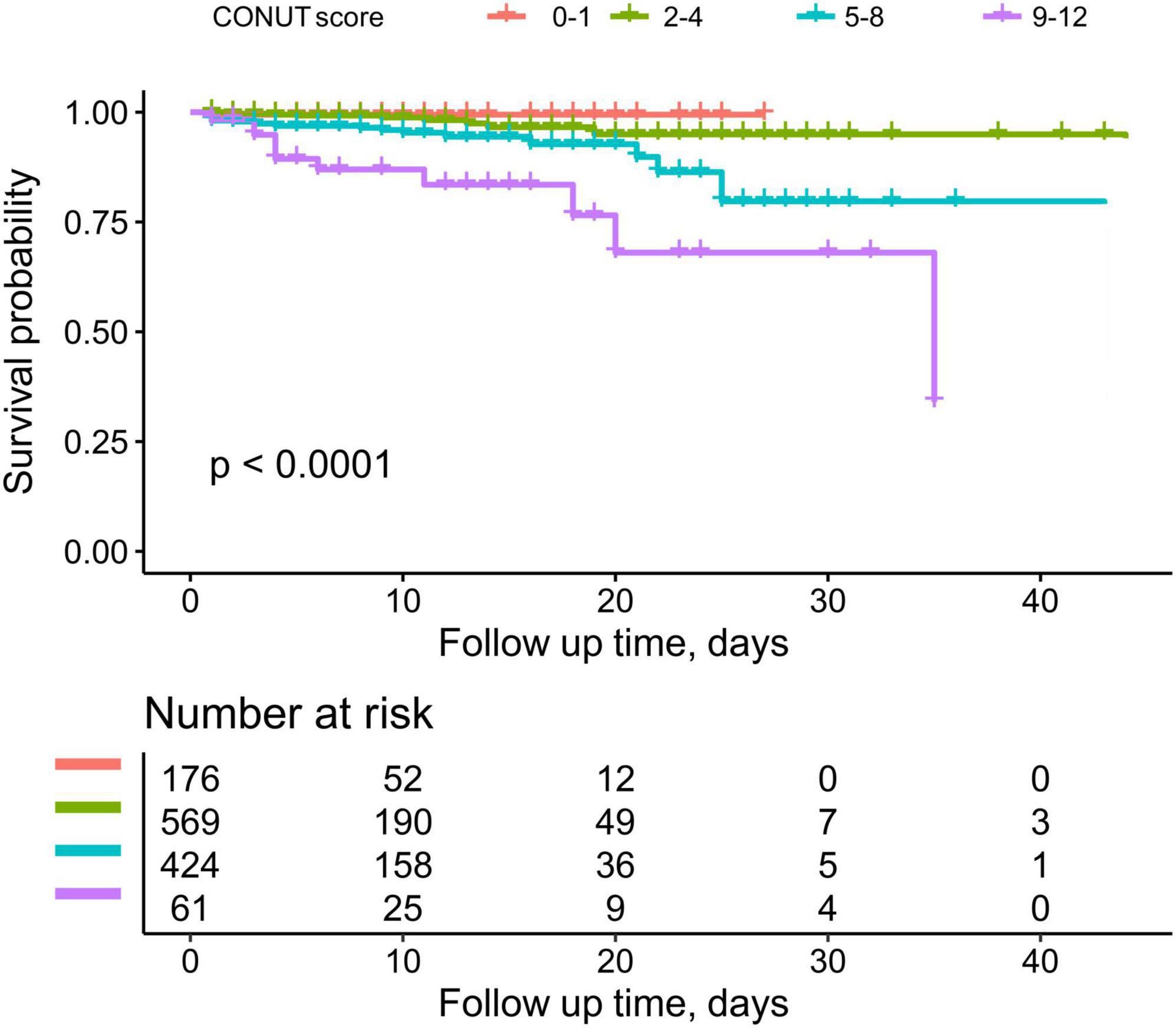
During the observation period, 44 participants experienced death during hospitalization, of which 37 were classified as deaths from cardiovascular causes. Figure 2 depicts the trend of cumulative survival rates for ADHF patients, showing lower survival rates in the severe malnutrition group over time.
Association between CONUT score and in-hospital mortality in ADHF patients in China
Before establishing multivariable Cox regression models, all covariates underwent collinearity diagnostics. Variables such as ALT and TC with variance inflation factor values greater than 3 were considered to have high collinearity and were not included in subsequent adjustment models (Supplementary Table 1). Four progressively adjusted Cox regression models were established to assess the relationship between CONUT score and in-hospital mortality in ADHF patients (Table 4). It was found that in all models, the CONUT score were significantly and positively associated with both in-hospital all-cause mortality and cardiovascular death In the fully adjusted Model 4, each additional unit increase in the CONUT score was associated with a 25% (HR 1.25, 95% CI: 1.06,1.47) increase in the risk of all-cause mortality and a 22% (HR 1.22, 95% CI: 1.01,1.48) increased risk of cardiovascular death during hospitalization in ADHF patients. When treating the CONUT score as a categorical variable and using the no malnutrition category as the reference, the severe malnutrition category had the highest risk of in-hospital all-cause/cardiovascular mortality in ADHF patients. Additionally, it’s noteworthy that after fitting with smooth splines, we observed a potential threshold effect in the relationship between the CONUT score and all-cause/cardiovascular mortality during hospitalization in ADHF patients (Figure 3), estimating a rapid increase in mortality risk when the CONUT score exceeded 5.
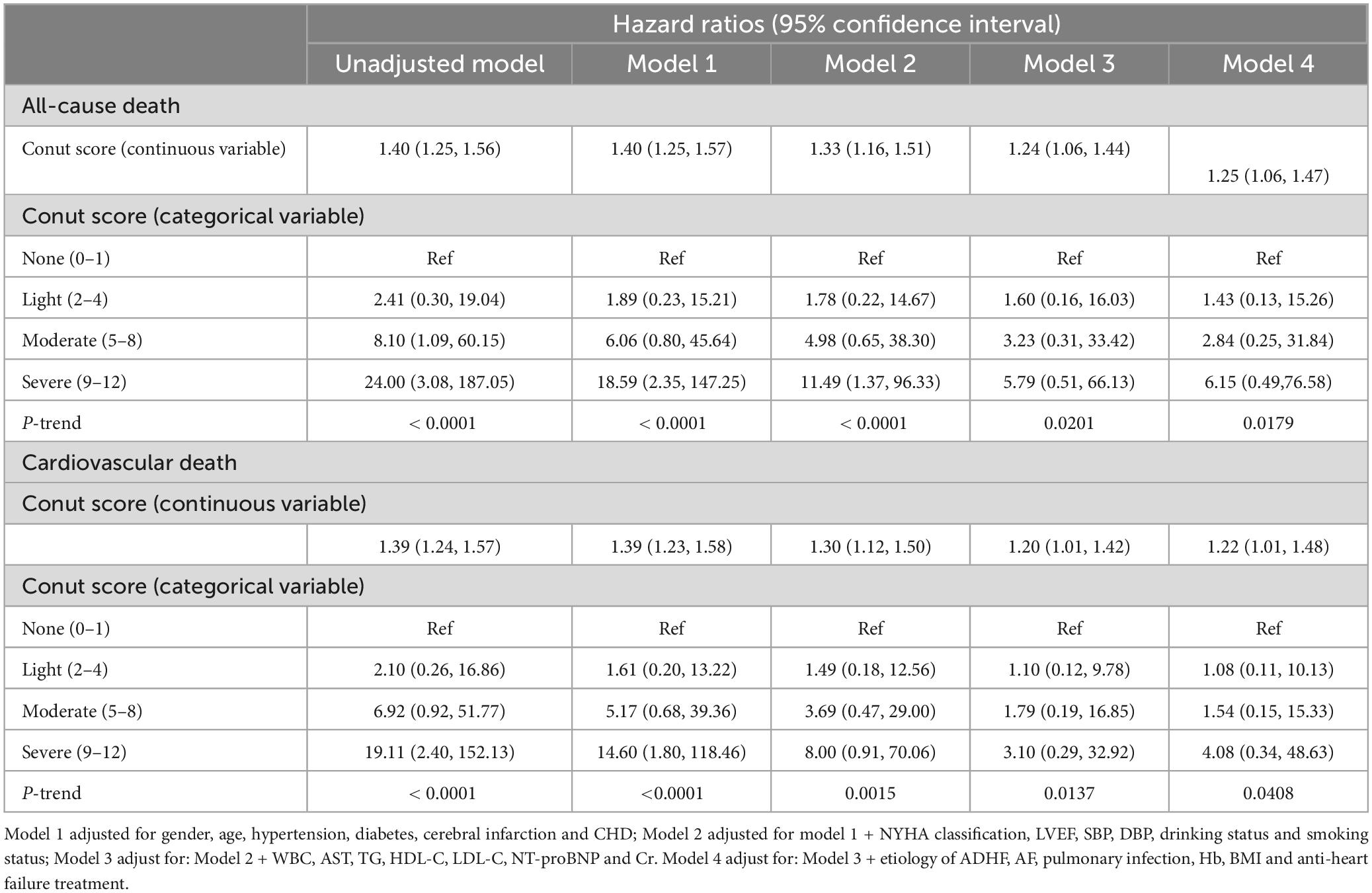
Table 4. Multivariable Cox regression analysis of the relationship between CONUT score and in-hospital all-cause mortality in patients with acute decompensated heart failure.
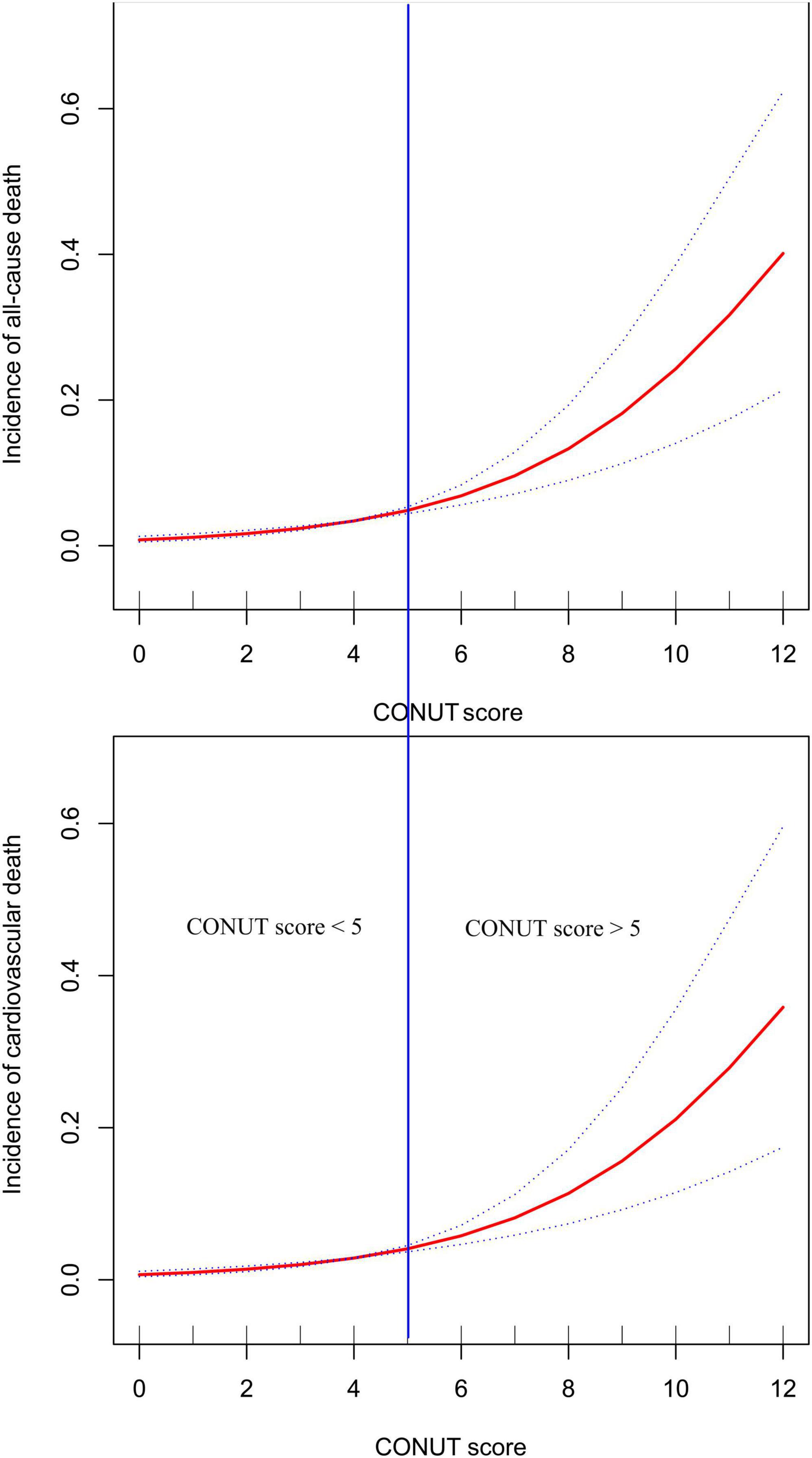
Figure 3. Fitting the dose-response relationship between CONUT score and all-cause mortality and cardiovascular mortality in ADHF patients with 3 knots restricted cubic spline.
Accuracy of CONUT score in identifying all-cause mortality events during hospitalization in ADHF patients
ROC curves were utilized to evaluate the ability of the CONUT score and its components (TL, ALB, and TC) to identify all-cause mortality during hospitalization in ADHF patients (Table 5). As depicted in Figure 4, the CONUT score demonstrated the highest AUC value for predicting all-cause mortality during hospitalization in ADHF patients compared to TL, ALB, and TC (AUC: CONUT score 0.7625, TL 0.7359, ALB: 0.6926, TC 0.6257; All DeLong P < 0.0001), with the optimal threshold identified as 5.5.
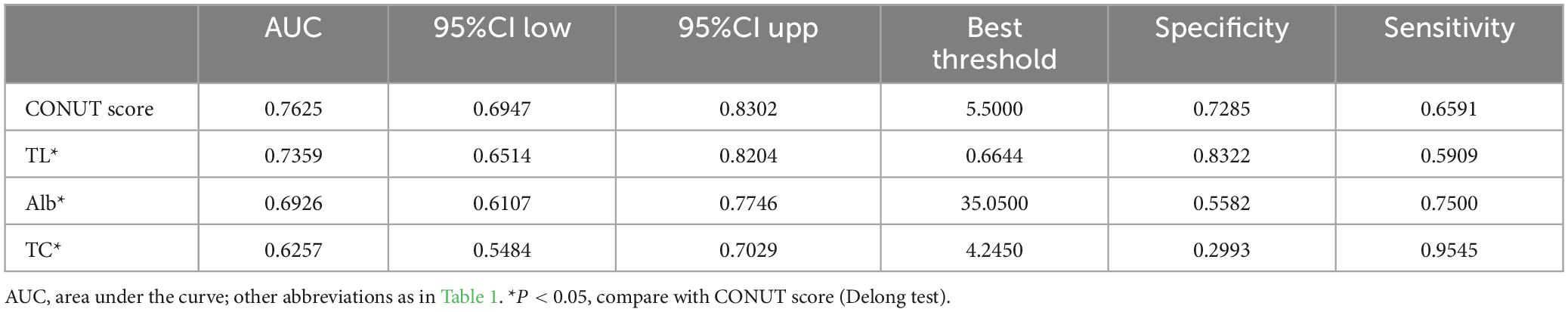
Table 5. Area under the receiver operating characteristic curve of the CONUT score and its components on in-hospital all-cause mortality in patients with acute decompensated heart failure.
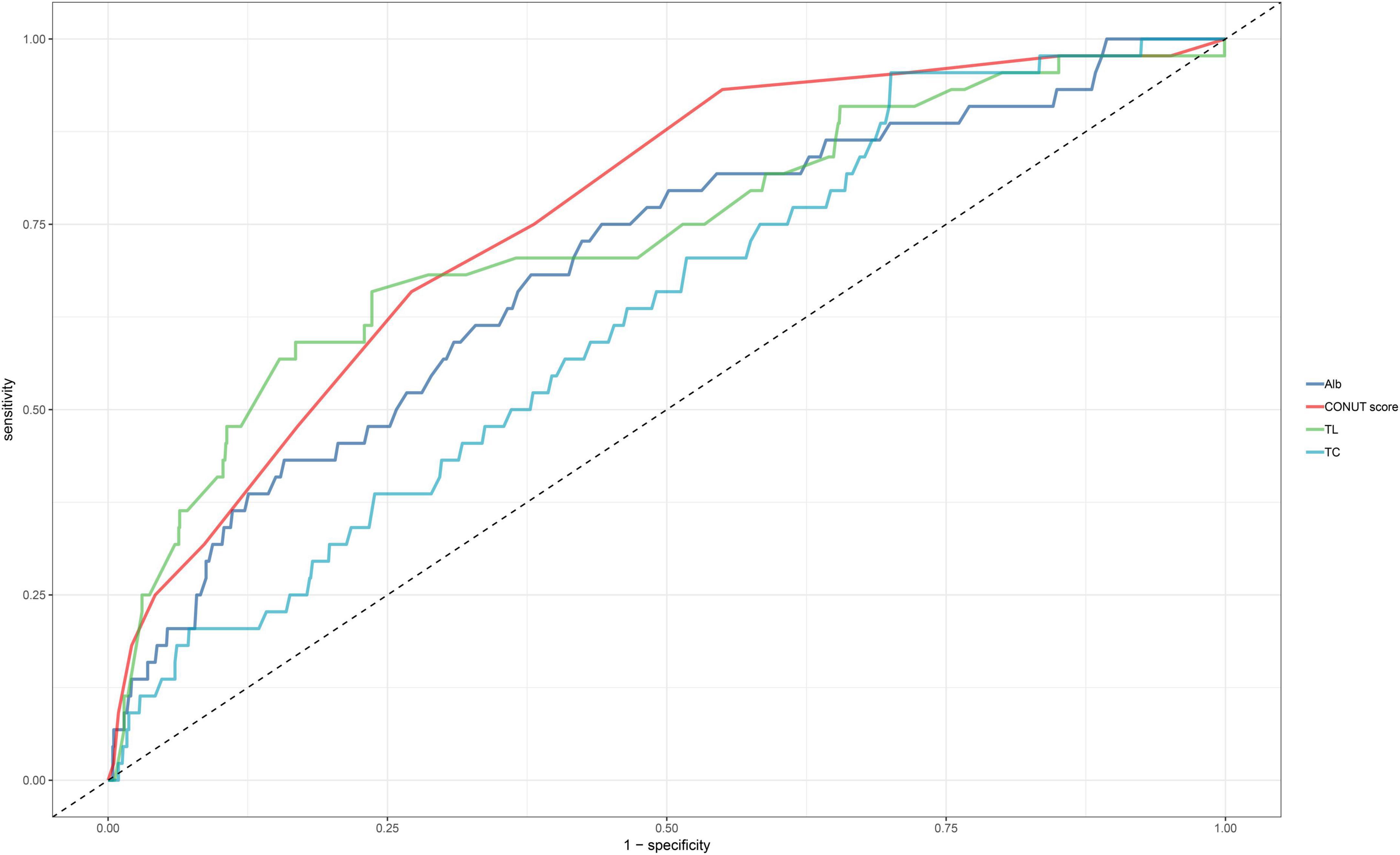
Figure 4. ROC analysis shows the predictive value of CONUT score and its components for all-cause mortality in patients with ADHF.
Subgroup analysis
Stratified analyses were conducted based on age, gender, NYHA classification, LVEF, and the presence of hypertension, diabetes, cerebral infarction, and coronary heart disease to explore potential specific populations affecting the relationship between the CONUT score and all-cause mortality during hospitalization in ADHF patients (Table 6). These parameters were stratified according to clinically common critical points. The results indicated that, except for the gender and history of cerebral infarction subgroups, there were no significant interactions between other subgroup factors and CONUT score-related in-hospital all-cause mortality. In the gender subgroup, compared to females, male ADHF patients had a significantly higher risk of CONUT score-related in-hospital all-cause mortality (HR: 1.34 vs 1.06). In the subgroup with a history of cerebral infarction, ADHF patients with concomitant cerebral infarction had a significantly higher risk of CONUT score-related in-hospital all-cause mortality compared to those without cerebral infarction (HR: 1.71 vs 1.05).
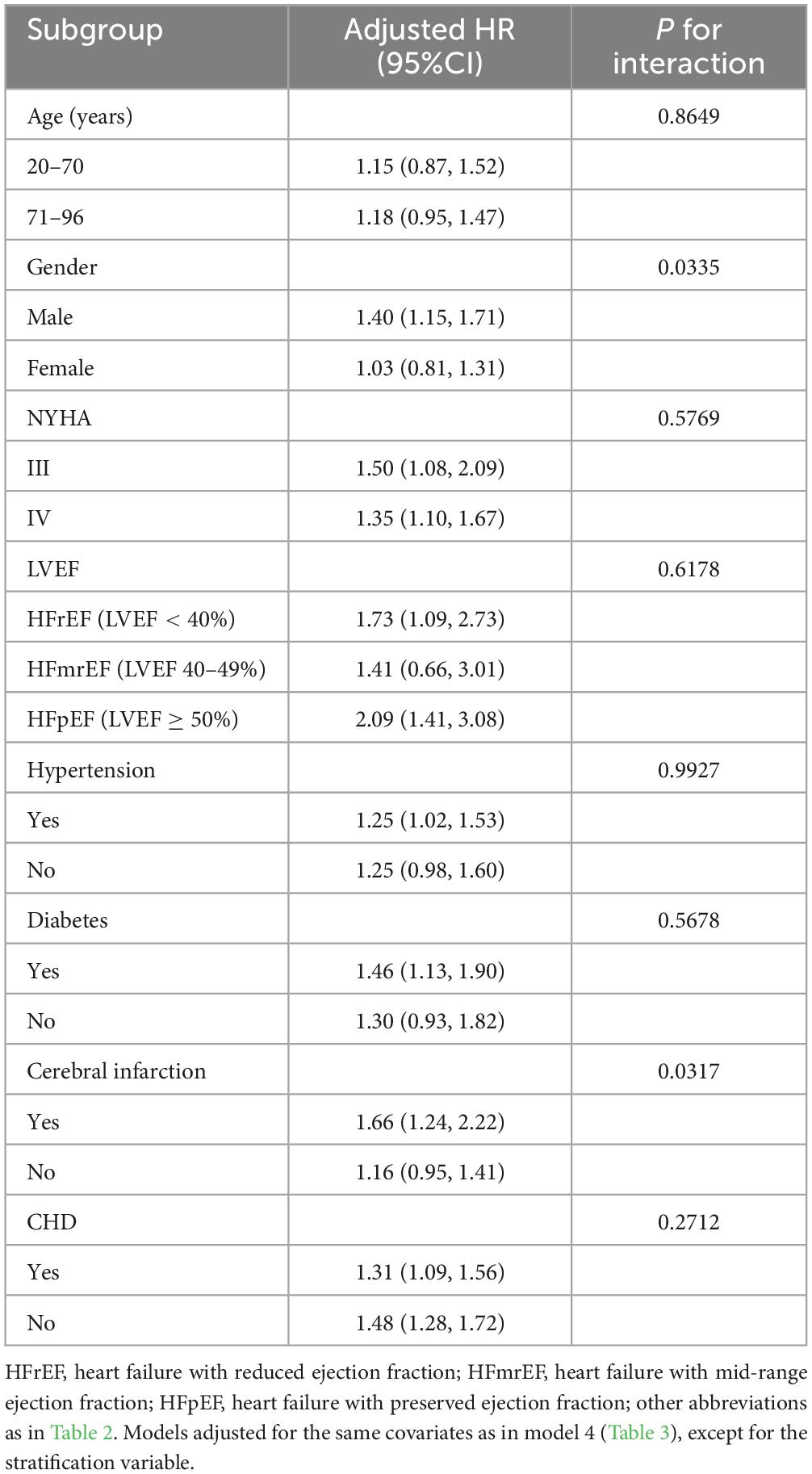
Table 6. Stratified analysis showed the relationship between CONUT score and in-hospital all-cause mortality in patients with acute decompensated heart failure in different age, gender, NYHA class, LVEF and whether combined with hypertension/diabetes/cerebral infarction/CHD.
Discussion
This study analyzed data from the JX-ADHF1 project spanning 2019–2022 to explore the association between nutritional status, as assessed by the CONUT score, and mortality during hospitalization in patients with ADHF. We found that the CONUT score could predict the risk of in-hospital all-cause/cardiovascular mortality in ADHF patients in the Jiangxi population of China. Notably, a CONUT score of 5 appeared to be a threshold effect point for poor in-hospital prognosis, with a rapid increase in mortality risk when the score exceeded this value.
ADHF is a worsening state of heart failure (12), primarily related to systemic congestion. Reduced cardiac output in ADHF can lead to insufficient perfusion of organs such as the gastrointestinal tract, lungs, liver, and kidneys, causing significant adverse effects (32, 33). Epidemiological studies have shown a high mortality rate during hospitalization for ADHF patients (34), highlighting the importance of assessing all-cause mortality risk at admission. Previous studies have indicated that a high CONUT score is an independent risk factor for all-cause mortality during hospitalization in ADHF patients in Japan and is closely associated with adverse outcomes in chronic heart failure patients in the United States (35). Considering national and ethnic differences and disease mechanisms, it was unclear whether this score would have the same effect in the Chinese ADHF population. Our study demonstrated a significant positive correlation between the CONUT score and all-cause mortality in ADHF patients in China, with every unit increase in the CONUT score increasing the risk of all-cause mortality during hospitalization by 24%, even after adjusting for confounding factors.
The CONUT score is composed of TL, ALB, and TC, with each component’s reduction and an increase in CONUT score associated with poorer nutritional status (36). Malnutrition and ADHF are interrelated in a bidirectional manner. Hormonal imbalances, metabolic disturbances, and immune dysregulation are key pathophysiological mechanisms in ADHF (33, 37–39). These changes lead to increased catecholamines, TNF-α, and natriuretic peptides, further accelerating the breakdown of ALB and fats, leading to malnutrition (40–44). Moreover, hypoalbuminemia exacerbates myocardial dysfunction by promoting pulmonary congestion, fluid retention, and myocardial edema (45–48). Hyperlipidemia is a risk factor for cardiovascular diseases, obesity, diabetes, and other metabolic disorders (49, 50), with various measures developed to reduce lipid levels (51–53). However, low lipid levels have been identified as a marker of increased mortality in heart failure patients (54–56), and reduced TC can increase the risk of death in ADHF patients (57). This lipid paradox might be related to inflammatory responses and nutritional status (58); decreased TC can elevate endotoxins, exacerbating inflammation (59, 60). Additionally, TL plays a protective role in cardiac homeostasis and response to injury (61–63). A reduction in TL count signifies a precarious state for the heart. The Seattle Heart Failure Model also identified TL as an independent predictor of heart failure prognosis (64), a finding further validated by our study.
Our analysis revealed a potential threshold effect in the dose-response relationship between the CONUT score and all-cause/cardiovascular mortality in ADHF patients. The risk of mortality increased rapidly when the CONUT score exceeded 5. Combined with the ROC analysis threshold result, we suggest that a CONUT score of 5 is significant for ADHF patients; scores exceeding this value indicate a potential for significant adverse events in the short term, warranting increased attention to improving nutritional status. Previous studies have also shown that nutritional status is related to the prognosis of cardiovascular diseases (36, 65–67), with patients suffering from moderate to severe malnutrition at a greater risk of adverse cardiovascular events, aligning with the findings of our study.
Our subgroup analysis observed some meaningful results: compared to female patients and those without cerebral infarction, male ADHF patients with cerebral infarction exhibited a relatively higher risk of in-hospital all-cause mortality associated with CONUT scores. This finding is consistent with the results summarized in the baseline characteristics table. Table 3 shows that as the severity of malnutrition increases, the proportion of male patients and those with cerebral infarction significantly rises. We have the following considerations for these particular results in male patients and those with cerebral infarction: (1) Compared to females, males generally tend to have poorer lifestyle habits, such as smoking, alcohol consumption, and worse dietary control (68), which can lead to various cardiometabolic diseases and exacerbate malnutrition (69–71). Additionally, patients with cerebral infarction often experience varying degrees of physical activity impairment, gastrointestinal dysfunction, and even feeding difficulties. These sequelae significantly aggravate the malnutrition in these patients (72). Therefore, the higher all-cause mortality related to CONUT scores in male and cerebral infarction patients may be associated with further malnutrition secondary to comorbidities. (2) From the perspective of CONUT score calculation, decreases in TL, ALB, and TC lead to higher CONUT scores, subsequently increasing the risk of adverse outcomes. In our study population, which mainly consisted of elderly individuals (mean age 68 years), postmenopausal women showed significantly reduced estrogen levels, leading to fat accumulation and elevated TC levels (73). It is noteworthy that high TC levels are associated with low CONUT scores, which might explain why the mortality risk related to CONUT scores is lower in females than in males. For patients with cerebral infarction, post-ischemic brain damage can easily trigger oxidative stress and inflammatory responses, disrupting the gut-brain axis and the hypothalamic-pituitary-adrenal axis, resulting in reduced TL production, decreased ALB synthesis, increased catabolism, and increased TC breakdown, leading to malnutrition (74–78).
Study strengths and limitations
Strengths: (1) This study is the first to confirm the association between the CONUT score and in-hospital mortality in ADHF patients in the Chinese population, establishing its predictive value. (2) The CONUT score comprises easily accessible indicators, making it simple, convenient, and easily adoptable. (3) We identified population dependencies in the risk of in-hospital all-cause mortality related to the CONUT score in ADHF patients, highlighting the importance of identifying these specific populations for assessment.
Limitations: (1) The study population primarily comes from various regions in Jiangxi, and caution should be exercised when generalizing these findings to other regions in China and other countries. (2) As a retrospective study, it is subject to inherent statistical limitations, and our researchers had limited access to historical data, such as lipid-lowering medication use and family history. (3) This study assessed the impact of the CONUT score at admission on in-hospital mortality outcomes in ADHF patients, and the relationship between dynamic changes in the CONUT score and in-hospital adverse outcomes remains unclear, necessitating further research.
Conclusion
The CONUT score is an independent predictor of all-cause mortality during hospitalization in patients with ADHF. Our study indicates that a CONUT score of 5 may represent a threshold effect point for the occurrence of all-cause mortality during hospitalization in ADHF patients. When the CONUT score exceeds 5, there is a rapid increase in the risk coefficient for all-cause mortality. Additionally, the relationship between the CONUT score and in-hospital mortality in ADHF patients shows a dependency on specific population subgroups, with male patients and those with a history of cerebral infarction requiring particular attention to their CONUT score results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of Jiangxi Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XH: Formal analysis, Investigation, Software, Validation, Writing−original draft. JQ: Investigation, Software, Writing−original draft. MK: Formal analysis, Investigation, Software, Validation, Writing−original draft. CW: Investigation, Writing−review and editing. SH: Investigation, Writing−review and editing. CY: Investigation, Writing−review and editing. GX: Investigation, Writing−review and editing. GS: Conceptualization, Methodology, Project administration, Supervision, Writing−review and editing. YZ: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Writing−review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. This work was supported by the Natural Science Foundation of Jiangxi Province [No. 20232BAB216004 to YZ].
Acknowledgments
We would like to thank Jiangxi Provincial People’s Hospital for its strong support to the research project and the members of the JX-ADHF1 research team for their great efforts in the data collection process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1392268/full#supplementary-material
References
1. Ziaeian B, Fonarow G. Epidemiology and aetiology of heart failure. Nat Rev Cardiol (2016) 13:368–78. doi: 10.1038/nrcardio.2016.25
3. Boorsma E, Ter Maaten J, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol (2020) 17:641–55. doi: 10.1038/s41569-020-0379-7
4. Parikh K, Sharma K, Fiuzat M, Surks H, George J, Honarpour N, et al. Heart Failure With Preserved Ejection Fraction Expert Panel Report: Current Controversies and Implications for Clinical Trials. JACC Heart Fail (2018) 6:619–32. doi: 10.1016/j.jchf.2018.06.008
5. Tsao C, Aday A, Almarzooq Z, Anderson C, Arora P, Avery C, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation (2023) 147:e93–621. doi: 10.1161/CIR.0000000000001123
6. Ambrosy A, Fonarow G, Butler J, Chioncel O, Greene S, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol (2014) 63:1123–33. doi: 10.1016/j.jacc.2013.11.053
7. Crespo-Leiro M, Anker S, Maggioni A, Coats A, Filippatos G, Ruschitzka F, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail (2016) 18:613–25. doi: 10.1002/ejhf.566
8. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
9. Arrigo M, Jessup M, Mullens W, Reza N, Shah A, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers (2020) 6:16. doi: 10.1038/s41572-020-0151-7
10. Cotter G, Felker G, Adams K, Milo-Cotter O, O’Connor C. The pathophysiology of acute heart failure–is it all about fluid accumulation? Am Heart J (2008) 155:9–18. doi: 10.1016/j.ahj.2006.02.038
11. Gheorghiade M, Pang P. Acute heart failure syndromes. J Am Coll Cardiol (2009) 53:557–73. doi: 10.1016/j.jacc.2008.10.041
12. Spinar J, Parenica J, Vitovec J, Widimsky P, Linhart A, Fedorco M, et al. Baseline characteristics and hospital mortality in the Acute Heart Failure Database (AHEAD) Main registry. Crit Care (2011) 15:R291. doi: 10.1186/cc10584
13. Lepage S. Acute decompensated heart failure. Can J Cardiol (2008) 24 Suppl B:6B–8B. doi: 10.1016/s0828-282x71022-5
14. Lagu T, Pekow P, Shieh M, Stefan M, Pack Q, Kashef M, et al. Validation and Comparison of Seven Mortality Prediction Models for Hospitalized Patients With Acute Decompensated Heart Failure. Circ Heart Fail (2016) 9:e002912. doi: 10.1161/CIRCHEARTFAILURE.115.002912
15. Adams K Jr., Fonarow G, Emerman C, LeJemtel T, Costanzo M, Abraham W, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J (2005) 149:209–16. doi: 10.1016/j.ahj.2004.08.005
16. Sandek A, Doehner W, Anker S, von Haehling S. Nutrition in heart failure: an update. Curr Opin Clin Nutr Metab Care (2009) 12:384–91. doi: 10.1097/MCO.0b013e32832cdb0f
17. Müller T, Nogueiras R, Andermann M, Andrews Z, Anker S, Argente J, et al. Ghrelin. Mol Metab (2015) 4:437–60. doi: 10.1016/j.molmet.2015.03.005
18. Bonilla-Palomas J, Gámez-López A, Castillo-Domínguez J, Moreno-Conde M, López Ibáñez M, Alhambra Expósito R, et al. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch Med Res (2016) 47:535–40. doi: 10.1016/j.arcmed.2016.11.005
19. Gámez-López A, Bonilla-Palomas J, Anguita-Sánchez M, Moreno-Conde M, López-Ibáñez C, Alhambra-Expósito R, et al. Rationale and design of PICNIC study: nutritional intervention program in hospitalized patients with heart failure who are malnourished. Rev Esp Cardiol (Engl Ed) (2014) 67:277–82. doi: 10.1016/j.rec.2013.07.013
20. Al-Najjar Y, Clark A. Predicting outcome in patients with left ventricular systolic chronic heart failure using a nutritional risk index. Am J Cardiol (2012) 109:1315–20. doi: 10.1016/j.amjcard.2011.12.026
21. Bonilla-Palomas J, Gámez-López A, Moreno-Conde M, López-Ibáñez M, Anguita-Sánchez M. Hypoalbuminemia in acute heart failure patients: causes and its impact on hospital and long-term mortality. J Card Fail (2014) 20:350–8. doi: 10.1016/j.cardfail.2014.01.016
22. Suzuki N, Kida K, Suzuki K, Harada T, Akashi Y. Assessment of transthyretin combined with mini nutritional assessment on admission provides useful prognostic information in patients with acute decompensated heart failure. Int Heart J (2015) 56:226–33. doi: 10.1536/ihj.14-255
23. Parahiba S, Spillere S, Zuchinali P, Padilha G, Duarte M, da Silveira I, et al. Handgrip strength in patients with acute decompensated heart failure: Accuracy as a predictor of malnutrition and prognostic value. Nutrition (2021) 9(1–92):111352. doi: 10.1016/j.nut.2021.111352
24. Agnoletti D, Arcaro G, Scaturro G, Turcato E, Grison E, Ferrari E, et al. Controlling nutritional status score predicts 2-year outcomes in elderly patients admitted for acute heart failure. Intern Emerg Med (2023) 18:1031–9. doi: 10.1007/s11739-023-03230-x
25. Iwakami N, Nagai T, Furukawa T, Sugano Y, Honda S, Okada A, et al. Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int J Cardiol (2017) 230:529–36. doi: 10.1016/j.ijcard.2016.12.064
26. Ignacio de Ulíbarri J, González-Madroño A, de Villar N, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp (2005) 20:38–45.
27. Chen L, Sun H, Zhao R, Huang R, Pan H, Zuo Y, et al. Controlling Nutritional Status (CONUT) Predicts Survival in Gastric Cancer Patients With Immune Checkpoint Inhibitor (PD-1/PD-L1) Outcomes. Front Pharmacol (2022) 13:836958. doi: 10.3389/fphar.2022.836958
28. Une M, Ito M, Suzuki H, Toide M, Kobayashi S, Fukushima H, et al. Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma. Cancers (Basel) (2022) 14:5075. doi: 10.3390/cancers14205075
29. Arero G, Arero A, Mohammed S, Vasheghani-Farahani A. Prognostic Potential of the Controlling Nutritional Status (CONUT) Score in Predicting All-Cause Mortality and Major Adverse Cardiovascular Events in Patients With Coronary Artery Disease: A Meta-Analysis. Front Nutr (2022) 9:850641. doi: 10.3389/fnut.2022.850641
30. Takagi K, Takahashi H, Miura T, Yamagiwa K, Kawase K, Muramatsu-Maekawa Y, et al. Prognostic Value of the Controlling Nutritional Status (CONUT) Score in Patients at Dialysis Initiation. Nutrients (2022) 14:2317. doi: 10.3390/nu14112317
31. Kato T, Yaku H, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, et al. Association with Controlling Nutritional Status (CONUT) Score and In-hospital Mortality and Infection in Acute Heart Failure. Sci Rep (2020) 10:3320. doi: 10.1038/s41598-020-60404-9
32. Verbrugge F, Guazzi M, Testani J, Borlaug B. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation (2020) 142:998–1012. doi: 10.1161/CIRCULATIONAHA.119.045409
33. Njoroge J, Teerlink J. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ Res (2021) 128:1468–86. doi: 10.1161/CIRCRESAHA.121.318186
34. Rider I, Sorensen M, Brady W, Gottlieb M, Benson S, Koyfman A, et al. Disposition of acute decompensated heart failure from the emergency department: An evidence-based review. Am J Emerg Med (2021) 50:459–65. doi: 10.1016/j.ajem.2021.08.070
35. Sze S, Pellicori P, Zhang J, Weston J, Clark A. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr (2021) 113:695–705. doi: 10.1093/ajcn/nqaa311
36. Miano N, Di Marco M, Alaimo S, Coppolino G, L’Episcopo G, Leggio S, et al. Controlling Nutritional Status (CONUT) Score as a Potential Prognostic Indicator of In-Hospital Mortality, Sepsis and Length of Stay in an Internal Medicine Department. Nutrients (2023) 15:1554. doi: 10.3390/nu15071554
37. Doehner W, Frenneaux M, Anker S. Metabolic impairment in heart failure: the myocardial and systemic perspective. J Am Coll Cardiol (2014) 64:1388–400. doi: 10.1016/j.jacc.2014.04.083
38. Tang S, Li R, Ma W, Lian L, Gao J, Cao Y, et al. Cardiac-to-adipose axis in metabolic homeostasis and diseases: special instructions from the heart. Cell Biosci (2023) 13:161. doi: 10.1186/s13578-023-01097-1
39. Levine B, Kalman J, Mayer L, Fillit H, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med (1990) 323:236–41. doi: 10.1056/NEJM199007263230405
40. Rydén M, Arner P. Fat loss in cachexia–is there a role for adipocyte lipolysis? Clin Nutr (2007) 26:1–6. doi: 10.1016/j.clnu.2006.09.009
41. Rydén M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, Arner P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem Biophys Res Commun (2004) 318:168–75. doi: 10.1016/j.bbrc.2004.04.010
42. Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J (2000) 14:1345–51.
43. Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab (2008) 19:130–7. doi: 10.1016/j.tem.2007.11.006
44. Polak J, Kotrc M, Wedellova Z, Jabor A, Malek I, Kautzner J, et al. Lipolytic effects of B-type natriuretic peptide 1-32 in adipose tissue of heart failure patients compared with healthy controls. J Am Coll Cardiol (2011) 58:1119–25. doi: 10.1016/j.jacc.2011.05.042
45. Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail (2011) 17:451–8. doi: 10.1016/j.cardfail.2011.02.010
46. Dongaonkar R, Stewart R, Geissler H, Laine G. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc Res (2010) 87:331–9. doi: 10.1093/cvr/cvq145
47. Jacob M, Bruegger D, Rehm M, Welsch U, Conzen P, Becker B. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology (2006) 104:1223–31. doi: 10.1097/00000542-200606000-00018
48. Jacob M, Paul O, Mehringer L, Chappell D, Rehm M, Welsch U, et al. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation (2009) 87:956–65. doi: 10.1097/TP.0b013e31819c83b5
49. Bertolotti M, Maurantonio M, Gabbi C, Anzivino C, Carulli N. Review article: hyperlipidaemia and cardiovascular risk. Aliment Pharmacol Ther (2005) 22(Suppl 2):28–30. doi: 10.1111/j.1365-2036.2005.02591.x
50. Su X, Chen X, Wang B. Pathology of metabolically-related dyslipidemia. Clin Chim Acta (2021) 521:107–15. doi: 10.1016/j.cca.2021.06.029
51. Gong F, Shi Q, Mou X, Wang K, Wang Q, Wang H. Atorvastatin mitigates memory deficits and brain monocyte infiltration in chronic hypercholesterolemia. Aging (Albany NY) (2023) 15:13669–79. doi: 10.18632/aging.205217
52. Villaño D, Marhuenda J, Arcusa R, Moreno-Rojas J, Cerdá B, Pereira-Caro G, et al. Effect of Black Garlic Consumption on Endothelial Function and Lipid Profile: A Before-and-After Study in Hypercholesterolemic and Non-Hypercholesterolemic Subjects. Nutrients (2023) 15:3138. doi: 10.3390/nu15143138
53. Lan N, Bajaj A, Watts G, Cuchel M. Recent advances in the management and implementation of care for familial hypercholesterolaemia. Pharmacol Res (2023) 194:106857. doi: 10.1016/j.phrs.2023.106857
54. Degoricija V, Potočnjak I, Gastrager M, Pregartner G, Berghold A, Scharnagl H, et al. HDL subclasses and mortality in acute heart failure patients. Clin Chim Acta (2019) 490:81–7. doi: 10.1016/j.cca.2018.12.020
55. Mehra M, Uber P, Lavie C, Milani R, Park M, Ventura H. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant (2009) 28:876–80. doi: 10.1016/j.healun.2009.04.026
56. Rauchhaus M, Clark A, Doehner W, Davos C, Bolger A, Sharma R, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol (2003) 42:1933–40. doi: 10.1016/j.jacc.2003.07.016
57. Horwich T, Hernandez A, Dai D, Yancy C, Fonarow G. Cholesterol levels and in-hospital mortality in patients with acute decompensated heart failure. Am Heart J (2008) 156:1170–6. doi: 10.1016/j.ahj.2008.07.004
58. Liu Y, Coresh J, Eustace J, Longenecker J, Jaar B, Fink N, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA (2004) 291:451–9. doi: 10.1001/jama.291.4.451
59. Rauchhaus M, Coats A, Anker S. The endotoxin-lipoprotein hypothesis. Lancet (2000) 356:930–3. doi: 10.1016/S0140-673602690-8
60. Mueller C, Laule-Kilian K, Christ A, Brunner-La Rocca H, Perruchoud A. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J (2006) 151:845–50. doi: 10.1016/j.ahj.2005.06.046
61. Rurik J, Aghajanian H, Epstein J. Immune Cells and Immunotherapy for Cardiac Injury and Repair. Circ Res (2021) 128:1766–79. doi: 10.1161/CIRCRESAHA.121.318005
62. Rieckmann M, Delgobo M, Gaal C, Büchner L, Steinau P, Reshef D, et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest (2019) 129:4922–36. doi: 10.1172/JCI123859
63. Schwartz M, Moalem G, Leibowitz-Amit R, Cohen I. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci (1999) 22:295–9. doi: 10.1016/s0166-223601405-8
64. Levy W, Mozaffarian D, Linker D, Sutradhar S, Anker S, Cropp A, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation (2006) 113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102
65. Tonet E, Campo G, Maietti E, Formiga F, Martinez-Sellés M, Pavasini R, et al. Nutritional status and all-cause mortality in older adults with acute coronary syndrome. Clin Nutr (2020) 39:1572–9. doi: 10.1016/j.clnu.2019.06.025
66. Lew C, Wong G, Cheung K, Fraser R, Chua A, Chong M, et al. The association between nutritional adequacy and 28-day mortality in the critically ill is not modified by their baseline nutritional status and disease severity. Crit Care (2019) 23:222. doi: 10.1186/s13054-019-2500-z
67. Czapla M, Karniej P, Juárez-Vela R, Łokieć K. The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome-A Result of the Retrospective Nutritional Status Heart Study (NSHS). Nutrients (2020) 12:3091. doi: 10.3390/nu12103091
68. Minami M, Demura S, Nagasawa Y. [Gender and age-related differences in life style characteristics, health condition, and unidentified complaints in healthy old-aged people]. Nihon Eiseigaku Zasshi (2002) 56:682–92. doi: 10.1265/jjh.56.682
69. Li C, Mao Z, Yu C. The effects of smoking, regular drinking, and unhealthy weight on health care utilization in China. BMC Public Health (2021) 21:2268. doi: 10.1186/s12889-021-12309-z
70. Ding N, Shah A, Blaha M, Chang P, Rosamond W, Matsushita K. Cigarette Smoking, Cessation, and Risk of Heart Failure With Preserved and Reduced Ejection Fraction. J Am Coll Cardiol (2022) 79:2298–305. doi: 10.1016/j.jacc.2022.03.377
71. Zhang X, Shan C, Hu K, Fang B, Zhang Z, Xie Q, et al. Prognostic value of metabolic syndrome in patients with heart failure and malnutrition. BMC Cardiovasc Disord (2024) 24:136. doi: 10.1186/s12872-024-03767-5
72. Zielińska-Nowak E, Cichon N, Saluk-Bijak J, Bijak M, Miller E. Nutritional Supplements and Neuroprotective Diets and Their Potential Clinical Significance in Post-Stroke Rehabilitation. Nutrients (2021) 13:2704. doi: 10.3390/nu13082704
73. Ko S, Kim H. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients (2020) 12:202. doi: 10.3390/nu12010202
74. Kelly P, Lemmens R, Tsivgoulis G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke (2021) 52:2697–706. doi: 10.1161/STROKEAHA.121.034388
75. Soeters P, Wolfe R, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr (2019) 43:181–93. doi: 10.1002/jpen.1451
76. Carpentier Y, Scruel O. Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr Opin Clin Nutr Metab Care (2002) 5:153–8. doi: 10.1097/00075197-200203000-00006
77. Courties G, Frodermann V, Honold L, Zheng Y, Herisson F, Schloss M, et al. Glucocorticoids Regulate Bone Marrow B Lymphopoiesis After Stroke. Circ Res (2019) 124:1372–85. doi: 10.1161/CIRCRESAHA.118.314518
Keywords: predictive value, controlling nutritional status score, acute decompensated heart failure, CONUT score, ADHF, in-hospital mortality
Citation: Huang X, Qiu J, Kuang M, Wang C, He S, Yu C, Xie G, Sheng G and Zou Y (2024) Assessing the predictive value of the controlling nutritional status score on all-cause mortality during hospitalization in patients with acute decompensated heart failure: a retrospective cohort study from Jiangxi, China. Front. Nutr. 11:1392268. doi: 10.3389/fnut.2024.1392268
Received: 01 March 2024; Accepted: 24 June 2024;
Published: 05 July 2024.
Edited by:
Filippo Valbusa, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyReviewed by:
Ramdas G. Pai, University of California, Riverside, United StatesXinyi Lu, Nanjing Medical University, China
Copyright © 2024 Huang, Qiu, Kuang, Wang, He, Yu, Xie, Sheng and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guotai Sheng, dGdzMjAwNTA5QDE2My5jb20=; Yang Zou, anh5eHl6eUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xin Huang
Xin Huang Jiajun Qiu
Jiajun Qiu Maobin Kuang
Maobin Kuang Chao Wang
Chao Wang Shiming He
Shiming He Changhui Yu
Changhui Yu Guobo Xie
Guobo Xie Guotai Sheng
Guotai Sheng Yang Zou
Yang Zou