- 1Scientific Society for Vegetarian Nutrition, Venice, Italy
- 2Earth Philosophical Society “Melodia Vitae”, International, Toronto, CA, Canada
- 3Independent Researcher, Messina, Italy
- 4School of Chemical Engineering, Aalto University, Espoo, Finland
- 5I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 6Division of Gastroenterology, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy
- 7NUTRAFOOD, Interdepartmental Center for Nutraceutical Research and Nutrition for Health, University of Pisa, Pisa, Italy
Introduction: The growing prevalence of vegetarianism determines the need for comprehensive study of the impact of these diets on health and particularly on bone metabolism. We hypothesized that significant dietary differences between vegans, lacto-ovo-vegetarians, and omnivores also cause significant differences in their nutrient status, which may affect bone health.
Methods: The study assessed dual-energy X-ray absorptiometry parameters in lumbar spine and femoral neck, average nutrient intake, serum nutrient concentrations, serum PTH levels, and urinary pH among 46 vegans, 38 lacto-ovo-vegetarians, and 44 omnivores.
Results: There were no differences in bone mineral density (BMD) between the groups. However, the parathyroid hormone (PTH) levels were still higher in vegans compared to omnivores, despite the same prevalence of hyperparathyroidism in all groups. These findings may probably be explained by the fact that each group had its own “strengths and weaknesses.” Thus, vegans and, to a lesser extent, lacto-ovo-vegetarians consumed much more potassium, magnesium, copper, manganese, and vitamins B6, B9, and C. At the same time, the diet of omnivores contained more protein and vitamins D and B12. All the subjects consumed less vitamin D than recommended. More than half of vegans and omnivores had insufficiency or even deficiency of vitamin D in the blood. Low serum concentrations of manganese with its quite adequate intake are also noteworthy: its deficiency was observed in 57% of vegans, 79% of lacto-ovo-vegetarians, and 63% of omnivores.
Discussion: Currently, it is no longer possible to conclude that lacto-ovo-vegetarians have lower BMD than omnivores, as our research supported. Vegans in our study also did not demonstrate lower BMD values, only higher PTH blood concentrations, compared to omnivores, however, a large number of studies, including recent, show the opposite view. In this regard, further large-scale research is required. Vegans and lacto-ovo-vegetarians now have a variety of foods fortified with vitamins D and B12, as well as calcium. There is also a great diversity of ethically sourced dietary supplements. The found low concentrations of manganese require further investigation.
1 Introduction
More and more people exclude various products of animal origin from their diet for many reasons such as health, moral, ethical, religious, cultural, ecological and other beliefs (1–4). According to the SuperJob sociological survey (5), which involved 1,600 economically active respondents over 18 years old, in 2008, there were 3% of lacto-ovo-vegetarians in Russia. By 2013, this value had risen to 4%, but in 2021 returned to the previous value: the observed changes could be attributed to bias or related to some other reasons. Limiting the consumption of meat and offal may be, for instance, a necessary measure for cardiological and nephrological patients (6, 7). At the same time, there is an evidence (8) that this type of diets, if not properly planned, can lead to deficiency of certain nutrients (as other diets certainly can), which may contribute to metabolic disorders, including bone loss, that is, osteopenia and osteoporosis, because nutritional status has a great influence on bone metabolism.
Protein, in addition to its structural function (9), further performs a signaling role (10) by regulating the activity of osteoclast proteasomes and the balance of somatotropin and insulin-like growth factor-1 (9), which promote osteoblast activity (11) and stimulate intestinal calcium and phosphate uptake and phosphate reabsorption in the kidney (12, 13).
The inorganic component of bone is mainly represented by calcium and phosphorus (14, 15). Therefore, insufficient dietary intake of these elements can lead to demineralization of bone tissue. The composition of hydroxyapatite can also include magnesium (16–18) and zinс (19). Zinc reduces osteoclasts’ activity (20, 21) and at the same time promotes osteoblasts’ differentiation due to runt-related transcription factor 2 (RUNX2) stimulation (22). Magnesium inhibits parathyroid hormone (PTH) secretion (12) and induces osteoblasts’ proliferation (13). Moreover, copper inhibits bone resorption (23, 24) and contributes to the differentiation of stem cells into osteoblasts (23). Finally, the maturation of collagen, which is the main protein of bone tissue, occurs under the action of glycosyl and xylosyl transferases, whereas manganese serves as a cofactor for these enzymes (25).
Food has a significant impact on the acid–base state of the body. With a large acid load, reserves of alkali and alkaline earth metals are used to compensate for it, primarily calcium, the main store of which is bone tissue (26, 27). Ensuring a good supply with potassium can reduce the effect of the acid load on bone tissue (28), although the effect of acid load on calcium losses remains controversial (29). In addition, a sufficient intake of potassium reduces the body’s ability to accumulate lithium (30), an excess of which, for example, due to long-term lithium therapy for bipolar affective disorder patients, is associated with a decrease in bone mineral density (BMD) (31).
Vitamins В6, В9, and В12 are involved in folate cycle. Their inadequate blood levels cause the accumulation of an intermediate product, the amino acid homocysteine, in the body (32, 33). Hyperhomocysteinemia leads to a weakening of collagen bonds in bone tissue (34, 35) and a decrease in BMD can result (36). Vitamin C acts as a co-factor for prolyl and lysyl transferases engaged in collagen synthesis (37). Carotenoids reduce bone resorption (38) by inhibiting osteoclast formation (39, 40). They also constitute biological precursors of vitamin A, the alcohol form of which is retinol. The latter, in turn, stimulates the proliferation of osteoclasts (41) and can stimulate the release of lysosomal enzymes in them, eliciting bone resorption (42). Vitamin D regulates calcium and phosphate metabolism (43, 44). In addition, it stimulates osteoblast differentiation and inhibits osteoclastogenesis (45).
PTH has a significant impact on bone health. Intermittent action of this hormone enhances bone formation. However, persistently high concentrations of PTH in the blood have the opposite effect (46, 47). PTH receptors are located on both osteoblasts and osteoclasts. Activation of the former can prolong the life of osteoblasts and increase their activity. Signal transmission to the osteoclasts is slower than osteoblast stimulation. That is the reason why intermittent exposure to PTH stimulates anabolic processes (48, 49).
As a consequence, the presence of nutritional differences (status of vitamins, major and trace elements, and dietary acid load) in vegans, lacto-ovo-vegetarians, and omnivores may affect bone metabolism and increase fracture risk, as reported by other authors (50, 51).
We have not found any studies on BMD and bone health in vegetarians conducted in the Russian or any post-Soviet population, so we had a goal to represent it. The expansion of such research geography seems crucial because every region has its own climatogeographic features, and moreover, specifics of economic development, material well-being, national mentality, availability of food, and culinary preferences. Thus, the aim of our study was to crossectionally assess the state of nutritional factors potentially affecting bone metabolism in vegetarian diets (lacto-ovo and vegan), and to evaluate BMD itself in comparison to omnivorous in Russian adults.
In order to save simplicity and readability of the paper, individuals following vegan, lacto-ovo-vegetarian, or omnivorous diets will be presented as vegans, lacto-ovo-vegetarians, and omnivores, respectively. Please note that by these names authors mean nothing but dietary preferences.
2 Materials and methods
2.1 Study population
The present study included 128 adult patients: 84 vegetarians (46 vegans and 38 lacto-ovo-vegetarians) and 44 omnivorous, who were examined at the Federal Research Center for Nutrition, Biotechnology and Food Safety (Moscow) from February 2018 to December 2019. The appropriate total sample size was calculated using the G*Power software (Kiel University, Germany), with 5% error probability and 80% power (52).
We did not include to the samples subjects affected by serious somatic, neurological, and psychiatric pathologies. Since bone loss intensifies significantly with the end of reproductive age (53), especially in women (54), patients older than 50 years were also not included in the study.
Participants were divided into groups based on their own self-reported dietary pattern. The group of lacto-ovo-vegetarians included subjects who did not consume animal flesh for at least 1 year (the sample also included 3 lacto-vegetarians), and the group of vegans included subjects who did not consume any product of animal origin for at least 1 year, which corresponds to the generally accepted definitions of these dietary patterns (2).
Voluntary written informed consent was obtained from all study participants. The study was approved by the Ethics Committee of the Federal Research Center for Nutrition, Biotechnology and Food Safety (Protocol of the Ethics Committee No. 6 of December 22, 2017). This study was carried out in accordance with the World Medical Association Declaration of Helsinki (1964) and its subsequent amendments.
2.2 Densitometry
Dual energy X-ray absorptiometry (DXA) was chosen for the bone tissue examination. It is the gold standard for osteoporosis diagnosis, due to the relatively low radiation exposure. DXA results can be expressed as “areal bone mineral density” (g/cm2), which we shall henceforth call “BMD,” or as a ratio to two norms such as the BMD of the reference population by age and ethnicity (Z-score) or the BMD of the reference population of young people aged 20–30 (T-score) (55). According to the Russian clinical guidelines on diagnosis of osteoporosis, T-score should be used when working with peri and postmenopausal women and men aged over 50, who were excluded from our study (54). For this reason, we used Z-score, which is recommended for the other cases. The World Health Organization defines osteopenia when Z-score ranges from −1.0 to −2.5, while Z-score below −2.5 is a characteristic of osteoporosis (similar values are used for T-score) (56). DXA parameters such as Z-score and bone BMD were measured with a Stratos X-ray osteodensitometer (DMS, France, 2018) at the lumbar spine and at the femoral neck.
2.3 Laboratory analyzes
The blood samples were inoculated to Lab-Vac test tubes and then centrifuged, which allowed to separate serum from formed elements. After that, serum was transferred to micro-centrifuge tubes and frozen at a temperature of −80 degrees Celsius for up to 3 months. PTH analysis was performed immediately after the samples were obtained.
The serum concentrations of elements were determined by mass spectrometry with inductively coupled plasma (ICP-MS). For analysis, serum samples weighing about 0.8 g were placed in polypropylene centrifuge tubes and brought to a volume of 15 mL with a solution based on deionized water with a resistance of at least 15 MΩ * cm2 and with added 1% 1-butanol (# 1.00988, Merck KGaA, Germany), 0.1% Triton X-100 (Sigma #T9284 Sigma-Aldrich, United States), 0.07% nitric acid (Fluka #02650 TraceSELECT™, United States). Thus, the prepared sample was analyzed by ICP-MS on a NexION 300D quadrupole mass spectrometer (PerkinElmer, United States, s/n 81DN2062801) complete with a computer and specialized software.
Folate and vitamin B12 concentrations in serum were determined by chemiluminescent immunoassay using standard kits “Immulite 2000 Folic acid” and “Immulite 2000 B12” on Immulite 2000 analyzer (Siemens, Germany). The level of vitamin B6 was determined by microbiological method using the “ID-Vit® Vitamin B6” and “Immundiagnostik AG” (Germany) kits. To determine the concentration of 25-hydroxyvitamin D in blood, chemiluminescent immunoassay was performed on ADVIA Centaur automatic analyzer with “Siemens 25-OH Vitamin D” kits (Germany). Vitamin C was determined using a Surveyor high-performance liquid chromatographic system with a PDA Plus detector and XCalibur software (Thermo, United States).
PTH level was determined using enzyme-linked immunosorbent assay (ELISA) on Immulite 2000 analyzer (Siemens, Germany).
Urine acidity was analyzed using an automated LabUMat test strip meter.
2.4 Dietary assessment
The nutritional assessment of the subjects was carried out using the Nutrilogic program (Nutrilogic LLC, Russia), which was developed in cooperation with the Federal Research Center for Nutrition, Biotechnology and Food Safety for nutrition specialists to evaluate the patients` dietary anamneses (57). The effectiveness of the Nutrilogic service for analyzing human nutrition and the diet’s chemical composition and its validity were confirmed in 2018 at the Moscow State University of Food Production through comparison with the results of classic calculation procedure with food tables (58).
Since we assessed the yearly nutrition of the subjects, the food-frequency method was chosen. One interview lasting 30–70 min was conducted with each subject in the format of food frequency (and amount) questionnaires. The collection of information on nutrition was carried out under the supervision of the specialist (A.G.) at the Federal Research Center for Nutrition, Biotechnology and Food Safety. The interview was fully structured. A few days before the interview, each subject was asked to recall the features of their diet and the frequency of consumption of dishes and products. During the conversation, the interviewer invited the participant to evaluate their consumption of each group of products separately: whether the subject had consumed this product during the year, how often, and in which quantity. The following intervals were used to assess the frequency of food consumption: “once a day,” “2–4 times a week,” “once a week,” “2 times a month,” “once a month,” “2–4 times a year.”
The amount of food was estimated in grams using photographic measures. For this, color images of portions of various products and dishes with a known weight from the Nutrilogic program package were used. Portions of various weights were presented in life-size photographs on a screen, and the subject determined how much their average portion was commensurate with the one depicted. The Russian national food composition database “Chemical composition and calorie content of Russian food” by V.A. Tutel’yan was used as a food composition database for the analysis (59). The handbook contains data both on raw products and cooked dishes, thus, all the technological loses were taken into account.
In the Russian Federation, food fortification is not mandatory and not widely spread overall, especially in adults, so we did not evaluate fortified food consumption. Supplements were also not considered in our study.
2.5 Statistical analysis
Absolute values of nutrient intake were determined for each group. The levels of PTH, chemical elements, and vitamins in the blood, and the values of pH and DXA parameters were evaluated. The results obtained by the dietary assessment were compared with the daily nutritional requirements set in the Russian Federation (60, 61). The blood levels of chemical elements were compared with the normative values calculated by the laboratory “Micronutrients LLC” (Moscow), the other blood tests` reference values were taken as set by the Federal Research Center for Nutrition, Biotechnology, and Food Safety (Moscow). In each group, the percentage of people who consumed an inadequate amount of nutrients, with an inappropriate concentration of PTH or abnormal levels of nutrients in the serum was calculated. Densitometry criteria were compared with reference values for osteopenia and osteoporosis, and the proportion of people with these conditions was calculated.
The normality of continuous variables was checked by Shapiro–Wilk test. If normal, a variable was shown as mean (SD) and the t-test for independent samples was used to compare 2 groups. If not normal, a variable was presented as median (interquartile range) and the Mann–Whitney test was applied to compare 2 groups. Bonferroni post-hoc correction was applied to reduce the risk of false-positive results in multiple comparisons. Correlations were estimated by Pearson’s coefficient or Spearman rank correlation tests, where appropriate. The comparison of proportions was performed by Pearson’s χ2 test, Pearson’s χ2 test with Yates’s continuity correction, or Fisher’s exact test. p-value less than 0.05 was considered as statistically significant. All analyses were performed with SPSS 23 (IBM, United States).
3 Results
There were no significant differences in age and BMI between the groups. Anthropometric characteristics of the groups are shown in Table 1.
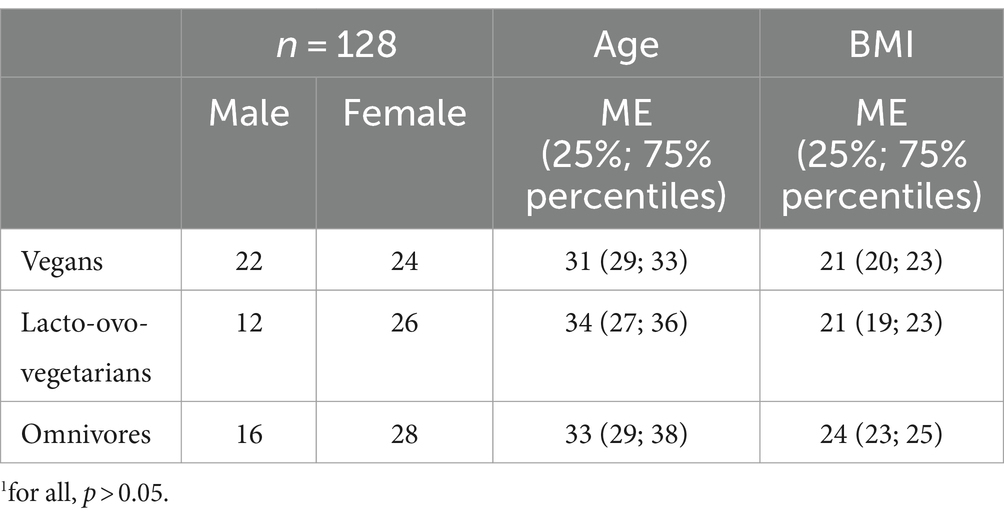
Table 1. Demographic and anthropometric characteristics of the vegan, lacto-ovo-vegetarian and omnivore participants.1
3.1 Densitometry
No significant differences in mean DXA parameters were found among the dietary groups. Twenty-six percent of vegans were diagnosed with osteopenia following the lumbar spine investigation. The same condition was detected in 19% of vegans, when examining the femoral neck. In 43% of lacto-ovo-vegetarians, the level of mineralization at the lumbar spine corresponded to osteopenia. The left femoral neck DXA showed a similar result in 20% of lacto-ovo-vegetarians. Among omnivores, osteopenia was found in 32% at the lumbar spine and in 13% at the left femur. Also, only among lacto-ovo-vegetarians, it was observed 4% of subjects with osteoporosis at the femoral neck. However, there were no statistically significant differences between the groups (Table 2).
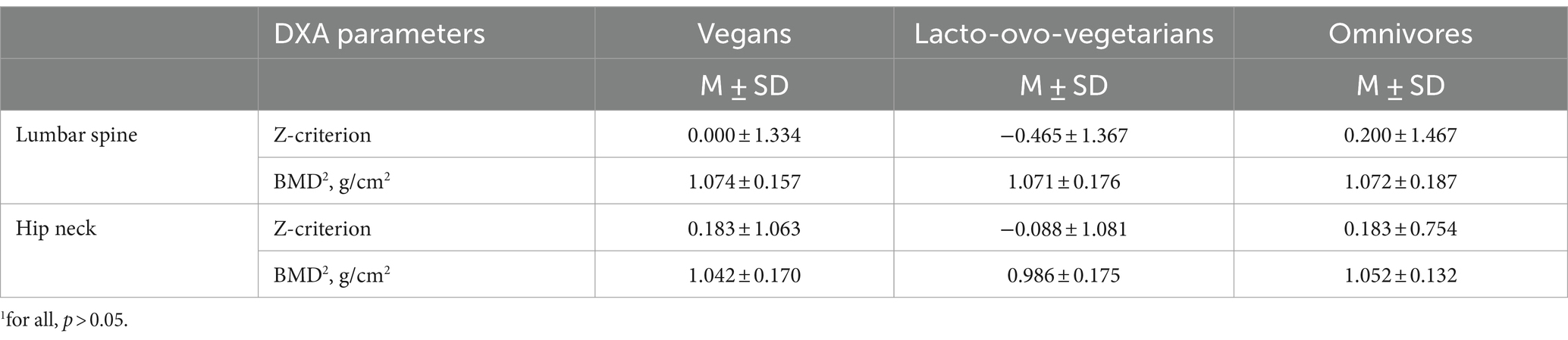
Table 2. DXA parameters among vegan, lacto-ovo-vegetarian, and omnivore groups measured at the left femoral neck and at the lumbar spine.1
3.2 PTH
The PTH levels in vegans were significantly higher than in omnivores (p < 0.001), although the mean (median) values fell within the normal range. No other significant differences between the groups were observed. The prevalence of hyperparathyroidism also did not differ (Table 3).
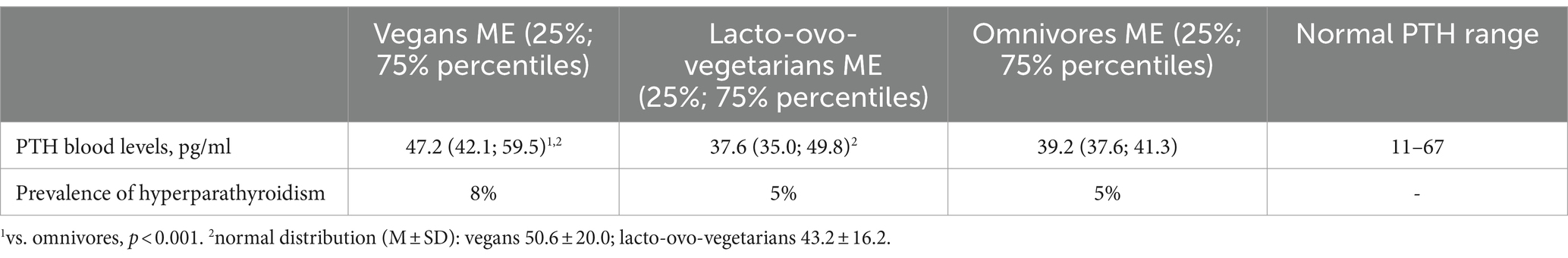
Table 3. PTH blood levels and prevalence of hyperparathyroidism in vegans, lacto-ovo-vegetarians, and omnivores.
3.3 Nutritional intake
Omnivores consumed significantly more protein: a mean of 70 g/day, compared to 54 g/day among lacto-ovo-vegetarians and 58 g/day among vegans. While calcium intake did not differ significantly between the groups (Table 4), the percentage of people intaking calcium less than the recommended 1,000 mg per day was higher among omnivores than among lacto-ovo-vegetarians (Table 5).
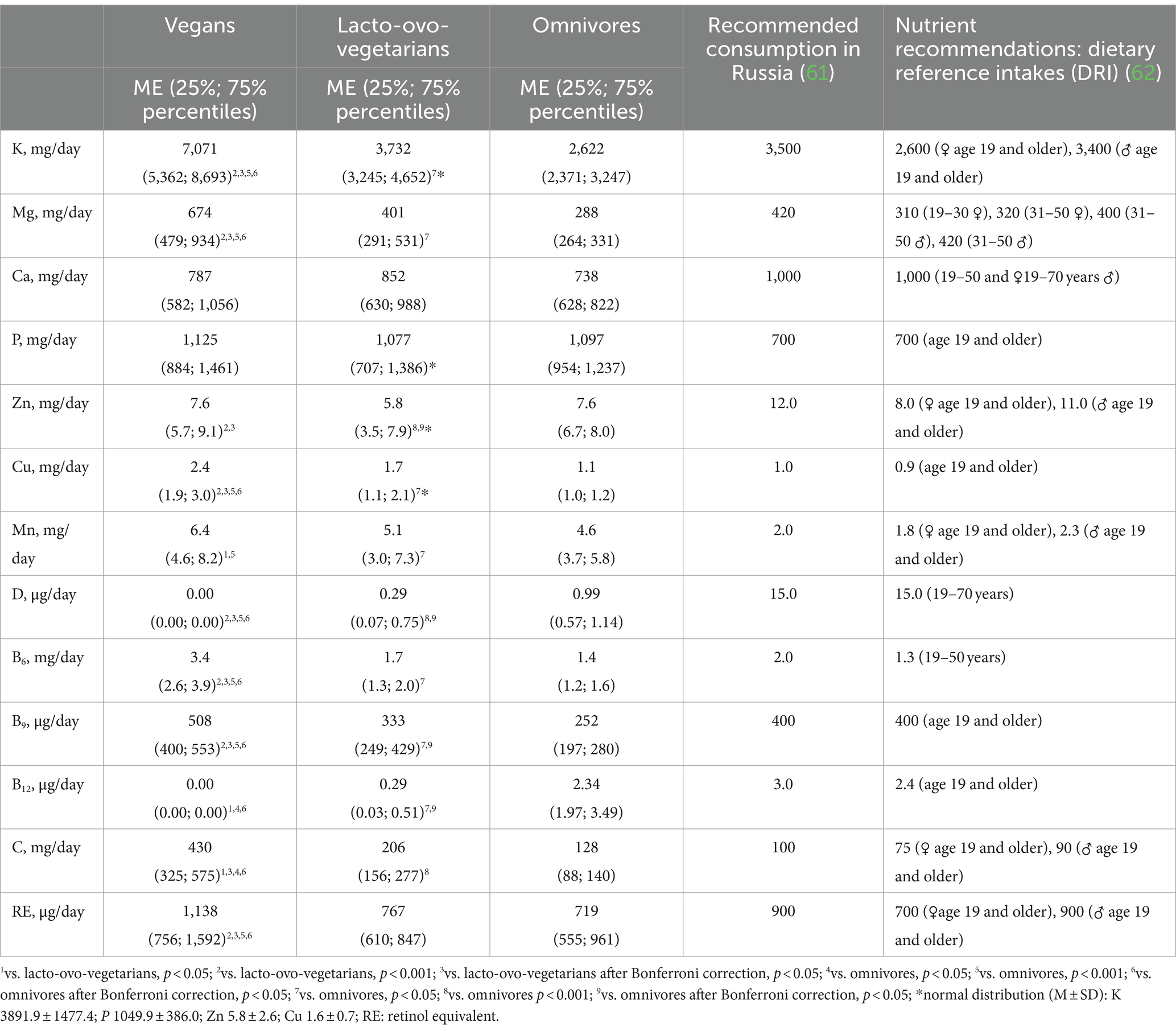
Table 4. Intake of chemical elements and vitamins among vegans, lacto-ovo-vegetarians, and omnivores.
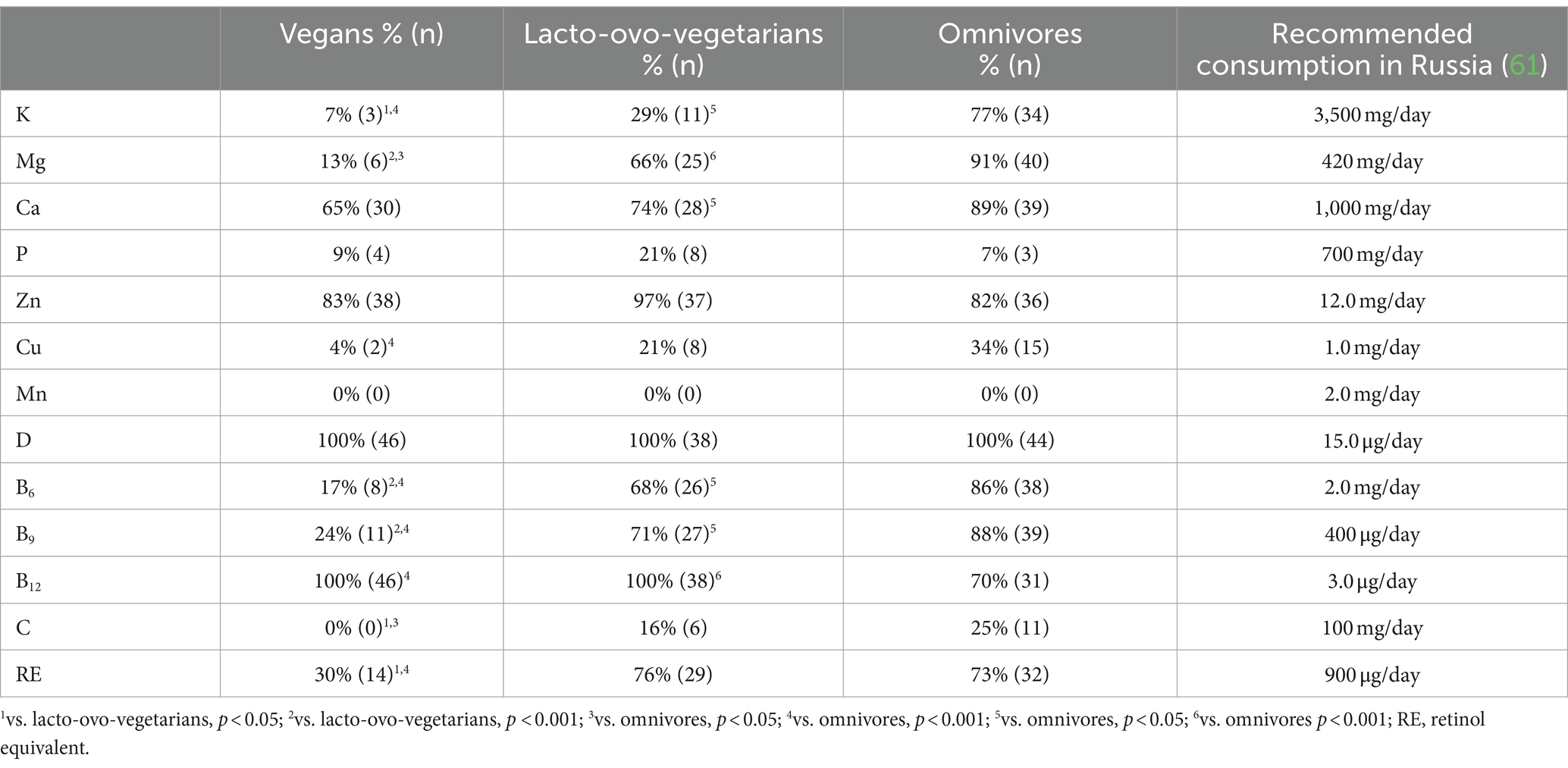
Table 5. Prevalence of chemical elements and vitamins intake insufficiency among vegans, lacto-ovo-vegetarians, and omnivores.
Vegans consumed significantly more potassium, magnesium, and copper than lacto-ovo-vegetarians and omnivores and had a lower risk of deficiency of these elements in their diet. Lacto-ovo-vegetarians occupied an intermediate position (whether any significant differences were observed) in terms of intake of all chemical elements except zinc: they consumed it less than other groups, but there were no significant differences among the groups in the occurrence of zinc intake below the recommended level (Tables 4, 5).
The consumption of almost all vitamins, except D and B12, increased with the decrease in the proportion of animal-derived food in the diet. Vitamins D and B12 were maximally represented in the diet of omnivores (Table 4). However, the risk of insufficient intake of these vitamins was present in the overwhelming number of the subjects from all groups (Table 5).
3.4 Serum nutrient levels
Despite the higher amount of copper in the diet of vegans, its concentrations in the blood were, on the contrary, lower than in omnivores, although after using Bonferroni correction, the differences were not supported (Table 6).
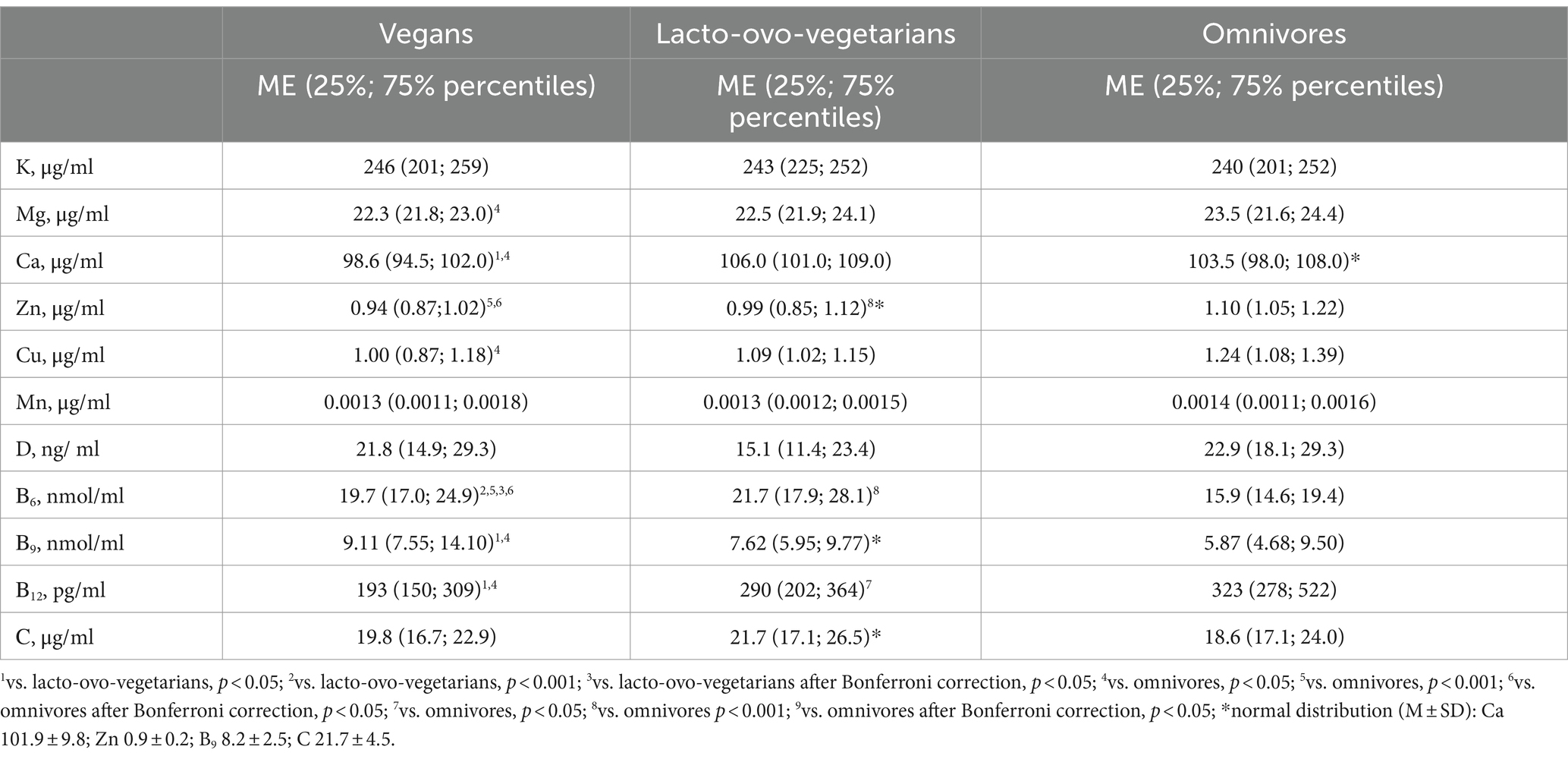
Table 6. Serum levels of chemical elements and vitamins among vegans, lacto-ovo-vegetarians, and omnivores.
In general, after correction, only zinc levels in the blood of omnivores remained significantly higher compared to vegans (Table 6). The risk of deficiency of major and trace elements in the serum did not differ between the dietary groups (Table 7). Deficiency of any element in the blood, except manganese, was observed in no more than 11% of vegans, 5% of lacto-ovo-vegetarians, and 5% of omnivores. Manganese deficiency was extremely widespread. It was found in more than half of subjects from all groups.
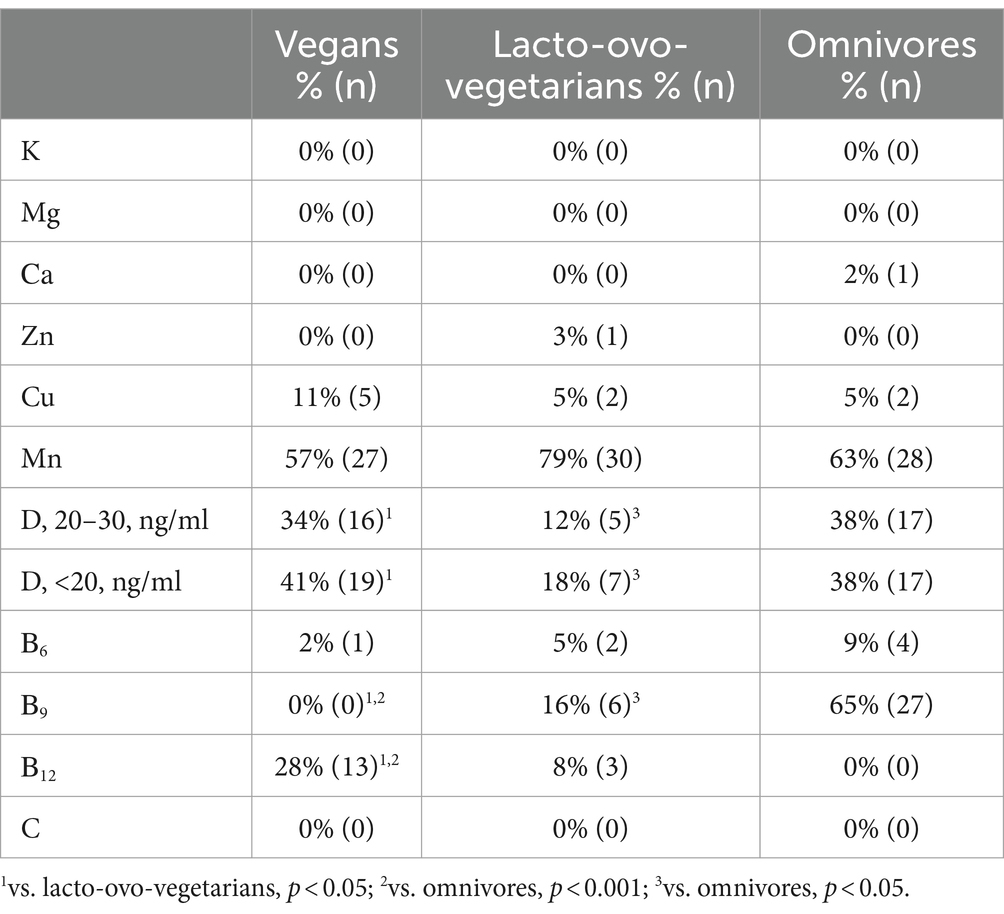
Table 7. Prevalence of chemical elements and vitamins deficiency in the blood of vegans, lacto-ovo-vegetarians and omnivores.
Serum vitamin D concentrations did not differ among the subjects (Table 6), and most were below the recommended level. According to the recommendations of the Union of Pediatricians of Russia (63), insufficient vitamin D level is defined with a calcidiol level from 20 to 30 ng/mL. Vitamin D deficiency is defined if 25(ОН)D concentration falls below 20 ng/mL. Interestingly, despite the absence of significant differences in serum concentrations, lacto-ovo-vegetarians had the lowest prevalence of both vitamin D insufficiency and deficiency in the blood (Table 7). The latter could be due to their consumption of milk fortified with vitamin D, although, as already mentioned, such products are not widely spread in the region of study.
The level of vitamin B9 in the serum increased with an increase in the proportion of plant foods in the diet. The reverse trend was observed for vitamin B12. Vitamin B6 concentrations were highest in lacto-ovo-vegetarians, but the frequency of deficiencies remained similar to the vitamin B9 trend (Tables 6, 7).
3.5 Urinalysis
The urine pH levels were significantly lower in omnivores than those in vegans and lacto-ovo-vegetarians were (p = 0.000 and p = 0.02, respectively) and amounted to 5.6 (Me; 4.8 (25th percentile); 6.2 (75th percentile)). pH values among vegans and lacto-ovo-vegetarians were 6.4 (6.0, 7.4) and 6.3 (5.8, 6.9), respectively. There were no significant differences in the results of the analysis between vegans and lacto-ovo-vegetarians.
3.6 Correlation analysis
Neither consumption nor serum levels of the assessed nutrients were associated with the BMD or PTH levels. It might be explained by the complexity of the multifactorial influence of nutrition on BMD: a good supply with some important for bone health nutrients was accompanied by a poor supply with the other ones.
4 Discussion
4.1 BMD
Although no significant differences in DXA indicators were found among our dietary groups, it is worth noting that Z-criteria in lacto-ovo-vegetarians were slightly lower than in the other groups, and the percentage of people with osteopenia was higher. Lacto-ovo-vegetarians were also the only group where osteoporosis was found. However, lacto-ovo-vegetarians, although not statistically significant, were still somewhat older than their counterparts.
Research on bone health in vegetarian and plant-based diets is overall not consistent. In a 2009 meta-analysis, the BMD of vegetarians (lacto-ovo-vegetarians and vegans) was lower than that of omnivores (64), which was further found in 2018 meta-analysis (65). Movassagh et al. even showed with DXA that the BMD of lacto-ovo-vegetarians may be higher than that of omnivores (66) and, moreover, that lacto-ovo-vegetarian diet has long-term positive impact on BMD. The opposite view was presented in a 2022 study by Chuang et al. (67), in which they described larger reduction in lumbar spine BMD for 3 years in vegetarian (lacto-ovo and vegan) woman, compared with non-vegetarians, aged 40–55.
In a 2019 study by Xie et al., BMD of lacto-ovo-vegetarians and omnivores, measured via ultrasound densitometer, did not differ (68), yet the BMD of vegans was still significantly lower. In a 2021 cross-sectional study, vegans also had lower ultrasound BMD parameters than omnivores (69). It seems therefore, that some components of animal origin, which are characteristic of lacto-ovo-vegetarian diet, may play a special role in BMD formation. Thus, positive association was observed between whole-body T-score and egg consumption (70). A 2021 meta-analysis (71) provided that dairy products also can increase BMD, however, in the latest study (72) dairy food intake was not associated with bone texture.
In our study, the BMD of lacto-ovo-vegetarians did not significantly differ from that of omnivores, which is consistent with results of some recent studies. The BMD of vegans also was not significantly lower, in contrast to most other results. In any case, great assortment of participants’ ages in every single study makes it difficult to compare the outcomes. They are difficult to compare not only because participants of different ages have, as expected, different BMDs. It is known that bone mass gain occurs until the age of 30 years (predicted medians are 22.31 (21.95; 22.59) for females and 26.86 (25.14; 27.98) for males) (73), and after this age, its decrease begins. Accordingly, studies involving participants under 20 years of age evaluate the effect of diet or lifestyle primarily on the processes of osteosynthesis, while studies with an age group of 30 years and older assess the effect of vegetarianism (lacto-ovo and/or veganism) primarily on the processes of bone resorption. Moreover, the improvement in the quality of vegetarian diets, due to an increase of the knowledge in recent times of the correct planning of such diets and the role of nutrients in human health (8) may be responsible of better outcomes on previous pitfalls.
4.2 PTH
In most studies, PTH levels among lacto-ovo-vegetarians and vegans were significantly higher than those of omnivores (74–76). We observed significantly higher concentrations of PTH in vegans in contrast to omnivores, though prevalence of hyperparathyroidism did not differ between the groups. This is most likely due to the fact that they consume significantly less vitamin D and have a higher risk of its deficiency (77). However, some authors found the opposite picture. In their studies, PTH blood concentrations were lower in the people with plant-based diets (78, 79).
4.3 Nutritional status
Low protein intake usually increases the risk of fractures (80–82). On the other hand, the Nurse’s Health Study found no association between protein consumption and fracture risk (83). Moreover, some studies have revealed that people with a high proportion of protein in their diet had a higher rate of bone resorption (84). Lacto-ovo-vegetarian and vegan diets usually satisfy the body’s need for protein and all 20 amino acids (85–87). At the same time, vegans still need to pay attention to protein and, especially, sulfur-containing amino acids consumption, since even with an adequate amount of total protein intake, they may sometimes be deficient in methionine and cysteine due to their lower content in some staple protein-rich plant foods (88).
In our study, the amounts of protein in the diet of lacto-ovo-vegetarians and vegans were at the same level, lower than those of omnivores. The same has been found in other studies (89–93). The recommended daily protein intake according to the Russian prescriptions equals to 75–114 g/day for men and 60–90 g/day for women (according to the US RDA, 0.8 g/kg of body weight per day for both sexes). However, individual protein requirements are highly variable and depend on plenty of factors that were not estimated in this study (94), Thus, we decided not to use these reference values to compare inadequate protein intake between the groups. We should also note, that, naturally, the recommended intake levels usually mean the amount of protein, as well as any other nutrient, constant daily consumption of which with food suffices to prevent the development of its deficit in 97.5% of the almost healthy population, given its bioavailability (95). Accordingly, average nutrient requirements are significantly lower than the recommended daily intakes.
Insufficient calcium intake can lead to bone demineralization and a decreased BMD (8, 96, 97). As a recent meta-analysis (98) demonstrates, vegan diets usually contain less calcium, which may increase the risk of osteoporosis (99), although in 2021 the study by Jakše et al. showed the reverse picture (100). Additionally, 2020 review concluded that plant-based diets with adequate calcium and vitamin D levels do not have any detrimental effects on bone health (101). In principle, lower-than-optimal calcium intake is common in most diets. In our study, there were no significant differences in calcium intake among dietary groups, although its levels in the blood were significantly lower in vegans. Similar serum test results were found in other studies (69, 76, 91, 102, 103). However, circulating calcium concentration cannot be considered as the best indicator of calcium status in the body, since its blood levels, as other electrolytes’ (including potassium and magnesium), vary not much because of strict homeostatic control (104). The bioavailability of calcium is reduced in many products due to the presence of phytic and oxalic acids that inhibit its absorption (14). Low content of these acids in some green leafy vegetables that contain low oxalate (such as kale, Brussel sprouts, broccoli) and tofu makes them a good source of calcium for vegans, while for lacto-ovo-vegetarians it is also dairy products (1, 105–110). Mineral waters may be considered as another good source of calcium for any dietary group (111).
Phosphorus in food products is distributed very widely and evenly (105), therefore, its deficiency is rare among all dietary groups (90, 91, 103, 112–115). In addition, phosphorus intake is quite difficult to estimate today, since phosphate supplements are widespread (44).
Potassium and magnesium are usually found in adequate quantities in plant-based diets. Often, a decrease in animal-derived food consumption rises potassium and magnesium intake (114, 116–119). Among omnivores, magnesium deficiency in Western societies is common (8, 120–122).
A number of studies support the higher intake of copper by lacto-ovo-vegetarians and vegans (91, 123). It is important to note that copper absorption occurs mainly in the stomach, and dietary phytates do not interfere with this process (124). However, the bioavailability of copper in plant-based diets is still somewhat lower than in omnivorous diets due to the reduction potential of larger quantities of vitamin C (125). This explains the lower concentration of copper in the blood of vegans compared to omnivores.
In a plant-based diet, manganese deficiency is unusual (124, 126, 127), and in our study high enough intake was observed. Surprisingly, at the same time, its deficiency was widely distributed. Since manganese is an essential trace element, its status in different populations deserves further studies.
Low intake of zinc is widespread. In our study, zinc consumption was the lowest among lacto-ovo-vegetarians, as well as its serum levels among subjects who followed plant-based diets were, because the absorption of this element is inhibited by oxalic and phytic acids (1, 115, 128, 129). Despite the fact that activity of phytates decreases during cooking (130, 131), recommended values of zinc intake by vegetarians (lacto-ovo and vegans) are about 1.5 times higher (14, 132).
30% of all the subjects in our study had vitamin D insufficiency (20–30 ng/mL) and 34% had deficiency (<20 ng/mL) of this nutrient. 100% of the participants consumed insufficient quantities of Vitamin D. Many studies support that with an increase in the proportion of products of animal origin, the intake of this vitamin increases (91, 103, 114, 116, 119, 123). The level of vitamin D in the serum of omnivores has been reported higher compared to vegans (53, 76, 133). However, we observed almost identical calcidiol concentrations in vegans and omnivores. Similar results were obtained in some other studies (80, 134). Most of the subjects in all studies had vitamin D deficiency, which indicates the mass nature of this problem, regardless of the type of diet. Moreover, the prevalence of vitamin D deficiency increases among countries without mandatory fortification, which is one of the main determinants of the vitamin status (135) and with increasing latitude, as the cutaneal synthesis of calciferol becomes impossible in areas with low insolation (136). It seems therefore that vitamin D status relies predominantly on the lifestyle and insolation regime, but not on the type of diet (77). Thus, in the Vietnamese study (53), vitamin D levels among all subjects were slightly higher than in other investigations, while as our recent study shows, even mild supplementation is insufficient for vegans in Russia in most cases (137).
Lacto-ovo-vegetarians and vegans usually consume adequate (113, 123) and often higher (138) amounts of vitamin В6 than omnivores do. Serum concentrations of this vitamin are often similar in people with different eating behaviors (103, 134). Vegans had the highest intake of vitamin B6 in our study. Folate intake in plant-based diets (89–91, 103, 112, 119, 123, 139) and the blood levels of this vitamin in vegetarians (lacto-ovo and vegans) (103, 126, 140) are usually higher than in omnivorous ones, which corresponds to our results. In many studies, the intake of vitamin B12 in all dietary groups was noticeably higher than it was in our study (91, 103, 116, 123). Cobalamin consumption is limited in a diet including few animal-derived products or excluding them, since plants do not accumulate (or synthesize) it. As a result, vegetarians (lacto-ovo and, especially, vegans) are more predisposed to lower concentrations of vitamin B12 in the serum if not supplement it (103).
Vitamin C intake in all groups in our study often exceeded other results (103, 114, 116, 117, 133). In general, among lacto-ovo-vegetarians and vegans, vitamin C consumption is higher than among omnivores. This also reflects the level of this vitamin in the blood (103, 134).
Vegetarians (lacto-ovo and vegans) consume a bit of retinol (118, 123, 140), unlike omnivores do (141), but the large amount of carotenoids ingested by them provides high total values of retinol equivalent (RE) (118, 140). As for retinol concentrations, there are usually no significant differences between the groups (103, 134, 142).
4.4 Acid load
Barzel et al. believe that animal protein significantly enlarges acid load, which increases bone resorption (143). The negative effect of acidity on bone tissue is explained by the fact that extracellular H+ stimulate activity of osteoclasts (144), which in turn produce hydrogen ions for bone resorption (145). The pH of the urine that we measured might have reflected the pH of the blood, since when the acidity of the serum increases, excess protons are excreted by the kidneys (146). Thus, the higher acidity of urine we found in omnivores may have indicated the higher acid load of serum in their diet.
In Western countries, typical omnivorous diets mainly reduce blood pH by about 50–70 mEq/day (53). Plant-based diets, on the contrary, have an alkalizing effect (117, 147–149). The effect of different diets on serum pH was compared by Knurick et al.: +19.6 mEq/day for omnivores, −1.5 mEq/day for lacto-ovo-vegetarians, and − 15.2 mEq/day for vegans (89). A significant alkalizing effect of food was also found in vegans in Germany (−39 mEq/day) (117). The vegan diet, which has the most pronounced alkalizing effect, could slow down the resorption of bone tissue and promote its synthesis. In general, sufficient consumption of vegetables has a beneficial effect on bone tissue (147).
Nevertheless, a meta-analysis by Fenton et al. found no evidence that increasing the diet acid load promotes skeletal bone mineral loss or osteoporosis (29).
4.5 Strengths and limitations
Our study has several strengths: the first one relies on having separately examined vegetarians as lacto-ovo-vegetarians and vegans, since we could dispose of dietary samples of comparable size. The second strength is that a large array of the most important parameters linked to bone metabolism has been evaluated. Another strength is the geography of the study. All the previous studies on this topic have been conducted in Western Europe, North America or South-East Asia. Thus, we spread the study geography quite far from those 3 parts of world.
Some limitations are also present. No statistically significant difference in BMD was detected in the different dietary groups, but the results have not been adjusted for other confounding variables, which were not evaluated, such as lifestyle habits, physical activity, use of supplements, state of microbiota (12). These factors can vary between groups and elicit a significant impact on the research outcomes, as was mentioned earlier (150). The cross-sectional design of the study could not provide a causal effect of diet on BMD and osteoporosis. To have any chance to speculate about the cause-effect relationships, a longitudinal study is required, and its duration probably should last for decades. As BMD changes gradually over the course of life, another crucial aspect for dietary effects assessment is the study design that contemplates inclusion of participants who have been following their diets for many years. In our study the minimum acceptable period of following the diet was quite short (1 year). The effectiveness of statistical data processing decreased as a result of the fact that the groups were rather small and were not divided by sex or age. Besides, although there were no differences in the age composition between the groups, the age variation was rather wide.
Another important limitation is the method we used to estimate BMD. The most accurate method for osteoporosis diagnosis is quantitative computed tomography (QCT), as it evaluates the true BMD by showing the three-dimensional genuine BMD measured in g/cm3 and allows to assess the spongy bone condition, with changes in which osteoporosis begins (151). However, QCT could not be used in our study because it is associated with a high radiation exposure. Thus, DXA was used as the gold standard for primary diagnosis of osteoporosis and particularly because of its low cost. In this regard, it is important to mention that DXA can give false-positive results since it is sometimes impossible to distinguish between a progressive pathological process and congenital features of bone tissue development. For instance, the cortical bone (which DXA assesses) is often rather thin in asthenic people.
Furthermore, single bone scanning is not an indicator of progressive destructive process. The osteoporosis diagnosed in our study is nothing more than the correspondence of the measured BMD value to the accepted indicator, while osteoporosis is a pathological process accompanied by a gradual decrease in the bone’s mineral composition. The disease may be present in a person with BMD within the normal range, if the process has not yet affected the cortical bone but originated in the spongy one. The exact diagnosis can be made only by the results of several studies showing whether the BMD is decreasing at the expected rate. Nevertheless, the quantification of BMD by DXA is mainly used in research studies.
Our purpose was to evaluate nutrition of the subjects throughout the year. Since we had limited resources, our choice fell on the food-frequency method, which, undoubtedly, has a big margin of error, especially, when considering a one-year period of data collection. However, supervision of the specialist during the interview significantly increased the accuracy of the study. Although 24-h dietary recall or, moreover, prospective methods are much more accurate, their using for such a long period requires considerable resources and very high participants’ motivation. At the same time, validity of the food-frequency method was shown in a number of studies (152–154). Used by us food-frequency method is not very exact, but its bias affects all the groups in the same way, so presented differences may be considered as informative enough.
5 Conclusion
In our study, we found that vegetarians (lacto-ovo and vegans) did not have lower BMD than omnivores. Meanwhile, higher PTH levels were observed in vegans compared to omnivores, though prevalence of hyperparathyroidism was the same in all groups. Despite vegans are reported at an increased risk of bone loss, our study did not show it.
Omnivores were better provided with vitamins D and B12, vegans – with all other assessed vitamins. The RE consumption values in the vegan group were significantly higher than in others. Vegans` RE intake is predominantly made up of carotenoids consumption, which have a protective effect on bones. Instead, in omnivores retinol, able to intensify bone resorption, is the main contributor to the RE intake. Vegans and lacto-ovo-vegetarians consumed more potassium, magnesium, and copper and had a slightly higher urine pH, which, in general, prevented bone resorption. The risk of deficiency of major and trace elements in the serum did not differ between the dietary groups. Conversely, omnivores had more protein in their diets compared to plant-based diets. Lacto-ovo-vegetarians occupied an intermediate position in almost all cases, where significant differences were found. Results of the present study are in line with the most recent studies on this topic (8).
Bone metabolism is affected by many nutrients, and each dietary group could have its own strengths and weaknesses in this regard. Any dietary group of temperate climates is at extremely high risk for vitamin D deficiency, and latitude and supplementation have a much greater effect on vitamin D status than dietary type. Supplementing vitamin D is necessary for every people living in an extratropical region. All types of vegetarians ought to supplement vitamin B12, as well [as suggested in the VegPlate (155)]. Very poor calcium intake in all dietary groups directs our attention to products with high calcium and low oxalate content (Brassica vegetables, tofu, mineral waters) in the role of primary calcium sources, as well as raises a question of calcium mandatory supplementation and spread of fortification of products with it. Moreover, omnivores need to pay an extra attention to other micronutrients: insufficient intake of potassium, magnesium, and folate is rampant in developed countries. Zinc levels are recommended to be monitored by all groups, but especially by vegans and lacto-ovo-vegetarians. Manganese intake was high in all groups, although its deficiency was frequently observed. Thus, its status requires special study.
In light of the above, it makes sense for people from the high-risk groups (for instance, people over 50 years old, especially women) to conduct DXA with a certain frequency, depending on the results of the study. In a case of low DXA indicators, it is recommended to undergo QCT scanning (151). After osteoporosis has been diagnosed, it is also desirable to examine the peripheral bones, primarily the rays, since they are at high risk of low-traumatic fractures, as well. The assessment can be done with peripheral QCT, which irradiates a relatively small volume of tissues. Screening of peripheral bones condition may also include quantitative ultrasonography. This method is only applicable for evaluating of superficially located bones and does not have a high diagnostic value. However, quantitative ultrasonography uses non-ionizing radiation, it is quite cheap and often transportable, which makes it convenient for periodic assessment of the bones condition. Besides, bone metabolism can be indirectly tracked by studying the concentrations of bone tissue metabolites: ostase, osteocalcin, procollagen type 1 N-terminal propeptide, or C- and N-terminal telopeptides of type 1 collagen in serum, as well as pyridinoline and deoxypyridinoline in urine (156).
A regular physical activity is the basis of recommendations for the prevention of BMD reduction (12, 157). R. Wakolbinger-Habel et al. in their study found that BMD in non-exercising vegans was lower when compared with a similar group of people with an omnivore diet, although there were no differences in the microarchitecture of bone tissue between vegans and omnivores who had regular strength training (158).
Future studies should contain a comprehensive assessment of nutritional status with a larger number of subjects, divided into dietary, sex and age groups. In addition, further expansion of the geography of such studies is needed due to significant regional features of the chemical composition of soils, religious, cultural, culinary traditions, and economic status.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Federal Research Center for Nutrition, Biotechnology and Food Safety. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft. GR: Writing – review & editing. ESi: Data curation, Formal analysis, Validation, Writing – original draft. ESk: Data curation, Validation, Writing – original draft. LB: Writing – review & editing. PV: Supervision, Writing – review & editing. GG: Writing – review & editing. ND: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research was subsidized and conducted within the framework of the State assignment under the Program of fundamental scientific research of the Russian State Academies of Sciences for 2022–2024 (theme № FGMF-2022-0005).
Acknowledgments
The authors are grateful to Chiara Bonetto for her critical comments and recommendations which helped in improving the paper’s quality. The authors also would like to express their gratitude to the reviewers for their valuable time and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Agnoli, C, Baroni, L, Bertini, I, Ciappellano, S, Fabbri, A, Papa, M, et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr Metab Cardiovasc Dis. (2017) 27:1037–52. doi: 10.1016/j.numecd.2017.10.020
2. Melina, V, Craig, W, and Levin, S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. (2016) 116:1970–80. doi: 10.1016/j.jand.2016.09.025
3. Rizzo, G, and Baroni, L. Health and ecological implications of fish consumption: a deeper insight. Mediterr J Nutr Metab. (2016) 9:7–22. doi: 10.3233/MNM-160054
4. Baroni, L, Berati, M, Candilera, M, and Tettamanti, M. Total environmental impact of three Main dietary patterns in relation to the content of animal and plant food. Food Secur. (2014) 3:443–60. doi: 10.3390/foods3030443
5. SuperJob Research Center. Every second Russian evaluates vegetarianism positively, but eats meat. Superjobru (2023). Available at: https://www.superjob.ru/research/articles/113155/kazhdyj-vtoroj-rossiyanin-vegetarianstvo-ocenivaet-polozhitelno/ [Accessed February 9, 2024]
6. Kahleova, H, Levin, S, and Barnard, N. Cardio-metabolic benefits of plant-based diets. Nutrients. (2017) 9:848. doi: 10.3390/nu9080848
7. Zhubi-Bakija, F, Bajraktari, G, Bytyçi, I, Mikhailidis, DP, Henein, MY, Latkovskis, G, et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the international lipid expert panel (ILEP). Clin Nutr. (2021) 40:255–76. doi: 10.1016/j.clnu.2020.05.017
8. Galchenko, A, Gapparova, K, and Sidorova, E. The influence of vegetarian and vegan diets on the state of bone mineral density in humans. Crit Rev Food Sci Nutr. (2021) 63:845–61. doi: 10.1080/10408398.2021.1996330
9. Lv, C, Liu, S, Xia, J, Xu, L, Cheng, Y, Li, W, et al. The mechanism of dietary protein modulation of bone metabolism via alterations in members of the GH/IGF Axis. Curr Protein Pept Sci. (2019) 20:115–24. doi: 10.2174/1389203719666180514143828
10. Cosman, F, de Beur, SJ, LeBoff, MS, Lewiecki, EM, Tanner, B, Randall, S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. (2014) 25:2359–81. doi: 10.1007/s00198-014-2794-2
11. Schurch, M-A. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. (1998) 128:801–9. doi: 10.7326/0003-4819-128-10-199805150-00002
12. Rizzoli, R, Biver, E, and Brennan-Speranza, TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. (2021) 9:606–21. doi: 10.1016/S2213-8587(21)00119-4
13. Garach, A, García-Fontana, B, and Muñoz-Torres, M. Nutrients and dietary patterns related to osteoporosis. Nutrients. (2020) 12:1986. doi: 10.3390/nu12071986
14. Tucker, KL. Vegetarian diets and bone status. Am J Clin Nutr. (2014) 100:329S–35S. doi: 10.3945/ajcn.113.071621
15. Komoroski, M, Azad, N, and Camacho, P. Disorders of bone and bone mineral metabolism. Handb. Clin. Neurol. (2014). 120:865–87. doi: 10.1016/B978-0-7020-4087-0.00058-9
16. Li, M, Hasegawa, T, Masuki, H, Liu, Z, Guo, Y, Suzuki, R, et al. Ultrastructural assessment of mineral crystallization and collagen mineralization in bone. Journal of Oral Biosciences. (2010) 52:94–9. doi: 10.1016/S1349-0079(10)80037-4
17. Al Alawi, AM, Majoni, SW, and Falhammar, H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. (2018) 2018:1–17. doi: 10.1155/2018/9041694
18. Castiglioni, S, Cazzaniga, A, Albisetti, W, and Maier, J. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. (2013) 5:3022–33. doi: 10.3390/nu5083022
19. Kim, DE, Cho, SH, Park, HM, and Chang, YK. Relationship between bone mineral density and dietary intake of β-carotene, vitamin C, zinc and vegetables in postmenopausal Korean women: a cross-sectional study. J Int Med Res. (2016) 44:1103–14. doi: 10.1177/0300060516662402
20. Lowe, NM, Fraser, WD, and Jackson, MJ. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc Nutr Soc. (2002) 61:181–5. doi: 10.1079/PNS2002154
21. Palacios, C. The role of nutrients in bone health, from a to Z. Crit Rev Food Sci Nutr. (2006) 46:621–8. doi: 10.1080/10408390500466174
22. Molenda, M, and Kolmas, J. The role of zinc in bone tissue health and regeneration—a review. Biol Trace Elem Res. (2023) 201:5640–51. doi: 10.1007/s12011-023-03631-1
23. Qu, X, He, Z, Qiao, H, Zhai, Z, Mao, Z, Yu, Z, et al. Serum copper levels are associated with bone mineral density and total fracture. Journal of Orthopaedic Translation. (2018) 14:34–44. doi: 10.1016/j.jot.2018.05.001
24. Mahdavi-Roshan, M. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin. Cases Miner. Bone Metab. (2015) 12:18–21. doi: 10.11138/ccmbm/2015.12.1.018
25. Sobotka, L, Allison, S, and Stanga, Z. Basics in clinical nutrition: water and electrolytes in health and disease. e-SPEN, the European e-Journal of Clinical Nutrition and Metabolism. (2008) 3:e259–66. doi: 10.1016/j.eclnm.2008.06.004
26. Frassetto, L, Banerjee, T, Powe, N, and Sebastian, A. Acid balance, dietary acid load, and bone effects—a controversial subject. Nutrients. (2018) 10:517. doi: 10.3390/nu10040517
27. Arnett, TR. Acid–base regulation of bone metabolism. Int Congr Ser. (2007) 1297:255–67. doi: 10.1016/j.ics.2006.08.005
29. Fenton, TR, Lyon, AW, Eliasziw, M, Tough, SC, and Hanley, DA. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J Bone Miner Res. (2009) 24:1835–40. doi: 10.1359/jbmr.090515
30. Galchenko, AV, and Sherstneva, AA. Сonditionally essential ultra trace elements in nutrition of vegetarians and vegans. Nickel, lithium, vanadium, germanium. Trace Elements in Medicine (Moscow). (2021) 22:3–16. doi: 10.19112/2413-6174-2021-22-2-3-16
31. Eren, I, Yildiz, M, and İnanlı, İ. The effects of lithium treatment on bone mineral density in bipolar patients. Neurol Psychiatry Brain Res. (2006) 13:174–9.
32. Yang, Q, and He, G-W. Imbalance of homocysteine and H 2 S: significance, mechanisms, and therapeutic promise in vascular injury. Oxidative Med Cell Longev. (2019) 2019:1–11. doi: 10.1155/2019/7629673
33. Rizzo, G, and Laganà, AS. The link between homocysteine and Omega-3 polyunsaturated fatty acid: critical appraisal and future directions. Biomol Ther. (2020) 10:219. doi: 10.3390/biom10020219
34. Herrmann, M, Tami, A, Wildemann, B, Wolny, M, Wagner, A, Schorr, H, et al. Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone. (2009) 44:467–75. doi: 10.1016/j.bone.2008.10.051
35. Herrmann, M, Umanskaya, N, Wildemann, B, Colaianni, G, Widmann, T, Zallone, A, et al. Stimulation of osteoblast activity by homocysteine. J Cell Mol Med. (2008) 12:1205–10. doi: 10.1111/j.1582-4934.2008.00104.x
36. Tyagi, N, Kandel, M, Munjal, C, Vacek, JC, and Qipshidze, N. Homocysteine mediated decrease in bone blood flow and remodeling: role of folic acid. J Orthop Res. (2011) 29:1511–6. doi: 10.1002/jor.21415
37. Marini, JC, Cabral, WA, Barnes, AM, and Chang, W. Components of the collagen prolyl 3-hydroxylation complex are crucial for Normal bone development. Cell Cycle. (2007) 6:1675–81. doi: 10.4161/cc.6.14.4474
38. Sahni, S, Hannan, MT, Blumberg, J, Cupples, LA, Kiel, DP, and Tucker, KL. Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: the Framingham osteoporosis Study123. Am J Clin Nutr. (2009) 89:416–24. doi: 10.3945/ajcn.2008.26388
39. Regu, G, Kim, H, Kim, Y, Paek, J, Lee, G, Chang, N, et al. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30–75 years using data from the fourth and fifth Korean National Health and nutrition examination surveys (2008–2011). Nutrients. (2017) 9:1025. doi: 10.3390/nu9091025
40. Uchiyama, S, and Yamaguchi, M. Inhibitory effect of β-cryptoxanthin on osteoclast-like cell formation in mouse marrow cultures. Biochem Pharmacol. (2004) 67:1297–305. doi: 10.1016/j.bcp.2003.11.011
41. Marcucci, G, and Brandi, ML. Rare causes of osteoporosis. Clin Cases Miner Bone Metab. (2015) 12:151–6. doi: 10.11138/ccmbm/2015.12.2.151
42. Lind, T, Lind, PM, Jacobson, A, Hu, L, Sundqvist, A, Risteli, J, et al. High dietary intake of retinol leads to bone marrow hypoxia and diaphyseal endosteal mineralization in rats. Bone. (2011) 48:496–506. doi: 10.1016/j.bone.2010.10.169
43. Bergqvist, C, and Ezzedine, K. Vitamin D and the skin: what should a dermatologist know? G Ital Dermatol Venereol. (2019) 154:669–80. doi: 10.23736/S0392-0488.19.06433-2
44. Costanzo, PR, and Knoblovits, P. Vitamin D and male reproductive system. Horm Mol Biol Clin Invest. (2016) 28:151–9. doi: 10.1515/hmbci-2016-0049
45. Drissi, H, Pouliot, A, Koolloos, C, Stein, JL, Lian, JB, Stein, GS, et al. 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp Cell Res. (2002) 274:323–33. doi: 10.1006/excr.2002.5474
46. Rodan, GA, and Martin, TJ. Therapeutic approaches to bone diseases. Science. (2000) 289:1508–14. doi: 10.1126/science.289.5484.1508
47. Harada, S, and Rodan, G. Control of osteoblast function and regulation of bone mass. Nature. (2003) 423:349–55. doi: 10.1038/nature01660
48. Dobnig, H, and Turner, RT. The effects of programmed Administration of Human Parathyroid Hormone Fragment (1–34) on bone Histomorphometry and serum chemistry in rats. Endocrinology. (1997) 138:4607–12. doi: 10.1210/en.138.11.4607
49. Potts, JT. Parathyroid hormone: past and present. J Endocrinol. (2005) 187:311–25. doi: 10.1677/joe.1.06057
50. Oussalah, A, Levy, J, Berthezène, C, Alpers, DH, and Guéant, J-L. Health outcomes associated with vegetarian diets: an umbrella review of systematic reviews and meta-analyses. Clin Nutr. (2020) 39:3283–307. doi: 10.1016/j.clnu.2020.02.037
51. Tong, TYN, Appleby, PN, Armstrong, MEG, Fensom, GK, Knuppel, A, Papier, K, et al. Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study. BMC Med. (2020) 18:353. doi: 10.1186/s12916-020-01815-3
52. Faul, F, Erdfelder, E, Lang, A-G, and Buchner, A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
53. Ho-Pham, LT, Vu, BQ, Lai, TQ, Nguyen, ND, and Nguyen, TV. Vegetarianism, bone loss, fracture and vitamin D: a longitudinal study in Asian vegans and non-vegans. Eur J Clin Nutr. (2012) 66:75–82. doi: 10.1038/ejcn.2011.131
54. Belaya, ZE, Belova, KY, Biryukova, EV, Dedov, II, Dzeranova, LK, Drapkina, OM, et al. Federal clinical guidelines for diagnosis, treatment and prevention of osteoporosis. Osteopor Bone Dis. (2021) 24:4–47. doi: 10.14341/osteo12930
55. Oral, A, Esmaeilzadeh, S, Yalıman, A, Sindel, D, Kürsüz Köseoğlu, P, and Aydın, T. The ability of calcaneal and multisite quantitative ultrasound variables in the identification of osteoporosis in women and men. Turk J Phys Med Rehabil. (2019) 65:203–15. doi: 10.5606/tftrd.2019.1894
56. Karaguzel, G, and Holick, MF. Diagnosis and treatment of osteopenia. Rev Endocr Metab Disord. (2010) 11:237–51. doi: 10.1007/s11154-010-9154-0
57. LLC “Nutrilogic.” Nutrilogic. (2018). Available at: https://www.elibrary.ru/download/elibrary_39293991_57637765.PDF [Accessed May 7, 2024]
58. Dubcov, ГГ. Zaklyuchenie ob effektivnosti Nauchno-tekhnicheskoj razrabotki «Metod analiza haraktera pitaniya cheloveka v domashnih usloviyah i fakticheskogo himicheskogo sostava dietologicheskih racionov, generirovannyh s ispol’zovaniem servisa Nutrilogic» [Conclusion on the effectiveness of the scientific and technical development “A method for analyzing the nature of human nutrition at home and the actual chemical composition of food rations obtained using the Nutrilogic service”]. (2018). Available at: https://nutrilogic.ru/f/2020-02/zaklyuchenie.pdf?08f53d6231 [Accessed May 7, 2024]
59. Tutel’yan, VA. Himicheskij sostav i kalorijnost’ rossijskih produktov pitaniya: Spravochnik [chemical composition and calorie content of Russian food products. Handbook]. Moscow: DeLi Plyus (2012). 284 p.
60. Tutel’yan, VA, Baturni, AK, Vasil’ev, AV, Vrzhesinskaya, OA, Vysockij, VG, and Gapparov, MM. Rekomenduemye urovni potrebleniya pishchevyh i biologicheski aktivnyh veshchestv: Metodicheskie rekomendacii [recommended consumption levels of food and biologically active substances: Guidelines]. Moscow: Federal Service for the Oversight of Consumer Protection and Welfare of the Russian Federation. (2004).
61. Chief State Sanitary Doctor of the Russian Federation. Metodicheskie rekomendacii MP 2.3.1.0253–21 “Normy fiziologicheskih potrebnostej v energii i pishchevyh veshchestvah dlya razlichnyh grupp naseleniya Rossijskoj Federacii” [Guidelines MP 2.3.1.0253–21 “Norms of physiological requirements in energy and nutrients of various groups of the population of the Russian Federation”]. (2021). Available at: http://base.garant.ru/402816140/ [Accessed February 14, 2024]
62. Office of Dietary Supplements. Nutrient Recommendations and Databases. Available at: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx [Accessed January 29, 2024]
63. FGAU “NMIT of Children’s health” Ministry of Health of Russia, FSBEI FPE RMACPE Ministry of Health Russia, Union of Pediatricians of Russia, FRC of nutrition, biotechnology and food safety. Nedostatochnost’ vitamina D u detej i podrostkov Rossijskoj Federacii: sovremennye podhody k korrekcii In: Nacional’naya programma [vitamin D deficiency in children and adolescents of the Russian Federation: Modern approaches to correction. National program]. Moscow: Pediatr (2018). 96.
64. Ho-Pham, LT, Nguyen, ND, and Nguyen, TV. Effect of vegetarian diets on bone mineral density: a Bayesian meta-analysis. Am J Clin Nutr. (2009) 90:943–50. doi: 10.3945/ajcn.2009.27521
65. Iguacel, I, Miguel-Berges, ML, Gómez-Bruton, A, Moreno, LA, and Julián, C. Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev. (2019) 77:1–18. doi: 10.1093/nutrit/nuy045
66. Movassagh, EZ, Baxter-Jones, ADG, Kontulainen, S, Whiting, S, Szafron, M, and Vatanparast, H. Vegetarian-style dietary pattern during adolescence has long-term positive impact on bone from adolescence to young adulthood: a longitudinal study. Nutr J. (2018) 17:36. doi: 10.1186/s12937-018-0324-3
67. Chuang, T-L, Koo, M, Chuang, M-H, Lin, C-H, Huang, C-H, and Wang, Y-F. Changes in bone mineral density and trabecular bone score over time between vegetarian and non-vegetarian middle-aged and older women: a three-year retrospective medical record review. Int J Environ Res Public Health. (2022) 19:2445. doi: 10.3390/ijerph19042445
68. Xie, L, Wang, B, Cui, X, Tang, Q, Cai, W, and Shen, X. Young adult vegetarians in Shanghai have comparable bone health to omnivores despite lower serum 25(OH) vitamin D in vegans: A cross-sectional study. Asia Pac. J. Clin. Nutr. (2019). 28:383–8. doi: 10.6133/apjcn.201906_28(2).0021
69. Menzel, J, Abraham, K, Stangl, GI, Ueland, PM, Obeid, R, Schulze, MB, et al. Vegan diet and bone health—results from the cross-sectional RBVD study. Nutrients. (2021) 13:685. doi: 10.3390/nu13020685
70. Pujia, R, Ferro, Y, Maurotti, S, Mare, R, Arturi, F, Montalcini, T, et al. Relationship between osteoporosis, multiple fractures, and egg intake in healthy elderly. J Midlife Health. (2021) 12:287–93. doi: 10.4103/jmh.jmh_118_21
71. Shi, Y, Zhan, Y, Chen, Y, and Jiang, Y. Effects of dairy products on bone mineral density in healthy postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Arch Osteoporos. (2020) 15:48. doi: 10.1007/s11657-020-0694-y
72. Millar, CL, Kiel, DP, Hannan, MT, and Sahni, S. Dairy food intake is not associated with spinal trabecular bone score in men and women: the Framingham osteoporosis study. Nutr J. (2022) 21:26. doi: 10.1186/s12937-022-00781-1
73. Lu, J, Shin, Y, Yen, M-S, and Sun, SS. Peak bone mass and patterns of change in Total bone mineral density and bone mineral contents from childhood into young adulthood. J Clin Densitom. (2016) 19:180–91. doi: 10.1016/j.jocd.2014.08.001
74. Outila, TA, Kärkkäinen, MUM, Seppänen, RH, and Lamberg-Allardt, CJE. Dietary intake of vitamin D in premenopausal, healthy vegans was insufficient to maintain concentrations of serum 25-hydroxyvitamin D and intact parathyroid hormone within Normal ranges during the winter in Finland. J Am Diet Assoc. (2000) 100:434–41. doi: 10.1016/S0002-8223(00)00134-6
75. Baig, JA, Sheikh, SA, Islam, I, and Kumar, M. Vitamin D status among vegetarians and non- vegetarians. J Ayub Med Coll Abbottabad. (2013) 25:152–5. doi: 10.1080/00140130500101031
76. Hansen, T, Madsen, M, Jørgensen, N, Cohen, A, Hansen, T, Vestergaard, H, et al. Bone turnover, calcium homeostasis, and vitamin D status in Danish vegans. Eur J Clin Nutr. (2018) 72:1046–54. doi: 10.1038/s41430-017-0081-y
77. Galchenko, A, and Ranjit, R. Vitamin D and its status in vegetarians and vegans. Problems of Biological Medical and Pharmaceutical Chemistry. (2021) 24:20–27. doi: 10.29296/25877313-2021-11-04
78. Ciardella, F, Cupisti, A, Catapano, G, Guidi, A, Pasquinucci, A, Morelli, E, et al. Effects of a low-phosphorus, low-nitrogen diet supplemented with essential amino acids and Ketoanalogues on serum Beta-endorphin in chronic renal failure. Nephron. (1989) 53:129–32. doi: 10.1159/000185724
79. Moe, SM, Zidehsarai, MP, Chambers, MA, Jackman, LA, Radcliffe, JS, Trevino, LL, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. CJASN. (2011) 6:257–64. doi: 10.2215/CJN.05040610
80. Appleby, P, Roddam, A, Allen, N, and Key, T. Comparative fracture risk in vegetarians and nonvegetarians in EPIC-Oxford. Eur J Clin Nutr. (2007) 61:1400–6. doi: 10.1038/sj.ejcn.1602659
81. Misra, D, Berry, SD, Broe, KE, McLean, RR, Cupples, LA, Tucker, KL, et al. Does dietary protein reduce hip fracture risk in elders? The Framingham osteoporosis study. Osteoporos Int. (2011) 22:345–9. doi: 10.1007/s00198-010-1179-4
82. Hannan, MT, Tucker, KL, Dawson-Hughes, B, Cupples, LA, Felson, DT, and Kiel, DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham osteoporosis study. J Bone Miner Res. (2000) 15:2504–12. doi: 10.1359/jbmr.2000.15.12.2504
83. Feskanich, D, Willett, WC, Stampfer, MJ, and Colditz, GA. Protein consumption and bone fractures in women. Am J Epidemiol. (1996) 143:472–9. doi: 10.1093/oxfordjournals.aje.a008767
84. Sellmeyer, DE, Stone, KL, Sebastian, A, and Cummings, SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Am J Clin Nutr. (2001) 73:118–22. doi: 10.1093/ajcn/73.1.118
85. Mariotti, G. Dietary protein and amino acids in vegetarian diets—a review. Nutrients. (2019) 11:2661. doi: 10.3390/nu11112661
86. Clarys, P, Deliens, T, Huybrechts, I, Deriemaeker, P, Vanaelst, B, De Keyzer, W, et al. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, Pesco-vegetarian and omnivorous diet. Nutrients. (2014) 6:1318–32. doi: 10.3390/nu6031318
87. Gardner, CD, Hartle, JC, Garrett, RD, Offringa, LC, and Wasserman, AS. Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States. Nutr Rev. (2019) 77:197–215. doi: 10.1093/nutrit/nuy073
88. Galchenko, AV, Morozova, LD, and Zaletova, ТS. Evaluation of protein and amino acid requirements, based on biosynthetic needs and nitrogen balance parameters. Vopr dietol. (2017) 7:64–8. doi: 10.20953/2224-5448-2017-2-64-68
89. Knurick, J, Johnston, C, Wherry, S, and Aguayo, I. Comparison of correlates of bone mineral density in individuals adhering to lacto-Ovo, vegan, or omnivore diets: a cross-sectional investigation. Nutrients. (2015) 7:3416–26. doi: 10.3390/nu7053416
90. Kristensen, NB, Madsen, ML, Hansen, TH, Allin, KH, Hoppe, C, Fagt, S, et al. Intake of macro- and micronutrients in Danish vegans. Nutr J. (2015) 14:115. doi: 10.1186/s12937-015-0103-3
91. Allès, B, Baudry, J, Méjean, C, Touvier, M, Péneau, S, Hercberg, S, et al. Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the NutriNet-Santé study. Nutrients. (2017) 9:1023. doi: 10.3390/nu9091023
92. Fallon, N, and Dillon, SA. Low intakes of iodine and selenium amongst vegan and vegetarian women highlight a potential nutritional vulnerability. Front Nutr. (2020) 7:72. doi: 10.3389/fnut.2020.00072
93. Garcia-Maldonado, E, Zapatera, B, Alcorta, A, and Vaquero, M. Metabolic and nutritional biomarkers in adults consuming lacto-ovo vegetarian, vegan and omnivorous diets in Spain. A cross-sectional study. Food Funct. (2023) 14:1608–16. doi: 10.1039/D2FO03167A
94. Lonnie, M, Hooker, E, Brunstrom, JM, Corfe, BM, Green, MA, Watson, AW, et al. Protein for life: review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients. (2018) 10:360. doi: 10.3390/nu10030360
95. Galchenko, AV. Sulfur: metabolic role, physiological need, manifestations of deficiency. Trace Elements in Medicine. (2022) 23:14–7. doi: 10.19112/2413-6174-2022-23-4-14-17
96. Warensjo, E, Byberg, L, Melhus, H, Gedeborg, R, Mallmin, H, Wolk, A, et al. Dietary calcium intake and risk of fracture and osteoporosis: prospective longitudinal cohort study. BMJ. (2011) 342:d1473–3. doi: 10.1136/bmj.d1473
97. Burckhardt, P. The role of low acid load in vegetarian diet on bone health: a narrative review. Swiss Med Wkly. (2016) 146:w14277. doi: 10.4414/smw.2016.14277
98. Bickelmann, F, Leitzmann, M, Keller, M, Baurecht, H, and Jochem, C. Calcium intake in vegan and vegetarian diets: a systematic review and Meta-analysis. Crit Rev Food Sci Nutr. (2022) 63:10659–77. doi: 10.1080/10408398.2022.2084027
99. New, S. Do vegetarians have a normal bone mass? Osteoporos Int. (2004) 15:679–88. doi: 10.1007/s00198-004-1647-9
100. Jakše, B, Jakše, B, Godnov, U, and Pinter, S. Nutritional, cardiovascular health and lifestyle status of ‘health conscious’ adult vegans and non-vegans from Slovenia: a cross-sectional self-reported survey. IJERPH. (2021) 18:5968. doi: 10.3390/ijerph18115968
101. Hsu, E. Plant-based diets and bone health: sorting through the evidence. Curr Opin Endocrinol Diabetes Obes. (2020) 27:248–52. doi: 10.1097/MED.0000000000000552
102. Kohlenberg-Mueller, K, and Raschka, L. Calcium balance in young adults on a vegan and lactovegetarian diet. J Bone Miner Metab. (2003) 21:28–33. doi: 10.1007/s007740300005
103. Schüpbach, R, Wegmüller, R, Berguerand, C, Bui, M, and Herter-Aeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur J Nutr. (2017) 56:283–93. doi: 10.1007/s00394-015-1079-7
104. Jeon, US. Kidney and calcium homeostasis. Electrolyte Blood Press. (2008) 6:68–76. doi: 10.5049/EBP.2008.6.2.68
105. Galchenko, AV, and Ranjit, R. Calcium status among vegetarians and vegans. In: Fundamentals of technological development of agriculture, 24–25 October 2019. Orenburg, Russia, Russia: Federal State Budgetary Scientific Institution «Federal Scientific Center of Biological Systems and Agrotechnologies of the Russian Academy of Sciences» (2019). p. 209–212.
106. Heaney, RP, and Weaver, CM. Calcium absorption from kale. Am J Clin Nutr. (1990) 51:656–7. doi: 10.1093/ajcn/51.4.656
107. Heaney, RP. Protein intake and bone health: the influence of belief systems on the conduct of nutritional science. Am J Clin Nutr. (2001) 73:5–6. doi: 10.1093/ajcn/73.1.5
108. Heaney, RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr. (2000) 19:83S–99S. doi: 10.1080/07315724.2000.10718088
109. Heaney, RP, Recker, RR, and Weaver, CM. Absorbability of calcium sources: the limited role of solubility. Calcif Tissue Int. (1990) 46:300–4. doi: 10.1007/BF02563819
110. Heaney, RP, Dowell, MS, Rafferty, K, and Bierman, J. Bioavailability of the calcium in fortified soy imitation milk, with some observations on method. Am J Clin Nutr. (2000) 71:1166–9. doi: 10.1093/ajcn/71.5.1166
111. Heaney, RP. Absorbability and utility of calcium in mineral waters. Am J Clin Nutr. (2006) 84:371–4. doi: 10.1093/ajcn/84.2.371
112. Papadaki, A, Vardavas, C, Hatzis, C, and Kafatos, A. Calcium, nutrient and food intake of Greek orthodox Christian monks during a fasting and non-fasting week. Public Health Nutr. (2008) 11:1022–9. doi: 10.1017/S1368980007001498
113. Rizzo, NS, Jaceldo-Siegl, K, Sabate, J, and Fraser, GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. (2013) 113:1610–9. doi: 10.1016/j.jand.2013.06.349
114. García-Morant, A, Cortés-Castell, E, Palazón-Bru, A, Martínez-Amorós, N, Gil-Guillén, VF, and Rizo-Baeza, M. Macronutrients and micronutrients in Spanish adult vegans (Mediterranean population). Nutr Hosp. (2020) 34:549–58. doi: 10.20960/nh.02939
115. Deriemaeker, P, Alewaeters, K, Hebbelinck, M, Lefevre, J, Philippaerts, R, and Clarys, P. Nutritional status of Flemish vegetarians compared with non-vegetarians: a matched samples study. Nutrients. (2010) 2:770–80. doi: 10.3390/nu2070770
116. Lindqvist, HM, Rådjursöga, M, Torstensson, T, Jansson, L, Ellegård, L, and Winkvist, A. Urine metabolite profiles and nutrient intake based on 4-day weighed food diary in habitual vegans, vegetarians, and omnivores. J Nutr. (2021) 151:30–9. doi: 10.1093/jn/nxaa019
117. Ströhle, A, Waldmann, A, Koschizke, J, Leitzmann, C, and Hahn, A. Diet-dependent net endogenous acid load of vegan diets in relation to food groups and bone health-related nutrients: results from the German vegan study. Ann Nutr Metab. (2011) 59:117–26. doi: 10.1159/000331572
118. Blaurock, J, Kaiser, B, Stelzl, T, Weech, M, Fallaize, R, Franco, RZ, et al. Dietary quality in vegetarian and omnivorous female students in Germany: a retrospective study. IJERPH. (2021) 18:1888. doi: 10.3390/ijerph18041888
119. Neufingerl, N, and Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. (2021) 14:29. doi: 10.3390/nu14010029
120. Galchenko, A, and Nazarova, AM. Macroelements in nutrition of vegetarians and vegans. Trace Elements in Medicine (Moscow). (2019) 20:3–17. doi: 10.19112/2413-6174-2019-20-2-3-17
121. Ford, ES, and Mokdad, AH. Dietary magnesium intake in a National Sample of U.S. adults. J Nutr. (2003) 133:2879–82. doi: 10.1093/jn/133.9.2879
122. Workinger, J, Robert, D, and Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients. (2018) 10:1202. doi: 10.3390/nu10091202
123. Sobiecki, JG, Appleby, PN, Bradbury, KE, and Key, TJ. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European prospective investigation into Cancer and nutrition–Oxford study. Nutr Res. (2016) 36:464–77. doi: 10.1016/j.nutres.2015.12.016
124. Galchenko, A, and Nazarova, AM. Essential trace and ultra trace elements in nutrition of vegetarians and vegans. Part 1. IRON, zinc, copper, manganese. Trace Elements in Medicine (Moscow). (2019) 20:14–23. doi: 10.19112/2413-6174-2019-20-4-14-23
125. Hunt, JR, and Vanderpool, RA. Apparent copper absorption from a vegetarian diet. Am J Clin Nutr. (2001) 74:803–7. doi: 10.1093/ajcn/74.6.803
126. Haddad, EH, Berk, LS, Kettering, JD, Hubbard, RW, and Peters, WR. Dietary intake and biochemical, hematologic, and immune status of vegans compared with nonvegetarians. Am J Clin Nutr. (1999) 70:586s–93s. doi: 10.1093/ajcn/70.3.586s
127. Skalnaya, AA, Skalnaya, OA, Cheng-Chi, W, and Demidov, VA. Hair essential trace elements in BANGLADESH women: influence of vegetarianism. TEM (Moscow). (2016) 17:36–40. doi: 10.19112/2413-6174-2016-17-3-36-40
128. Davey, GK, Spencer, EA, Appleby, PN, Allen, NE, Knox, KH, and Key, TJ. EPIC–Oxford:lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. (2003) 6:259–68. doi: 10.1079/PHN2002430
129. Mangels, AR. Bone nutrients for vegetarians. Am J Clin Nutr. (2014) 100:469S–75S. doi: 10.3945/ajcn.113.071423
130. Saunders, AV, Craig, WJ, and Baines, SK. Zinc and vegetarian diets. Med J Aust. (2013) 199:S17–S21. doi: 10.5694/mja11.11493
131. Silva, S, Pinho, J, Borges, C, Santos, C, Santos, A, and Graca, P. Guidelines for a healthy vegetarian diet. Lisbon: Direção-Geralda Saúde (2015). 45 p.
132. Holt, R, Uriu-Adams, J, and Keen, C. Zinc In: J Erdman, I Macdonald, and S Zeisel, editors. Present knowledge in nutrition. Washington, D.C.: ILSI International Life Sciences Institute (2012)
133. Elorinne, A-L, Alfthan, G, Erlund, I, Kivimäki, H, Paju, A, Salminen, I, et al. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PLoS One. (2016) 11:e0148235. doi: 10.1371/journal.pone.0148235
134. Millet, P, Guilland, JC, Fuchs, F, and Klepping, J. Nutrient intake and vitamin status of healthy French vegetarians and nonvegetarians. Am J Clin Nutr. (1989) 50:718–27. doi: 10.1093/ajcn/50.4.718
135. Chan, J, Jaceldo-Siegl, K, and Fraser, GE. Serum 25-hydroxyvitamin D status of vegetarians, partial vegetarians, and nonvegetarians: the Adventist health Study-2. Am J Clin Nutr. (2009) 89:1686S–92S. doi: 10.3945/ajcn.2009.26736X
136. Rostand, SG, McClure, LA, Kent, ST, Judd, SE, and Gutiérrez, OM. Associations of blood pressure, sunlight, and vitamin D in community-dwelling adults. J Hypertens. (2016) 34:1704–10. doi: 10.1097/HJH.0000000000001018
137. Galchenko, AV, Gapparova, KM, Revyakina, VA, Korotkova, ТN, Chekhonina, YG, and Sidorova, EI. Self-administration of vitamin D by vegans to prevent its deficiency and decrease in bone mineral density: does it bring any benefits. Nutrition. (2022) 12:12–7. doi: 10.20953/2224-5448-2022-1-12-17
138. Janelle, KC, and Barr, SI. Nutrient intakes and eating behavior see of vegetarian and nonvegetarian women. J Am Diet Assoc. (1995) 95:180–9. doi: 10.1016/S0002-8223(95)00045-3
139. Trefflich, I, Marschall, H-U, Giuseppe, R, Ståhlman, M, Michalsen, A, Lampen, A, et al. Associations between dietary patterns and bile acids—results from a cross-sectional study in vegans and omnivores. Nutrients. (2019) 12:47. doi: 10.3390/nu12010047
140. Li, D, Sinclair, AJ, Mann, NJ, Turner, A, and Ball, MJ. Selected micronutrient intake and status in men with differing meat intakes, vegetarians and vegans. Asia Pac J Clin Nutr. (2000) 9:18–23. doi: 10.1046/j.1440-6047.2000.00129.x
141. Galchenko, A, and Ranjit, R. Vitamin a and its status in vegetarians and vegans. Problems of Biological Medical and Pharmaceutical Chemistry. (2021) 24:40–8. doi: 10.29296/25877313-2021-03-06
142. Krajcovicová-Kudlácková, M, Simoncic, R, Babinska, K, Bederova, A, Brtkova, A, Magalova, T, et al. Selected vitamins and trace elements in blood of vegetarians. Ann Nutr Metab. (1995) 39:334–9. doi: 10.1159/000177882
143. Barzel, US, and Massey, LK. Excess dietary protein can adversely affect bone. J Nutr. (1998) 128:1051–3. doi: 10.1093/jn/128.6.1051
144. Komarova, SV, Pereverzev, A, Shum, JW, Sims, SM, and Dixon, SJ. Convergent signaling by acidosis and receptor activator of NF- B ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc Natl Acad Sci. (2005) 102:2643–8. doi: 10.1073/pnas.0406874102
145. Riggs, BL, and Melton, LJ. Osteoporosis: Etiology, diagnosis, and management. Saint Petersburg: BINOM (2000). 558 p.
146. Bobulescu, IA, Park, SK, Xu, LHR, Blanco, F, Poindexter, J, Adams-Huet, B, et al. Net acid excretion and urinary organic anions in idiopathic uric acid nephrolithiasis. CJASN. (2019) 14:411–20. doi: 10.2215/CJN.10420818
147. Cao, JJ, Roemmich, JN, Sheng, X, and Jahns, L. Increasing vegetable intake decreases urinary acidity and bone resorption marker in overweight and obese adults: an 8-week randomized controlled trial. J Nutr. (2021) 151:3413–20. doi: 10.1093/jn/nxab255
148. Ausman, LM, Oliver, LM, Goldin, BR, Woods, MN, Gorbach, SL, and Dwyer, JT. Estimated net acid excretion inversely correlates with urine pH in vegans, lacto-Ovo vegetarians, and omnivores. J Ren Nutr. (2008) 18:456–65. doi: 10.1053/j.jrn.2008.04.007
149. Penczynski, KJ, Remer, T, Menzel, J, Abraham, K, and Weikert, C. Urinary potential renal acid load (uPRAL) among vegans versus omnivores and its association with bone health in the cross-sectional risks and benefits of a vegan diet study. Nutrients. (2022) 14:4468. doi: 10.3390/nu14214468
150. Chung, P-C, and Chan, T-C. Environmental and personal factors for osteoporosis or osteopenia from a large health check-up database: a retrospective cohort study in Taiwan. BMC Public Health. (2022) 22:1531. doi: 10.1186/s12889-022-13938-8
151. Shkaraburov, AS, Kolpinskiy, GI, Zakharov, IS, Shkaraburov, SP, and Mozes, VG. X-ray techniques for diagnostics of postmenopausal osteoporosis. Fundamental and Clinical Medicine. (2017) 2:70–6. doi: 10.23946/2500-0764-2017-2-2-70-76
152. El Kinany, K, Garcia-Larsen, V, Khalis, M, Deoula, MMS, Benslimane, A, Ibrahim, A, et al. Adaptation and validation of a food frequency questionnaire (FFQ) to assess dietary intake in Moroccan adults. Nutr J. (2018) 17:61. doi: 10.1186/s12937-018-0368-4
153. Noor Hafizah, Y, Ang, LC, Yap, F, Nurul Najwa, W, Cheah, WL, Ruzita, AT, et al. Validity and reliability of a food frequency questionnaire (FFQ) to assess dietary intake of preschool children. Int J Environ Res Public Health. (2019) 16:4722. doi: 10.3390/ijerph16234722
154. Yaghi, N, Boulos, C, Baddoura, R, Abifadel, M, and Yaghi, C. Validity and reliability of a food frequency questionnaire for community dwelling older adults in a Mediterranean country: Lebanon. Nutr J. (2022) 21:40. doi: 10.1186/s12937-022-00788-8
155. Baroni, L, Goggi, S, and Battino, M. VegPlate: a Mediterranean-based food guide for Italian adult, pregnant, and lactating vegetarians. J Acad Nutr Diet. (2018) 118:2235–43. doi: 10.1016/j.jand.2017.08.125
156. Galchenko, AV. Influence of lifestyle factors on bone metabolism and the risk of osteoporosis. Profilakticheskaya meditsina. (2022) 25:96. doi: 10.17116/profmed20222506196
157. Sinaki, M, Pfeifer, M, Preisinger, E, Itoi, E, Rizzoli, R, Boonen, S, et al. The role of exercise in the treatment of osteoporosis. Curr Osteoporos Rep. (2010) 8:138–44. doi: 10.1007/s11914-010-0019-y
Keywords: dual energy X-ray absorptiometry (DXA), osteoporosis, osteopenia, bone metabolism, parathyroid hormone (PTH), vitamin, trace element, acid load
Citation: Galchenko A, Rizzo G, Sidorova E, Skliar E, Baroni L, Visaggi P, Guidi G and de Bortoli N (2024) Bone mineral density parameters and related nutritional factors in vegans, lacto-ovo-vegetarians, and omnivores: a cross-sectional study. Front. Nutr. 11:1390773. doi: 10.3389/fnut.2024.1390773
Edited by:
Rakesh Bhardwaj, Indian Council of Agricultural Research (ICAR), IndiaReviewed by:
Mojca Korošec, University of Ljubljana, SloveniaNeal Barnard, Physicians Committee for Responsible Medicine, United States
Copyright © 2024 Galchenko, Rizzo, Sidorova, Skliar, Baroni, Visaggi, Guidi and de Bortoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey Galchenko, YWxleGV5LmdhbGNoZW5rb0BzY2llbnphdmVnZXRhcmlhbmEuaXQ=
 Alexey Galchenko
Alexey Galchenko Gianluca Rizzo
Gianluca Rizzo Elizaveta Sidorova
Elizaveta Sidorova Elena Skliar
Elena Skliar Luciana Baroni
Luciana Baroni Pierfrancesco Visaggi
Pierfrancesco Visaggi Giada Guidi6
Giada Guidi6 Nicola de Bortoli
Nicola de Bortoli