- 1College of Life Sciences, Hebei Normal University, Shijiazhuang, China
- 2Institute of Biology, Hebei Academy of Science, Shijiazhuang, China
- 3College of Civil Engineering and Architecture, North China Institute of Aerospace Engineering, Langfang, China
- 4Hebei Collaborative Innovation Center for Eco-Environment, Hebei Normal University, Shijiazhuang, China
Edible mushrooms are an important source of nutraceuticals and for the discovery of bioactive metabolites as pharmaceuticals. In this work, six new polyphenolic metabolites suillusol A-D (1–4), suillusinoic acid (5), ethyl suillusinoate (6), were isolated from the Suillus granulatus. The structures of new compounds were elucidated using high-resolution electrospray ionization mass spectroscopy, nuclear magnetic resonance data, and single-crystal X-ray diffraction analysis. As far as we know, compound 1 represents an unprecedented type of natural product and compound 3 represents a new type of polyphenol fungal pigment, which may be biosynthetically related to thelephoric acid. The cytotoxicity against HepG2 cells of the new compounds were also evaluated. Compound 2 demonstrate significant inhibitory activity against HepG2 cells with IC50 values of 10.85 μM, surpassing that of positive control cisplatin. Moreover, compound 1 and 3 also exhibited moderate cytotoxic activity with their IC50 values measured at 35.60 and 32.62 μM, respectively. Our results indicate that S. granulatus is a rich source of chemical constituents that may provide new lead compounds for the development of anticancer agents.
Introduction
The genus Suillus, a type of ectomycorrhizal fungi known for its high host specificity and commonly found in symbiosis with trees such as pines and firs, is classified within the Boletales order of the Basidiomycetes class (1). In some regions of China, it is commonly referred to as “pine mushrooms.” The Suillus genus has attracted growing research interest due to its nutritional value, capacity to produce biologically active secondary metabolites, and potential applications. Since the 1960s, studies have been initiated on the extracts, chemical constituents, and biological activities of this genus (2, 3). Studies have shown that the extracts derived from Suillus genus exhibit notable antioxidant, antineoplastic, antimicrobial properties and others (4–13). This implies that they integrate culinary and medicinal properties, being a valuable forest resource with significant economic and ecological benefits (14).
Suillus granulatus, a species of edible fungus from the genus Suillus, is widely distributed around the world (15). Current investigations into the chemical composition of S. granulates have been primarily confined to the analysis of polyprenylphenols and fatty acids, with the notable absence of studies on the complex phenolic metabolites (16–20).
Liver cancer is a malignant tumor with a global distribution, and it is estimated that mortality rate attributed to liver cancer will surpass one million by the year 2030 (21–23). Chemotherapy is a pivotal method in the treatment of liver cancer, especially for patients who are not suitable candidates for surgical removal of the tumor. Nevertheless, given the protracted nature of such regimens, associated adverse reactions, and the propensity for chemoresistance development, the therapeutic effectiveness of chemotherapy often falls short of expectations (24, 25). Therefore, natural products with a reliable anti-hepatocarcinoma effect that are less toxic and have fewer side effects have received increasing attention.
To identify new natural products with anti-hepatocarcinoma activity, we investigated chemical constituents of S. granulatus and isolated six novel polyphenolic compounds Subsequently, these compounds were evaluated for their cytostatic potential against hepatoma cell lines HepG2. Remarkably, all six compounds exhibited varying degrees of cytotoxic effects, with compound 2 exhibiting notably higher activity compared to the positive control cisplatin. The present study delineates the isolation, structural characterization, and antitumor activity against HepG2 cells of these novel polyphenolic compounds.
Materials and methods
General experimental procedure
HR-ESI-MS spectra were acquired on a Waters Xevo G2 Q-TOF mass spectrometer (Waters Co., Milford, MA, USA). NMR spectra were recorded on n a Bruker AM-600 spectrometer with TMS as an internal standard (Bruker, Ettlingen, German). NMR spectra were recorded at 25°C on a Bruker AM-600 spectrometer equipped with a cryoprobe, and deuterated solvents signal was used as an internal standard. The column chromatography (CC) was performed on YMC RP-18 gel (Fuji Silysia Chemical Ltd., Kasugai, Japan) and silica gel (200–300 mesh, Qingdao Marine Chemical Ltd., Qingdao, China). High-performance liquid chro-matography (HPLC) was performed on Waters 2,535 chromatography system (Waters, Milford, MA, USA) equipped with a Waters 2,489 UV/visible detector with a YMC-Pack ODS-A (250 × 10 mm, 5 μm) column (YMC Co., Ltd., Kyoto, Japan).
Mushroom material
The fresh fruiting bodies of S. granulatus were collected from the pine forests in Maojinba National Forest Park in Longhua Country, Chengde City, Hebei Province, China, in September 2022. It was morphologically identified by Prof. Li-an Wang (Hebei Normal University, China), and then verified by molecular analysis.
Extraction and isolation
The fruiting bodies of S. granulatus were subjected to air-drying for 48 h (dry weight, 20.5 kg) followed by extraction using 95% ethanol (60 L, three times) for 24 h to obtain the crude extract weighing 3,025 g. The crude extract was suspended in 3 L of water and then sequentially partitioned with petroleum ether (2 × 3 L, 2 h each), ethyl acetate (3 × 3 L, 2 h each), and n-butanol (2 × 3 L, 2 h each).
The petroleum ether fraction (PE, 137 g) was separated into eleven fractions (PE1–PE11) using silica gel CC eluted with petroleum ether/ethyl acetate (100:1–1:1). PE2 (1.4 g) was further purified by semi-preparative RP-HPLC (YMC ODS-A column, 250 × 10 mm, 5 μm, 2.5 mL/min, acetonitrile/water, 95:5) to obtain compound 5 (8 mg, tR = 32 min).
The ethyl acetate fraction (EA, 380 g) was separated into eight fractions (EA1–EA8) using silica gel CC eluted with dichloromethane/methanol (100:1–1:1). EA4 (31 g) and EA5 (43 g) were pooled and further purified by ODS reversed-phase silica gel CC eluting with a methanol/water (1:9–8:2) gradient system, resulting in the isolation of six sub-fractions designated as EA4A-EA4F. From EA4D (6 g), compounds 1 (acetonitrile/water, 25:75, 4 mg, tR = 20.5 min), 2 (acetonitrile/water, 30:70, 6 mg, tR = 13.3 min), 4 (acetonitrile/water, 30:70, 6 mg, tR = 29.7 min), and 6 (acetonitrile/water, 25:75, 16 mg, tR = 27.1 min) were isolated by semi-preparative RP-HPLC (2.5 mL/min).
The n-butanol fraction (54 g) was separated by ODS reversed-phase silica gel CC eluting with a methanol/water (1:9–8:2) gradient system, resulting in the isolation of six fractions designated as BA1–BA6. Compound 3 was separated as crystals from the BA6 fraction.
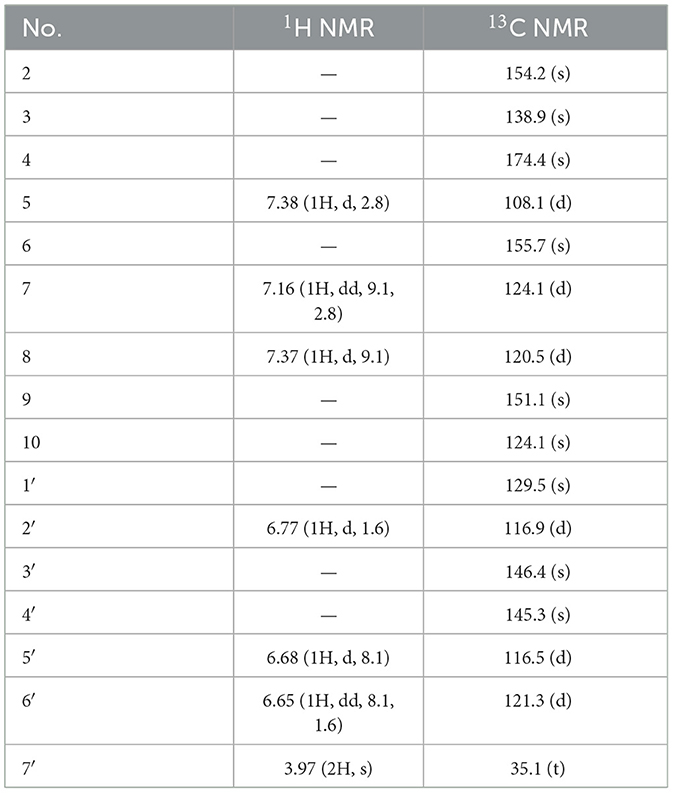
Suillusol A (1): light yellow powder; [α] 0 (c 0.1, MeOH); UV (MeOH) λmax 285, 375 nm; IRmax 3,319, 1,739, 1,615, 1,515, 1,460, 1,438, 1,384, 1,317, 1,260, 1,173, 1,124 cm−1, 1H and 13C NMR data see Table 1; HR-ESI-MS (m/z 311.0555 [M–H]−, calcd. 311.0561).
Suillusol B (2): light yellow powder; UV (MeOH) λmax 289, 347 nm; IRmax 3,305, 1,685, 1,612, 1,589, 1,509, 1,438, 1,383, 1,235, 1,182, 1,117 cm−1, 1H and 13C NMR data see Table 2; HR-ESI-MS (m/z 299.0555 [M–H]−, calcd. 299.0561).
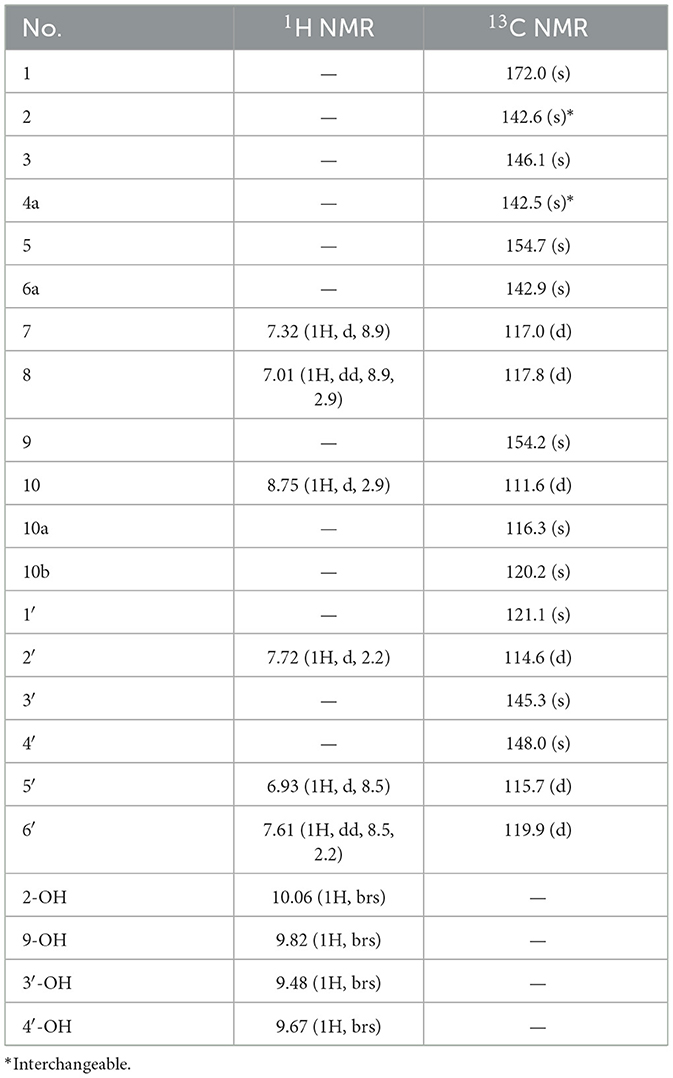
Suillusol C (3): yellow crystals (methanol); UV (MeOH) λmax 291, 377 nm; IRmax 3,413, 1,691, 1,510, 1,438, 1,376, 1,347, 1,251, 1,174, 1,084 cm−1, 1H and 13C NMR data see Table 3; HR-ESI-MS (m/z 353.0295 [M–H]−, calcd. 353.0303).
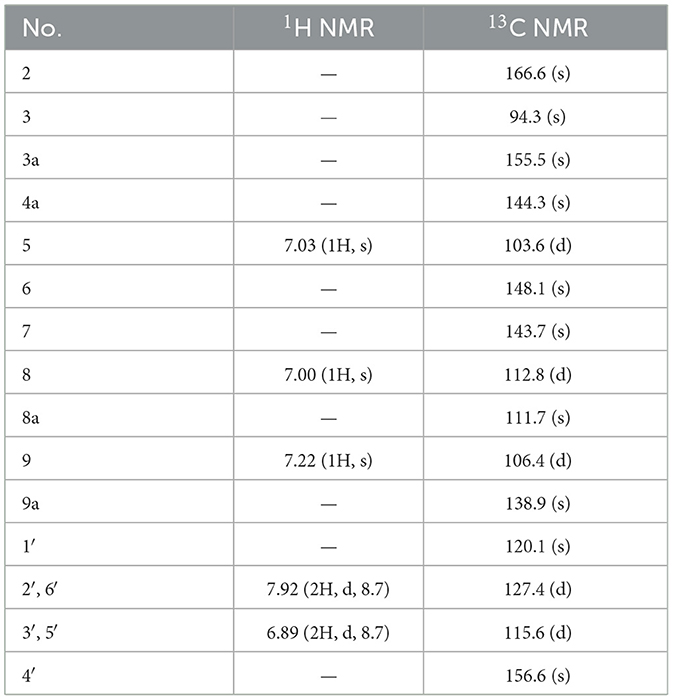
Suillusol D (4): yellow powder; UV (MeOH) λmax 271, 385 nm; IRmax 3,380, 1,735, 1,655, 1,616, 1,512, 1,465, 1,265 cm−1, 1H and 13C NMR data see Table 4; HR-ESI-MS (m/z 311.0542 [M+H]+, calcd. 311.0550).
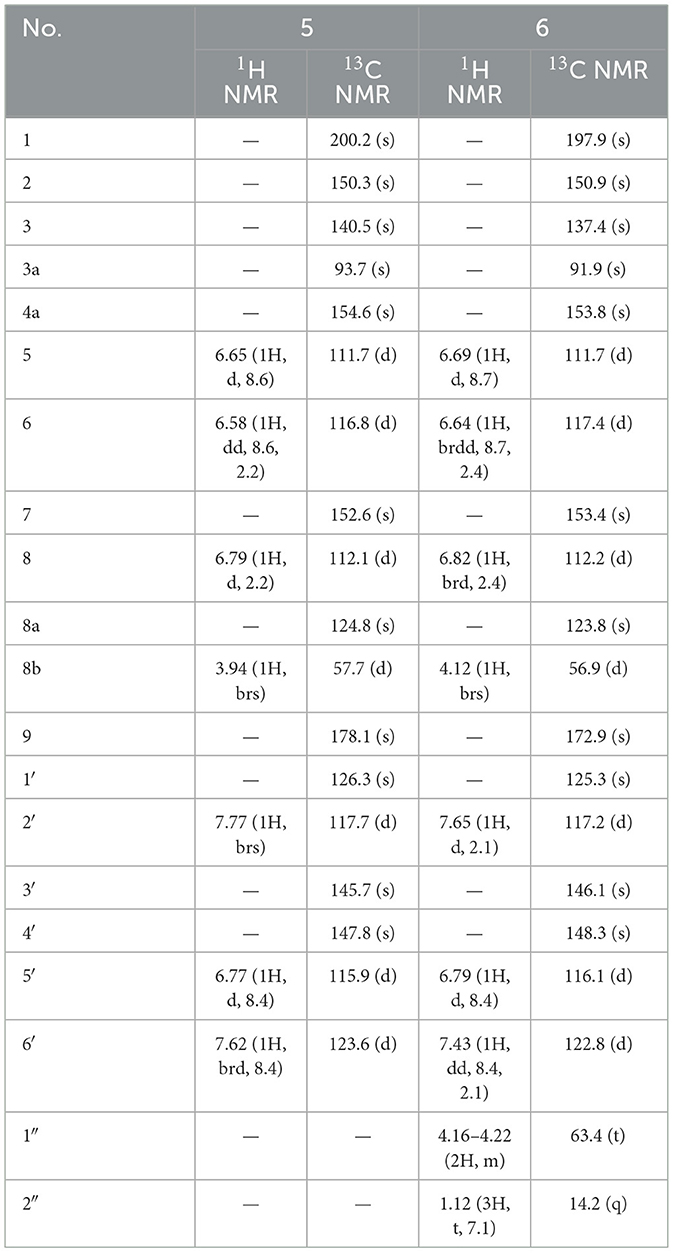
Suillusinoic acid (5): light yellow powder; UV (MeOH) λmax 311, 368 nm; IRmax 3,296, 1,716, 1,684, 1,608, 1,508, 1,458, 1,383, 1,237, 1,174 cm−1, 1H and 13C NMR data see Table 5; HR-ESI-MS (m/z 355.0458 [M–H]−, calcd. 355.0459).
Ethyl suillusinoate (6): light yellow powder; UV (MeOH) λmax 289, 370 nm; IRmax 3,295, 1,711, 1,685, 1,609, 1,509, 1,439, 1,384, 1,239, 1,175 cm−1, 1H and 13C NMR data see Table 5; HR-ESI-MS (m/z 383.0762 [M–H]−, calcd. 383.0772).
Cytotoxic activity
Cell viability assay was determined by the Cell Counting Kit-8 Assay Kit (CCK8, Bioss, China). The experiment was determined based on the method of Liu et al. (26). HepG2 cells (100 μL, 5 × 104/mL) were seeded onto a 96-well plate overnight. The cells were treated with different concentrations (5, 10, 20, 40, 80, 160 μM) of samples (100 μL) dissolved in medium for 72 h at 37°C and 5% CO2. Then, 10 μL CCK-8 solution was added to each well, and the plate was protected from light and incubated at 37°C for 1 h. Afterwards, the optical density was measured by a microplate reader at 450 nm. Each experiment was repeated three times. Cisplatin was used as a positive control, and the above operation was repeated.
Results and discussion
Structure elucidation
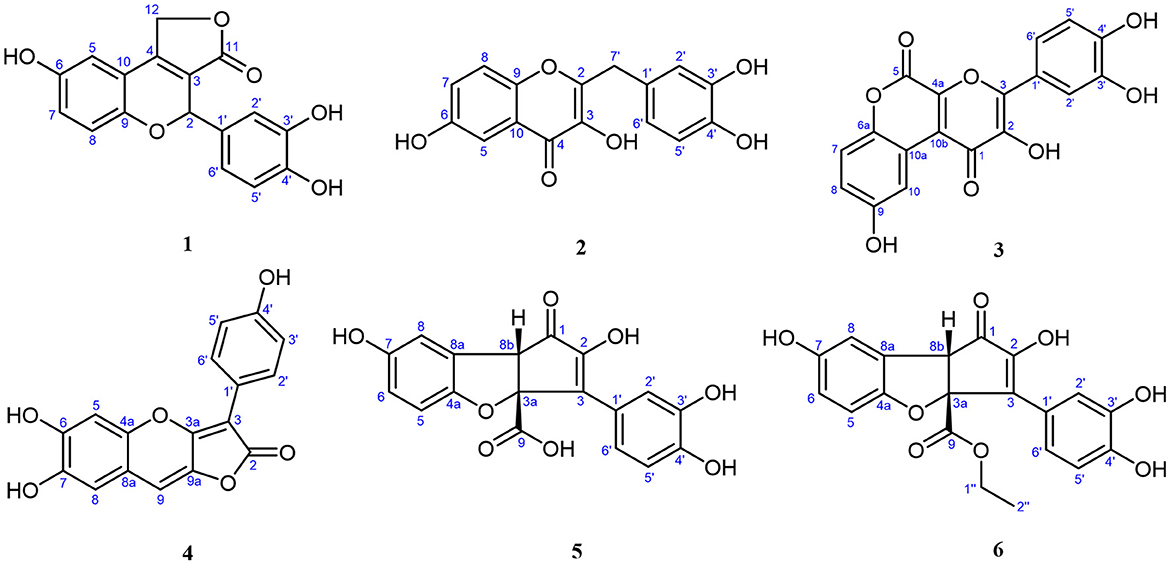
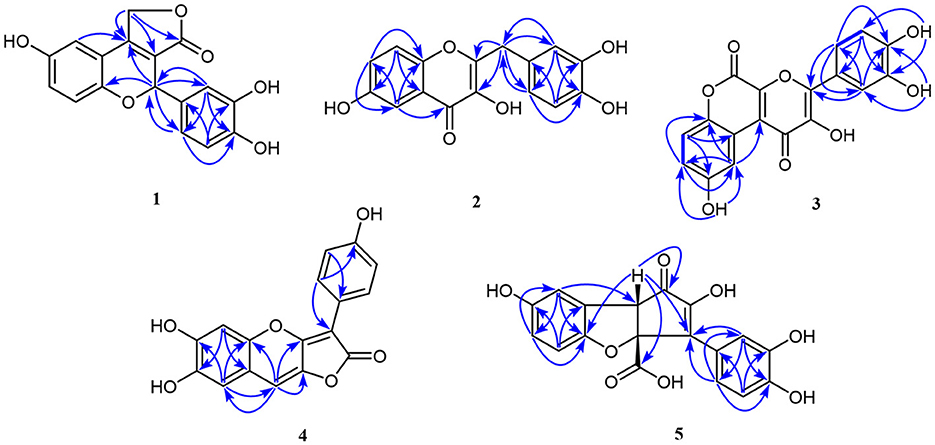
Compound 1, light yellow powder, possessed a molecular formula of C17H12O6 by the negative HR-ESI-MS (m/z 311.0555 [M–H]−, calcd. 311.0561), accounting for 12 degrees of unsaturation. The 1H NMR spectrum (Table 1) in CD3OD of 1 showed two sets of characteristic 1,3,4-trisubstituted benzene ring signals at δH 6.80 (1H, dd, J = 8.8, 2.8 Hz, H-7), 6.74 (1H, d, J = 8.8 Hz, H-8), 6.69 (1H, d, J = 2.8 Hz, H-5), 6.77 (1H, d, J = 1.8 Hz, H-2′), 6.69 (1H, d, J = 8.2 Hz, H-5′), and 6.67 (1H, dd, J = 8.2, 1.8 Hz, H-6′), one downfield oxygen-bearing methine proton at δH 6.00 (1H, d, J = 2.0 Hz, H-2), and one downfield oxygenated methylene signal at δH 5.21 (1H, dd, J = 17.3, 2.0 Hz, H-12a) and 5.39 (1H, d, J = 17.3 Hz, H-12b), which hinted the existence of three phenolic hydroxy protons. The 13C NMR spectrum (Table 1) showed a total of 17 carbon resonances, including one lactone carbonyl carbon at δC 173.3 (s, C-11), 14 aromatic or olefinic carbons due to two benzene rings and a double bond group, as well as two oxygenated carbons at δC 75.6 (d, C-2) and 70.1 (t, C-12). The analysis of the degrees of unsaturation suggested that there were two aliphatic rings in 1. A detailed analysis of the HMBC correlations (Figure 2) allowed to infer the presence of a 3,4-dihydroxyphenyl moiety, which was attached to the oxygenated methine carbon at δC 75.6 (d, C-2). While the other 1,3,4-trisubstituted benzene ring was located on a chromene nucleus by the observable HMBC correlations from H-5 [δH 6.69 (1H, d, J = 2.8 Hz)] and H-2 [δH 6.00 (1H, d, J = 2.0 Hz] to C-4 [δC 155.7 (s)] and C-9 [δC 148.2 (s)]. Furthermore, the 4J coupling constant of H-5 indicated a phenolic hydroxy group at C-6, which was confirmed by analysis of the HMBC correlations (Figure 2). By consideration of the NMR shifts of the two unsolved carbons (C-11 and C-12) and the degrees of unsaturation, the other aliphatic ring system was assigned to a γ-lactone ring, which was confirmed by the HMBC correlations from H2-12 [δH 5.21 (1H, dd, J = 17.3, 2.0 Hz) and 5.39 (1H, d, J = 17.3 Hz)] to C-3 [δC 121.5 (s)], C-4 [δC 155.7 (s)] and C-11 [δC 173.3 (s)]. The lack of significant optical rotation observed for compound 1, indicated that the compound may in fact be present as racemic mixtures. Therefore, the structure of 1 was established as 4-(3,4-dihydroxyphenyl)-8-hydroxy-1,4-dihydro-3H-furo[3,4-c]chromen-3-one shown in Figure 1 and named as suillusol A. As far as we know, the novel structure represents an unprecedented type of natural product.
Compound 2, obtained as light yellow powder, had a molecular formula of C16H12O6 by the negative HR-ESI-MS (m/z 299.0555 [M–H]−, calcd. 299.0561), accounting for 11 degrees of unsaturation. The 1H NMR spectrum (Table 2) in CD3OD of 2 exhibited two sets of characteristic 1,3,4-trisubstituted benzene ring signals at δH 7.38 (1H, d, J = 2.8 Hz, H-5), 7.37 (1H, d, J = 9.1 Hz, H-8), 7.16 (1H, dd, J = 9.1, 2.8 Hz, H-7), 6.77 (1H, d, J = 1.6 Hz, H-2′), 6.68 (1H, d, J = 8.1 Hz, H-5′), and 6.65 (1H, dd, J = 8.1, 1.6 Hz, H-6′), as well as a methylene singlet at δH 3.97 (2H, s, H-7′), which suggested the existence of four exchangeable protons. The 13C NMR spectrum (Table 2) showed a total of 16 carbon resonances, including one carbonyl carbon at δC 174.4 (s, C-4), 14 aromatic or olefinic carbons due to two benzene rings and a double bond group, as well as one aliphatic methylene carbon at δC 35.1 (t, C-7′). One carbonyl, two benzene rings, and one double bond occupied 10 degrees of unsaturation, indicative of the existence of one aliphatic ring in 2. A detailed analysis of the HMBC correlations (Figure 2) allowed to infer the presence of a 3,4-dihydroxybenzyl moiety, which was attached to a double bond group. While the other 1,3,4-trisubstituted benzene ring was connected with the carbonyl group by the HMBC correlation from H-5 [δH 7.38 (1H, d, J = 2.8 Hz)] to C-4 [δC 174.4 (s)]. In addition, the 4J coupling constant of H-5 suggested a phenolic hydroxy group at C-6, which was further confirmed by analysis of the HMBC correlations (Figure 2). Finally, a comprehensive consideration of the degrees of unsaturation and the 13C NMR shift of C-2, C-3, and C-4 allowed us to deduce that there must be a 3-hydroxy-4H-pyran-4-one moiety in the structure. Therefore, the structure of 2 was established as 2-(3,4-dihydroxybenzyl)-3,6-dihydroxy-4H-chromen-4-one shown in Figure 1 and named as suillusol B. It is worth mentioning that compound 2, as a representative of 2-benzylchromen-4-one, is a rare type of natural product (27).
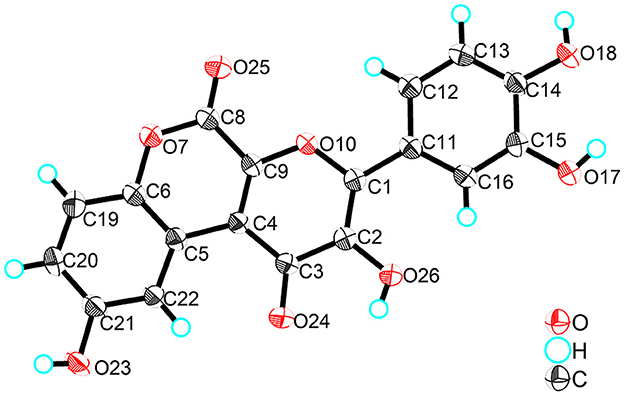
Compound 3, obtained as yellow crystals (methanol), had a molecular formula of C18H10O8 as determined by the negative HR-ESI-MS (m/z 353.0295 [M–H]−, calcd. 353.0303), accounting for 14 degrees of unsaturation. The 1H NMR spectrum (Table 3) in DMSO-d6 of 3 exhibited four phenolic hydroxy signals at δH 10.06 (1H, brs, 2-OH), 9.82 (1H, brs, 9-OH), 9.67 (1H, brs, 4′-OH), and 9.48 (1H, brs, 3′-OH), two sets of characteristic 1,3,4-trisubstituted benzene ring signals at δH 8.75 (1H, d, J = 2.9 Hz, H-10), 7.32 (1H, d, J = 8.9 Hz, H-7), 7.01 (1H, dd, J = 8.9, 2.9 Hz, H-8), 7.72 (1H, d, J = 2.2 Hz, H-2′), 7.61 (1H, dd, J = 8.5, 2.2 Hz, H-6′), and 6.93 (1H, d, J = 8.5 Hz, H-5′). The 13C NMR spectrum (Table 3) showed a total of 18 carbon resonances, and all were in the range of 111.6–172.0 ppm, including six aromatic methine carbons and 12 quaternary ones. The aforementioned NMR features suggested that 3 should be a polyphenol containing two benzene rings. A detailed analysis of the HMBC correlations (Figure 2) allowed to establish the specific substitutions of two benzene ring moieties. However, the four carbon signals at δC 172.0, 154.7, 142.6, and 142.5 were still unsolved, since no correlations were observed in the HMBC spectrum. Thereupon we had to resort to using single crystal X-ray diffraction method. Fortunately, its single crystals were eventually obtained through multiple attempts, and the structure was conclusively determined to be 3-(3,4-dihydroxyphenyl)-2,9-dihydroxypyrano[2,3-c]chromene-1,5-dione by single crystal X-ray crystallographic analysis (Figure 3). Thus, the structure of 3 was established as shown in Figure 1 and named as suillusol C. As far as we know, compound 3 represents a new type of polyphenol fungal pigment, which may be biosynthetically related to thelephoric acid.
Compound 4, isolated as a yellow powder, possessed a molecular formula of C17H10O6 by the positive HR-ESI-MS (m/z 311.0542 [M+H]+, calcd. 311.0550), indicating 13 degrees of unsaturation. The 1H NMR spectrum (Table 4) in DMSO-d6 of 4 showed a group of p-substituted benzene ring signals at δH 7.92 (2H, d, J = 8.7 Hz, H-2′/6′) and 6.89 (2H, d, J = 8.7 Hz, H-3′/5′), as well as three aromatic or olefinic proton singlets at δH 7.22 (1H, s, H-9), 7.03 (1H, s, H-5), and 7.00 (1H, s, H-8), which hinted the existence of three phenolic hydroxy protons. The 13C NMR spectrum (Table 4) showed a total of 17 carbon resonances, including a conjugated lactone carbonyl carbon at δC 166.6 (s, C-2), as well as 16 aromatic or olefinic carbons assignable to two benzene rings and two double bond groups. The analysis of the degrees of unsaturation hinted the existence of two additional rings. An analysis of the HMBC correlations (Figure 2) revealed the presence of a 4-hydroxyphenyl moiety, which was located at an up-field sp carbon at δC 94.3 (s, C-3). According to the peak shape of the remaining proton signals, the other benzene ring was inferred as 1,2,4,5-tetrasubstituted and located on a chromene nucleus, which was supported by the observable HMBC correlations from H-5 [δH 7.03 (1H, s)] to C-7 [δC 143.7 (s)] and C-8a [δC 111.7 (s)], from H-8 [δH 7.00 (1H, s)] to C-4a [δC 144.3 (s)], C-6 [δC 148.1 (s)] and C-9 [δC 106.4 (d)], and from H-9 [δH 7.22 (1H, s)] to C-3a [δC 155.5 (s)], C-4a and C-8 [δC 112.8 (d)]. By consideration of the 13C NMR shifts of the unsolved carbons and the degrees of unsaturation, the other ring system was assigned to a conjugated γ-lactone unit, which was further fused by the chromene nucleus to form an unusual 2H-furo[3,2-b]chromen-2-one core. And then two phenolic hydroxy groups were necessarily attached at C-6 and C-7. Therefore, the structure of 4 was established as 6,7-dihydroxy-3-(4-hydroxyphenyl)-2H-furo[3,2-b]chromen-2-one, shown in Figure 1 and named as suillusol D.
Compound 5, isolated as light yellow powder, possessed a molecular formula of C18H12O8 by the negative HR-ESI-MS (m/z 355.0458 [M–H]−, calcd. 355.0459), indicating 13 degrees of unsaturation. The 1H NMR spectrum (Table 5) in CD3OD of 5 exhibited two groups of 1,3,4-trisubstituted benzene ring signals at δH 7.77 (1H, brs, H-2′), 7.62 (1H, brd, J = 8.4 Hz, H-6′), 6.77 (1H, d, J = 8.4 Hz, H-5′), 6.79 (1H, d, J = 2.2 Hz, H-8), 6.65 (1H, d, J = 8.6 Hz, H-5), and 6.58 (1H, dd, J = 8.6, 2.2 Hz, H-6), as well as one downfield aliphatic methine singlet at δH 3.94 (1H, brs, H-8b), which hinted the existence of five exchangeable protons. The 13C NMR spectrum (Table 5) showed a total of 18 carbon resonances, including one conjugated ketone carbon at δC 200.2 (s, C-1), one carboxylic carbon at δC 178.1 (s, C-9), 14 aromatic or olefinic carbons assignable to two benzene rings and a double bond group, one downfield oxygenated sp3 quaternary carbon at δC 93.7 (s, C-3a), as well as one aliphatic methine carbon at δC 57.7 (d, C-8b). The analysis of the degrees of unsaturation was indicative of the presence of two aliphatic rings in 5. The aforementioned NMR features were very similar to those of suillusin, a unique cyclopenta[b]benzofuran derivative isolated from the same genus (19). Comparison of the NMR data of 5 with those of suillusin revealed that the signal differences were mainly from the carboxylic acid group at C-3a. The absence of the methoxy signals and the obvious downfield shift of the carboxylic carbon indicated that the methoxycarbonyl group was replaced by a carboxylic one in 5. The resulting structure was further verified by the careful HMBC analysis (Figure 2). While the relative configuration of two chiral centers was deduced to be the same as that of suillusin by comparison of their NMR data. Therefore, the structure of 5 was established as shown in Figure 1 and named as suillusinoic acid.
Compound 6, light yellow powder, possessed a molecular formula of C20H16O8 by the negative HR-ESI-MS (m/z 383.0762 [M–H]−, calcd. 383.0772). The 1H and 13C NMR spectra (Table 5) in CD3OD of 6 were very similar to those of suillusinoic acid (5). Comparison of the NMR data of 6 with those of 5 revealed that the signal differences were also from the carboxylic acid moiety at C-3a. The appearance of the ethoxy signals and the obvious upfield shift of the carboxylic carbon indicated that the carboxylic group was ethylated in 6, which was confirmed by the observable HMBC correlations from H2-1′' [δH 4.16–4.22 (2H, m)] and H-8b [δH 4.12 (1H, brs)] to the ester carbonyl carbon [δC 172.9 (s, C-9)]. Similarly, the relative configuration was the same as that of suillusinoic acid by comparison of their NMR data. Thus, the structure of 6 was established as shown in Figure 1 and named as ethyl suillusinoate.
Cytotoxicity against HepG2 cells
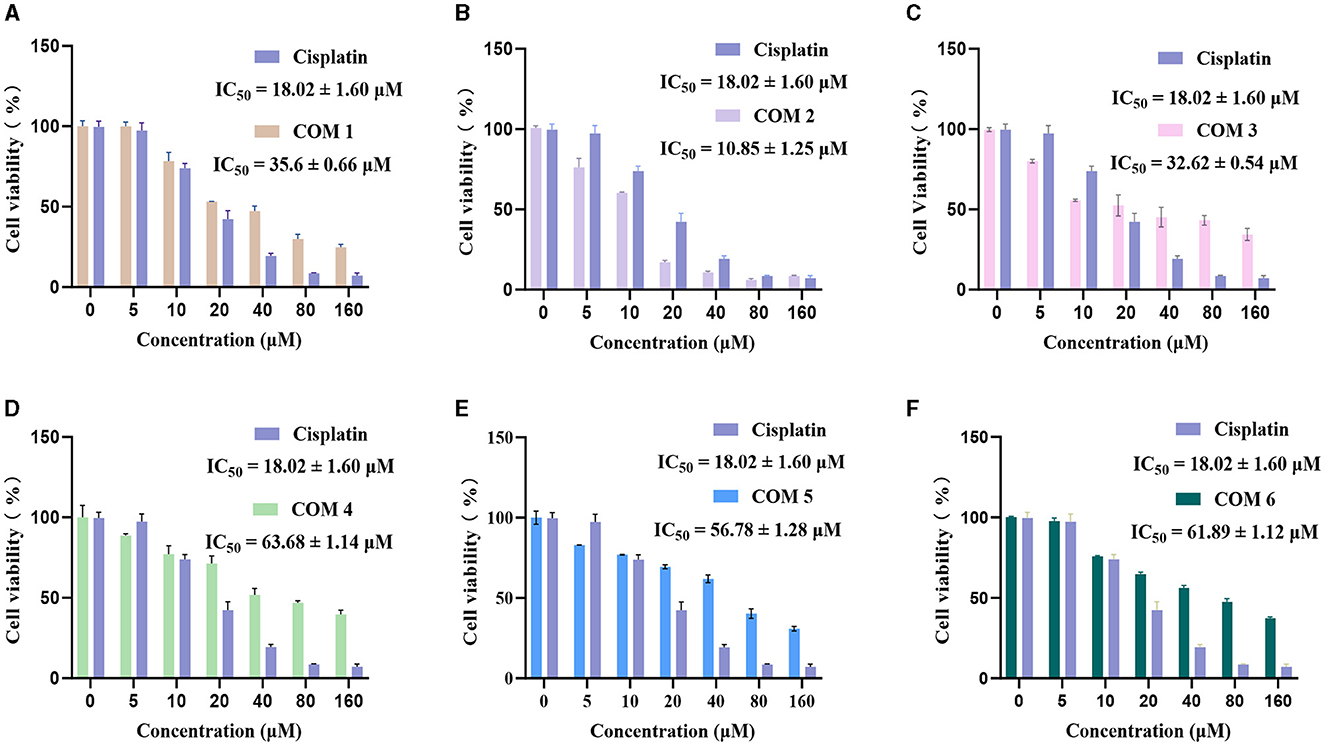
We tested the antitumor activity against HepG2 human liver carcinoma cells of the isolated compounds 1–6. The results shown in Figure 4 indicated that compound 2 exhibit higher efficacy than cisplatin, a commonly used anticancer chemotherapeutic agent, and demonstrate exceptional antitumor activity, with the half-maximal inhibitory concentrations (IC50) measured at 10.85 μM. Additionally, compounds 1 and 3 also exhibit moderate antitumor activity, with IC50 values of 35.60 and 32.62 μM. Compounds 5 and 6 exhibited the lowest inhibitory activity, with their IC50 values showing no significant difference due to their structural similarity. But during the experimental process, compounds 5 and 6 displayed color-changing phenomena, suggesting that they may have a more pronounced effect in antioxidant activity.
Conclusion
Six new polyphenolic compounds, named suillusol A-D (1–4), suillusinoic acid (5), ethyl suillusinoate (6), were isolated from the macrofungus S. granulatus. All the isolated compounds exhibited some degree of cytotoxicity against HepG2 cells. Compound 2 showed improved inhibitory activities than positive control cisplatin. Moreover, compounds 1 and 3 also exhibited moderate cytotoxic activity against HepG2 cells. Those results suggested that the polyphenolic compounds isolated from the S. granulatus could be investigated as natural anticancer agent in the pharmaceutical and food industries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HZ: Conceptualization, Project administration, Visualization, Writing – review & editing. MX: Resources, Validation, Visualization, Writing – review & editing. XY: Conceptualization, Formal analysis, Methodology, Software, Writing – review & editing. LY: Investigation, Methodology, Resources, Validation, Writing – original draft. ZW: Investigation, Writing – review & editing. L-aW: Formal analysis, Methodology, Resources, Software, Validation, Writing – original draft. ZL: Conceptualization, Formal analysis, Supervision, Visualization, Writing – review & editing. JZ: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. JL: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science Research Start-up Fund for Doctor of Hebei Normal University (L2023B22 and L2024B16) and Modern Seed-industry Technology Innovation Team for Edible Fungi (21326315D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1390256/full#supplementary-material
References
1. Min YJ, Park MS, Fong JJ, Seok SJ, Han SK, Lim YW. Molecular taxonomical re-classification of the genus Suillus micheli ex S. F Gray in South Korea. Mycobiology. (2014) 42:221–8. doi: 10.5941/MYCO.2014.42.3.221
2. Minami K, Asawa K, Sawada M. The structure of amitenone. Tetrahedron Lett. (1968) 9:5067–70. doi: 10.1016/S0040-4039(00)72286-6
3. Beaumont PC, Edwards RL. Constituents of the higher fungi. IX. Bovinone, 2,5-dihydroxy-3-geranylgeranyl-1,4-benzoquinone from Boletus (Suillus) bovinus (linn. ex Fr.) Kuntze. J Chem Soc Perkin Trans. (1969) 18:2398–2403. doi: 10.1039/j39690002398
4. Leon F, Brouard I, Torres F, Quintana J, Rivera A, Estevez F, et al. A new ceramide from Suillus luteus and its cytotoxic activity against human melanoma cells. Chem Biodivers. (2008) 5:120–5. doi: 10.1002/cbdv.200890002
5. Kalogeropoulos N, Yanni AE, Koutrotsios G, Aloupi M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem Toxicol. (2013) 55:378–85. doi: 10.1016/j.fct.2013.01.010
6. Santos TD, Tavares C, Sousa D, Vaz JA, Calhelha RC, Martins A, et al. Suillus luteus methanolic extract inhibits cell growth and proliferation of a colon cancer cell line. Food Res Int. (2013) 53:476–81. doi: 10.1016/j.foodres.2013.05.037
7. Radzki W, Slawinska A, Jablonska-Rys E, Gustaw W. Antioxidant capacity and polyphenolic content of dried wild edible mushrooms from Poland. Int J Med Mushrooms. (2014) 16:65–75. doi: 10.1615/IntJMedMushr.v16.i1.60
8. Vanyolos A, Orban-Gyapai O, Hohmann J. Xanthine oxidase inhibitory activity of Hungarian wild-growing mushrooms. Phytother Res. (2014) 28:1204–10. doi: 10.1002/ptr.5115
9. Morel S, Arnould S, Vitou M, Boudard F, Guzman C, Poucheret P, et al. Antiproliferative and antioxidant activities of wild Boletales mushrooms from France. Int J Med Mushrooms. (2018) 20:13–29. doi: 10.1615/IntJMedMushrooms.2018025329
10. Murray AF, Wickramasinghe PCK, Munafo JP. Key odorants from the fragrant Bolete, Suillus punctipes. J Agr Food Chem. (2020) 68:8621–8. doi: 10.1021/acs.jafc.0c03389
11. Volcão LM, Fernandes CLF, Ribeiro AC, Brum RDL, Eslabão CF, Badiale-Furlong E, et al. Bioactive extracts of Russula xerampelina and Suillus granulatus in the in vitro control of Pseudomonas aeruginosa phytopathogenic. S Afr J Bot. (2021) 140:218–25. doi: 10.1016/j.sajb.2021.03.043
12. Zhang J, Lu Y, Zhao L, Wang L-A. Hypoglycemic activity of total flavonoids from slippery jack mushroom, Suillus luteus (Agaricomycetes), in vitro and in vivo. Int J Med Mushrooms. (2021) 23:17–26. doi: 10.1615/IntJMedMushrooms.2021040384
13. Yao L, Lv JH, Li JP, An XY, Cheng GH, Li CT, et al. Chemical constituents from mushroom Suillus luteus (Agaricomycetes) and their bioactivities. Int J Med Mushrooms. (2022) 24:63–71. doi: 10.1615/IntJMedMushrooms.2022045041
14. Deng XJ, Li MQ, Chen CF, Kang B, Yang F, Ma H, et al. Resources and functional components of wild edible and medicinal mushrooms in Ningxia province. Curr Topics Nutraceut R. (2023) 21:553–61. doi: 10.37290/ctnr2641-452X.21:553-561
15. Reis FS, Stojković D, Barros L, Glamočlija J, Cirić A, Soković M, et al. Can Suillus granulatus (L.) Roussel be classified as a functional food? Food Funct. (2014) 5:2861–2869. doi: 10.1039/C4FO00619D
16. Tringali C, Geraci C, Nicolosi G, Verbist JF, Roussakis C. An antitumor principle from Suillus granulatus. J Nat Prod. (1989) 52:844–5. doi: 10.1021/np50064a030
17. Tringali C, Piattelli M, Geraci C, Nicolosi G. Antimicrobial tetraprenylphenols from Suillus granulatus. J Nat Prod. (1989) 52:941–7. doi: 10.1021/np50065a005
18. Geraci C, Piattelli M, Tringali C, Verbist JF, Roussakis C. Cytotoxic activity of tetraprenylphenols related to suillin, an antitumor principle from Suillus granulatus. J Nat Prod. (1992) 55:1772–5. doi: 10.1021/np50090a010
19. Yun BS, Kang HC, Koshino H, Yu SH, Yoo ID. Suillusin, a unique benzofuran from the mushroom Suillus granulatus. J Nat Prod. (2001) 64:1230–1. doi: 10.1021/np010138a
20. Ribeiro B, Rangel J, Valentao P, Baptista P, Seabra RM, Andrade PB. Contents of carboxylic acids and two phenolics and antioxidant activity of dried portuguese wild edible mushrooms. J Agric Food Chem. (2006) 54:8530–7. doi: 10.1021/jf061890q
21. Villanueva A. Hepatocellular carcinoma. N Engl J Med. (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
22. Shek D, Chen D, Read SA, Ahlenstiel G. Examining the gut-liver axis in liver cancer using organoid models. Cancer Lett. (2021) 510:48–58. doi: 10.1016/j.canlet.2021.04.008
23. Yang N, Wang T, Li Q, Han F, Wang Z, Zhu R, et al. HBXIP drives metabolic reprogramming in hepatocellular carcinoma cells via METTL3-mediated m6A modification of HIF-1α. J Cell Physiol. (2021) 236:3863–80. doi: 10.1002/jcp.30128
24. Li TT, Li T, Wang ZQ, Jin YX. Cyclopeptide-based anti-liver cancer agents: a mini-review. Protein Peptide Lett. (2023) 30:201–13. doi: 10.2174/0929866530666230217160717
25. Li Y, Wu Q, Yu G, Li L, Zhao X, Huang X, et al. Polypyridyl Ruthenium (II) complex-induced mitochondrial membrane potential dissipation activates DNA damage-mediated apoptosis to inhibit liver cancer. Eup J Med Chem. (2019) 164:282–91. doi: 10.1016/j.ejmech.2018.12.041
26. Liu X, Dong J, Cai W, Pan Y, Li R, Li B. The effect of thymoquinone on apoptosis of SK-OV-3 ovarian cancer cell by regulation of Bcl-2 and Bax. Int J Gynecol Cancer. (2017) 27:1596–601. doi: 10.1097/IGC.0000000000001064
Keywords: Suillus granulatus, phenolic metabolites, mushrooms, macro fungi, cytotoxicity
Citation: Zhao H, Xiong M, Yang X, Yao L, Wang Z, Wang L-a, Li Z, Zhang J and Lv J (2024) Six new polyphenolic metabolites isolated from the Suillus granulatus and their cytotoxicity against HepG2 cells. Front. Nutr. 11:1390256. doi: 10.3389/fnut.2024.1390256
Received: 12 March 2024; Accepted: 08 April 2024;
Published: 24 April 2024.
Edited by:
Uroš M. Gašić, University of Belgrade, SerbiaReviewed by:
Ivana Sofrenic, University of Belgrade, SerbiaDejan S. Stojkovic, University of Belgrade, Serbia
Copyright © 2024 Zhao, Xiong, Yang, Yao, Wang, Wang, Li, Zhang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Lv, bHZqaWFuaHVhQGhlYnR1LmVkdS5jbg==; Jinxiu Zhang, eGl1ZG91ODgyMDAzQDE2My5jb20=
 Hanyu Zhao
Hanyu Zhao Miaomiao Xiong1
Miaomiao Xiong1 Zhuang Li
Zhuang Li Jianhua Lv
Jianhua Lv