
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 03 July 2024
Sec. Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1390232
This article is part of the Research TopicHuman Milk, Nutrition and Infant DevelopmentView all 27 articles
Background: Breast milk is the gold standard for infant feeding. It is a dynamic biological fluid rich in numerous bioactive components. Emerging research suggests that these components, including hormones, may serve as signals between mother and offspring. From an evolutionary perspective, maternal hormonal signals could allow co-adaptation of maternal and offspring phenotype, with implications for their Darwinian fitness. However, a series of steps need to be considered to establish the role of a component as a signal and this systematic review focuses on one step: ‘Do maternal factors influence the concentration of milk hormones?’
Objective: To systematically review human studies which analyze the association between maternal factors and the concentration of hormones in breast milk.
Methods: Three databases were searched for studies reporting the association of maternal factors including body mass index (BMI), weight, fat mass, age, ethnicity, smoking with hormones such as adiponectin, leptin, insulin, ghrelin, and cortisol in breast milk.
Results: Thirty-three studies were eligible for inclusion. Maternal BMI was positively associated with milk leptin (20/21 studies) and with milk insulin (4/6 studies). Maternal weight also displayed a positive correlation with milk leptin levels, and maternal diabetes status was positively associated with milk insulin concentrations. Conversely, evidence for associations between maternal fat mass, smoking, ethnicity and other maternal factors and hormone levels in breast milk was inconclusive or lacking.
Conclusion: Current evidence is consistent with a signaling role for leptin and insulin in breast milk, however other steps need to be investigated to understand the role of these components as definitive signals. This review represents a first step in establishing the role of signaling components in human milk and highlights other issues that need to be considered going forward.
The World Health Organisation (WHO) recognizes breast milk as the gold standard for infant feeding. It offers evident short-term advantages for infants, including a decrease in the incidence of mortality and morbidity related to infectious diseases (1). However, breast-feeding is also a mode of nutrition that exhibits substantial variability among mothers, for example in the volume and composition of the milk produced (2).
In this context, breast milk can be considered a medium through which the mother can communicate through different biological pathways with her offspring, potentially regulating the offspring’s growth and development. From an evolutionary perspective, a mother could optimize her Darwinian fitness if her investment in lactation can adapt in response to ongoing environmental factors, whether these relate directly to the mother (e.g., her energy reserves, which support lactation), or to external factors such as the supply of food (e.g., famine), or to psychosocial stress, which could divert maternal metabolic resources to the stress response, and thus reduce nutritional supply to the offspring. Beyond transferring macronutrients and micronutrients to the offspring, other biological molecules could influence how the offspring utilizes its nutritional supply, and hence its developmental trajectory. However, the volume and composition (including hormone content) of breast milk that maximizes maternal Darwinian fitness is not the same that maximizes the offspring’s fitness (3). The offspring may also influence maternal biology, for example through the strength of the suckling response.
Therefore, breastfeeding can be viewed as a dynamic process which involves complex physiological and psychosocial signaling or communication between the mother and the offspring (4).
Human milk contains numerous components that could act as signals between the mother and offspring including hormones, bacteria, nutrients, and growth factors. These components may interact with the infant’s cells, tissues, and organs, triggering various signaling pathways and physiological responses. Among the underlying mechanisms could be epigenetic modifications, including DNA methylation, histone modification, and microRNA effects, and impacts on the establishment of the infant microbiome (5). There are various steps that apply to any component being considered as a signal in milk such as the origin of milk components, whether they come from the mother’s circulation or are synthesized in the breast (or both), if the milk components reach the infant intestine or if they have specific gut receptors. Additional steps include if the milk components are absorbed and influence infant outcomes and whether maternal or environmental factors influence the concentration of milk components. This systematic review focuses on whether maternal and environmental factors influence the concentration of one group of milk components – milk hormones.
Previously, Andreas et al. (6) conducted a systematic review which indicated that there was an association between the concentration of leptin in breast milk and maternal BMI in ten out of fifteen studies (6). Furthermore, a narrative review undertaken in 2016 highlighted reported evidence to support the role of specific bioactive components and explained that several maternal factors such as BMI have been proposed to influence levels of these bioactive components in breast milk (7). In this review, leptin provided the clearest indication that maternal BMI was positively associated with leptin concentrations in breast milk.
The purpose of this review was to systematically search the literature for evidence on maternal and environmental factors that influence the concentration of hormones in human milk.
Observational studies and randomized controlled trials (RCTs) that reported on the association between maternal factors and breast milk hormones were eligible for inclusion. Data from RCTs were included only if they reported associations between an exposure (maternal factors) and subsequent measures of milk hormones. Full text studies published in English were included. Studies reported only in abstract form were omitted.
Eligible participants included human mother-infant pairs. Studies in which most (>50%) of the infants were exclusively or predominantly breast-fed at the time of sampling were eligible in order to isolate the potential association of maternal factors on breast milk composition. There were no restrictions on participant health status; this was due to the research interest being in how mothers signal their condition and experiences to their offspring, including markers of living conditions, lifestyle, ill health, and good health. Studies in animals were excluded.
The exposure was any maternal factor related to the mother’s condition, health, lifestyle, living condition or environment at any time period. It was expected that this would include factors such as maternal anthropometry, adiposity, gestational diabetes, age, ethnicity, socioeconomic status, stress, smoking or climate. Any time period was chosen because of the potential of the factors influencing outcomes via various mechanisms such as epigenetic programming and regulation of gene expression which can potentially affect human milk composition.
Studies were considered eligible if they reported on at least one breast milk hormone. It was expected that this would include cortisol, leptin, insulin, ghrelin, adiponectin, prolactin, oxytocin or resistin. Studies analyzing these hormones in colostrum, transitional or mature milk at any time-point were considered eligible.
Studies were identified by searching electronic databases with no limits on date of publication. The electronic databases searched were MEDLINE Ovid (from 1946), EMBASE Ovid (from 1974) and Cumulative Index of Nursing and Allied Health Literature (CINAHL).
The search strategy included database-specific search terms and medical subject headings (MeSH) terms with Boolean operators (NOT, AND, OR) were used on synonyms and variations of the terms relating to human milk, hormones, and maternal factors. The search terms were: “breast milk” OR “breastmilk” OR “human milk” OR “breastfeeding” OR “lactation” OR “breastfed” OR “breastfeed” OR “breast fed” OR “breast feed” OR “milk, human” OR “breast feeding” AND “hormones” OR “hormone concentrations” OR “hormonal concentrations” OR “hormone profile” OR “hormones profile” OR “hormone” OR “leptin” OR “insulin” OR “ghrelin” OR “cortisol” OR “prolactin” OR “oxytocin” OR “resistin” OR “adiponectin” OR “thyroid” OR “interleukin-6” OR “tum?r necrosis factor-a” OR “hydrocortisone” AND “parental factors” OR “maternal factors” OR “environmental factors” OR “stress” OR “inflammation” OR “BMI” OR “body mass index” OR “maternal BMI” OR “maternal body mass index” OR “obesity” OR “type 2 diabetes” OR “type 2 diabetes mellitus” OR “T2D” OR “diabetes” OR “diabetes mellitus” OR “university education” OR “weight” OR “height” OR “maternal weight” OR “maternal height.” Searches were limited to human studies. The search was run on 19th June 2023. An example search of MEDLINE can be found in Appendix A.
Duplications were removed and references were imported to Covidence for screening according to the eligibility criteria. Titles were screened by RQ, then abstracts and full-texts were screened by two authors independently (RQ and SD). Any discrepancies between the reviewers were discussed to reach consensus.
Data to be extracted was agreed by the research team. One reviewer (RQ) independently extracted the data from each study. Extracted data included author name, date of publication, sample size, location of study, study design, feeding type and duration, human milk hormones assessed, stage and type of milk analyzed, how the milk sample was processed for analysis, how the samples were obtained, the maternal factors assessed, results of associations, correlation coefficients and confounders adjusted.
One independent reviewer (RQ) used the revised Downs and Black Quality Index score system (Appendix B), known to be a reliable and valid tool for assessing bias in observational and randomized studies to assess the quality of individual publications (8). The quality assessment tool provided an overall score based on four assessed domains; reporting, external validity, internal validity bias and internal validity confounding. Each domain had an overall total score out of 10, 3, 7 and 6, respectively. Item 27 relating to the statistical power was given a score of 1when a power analysis had been conducted. Thus, the highest possible score for the checklist was 28 (9). The quality assessment can be found in Appendix C.
It was not possible to conduct a meta-analysis on the relationship between maternal factors and human milk composition due to highly heterogenous data for both maternal factors and the hormonal composition of human milk. As a result, the data was synthesized narratively and presented in tables.
The database searches identified 6,493 studies, of which 4,994 titles were screened following the removal of duplicates. Four hundred ninety abstracts were screened for appropriateness; 398 studies were excluded based on inappropriate title. The full text of 92 studies were reviewed; 59 studies were excluded after the review. This left 33 studies suitable for inclusion in the systematic review. A Preferred Reporting for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram is presented in Figure 1.
Study characteristics are summarized in Table 1. All included studies were conducted in high-income countries with the exception of two conducted in Turkey (11, 35) and one in Iran (20), both classified as upper-middle-income economies. The majority of studies were published in the last 8 years (n = 29), except for 5 studies published in 2002, 2006, 2007, 2011 and 2014 (10, 25, 34–36). The main type of study design was observational (n = 29), with 4 studies consisting of data from randomized controlled trials (10, 13, 18, 24). Most of the studies had a sample size of 100 or less (n = 26) with the largest study including 767 participants (36).
Human milk components analyzed included concentrations of adiponectin (n = 15), leptin (n = 26), insulin (n = 12), cortisol (n = 4) and ghrelin (n = 2). Maternal factors assessed included BMI (n = 23), fat mass (n = 7), weight (n = 6), maternal age (n = 3), gestational diabetes (n = 4), smoking (n = 2) and ‘other’ including hip, waist and mid-upper arm circumference, ethnicity, carbohydrate intake, energy intake, psychological stress, and mode of delivery (n = 7). Data for all eligible studies including the methods and analysis of sample collection are summarized in Table 1. Mature milk was the main type of milk sample analyzed (n = 33), with three studies also analyzing colostrum and one study analyzing transitional milk.
Twenty-three studies examined the relationship between maternal body mass index (BMI) and human milk composition in exclusively or predominantly breastfed infants. The studies investigated associations between maternal BMI pre-, during- and post-pregnancy and adiponectin, leptin, insulin, cortisol, and ghrelin concentrations in milk as shown in Table 2. Among the ten studies focusing on adiponectin, two demonstrated a positive association at 2 weeks and 1–3 months (38, 39), while one reported a negative association stronger at 1 month than 3 months (39). In 20 studies, leptin concentrations, analyzed in twenty-one studies, generally showed a positive correlation with maternal BMI post-pregnancy with higher levels in overweight and obese mothers. For insulin, six studies indicated a positive correlation with maternal BMI post-pregnancy, particularly strong at 3 months, with higher levels in overweight and obese mothers. Cortisol, analyzed in two studies, exhibited a positive correlation with maternal BMI. Ghrelin concentrations, studied in two investigations, displayed an inverse association in one study, while the other found no significant correlation with maternal BMI post-pregnancy. Only three studies among the twenty-three adjusted for potential confounding factors (12, 14, 32).
Seven studies examined the relationship between maternal fat mass and human milk composition, focusing on adiponectin (n = 5), leptin (n = 6), insulin (n = 2), cortisol (n = 1), and ghrelin (n = 1) as summarized in Table 3. Notably, none of the studies adjusted for potential confounding factors. Regarding adiponectin, only one study reported a significant negative association with fat mass at 6 months (20), while the 4 others found no significant correlations. In the case of leptin, all six studies identified significant associations, with three indicating a positive link between maternal fat mass and leptin concentrations (20, 21, 33). For insulin, one study reported a positive association, while another found no correlation (17). The sole study on cortisol showed no association with maternal fat mass, and the only study on ghrelin also found no correlation.
Six studies explored the relationship between maternal weight and human milk composition in predominantly breastfed infants, focusing on adiponectin (n = 2), leptin (n = 3), insulin (n = 4), and cortisol (n = 1), as detailed in Table 4. Only three studies accounted for potential confounding factors (17, 28, 33). Concerning adiponectin, the two available studies found no correlation with maternal weight. Regarding leptin, all three studies identified significant associations. For insulin, two out of four studies reported a significant association between maternal weight and insulin levels, with one study noting elevated insulin levels in overweight mothers (17). The lone study on cortisol found that normal-weight mothers had higher levels of milk cortisol than mothers with overweight/obesity.
Three studies investigated the relationship between maternal age and human milk composition, specifically examining adiponectin (n = 1), leptin (n = 2), insulin (n = 1), and cortisol (n = 2), as shown in Table 5. Only two studies accounted for potential confounding factors (12, 28). Regarding adiponectin, a single study found no correlation between maternal age and adiponectin concentrations in human milk (12). In the case of leptin, one study revealed that the concentration of leptin was lower in older women. For insulin, a lone study found no correlation between maternal age and insulin levels in human milk. Two studies explored the association between maternal age and cortisol, with both studies observing no correlation (28, 41).
Four studies investigated the link between pre-gestational and gestational maternal diabetes and human milk composition, examining adiponectin (n = 2), leptin (n = 2), insulin (n = 3), cortisol (n = 1), and ghrelin (n = 1), as detailed in Table 6. Notably, only two studies adjusted for potential confounding factors (13, 28). Regarding adiponectin, one study found a significant negative association with gestational diabetes mellitus in both colostrum and mature milk, while another study found no association (13, 39). For leptin, both studies observed no correlation with maternal diabetes (13, 39). All three studies exploring insulin concentrations reported significant associations with maternal diabetes, with one study revealing lower insulin levels in mothers with gestational diabetes (13) and another indicating that women with type 2 diabetes mellitus had twice the milk insulin levels compared to those with gestational diabetes and normal glucose tolerance (29). The sole study on cortisol found no correlation between maternal diabetes and cortisol in human milk (28) while the single study on ghrelin reported a significant negative correlation with gestational diabetes in both colostrum and mature milk (39).
Two studies investigated the relationship between maternal smoking and human milk composition, focusing on adiponectin (n = 2), leptin (n = 2), and insulin (n = 1), as summarized in Table 7. Both studies adjusted for potential confounding factors. However, no significant associations were observed between maternal smoking and adiponectin, leptin, or insulin. Notably, one of the studies found that levels of adiponectin were higher in the milk of non-smoking mothers, suggesting a potential impact of smoking on this specific milk component (36).
Table 8 presents a summary of findings on the associations between the hormonal composition of human milk and other maternal factors. Seven studies explored the relationships between these factors and adiponectin (n = 3), leptin (n = 4), insulin (n = 2), and cortisol (n = 3), with two studies adjusting for potential confounding factors. Regarding adiponectin, a study found lower levels in Asian mothers compared to Caucasian mothers (12), while another reported a positive association with changes in carbohydrate and total energy intake (24). However, a study observed no significant association with maternal body composition (19). For leptin, previous research identified positive correlations with hip, waist, and mid-upper arm circumferences at different postpartum time points (11). No associations were observed with ethnicity, maternal body composition, and carbohydrate and energy intake in other studies. In terms of insulin, Chan et al. (12) reported higher levels in Asian mothers compared to Caucasians, while another study found no association with maternal carbohydrate and energy intake (12). For cortisol, Romijn et al. (30) noted lower levels in mothers seeking psychiatric consultation compared to healthy controls, and another study found no association with postpartum depression score or perceived stress (41). Pundir et al. (41) observed no association between mode of delivery and cortisol levels at 3 months.
This systematic review explored the relationship between different maternal factors and hormones in breast milk, as a first step to establishing their role in signaling mechanisms between mother and infant. The review of 33 papers suggests a positive association between maternal adiposity (BMI and weight either pre-pregnancy and during lactation) and breast milk leptin concentrations (Figure 2). However, the evidence regarding maternal fat mass, age, smoking, and other factors was inconclusive. The review underscores the need for more research in this area, emphasizing the inconsistency in findings, likely due to variations in data collection and sampling methods across studies.
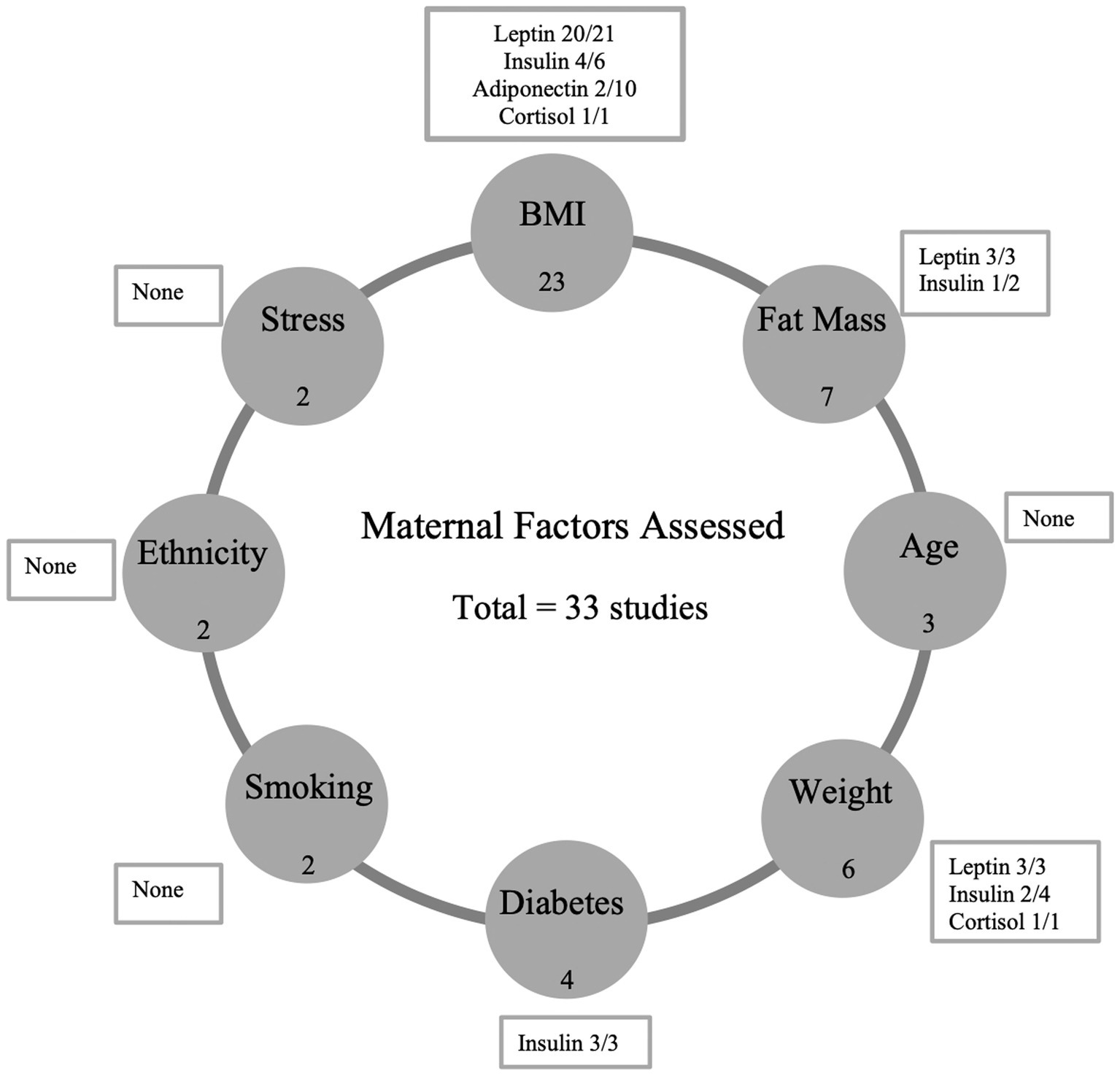
Figure 2. Summary of results. This figure shows the positive associations found between specific maternal factors and the concentration of breast milk hormones in this systematic review. The number of studies included which reported each maternal factor (i.e., BMI -23) are represented in filled circles. The number of studies that found a positive association between the maternal factor and hormone are shown in textboxes (i.e., Leptin 20 studies found a positive association out of a total of 21 studies). BMI, body mass index.
Maternal adiposity was assessed using a variety of measures in different studies. There was a positive association between maternal BMI and breast milk leptin and insulin in the majority of studies included with only 2/10 studies showing a positive association between maternal BMI and adiponectin. This aligns with a previous systematic review (6) by Andreas et al. (6) which also found an association of maternal BMI with breast milk leptin but not adiponectin (6). A 2016 narrative review further highlighted the positive association between maternal BMI and leptin and insulin levels in human breast milk (7). We also found that maternal weight was positively associated with milk leptin in all included studies, but its relationship with milk insulin was less clear. Inconsistent associations were also observed between maternal fat mass and milk leptin. It is possible that the associations of milk hormones with BMI were stronger than with fat mass due to the ease with which BMI could be assessed and thus the larger sample sizes in studies using this outcome.
Leptin in breast milk could serve as a signaling mechanism for the infant, affecting aspects such as metabolism, appetite, and fat storage. The leptin gene (LEP), which is responsible for the production of leptin, is expressed in mammary epithelial cells and can be influenced by many factors including maternal diet, nutritional status and hormone regulation (42). Since leptin signals the level of fat reserves to the brain, maternal leptin transfer to the infant could act to inflate such signals, effectively manipulating the infant’s brain into over-estimating its fat stores and thus impact its appetite. While studies have indicated that higher leptin in breast milk might indeed reduce infant appetite, this could have both positive and negative implications (32). Positive outcomes include better self-regulation, more appropriate feeding patterns and reduced long term obesity risk, while negatives could be inadequate nutrition and suboptimal growth. Similarly, higher insulin levels in milk were previously found to be associated with lower infant weight and weight-for-length z-scores (14). However, other studies found no link between milk insulin and infant anthropometrics.
This review confirmed that maternal diabetes is associated with significantly higher insulin levels in breast milk. Maternal insulin regulation can be disrupted in type 2 diabetes, leading to increased insulin levels in maternal circulation and potentially influencing its presence in breast milk. A study showed that women with type 2 diabetes had significantly higher insulin levels in their breast milk compared to those with gestational diabetes and normal glucose tolerance, possibly due to insulin therapy and injections (28). Limited research exists on the impact of breast milk constituents on the growth of infants born to mothers with diabetes during pregnancy, especially gestational diabetes. Previous data suggests that breast milk from diabetic mothers may lead to increased relative body weight and obesity at two years of age, while milk from healthy non-diabetic women had a beneficial effect on later body weight and glucose tolerance in childhood (43).
Aging is associated with many changes in the levels of several hormones in maternal plasma which could have an effect on breast milk hormone levels. For example, Isidori et al. (44) indicated that serum leptin gradually declines during aging but is independent from BMI and other age-related endocrine changes. However, only one study included in this review found that the concentration of leptin was lower in older women (12) with the remaining studies reporting no association between milk hormones and maternal age.
The effect of smoking on breast milk hormones might be expected to vary depending on factors such as smoking intensity, duration, and individual differences (45). Research indicates that smoking ten or more cigarettes a day can adversely affect lactation by reducing milk production and altering macronutrient content (45). Smoking during pregnancy and lactation can also impact maternal health, increasing stress and anxiety levels, potentially altering breast milk composition and hormone levels (43). Nonetheless, this review only included two studies on maternal smoking and breast milk hormones, one reporting higher levels of adiponectin in the milk of non-smoking mothers (36), while the other (14) found no association, likely due to low smoking rates in their study population. This highlights the need for further research to comprehensively understand how smoking influences breast milk hormonal composition and its impact on infant outcomes.
Ethnicity could theoretically influence hormone levels in breast milk due to a combination of genetic, cultural, environmental and lifestyle factors that vary among different ethnic groups. However, this review only found one study that mentioned ethnicity in relation to milk hormone levels, concluding that Asian mothers had lower levels of adiponectin and higher levels of insulin in their milk when compared to Caucasian mothers (12). This difference could reflect factors such as body composition as ethnic groups can have distinct body composition characteristics which could impact hormone production and metabolism (46). In addition, cultural dietary practices and certain foods and nutrients vary among different ethnic groups. Diets with anti-inflammatory properties, such as the Mediterranean diet and others emphasizing plant-based foods and healthy fats, have demonstrated the ability to lower leptin levels in circulating blood and enhance leptin sensitivity (47). Conversely, heightened intakes of saturated fatty acids have been linked to inducing leptin resistance by interrupting leptin signaling after chronic overstimulation of the leptin receptor (48). However, research on the specific differences in breast milk composition among various ethnicities is limited.
The review included two studies on post-partum depression and stress in relation to breast milk cortisol levels. One study reported that mothers in a psychiatry-obstetric-pediatric clinic had lower milk cortisol compared to those not in the clinic (30). However, this study had limitations, as it focused on a specific population with a higher risk of psychological distress during pregnancy. On the other hand, another study did not find a significant association between maternal depression and breast milk cortisol (29). Chronic stress can affect the hypothalamic–pituitary–adrenal (HPA) axis, potentially leading to elevated cortisol levels in breast milk. While previous studies have explored the connection between maternal psychological factors and milk cortisol levels, they have yielded inconsistent results. Objective assessments of maternal cortisol levels in plasma appear to provide a more reliable measure of chronic stress compared to subjective methods used in the studies reviewed.
The results from this systematic review suggest that the mother may communicate important cues through the hormonal composition of breast milk. The studies consistently revealed the presence of diverse hormones such as adiponectin, leptin, insulin, cortisol, and ghrelin in breast milk. The varying concentration of these hormones (specifically leptin and insulin) in relation to maternal factors such as BMI, weight and other health indicators highlight an intricate interplay between maternal physiology and breast milk composition. This supports the notion that maternal factors are somewhat linked to the hormonal makeup of breast milk in particular maternal BMI and weight, which, in turn, could potentially impact infant outcomes such as growth, development and overall health. Notably, associations between maternal nutritional status and offspring development, mediated by milk hormones, have recently been reported in primates (49). Breast milk hormones could potentially influence infant outcomes through epigenetic mechanisms such as changes in the gene expression that could be triggered by various environmental, nutritional and hormonal factors during pregnancy and lactation (50).
However, there are six steps that should be fulfilled for a component to act as a signal (Figure 3), with the results from this review contributing evidence toward one step only in establishing whether a hormone is a signaling component, by assessing which maternal factors affect their concentration in human breast milk. This review did not find any studies assessing the association between maternal factors and hormones such as prolactin, oxytocin and resistin. The lack of current evidence found does not rule out a signaling role of these hormones. Instead, it highlights a notable gap in the existing literature in relation to other hormones within breast milk. In addition, this review solely focused on hormones as potential signaling components, but there are many other possible signals in milk such as bacteria, nutrients, and growth factors. These other components could be investigated using the same approach as this systematic review.
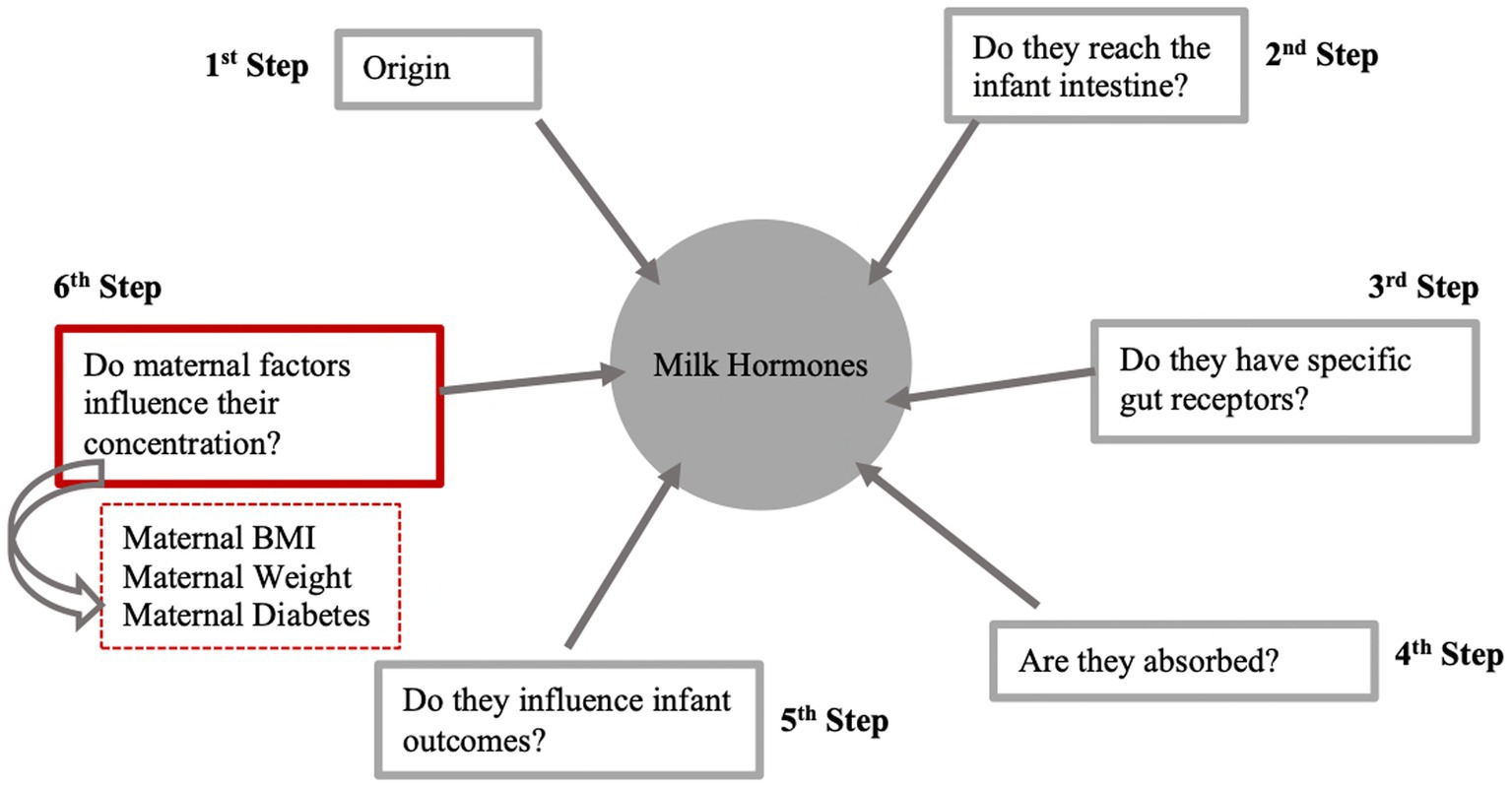
Figure 3. Six steps to determine whether a milk hormone acts as a signal between mother and offspring. This figure summarizes the results from this systematic review which aimed to obtain evidence for the sixth stage of the process. BMI, body mass index. Figure adapted from Fewtrell et al., (4), licensed under CC BY 4.0.
This systematic review has highlighted limitations stemming from inadequate study design, hindering our understanding of how maternal factors affect the composition of bioactive components in breast milk. The complexity of maternal influences, both genetic and environmental, makes it challenging to draw causal inferences, as randomizing subjects based on many relevant factors is practically impossible. New study designs are needed, such as long-term observational studies that follow mother-infant pairs over time or clustering them by similar characteristics like BMI. Targeted interventions, focusing on factors like maternal diet or stress reduction, can provide insights into how specific changes impact breast milk composition.
Additionally, the variability in sampling methods among different studies, including lactation stage, feeding frequency, and time of day, complicates direct comparisons of findings. Inconsistencies may arise from the lack of standardization in collecting and processing human milk samples. For instance, some hormones show diurnal patterns influenced by the time of day, and variations in sampling techniques, such as analyzing foremilk or hindmilk, can affect results. Notably, the quality of the studies reviewed was generally low or fair, and many studies lacked reporting on maternal factors or breastfeeding patterns, making it difficult to assess the strength of the associations between maternal factors and breast milk hormone concentrations.
This systematic review suggests that higher maternal BMI is linked to increased breast milk leptin and maternal diabetes to higher breast milk insulin. However, it fails to establish clear associations between maternal fat mass, age, smoking, ethnicity, stress, and breast milk hormonal composition due to insufficient data and methodological limitations in prior studies. The use of standardized protocols for sample collection and analysis in future studies would enable more meaningful cross-study comparisons. Further research is required to understand how breast milk hormones affect infant outcomes and their role as signaling components. In conclusion, this study underscores the complex relationship between maternal factors, breast milk composition, and potential infant signaling mechanisms, serving as a starting point for future investigations.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
RQ: Writing – original draft. MF: Writing – review & editing. JW: Writing – review & editing. SD: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1390232/full#supplementary-material
1. Horta, B., and Victora, C. (2013). Short-term effects of breastfeeding – a systematic review on the benefits of breastfeeding on diarrhoea and pneumonia mortality. Available at: https://apps.who.int/iris/bitstream/handle/10665/95585/9789241506120_eng.pdf
2. Golan, Y, and Assaraf, YG. Genetic and physiological factors affecting human Milk production and composition. Nutrients. (2020) 12:1500. doi: 10.3390/nu12051500
3. Wells, JCK. Breast-feeding as ‘personalized nutrition’. Eur J Clin Nutr. (2018) 72:1234–8. doi: 10.1038/s41430-018-0206-y
4. Fewtrell, MS, Mohd Shukri, NH, and Wells, JCK. “Optimising” breastfeeding: what can we learn from evolutionary, comparative and anthropological aspects of lactation? BMC Med. (2020) 18:4. doi: 10.1186/s12916-019-1473-8
5. Woo, V, and Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes. (2022) 14:2407. doi: 10.1080/19490976.2021.2022407
6. Andreas, NJ, Hyde, MJ, Gale, C, Parkinson, J, Jeffries, S, Holmes, E, et al. Effect of maternal body mass index on hormones in breast Milk: a systematic review. PLoS One. (2014) 9:e115043–3. doi: 10.1371/journal.pone.0115043
7. Fields, DA, Schneider, CR, and Pavela, G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity. (2016) 24:1213–21. doi: 10.1002/oby.21519
8. Downs, SH, and Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. (1998) 52:377–84. doi: 10.1136/jech.52.6.377
9. Hindiyeh, NA, Zhang, N, Farrar, M, Banerjee, P, Lombard, L, and Aurora, SK. The role of diet and nutrition in migraine triggers and treatment: a systematic literature review. Pain. (2020) 60:1300–16. doi: 10.1111/head.13836
10. Brunner, S, Schmid, D, Zang, K, Much, D, Knoeferl, B, Kratzsch, J, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. (2014) 10:67–73. doi: 10.1111/j.2047-6310.2014.222.x
11. Çağiran Yilmaz, F, and Özçelik, AÖ. The relationships between leptin levels in maternal serum and breast milk of mothers and term infants. Ann Med. (2021) 53:1310–6. doi: 10.1080/07853890.2021.1964037
12. Chan, D, Goruk, S, Becker, AB, Subbarao, P, Mandhane, PJ, Turvey, SE, et al. Adiponectin, leptin and insulin in breast milk: associations with maternal characteristics and infant body composition in the first year of life. Int J Obes. (2017) 42:36–43. doi: 10.1038/ijo.2017.189
13. Choi, Y, Nagel, E, Kharoud, H, Johnson, K, Gallagher, T, Duncan, K, et al. Gestational diabetes mellitus is associated with differences in human Milk hormone and cytokine concentrations in a fully breastfeeding United States cohort (2022) 14:667–7. doi: 10.3390/nu14030667
14. Christensen, SH, Lewis, JI, Larnkjær, A, Frøkiær, H, Allen, LH, Mølgaard, C, et al. Associations between maternal adiposity and appetite-regulating hormones in human milk are mediated through maternal circulating concentrations and might affect infant outcomes. Front Nutr. (2022) 9:1025439. doi: 10.3389/fnut.2022.1025439
15. Cortés-Macías, E, Selma-Royo, M, Rio-Aige, K, Bäuerl, C, Rodríguez-Lagunas, MJ, Martínez-Costa, C, et al. Distinct breast milk microbiota, cytokine, and adipokine profiles are associated with infant growth at 12 months: an in vitro host–microbe interaction mechanistic approach. Food Funct. (2023) 14:148–59. doi: 10.1039/d2fo02060b
16. de Luca, A, Frasquet-Darrieux, G, Gaud, M-A, Christin, P, Boquien, C-Y, Millet, C, et al. Higher leptin but not human Milk macronutrient concentration distinguishes Normal-weight from obese mothers at 1-month postpartum. PLoS One. (2016) 11:e0168568–8. doi: 10.1371/journal.pone.0168568
17. Ellsworth, L, Perng, W, Harman, E, Das, A, Pennathur, S, and Gregg, B. Impact of maternal overweight and obesity on milk composition and infant growth. Matern Child Nutr. (2020) 16:e12979. doi: 10.1111/mcn.12979
18. Enstad, S, Cheema, S, Thomas, R, Fichorova, RN, Martin, CR, O’Tierney-Ginn, P, et al. The impact of maternal obesity and breast milk inflammation on developmental programming of infant growth. Eur J Clin Nutr. (2020) 75:180–8. doi: 10.1038/s41430-020-00720-5
19. Gridneva, Z, Kugananthan, S, Rea, A, Lai, C, Ward, L, Murray, K, et al. Human milk adiponectin and leptin and infant body composition over the first 12 months of lactation. Nutrients. (2018) 10:1125. doi: 10.3390/nu10081125
20. Khodabakhshi, A, Mehrad-Majd, H, Vahid, F, and Safarian, M. Association of maternal breast milk and serum levels of macronutrients, hormones, and maternal body composition with infant’s body weight. Eur J Clin Nutr. (2018) 72:394–400. doi: 10.1038/s41430-017-0022-9
21. Kugananthan, S, Gridneva, Z, Lai, C, Hepworth, A, Mark, P, Kakulas, F, et al. Associations between maternal body composition and appetite hormones and macronutrients in human Milk. Nutrients. (2017) 9:252. doi: 10.3390/nu9030252
22. Larson-Meyer, DE, Schueler, J, Kyle, E, Austin, KJ, Hart, AM, and Alexander, BM. Appetite-regulating hormones in human milk: a plausible biological factor for obesity risk reduction? J Human Lactation. (2021) 37:603–14. doi: 10.1177/0890334420954160
23. Larsson, M, Lind, M, Larnkjær, A, Due, A, Blom, I, Wells, J, et al. Excessive weight gain followed by catch-down in exclusively breastfed infants: an exploratory study. Nutrients. (2018) 10:1290. doi: 10.3390/nu10091290
24. Leghi, GE, Netting, MJ, Lai, CT, Narayanan, A, Dymock, M, Rea, A, et al. Reduction in maternal energy intake during lactation decreased maternal body weight and concentrations of leptin, insulin and adiponectin in human milk without affecting milk production, milk macronutrient composition or infant growth. Nutrients. (2021) 13:1892. doi: 10.3390/nu13061892
25. Miralles, O, Sánchez, J, Palou, A, and Picó, C. A physiological role of breast Milk leptin in body weight control in developing infants*. Obesity. (2006) 14:1371–7. doi: 10.1038/oby.2006.155
26. Nuss, H, Altazan, AD, Zabaleta, J, Sothern, M, and Redman, LM. Maternal pre-pregnancy weight status modifies the influence of PUFAs and inflammatory biomarkers in breastmilk on infant growth. PLoS One. (2019) 14:e0217085–5. doi: 10.1371/journal.pone.0217085
27. Pundir, S, Gridneva, Z, Pillai, A, Thorstensen, EB, Wall, CR, Geddes, DT, et al. Human milk glucocorticoid levels are associated with infant adiposity and head circumference over the first year of life. Front Nutr. (2020) 7:166. doi: 10.3389/fnut.2020.00166
28. Pundir, M, Mäkelä, J, Nuora, A, Junttila, N, Wall, CR, Linderborg, K, et al. Maternal influences on the glucocorticoid concentrations of human milk: the STEPS study. Clin Nutr. (2019) 38:1913–20. doi: 10.1016/j.clnu.2018.06.980
29. Rodel, R, Farabi, SS, Hirsch, N, Rolloff, KP, McNair, B, Hernandez, TL, et al. Human milk imparts higher insulin concentration in infants born to women with type 2 diabetes mellitus. J Maternal Fetal Neonatal Med. (2021) 35:7676–84. doi: 10.1080/14767058.2021.1960967
30. Romijn, M, Tilburg, V, Hollanders, JJ, Van, PDG, Dolman, KM, Heijboer, AC, et al. The association between maternal stress and glucocorticoid rhythmicity in human Milk. Nutrients. (2021) 13:1608–8. doi: 10.3390/nu13051608
31. Sadr Dadres, G, Whitaker, KM, Haapala, JL, Foster, L, Smith, KD, Teague, AM, et al. Relationship of maternal weight status before, during, and after pregnancy with breast milk hormone concentrations. Obesity. (2019) 27:621–8. doi: 10.1002/oby.22409
32. Savino, F, Sardo, A, Rossi, L, Benetti, S, Savino, A, and Silvestro, L. Mother and infant body mass index, breast Milk leptin and their serum leptin values. Nutrients. (2016) 8:383. doi: 10.3390/nu8060383
33. Schneider-Worthington, CR, Bahorski, JS, Fields, DA, Gower, BA, Fernández, JR, and Chandler-Laney, PC. Associations among maternal adiposity, insulin, and Adipokines in circulation and human milk. J Hum Lact. (2020) 37:714–22. doi: 10.1177/0890334420962711
34. Schuster, S, Hechler, C, Gebauer, C, Kiess, W, and Kratzsch, J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. (2011) 70:633–7. doi: 10.1203/pdr.0b013e31823214ea
35. Uysal, FK, Önal, EE, Aral, YZ, Adam, B, Dilmen, U, and Ardiçolu, Y. Breast milk leptin: its relationship to maternal and infant adiposity. Clin Nutr. (2002) 21:157–60. doi: 10.1054/clnu.2001.0525
36. Weyermann, M, Brenner, H, and Rothenbacher, D. Adipokines in human Milk and risk of overweight in early childhood. Epidemiology. (2007) 18:722–9. doi: 10.1097/ede.0b013e3181567ed4
37. Young, B, Levek, C, Reynolds, R, Rudolph, MC, MacLean, PS, Hernandez, TL, et al. Bioactive components in human milk are differentially associated with rates of lean and fat mass deposition in infants of mothers with normal vs. elevated BMI. Pediatr Obes. (2018) 13:598–606. doi: 10.1111/ijpo.12394
38. Young, BE, Patinkin, Z, Palmer, C, de la Houssaye, B, Barbour, LA, Hernandez, T, et al. Human milk insulin is related to maternal plasma insulin and BMI: but other components of human milk do not differ by BMI. Eur J Clin Nutr. (2017) 71:1094–100. doi: 10.1038/ejcn.2017.75
39. Yu, X, Rong, SS, Sun, X, Ding, G, Wan, W, Zou, L, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: a longitudinal study. British J Nutr. (2018) 120:1380–7. doi: 10.1017/S0007114518002933
40. Zamanillo, R, Sánchez, J, Serra, F, and Palou, A. Breast Milk supply of MicroRNA associated with leptin and adiponectin is affected by maternal overweight/obesity and influences infancy BMI. Nutrients. (2019) 11:2589. doi: 10.3390/nu11112589
41. Zielinska-Pukos, M, Bryś, J, Kucharz, N, Chrobak, A, Wesolowska, A, Grabowicz-Chądrzyńska, I, et al. Factors influencing cortisol concentrations in breastmilk and its associations with breastmilk composition and infant development in the first six months of lactation. Int J Environ Res Public Health. (2022) 19:14809. doi: 10.3390/ijerph192214809
42. Sinkiewicz-Darol, E, Adamczyk, I, Łubiech, K, Pilarska, G, and Twarużek, M. Leptin in human milk—one of the key regulators of nutritional programming. Molecules. (2022) 27:3581. doi: 10.3390/molecules27113581
43. Plagemann, A, Harder, T, Franke, K, and Kohlhoff, R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. (2002) 25:16–22. doi: 10.2337/diacare.25.1.16
44. Isidori, AM, Strollo, F, Morè, M, Caprio, M, Aversa, A, Moretti, C, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metabol. (2000) 85:1954–62. doi: 10.1210/jcem.85.5.6572
45. Mennella, JA, Yourshaw, LM, and Morgan, LK. Breastfeeding and smoking: short-term effects on infant feeding and sleep. Pediatrics. (2007) 120:497–502. doi: 10.1542/peds.2007-0488
46. Pereira, B, Figueiredo, B, Pinto, TM, and Míguez, MC. Effects of tobacco consumption and anxiety or depression during pregnancy on maternal and neonatal health. Int J Environ Res Public Health. (2020) 17:8138. doi: 10.3390/ijerph17218138
47. Heymsfield, SB, Peterson, CM, Thomas, DM, Heo, M, and Schuna, JM. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. (2015) 17:262–75. doi: 10.1111/obr.12358
48. Engin, A. Diet-induced obesity and the mechanism of leptin resistance. Obesity Lipotoxicity. (2017):381–97. doi: 10.1007/978-3-319-48382-5_16
49. Petrullo, L, Hinde, K, and Lu, A. Steroid hormone concentrations in milk predict sex-specific offspring growth in a nonhuman primate. Am J Hum Biol. (2019) 31:e23315. doi: 10.1002/ajhb.23315
Keywords: breast milk, maternal factors, breast milk hormones, parent-offspring signaling, breast milk composition
Citation: Qureshi R, Fewtrell M, Wells JCK and Dib S (2024) The association between maternal factors and milk hormone concentrations: a systematic review. Front. Nutr. 11:1390232. doi: 10.3389/fnut.2024.1390232
Received: 22 February 2024; Accepted: 14 June 2024;
Published: 03 July 2024.
Edited by:
Francisco José Pérez-Cano, University of Barcelona, SpainReviewed by:
Estefanía Diéguez, Ghent University, BelgiumCopyright © 2024 Qureshi, Fewtrell, Wells and Dib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary Fewtrell, bS5mZXd0cmVsbEB1Y2wuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.