
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 04 July 2024
Sec. Nutrition, Psychology and Brain Health
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1384489
Background: Growing evidence suggests a link between vitamin K (VK) intake and depression, although the underlying mechanisms remain unclear. We aimed to investigate whether oxidative balance scores (OBS) mediate the association between VK intake and depression in participants from the National Health and Nutrition Examination Survey (NHANES) 2007–2018.
Methods: We analyzed data from 30,408 individuals. Dietary VK intake served as the independent variable, depression symptoms as the outcome variable, and OBS as the mediator. Multivariable logistic regression and restricted cubic splines assessed the associations. Mediation analysis was conducted to evaluate the potential mediating role of OBS.
Results: Higher dietary VK intake was associated with lower depression risk in the multivariate model. Compared to the lowest log2 VK quartile, those in the higher quartiles had significantly lower depression odds (Q3: OR 0.66, 95% CI 0.55–0.78; Q4: OR 0.64, 95% CI 0.52–0.78). Additionally, a 1-unit increase in log2 VK intake was associated with a 15% decrease in depression odds (OR 0.85, 95% CI 0.81–0.90). Restricted cubic splines revealed a non-linear relationship between log2 VK and depression (p for non-linearity <0.001). Notably, OBS mediated 26.09% (p < 0.001) of the association between log2 VK and depression.
Conclusion: Higher VK intake is associated with reduced depression risk, potentially mediated by oxidative balance. Further research is warranted to confirm causality and elucidate the underlying mechanisms.
Current evidence indicates that depression has emerged as a significant contributor to the global burden of disease, with a lifetime risk ranging from 15–18% (1). According to the World Health Organization (WHO), depression is poised to become the predominant cause of years lived with disability worldwide by 2030 (2). Despite notable advancements in medical treatment, approximately one-third of patients do not achieve remission, despite the wide range of antidepressants available (1, 3). In recent years, there has been a growing interest in preventive approaches centered around dietary interventions, particularly involving vitamins (4, 5). In this respect, there has been an increased focus on vitamin K (VK) and its potential health benefits. A study involving female and overweight elderly Japanese individuals found an association between a deficiency in vitamin K and depressive symptoms (6). Additionally, high vitamin K supplementation has been demonstrated to significantly reduce the risk of depression in older North American individuals (7). Another study in Japan, focusing on the elderly, indicated a correlation between depression and insufficient vitamin K levels (8). A recent extensive study involving a large sample found a negative and independent correlation between vitamin K intake and the likelihood of experiencing depressive symptoms among adults in the United States (9). However, the specific mechanism through which vitamin K influences depression remains inadequately explored.
The burgeoning interest in the link between oxidative stress (OS) and depression centers upon the oxidative stress hypothesis. This hypothesis posits that an imbalance between the generation of reactive oxygen species (ROS) and the body’s antioxidant defenses can negatively impact brain structure and contribute to the development of depression (10–12). This imbalance, or level of oxidative stress, arises from a complex interplay of various factors, including dietary, lifestyle, and genetic elements. To holistically capture the combined effect of these diverse factors, the oxidative balance score (OBS) metric was developed (13). Higher OBS values generally correspond to greater antioxidant capacity within the body. Vitamin K possesses documented antioxidant and anti-inflammatory properties, shielding cells from oxidative stress (14, 15). Based on these properties, we hypothesized that vitamin K intake could influence depressive symptoms by modulating the level of oxidative stress reflected by oxidative balance scores as a marker of oxidative balance.
To validate the hypothesis that vitamin K intake is inversely associated with the risk of depressive symptoms in adults in the US, the present study utilized data from the National Health and Nutrition Examination Survey (NHANES). Furthermore, our study aimed to investigate whether oxidative balance scores mediate this association.
This study utilized data from the NHANES, an ongoing investigation into the nutrition and health status of the American population (Retrieved from https://www.cdc.gov/nchs/nhanes.htm on 15 January 2024). Before participating in the study, all individuals provided written consent. The analysis focused on information gathered from 59,842 participants during the NHANES surveys conducted from 2007 to 2018. Following the exclusion of participants under 18 years old and those lacking data on vitamin K intake, Patient Health Questionnaire-9 (PHQ-9), and observation parameters (OBS), the final cohort comprised 30,408 participants (Figure 1).
In this study, we assessed depressive symptoms using PHQ-9, which aligns with the diagnostic criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (16). Participants were rated on a scale ranging from 0 to 3, where 0 represented “not at all,” 1 denoted “several days,” 2 indicated “more than half the days,” and 3 reflected “nearly every day” (17). A cumulative PHQ-9 score equal to or greater than 10 was indicative of depression (18). In the present study the Cronbach’s α value was 0.839.
To determine the daily dietary intake of vitamin K, we computed the average intake based on two 24-h dietary recall interviews sourced from the NHANES database (19). In instances where participants only provided data for either the first or second 24-h dietary recall, this study relied on the available one-day data. The assessment of data quality and integrity involved using the “Dietary recall status code” in NHANES, and the total VK intake data were extracted from the “Total Nutrient Intakes Files.”
The Oxidative Balance Score (OBS), adapted from Zhang et al. (20), assesses overall oxidative stress levels by analyzing sixteen dietary and four lifestyle components known to influence it (13). The dietary components are divided into two groups: dietary antioxidants and dietary pro-oxidants. The dietary antioxidants include fiber, β-carotene, riboflavin, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, and selenium. Participants are categorized into tertiles based on the distribution of these components in the study population. Those in tertile 1 to tertile 3 are assigned 0 to 2 points, respectively. Conversely, for dietary pro-oxidants such as total fat and iron, the scoring is reversed, with participants in tertile 1 receiving 2 points and those in tertile 3 scoring 0 points. The lifestyle components also consist of two groups: lifestyle antioxidants and lifestyle pro-oxidants. Physical activity is considered a lifestyle antioxidant, and participants are categorized as having low, moderate, or high physical activity levels based on the 2018 Physical Activity Guidelines for Americans (21). They are assigned 0, 1, or 2 points accordingly. Alcohol consumption is a lifestyle pro-oxidant and is scored based on sex-specific levels: consuming nonalcoholic drinks is assigned 2 points, consuming 0-15 g per day for women or 0-30 g per day for men is assigned 1 point, and consuming ≥15 g per day for women or ≥ 30 g per day for men is assigned 0 points. Smoking status is estimated using cotinine levels and assigned points ranging from 0 to 2, with higher scores indicating a higher level of smoking. Body Mass Index (BMI) is categorized as normal, overweight, or obese and assigned 2, 1, or 0 points, respectively.
In this study, we identified several variables that could potentially influence the relationship between vitamin K intake, OBS, and depressive symptoms, including gender, age, race, education level, marital status, family income, diabetes, hypertension, and total energy intake. The variable representing family income in this study was expressed as the ratio of family income to poverty. “Has your doctor diagnosed you with diabetes?” was used to assess diabetes prevalence. “Has your physician diagnosed you with hypertension?” was used as to ascertain the presence/absence of hypertension.
Sample weights were computed based on the NHANES analysis guide. Continuous variables with normal distributions were summarized as mean ± standard deviation, while those with non-normal distributions were presented as median (interquartile range). Categorical variables were expressed as percentages. Due to the skewed distribution of dietary VK intake, the data were log2-transformed and then categorized into quartiles (Q1, Q2, Q3, and Q4). Differences between continuous variables were assessed for statistical significance using one-way analysis of variance (ANOVA) for normally distributed data or the Kruskal-Wallis test for skewed distributions. The rank-sum test evaluated differences between groups of categorical variables. A p-value <0.05 was statistically significant. Logistic regression models were employed to investigate the relationship between VK intake and depressive symptoms. Linear regression models explored the associations between OBS and depression, as well as VK and OBS. Three regression models were developed: Model 1 (unadjusted), Model 2 (adjusted for age, gender, race, education level, marital status, and family income), and Model 3 (adjusted for all covariables). Restricted cubic splines (RCS) were further utilized to identify potential non-linear associations between VK intake and depression.
To assess the potential mediating role of OBS in the association between log2VK and depression, we employed mediation analysis through the R package “mediation.” Five thousand bootstrap iterations, a well-established method for robust confidence interval estimation, minimized bias. We controlled for potential confounders by adjusting our analysis for gender, age, race, education level, marital status, family income, diabetes, hypertension, and total energy intake. Our analysis estimated the indirect effect size (βindirect), direct effect size (βdirect), total effect size (βtotal), proportion mediated (PM), and associated p values.
Data analysis was conducted using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was determined using a two-tailed alpha level of 0.05.
Our analysis of 30,408 individuals (representing 222,496,148 US population) revealed a mean age of 46.6 years (SE = 0.26; range: 18–80) with female predominance (51.2%). The prevalence of depression was 9.08%. Compared to higher intake quartiles, participants in the lowest dietary vitamin K quartile (Q1) were younger. While the prevalence of diabetes and hypertension remained comparable across quartiles, statistically significant differences emerged in terms of gender, race, education levels, marital status, and family income (Table 1).
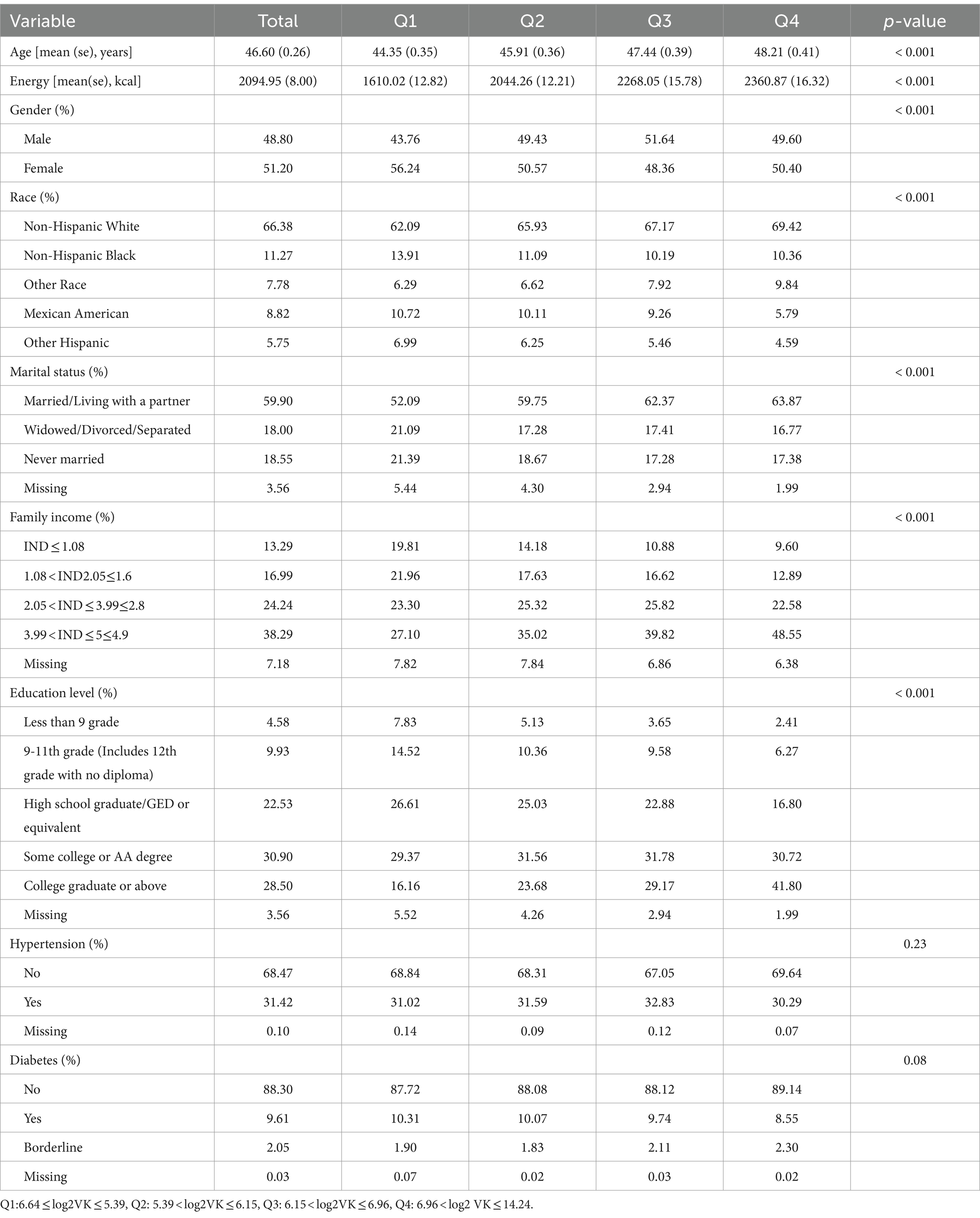
Table 1. Characteristics of 30,408 participants, National Health and Nutrition Examination Survey, the United States, 2007–2018.
To assess the association between vitamin K supplementation and depression, three statistical models were implemented. Each model yielded consistent findings, albeit with varying levels of granularity. In all models, increased log2 dietary VK intake was associated with a significant decrease in depression risk. Model 1 revealed a 22% reduction (OR: 0.78, 95% CI: 0.75–0.81) per unit increase in log2 VK intake, while Models 2 and 3 demonstrated a 14% (OR: 0.86, 95% CI: 0.83–0.90) and 15% (OR: 0.85, 95% CI: 0.81–0.90) decrease, respectively. Interestingly, Model 1 and Model 3 further indicated a dose-dependent effect, with higher quartiles of VK intake associated with progressively larger reductions in depression risk: [Model 1: Q2 (25%), Q3 (47%), and Q4 (55%)], [Model 3: Q2 (12%), Q3 (34%), and Q4 (36%)]. However, Models 2 did not show a statistically significant decrease in depression for the Q2 group compared to Q1 (Table 2).
Restricted cubic splines showed a nonlinear trend (L-shape) in the association of log2VK with the risk of depressive symptoms (P for nonlinearity <0.001; Figure 2).
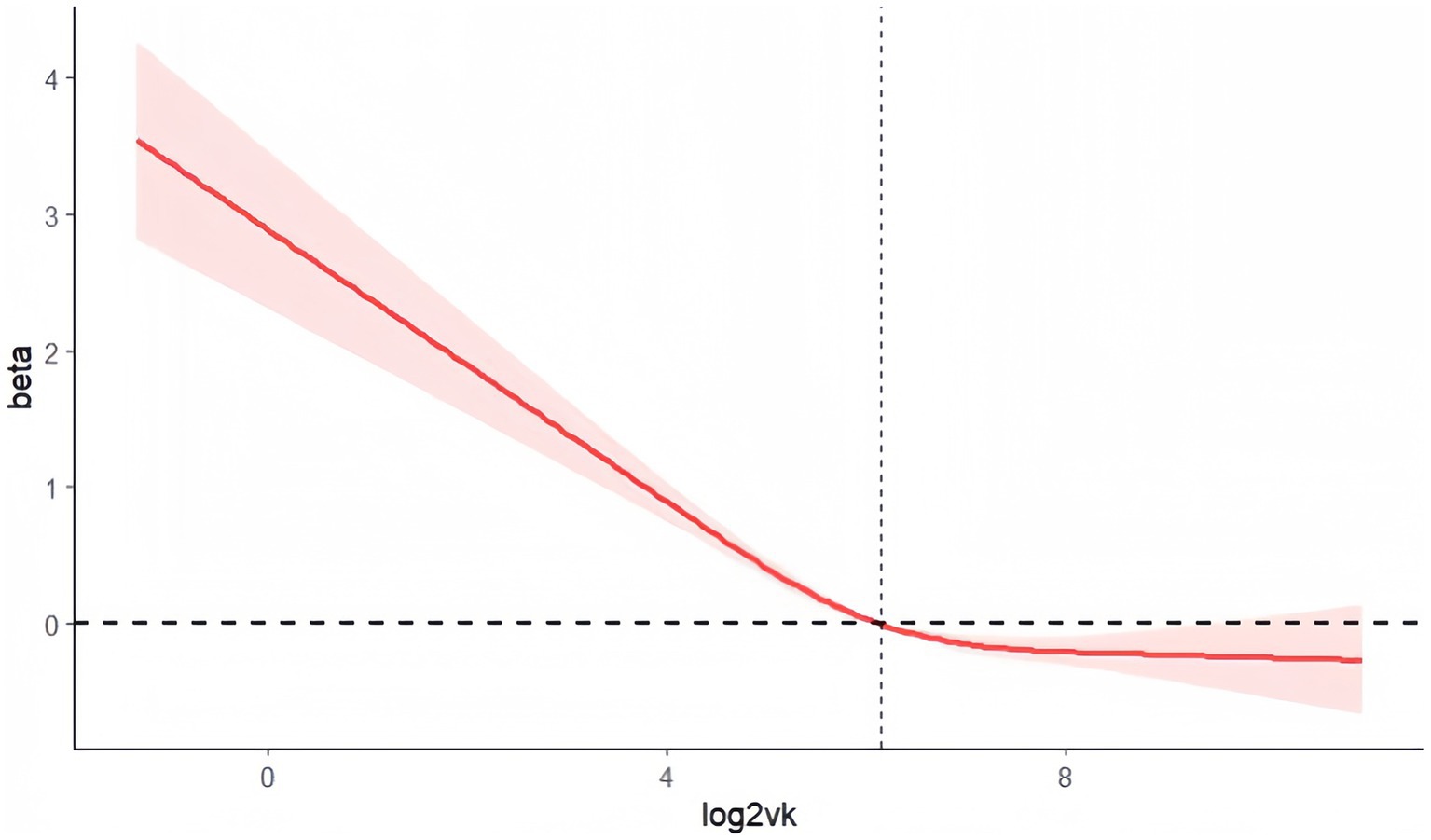
Figure 2. Analysis of restricted cubic spline regression. Legend: Adjusted restricted cubic spline models adjusted for Analysis of restricted cubic spline regression. Adjusted restricted cubic spline models adjusted for age, sex, race, marital status, education, poverty, diabetes, hypertension, and total energy intake. Label: P for nonlinear <0.001.
As shown in Table 3, linear regression analysis revealed a significant inverse association between OBS and depressive scores. Increased OBS scores were associated with lower depression scores in both the multivariable model (β coefficient − 0.04, 95% CI −0.05 to −0.03, p < 0.001) and individual quartile comparisons. Compared to the lowest OBS quartile (Q1), participants in Q2, Q3, and Q4 exhibited progressively lower depression scores (Q2: β −0.30, 95% CI −0.52 to −0.09, p = 0.01; Q3: β −0.54, 95% CI −0.77 to −0.31, p < 0.001; Q4: β −0.96, 95% CI −1.21 to −0.72, p < 0.001). These findings suggest a stronger protective effect of higher OBS against depressive symptoms.
Table 4 shows the associations between log2VK and OBS based on linear regressions. We found that higher levels of log2VK were associated with higher OBS.
OBS was found to be a significant mediator (accounting for 26.09%) of the association between log2VK intake and depression scores, as demonstrated through mediation analyses (p < 0.001) (Table 5; Figure 3).
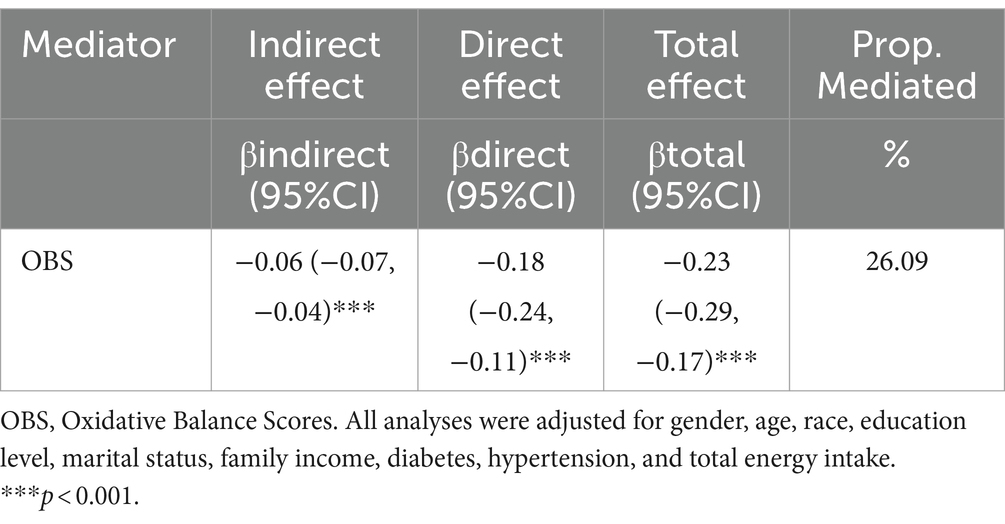
Table 5. Mediation analyses with OBS between log2vk and depression scores in US adult population, NHANES 2007–2018.
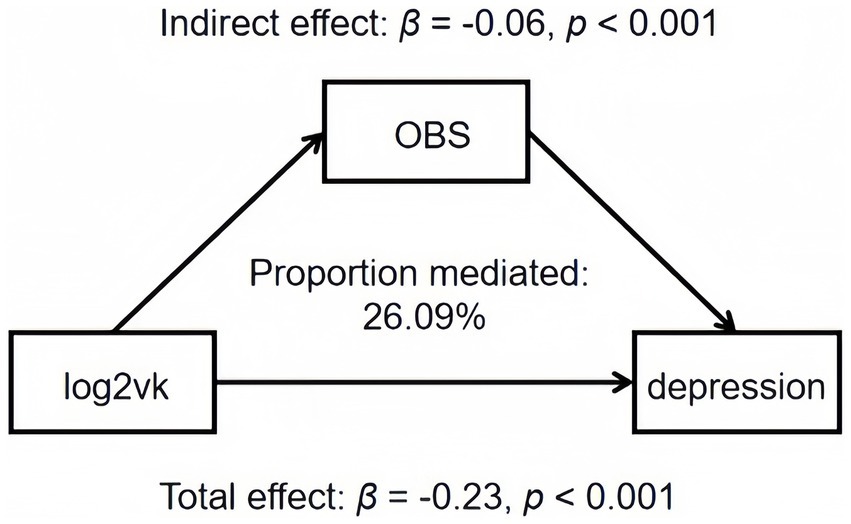
Figure 3. Estimated proportion of the association between log2vk and depression mediated by OBS. Models were adjusted for agender, age, race, education level, marital status, family income, diabetes, hypertension, and total energy intake. Proportion mediated (PM) = βindirect/βtotal. Abbreviation: OBS, oxidative balance score.
The present study sheds preliminary light on the protective effect of dietary vitamin K against depression in adults aged 18–80. Despite controlling for confounding factors, the observed negative correlation between VK intake and depression displayed an L-shaped curve, indicating diminishing protective effects beyond a specific intake threshold. Moreover, this study provides the first evidence that OBS mediates the relationship between vitamin K and depression. This pioneering study opens avenues for further exploration of the interplay between VK intake, OBS, and depressive symptoms.
An increasing body of literature suggests a link between dietary vitamin K intake and depression across distinct populations. Large-scale studies have observed this negative association in adults aged 45–49 (7) and the Japanese elderly (8), with recent data further confirming a similar inverse correlation in adults in the US (9). This study contributes by extending this association to a broader age range, encompassing adults from 18 to 80 years old. However, conflicting observations exist, with one study in Spanish children reporting a positive association between vitamin K supplementation and depression (22), potentially highlighting the influence of age-specific effects and confounding factors.
There are several potential mechanisms underlying vitamin K’s antidepressant effect: Firstly, growing body of evidence highlighting elevated proinflammatory cytokine expression in both peripheral and central tissues of depression patients (23–26). Notably, the NF-kB signaling pathway plays a pivotal role in governing cytokine release (27), and interestingly, VK’s inhibitory effect on NF-kB activation has been documented (28). This suggests that VK’s anti-inflammatory properties may partially explain its protective effect against depression. Secondly, vitamin K plays a role in sphingolipid metabolism (29), with sphingolipids, particularly ceramides, recognized as markers of depression (30). Enhancing sphingolipid metabolism may ameliorate depressive symptoms (31). Research in animals indicates that inadequate vitamin K intake correlates with elevated ceramide levels in the hippocampus (32). Therefore, vitamin K may alleviate depression by enhancing the metabolism of sphingolipids, particularly ceramide. Thirdly, VK’s influence on intestinal flora could potentially regulate its antidepressant activity. VK has been shown to modulate gut microbiota (33), with emerging evidence emphasizing the crucial role of intestinal flora in modulating depressive symptoms via the brain-gut axis (34). Fourthly, VK3 has been demonstrated to inhibit monoamine oxidase (MAO) (35), a key player in depression pathogenesis (1). However, the L-shaped association observed between log2 VK and depression risk may be attributed to the synergistic effects of VK’s mitigation of NF-kB signaling and potential saturation of MAO activity. Finally, Gas-6 is a vitamin K-dependent protein extensively expressed in the nervous system, exerting anti-inflammatory effects, promoting the survival of hippocampal neurons, and regulating microglial survival (29, 36). Gas-6 induced neuronal pathways therapy holds promise for clinical significance in treating depression (37).
Numerous studies have explored the relationship between diet and depression. A recent meta-analysis has uncovered a significant inverse relationship between total dietary fiber consumption and the likelihood of depression in adults. Specifically, for every additional 5 grams of dietary fiber intake, there is a corresponding 5% decrease in the risk of developing depression (38). Another meta-analysis confirmed that both vitamin C and E supplementation are inversely associated with depression (39). Ferriani’s study of Brazilian adults identified that lower intake of vitamin B complex (B6, folate and B12) was associated with depression (40). In additional, intake of carotene and vitamin A have been proven to be negatively correlated with depression (41). Research on the relationship between dietary calcium and depression is relatively limited. However, a recent large-scale study in the American population has found a negative correlation between dietary calcium intake and depression (42). Dietary magnesium is negatively correlated with depression in a dose–response manner (43). Moreover, insufficient magnesium intake has been confirmed as a significant cause of treatment-resistant depression (44). Additionally, zinc, copper, iron and selenium have each been found to reduce the risk of depression (45, 46). The connection between a pro-oxidant lifestyle and the onset of depression has been well-established. The relationship between alcohol consumption levels and the risk of depression appears to follow a J- or U-shaped pattern. Moderate alcohol intake may alleviate depressive symptoms, whereas excessive consumption can heighten the risk of depression (47). Smoking increases the risk of depression (48), and quitting smoking can alleviate depression in individuals with or without psychiatric disorders (49). The relationship between obesity and depression is bidirectionalthe presence of one increases the risk for developing the other (50). Recent Mendelian studies have found a positive correlation and causal relationship between BMI and depression (51). On the contrary, a meta-analysis reveals that engaging in physical activity bestows substantial mental health advantages, affirming that even activity levels that fall short of public health guidelines can significantly mitigate depression (52).
Our mediation analysis revealed a compelling link between vitamin K intake and reduced depression scores, mediated by a 26.09% reduction in oxidative stress as measured by the OBS. This finding supports the hypothesis that increased vitamin K intake may alleviate depression by mitigating oxidative stress. Evidence substantiates this mechanism: Yuan et al. (53) observed in vivo increases in antioxidant capacity through vitamin K’s regulation of pro-oxidant and antioxidant enzymes. In another study, continuous regeneration of KH2, a potent free radical scavenger, was documented upon vitamin K supplementation in vivo (54). Additionally, activation of the Nrf2 antioxidant pathway, a cellular defense system against oxidative stress, has been documented following vitamin K administration (55, 56). The Nrf2 pathway is a cellular defense mechanism that helps protect cells from oxidative stress by increasing the expression of genes involved in antioxidant defense and detoxification (57). As mentioned previously, oxidative stress are important cause of depression. In recent large-scale studies, Liu et al. and Li et al. found negative correlations between OBS and depression (12, 58). Several studies have found that depressed patients and animals have significantly reduced levels of antioxidants, and increased peroxidation biomarkers (59–61). In addition, antidepressant agents can mitigate the pathogenesis of depressive disorders by upregulating the expression of antioxidant defense enzymes, further confirming the protective effect of antioxidant capacity on depression (62). This study provides valid evidence that antioxidant capacity may mediate the association between vitamin K and depression.
This study boasts several unique strengths: it pioneers the investigation of OBS mediation in the vitamin K-depression link, leverages a nationally representative sample for robust generalizability, and employs a comprehensive OBS metric capturing the influence of both dietary and lifestyle factors on individuals’ antioxidant status.
Despite the promising findings, recognizing the study’s limitations is crucial. The cross-sectional design precludes definitive conclusions about causality between dietary vitamin K and depression. Additionally, our reliance on 24-h dietary and the data collection method of OBS recalls introduces potential recall and measurement bias that may cloud the accuracy of the observed associations. Lastly, the possibility of unaddressed confounders such as pollutants, drugs, fatty acids and other diseases influencing the results cannot be dismissed. This underscores the need for further research employing robust methodologies to clarify the causal relationship and elucidate the underlying mechanisms.
This study demonstrates an L-shaped relationship between dietary vitamin K supplementation and depression in adults aged 18–80. Notably, OBS appears to partially mediate this association, suggesting a potential mechanism that warrants further investigation. The study suggests that dietary supplementation of VK may help reduce depressive symptoms. Additionally, maintaining an antioxidant-rich diet and behavioral patterns can contribute to the antidepressant effects of VK. To establish this link and elucidate its underlying biological pathways, rigorous longitudinal studies are imperative.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/nhanes.htm.
The studies involving humans were approved by National Health and Nutrition Examination Survey. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
LW: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. SH: Writing – review & editing. ZF: Data curation, Resources, Writing – review & editing. JX: Data curation, Software, Writing – original draft. GL: Project administration, Supervision, Writing – review & editing. YZ: Supervision, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We acknowledge the staff of the National Center for Health Statistics at the CDC, who designed, collected, and administered the NHANES data and released the data available for public use. We are thankful to all study participants for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malhi, GS, and Mann, JJ. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
2. Collaborators, G, and Nomura, S. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
3. Köhler, S, Sterzer, P, Normann, C, Berger, M, and Brakemeier, E. Overcoming treatment resistance in chronic depression: the role of inpatient psychotherapy. Nervenarzt. (2016) 87:701–7. doi: 10.1007/s00115-015-0034-4
4. Ano, Y, Kitaoka, S, Ohya, R, Kondo, K, and Furuyashiki, T. Hop bitter acids increase hippocampal dopaminergic activity in a mouse model of social defeat stress. Int J Mol Sci. (2020) 21:9612. doi: 10.3390/ijms21249612
5. Huang, X, Fan, Y, Han, X, Huang, Z, Yu, M, Zhang, Y, et al. Association between serum Vitamin levels and depression in U.S. adults 20 years or older based on National Health and nutrition examination survey 2005(−)2006. Int J Environ Res Public Health. (2018) 15:15. doi: 10.3390/ijerph15061215
6. Nguyen, TTT, Tsujiguchi, H, Kambayashi, Y, Hara, A, Miyagi, S, Yamada, Y, et al. Relationship between Vitamin intake and depressive symptoms in elderly Japanese individuals: differences with gender and body mass index. Nutrients. (2017) 9:1319. doi: 10.3390/nu9121319
7. Bolzetta, F, Veronese, N, Stubbs, B, Noale, M, Vaona, A, Demurtas, J, et al. The relationship between dietary Vitamin K and depressive symptoms in late adulthood: a cross-sectional analysis from a large cohort study. Nutrients. (2019) 11:11. doi: 10.3390/nu11040787
8. Azuma, K, Osuka, Y, Kojima, N, Sasai, H, Kim, H, and Inoue, S. Association of Vitamin K Insufficiency as evaluated by serum Undercarboxylated osteocalcin with depressive symptoms in community-dwelling older adults. Am J Geriatr Psychiatry. (2022) 30:1051–2. doi: 10.1016/j.jagp.2022.04.012
9. Zhang, Y, Tan, W, Xi, X, Yang, H, Zhang, K, Li, S, et al. Association between vitamin K intake and depressive symptoms in US adults: data from the National Health and nutrition examination survey (NHANES) 2013-2018. Front Nutr. (2023) 10:1102109. doi: 10.3389/fnut.2023.1102109
10. Bhatt, S, Nagappa, AN, and Patil, CR. Role of oxidative stress in depression. Drug Discov Today. (2020) 25:1270–6. doi: 10.1016/j.drudis.2020.05.001
11. Shafiee, M, Ahmadnezhad, M, Tayefi, M, Arekhi, S, Vatanparast, H, Esmaeili, H, et al. Depression and anxiety symptoms are associated with prooxidant-antioxidant balance: a population-based study. J Affect Disord. (2018) 238:491–8. doi: 10.1016/j.jad.2018.05.079
12. Li, H, Song, L, Cen, M, Fu, X, Gao, X, Zuo, Q, et al. Oxidative balance scores and depressive symptoms: mediating effects of oxidative stress and inflammatory factors. J Affect Disord. (2023) 334:205–12. doi: 10.1016/j.jad.2023.04.134
13. Hernández-Ruiz, Á, García-Villanova, B, Guerra-Hernández, EJ, Carrión-García, CJ, Amiano, P, Sánchez, M-J, et al. Oxidative balance scores (OBSs) integrating nutrient, food and lifestyle dimensions: development of the NutrientL-OBS and FoodL-OBS. Antioxidants. (2022) 11:11. doi: 10.3390/antiox11020300
14. Nuszkiewicz, J, Sutkowy, P, Wróblewski, M, Pawłowska, M, Wesołowski, R, Wróblewska, J, et al. Links between Vitamin K, Ferroptosis and SARS-CoV-2 infection. Antioxidants. (2023) 12:733. doi: 10.3390/antiox12030733
15. Ivanova, D, Zhelev, Z, Getsov, P, Nikolova, B, Aoki, I, Higashi, T, et al. Vitamin K: redox-modulation, prevention of mitochondrial dysfunction and anticancer effect. Redox Biol. (2018) 16:352–8. doi: 10.1016/j.redox.2018.03.013
16. Costantini, L, Pasquarella, C, Odone, A, Colucci, ME, Costanza, A, Serafini, G, et al. Screening for depression in primary care with patient health Questionnaire-9: a systematic review. J Affect Disord. (2021) 279:473–83. doi: 10.1016/j.jad.2020.09.131
17. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
18. McCall, WV, Blocker, JN, D'Agostino, R Jr, Kimball, J, Boggs, N, Lasater, B, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. (2010) 6:322–9. doi: 10.5664/jcsm.27872
19. Wang, A, Zhao, M, Luo, J, Zhang, T, and Zhang, D. Association of Dietary Vitamin K Intake with Cognition in the elderly. Front Nutr. (2022) 9:900887. doi: 10.3389/fnut.2022.900887
20. Zhang, W, Peng, SF, Chen, L, Chen, HM, Cheng, XE, and Tang, YH. Association between the oxidative balance score and telomere length from the National Health and nutrition examination survey 1999-2002. Oxidative Med Cell Longev. (2022) 2022:1345071–11. doi: 10.1155/2022/1345071
21. Piercy, KL, Troiano, RP, Ballard, RM, Carlson, SA, Fulton, JE, Galuska, DA, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
22. Rubio-López, N, Morales-Suárez-Varela, M, Pico, Y, Livianos-Aldana, L, and Llopis-González, A. Nutrient intake and depression symptoms in Spanish children: the ANIVA study. Int J Environ Res Public Health. (2016) 13:13. doi: 10.3390/ijerph13030352
23. Köhler, CA, Freitas, TH, Maes, M, de Andrade, NQ, Liu, CS, Fernandes, BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
24. Syed, SA, Beurel, E, Loewenstein, DA, Lowell, JA, Craighead, WE, Dunlop, BW, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. (2018) 99:914–924.e3. doi: 10.1016/j.neuron.2018.08.001
25. Miller, AH, and Raison, CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2015) 16:22–34. doi: 10.1038/nri.2015.5
26. Cui, W, Ning, Y, Hong, W, Wang, J, Liu, Z, and Li, MD. Crosstalk between inflammation and glutamate system in depression: signaling pathway and molecular biomarkers for Ketamine’s antidepressant effect. Mol Neurobiol. (2019) 56:3484–500. doi: 10.1007/s12035-018-1306-3
27. Jiang, Y, Xu, S, Lan, J, Zhang, J, and Chen, T. Dietary Vitamin K intake and HPV-infection status among American women: a secondary analysis from National Health and nutrition examination survey data from 2003 to 2016. Int J Public Health. (2022) 67:1604616. doi: 10.3389/ijph.2022.1604616
28. Xia, J, Matsuhashi, S, Hamajima, H, Iwane, S, Takahashi, H, Eguchi, Y, et al. The role of PKC isoforms in the inhibition of NF-κB activation by vitamin K2 in human hepatocellular carcinoma cells. J Nutr Biochem. (2012) 23:1668–75. doi: 10.1016/j.jnutbio.2011.11.010
29. Ferland, G. Vitamin K and brain function. Semin Thromb Hemost. (2013) 39:849–55. doi: 10.1055/s-0033-1357481
30. Tomasik, J, Harrison, SJ, Rustogi, N, Olmert, T, Barton-Owen, G, Han, SYS, et al. Metabolomic biomarker signatures for bipolar and unipolar depression. Jama. Psychiatry. (2024) 81:101–6. doi: 10.1001/jamapsychiatry.2023.4096
31. Dinoff, A, Herrmann, N, and Lanctôt, KL. Ceramides and depression: a systematic review. J Affect Disord. (2017) 213:35–43. doi: 10.1016/j.jad.2017.02.008
32. Carri, I, Blanger, E, Portoukalian, J, Rochford, J, and Ferland, G. Lifelong low-Phylloquinone intake is associated with cognitive impairments in old rats. J Nutr. (2011) 141:1495–501. doi: 10.3945/jn.110.137638
33. Lai, Y, Masatoshi, H, Ma, Y, Guo, Y, and Zhang, B. Role of Vitamin K in intestinal health. Front Immunol. (2022) 12:12. doi: 10.3389/fimmu.2021.791565
34. Hao, WZ, Li, XJ, Zhang, PW, and Chen, JX. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. (2020) 284:112691. doi: 10.1016/j.psychres.2019.112691
35. Coelho Cerqueira, E, Netz, PA, Diniz, C, Petry Do Canto, V, and Follmer, C. Molecular insights into human monoamine oxidase (MAO) inhibition by 1,4-naphthoquinone: evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Bioorg Med Chem. (2011) 19:7416–24. doi: 10.1016/j.bmc.2011.10.049
36. Ferland, G. Vitamin K, an emerging nutrient in brain function. Biofactors. (2012) 38:151–7. doi: 10.1002/biof.1004
37. Reemst, K, Kracht, L, Kotah, JM, Rahimian, R, van Irsen, AAS, Congrains Sotomayor, G, et al. Early-life stress lastingly impacts microglial transcriptome and function under basal and immune-challenged conditions. Transl Psychiatry. (2022) 12:507. doi: 10.1038/s41398-022-02265-6
38. Saghafian, F, Hajishafiee, M, Rouhani, P, and Saneei, P. Dietary fiber intake, depression, and anxiety: a systematic review and meta-analysis of epidemiologic studies. Nutr Neurosci. (2023) 26:108–26. doi: 10.1080/1028415X.2021.2020403
39. Ding, J, and Zhang, Y. Associations of dietary Vitamin C and E intake with depression. A Meta-analysis of observational studies. Front Nutr. (2022) 9:857823. doi: 10.3389/fnut.2022.857823
40. Ferriani, LOSDMM. Associations of depression and intake of antioxidants and vitamin B complex: results of the Brazilian longitudinal study of adult health (ELSA-Brasil). J Affect Disord. (2022) 297:259–68. doi: 10.1016/j.jad.2021.10.027
41. Zhang, Y, Ding, J, and Liang, J. Associations of dietary Vitamin a and Beta-carotene intake with depression. A Meta-analysis of observational studies. Front Nutr. (2022) 9:881139. doi: 10.3389/fnut.2022.881139
42. Shen, X, Gu, X, Liu, Y-Y, Yang, L, Zheng, M, and Jiang, L. Association between dietary calcium and depression among American adults: national health and nutrition examination survey. Front Nutr. (2023) 10:1042522. doi: 10.3389/fnut.2023.1042522
43. Hajhashemy, Z, Shirani, F, and Askari, G. Dietary magnesium intake in relation to depression in adults: a GRADE-assessed systematic review and dose-response Meta-analysis of epidemiologic studies. Nutr Rev. (2024):nuae056. doi: 10.1093/nutrit/nuae056
44. Eby, GA, and Rd, EK. Magnesium for treatment-resistant depression: a review and hypothesis. Med Hypotheses. (2010) 74:649–60. doi: 10.1016/j.mehy.2009.10.051
45. Ding, J, and Zhang, Y. Associations of dietary copper, selenium, and manganese intake with depression: a Meta-analysis of observational studies. Front Nutr. (2022) 9:854774. doi: 10.3389/fnut.2022.854774
46. Li, Z, Li, B, Song, X, and Zhang, D. Dietary zinc and iron intake and risk of depression: a meta-analysis. Psychiatry Res. (2017) 251:41–7. doi: 10.1016/j.psychres.2017.02.006
47. Nunes, EV. Alcohol and the etiology of depression. Am J Psychiatry. (2023) 180:179–81. doi: 10.1176/appi.ajp.20230004
48. Bakhshaie, J, Zvolensky, MJ, and Goodwin, RD. Cigarette smoking and the onset and persistence of depression among adults in the United States: 1994-2005. Compr Psychiatry. (2015) 60:142–8. doi: 10.1016/j.comppsych.2014.10.012
49. Wu, AD, Gao, M, Aveyard, P, and Taylor, G. Smoking cessation and changes in anxiety and depression in adults with and without psychiatric disorders. JAMA Netw Open. (2023) 6:e2316111. doi: 10.1001/jamanetworkopen.2023.16111
50. Milaneschi, Y, Simmons, WK, van Rossum, EFC, and Penninx, BWJH. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
51. Karageorgiou, V, Casanova, F, O’Loughlin, J, Green, H, McKinley, TJ, Bowden, J, et al. Body mass index and inflammation in depression and treatment-resistant depression: a Mendelian randomisation study. BMC Med. (2023) 21:355. doi: 10.1186/s12916-023-03001-7
52. Pearce, M, Garcia, L, Abbas, A, Strain, T, Schuch, FB, Golubic, R, et al. Association between physical activity and risk of depression: a systematic review and Meta-analysis. JAMA Psychiatry. (2022) 79:550–9. doi: 10.1001/jamapsychiatry.2022.0609
53. Yuan, JM, Feng, L, Jiang, WD, Liu, Y, Jiang, J, Li, SH, et al. Effects of dietary vitamin K levels on growth performance, enzyme activities and antioxidant status in the hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Nutr. (2016) 22:352–66. doi: 10.1111/anu.12264
54. Chen, A, Li, J, Shen, N, Huang, H, and Hang, Q. Vitamin K: new insights related to senescence and cancer metastasis. Biochim Biophys Acta Rev Cancer. (2023) 1879:189057. doi: 10.1016/j.bbcan.2023.189057
55. El-Sherbiny, M, Atef, H, Helal, GM, Al-Serwi, RH, Elkattawy, HA, Shaker, GA, et al. Vitamin K2 (MK-7) intercepts Keap-1/Nrf-2/HO-1 pathway and hinders inflammatory/apoptotic signaling and liver aging in naturally aging rat. Antioxidants. (2022) 11:2150. doi: 10.3390/antiox11112150
56. Lv, J, Hou, B, Song, J, Xu, Y, and Xie, S. The relationship between Ferroptosis and diseases. J Multidiscip Healthc. (2022) 15:2261–75. doi: 10.2147/JMDH.S382643
57. Muchtaridi, M, Amirah, SR, Harmonis, JA, and Ikram, EHK. Role of nuclear factor erythroid 2 (Nrf2) in the recovery of long COVID-19 using natural antioxidants: a systematic review. Antioxidants. (2022) 11:11. doi: 10.3390/antiox11081551
58. Liu, X, Liu, X, Wang, Y, Zeng, B, Zhu, B, and Dai, F. Association between depression and oxidative balance score: National Health and nutrition examination survey (NHANES) 2005-2018. J Affect Disord. (2023) 337:57–65. doi: 10.1016/j.jad.2023.05.071
59. Visentin, APV, Colombo, R, Scotton, E, Fracasso, DS, da Rosa, AR, Branco, CS, et al. Targeting inflammatory-mitochondrial response in major depression: current evidence and further challenges. Oxidative Med Cell Longev. (2020) 2020:1–20. doi: 10.1155/2020/2972968
60. Juszczyk, G, Mikulska, J, Kasperek, K, Pietrzak, D, Mrozek, W, and Herbet, M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer's disease: The role of antioxidants in prevention and treatment. Antioxidants. (2021) 10:10. doi: 10.3390/antiox10091439
61. Ji, N, Lei, M, Chen, Y, Tian, S, Li, C, and Zhang, B. How oxidative stress induces depression? ASN Neuro. (2023) 15:15. doi: 10.1177/17590914231181037
Keywords: depression, vitamin K, oxidative balance scores (OBS), NHANES, mediation analysis
Citation: Wang L, Huang S, Feng Z, Xiao J, Luo G and Zhang Y (2024) Assessing the role of antioxidant and pro-oxidant balance in mediating the relationship between vitamin K intake and depressive symptoms in adults. Front. Nutr. 11:1384489. doi: 10.3389/fnut.2024.1384489
Received: 09 February 2024; Accepted: 25 June 2024;
Published: 04 July 2024.
Edited by:
Khadijeh Irandoust, Imam Khomeini International University, IranReviewed by:
Seydi Yıkmış, Namik Kemal University, TürkiyeCopyright © 2024 Wang, Huang, Feng, Xiao, Luo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Zhang, MjY2OTI5NzM5OUBxcS5jb20=; Gaoquan Luo, bHVvaG9yc2UxMkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.