- 1College of Pharmacy, Gannan Medical University, Ganzhou, China
- 2Department of Clinical Medicine Research Center, The First Affiliated Hospital of Gannan Medical University, Ganzhou, China
- 3Ganzhou Key Laboratory of Antitumor Effects of Natural Products, Ganzhou, China
Zein is the main vegetable protein from maize. In recent years, Zein has been widely used in pharmaceutical, agriculture, food, environmental protection, and other fields because it has excellent biocompatibility and biosafety. However, there is still a lack of systematic review and research on Zein-based nano-delivery systems. This paper systematically reviews preparation and modification methods of Zein-based nano-delivery systems, based on the basic properties of Zein. It discusses the preparation of Zein nanoparticles and the influencing factors in detail, as well as analyzing the advantages and disadvantages of different preparation methods and summarizing modification methods of Zein nanoparticles. This study provides a new idea for the research of Zein-based nano-delivery system and promotes its application.
1 Introduction
The low solubility and poor stability of drugs have become the obstacles to their current application (1–4). In recent years, advanced materials such as micelles, liposomes, proteins, and metal nanomaterials have obvious advantages in maintaining drug stability, prolonging blood circulation time, controlling drug release, improving bioavailability, reducing toxicity, and enhancing cell absorption (5–9). Natural protein is promising alternatives material to traditional synthetic materials due to environment-friendliness (10, 11). Besides, compared with animal protein, plant protein has received a lot of attention from researchers due to their advantages of inexpensiveness and ready accessibility (12).
Maize is considered the third most important cereal in the world (13). Zein is a kind of natural plant protein extracted from maize and is the second most important nutrient in maize (14, 15). Zein lacks lysine and tryptophan, two essential amino acids for humans, which means that maize kernels contain relatively poor-quality protein as food (16). However, Zein has the characteristics of ideal drug delivery carrier such as self-assembly, low immunogenicity, good biocompatibility, and easily modifiable (12, 17). Based on the long history of Zein usage in food and related evaluation data, Zein has been used as an ideal material for food technology and drug delivery since 1939. In 1985, Zein had been granted Generally Recognized As Safe (GRAS) status by US-FDA (5, 18). In recent years, the research on Zein-based nano-delivery system has covered many fields such as food nutrition (19, 20), drug delivery (5, 21–25), agriculture (26), and environmental protection (27, 28). In addition, Zein as a carrier material has been designed into nanoparticles, nanocapsule, films, and nanofibres. However, the systematic summary and comprehensive review of Zein-based nano-delivery system is still lacking.
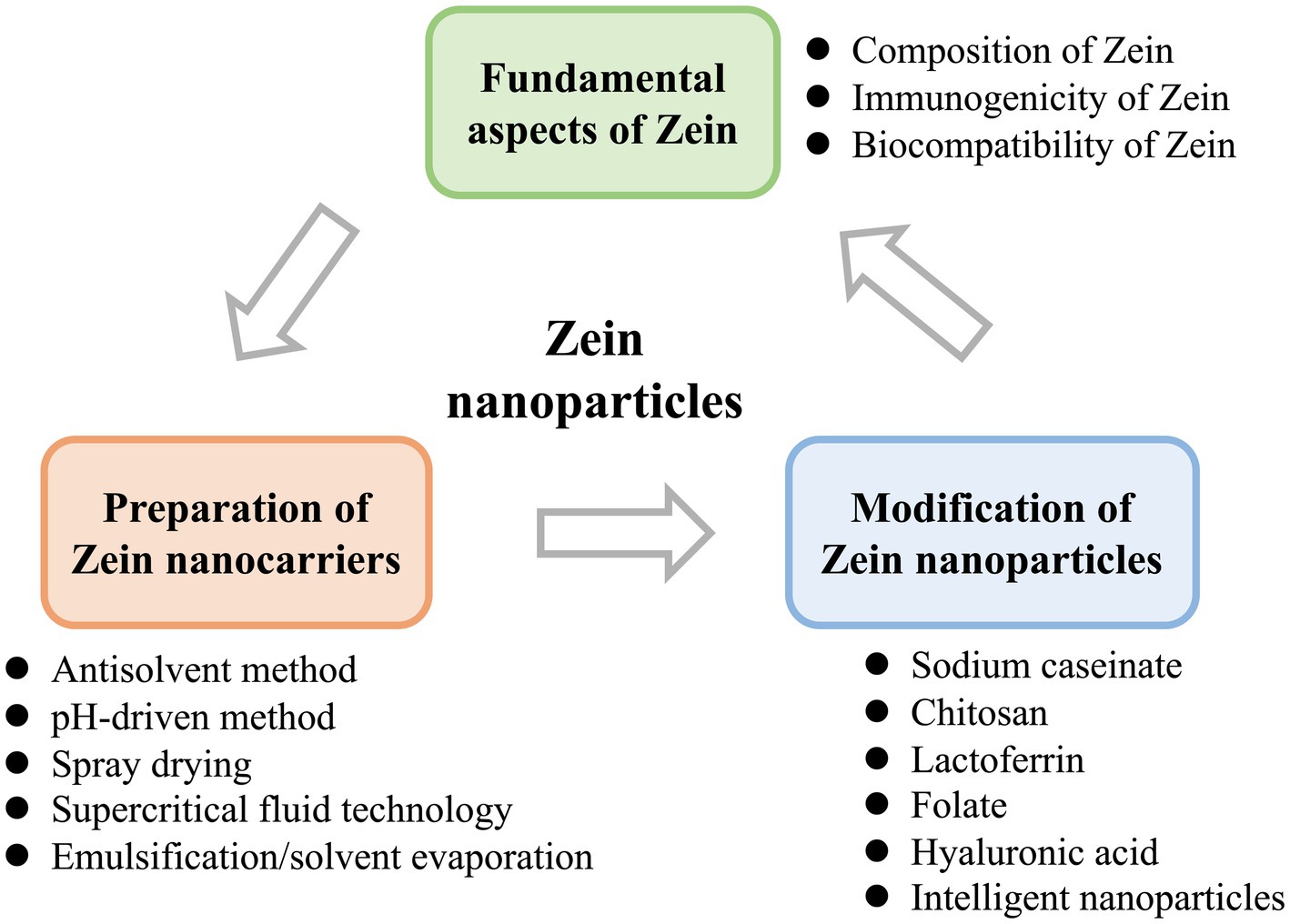
In first part, the composition, immunogenicity, biocompatibility, and other basic properties of Zein were summarized. Then, the principles, influencing factors, advantages and disadvantages of different preparation methods of Zein nanoparticles are discussed. Finally, the optimization effect of different modification methods on the function of Zein nanoparticles was researched (Graphical Abstract). The purpose of this paper is to summarize and discuss the research of Zein-based nano-delivery system in recent years, and to lay a foundation for further research of Zein-based nano-delivery system and provide a new research idea.
2 Fundamental aspects of Zein
2.1 Composition of Zein
Zein is a prolamin protein isolated from the endosperm of maize and makes up about 80% of the entire protein in maize (23). Zein is rich in hydrophobic and neutral amino acids (such as leucine 20%, proline 10%, and alanine 10%), but lacks lysine and tryptophan. Furthermore, the small number of arginine and histidine residues in the structure of Zein are the main differences between Zein and other proteins (5). The fixed amino acid composition provides Zein with unique solubility (25). Therefore, Zein is insoluble in water, but soluble in alkaline solutions (pH ≥ 11.5), 70–95% aqueous ethanol solutions and water-acetone solutions (17).
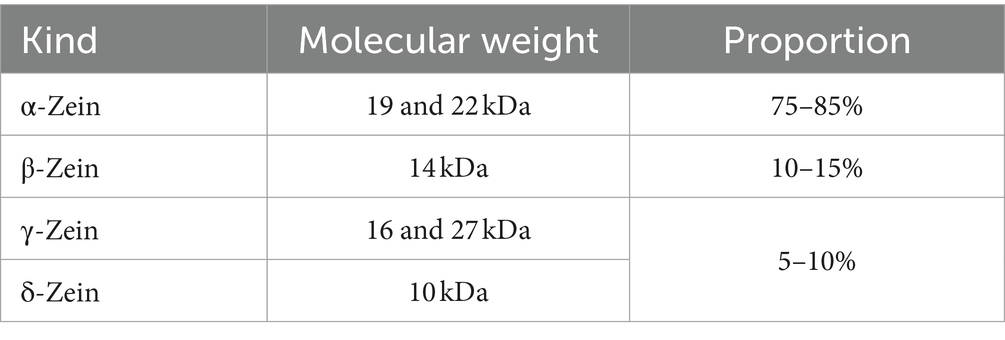
Zein is classified into four categories (α-Zein, β-Zein, γ-Zein, and δ-Zein) based on their different conformational arrangements, molecular sizes, molecular weights, and solubilities (12). The specific proportions and molecular weights of the four Zein species are shown in Table 1. The primary structure and secondary structure of Zein can be determined by chromatography, circular dichroism, Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR) (29–31). At present, studies on the structure of Zein mainly focus on α-Zein and γ-Zein. The α-Zein consists of highly homologous repeating units and has a high α-helix content of 35–60% (32, 33). Several different models of the α-Zein tertiary structure have been proposed: cylindrical model, ribbon model, hairpin model, and super helix model (5, 23, 34). In addition, the secondary and tertiary structures of α-Zein were affected by different solvent types, for example, Daniel et al. showed that the α-helix structure in α-Zein decreased with the increase of water content in the ethanol-water system, and the β-sheet content increased with the increase of the proportion of hydrophilic solvent (35). Research by Wang and Padua also supports this argument (36, 37). Moreover, both α-Zein and γ-Zein are rich in glutamine, alanine, leucine, and proline, but differ in their cysteine content. Specifically, α-Zein contains 1–3 cysteine residues, while γ-Zein contains 12–15 cysteine residues, which can form disulfide bonds to stabilize protein structures (38, 39). The glutamine-rich structure of γ-Zein with molecular weight of 27 kDa may be involved in protein interaction and protein oligomerization. The N-terminal repeat domain (VHLPPP) of γ-Zein is related to membrane permeability and has the ability to cross cell membranes, which allows it to use as a carrier peptide to facilitate drug absorption (40–42).
2.2 Immunogenicity of Zein
Immunogenicity is a key factor to be considered in the preparation of drug delivery systems in vivo. Compared with animal protein, Zein is more readily available and has less immunogenic potential (43). Certainly, the route of administration affects antigenicity of Zein (5). Through mice experiments, Pepi et al. proved that intramuscular injection of Zein triggers a systemic immune response. The oral administration of Zein nanoparticles did not cause systemic immune response but induced systemic tolerance without mucosal tolerance (44). Park et al. (45) showed that inhaling Zein dust could induce type 1 hypersensitivity reaction leading to asthma. Zein is hydrolyzed by gastrointestinal proteases and still causes allergic reactions in celiac patients, which is related to IgA antibodies in celiac disease patients recognizing digested α-Zein as celiac disease antigens (46). The 50 kDa protein in maize is a reduced soluble protein as defined by Wilson et al. and this protein has been identified as potential allergen for maize allergy sufferers by Pasini’s study. Moreover, Lee et al. identified the 50 kDa protein in maize as 50 kDa γ-Zein (47–49). Immunological experiments in mice showed that Zein nanoparticles with particle size between 100 and 400 nm had no immune response, but the particle size greater than 400 nm can lead to an immune response two to four times higher than the saline group (50). In addition, the cells involved in nanoparticle-triggered immune responses are mainly phagocytes (51). Easier adsorption of proteins on the surface of hydrophobic nanoparticles leads to their easier uptake by phagocytes (52). The immune response induced by Zein may be related to hydrophobic amino acids such as glutamine, leucine and alanine in Zein. Therefore, the immunogenicity of Zein nanoparticles was related to the route of administration, particle size, and hydrophobicity.
2.3 Biocompatibility of Zein
Biocompatibility was redefined in 1987 as the ability of a material to perform with an appropriate host response in a specific situation (53). Materials with excellent biocompatibility should satisfy the requirements of non-toxicity, non-immunogenicity, non-thrombogenicity, and non-carcinogenicity of themselves and their metabolites (54–56). The safety of biological materials is crucial for drug delivery, so biocompatibility is a necessary area in the material research process (57). In the past few years, due to the biocompatibility and degradability of Zein, the application of Zein polymer in drug delivery and tissue engineering has been widely studied (58). Dong et al. investigated the effects of Zein on the attachment, morphology, and proliferation of HL-7702 cells and NIH3T3 cells by microscopic observation and MTT experiment, and the results showed that Zein film with low concentration and small particles had better cell proliferation ability, which proved that Zein had good biocompatibility (59). Liu et al. (60) showed that Zein-fucoidan complex nanoparticles had good biocompatibility. In addition, hemolysis test and cell culture experiments demonstrated that Zein had no hemolysis effect and low cytotoxicity (61) Studies by Gong (62), Wang (63), and Kim (64) also prove this view.
3 Preparation of Zein nanocarriers
3.1 Antisolvent method
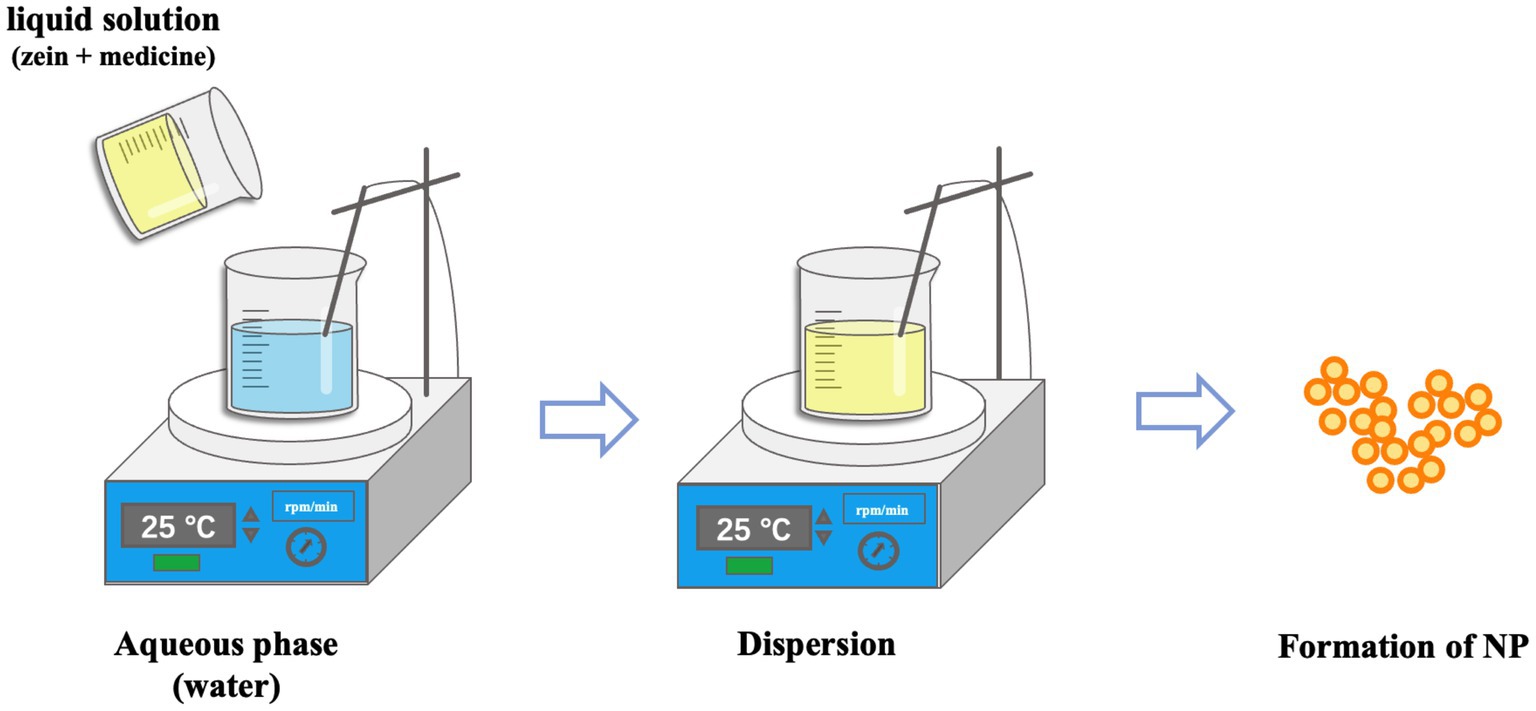
Antisolvent precipitation, also known as phase separation or liquid–liquid dispersion, is a common preparation method for Zein nanoparticles (65). Deionized water is usually used as the antisolvent, and ethanol aqueous solution is used as the solvent of Zein (66). The basic principle of this method is to prepare nanoparticles by changing the polarity of the solvent around Zein. In this process, the proportion of organic solvent is reduced, the solubility of Zein is reduced, and the nanoparticles are self-assembled (67, 68). The unique solubility of Zein makes it to self-assemble into nucleus-like particles in the antisolvent and the nucleus can grow further by trapping non-aggregated solute molecules, but the particle growth will stop when the concentration of Zein in the solvent is too low (69–71). The preparation method is shown in Figure 1. Lou et al. prepared Zein nanoparticles with a particle size of 252.8 ± 7.3 nm by dissolving Zein in 70% aqueous alcohol solution and injecting anti-solvent under vigorous stirring. The particle size of the nanoparticles prepared by adding the stabilizer carboxymethyl chitosan was 113 nm, which is a decrease in particle size compared to the nanoparticles without stabilize (72). Ye et al. dissolved Zein in 80% ethanol solution and added it into water at a rotational speed of 1,200 rpm to prepare nanoparticles with a particle size of 209.2 ± 1.9 nm and Zeta-potentials of-19.0 ± 0.7 mV (73). Lonare et al. (74) found that polymer concentration was negatively correlated with nanoparticle size, and the higher the polymer solution concentration was, the smaller the nanoparticle size was obtained. Therefore, when nanoparticles were prepared by antisolvent methods, the polymer content, the type of solvent and non-solvent, the ratio of solvent to non-solvent, the addition rate of solvent to non-solvent, the action of stabilizer, and the stirring speed all affected the particle size of the nanoparticles.
3.2 pH-driven method
The preparation of Zein nanoparticles by antisolvent method is simple and efficient, but it may bring some safety risks due to the large amount of organic solvents involved (75). The principle of the pH-driven method is based on that Zein is soluble in alkaline solutions and insoluble in neutral or acidic solutions. Therefore, Zein and the drug can be dissolved in deionized water under magnetic agitation at pH 12.0, then NaOH and HCl are used to adjust the pH of the deionized water. Finally, Zein nanoparticles were formed when the pH of the solution changed from alkaline to neutral under agitation (76). The preparation method is shown in Figure 2. At present, the pH-driven method has been widely used to prepare Zein nanoparticles (77). For example, the natamycin-loaded Zein-casein nanoparticles (N-Z/C NPs) were prepared by pH driven method. The average particle size of the nanoparticles was <100 nm and the Zeta potential < −30 mV (78); Yuan et al. prepared Zein/Tea saponin composite nanoparticles (Z/TSNPs) using pH-driven method. Zein and Tea saponin (TS) were dissolved at pH 12.0, after which the above solution was adjusted to pH 7.0 using HCl, and Zein nanoparticles were obtained by centrifugation at 2,000 g for 10 min, with encapsulation efficiency of 83.73% and loading capacity of 22.33%. Besides, Z/TSNPs increased the solubility of curcumin by about 290 times (79). However, nanoparticles prepared by the pH-driven method tend to aggregate and even form irregular precipitates (80). In practical applications, it is not only necessary to select drugs and carrier materials whose solubility varies with pH, but also to consider whether high-alkali solutions will cause drug degradation (81, 82).
3.3 Spray drying
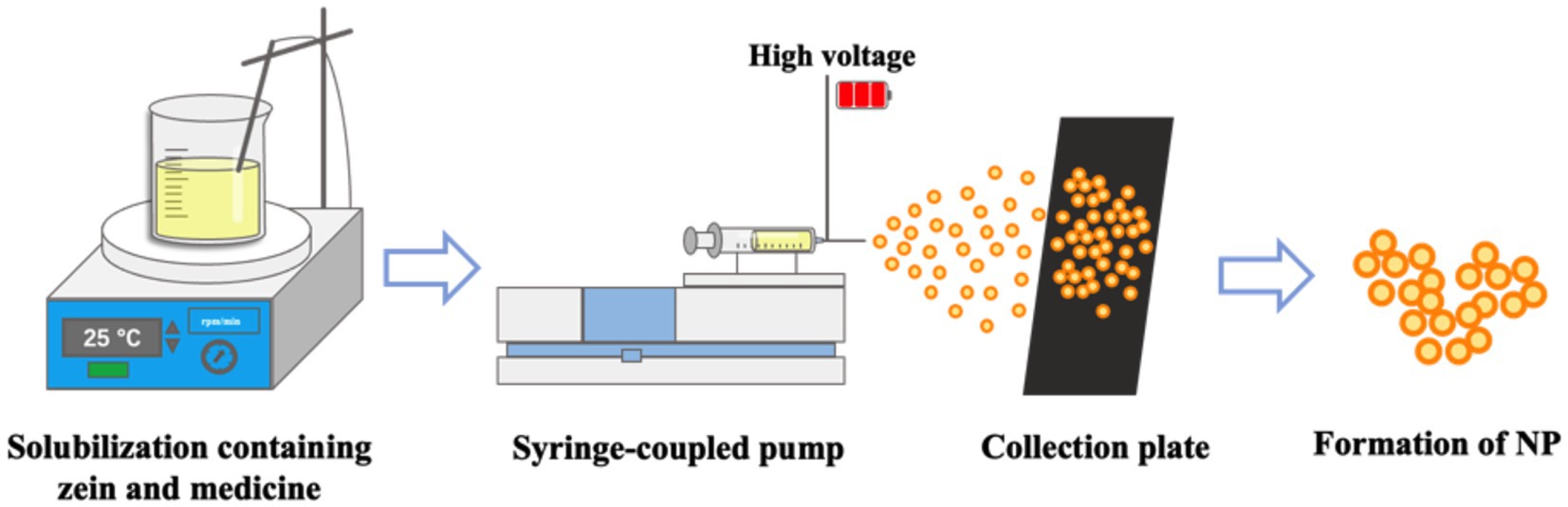
Spray drying, also known as electrohydrodynamic atomization, is a frequently used technology for producing dry powder in pharmaceutical industry (83). The four key procedure of spray drying are atomization, droplet–to–drying gas contact, particle drying, and particle collection (84). The method mainly depends on spraying the material to be dried in hot air by mechanical action, the solvent is evaporated, and the resulting ultrafine powder is collected (85). The preparation method is shown in The preparation method is shown in Figure 3. According to the literatures, the solubility of the drug and polymer, the choice of solvent, and the temperature affect the prepared nanoparticles. Especially the increase of temperature can increase the solubility of the drug and polymer, and make the prepared nanoparticles more homogeneous (86–88). Zein based nanoparticles can also be produced by spray drying liquids containing already-formed Zein nanoparticles or by spray drying an ethanol aqueous solution of Zein. Francisco et al. dissolved Zein in 80% ethanol solution and prepared Zein nanoparticles loaded with quercetin by spray drying method. The parameters of the equipment used for the preparation of nanoparticles: electrical potential of 15 kV; flow rate of 0.1 mL/h; and distance from the needle to the collector of 15 cm. The encapsulation efficiency of quercetin in the prepared Zein nanoparticles reached 87.9 ± 1.5 to 93.0 ± 2.6%, and the 4 h release of quercetin in the simulated experiment of gastrointestinal tract was 79.1% (89). Baspinar et al. prepared Zein nanoparticles loaded with curcumin by spray drying method, with a particle size of about 500 nm (90, 91). However, the high temperatures during production of this method may have an impact on drug stability, and the technique should be used with caution when preparing drug delivery systems loaded with heat-sensitive compounds (92, 93).
3.4 Supercritical fluid technology
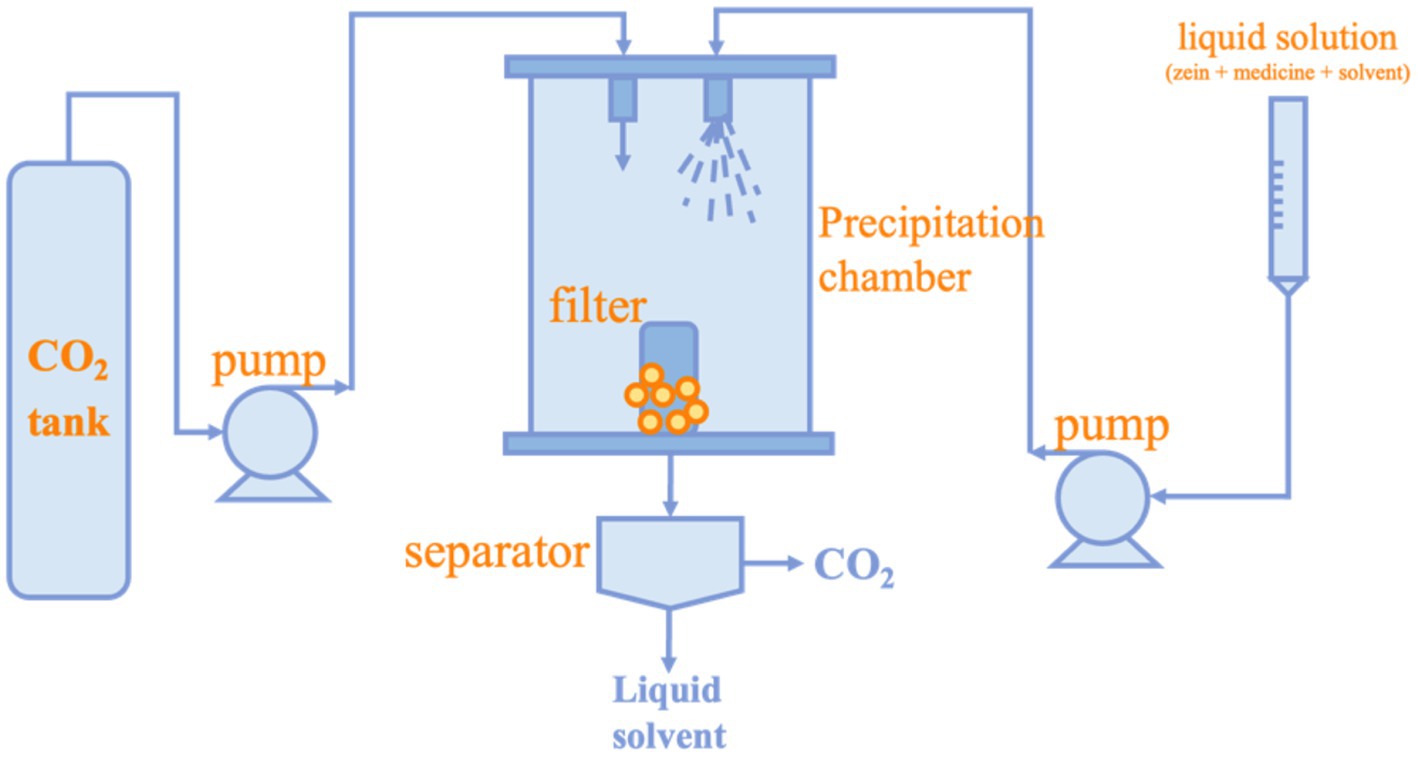
The supercritical fluid (SCF) technology is an efficient and environmentally friendly method for preparing nanoparticles (94). The simple process of supercritical fluid technology is to extract the co-solvent in the atomized droplet by supercritical CO2 after continuous injection of the feedstock. Since most polymers are insoluble in CO2, the solubility of the polymer gradually decreases, thus forming atomic nuclei and gradually growing into nanoparticles or microparticles (95). The preparation method is shown in Figure 4. Compared with other preparation methods, supercritical fluid technology can effectively remove organic solvents and is friendly to heat-sensitive bioactive substances so that they do not degrade during particle formation (96). In recent years, with the improvement of science and technology, supercritical fluid technology can produce smaller and more controllable particles (97). The main factors that affect particle size when nanoparticles are prepared using SCF include temperature, pressure, properties of organic solvent, solute concentration, anti-solvent and solution flow rate, and the geometry of the chamber and nozzle. Hu et al. used this technique to prepare Lutein/Zein nanoparticles with a particle size of approximately 200 nm at a pressure of 10 MPa, the Lutein/Zein ratio of 1: 18 (w/w), the solution flow rate of 1.0 mL/min, and the temperature of 45°C. And it was demonstrated that lower temperatures and solution flow rates coupled with high pressures favored smaller, more regular nanoparticles (98). Li et al. successfully prepared Zein nanoparticles with a minimum size of 50 nm by SCF. The concentration of Zein was fixed at 10 mg/mL, and it was dissolved in a mixture of ethanol and dichloromethane at a volume ratio of 5: 7. The specific operating parameters of the apparatus were: temperature of 45°C; pressure of 10 MPa; and flow rate of the dosing solution of 1 mL/min. The study showed that the nozzle structure and CO2 flow rate affected the morphology and size of the nanoparticles (99).
3.5 Emulsification/solvent evaporation
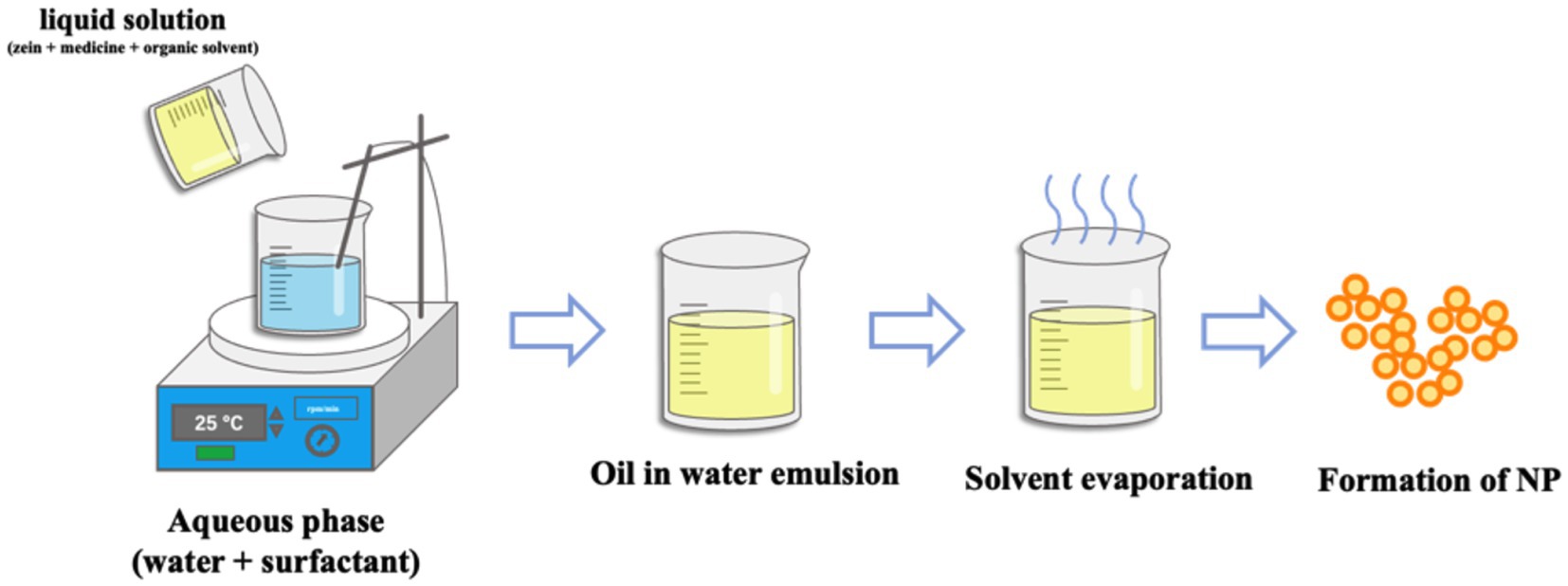
The preparation of nanoparticles by emulsification/solvent evaporation technology is mainly divided into two parts, the first step is to emulsify the polymer solution into an aqueous phase, and the second step is to evaporate the solvent. Specifically, the emulsification/solvent evaporation is the process of dissolving selected polymers in a volatile organic solvent (oil phase), and then injecting the oil-phase solution into an aqueous solution containing a surfactant to obtain a nanoemulsion. Upon evaporation of the organic solvent, drug-loaded nanoparticles in the aqueous surfactant solution are obtained (100–102). Therefore, the diameter of nanoparticles can be controlled by adjusting the stirring speed, the viscosity of aqueous phase and organic phase, the type and concentration of dispersing agent when emulsified solvent evaporation is used to prepare nanoparticles (103). The preparation method is shown in The preparation method is shown in Figure 5. Yang et al. prepared Zein nanoparticles loaded with resveratrol by emulsification/solvent evaporation method. The particle size of the nanoparticles was 389.90 ± 3.02 nm, the Zeta potential was −13.37 ± 0.26 mV, the encapsulation efficiency was 64.17 ± 0.07%, and the drug loading was 5.78 ± 0.01% (104).
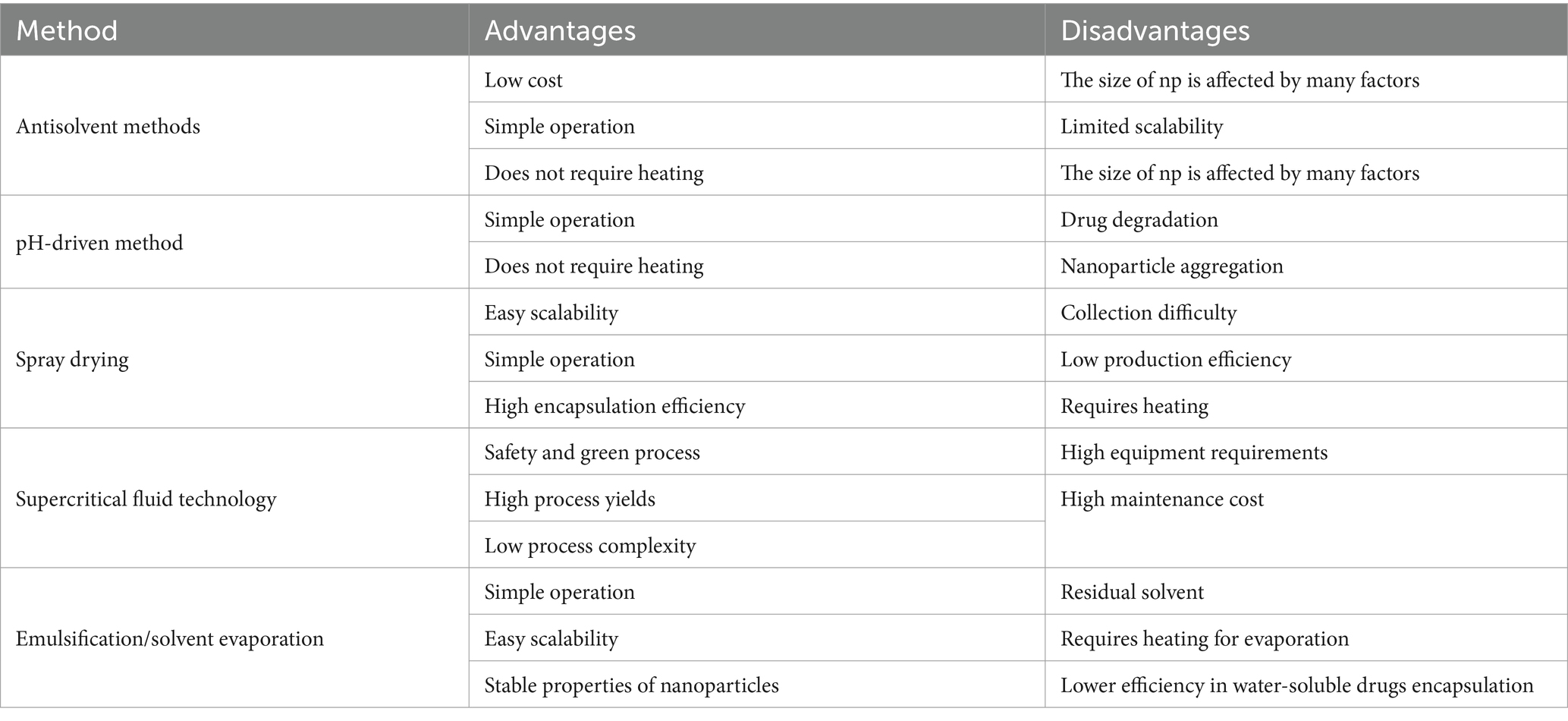
The advantages and disadvantages of different preparation methods are summarized in Table 2.
4 Modification of Zein nanoparticles
4.1 Sodium caseinate
Casein is the main protein of milk in the form of macromolecular aggregates (105, 106). Sodium tyrosine is a commonly used nutrient and functional component in emulsion preparation due to its good solubility and emulsification properties (107). Caseinate moves the isoelectric point of the colloidal particles from 6.0 to around pH 5.0, thus preventing Zein from accumulating near its native IEP (pH 6.2) and is the most commonly used stabilizer for Zein nanoparticles (108, 109). In addition, the addition of sodium caseinate can provide spatial stability for Zein nanoparticles and improve the poor redispersibility of Zein after lyophilization (110). Some studies suggest that sodium caseinate modifies Zein nanoparticles by electrostatic adsorption (111). Patel et al. studied the FT-IR spectra of Zein nanoparticles and caseinate stabilized Zein nanoparticles and found that the FT-IR spectra of Zein nanoparticles modified with sodium caseinate did not show significant peak shift or the appearance of new peaks. Meanwhile, the DSC thermograms of Zein colloidal particles and caseinate stabilized Zein colloidal particles demonstrated the absence of any chemical interaction between Zein and caseinates (109). Chang et al. used the pH-driven method to prepare Zein/caseinate/pectin composite nanoparticles with particle size of less than 200 nm, narrow distribution, spherical distribution, and strong negative charge. And Zein nanoparticles modified with sodium caseinate have good redispersability (112). Huang et al. verified the stability of Curcumin-loaded Zein/sodium caseinate-alginate nanoparticles by dispersing the nanoparticles in a solution with a pH of 2.0–8.0 and a salt concentration of 0–1.6 M NaCl. The results show that the nanoparticles have stable anti-aggregation properties in the pH range of 2.0–7.3 and at a high salt concentration of 1.6 M (113). Mohamed et al. designed and prepared Zein/sodium caseinate nanoparticles encapsulated with celecoxib (CXB) and prodigiosin (PDG) for the treatment of triple-negative breast cancer. The increased cytotoxicity of nanoparticles may be related to the nanoparticles stability and the cell absorption capacity of Zein nanoparticles enhanced by the addition of sodium caseinate (114).
4.2 Chitosan
Chitosan is a natural linear polysaccharide cationic and hydrophilic polymer, obtained by alkaline hydrolysis of chitin, which is not only non-toxic but also has good biocompatibility (115). Recent studies have shown that the embedding material of Zein nanoparticles without any modification is easy to release quickly in a short time (116, 117). To overcome this problem, polysaccharides were used as colloidal stabilizers to coat Zein nanoparticles (118, 119). Chitosan has rich hydroxyl (−OH) and amine (−NH2) functional groups, which can be used to react with crosslinking agents for in situ chemical crosslinking (120). Hence, chitosan as a cationic polysaccharide has attracted much attention due to its great potential in the development of Zein nanoparticles (121). Zhang et al. (122) designed liver-specific targeted nanoparticles naringenin-Zein-sodium caseinate-chitosan nanoparticles to improve the stability of Zein nanoparticles and improve the lipid-lowering activity of Naringenin. Cai et al. (123) found that chitosan modified Zein nanoparticles could achieve sustained antibacterial effect. Liu et al. (124) prepared chitosan modified Zein nanoparticles with a particle size of about 138 nm, which effectively improved the stability of Zein nanoparticles at high ionic strength, and effectively improved the thermal stability and light stability of curcumin. Li et al. (125) found that modifying Zein nanoparticles with Chitosan can not only improve the stability of the nanoparticles, but also make the nanoparticles have excellent antibacterial effect.
4.3 Lactoferrin
Lactoferrin is a nutrient that is typically found in mammalian milk and has antibacterial and antiviral effects (126). Lactoferrin is thought to exert its primary biological activity after interacting with receptors on target cells. Lactoferrin receptors include CD14 (127), intestinal epithelial cells and lymphocytes (128), and LDL receptor-related protein-1 (LRP-1/CD91, 129). Importantly, lactoferrin can also bind to heparan sulfate proteoglycans (HSPGs) on the cell surface (130). This can be used in targeting strategies where overexpressed receptors on the cell surface favor receptor-mediated endocytosis of nanoparticles, leading to enhanced substance delivery (131). In addition, lactoferrin can be used as a stabilizer to improve the instability of Zein nanoparticles under high salt conditions. Chen et al. prepared glycosylated lactoferrin by Maillard reaction as a stabilizer, established Zein/glycosylated LF nanoparticles and successfully encapsulated 7, 8-dihydroxyflavone (7, 8-DHF). Meanwhile, the bioaccessibility of DHF-Zein/LF was three times higher than free 7, 8-DHF through in vitro simulation of gastrointestinal digestion experiment (132). Sarah et al. designed active targeting nanoparticles based on the anticancer activity of lactoferrin itself and the high expression of lactoferrin receptor (LDL receptor) in breast cancer cells, and the study showed that Lactoferrin modification may enhance the targeting and internalization behavior of nanocarriers entering cancer cells (133). Wang et al. (134) found that lactoferrin can promote the absorption of NPs by intestinal epithelial cells to improve the brain permeability of CF3CN. According to these literatures, lactoferrin mainly modifies Zein nanoparticles by forming hydrogen bonds.
4.4 Folate
Folate, also known as vitamin B9, plays a crucial role in one-carbon transfer reactions, cell division, growth, and survival, especially in rapidly dividing cells (135, 136). Folate receptor (FR) is a 38 kDa glycosylphosphatidylinositol-anchored that binds to the vitamin folate with high affinity (137). Folate receptors are most widely expressed at very low levels in normal tissue, but it is overexpressed in many cancers, including stomach, ovarian, and breast cancers (138, 139). Many studies have shown that folate increases tumor accumulation of nanoparticles. And folate modified nanoparticles show effective targeting ability in tumor diagnosis and therapy (140–142). The methods of folic acid modification of Zein nanoparticles mainly include directly forming amide bond with Zein, forming amide bond with other compounds to modify Zein nanoparticles and some modifications without chemical reaction. For instance, Wu et al. (143) used an amide coupling agent to activate the carboxyl group of folate, which combined with the amino group of Zein to form an amide bond, and used this material to prepare targeted nanoparticles. Zar et al. (144) first reacted folic acid with PEG to obtain FA-PEG-COOH and then reacted this substance with Zein to prepare nanoparticles. In addition, Wang et al. (145) and Wusigale et al. (146) did not use chemical coupling to design folate-modified Zein targeting nanoparticles. The variety of modification methods of folic acid make it widely used in the modification of targeted nanoparticles. Folic acid-modified Zein nanoparticles FA-NP-DOX were prepared by Hou et al. The results in vivo pharmacokinetic studies showed that DOX was cleared from the circulation after 4 h, DOX from NP-DOX was completely released and cleared after 7 h, while DOX from FA-NP-DOX remained in the circulation after 24 h. This demonstrated that FA-NP-DOX could effectively prolong the release of antitumor drugs. Besides, in vivo anticancer studies demonstrated that FA-NP-DOX significantly inhibited tumor growth (147). Research has shown that folate receptor levels are significantly higher in inflammatory sites than in normal tissues. Wu et al. modified Zein nanoparticles with folate and observed the distribution of nanoparticles in colonic inflammatory tissues by using frozen section technology combined with laser confocal microscopy. The results showed that the fluorescence intensity of Zein nanoparticles modified with folate was stronger than that of unmodified Zein nanoparticles, which demonstrated that folate modification could target and enrich nanoparticles at the site of inflammation (148).
4.5 Hyaluronic acid
Hyaluronic acid is an anionic non-sulfated glycosaminoglycan, and it is widely distributed in connective tissue and epithelial tissue (149). Hyaluronic acid is essential component of extracellular matrix (150, 151). Meanwhile, it can bind specifically to receptors such as CD44, GHAP (glionic acid binding protein), and TSG6 (TNF-stimulating gene 6, 152). Current studies have shown that CD44 is of major significance (153). CD44 is a non-kinase transmembrane glycoprotein and is overexpressed in several cell types, including cancer stem cells (154). Hyaluronic acid is the main ligand of CD44, which binds to CD44 and activates CD44, leading to cell proliferation, adhesion, migration, and invasion (155, 156). CD44 may be a molecular target for cancer therapy and an important prognostic marker (157). Hydrogen bonding, electrostatic and hydrophobic interactions between Zein and hyaluronic acid molecules may be responsible for the formation of stable complexes in the nanoparticles (158). Seok et al. (159) successfully developed hyaluronic acid cross-linked Zein nanoparticles to deliver curcumin to CD44-expressing cancer cells. Zhang et al. (160) designed Zein nanoparticles loaded with the anticancer drug Honokiol (HNK) and modified them with hyaluronic acid. HA-Zein-HNK could be delivered in a targeted manner to improve the therapeutic efficacy in breast cancer treatment.
4.6 Intelligent nanoparticles
4.6.1 pH-responsive nanoparticles
The rapid growth of tumor tissue can lead to hypoxia (161). Therefore, glycolysis occurs at the tumor site to produce acidic metabolites, resulting in a tumor site pH lower than the physiological pH value (162). Kaushik et al. designed pH-dependent hydrogel-modified Zein nanoparticles, which could effectively release adriamycin into the cellular acidic environment of HeLa cells. In addition to their application in cancer targeting, the research on targeted delivery of pH-response nanoparticles in the gastrointestinal tract is also of great significance. Li et al. proposed glycyrrhizic acid (GA) as pH-responsive substances to functionalize curcumin-loaded Zein nanoparticles. As the pH increases from 3 to 7. The release of GA from the surface of nanoparticles leads to a change in the stability of Zein nanoparticles, and the curcumin is released from the nanoparticles, achieving the effect of intelligent control of drug release (163). At the same time, the PH-responsive Zein nanoparticles have been applied in the treatment of diabetes (164), food preservation (19, 165), and improving the water dispersity of pesticides (166).
4.6.2 Magnetically responsive nanoparticles
Magnetic nanoparticles (MNP) are prepared by metal materials or magnetic nanoparticle composite materials. Under the guidance of magnetic field in vitro, the magnetically responsive nanoparticles can accumulate at the target site (167). Nanoparticles with magnetic response usually consist of two structures, the first one is modification on the surface of magnetic nanoparticles and the second one is encapsulation of magnetic material in nanoparticles (168, 169). Pang et al. prepared nanoparticles from superparamagnetic iron oxide nanoparticles (SPIONs) and gefitinib (GEF) encapsulated in folate-conjugated Zein (Fa-Zein). The experimental results showed that the uptake of GEF into A549 cells was facilitated by utilizing the magnetic response property of SPIONs and the active targeting of folate, which enhanced the toxicity of GEF to A549 cells (170). In addition to the research of cancer-targeting nanoparticles, magnetically responsive Zein nanoparticles are also of great significance in environmental protection and food detection (171, 172).
4.6.3 Photo-responsive nanoparticles
Currently, photodynamic/photothermal therapy of photosensitizers is currently undergoing intensive preclinical and clinical studies (173). Lee et al. used the Zein-phosphatidylcholine hybrid nanoparticles (Z/PC-NP) as drug carrier to prepare light-sensitive nanoparticles loaded with near-infrared dye indocyanine green (ICG). ICG encapsulated in Z/PC-NP is twice as phototoxic to cancer cells as PC-NP (174). Abdelsalam et al. (175) designed light-responsive nanoparticles of Zein modified with the natural photosensitizer hypericin and investigated its active targeting of HepG2 cells by apoptosis assay, the results showed that the apoptosis was more obvious in hypericin-modified Zein nanoparticles group.
5 Conclusion and outlook
Zein is a plant protein with unique solubility, biocompatibility and low immunogenicity. In recent years, Zein-based nano-delivery system has been widely used in pharmaceutical, food, agriculture, environmental protection and other fields. Emerging technologies for the preparation of Zein-based nano-delivery systems play an important role in the preparation of nanoparticles that are stable, actively targeted, intelligently responsive, and multifunctional hybrids. This paper reviews the current status of research on different preparation methods and modifications of Zein-based nano-delivery systems based on the basic properties of Zein. The low immunogenicity and good biocompatibility of Zein are essential parameters for its wide range of applications in biomedical, pharmaceutical and other fields. Evidence in the literatures suggest that Zein nanoparticles with different particle sizes and size distributions can be produced by using different preparation methods. Although Zein nanoparticles with narrow particle size distribution, higher encapsulation efficiency and drug loading capacity can be obtained by different preparation methods, Zein-based nano-delivery systems are widely known for their poor colloidal stability. The selection of different additives to enhance its stability has become a hot research topic.
The development trend of Zein-based nano-delivery system in the future is mainly the design of active targeting and the expansion of industrialization. Firstly, although unmodified Zein nanoparticles can improve the problem of low drug solubility and poor stability, their entrapment efficiency at the targeting site is very limited. The design of Zein nanoparticles with active targeting function can effectively improve the enrichment efficiency of drugs and improve drug utilization efficiency. Secondly, Zein is easy to modify and has favorable biocompatibility and degradability, but the effect of biodegradation of modified Zein derivatives is less studied at present. The last is to realize the expansion of Zein nanoparticle preparation technology from laboratory to industrialization. With the rapid development of nanoparticle preparation technology, small-scale preparation of Zein nanoparticles in the laboratory has achieved satisfactory stability and reproducibility, but how to industrialize its large-scale production has become a major obstacle limiting the application of Zein nanoparticles at present. Therefore, the design and development of software, which can predict the parameters of nanoparticles may be a solution to solve the difficulties in the industrialization of nanoparticle preparation technology. The fact that Zein is cheap and easily available as a biomaterial and the presence of different groups in its polymer chain (amine, amide, hydroxyl, carboxylate, and phenol) gives it a wide range of modification possibilities. In addition, the material can be prepared into drug delivery systems of different sizes and shapes, which has a very high potential for applications.
Author contributions
XL: Writing – original draft, Writing – review & editing. MZ: Writing – review & editing. XZ: Writing – review & editing. MW: Writing – review & editing. AC: Writing – review & editing. BX: Writing – review & editing. JY: Supervision, Writing – review & editing. HL: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82260985), Starting Fund for Scientific Research of High-level Talents of Gannan Medical University (QD202207), and the Science and Technology Project of Ganzhou (2022DSYS9969).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kawabata, Y, Wada, K, Nakatani, M, Yamada, S, and Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. (2011) 420:1–10. doi: 10.1016/j.ijpharm.2011.08.032
2. Loftsson, T, and Brewster, ME. Pharmaceutical applications of Cyclodextrins: basic science and product development. J Pharm Pharmacol. (2010) 62:1607–21. doi: 10.1111/j.2042-7158.2010.01030.x
3. Aucamp, M, and Milne, M. The physical stability of drugs linked to quality-by-design (Qbd) and in-process technology (pat) perspectives. Eur J Pharm Sci. (2019) 139:105057. doi: 10.1016/j.ejps.2019.105057
4. Masimirembwa, CM, Bredberg, U, and Andersson, TB. Metabolic stability for drug discovery and development: pharmacokinetic and biochemical challenges. Clin Pharmacokinet. (2003) 42:515–28. doi: 10.2165/00003088-200342060-00002
5. Zhang, Y, Cui, L, Li, F, Shi, N, Li, C, Yu, X, et al. Design, fabrication and biomedical applications of Zein-based Nano/Micro-carrier systems. Int J Pharm. (2016) 513:191–210. doi: 10.1016/j.ijpharm.2016.09.023
6. Chauhan, DS, Arunkumar, P, Prasad, R, Mishra, SK, Reddy, BPK, De, A, et al. Facile synthesis of plasmonic zein nanoshells for imaging-guided photothermal cancer therapy. Mater Sci Eng C Mater Biol Appl. (2018) 90:539–48. doi: 10.1016/j.msec.2018.04.081
7. Huang, W, Deng, Y, Ye, L, Xie, Q, and Jiang, Y. Enhancing Hemocompatibility and the performance of au@silica nanoparticles by coating with Crgd functionalized Zein. Mater Sci Eng C Mater Biol Appl. (2021) 125:112064. doi: 10.1016/j.msec.2021.112064
8. Jiang, L, Li, L, He, X, Yi, Q, He, B, Cao, J, et al. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and Ph-response. Biomaterials. (2015) 52:126–39. doi: 10.1016/j.biomaterials.2015.02.004
9. Gaber, M, Elhasany, KA, Sabra, S, Helmy, MW, Fang, JY, Khattab, SN, et al. Co-administration of tretinoin enhances the anti-cancer efficacy of etoposide via tumor-targeted green nano-micelles. Colloids Surf B: Biointerfaces. (2020) 192:110997. doi: 10.1016/j.colsurfb.2020.110997
10. Aljabali, AA, Rezigue, M, Alsharedeh, RH, Obeid, MA, Mishra, V, Serrano-Aroca, Á, et al. Protein-based nanomaterials: a new tool for targeted drug delivery. Ther Deliv. (2022) 13:321–38. doi: 10.4155/tde-2021-0091
11. Oleandro, E, Stanzione, M, Buonocore, GG, and Lavorgna, M. Zein-based nanoparticles as active platforms for sustainable applications: recent advances and perspectives. Nanomaterials. (2024) 14:414. doi: 10.3390/nano14050414
12. De Marco, I . Zein microparticles and nanoparticles as drug delivery systems. Polymers. (2022) 14:2172. doi: 10.3390/polym14112172
13. Van Hung, P . Phenolic compounds of cereals and their antioxidant capacity. Crit Rev Food Sci Nutr. (2016) 56:25–35. doi: 10.1080/10408398.2012.708909
14. Li, C, and Song, R. The regulation of Zein biosynthesis in maize endosperm. Theor Appl Genet. (2020) 133:1443–53. doi: 10.1007/s00122-019-03520-z
15. Kikushima, K, and Kamiya, R. Clockwise translocation of microtubules by flagellar inner-arm Dyneins in vitro. Biophys J. (2008) 94:4014–9. doi: 10.1529/biophysj.107.123083
16. Salvador-Reyes, R, Rebellato, AP, Lima Pallone, JA, Ferrari, RA, and Clerici, M. Kernel characterization and starch morphology in five varieties of Peruvian Andean maize. Food Res Int. (2021) 140:110044. doi: 10.1016/j.foodres.2020.110044
17. Sousa, FF, Luzardo-Alvarez, A, Blanco-Mendez, J, Otero-Espinar, FJ, Martin-Pastor, M, and Sandez, MI. Use of 1h Nmr Std, Waterlogsy, and Langmuir monolayer techniques for characterization of drug-Zein protein complexes. Eur J Pharm Biopharm. (2013) 85:790–8. doi: 10.1016/j.ejpb.2013.07.008
18. Paliwal, R, and Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J Control Release. (2014) 189:108–22. doi: 10.1016/j.jconrel.2014.06.036
19. Aytac, Z, Xu, J, Raman Pillai, SK, Eitzer, BD, Xu, T, Vaze, N, et al. Enzyme-and relative humidity-responsive antimicrobial fibers for active food packaging. ACS Appl Mater Interfaces. (2021) 13:50298–308. doi: 10.1021/acsami.1c12319
20. Lan, X, Zhang, X, Wang, L, Wang, H, Hu, Z, Ju, X, et al. A review of food preservation based on Zein: the perspective from application types of coating and film. Food Chem. (2023) 424:136403. doi: 10.1016/j.foodchem.2023.136403
21. Hou, H, Zhang, D, Lin, J, Zhang, Y, Li, C, Wang, Z, et al. Zein-paclitaxel prodrug nanoparticles for redox-triggered drug delivery and enhanced therapeutic efficiency. J Agric Food Chem. (2018) 66:11812–22. doi: 10.1021/acs.jafc.8b04627
22. Huang, W, Yao, F, Tian, S, Liu, M, Liu, G, and Jiang, Y. Recent advances in zein-based nanocarriers for precise cancer therapy. Pharmaceutics. (2023) 15:1820. doi: 10.3390/pharmaceutics15071820
23. Zhang, Y, Cui, L, Che, X, Zhang, H, Shi, N, Li, C, et al. Zein-based films and their usage for controlled delivery: origin, classes and current landscape. J Control Release. (2015) 206:206–19. doi: 10.1016/j.jconrel.2015.03.030
24. Zhang, Y, Cui, L, Chen, Y, Zhang, H, Zhong, J, Sun, Y, et al. Zein-based nanofibres for drug delivery: classes and current applications. Curr Pharm Des. (2015) 21:3199–207. doi: 10.2174/1381612821666150531170448
25. Reyes, FC, Chung, T, Holding, D, Jung, R, Vierstra, R, and Otegui, MS. Delivery of prolamins to the protein storage vacuole in maize aleurone cells. Plant Cell. (2011) 23:769–84. doi: 10.1105/tpc.110.082156
26. Monteiro, RA, Camara, MC, de Oliveira, JL, Campos, EVR, Carvalho, LB, Proença, PLF, et al. Zein based-nanoparticles loaded botanical pesticides in Pest control: An enzyme stimuli-responsive approach aiming sustainable agriculture. J Hazard Mater. (2021) 417:126004. doi: 10.1016/j.jhazmat.2021.126004
27. Xu, H, Zhang, Y, Jiang, Q, Reddy, N, and Yang, Y. Biodegradable hollow Zein nanoparticles for removal of reactive dyes from wastewater. J Environ Manag. (2013) 125:33–40. doi: 10.1016/j.jenvman.2013.03.050
28. Fabrikov, D, Varga, ÁT, García, MCV, Bélteky, P, Kozma, G, Kónya, Z, et al. Antimicrobial and antioxidant activity of encapsulated tea polyphenols in chitosan/alginate-coated Zein nanoparticles: A possible supplement against fish pathogens in aquaculture. Environ Sci Pollut Res Int. (2024) 31:13673–87. doi: 10.1007/s11356-024-32058-x
29. Forato, LA, Doriguetto, AC, Fischer, H, Mascarenhas, YP, Craievich, AF, and Colnago, LA. Conformation of the Z19 Prolamin by Ftir, Nmr, and Saxs. J Agric Food Chem. (2004) 52:2382–5. doi: 10.1021/jf035020+
30. Cabra, V, Arreguin, R, Vazquez-Duhalt, R, and Farres, A. Effect of temperature and Ph on the secondary structure and processes of oligomerization of 19 Kda alpha-Zein. Biochim Biophys Acta. (2006) 1764:1110–8. doi: 10.1016/j.bbapap.2006.04.002
31. Forato, LA, Bicudo Tde, C, and Colnago, LA. Conformation of alpha Zeins in solid state by Fourier transform Ir. Biopolymers. (2003) 72:421–6. doi: 10.1002/bip.10481
32. Bugs, MR, Forato, LA, Bortoleto-Bugs, RK, Fischer, H, Mascarenhas, YP, Ward, RJ, et al. Spectroscopic characterization and structural modeling of Prolamin from maize and pearl millet. Eur Biophys J. (2004) 33:335–43. doi: 10.1007/s00249-003-0354-3
33. Cabra, V, Arreguin, R, Galvez, A, Quirasco, M, Vazquez-Duhalt, R, and Farres, A. Characterization of a 19 Kda alpha-Zein of high purity. J Agric Food Chem. (2005) 53:725–9. doi: 10.1021/jf048530s
34. Yan, X, Li, M, Xu, X, Liu, X, and Liu, F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: a review. Front Nutr. (2022) 9:999373. doi: 10.3389/fnut.2022.999373
35. Erickson, DP, Ozturk, OK, Selling, G, Chen, F, Campanella, OH, and Hamaker, BR. Corn Zein undergoes conformational changes to higher Beta-sheet content during its self-assembly in an increasingly hydrophilic solvent. Int J Biol Macromol. (2020) 157:232–9. doi: 10.1016/j.ijbiomac.2020.04.169
36. Wang, Y, and Padua, GW. Formation of Zein microphases in ethanol-water. Langmuir. (2010) 26:12897–901. doi: 10.1021/la101688v
37. Wang, Y, and Padua, GW. Nanoscale characterization of Zein self-assembly. Langmuir. (2012) 28:2429–35. doi: 10.1021/la204204j
38. Sturgeon, SR, Brinton, LA, Devesa, SS, and Kurman, RJ. In situ and invasive vulvar cancer incidence trends (1973 to 1987). Am J Obstet Gynecol. (1992) 166:1482. doi: 10.1016/0002-9378(92)91623-i
39. Dong, SR, Xu, HH, Tan, JY, Xie, MM, and Yu, GP. The structure and Amphipathy characteristics of modified gamma-Zeins by Sds or alkali in conjunction with heating treatment. Food Chem. (2017) 233:361–8. doi: 10.1016/j.foodchem.2017.04.128
40. Chen, HI, Einbond, A, Kwak, SJ, Linn, H, Koepf, E, Peterson, S, et al. Characterization of the Ww domain of human yes-associated protein and its Polyproline-containing ligands. J Biol Chem. (1997) 272:17070–7. doi: 10.1074/jbc.272.27.17070
41. Hughes, RE, and Olson, JM. Therapeutic opportunities in Polyglutamine disease. Nat Med. (2001) 7:419–23. doi: 10.1038/86486
42. Fernandez-Carneado, J, Kogan, MJ, Castel, S, and Giralt, E. Potential peptide carriers: amphipathic proline-rich peptides derived from the N-terminal domain of gamma-Zein. Angew Chem Int Ed Eng. (2004) 43:1811–4. doi: 10.1002/anie.200352540
43. Reddy, N, and Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. (2011) 29:490–8. doi: 10.1016/j.tibtech.2011.05.003
44. Hurtado-Lopez, P, and Murdan, S. An investigation into the Adjuvanticity and immunogenicity of Zein microspheres being researched as drug and vaccine carriers. J Pharm Pharmacol. (2006) 58:769–74. doi: 10.1211/jpp.58.6.0007
45. Park, HS, and Nahm, DH. Identification of Ige-binding components in occupational asthma caused by corn dust. Ann Allergy Asthma Immunol. (1997) 79:75–9. doi: 10.1016/S1081-1206(10)63089-X
46. Cabrera-Chavez, F, Iametti, S, Miriani, M, de la Barca, AM, Mamone, G, and Bonomi, F. Maize prolamins resistant to peptic-tryptic digestion maintain immune-recognition by Iga from some celiac disease patients. Plant Foods Hum Nutr. (2012) 67:24–30. doi: 10.1007/s11130-012-0274-4
47. Pasini, G, Simonato, B, Curioni, A, Vincenzi, S, Cristaudo, A, Santucci, B, et al. Ige-mediated allergy to corn: A 50 Kda protein, belonging to the reduced soluble proteins is a major allergen. Allergy. (2002) 57:98–106. doi: 10.1034/j.1398-9995.2002.1o3413.x
48. Lee, SH, Benmoussa, M, Sathe, SK, Roux, KH, Teuber, SS, and Hamaker, BR. A 50 Kda maize gamma-Zein has marked cross-reactivity with the almond major protein. J Agric Food Chem. (2005) 53:7965–70. doi: 10.1021/jf0479618
49. American Association of Cereal Chemists . Maize endosperm proteins compared by sodium dodecyl sulfate gel electrophoresis and isoelectric focusing. Cereal Chem. (1981) 58:275–81.
50. Perumal, OP, Podaralla, SK, and Kaushik, RS (2009). Method of forming non-immunogenic hydrophobic protein nanoparticles and uses therefor. US.
51. Boraschi, D, Italiani, P, Palomba, R, Decuzzi, P, Duschl, A, Fadeel, B, et al. Nanoparticles and innate immunity: new perspectives on host defence. Semin Immunol. (2017) 34:33–51. doi: 10.1016/j.smim.2017.08.013
52. Esmaeili, F, Ghahremani, MH, Esmaeili, B, Khoshayand, MR, Atyabi, F, and Dinarvand, R. Plga nanoparticles of different surface properties: preparation and evaluation of their body distribution. Int J Pharm. (2008) 349:249–55. doi: 10.1016/j.ijpharm.2007.07.038
53. Williams, DF (1987). “Definitions in biomaterials” in Proceedings of a Consensus Conference of the European Society for Biomaterials; Proceedings.
54. Fournier, E, Passirani, C, Montero-Menei, CN, and Benoit, JP. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. (2003) 24:3311–31. doi: 10.1016/s0142-9612(03)00161-3
55. Williams, DF . On the mechanisms of biocompatibility. Biomaterials. (2008) 29:2941–53. doi: 10.1016/j.biomaterials.2008.04.023
56. Middleton, JC, and Tipton, AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. (2000) 21:2335–46. doi: 10.1016/s0142-9612(00)00101-0
57. Crawford, L, Wyatt, M, Bryers, J, and Ratner, B. Biocompatibility evolves: phenomenology to toxicology to regeneration. Adv Healthcare Mater. (2021) 10:e2002153. doi: 10.1002/adhm.202002153
58. Demir, M, Ramos-Rivera, L, Silva, R, Nazhat, SN, and Boccaccini, AR. Zein-based composites in biomedical applications. J Biomed Mater Res A. (2017) 105:1656–65. doi: 10.1002/jbm.a.36040
59. Dong, J, Sun, Q, and Wang, JY. Basic study of corn protein, Zein, as a biomaterial in tissue engineering. Surf Morphol Biocompatib Biomater. (2004) 25:4691–7. doi: 10.1016/j.biomaterials.2003.10.084
60. Liu, Q, Qin, Y, Jiang, B, Chen, J, and Zhang, T. Development of self-assembled Zein-Fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf B: Biointerfaces. (2022) 216:112529. doi: 10.1016/j.colsurfb.2022.112529
61. Liu, X, Xie, Y, Li, W, Sheng, W, Li, Y, Tong, Z, et al. Structure, physical properties, hemocompatibility and cytocompatibility of starch/zein composites. Biomed Mater Eng. (2015) 25:47–55. doi: 10.3233/BME-141227
62. Gong, S, Wang, H, Sun, Q, Xue, ST, and Wang, JY. Mechanical properties and in vitro biocompatibility of porous Zein scaffolds. Biomaterials. (2006) 27:3793–9. doi: 10.1016/j.biomaterials.2006.02.019
63. Wang, HJ, Gong, SJ, Lin, ZX, Fu, JX, Xue, ST, Huang, JC, et al. In vivo biocompatibility and mechanical properties of porous Zein scaffolds. Biomaterials. (2007) 28:3952–64. doi: 10.1016/j.biomaterials.2007.05.017
64. Kim, Y, Park, CH, An, JS, Choi, SH, and Kim, TW. Biocompatible artificial synapses based on a Zein active layer obtained from maize for neuromorphic computing. Sci Rep. (2021) 11:20633. doi: 10.1038/s41598-021-00076-1
65. Song, J, Sun, C, Gul, K, Mata, A, and Fang, Y. Prolamin-based complexes: structure design and food-related applications. Compr Rev Food Sci Food Saf. (2021) 20:1120–49. doi: 10.1111/1541-4337.12713
66. Caicedo Chacon, WD, Verruck, S, Monteiro, AR, and Valencia, GA. The mechanism biopolymers and active compounds for the production of nanoparticles by anti-solvent precipitation: a review. Food Res Int. (2023) 168:112728. doi: 10.1016/j.foodres.2023.112728
67. Lepeltier, E, Bourgaux, C, and Couvreur, P. Nanoprecipitation and the "ouzo effect": application to drug delivery devices. Adv Drug Deliv Rev. (2014) 71:86–97. doi: 10.1016/j.addr.2013.12.009
68. Vratsanos, MA, Xue, W, Rosenmann, ND, Zarzar, LD, and Gianneschi, NC. Ouzo effect examined at the nanoscale via direct observation of droplet nucleation and morphology. ACS Cent Sci. (2023) 9:457–65. doi: 10.1021/acscentsci.2c01194
69. Aubry, J, Ganachaud, F, Cohen Addad, JP, and Cabane, B. Nanoprecipitation of polymethylmethacrylate by solvent shifting: 1. Bound Langmuir. (2009) 25:1970–9. doi: 10.1021/la803000e
70. Tortorella, S, Maturi, M, Vetri Buratti, V, Vozzolo, G, Locatelli, E, Sambri, L, et al. Zein as a versatile biopolymer: different shapes for different biomedical applications. RSC Adv. (2021) 11:39004–26. doi: 10.1039/d1ra07424e
71. Lince, F, Marchisio, DL, and Barresi, AA. Strategies to control the particle size distribution of poly-epsilon-caprolactone nanoparticles for pharmaceutical applications. J Colloid Interface Sci. (2008) 322:505–15. doi: 10.1016/j.jcis.2008.03.033
72. Luo, Y, Wang, TT, Teng, Z, Chen, P, Sun, J, and Wang, Q. Encapsulation of indole-3-carbinol and 3, 3'-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. (2013) 139:224–30. doi: 10.1016/j.foodchem.2013.01.113
73. Ye, G, Wu, T, Li, Z, Teng, M, Ma, L, Qin, M, et al. Preparation and characterization of novel composite nanoparticles using zein and hyaluronic acid for efficient delivery of Naringenin. Food Chem. (2023) 417:135890. doi: 10.1016/j.foodchem.2023.135890
74. Lonare, AA, and Patel, SR. Antisolvent crystallization of poorly water soluble drugs. Int J Chem Eng Appl. (2013) 4:337–41. doi: 10.7763/IJCEA.2013.V4.321
75. Yu, X, Han, N, Dong, Z, Dang, Y, Zhang, Q, Hu, W, et al. Combined chemo-immuno-photothermal therapy for effective cancer treatment via an all-in-one and one-for-all nanoplatform. ACS Appl Mater Interfaces. (2022) 14:42988–3009. doi: 10.1021/acsami.2c12969
76. Liu, G, An, D, Li, J, and Deng, S. Zein-based nanoparticles: preparation, characterization, and pharmaceutical application. Front Pharmacol. (2023) 14:1120251. doi: 10.3389/fphar.2023.1120251
77. Zheng, H, Wang, J, You, F, Zhou, M, and Shi, S. Fabrication, characterization, and antimicrobial activity of carvacrol-loaded zein nanoparticles using the Ph-driven method. Int J Mol Sci. (2022) 23:9227. doi: 10.3390/ijms23169227
78. Xu, X, Peng, X, Huan, C, Chen, J, Meng, Y, and Fang, S. Development of Natamycin-loaded Zein-casein composite nanoparticles by a Ph-driven method and application to postharvest fungal control on peach against Monilinia Fructicola. Food Chem (2023) 404::134659. doi: 10.1016/j.foodchem.2022.134659
79. Yuan, Y, Xiao, J, Zhang, P, Ma, M, Wang, D, and Xu, Y. Development of Ph-driven zein/tea saponin composite nanoparticles for encapsulation and oral delivery of curcumin. Food Chem. (2021) 364:130401. doi: 10.1016/j.foodchem.2021.130401
80. Zhang, R, Han, Y, Xie, W, Liu, F, and Chen, S. Advances in protein-based nanocarriers of bioactive compounds: from microscopic molecular principles to macroscopical structural and functional attributes. J Agric Food Chem. (2022) 70:6354–67. doi: 10.1021/acs.jafc.2c01936
81. Peng, S, Zou, L, Zhou, W, Liu, W, Liu, C, and McClements, DJ. Encapsulation of lipophilic polyphenols into nanoliposomes using Ph-driven method: advantages and disadvantages. J Agric Food Chem. (2019) 67:7506–11. doi: 10.1021/acs.jafc.9b01602
82. Li, M, Liu, Y, Liu, Y, Zhang, X, Han, D, and Gong, J. Ph-driven self-assembly of alcohol-free curcumin-loaded Zein-propylene glycol alginate complex nanoparticles. Int J Biol Macromol. (2022) 213:1057–67. doi: 10.1016/j.ijbiomac.2022.06.046
83. Sosnik, A, and Seremeta, KP. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv Colloid Interf Sci. (2015) 223:40–54. doi: 10.1016/j.cis.2015.05.003
84. Baumann, JM, Adam, MS, and Wood, JD. Engineering advances in spray drying for pharmaceuticals. Annu Rev Chem Biomol Eng. (2021) 12:217–40. doi: 10.1146/annurev-chembioeng-091720-034106
85. Salama, AH . Spray drying as an advantageous strategy for enhancing pharmaceuticals bioavailability. Drug Deliv Transl Res. (2020) 10:1–12. doi: 10.1007/s13346-019-00648-9
86. Bhujbal, SV, Mitra, B, Jain, U, Gong, Y, Agrawal, A, Karki, S, et al. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm Sin B. (2021) 11:2505–36. doi: 10.1016/j.apsb.2021.05.014
88. Ziaee, A, Albadarin, AB, Padrela, L, Femmer, T, O'Reilly, E, and Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: critical parameters and experimental process optimization approaches. Eur J Pharm Sci. (2019) 127:300–18. doi: 10.1016/j.ejps.2018.10.026
89. Rodriguez-Felix, F, Del-Toro-Sanchez, CL, Javier Cinco-Moroyoqui, F, Juarez, J, Ruiz-Cruz, S, Lopez-Ahumada, GA, et al. Preparation and characterization of quercetin-loaded zein nanoparticles by electrospraying and study of in vitro bioavailability. J Food Sci. (2019) 84:2883–97. doi: 10.1111/1750-3841.14803
90. Baspinar, Y, Ustundas, M, Bayraktar, O, and Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: development and in-vitro characterisation. Saudi Pharm J. (2018) 26:323–34. doi: 10.1016/j.jsps.2018.01.010
91. Douroumis, D, Ross, SA, and Nokhodchi, A. Advanced methodologies for cocrystal synthesis. Adv Drug Deliv Rev. (2017) 117:178–95. doi: 10.1016/j.addr.2017.07.008
92. Ozkan, G, Franco, P, De Marco, I, Xiao, J, and Capanoglu, E. A review of microencapsulation methods for food antioxidants: principles, advantages, drawbacks and applications. Food Chem. (2019) 272:494–506. doi: 10.1016/j.foodchem.2018.07.205
93. Beck-Broichsitter, M, Schweiger, C, Schmehl, T, Gessler, T, Seeger, W, and Kissel, T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J Control Release. (2012) 158:329–35. doi: 10.1016/j.jconrel.2011.10.030
94. Kalani, M, and Yunus, R. Effect of supercritical fluid density on nanoencapsulated drug particle size using the supercritical antisolvent method. Int J Nanomedicine. (2012) 7:2165–72. doi: 10.2147/IJN.S29805
95. Kakran, M, Sahoo, NG, Antipina, MN, and Li, L. Modified supercritical antisolvent method with enhanced mass transfer to fabricate drug nanoparticles. Mater Sci Eng C Mater Biol Appl. (2013) 33:2864–70. doi: 10.1016/j.msec.2013.03.002
96. Liu, M, Liu, Y, Ge, Y, Zhong, Z, Wang, Z, Wu, T, et al. Solubility, antioxidation, and oral bioavailability improvement of mangiferin microparticles prepared using the supercritical antisolvent method. Pharmaceutics. (2020) 12:90. doi: 10.3390/pharmaceutics12020090
97. Abuzar, SM, Hyun, SM, Kim, JH, Park, HJ, Kim, MS, Park, JS, et al. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical Antisolvent (Sas) process. Int J Pharm. (2018) 538:1–13. doi: 10.1016/j.ijpharm.2017.12.041
98. Hu, D, Lin, C, Liu, L, Li, S, and Zhao, Y. Preparation, characterization, and in vitro release investigation of lutein/Zein nanoparticles via solution enhanced dispersion by supercritical fluids. J Food Eng. (2012) 109:545–52. doi: 10.1016/j.jfoodeng.2011.10.025
99. Li, S, and Zhao, Y. Preparation of zein nanoparticles by using solution-enhanced dispersion with supercritical co (2) and elucidation with computational fluid dynamics. Int J Nanomedicine. (2017) 12:3485–94. doi: 10.2147/IJN.S135239
100. Soppimath, KS, Aminabhavi, TM, Kulkarni, AR, and Rudzinski, WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. (2001) 70:1–20. doi: 10.1016/s0168-3659(00)00339-4
101. Szczech, M, and Szczepanowicz, K. Polymeric core-shell nanoparticles prepared by spontaneous emulsification solvent evaporation and functionalized by the layer-by-layer method. Nanomaterials. (2020) 10:496. doi: 10.3390/nano10030496
102. Paswan, SK, and Saini, TR. Purification of drug loaded Plga nanoparticles prepared by emulsification solvent evaporation using stirred cell ultrafiltration technique. Pharm Res. (2017) 34:2779–86. doi: 10.1007/s11095-017-2257-5
103. Pulingam, T, Foroozandeh, P, Chuah, JA, and Sudesh, K. Exploring various techniques for the chemical and biological synthesis of polymeric nanoparticles. Nano. (2022) 12:576. doi: 10.3390/nano12030576
104. Wei, Y, Yu, Z, Lin, K, Sun, C, Dai, L, Yang, S, et al. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. (2019) 95:336–48. doi: 10.1016/j.foodhyd.2019.04.048
105. Cervato, G, Cazzola, R, and Cestaro, B. Studies on the antioxidant activity of Milk caseins. Int J Food Sci Nutr. (1999) 50:291–6. doi: 10.1080/096374899101175
106. Khan, IT, Nadeem, M, Imran, M, Ullah, R, Ajmal, M, and Jaspal, MH. Antioxidant properties of milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. (2019) 18:41. doi: 10.1186/s12944-019-0969-8
107. Liao, W, Gharsallaoui, A, Dumas, E, and Elaissari, A. Understanding of the key factors influencing the properties of emulsions stabilized by sodium caseinate. Compr Rev Food Sci Food Saf. (2022) 21:5291–317. doi: 10.1111/1541-4337.13062
108. Andre de Almeida Campos, L, Francisco Silva Neto, A, Cecilia Souza Noronha, M, Ferreira de Lima, M, Macario Ferro Cavalcanti, I, and Stela Santos-Magalhaes, N. Zein nanoparticles for drug delivery: preparation methods and biological applications. Int J Pharm. (2023) 635:122754. doi: 10.1016/j.ijpharm.2023.122754
109. Patel, AR, Bouwens, EC, and Velikov, KP. Sodium caseinate stabilized zein colloidal particles. J Agric Food Chem. (2010) 58:12497–503. doi: 10.1021/jf102959b
110. Zhang, Y, Niu, Y, Luo, Y, Ge, M, Yang, T, Yu, LL, et al. Fabrication, characterization and antimicrobial activities of thymol-loaded Zein nanoparticles stabilized by sodium Caseinate-chitosan hydrochloride double layers. Food Chem. (2014) 142:269–75. doi: 10.1016/j.foodchem.2013.07.058
111. Liu, J, Zhang, Y, Liu, W, Gao, B, and Yu, LL. A novel Zein-based composite nanoparticles for improving bioaccessibility and anti-inflammatory activity of resveratrol. Food Secur. (2021) 10:269–75. doi: 10.3390/foods10112773
112. Chang, C, Wang, T, Hu, Q, and Luo, Y. Zein/Caseinate/pectin complex nanoparticles: formation and characterization. Int J Biol Macromol. (2017) 104:117–24. doi: 10.1016/j.ijbiomac.2017.05.178
113. Huang, Y, Zhan, Y, Luo, G, Zeng, Y, McClements, DJ, and Hu, K. Curcumin encapsulated Zein/Caseinate-alginate nanoparticles: release and antioxidant activity under in vitro simulated gastrointestinal digestion. Curr Res Food Sci. (2023) 6:100463. doi: 10.1016/j.crfs.2023.100463
114. Mohamed, WA, El-Nekhily, NA, Mahmoud, HE, Hussein, AA, and Sabra, SA. Prodigiosin/celecoxib-loaded into Zein/sodium Caseinate nanoparticles as a potential therapy for triple negative breast Cancer. Sci Rep. (2024) 14:181. doi: 10.1038/s41598-023-50531-4
115. Rizeq, BR, Younes, NN, Rasool, K, and Nasrallah, GK. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int J Mol Sci. (2019) 20:5776. doi: 10.3390/ijms20225776
116. Luo, Y, Zhang, B, Whent, M, Yu, LL, and Wang, Q. Preparation and characterization of Zein/chitosan complex for encapsulation of alpha-tocopherol, and its in vitro controlled release study. Colloids Surf B: Biointerfaces. (2011) 85:145–52. doi: 10.1016/j.colsurfb.2011.02.020
117. Luo, Y, Teng, Z, and Wang, Q. Development of Zein nanoparticles coated with Carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem. (2012) 60:836–43. doi: 10.1021/jf204194z
118. Liang, J, Yan, H, Wang, X, Zhou, Y, Gao, X, Puligundla, P, et al. Encapsulation of epigallocatechin Gallate in Zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. (2017) 231:19–24. doi: 10.1016/j.foodchem.2017.02.106
119. Shehzad, Q, Liu, Z, Zuo, M, and Wang, J. The role of polysaccharides in improving the functionality of Zein coated Nanocarriers: implications for colloidal stability under environmental stresses. Food Chem. (2024) 431:136967. doi: 10.1016/j.foodchem.2023.136967
120. Mohebbi, S, Nezhad, MN, Zarrintaj, P, Jafari, SH, Gholizadeh, SS, Saeb, MR, et al. Chitosan in biomedical engineering: a critical review. Curr Stem Cell Res Ther. (2019) 14:93–116. doi: 10.2174/1574888X13666180912142028
121. Li, MF, Chen, L, Xu, MZ, Zhang, JL, Wang, Q, Zeng, QZ, et al. The formation of Zein-chitosan complex Coacervated particles: relationship to encapsulation and controlled release properties. Int J Biol Macromol. (2018) 116:1232–9. doi: 10.1016/j.ijbiomac.2018.05.107
122. Zhang, H, Liu, R, Wang, J, Cui, SW, Wang, S, Wang, B, et al. Fabrication, characterization, and lipid-lowering effects of Naringenin-Zein-sodium Caseinate-Galactosylated chitosan nanoparticles. Int J Biol Macromol. (2023) 230:123150. doi: 10.1016/j.ijbiomac.2023.123150
123. Cai, Z, Chen, L, Yu, X, Yagoub, AEA, Okonkwo, CE, and Zhou, C. Effect of molecular weight of chitosan on the formation and properties of Zein-Nisin-chitosan Nanocomplexes. Carbohydr Polym. (2022) 292:119664. doi: 10.1016/j.carbpol.2022.119664
124. Liu, J, Li, Y, Zhang, H, Liu, S, Yang, M, Cui, M, et al. Fabrication, characterization and functional attributes of Zein-egg White derived peptides (Ewdp)-chitosan ternary nanoparticles for encapsulation of curcumin: role of Ewdp. Food Chem. (2022) 372:131266. doi: 10.1016/j.foodchem.2021.131266
125. Li, S, Liu, X, Zhang, X, Fan, L, Wang, F, Zhou, J, et al. Preparation and characterization of Zein-tannic acid nanoparticles/chitosan composite films and application in the preservation of sugar oranges. Food Chem. (2024) 437:137673. doi: 10.1016/j.foodchem.2023.137673
126. Kell, DB, Heyden, EL, and Pretorius, E. The biology of Lactoferrin, an Iron-binding protein that can help defend against viruses and Bacteria. Front Immunol. (2020) 11:1221. doi: 10.3389/fimmu.2020.01221
127. Rawat, P, Kumar, S, Sheokand, N, Raje, CI, and Raje, M. The multifunctional glycolytic protein Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) is a novel macrophage Lactoferrin receptor. Biochem Cell Biol. (2012) 90:329–38. doi: 10.1139/o11-058
128. Jiang, R, Lopez, V, Kelleher, SL, and Lonnerdal, B. Apo-and Holo-Lactoferrin are both internalized by Lactoferrin receptor via Clathrin-mediated endocytosis but differentially affect Erk-signaling and cell proliferation in Caco-2 cells. J Cell Physiol. (2011) 226:3022–31. doi: 10.1002/jcp.22650
129. Fillebeen, C, Descamps, L, Dehouck, MP, Fenart, L, Benaissa, M, Spik, G, et al. Receptor-mediated transcytosis of Lactoferrin through the blood-brain barrier. J Biol Chem. (1999) 274:7011–7. doi: 10.1074/jbc.274.11.7011
130. Milewska, A, Zarebski, M, Nowak, P, Stozek, K, Potempa, J, and Pyrc, K. Human coronavirus Nl63 utilizes Heparan sulfate proteoglycans for attachment to target cells. J Virol. (2014) 88:13221–30. doi: 10.1128/JVI.02078-14
131. Ando, K, Hasegawa, K, Shindo, K, Furusawa, T, Fujino, T, Kikugawa, K, et al. Human Lactoferrin activates Nf-Kappab through the toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated Tlr 4 signaling. FEBS J. (2010) 277:2051–66. doi: 10.1111/j.1742-4658.2010.07620.x
132. Chen, Y, Zhao, Z, Xia, G, Xue, F, Chen, C, and Zhang, Y. Fabrication and characterization of Zein/Lactoferrin composite nanoparticles for encapsulating 7, 8-Dihydroxyflavone: enhancement of stability, water solubility and bioaccessibility. Int J Biol Macromol. (2020) 146:179–92. doi: 10.1016/j.ijbiomac.2019.12.251
133. El-Lakany, SA, Elgindy, NA, Helmy, MW, Abu-Serie, MM, and Elzoghby, AO. Lactoferrin-decorated vs Pegylated Zein Nanospheres for combined aromatase inhibitor and herbal therapy of breast Cancer. Expert Opin Drug Deliv. (2018) 15:835–50. doi: 10.1080/17425247.2018.1505858
134. Wang, G, Han, J, Meng, X, Kang, SS, Liu, X, Sun, YE, et al. Zein-based nanoparticles improve the therapeutic efficacy of a Trkb agonist toward Alzheimer's disease. ACS Chem Neurosci. (2023) 14:3249–64. doi: 10.1021/acschemneuro.3c00401
135. Xu, L, Bai, Q, Zhang, X, and Yang, H. Folate-mediated chemotherapy and diagnostics: An updated review and outlook. J Control Release. (2017) 252:73–82. doi: 10.1016/j.jconrel.2017.02.023
136. Choi, SW, and Mason, JB. Folate and carcinogenesis: An integrated scheme. J Nutr. (2000) 130:129–32. doi: 10.1093/jn/130.2.129
137. Paulos, CM, Turk, MJ, Breur, GJ, and Low, PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. (2004) 56:1205–17. doi: 10.1016/j.addr.2004.01.012
138. Turk, MJ, Waters, DJ, and Low, PS. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. (2004) 213:165–72. doi: 10.1016/j.canlet.2003.12.028
139. Parker, N, Turk, MJ, Westrick, E, Lewis, JD, Low, PS, and Leamon, CP. Folate receptor expression in carcinomas and Normal tissues determined by a quantitative Radioligand binding assay. Anal Biochem. (2005) 338:284–93. doi: 10.1016/j.ab.2004.12.026
140. Handali, S, Moghimipour, E, Kouchak, M, Ramezani, Z, Amini, M, Angali, KA, et al. New folate receptor targeted Nano liposomes for delivery of 5-fluorouracil to cancer cells: strong implication for enhanced potency and safety. Life Sci. (2019) 227:39–50. doi: 10.1016/j.lfs.2019.04.030
141. Marko, AJ, Borah, BM, Siters, KE, Missert, JR, Gupta, A, Pera, P, et al. Targeted nanoparticles for fluorescence imaging of folate receptor positive tumors. Biomol Ther. (2020) 10:1651. doi: 10.3390/biom10121651
142. Zhang, DY, Zheng, Y, Zhang, H, Yang, GG, Tan, CP, He, L, et al. Folate receptor-targeted Theranostic Irs (X) nanoparticles for multimodal imaging-guided combined chemo-Photothermal therapy. Nanoscale. (2018) 10:22252–62. doi: 10.1039/c8nr08095j
143. Wu, Z, Li, J, Zhang, X, Li, Y, Wei, D, Tang, L, et al. Rational fabrication of folate-conjugated zein/soy lecithin/carboxymethyl chitosan core-shell nanoparticles for delivery of docetaxel. ACS Omega. (2022) 7:13371–81. doi: 10.1021/acsomega.2c01270
144. Soe, ZC, Ou, W, Gautam, M, Poudel, K, Kim, BK, Pham, LM, et al. Development of folate-functionalized Pegylated Zein nanoparticles for ligand-directed delivery of paclitaxel. Pharmaceutics. (2019) 11:562. doi: 10.3390/pharmaceutics11110562
145. Wang, H, Zhu, W, Huang, Y, Li, Z, Jiang, Y, and Xie, Q. Facile encapsulation of hydroxycamptothecin nanocrystals into zein-based nanocomplexes for active targeting in drug delivery and cell imaging. Acta Biomater. (2017) 61:88–100. doi: 10.1016/j.actbio.2017.04.017
146. Wusigale,, Wang, T, Hu, Q, Xue, J, Khan, MA, Liang, L, et al. Partition and stability of folic acid and caffeic acid in hollow zein particles coated with chitosan. Int J Biol Macromol. (2021) 183:2282–92. doi: 10.1016/j.ijbiomac.2021.05.216
147. Hou, H, Zhang, D, Zeng, J, Zhou, L, Wang, Z, Yao, M, et al. Bilayer Nanocarriers with protein-acid conjugation for prolonged release and enhanced anticancer effects. Langmuir. (2019) 35:3710–6. doi: 10.1021/acs.langmuir.8b02882
148. Wu, A, Chen, C, Lu, J, Sun, J, Xiao, M, Yue, X, et al. Preparation of oral core-shell zein nanoparticles to improve the bioavailability of glycyrrhizic acid for the treatment of ulcerative colitis. Biomacromolecules. (2022) 23:210–25. doi: 10.1021/acs.biomac.1c01233
149. Vasvani, S, Kulkarni, P, and Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol. (2020) 151:1012–29. doi: 10.1016/j.ijbiomac.2019.11.066
150. Toole, BP . Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. (2004) 4:528–39. doi: 10.1038/nrc1391
151. Gupta, RC, Lall, R, Srivastava, A, and Sinha, A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci. (2019) 6:192. doi: 10.3389/fvets.2019.00192
152. Tsuji, R, Ogata, S, and Mochizuki, S. Interaction between Cd44 and highly condensed hyaluronic acid through crosslinking with proteins. Bioorg Chem. (2022) 121:105666. doi: 10.1016/j.bioorg.2022.105666
153. McKee, CM, Penno, MB, Cowman, M, Burdick, MD, Strieter, RM, Bao, C, et al. Hyaluronan (ha) fragments induce chemokine gene expression in alveolar macrophages. The role of ha size and Cd44. J Clin Invest. (1996) 98:2403–13. doi: 10.1172/JCI119054
154. Gronthos, S, Franklin, DM, Leddy, HA, Robey, PG, Storms, RW, and Gimble, JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. (2001) 189:54–63. doi: 10.1002/jcp.1138
155. Ponta, H, Sherman, L, and Herrlich, PA. Cd44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. (2003) 4:33–45. doi: 10.1038/nrm1004
156. Zoller, M . Cd44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. (2011) 11:254–67. doi: 10.1038/nrc3023
157. Li, L, Hao, X, Qin, J, Tang, W, He, F, Smith, A, et al. Antibody against Cd44s inhibits pancreatic tumor initiation and Postradiation recurrence in mice. Gastroenterology. (2014) 146:1108–1118.e12. doi: 10.1053/j.gastro.2013.12.035
158. Liu, L, Yang, S, Chen, F, and Cheng, KW. Hyaluronic acid-Zein Core-Shell nanoparticles improve the anticancer effect of curcumin alone or in combination with Oxaliplatin against colorectal Cancer via Cd44-mediated cellular uptake. Molecules. (2022) 27:1498. doi: 10.3390/molecules27051498
159. Seok, HY, Sanoj Rejinold, N, Lekshmi, KM, Cherukula, K, Park, IK, and Kim, YC. Cd44 targeting biocompatible and biodegradable hyaluronic acid cross-linked Zein Nanogels for curcumin delivery to Cancer cells: in vitro and in vivo evaluation. J Control Release. (2018) 280:20–30. doi: 10.1016/j.jconrel.2018.04.050
160. Zhang, Q, Wang, J, Liu, D, Zhu, W, Guan, S, Fan, L, et al. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr Polym. (2020) 240:116325. doi: 10.1016/j.carbpol.2020.116325
161. Zhang, YR, Lin, R, Li, HJ, He, WL, Du, JZ, and Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2019) 11:e1519. doi: 10.1002/wnan.1519
162. Lee, ES, Gao, Z, and Bae, YH. Recent progress in tumor Ph targeting nanotechnology. J Control Release. (2008) 132:164–70. doi: 10.1016/j.jconrel.2008.05.003
163. Li, Z, Liu, W, Sun, C, Wei, X, Liu, S, and Jiang, Y. Gastrointestinal Ph-sensitive Pickering emulsions stabilized by Zein nanoparticles coated with bioactive Glycyrrhizic acid for improving Oral bioaccessibility of curcumin. ACS Appl Mater Interfaces. (2023). doi: 10.1021/acsami.2c21549
164. Razavi, R, Kenari, RE, Farmani, J, and Jahanshahi, M. Preparation of double-layer Nanoemulsions with controlled release of glucose as prevention of hypoglycemia in diabetic patients. Biomed Pharmacother. (2021) 138:111464. doi: 10.1016/j.biopha.2021.111464
165. Kong, J, Ge, X, Sun, Y, Mao, M, Yu, H, Chu, R, et al. Multi-functional Ph-sensitive active and intelligent packaging based on highly cross-linked Zein for the monitoring of pork freshness. Food Chem. (2023) 404:134754. doi: 10.1016/j.foodchem.2022.134754
166. Hao, L, Lin, G, Chen, C, Zhou, H, Chen, H, and Zhou, X. Phosphorylated Zein as biodegradable and aqueous Nanocarriers for pesticides with sustained-release and anti-Uv properties. J Agric Food Chem. (2019) 67:9989–99. doi: 10.1021/acs.jafc.9b03060
167. Levin, CS, Hofmann, C, Ali, TA, Kelly, AT, Morosan, E, Nordlander, P, et al. Magnetic-plasmonic core-shell nanoparticles. ACS Nano. (2009) 3:1379–88. doi: 10.1021/nn900118a
168. Nosrati, H, Salehiabar, M, Davaran, S, Danafar, H, and Manjili, HK. Methotrexate-conjugated L-lysine coated Iron oxide magnetic nanoparticles for inhibition of Mcf-7 breast Cancer cells. Drug Dev Ind Pharm. (2018) 44:886–94. doi: 10.1080/03639045.2017.1417422
169. Xu, JW, Cui, ZM, Xu, F, and Luo, YL. Preparation and self-assembly of au nanoparticles coordinated Fe3o4 graft block copolymer multifunctional Nanohybrids with Ph, electrochemical and magnetic stimuli responsiveness. J Mater Sci. (2018) 53:1945–61. doi: 10.1007/s10853-017-1675-4
170. Pang, J, Li, Z, Li, S, Lin, S, and Jiang, Y. Folate-conjugated Zein/Fe3o4 Nanocomplexes for the enhancement of cellular uptake and cytotoxicity of Gefitinib. J Mater Sci. (2018) 53:14907–21. doi: 10.1007/s10853-018-2684-7
171. Rahimi Moghadam, M, Zargar, B, and Rastegarzadeh, S. Novel magnetic hollow Zein nanoparticles for Preconcentration of Chlorpyrifos from water and soil samples prior to analysis via high-performance liquid chromatography (Hplc). Analyst. (2018) 143:2174–82. doi: 10.1039/c7an01526g
172. Tan, L, Li, QY, Li, YJ, Ma, RR, He, JY, Jiang, ZF, et al. Specific adsorption and determination of aspartame in soft drinks with a Zein magnetic molecularly imprinted modified Mgce sensor. RSC Adv. (2021) 11:13486–96. doi: 10.1039/d0ra10824c
173. Guo, R, Wang, S, Zhao, L, Zong, Q, Li, T, Ling, G, et al. Engineered nanomaterials for synergistic photo-immunotherapy. Biomaterials. (2022) 282:121425. doi: 10.1016/j.biomaterials.2022.121425
174. Lee, EH, Lee, MK, and Lim, SJ. Enhanced stability of Indocyanine green by encapsulation in Zein-phosphatidylcholine hybrid nanoparticles for use in the phototherapy of Cancer. Pharmaceutics. (2021) 13:305. doi: 10.3390/pharmaceutics13030305
Keywords: Zein, nano-delivery system, nanoparticles, self-assembly, active targeting, intelligent response nanoparticles
Citation: Liu X, Zhang M, Zhou X, Wan M, Cui A, Xiao B, Yang J and Liu H (2024) Research advances in Zein-based nano-delivery systems. Front. Nutr. 11:1379982. doi: 10.3389/fnut.2024.1379982
Edited by:
Xingyu Lin, Zhejiang University, ChinaReviewed by:
Sutapa Biswas Majee, NSHM Knowledge Campus, Kolkata, IndiaKhashayar Sarabandi, Research Institute of Food Science and Technology (RIFST), Iran
Copyright © 2024 Liu, Zhang, Zhou, Wan, Cui, Xiao, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Liu, bGl1aGFpdXNlckAxNjMuY29t
†These authors have contributed equally to this work
 Xiaoxuan Liu
Xiaoxuan Liu Minhong Zhang
Minhong Zhang Xuelian Zhou1
Xuelian Zhou1 Aiping Cui
Aiping Cui Bang Xiao
Bang Xiao Jianqiong Yang
Jianqiong Yang Hai Liu
Hai Liu