- 1The Seventh Clinical Medical College of Guangzhou University of Chinese Medicine, Shenzhen, Guangdong, China
- 2Shenzhen Bao’an Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, Shenzhen, Guangdong, China
Background: Epidemiological studies show dietary habits can have an impact on the risk of cholelithiasis, but the relationship is still unclear. We used a comprehensive Mendelian randomization (MR) study to explore the relationship between dietary habits and cholelithiasis.
Methods: The 18 dietary habits were divided into six categories: meat foods, cereals, vegetables, fruits, dairy products, beverages, and condiments. Cholelithiasis data came from a GWAS meta-analysis and the FinnGen consortium. The inverse variance weighted (IVW), the weighted median (WM), and MR-Egger approaches were used as the main MR analysis methods. In addition, multiple sensitivity analysis and meta-analysis were performed to verify the robustness of the results.
Results: Dried fruit intake [odds ratio (OR) = 0.568; 95% confidence interval (CI), 0.405–0.797; p = 0.001] was discovered to reduce the risk of cholelithiasis. The sensitivity analysis and meta-analysis showed reliable results for the relationship between dried fruit intake and cholelithiasis.
Conclusion: Our study found that dried fruit intake is a protective factor in the development of cholelithiasis. However, the mechanisms of action need to be further explored.
Introduction
Cholelithiasis is a common gastrointestinal disorder that usually has no clinical symptoms (1). Cholelithiasis affects up to 20% of the population in Europe and can cause a loss of up to $1.6 billion per year (2, 3). Studies have shown that 20% to 35% of asymptomatic patients will develop symptomatic cholelithiasis during their lifetime and more than 30,000 people are hospitalized for cholelithiasis each year (3–5). Cholecystectomy is the primary treatment for cholelithiasis, with over 830,000 cholecystectomies carried out annually in the United Kingdom and the United States (6, 7). However, many patients with cholelithiasis do not benefit from cholecystectomy, and the complications of this treatment may reduce the patient’s overall quality of life (6, 8, 9). Gastrointestinal dysfunction and chronic pain are common postoperative complications (10–13). In terms of medication, using generic medications to prevent gallstones is not recommended, even if predisposing factors are present (2). Ursodeoxycholic acid, a commonly used drug for the treatment of cholelithiasis, should only be used in patients with occasional small stones with symptoms (2). Meanwhile, there is controversy in different studies regarding ursodeoxycholic acid for cholelithiasis (14, 15). Therefore, it is necessary to prevent cholelithiasis through modifiable factors (2).
Nutrition intervention, as an important means to intervene in stone development, has the potential to reduce the occurrence of cholelithiasis and promote therapeutic intervention (16, 17). Many recent studies have indicated that dietary factors are linked to cholelithiasis (18, 19). Consuming carbohydrates and saturated fats may increase the risk of forming gallstones. Consumption of protein, fiber, nuts, coffee, and moderate amounts of alcohol may reduce this (20). Moreover, an animal study suggests that a phosphatidylcholine diet helps prevent the formation of gallstones (21). However, the results of observational studies may not be completely reliable because of reverse causality and confounding factors (22). Therefore, it is still necessary to explore the relationship between dietary habits and cholelithiasis. To correctly and reliably assess the relationship between diet and cholelithiasis, MR methods were performed.
MR is a method of epidemiologic investigation that relies on genetic variation to distinguish between observed correlation and causality (23). Meanwhile, MR analysis can generate robust evidence for which interventions should yield health benefits through modifiable exposure to closely related genetic variations (24). It overcomes the shortcomings of randomized controlled trials that are costly, time-consuming, and less feasible (25).
While MR designs have been used to explore the relationship between dietary factors and the risk of a diverse range of diseases, MR analyses of the relationship between dietary factors and cholelithiasis have not yet been performed (26, 27). A comprehensive exploration of the role of dietary habits in cholelithiasis is crucial for the development of nonpharmacologic interventions. This study used a comprehensive MR approach to assess the effects of dietary habits on cholelithiasis.
Study design
Figure 1 provides a flow chart of our study. A comprehensive MR approach was performed to explore the potential effects of dietary habits on cholelithiasis. The MR analysis should meet the three core hypotheses: (1) genetic variable tools are strongly correlated with dietary habits (28); (2) genetic variable tools should be independent of any confounding factors related to cholelithiasis (29); (3) genetic variable tools can only influence cholelithiasis through dietary habits (30). Notably, the sample size had an impact on the estimates of the MR analysis, we used two GWAS data, one for primary analysis and the other for repeated analyses to increase the confidence of the results.
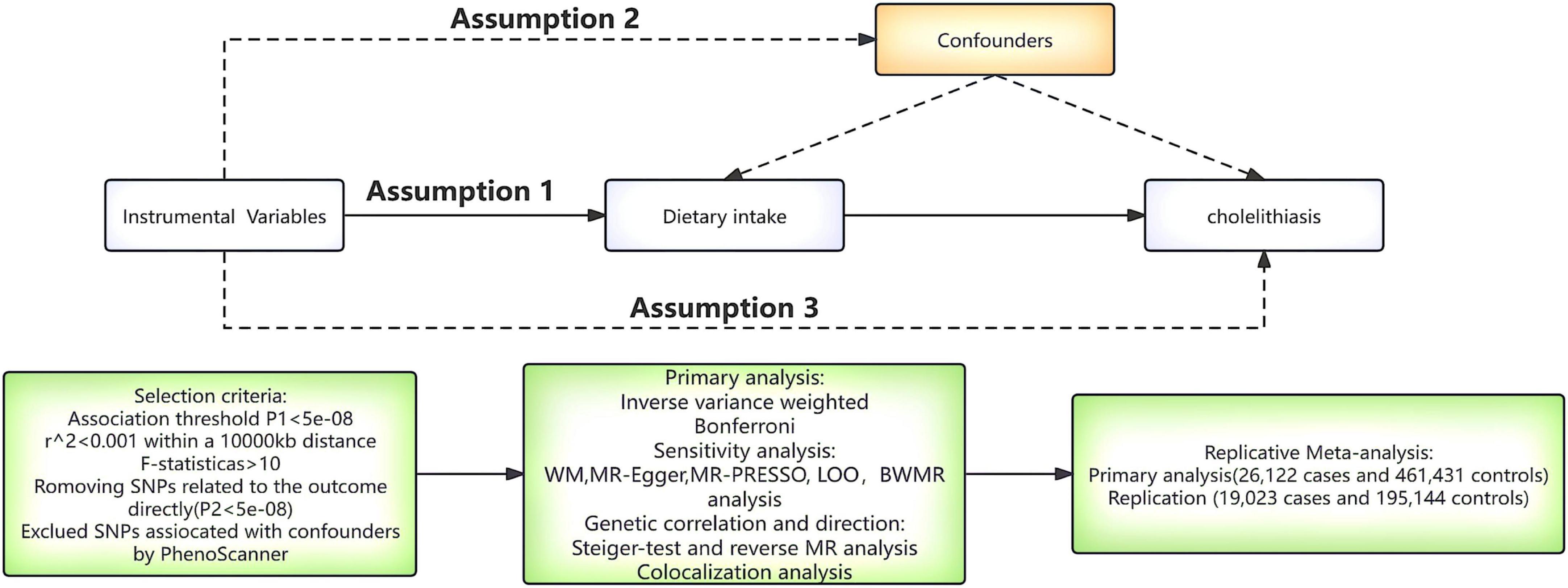
Figure 1. A flow chart of the study. WM, weighted median; MR-PRESSO, MR polytropic residual sums and outliers; LOO, leave-one-out; BWMR, Bayesian weighted Mendelian randomization.
Genome-wide association study (GWAS) data for dietary habits and cholelithiasis
We collected the GWAS data for dietary habits and cholelithiasis from the IEU Open GWAS Project.1
The GWAS data for 18 dietary habits were derived from the UK Biobank, a large population-based survey of genetic and non-genetic factors for disease in middle-aged and older adults (31). The 18 dietary habits were divided into six categories: meat foods (processed meat, beef, mutton, pork, non-oily fish, oily fish, poultry, Lamb/mutton); cereals (cereals, bread); vegetables (salad/raw vegetables, cooked vegetables); fruits (dried fruit, fresh fruit); dairy products (cheese); beverages (coffee, tea, alcohol), and condiments (salt). The GWAS data for cholelithiasis were obtained from two datasets: (1) the pooled data for cholelithiasis for the main analysis came from a GWAS meta-analysis of a mixed population including 26,122 cases and 461,431 controls (32). (2) The second cholelithiasis GWAS data were derived from the FinnGen consortium,2 including the number of 19,023 cases and 195,144 controls (Table 1).
Selection of instrumental variables (IVs)
We employed the following criteria to select the single nucleotide polymorphisms (SNPs) as the valid instrumental variables: (1) we selected SNPs associated with dietary habits (p < 5e-08), making sure they are independent of an aggregate distance of 10,000 kb (r2 < 0.001); (2) The SNPs strongly associated with cholelithiasis (p < 5e-08) were deleted; (3) We tested for associations between instrumental variables and dietary habits using formula F. When F is greater than 10, instrumental variables are considered to effectively avoid bias from weak instruments (33). (4) A palindromic SNP with an intermediate allele frequency was excluded from the analysis to maintain the consistency between the effects of the SNPs on the exposure and the outcome. (5) We removed those SNPs that came out by the MR polytropic residual sums and outliers (MR-PRESSO) test as potentially affecting the results. (6) Since body mass index (BMI) (34), diabetes (35), and cholesterol level (36) were risk factors for the formation of cholelithiasis, we excluded SNPs associated with BMI, diabetes, Triglycerides, and total cholesterol by the PhenoScanner database (Supplementary Table 1).3
Univariate MR analysis
The IVW method is the main method for MR analysis and provides reliable results in the absence of horizontal pleiotropy (37). To improve the reliability of the evaluation results, we used the WM method and the MR-Egger method as a complement to the IVW method (38, 39). The Cochran’s Q test was used to test for heterogeneity, and the MR-Egger intercept was used to assess horizontal pleiotropy (40–42). When heterogeneity or multiplicity was present (p < 0.05), We recognized potential outliers using the MR-PRESSO analysis. MR-PRESSO analysis is used to detect and attempt to reduce level pleiotropy by excluding significant outliers (43). After excluding the outliers, MR analysis was performed again. The leave-one-out (LOO) analysis was used to assess the effect of a single SNP on the outcome (40). Due to multiple testing, the Bonferroni correction (0.003, 0.05/18) was used to adjust the p-value (44).
Bayesian weighted Mendelian randomization (BWMR)
For the significant dietary habits, we performed the BWMR analysis for the evaluation. BWMR considers the uncertainty of weak effects due to the polygenic structure of complex traits, and the problem of violating IV assumptions due to polygenicity (45).
Directionality test and reverse MR analysis
We used the Steiger test and reverse MR analysis to assess whether cholelithiasis also influenced dietary habits. The Steiger test can be used to confirm whether the observed causality deviates due to reverse causality (46). Causal inference was not biased when SNP combinations were found to have no genetic risk for cholelithiasis compared to dietary habits (Steiger p < 0.05). The reverse MR analysis further assessed whether cholelithiasis showed a causal effect on dietary habits.
Multivariate MR analysis (MVMR) and colocalization analysis
Previous MR studies suggest that there may be reciprocal influences because dietary habits are not independent factors (47). We performed a multivariate analysis of the identified dietary factors to assess whether there was a mutual influence between each other. Furthermore, we applied colocalization analysis to test whether the identified dietary habits and cholelithiasis share common causal variants in a given region (48). Based on previous studies, the significant colocalization (posterior probability) was set to PP.H4 > 0.95. When PP.H4 > 0.95, exposure was considered a potential contributing factor (49).
Meta-analysis
For dietary habits significantly associated with cholelithiasis, we used two different GWAS-related data to assess the robustness of our results.
Statistical analysis
We used R software (version 4.3.2) to analyze. The TwoSampleMR (version 0.5.7), color (version 5.2.3), meta (version 6.5-0), and MR-PRESSO (version 1.0) packages were included.
Results
Following a rigorous instrument selection procedure, we performed an MR analysis of 18 dietary habits. All the F- statistics exceed the empirical threshold of 10 (Supplementary Table 2).
Univariate MR analysis
After the Bonferroni correction (p < 0.003), 4 dietary habits were initially identified by IVW as significantly related to cholelithiasis. Among them, intake of cheese (OR = 0.661; 95% CI, 0.542–0.808; p = 5.02 × 10−5), tea (OR = 0.707; 95% CI, 0.566–0.886; p = 0.002), and dried fruit (OR = 0.568; 95% CI, 0.405–0.797; p = 0.001) reduced cholelithiasis scores. In contrast, alcohol intake (OR = 1.272; 95% CI, 1.120–1.446; p = 0.0002) increased cholelithiasis scores (Figure 2 and Supplementary Table 3). The direction and amplitude of the WM and MR-Egger methods remained consistent with the IVW method, which supported the robustness of the causal relationships. The results of the scatter plots indicated the stability of the results (Supplementary Figure 1). The p-value of 4 dietary habits in the MR-Egger intercept were greater than 0.05, which implied that there was no horizontal pleiotropy (Supplementary Table 4). LOO analysis also did not find any SNP with a strong impact on the outcome (Supplementary Figure 2).
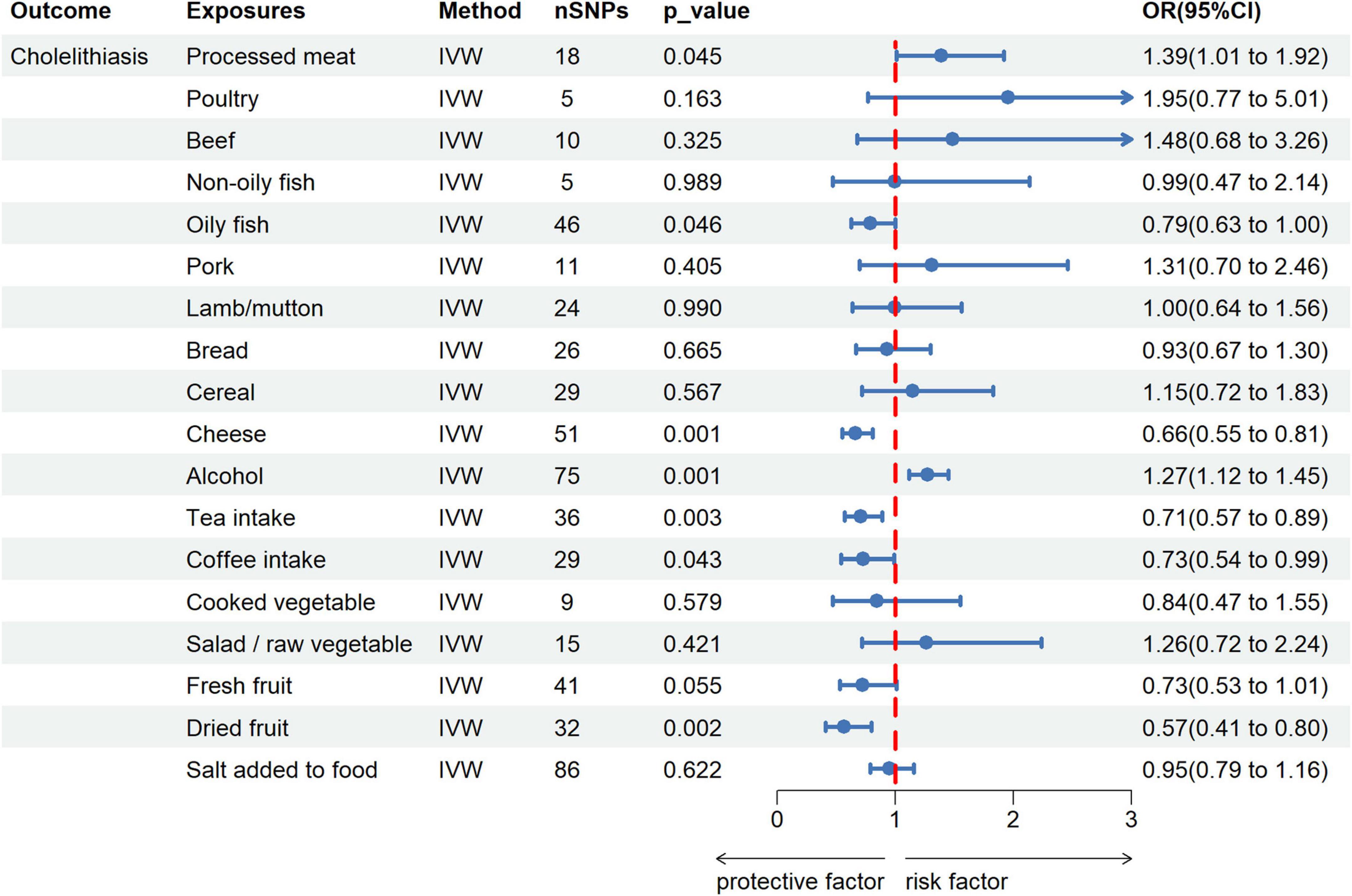
Figure 2. Forest plot for the causal effect of dietary habits on the risk of cholelithiasis. IVW, inverse variance weighted; SNPs, single nucleotide polymorphisms; OR, odds ratio; 95% CI, 95% confidence interval.
BWMR
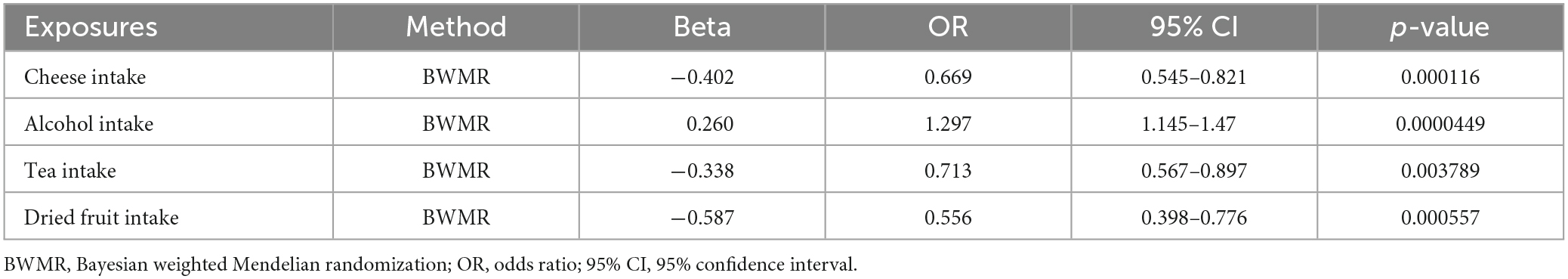
BWMR showed cheese intake (OR = 0.669; 95% CI, 0.545–0.821; p < 0.001), alcohol intake (OR = 1.297; 95% CI, 1.145–1.470; p < 0.001), tea intake (OR = 0.713; 95% CI, 0.567–0.897; p = 0.003) and dried fruit intake (OR = 0.556; 95% CI, 0.398–0.776; p < 0.001) were association with cholelithiasis (Table 2).
Directionality test and reverse MR analysis
The results of the Steiger test did not support a reverse causal effect between dietary habits and cholelithiasis (p < 0.05). Furthermore, reverse MR analysis indicated no association of cholelithiasis with cheese intake (p = 0.114), tea intake (p = 0.117), dried fruit intake (p = 0.424), and alcohol intake (p = 0.674) (Supplementary Table 5).
MVMR and colocalization analysis
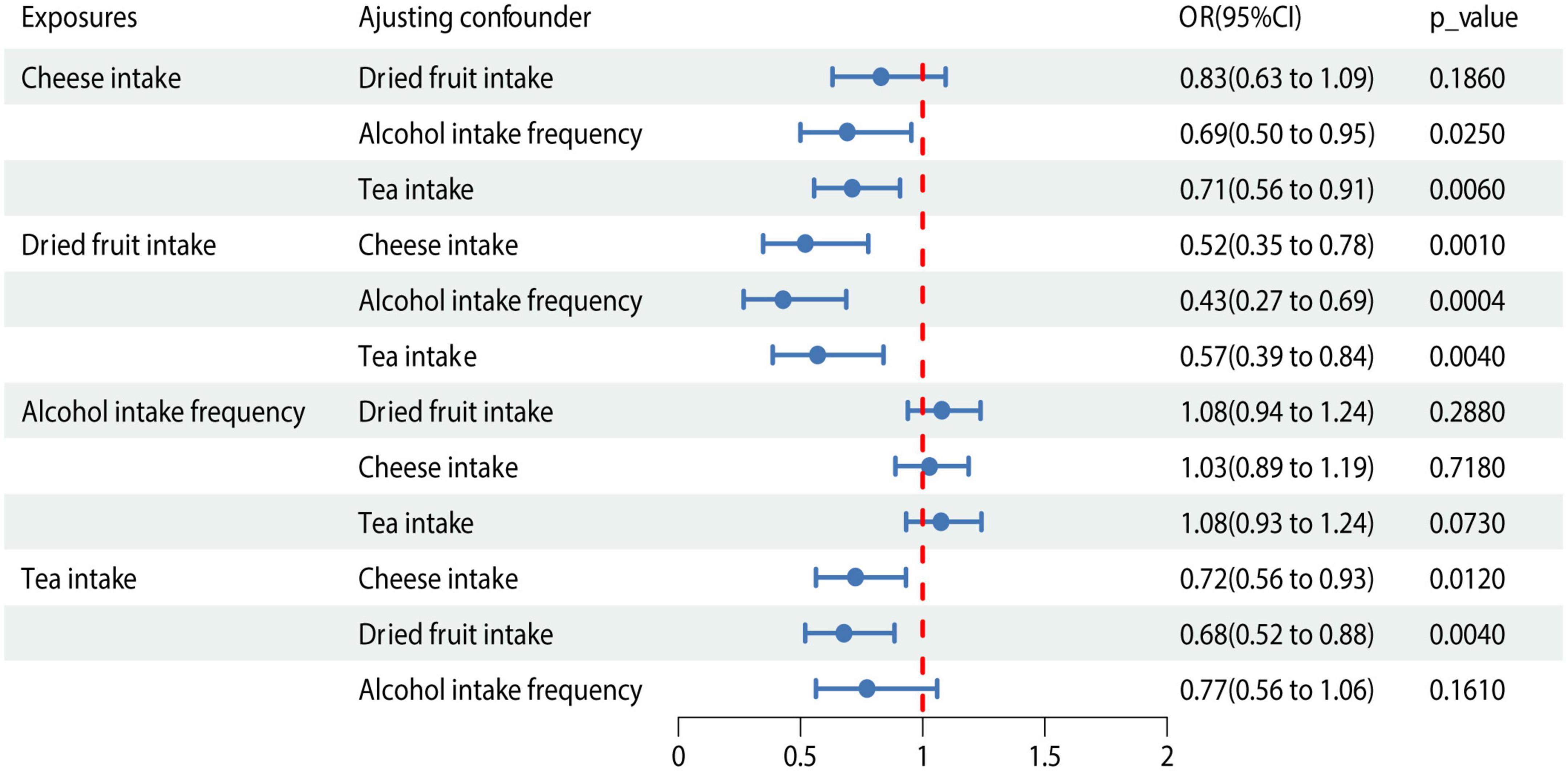
We conducted MVMR analysis of the 4 dietary habits according to the causality determined by the IVW method described above (Figure 3). The association between dried fruit intake and cholelithiasis was still significant in MVMR analysis when adjusted for cheese intake (OR = 0.519; 95% CI, 0.347–0.776; P = 0.001), alcohol intake (OR = 0.428; 95% CI, 0.267–0.687; P < 0.001), and tea intake (OR = 0.569; 95% CI, 0.386–0.839; P = 0.004). Colocalization analysis revealed that dried fruit intake and cholelithiasis shared a causal variant (PP.H4 = 0.952) within the gene region (± 500 kb). Meanwhile, no causal variant shared cheese intake (PP.H4 = 8.74 × 10−19), tea intake (PP.H4 = 0.002), and alcohol intake (PP.H4 = 0.007) with cholelithiasis (Supplementary Table 6).
Meta-analysis
We performed repeated validation using additional GWAS dataset to further confirm the causal relationship between dried fruit intake and cholelithiasis. The result showed that the higher intake of dried fruit intake (OR = 0.61; 95% CI, 0.47–0.79; p < 0.01) was associated with a lower risk of cholelithiasis (Figure 4).
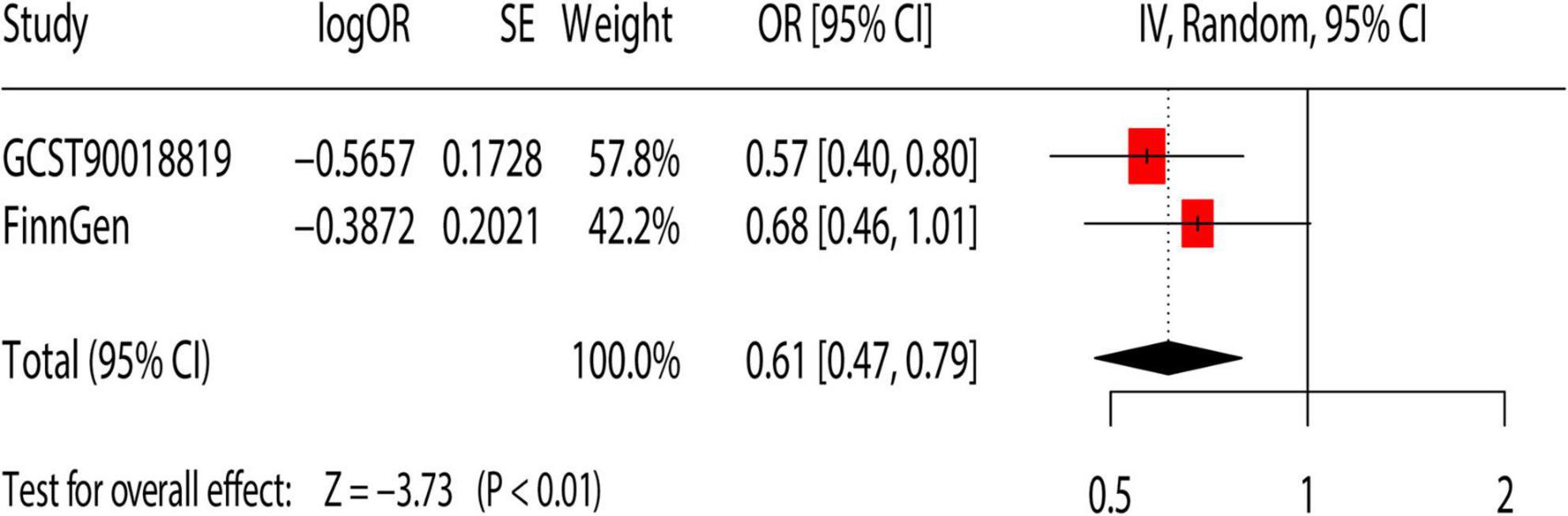
Figure 4. Meta-analysis of the causal association between dietary habits and cholelithiasis. OR, odds ratio; 95% CI, 95% confidence interval; SE, standard error. GCST90018819: Primary analysis of cholelithiasis GWAS; FinnGen: replication analysis of cholelithiasis.
Discussion
We used large-scale GWAS data to assess the impact of 18 dietary habits on the incidence of cholelithiasis. Among the 18 dietary habits, IVW analysis and Bonferroni correction initially identified causal associations between tea intake, cheese intake, dried fruit intake, and alcohol intake and cholelithiasis. MVMR analysis and colocalization analysis indicated that among the 4 dietary habits, dried fruit intake had the most reliable association with cholelithiasis. Finally, the result of the meta-analysis confirmed that a higher intake of dried fruits is associated with a reduced risk of cholelithiasis.
Early cholelithiasis is usually asymptomatic, which increases the difficulty of physician diagnosis and treatment (50). As an independent risk factor for gallbladder cancer, early prevention and intervention should be carried out (51). Dried fruits are healthy snacks for fresh fruit obtained through a variety of drying techniques (52). Dried fruit has a similar nutritional composition to fresh fruit, but it overcomes the defect of the short shelf life of fresh fruit (53). Dried fruit is rich in essential health-promoting substances and nutrients that have an impact on human health (52). Previous studies have linked dried fruit intake to cardiovascular disease, gastrointestinal health, cancer, bone health, etc (54, 55). Our study confirmed from the genetic level that the intake of dried fruit was negatively related to the incidence of cholelithiasis. Multiple sensitivity analyses strongly supported our findings. Therefore, it should be actively advocated that patients with cholelithiasis can appropriately increase their dried fruit intake through dietary intervention to reduce the risk of cholelithiasis.
Studies have indicated that dried fruits are rich in dietary fiber (56). Excretion of bile acids and cholesterol synthesis are crucial steps in the formation of cholelithiasis (57). By promoting the excretion of fecal neutral sterols, dietary fiber can reduce cholesterol (58). Furthermore, supplementation with dietary fibers diminishes the conversion of primary bile acids to secondary bile acids (59). A vitro study found that different types and shapes of raisins have the ability to have bile acids bound to them (60). Our study further confirms the relevance of dried fruit intake in reducing cholelithiasis at the genetic level. However, the potential mechanism of reducing cholelithiasis with dried fruit is currently unclear. More research is needed to further validate the protective mechanisms of dried fruit intake.
Our study is the first MR study to systematically assess the causal relationship between dietary intake and cholelithiasis. We performed strict quality control conditions and used a variety of models to assess causal effects. Furthermore, we used a meta-analysis to validate the credibility of the results. However, there are some shortcomings in our study: (1) All genomic analysis data on dietary factors and cholelithiasis were obtained from the Western populations, and the results would not be extended to other cohorts. (2) We only included 18 dietary factors as exposure, while other dietary factors were not included in the study due to the number of SNPs. (3) Despite our attempts to reduce the bias of confounding factors, some bias may still exist. (4) Due to database limitations, we were only able to determine that dried fruit intake was associated with a reduced risk of cholelithiasis at the genetic level, but we were unable to estimate the ideal amount of dried fruit. (5) The overlap of populations may have some impact on the effect values of the meta-analysis. (6) We found a potential link between dried fruits and cholelithiasis at the gene level. However, studies on dried fruit intake and cholelithiasis are lacking. Therefore, the MR findings should be further verified.
Conclusion
In conclusion, we found that high levels of dried fruit intake help reduce the incidence of cholelithiasis. Further exploration of conservation mechanisms for the intake of dried fruits is needed.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Due to publicly available GWAS summary statistics, there was no need to apply for ethical approval.
Author contributions
LX: Writing – original draft. MX: Methodology, Writing – original draft. YL: Methodology, Software, Writing – review and editing. JL: Software, Writing – original draft. JX: Supervision, Writing – review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Sanming Project of Medicine in Shenzhen (No. SZZYSM202206014).
Acknowledgments
We thank the developers and staff of IEU Open GWAS and the FinnGen consortium.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1377631/full#supplementary-material
Abbreviations
MR, Mendelian randomization; OR, odds ratio; CI, confidence interval; GWAS, genome-wide association study; IVW, inverse variance weighted; WM, weighted median; SNPs, single nucleotide polymorphisms; IVs, instrumental variables; MR-PRESSO, MR polytropic residual sums and outliers; LOO, leave-one-out; BWMR, Bayesian weighted Mendelian randomization.
Footnotes
- ^ https://gwas.mrcieu.ac.uk/, accessed on 12 December 2023
- ^ https://r5.finngen.fi/
- ^ http://www.phenoscanner.medschl.cam.ac.uk/, accessed on 12 December 2023
References
1. Shabanzadeh DM. Incidence of gallstone disease and complications. Curr Opin Gastroenterol. (2018) 34:81–9. doi: 10.1097/mog.0000000000000418
2. Gutt C, Schläfer S, Lammert F. The treatment of gallstone disease. Dtsch Arztebl Int. (2020) 117:148–58. doi: 10.3238/arztebl.2020.0148
3. Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. (2019) 156:254–72.e211. doi: 10.1053/j.gastro.2018.08.063
4. Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Prim. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
5. Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants. (2005) 15:329–38. doi: 10.1615/jlongtermeffmedimplants.v15.i3.90
6. Ransohoff DF, Gracie WA. Treatment of gallstones. Ann Intern Med. (1993) 119:606–19. doi: 10.7326/0003-4819-119-7_part_1-199310010-00010
7. Latenstein CSS, de Reuver PR. Tailoring diagnosis and treatment in symptomatic gallstone disease. Br J Surg. (2022) 109:832–8. doi: 10.1093/bjs/znac154
8. de Jong JJ, Latenstein CSS, Boerma D, Hazebroek EJ, Hirsch D, Heikens JT, et al. Functional dyspepsia and irritable bowel syndrome are highly prevalent in patients with gallstones and are negatively associated with outcomes after cholecystectomy: A prospective, multicenter, observational study (PERFECT – trial). Ann Surg. (2022) 275:e766–72. doi: 10.1097/sla.0000000000004453
9. Mertens MC, Roukema JA, Scholtes VP, De Vries J. Risk assessment in cholelithiasis: Is cholecystectomy always to be preferred? J Gastrointest Surg. (2010) 14:1271–9. doi: 10.1007/s11605-010-1219-6
10. Ros E, Zambon D. Postcholecystectomy symptoms. A prospective study of gall stone patients before and two years after surgery. Gut. (1987) 28:1500–4. doi: 10.1136/gut.28.11.1500
11. Lorusso D, Porcelli P, Pezzolla F, Lantone G, Zivoli G, Guerra V, et al. Persistent dyspepsia after laparoscopic cholecystectomy. The influence of psychological factors. Scand J Gastroenterol. (2003) 38:653–8. doi: 10.1080/00365520310002995
12. Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: A prospective follow-up analysis. Scand J Gastroenterol. (2005) 40:1358–64. doi: 10.1080/00365520510023675
13. Kim H, Han IW, Heo JS, Oh MG, Lim CY, Choi YS, et al. Postcholecystectomy syndrome: Symptom clusters after laparoscopic cholecystectomy. Ann Surg Treat Res. (2018) 95:135–40. doi: 10.4174/astr.2018.95.3.135
14. Venneman NG, Besselink MG, Keulemans YC, Vanberge-Henegouwen GP, Boermeester MA, Broeders IA, et al. Ursodeoxycholic acid exerts no beneficial effect in patients with symptomatic gallstones awaiting cholecystectomy. Hepatology. (2006) 43:1276–83. doi: 10.1002/hep.21182
15. Tomida S, Abei M, Yamaguchi T, Matsuzaki Y, Shoda J, Tanaka N, et al. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: A cohort analysis. Hepatology. (1999) 30:6–13. doi: 10.1002/hep.510300108
16. Ferraro PM, Bargagli M. Dietetic and lifestyle recommendations for stone formers. Arch Esp Urol. (2021) 74:112–22.
17. Martínez García RM, Jiménez Ortega AI, Salas-González MD, Bermejo López LM, Rodríguez-Rodríguez E. [Nutritional intervention in the control of gallstones and renal lithiasis]. Nutr Hosp. (2019) 36:70–4. doi: 10.20960/nh.02813
18. Cuevas A, Miquel JF, Reyes MS, Zanlungo S, Nervi F. Diet as a risk factor for cholesterol gallstone disease. J Am Coll Nutr. (2004) 23:187–96. doi: 10.1080/07315724.2004.10719360
19. Letona AZ, Niot I, Laugerette F, Athias A, Monnot MC, Portillo MP, et al. CLA-enriched diet containing t10,c12-CLA alters bile acid homeostasis and increases the risk of cholelithiasis in mice. J Nutr. (2011) 141:1437–44. doi: 10.3945/jn.110.136168
20. Tseng M, Everhart JE, Sandler RS. Dietary intake and gallbladder disease: A review. Public Health Nutr. (1999) 2:161–72. doi: 10.1017/s136898009900021x
21. Kasbo J, Tuchweber B, Perwaiz S, Bouchard G, Lafont H, Domingo N, et al. Phosphatidylcholine-enriched diet prevents gallstone formation in mice susceptible to cholelithiasis. J Lipid Res. (2003) 44:2297–303. doi: 10.1194/jlr.M300180-JLR200
22. Guo JZ, Xiao Q, Gao S, Li XQ, Wu QJ, Gong TT, et al. Review of Mendelian randomization studies on ovarian cancer. Front Oncol. (2021) 11:681396. doi: 10.3389/fonc.2021.681396
23. Burgess S, Foley CN, Allara E, Staley JR, Howson JMM, et al. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. (2020) 11:376. doi: 10.1038/s41467-019-14156-4
24. Davey Smith G, Hemani G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
25. Zuccolo L, Holmes MV. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int J Epidemiol. (2017) 46:962–5. doi: 10.1093/ije/dyw327
26. Lai W, Li G, Peng D, Li N, Wang W. Mendelian randomization study reveals the relationship between dietary factors and respiratory diseases. Sci Rep. (2023) 13:22601. doi: 10.1038/s41598-023-50055-x
27. Deng Y, Huang J, Wong MCS. Associations between six dietary habits and risk of hepatocellular carcinoma: A Mendelian randomization study. Hepatol Commun. (2022) 6:2147–54. doi: 10.1002/hep4.1960
28. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
29. König IR, Greco FMD. Mendelian randomization: Progressing towards understanding causality. Ann Neurol. (2018) 84:176–7. doi: 10.1002/ana.25293
30. Gagliano Taliun SA, Evans DM. ) Ten simple rules for conducting a mendelian randomization study. PLoS Comput Biol. (2021) 17:e1009238. doi: 10.1371/journal.pcbi.1009238
31. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
32. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
33. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
34. Kim MS, Song M, Kim S, Kim B, Kang W, Kim JY, et al. Causal effect of adiposity on the risk of 19 gastrointestinal diseases: A Mendelian randomization study. Obesity (Silver Spring). (2023) 31:1436–44. doi: 10.1002/oby.23722
35. Chen J, Yuan S, Fu T, Ruan X, Qiao J, Wang X, et al. Gastrointestinal consequences of type 2 diabetes mellitus and impaired glycemic homeostasis: A Mendelian randomization study. Diabetes Care. (2023) 46:828–35. doi: 10.2337/dc22-1385
36. Chen L, Yang H, Li H, He C, Yang L, Lv G. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatology. (2022) 75:785–96. doi: 10.1002/hep.32183
37. Burgess S, Thompson SG. Improving bias and coverage in instrumental variable analysis with weak instruments for continuous and binary outcomes. Stat Med. (2012) 31:1582–600. doi: 10.1002/sim.4498
38. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int J Epidemiol. (2016) 45:1961–74. doi: 10.1093/ije/dyw220
39. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
40. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
41. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
42. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
43. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
44. Dong H, Kong X, Wang X, Liu Q, Fang Y, Wang J. The causal effect of dietary composition on the risk of breast cancer: A Mendelian randomization study. Nutrients. (2023) 15:2586. doi: 10.3390/nu15112586
45. Zhao J, Ming J, Hu X, Chen G, Liu J, Yang C, et al. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics. (2019) 36:1501–8. doi: 10.1093/bioinformatics/btz749
46. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
47. Zou M, Liang Q, Zhang W, Zhu Y, Xu Y. Causal association between dietary factors and esophageal diseases: A Mendelian randomization study. PLoS One. (2023) 18:e0292113. doi: 10.1371/journal.pone.0292113
48. Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. (2014) 10:e1004383. doi: 10.1371/journal.pgen.1004383
49. Hu J, Lu J, Lu Q, Weng W, Guan Z, Wag Z, et al. Mendelian randomization and colocalization analyses reveal an association between short sleep duration or morning chronotype and altered leukocyte telomere length. Commun Biol. (2023) 6:1014. doi: 10.1038/s42003-023-05397-7
50. National Institute for Health and Care Excellence [NICE]. National institute for health and care excellence: Guidelines, in gallstone disease: Diagnosis and management of cholelithiasis, cholecystitis and choledocholithiasis. London: National Clinical Guideline Centre (2014).
51. Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol. (2014) 6:99–109. doi: 10.2147/clep.S37357
52. Alasalvar C, Salvadó JS, Ros E. Bioactives and health benefits of nuts and dried fruits. Food Chem. (2020) 314:126192. doi: 10.1016/j.foodchem.2020.126192
53. Carughi A, Feeney MJ, Kris-Etherton P, Fulgoni V III, Kendall CW, et al. Pairing nuts and dried fruit for cardiometabolic health. Nutr J. (2016) 15:23. doi: 10.1186/s12937-016-0142-4
54. Sadler MJ, Gibson S, Whelan K, Ha MA, Lovegrove J, Higgs J. Dried fruit and public health – what does the evidence tell us? Int J Food Sci Nutr. (2019) 70:675–87. doi: 10.1080/09637486.2019.1568398
55. Bolling BW, Aune D, Noh H, Petersen KS, Freisling H. Dried fruits, nuts, and cancer risk and survival: A review of the evidence and future research directions. Nutrients. (2023) 15:1443. doi: 10.3390/nu15061443
56. Alasalvar C, Chang SK, Kris-Etherton PM, Sullivan VK, Petersen KS, Guasch-Ferré M, et al. Dried fruits: Bioactives, effects on gut microbiota, and possible health benefits-an update. Nutrients. (2023) 15:1611. doi: 10.3390/nu15071611
57. Hu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun. (2022) 13:252. doi: 10.1038/s41467-021-27758-8
58. Arjmandi BH, Ahn J, Nathani S, Reeves RD. Dietary soluble fiber and cholesterol affect serum cholesterol concentration, hepatic portal venous short-chain fatty acid concentrations and fecal sterol excretion in rats. J Nutr. (1992) 122:246–53. doi: 10.1093/jn/122.2.246
59. Vahouny GV, Khalafi R, Satchithanandam S, Watkins DW, Story JA, Cassidy MM, et al. Dietary fiber supplementation and fecal bile acids, neutral steroids and divalent cations in rats. J Nutr. (1987) 117:2009–15. doi: 10.1093/jn/117.12.2009
Keywords: diet, dried fruit intake, cholelithiasis, Mendelian randomization, sensitivity
Citation: Xie L, Xu M, Lei Y, Li J and Xie J (2024) The causal relationship between diet habits and cholelithiasis: a comprehensive Mendelian randomization (MR) study. Front. Nutr. 11:1377631. doi: 10.3389/fnut.2024.1377631
Received: 28 January 2024; Accepted: 29 May 2024;
Published: 12 June 2024.
Edited by:
Rahul Gupta, Synergy Institute of Medical Sciences, IndiaReviewed by:
Salvatore Vaccaro, IRCCS Local Health Authority of Reggio Emilia, ItalyAzam Doustmohammadian, Iran University of Medical Sciences, Iran
Copyright © 2024 Xie, Xu, Lei, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajia Xie, eGllamlhamlhYmF6eXlAMTYzLmNvbQ==
 Lin Xie
Lin Xie Mingzhi Xu
Mingzhi Xu Yahan Lei
Yahan Lei Juan Li
Juan Li Jiajia Xie
Jiajia Xie