- 1Department of Clinical Nutrition, College of Applied Medical Science, King Faisal University, Al-Ahsa, Saudi Arabia
- 2Department of Clinical Nutrition, Nottingham University, Nottingham, United Kingdom
Background: Obesity is reaching epidemic proportions with 51% of the population expected to be obese by 2030. Recently, polyphenols have been highlighted as an effective approach to managing obesity and associated risks. Polyphenols are a large class of bioactive plant compounds classified into two major categories: flavonoids which are distinguished by the fundamental C6-C3-C6 skeleton and non-flavonoids.
Objective: This systematic review evaluated the effect of different polyphenol sources in overweight and obese people with and without type 2 diabetes. The primary outcome was lipid profile and the secondary outcomes were blood glucose, HbA1c (%), HOMA-IR, weight, and body mass index.
Method: A search was undertaken in PubMed, Web of Science, Medline, and Wiley for randomized control trials that assessed different sources of polyphenols in overweight and obese people with or without type 2 diabetes. The quality of the included studies was assessed using the National Heart, Lung, and Blood Institute Quality Assessment Tool.
Result: The search yielded 935 studies, of which six randomized control trials met the inclusion criteria. Five studies found no significant difference in lipid profile between the control and intervention groups in triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol. However, one study showed significant differences in triglycerides (p = 0.04) and HDL cholesterol (p = 0.05) between the two groups with no significant difference in total cholesterol and LDL cholesterol. There were no significant changes in blood glucose observed in the included studies, with only two studies reporting a significant difference in A1c between the groups. Four studies found no difference in HOMA-IR, while one study showed a significant decrease in HOMA-IR in the intervention group compared to the control group. Three studies reported no difference in BMI or weight between the two groups.
Conclusion: The data associated with the specific health benefits of polyphenols and their sources in people with overweight, obese, and type 2 diabetes are still limited, so further research is required to support their use and prove their benefits.
Introduction
Obesity has reached epidemic proportions, with the World Health Organization (WHO) estimating that there are more than 1.9 billion overweight adults worldwide, of which 650 million are obese (1). In addition, there are more than 340 million overweight or obese children and adolescents between the ages of 5 and 19 (1). By 2030, 51% of the population is expected to be obese (2). Excess weight is associated with a range of metabolic complications such as hypertension, insulin resistance, dyslipidaemia, and type 2 diabetes (3–5), as well as a significant economic burden. The direct costs include healthcare costs for treating related diseases, while indirect costs include lost productivity due to disability or premature death (2, 6). Altering lifestyles to reduce calorie consumption and increase physical activity are difficult to preserve in the long term, therefore, it is necessary to find methods to lower the prevalence of obesity and its associated diseases.
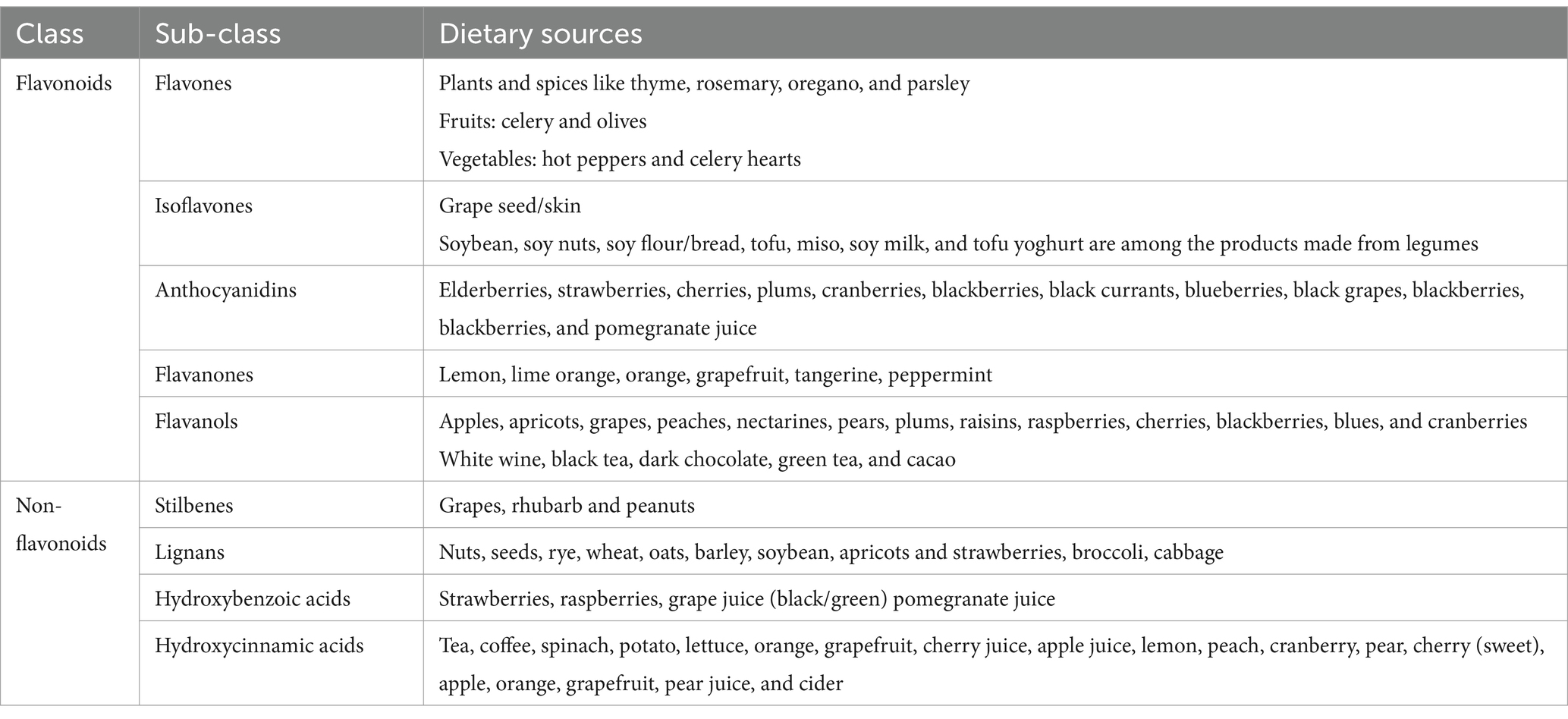
Recently, polyphenols have been highlighted as a practical approach to managing obesity and associated risks. Polyphenols are an enormous class of bioactive plant compounds classified into two major categories: flavonoids which are distinguished by the fundamental C6-C3-C6 skeleton, and non-flavonoids (particularly phenolic acids, stilbenes, and lignans) (7) as shown in Table 1. Flavones, flavonols, isoflavones, flavanones, anthocyanins, and flavanols, commonly known as catechins, are the subclasses that result from the heterocyclic ring connecting the two aromatic rings (7), the primary forms are either conjugated with acid-alcohol or with glycosides in plant food items (7, 8). In addition, polyphenols exist as monomers and oligomers, which are typically referred to as tannins (8). Condensed tannins and hydrolysable tannins are derived from catechin and are usually called procyanidins (8). Phenolic compounds are challenging to quantify because of their diversity and complexity (7, 8). As Polyphenols represent a diverse group of phytochemicals found abundantly in plant-based foods as shown in Table 1.
Flavonoids
This subgroup constitutes the largest and most studied class of polyphenols. Flavonoids are further categorized into subclasses such as flavonols, flavones, flavanones, flavan-3-ols (including catechins), anthocyanins, and isoflavones. They are found in fruits, vegetables, tea, wine, and various herbal remedies (9, 10).
Phenolic acids
These are aromatic compounds found in various plant foods, particularly in fruits (e.g., berries), vegetables, whole grains, and beverages like coffee. Major subclasses include hydroxybenzoic acids (e.g., gallic acid) and hydroxycinnamic acids (e.g., caffeic acid, ferulic acid) (9, 11).
Lignans
Lignans are polyphenolic compounds abundant in seeds, whole grains, fruits, and vegetables. They are converted by intestinal bacteria into enterolignans, which have been associated with various health benefits, including hormone regulation and antioxidant activity (9, 11).
Stilbenes
This subgroup includes compounds such as resveratrol, primarily found in grapes, red wine, peanuts, and berries. Resveratrol has gained attention for its potential health-promoting effects, including anti-inflammatory and cardioprotective properties (9, 11).
Others
This category encompasses a wide range of polyphenolic compounds, including tannins, stilbenoids, and curcuminoids. Tannins are commonly found in tea, wine, and certain fruits, and they contribute to the astringent taste of these foods. Stilbenoids, besides resveratrol, include pterostilbene, found in blueberries and grapes (9, 11). Curcuminoids are derived from turmeric and exhibit various biological activities, including antioxidant and anti-inflammatory properties.
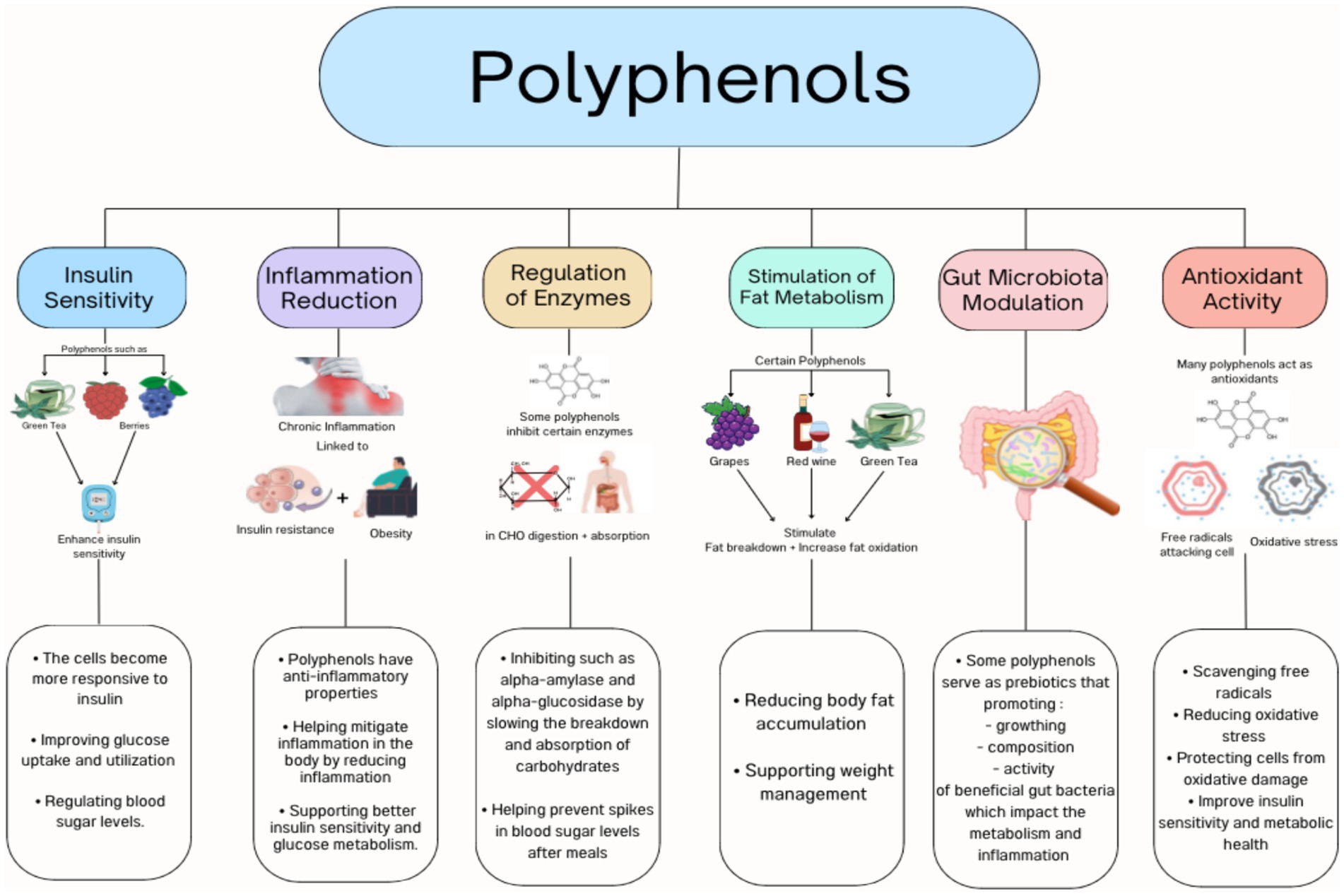
There are several possible mechanisms for the positive effect of polyphenols in obesity and associated risks as shown in Figure 1 including the inhibition of fat absorption from the gastrointestinal tract, glucose uptake by skeletal muscles, the regulation of fat production, appetite suppression, the modulation of the gut microbiota, and reduced chronic inflammation associated with obesity (12, 13).
Numerous epidemiological and clinical studies have investigated the effect of different polyphenols on obesity and associated risks. For example, Shrime et al. (14), revealed that consuming cocoa, which is high in flavanol, reduced insulin resistance. Also, insulin resistance was improved in healthy individuals who consumed pure-epicatechin for a month by alterations in fasting insulin levels without affecting fasting glucose levels (15). In a systematic analysis of six studies, the green coffee extract reduced fasting blood glucose, while only larger dosages helped enhance insulin resistance (16). Following a 12-week olive leaf polyphenols supplementation in middle-aged, overweight men had increased insulin action and secretion (17). A systematic review and meta-analysis of six studies reported that pistachio nut consumption enhanced insulin resistance (18). Pecan and almond eating appear to improve insulin resistance (19, 20). However, a systematic review of walnuts found no impact on fasting blood glucose or other measures of insulin resistance (21).
Habitual consumption of tea was linked to lower body fat in a cross-sectional human study in Taiwan (22). In addition, a longitudinal investigation from the Netherlands showed a lower body mass index among women with higher dietary flavone, flavonol, and catechin consumption (23). Furthermore, clinical trials have shown a positive effect of high doses of catechins in tea drinks, effectively reducing body fat and body weight, especially when combined with an exercise regimen (24, 25). Moreover, a recent meta-analysis showed that consuming resveratrol significantly reduces weight, body mass index, and fat mass (26). However, a systematic review and meta-analysis of 19 RCTs focusing on the effect of anthocyanin on cardiometabolic biomarkers showed that there is no significant effect of anthocyanin supplementation on body mass index, body weight, waist circumference, and blood pressure (27).
Inconsistencies in the findings and methodological limitations of individual studies, as well as inconsistencies in the results of previous systematic reviews and meta-analyses, justify the need for a comprehensive systematic review to assess the impact of polyphenols on obesity and its associated risks. In contrast to previous systematic reviews, different types of polyphenols were included and compared. In addition to the increasing volume of new studies, an updated systematic review is needed. Therefore, this systematic review compared different sources of polyphenols in overweight and obese individuals with or without type 2 diabetes. The primary outcome was lipid profile and the secondary outcomes were blood glucose, HbA1c (%), HOMA-IR, weight, and body mass index.
Methodology
This systematic review was conducted according to The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (28).
Search strategy
A systematic search of the PubMed, Web of Science, Medline and Wiley databases was performed from 13th May 2023 to Jane 2023 using the following terms: “(“resveratrol (3,5,4’trihydroxystilbene)” OR “epigallocatechin 3 gallate” OR “epigallocatechin gallate (ECG)” OR “epigallocatechin” OR “quercetin” OR “flavonoids” OR “Polyphenols” OR “tannins” OR “lignans” OR “stilbenes” OR “green tea catechins” OR “catechins” OR “epicatechin” OR “procyanidin” OR “genistein” OR “provinols” OR “phenolic acids” OR “apigenin” OR “blackberry polyphenols” OR “curcumin” OR “grape polyphenols” OR “cranberry polyphenols” OR “strawberry polyphenols”) AND (“insulin resistant” OR “insulin receptor substrate” OR “insulin receptor pathway” OR “GLUT” OR“insulin sensitivity”) AND (“Lipid” OR “HDL” OR “LDL” OR “Total cholesterol” OR “Triglycerides”) AND (“fat accumulation” OR “obesity” OR “overweight” OR “hyperlipidemia” AND “glucose” OR “blood sugar”) AND (“clinical trial”). In addition to manual search in references list. All identified studies were saved in EndNote and duplicates were removed.
The criterion for selecting the types of foods presented in the search terms was based on their known richness in polyphenols, as documented in scientific literature. We considered foods that are commonly recognized for their high polyphenol content and relevance to human consumption. Additionally, we aimed to include a diverse range of food sources to provide a comprehensive overview of polyphenol intake. However, there are many other sources of polyphenol not included in our search terms, including olives oil and that should be considered in future research.
Eligibility criteria
The current systematic review examined study eligibility using a population comparison and outcome as shown in Table 2:
• Study design: randomized control trial to address the research question
• Population: Overweight and obese adults with or without type 2 diabetes
• Intervention: different sources of polyphenols
• Outcomes: The primary outcome is lipid profile and secondary outcomes are blood glucose, HbA1c (%), weight and body mass index.
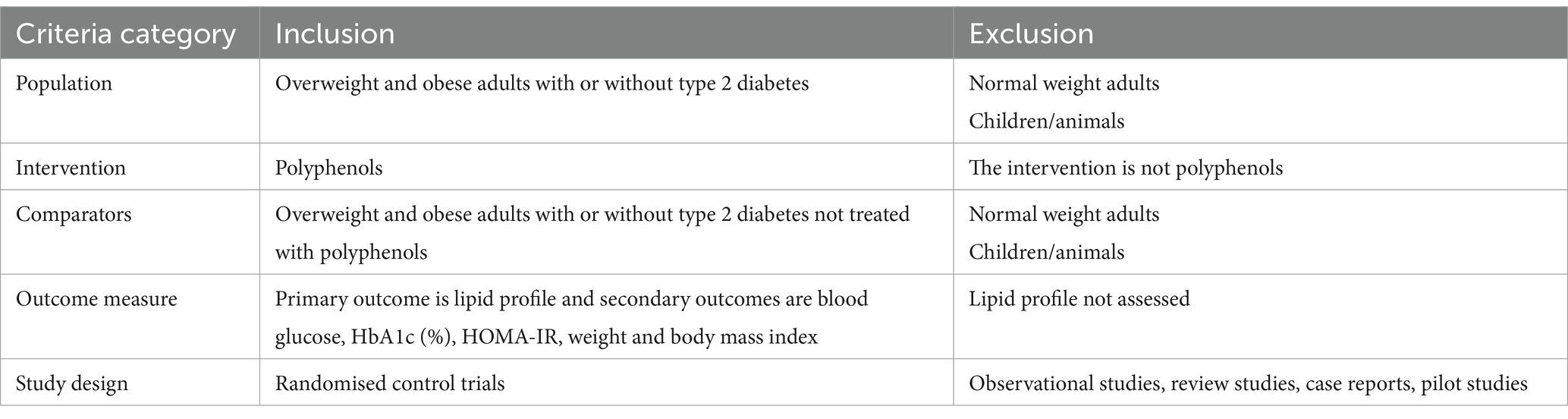
Table 2. Population–intervention–comparison–outcome (PICOS) criteria for study inclusion and exclusion.
Exclusion criteria
• Review studies
• Case reports
• Observation study
• Non-English studies
• The full text of the study was not available.
Data extraction and synthesis
The following data were extracted from the studies: the author’s name, the year of publication, the study location, the study design, the target population, the sample size, the baseline characteristics of participants, and the primary outcome lipid profile. The secondary outcomes were blood glucose, weight, and body mass index. A meta-analysis was not performed due to the heterogeneity of the data.
Quality assessment
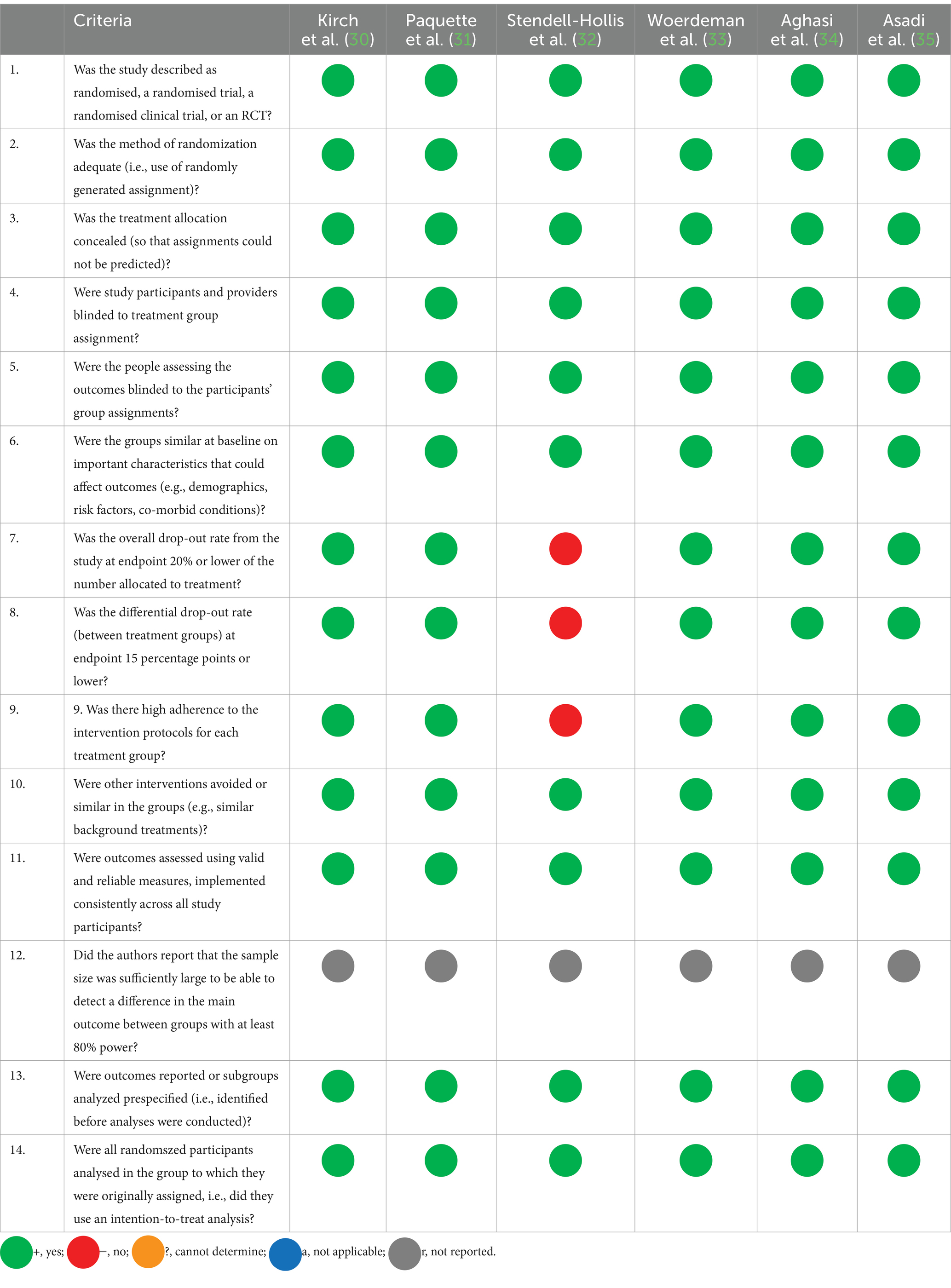
The study quality was evaluated using the National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for randomized controlled trials (29). The tool comprises 14 questions answered using the following options: yes, no, not reported, or not applicable.
Results
Eligibility of studies
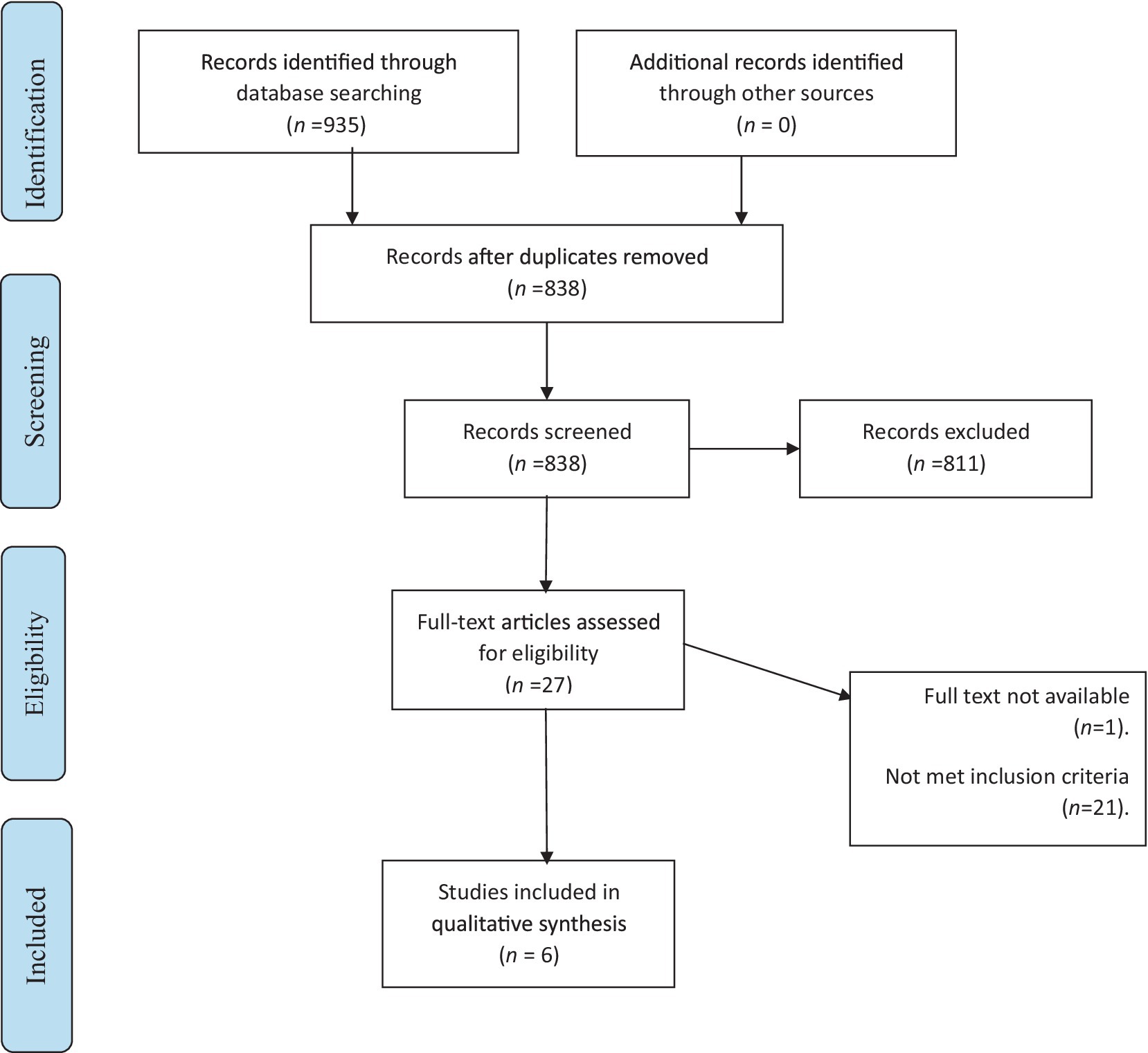
The literature search retrieved 935 studies, of which 97 were duplicate studies. Further screening of the title and abstract led to the exclusion of 811 studies, leaving 27 studies to be assessed against the inclusion/exclusion criteria and finally, six studies were included in this review (Figure 2).
Quality assessment
The quality of each study was evaluated using the NHLBI Quality Assessment Tool for randomized controlled trials (Table 3). Overall, the studies reported on the effect of different sources of polyphenols in overweight and obese people with and without type 2 diabetes seem convergent and reliable to indicate the effect of polyphenols.
Study characteristics
The six included studies, which involved a total of 301 adults, were published between 2010 and 2019. Four studies were conducted on people who were obese or overweight (30–33), and two studies were conducted on people with type 2 diabetes (34, 35).
Different sources of polyphenols were assessed including epicatechin (30), strawberry and cranberry (31), green tea (32), red wine (33), green cardamom (34), and Melissa officinalis (35). The study characteristics and results are shown in Tables 4, 5.
Primary outcomes
Polyphenols and lipid profile
Six studies evaluated the lipid profile, five of which found no significant difference in triglycerides, total cholesterol, LDL cholesterol and HDL cholesterol between the control and the intervention groups (30–34). However, Asadi et al. (35) showed significant differences in TG (p = 0.04) and HDL-c (p = 0.05) between the two groups at the end of the study.
Secondary outcomes
Polyphenols and glucose level
There were no significant changes in glucose levels between the groups (30–35).
Polyphenols and A1c
Only two studies assessed cumulative glucose and found significant differences between groups (34, 35).
Polyphenols and HOMA-IR
Five studies evaluated HOMA-IR, four of which found no difference between the two groups (30, 32, 33, 35) while Aghasi et al. (34) showed that there is a significant decrease in HOMA-IR (−1.7) in the intervention group compared to the control group.
Polyphenols and BMI
Three studies assessed BMI and found no difference between the two groups (30, 32, 34).
Polyphenols and weight
Three studies measured weight and showed that there is no difference between the two groups (30, 32, 33).
Discussion
This systematic review compared the effects of different sources of polyphenols in overweight and obese people with or without type 2 diabetes. The primary outcome was lipid profile and the secondary outcomes were blood glucose, HbA1c (%), HOMA-IR, weight, and body mass index. However, the effects of different sources of polyphenols for obese or overweight adults with or without type 2 diabetes remain unclear.
Epicatechin
Epicatechin is a polyphenol found in various plant-based foods and beverages such as cocoa, grapes, apples, berries, hazelnuts, walnuts, and tea. Kirch et al. (30) investigated whether regular consumption of 25 mg of pure epicatechin can affect glucose, HOMA-IR, LDL, HDL, total cholesterol, and triglycerides in overweight or obese people, showing that 2 weeks of epicatechin supplementation did not reduce cardiometabolic risk factors with no significant changes in glucose metabolism (glucose, HOMA-IR). Similarly, both Dower et al. (15) and Gutiérrez-Salmeán et al. (36) showed that regular intake of pure epicatechin (25–100 mg/day) was not effective or less effective on cardiovascular disease risk factors than comparative cocoa intake. Meta-analyses of randomized controlled trials found positive effects of cocoa consumption on glucose and lipid metabolism (14, 37) that could be predicted by epicatechin intake from cocoa (38). However, the results are inconsistent due to different dosing duration and may be due to adherence.
Strawberry and cranberry
There are several different types of phenolic compounds in strawberries and cranberries including phenolic acids, flavonoids, and polymerized molecules (39). Several vitro and animal studies have suggested that polyphenols may enhance peripheral glucose absorption in insulin-sensitive areas by enhancing GLUT4 translocation and activity and lowering oxidative stress and inflammation (40, 41). For example, Paquette et al. (31) evaluated the effectiveness of berries and strawberries on insulin sensitivity and lipid profile in overweight, obese, and non-diabetic subjects showing that a six-week intervention with 333 mg of polyphenols from strawberries and cranberries improved insulin sensitivity but was ineffective in improving cardiac risk factors. However, other studies have shown that eating freeze-dried strawberry powder or cranberry extract has a positive influence on lowering total cholesterol and LDL cholesterol (42, 43). This discrepancy may be due to the delivery of strawberries and cranberries in different forms (juice, dried strawberries, and powder) and the different quantities consumed, which makes it difficult to determine their effect.
Green tea
Epidemiologic and animal research supports the positive effect of regular green tea consumption in decreasing the risk of cardiovascular disease (44) and obesity (45). There are several hypothesized methods by which green tea and its naturally occurring polyphenolic catechins may modify body weight. For instance, green tea and its derivatives cause glucose malabsorption and downregulate fatty acid synthase (46, 47). Individuals with visceral obesity who consumed 583 mg of catechins daily for 12 weeks saw significant reductions in body weight and LDL cholesterol compared to the control group (48). However, Stendell-Hollis et al. (32) evaluated the effect of green tea consumption on weight, body mass index, triglycerides, total cholesterol, HDL and LDL cholesterol, glucose, and HOMA-IR in weight gain showing no significant differences between the participants who consumed 960 mL of decaffeinated green tea or placebo daily for 6 months.
Red wine
It has been reported that a moderate wine intake might inhibit metabolic syndrome and related medical consequences (49). According to preclinical research, red wine polyphenols are beneficial and improve insulin sensitivity in obese animal models. However, the effect of red wine on humans is minimal. Only one randomized control trial was conducted by Woerdeman et al. (33) to assess the effection of red wine consumption on total cholesterol, HDL and LDL cholesterol, glucose, and HOMA-IR. The participants were randomized to 600 mg/day of red wine polyphenols or placebo daily for 8 weeks and there were no significant changes in either total, LDL or HDL cholesterol or triglyceride levels, glucose, and HOMA-IR after red wine supplementation between groups (p > 0.5).
Green cardamom
Green cardamom is a good source of polyphenols with antioxidant properties (50). Cardamom supplementation enhanced insulin sensitivity and reduced total cholesterol and LDL-cholesterol in prediabetic women (51). However, these outcomes did not differ significantly from the placebo group. According to Azimi et al. (52), the lipid profiles of type 2 diabetes people who consumed three glasses of black tea along with 3 g of cardamom were improved when compared to the control group which only consumed three glasses of black tea. Furthermore, it has been shown that 3 g of cardamom significantly decreased serum HbA1C (−0.4%), HOMA-IR (−1.7), and TG (−39.9 mg dL − 1) in the cardamom group compared to placebo (34). However, there were no significant changes in serum glucose TC, HDL-c, and LDL-c levels between the two groups after 10 weeks (34).
Melissa officinalis (also known as lemon balm)
Melissa officinalis is a plant that can lower blood sugar and fat levels due to its abundance of flavonoids (53). Studies have shown the lipid-lowering and anti-inflammatory properties of M. officinalis (53, 54). Furthermore, Asadi et al. (35) assessed the effect of M. officinalis intake (700 mg/d) versus placebo on HbA1c, triglycerides, total cholesterol, HDL and LDL cholesterol, glucose, and HOMA-IR in individuals with type 2 diabetes, observing significant differences in HbA1c (p = 0.002), triglycerides (p = 0.04), and HDL-c (p = 0.05) between the two groups after 12 weeks. In vitro and animal experiments also demonstrated that M. officinalis reduced blood glucose and lipids (55, 56).
Potential mechanisms
Polyphenols are subject to a series of enzymatic reactions and microbial transformations upon ingestion, culminating in their absorption, distribution, metabolism, and excretion throughout various tissues and organs.
First, the chemical structure of polyphenolic compounds greatly influences their metabolic fate. For example, flavonoids, phenolic acids, and other subclasses exhibit distinct metabolic pathways, leading to the formation of diverse metabolites with different bioactivities (57). Phase I and II metabolic reactions, which are mainly facilitated by hepatic enzymes, modify the structure of the polyphenols, making them more water-soluble and leading to their elimination (58, 59).
Moreover, consuming food components together can significantly affect polyphenol metabolism. Interactions with macronutrients, such as lipids and proteins, may affect the absorption kinetics and bioavailability of polyphenols (60). In addition, the presence of other bioactive compounds within food matrices can modify polyphenol metabolism, which may enhance or inhibit their bioactivity through synergistic or antagonistic effects (61).
Furthermore, individual differences in the composition of the gut microbiota contribute to interindividual differences in polyphenol metabolism. Some microbial species possess the enzymatic machinery necessary for the degradation and biotransformation of polyphenolic compounds, resulting in metabolites that may exhibit distinct physiological effects compared to their parent compounds (62).
Understanding the metabolism of polyphenolic compounds is crucial to elucidating their health effects and therapeutic potential. By revealing the complex interplay between nutritional factors, host physiology, and gut microbiota, future research endeavors can pave the way for personalized nutritional interventions aimed at improving the bioavailability of polyphenols and harnessing their beneficial properties for human health and well-being.
Strength and limitation
The quality assessment of the included studies was conducted to evaluate the methodological rigor and reliability of the evidence presented. We utilized the NHLBI Quality Assessment for Randomized Control Trials (RCTs) which assesses various aspects of study design, conduct, and reporting.
Overall, the quality of the included polyphenol studies varied. Several studies demonstrated a high level of methodological rigor, including randomized controlled trials (RCTs) with appropriate randomization and blinding procedures. However, certain limitations were observed across the studies. Common issues included small sample sizes, lack of blinding, incomplete outcome data, and potential biases.
Despite these limitations, many studies provided valuable insights into the potential health effects of polyphenols.
It is important to interpret the findings of polyphenol studies with caution, considering the methodological limitations identified. Future research should prioritize high-quality study designs, including well-powered RCTs with long-term follow-up, and systematic reviews with rigorous methodology.
Conclusion
The current systematic review discussed the effect of different sources of polyphenols in overweight and obese people with or without type 2 diabetes, showing that cardamom significantly decreased serum HbA1C, HOMA-IR, and triglyceride and M. officinalis reduced blood sugar fat levels and lipids. However, the data associated with the specific health benefits of polyphenols and their sources in people who are overweight, obese, or have type 2 diabetes are still unclear and further research is required to support their use and prove their benefits.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Methodology, Writing – review & editing. BA: Methodology, Writing – review & editing. AbA: Methodology, Writing – review & editing. IA: Methodology, Writing – review & editing. AlA: Methodology, Writing – review & editing. AmA: Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO . (2021). Obesity and overweight. Available at:https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
2. Finkelstein, EA, Khavjou, OA, Thompson, H, Trogdon, JG, Pan, L, Sherry, B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. (2012) 42:563–70. doi: 10.1016/J.AMEPRE.2011.10.026
3. do Carmo, JM, da Silva, AA, Wang, Z, Fang, T, Aberdein, N, de Lara Rodriguez, CEP, et al. Obesity-induced hypertension: brain signaling pathways. Curr Hypertens Rep. (2016) 18:58. doi: 10.1007/S11906-016-0658-1
4. Eckel, RH, Kahn, SE, Ferrannini, E, Goldfine, AB, Nathan, DM, Schwartz, MW, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. (2011) 96:1654–63. doi: 10.1210/JC.2011-0585
5. Kinlen, D, Cody, D, and O’Shea, D. Complications of obesity. QJM. (2018) 111:437–43. doi: 10.1093/QJMED/HCX152
6. Tremmel, M, Gerdtham, UG, Nilsson, PM, and Saha, S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. (2017) 14:435. doi: 10.3390/IJERPH14040435
7. Di Lorenzo, C, Colombo, F, Biella, S, Stockley, C, and Restani, P. Polyphenols and human health: the role of bioavailability. Nutrients. (2021) 13:273. doi: 10.3390/NU13010273
8. Singla, RK, Dubey, AK, Garg, A, Sharma, RK, Fiorino, M, Ameen, SM, et al. Natural polyphenols: chemical classification, definition of classes, subcategories, and structures. J AOAC Int. (2019) 102:1397–400. doi: 10.5740/JAOACINT.19-0133
9. Pandey, KB, and Rizvi, SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev. (2009) 2:270–8. doi: 10.4161/OXIM.2.5.9498
10. Yao, LH, Jiang, YM, Shi, J, Tomás-Barberán, FA, Datta, N, Singanusong, R, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. (2004) 59:113–22. doi: 10.1007/s11130-004-0049-7
11. Manach, C, Scalbert, A, Morand, C, Rémésy, C, and Jiménez, L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. (2004) 79:727–47. doi: 10.1093/AJCN/79.5.727
12. Aloo, SO, Ofosu, FK, Kim, NH, Kilonzi, SM, and Oh, DH. Insights on dietary polyphenols as agents against metabolic disorders: obesity as a target disease. Antioxidants. (2023) 12:416. doi: 10.3390/antiox12020416
13. Meydani, M, and Hasan, ST. Dietary polyphenols and obesity. Nutrients. (2010) 2:737–51. doi: 10.3390/NU2070737
14. Shrime, MG, Bauer, SR, McDonald, AC, Chowdhury, NH, Coltart, CEM, and Ding, EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. (2011) 141:1982–8. doi: 10.3945/JN.111.145482
15. Dower, JI, Geleijnse, JM, Gijsbers, L, Zock, PL, Kromhout, D, and Hollman, PCH. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr. (2015) 101:914–21. doi: 10.3945/AJCN.114.098590
16. Nikpayam, O, Najafi, M, Ghaffari, S, Jafarabadi, MA, Sohrab, G, and Roshanravan, N. Effects of green coffee extract on fasting blood glucose, insulin concentration and homeostatic model assessment of insulin resistance (HOMA-IR): a systematic review and meta-analysis of interventional studies. Diabetology & Metabolic Syndrome. (2019) 11. doi: 10.1186/S13098-019-0489-8
17. de Bock, M, Derraik, JGB, Brennan, CM, Biggs, JB, Morgan, PE, Hodgkinson, SC, et al. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: a randomized, placebo-controlled, crossover trial. PloS One. (2013) 8. doi: 10.1371/JOURNAL.PONE.0057622
18. Nowrouzi-Sohrabi, P, Hassanipour, S, Sisakht, M, Daryabeygi-Khotbehsara, R, Savardashtaki, A, and Fathalipour, M. The effectiveness of pistachio on glycemic control and insulin sensitivity in patients with type 2 diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome. (2020) 14:1589–95. doi: 10.1016/J.DSX.2020.07.052
19. Dhillon, J, Thorwald, M, De La Cruz, N, Vu, E, Asghar, SA, Kuse, Q, et al. Glucoregulatory and Cardiometabolic Profiles of Almond vs. Cracker Snacking for 8 Weeks in Young Adults: A Randomized Controlled Trial. Nutrients. (2018) 10. doi: 10.3390/NU10080960
20. McKay, DL, Eliasziw, M, Oliver Chen, CY, and Blumberg, JB. A Pecan-Rich Diet Improves Cardiometabolic Risk Factors in Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients. (2018) 10. doi: 10.3390/NU10030339
21. Neale, EP, Guan, V, Tapsell, LC, and Probst, YC. Effect of walnut consumption on markers of blood glucose control: a systematic review and meta-analysis. The British Journal of Nutrition. (2020) 124:641–53. doi: 10.1017/S0007114520001415
22. Wu, CH, Lu, FH, Chang, CS, Chang, TC, Wang, RH, and Chang, CJ. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obesity Research. (2003) 11:1088–95. doi: 10.1038/OBY.2003.149
23. Hughes, LAE, Arts, ICW, Ambergen, T, Brants, HAM, Dagnelie, PC, Goldbohm, RA, et al. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study. The American Journal of Clinical Nutrition. (2008) 88:1341–52. doi: 10.3945/AJCN.2008.26058
24. Diepvens, K, Kovacs, EMR, Nijs, IMT, Vogels, N, and Westerterp-Plantenga, MS. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. British Journal of Nutrition. (2005) 94:1026–34. doi: 10.1079/BJN20051580
25. Nagao, T, Komine, Y, Soga, S, Meguro, S, Hase, T, Tanaka, Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. The American Journal of Clinical Nutrition. (2005) 81:122–9. doi: 10.1093/AJCN/81.1.122
26. Tabrizi, R, Tamtaji, OR, Lankarani, KB, Akbari, M, Dadgostar, E, Dabbaghmanesh, MH, et al. The effects of resveratrol intake on weight loss: a systematic review and meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition. (2020) 60:375–90. doi: 10.1080/10408398.2018.1529654
27. Daneshzad, E, Shab-Bidar, S, Mohammadpour, Z, and Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2019) 38:1153–65. doi: 10.1016/j.clnu.2018.06.979
28. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/BMJ.N71
29. NHLBI, NIH , Study Quality Assessment Tools. (2021). Available at:https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
30. Kirch, N, Berk, L, Liegl, Y, Adelsbach, M, Zimmermann, BF, Stehle, P, et al. A nutritive dose of pure (−)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults - a randomized, placebo-controlled, double-blind crossover study. Am J Clin Nutr. (2018) 107:948–56. doi: 10.1093/ajcn/nqy066
31. Paquette, M, Medina Larqué, AS, Weisnagel, SJ, Desjardins, Y, Marois, J, Pilon, G, et al. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial. Br J Nutr. (2017) 117:519–31. doi: 10.1017/S0007114517000393
32. Stendell-Hollis, NR, Thomson, CA, Thompson, PA, Bea, JW, Cussler, EC, and Hakim, IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet. (2010) 23:590–600. doi: 10.1111/j.1365-277X.2010.01078.x
33. Woerdeman, J, Del Rio, D, Calani, L, Eringa, EC, Smulders, YM, and Serné, EH. Red wine polyphenols do not improve obesity-associated insulin resistance: a randomized controlled trial. Diabetes Obes Metab. (2018) 20:206–10. doi: 10.1111/dom.13044
34. Aghasi, M, Koohdani, F, Qorbani, M, Nasli-Esfahani, E, Ghazi-Zahedi, S, Khoshamal, H, et al. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J Sci Food Agric. (2019) 99:3933–40. doi: 10.1002/jsfa.9617
35. Asadi, A, Shidfar, F, Safari, M, Hosseini, AF, Fallah Huseini, H, Heidari, I, et al. Efficacy of Melissa officinalis L. (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a randomized, double-blind, clinical trial. Phytother Res. (2019) 33:651–9. doi: 10.1002/ptr.6254
36. Gutiérrez-Salmeán, G, Meaney, E, Lanaspa, MA, Cicerchi, C, Johnson, RJ, Dugar, S, et al. A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: unique in vitro effects. Int J Cardiol. (2016) 223:500–6. doi: 10.1016/J.IJCARD.2016.08.158
37. Tokede, OA, Gaziano, JM, and Djoussé, L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. (2011) 65:879–86. doi: 10.1038/EJCN.2011.64
38. Ellinger, S, Reusch, A, Stehle, P, and Helfrich, HP. Epicatechin ingested via cocoa products reduces blood pressure in humans: a nonlinear regression model with a Bayesian approach. Am J Clin Nutr. (2012) 95:1365–77. doi: 10.3945/AJCN.111.029330
39. Szajdek, A, and Borowska, EJ. Bioactive compounds and health-promoting properties of berry fruits: a review. Plant Foods Hum Nutr. (2008) 63:147–56. doi: 10.1007/S11130-008-0097-5
40. Denis, MC, Desjardins, Y, Furtos, A, Marcil, V, Dudonné, S, Montoudis, A, et al. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin Sci. (2015) 128:197–212. doi: 10.1042/CS20140210
41. Nizamutdinova, IT, Jin, YC, Chung, J, Shin, SC, Lee, SJ, Seo, HG, et al. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res. (2009) 53:1419–29. doi: 10.1002/MNFR.200800526
42. Basu, A, Wilkinson, M, Penugonda, K, Simmons, B, Betts, NM, and Lyons, TJ. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: baseline and post intervention effects. Nutr J. (2009) 8:43. doi: 10.1186/1475-2891-8-43
43. Lee, IT, Chan, YC, Lin, CW, Lee, WJ, and Sheu, WHH. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet Med. (2008) 25:1473–7. doi: 10.1111/J.1464-5491.2008.02588.X
44. Wolfram, S . Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. (2007) 26:373S–88S. doi: 10.1080/07315724.2007.10719626
45. Zaveri, NT . Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. (2006) 78:2073–80. doi: 10.1016/J.LFS.2005.12.006
46. Zhang, R, Xiao, W, Wang, X, Wu, X, and Tian, W. Novel inhibitors of fatty-acid synthase from green tea (Camellia sinensis Xihu Longjing) with high activity and a new reacting site. Biotechnol Appl Biochem. (2006) 43:1–7. doi: 10.1042/BA20050064
47. Zhong, L, Furne, JK, and Levitt, MD. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am J Clin Nutr. (2006) 84:551–5. doi: 10.1093/AJCN/84.3.551
48. Nagao, T, Hase, T, and Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. (2007) 15:1473–83. doi: 10.1038/OBY.2007.176
49. Liu, L, Wang, Y, Lam, K, and Xu, A. Moderate wine consumption in the prevention of metabolic syndrome and its related medical complications. Endocr Metab Immune Disord Drug Targets. (2008) 8:89–98. doi: 10.2174/187153008784534385
50. Suneetha, WJ, and Krishnakantha, TP. Cardamom extract as inhibitor of human platelet aggregation. Phytother Res. (2005) 19:437–40. doi: 10.1002/ptr.1681
51. Fatemeh, Y, Siassi, F, Rahimi, A, Koohdani, F, Doostan, F, Qorbani, M, et al. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: a randomized controlled trial. J Diabetes Metab Disord. (2017) 16:40. doi: 10.1186/S40200-017-0320-8
52. Azimi, P, Ghiasvand, R, Feizi, A, Hariri, M, and Abbasi, B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud. (2014) 11:258–66. doi: 10.1900/RDS.2014.11.258
53. Shakeri, A, Sahebkar, A, and Javadi, B. Melissa officinalis L. - a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. (2016) 188:204–28. doi: 10.1016/J.JEP.2016.05.010
54. Jandaghi, P, Noroozi, M, Ardalani, H, and Alipour, M. Lemon balm: a promising herbal therapy for patients with borderline hyperlipidemia-a randomized double-blind placebo-controlled clinical trial. Complement Ther Med. (2016) 26:136–40. doi: 10.1016/J.CTIM.2016.03.012
55. Chung, MJ, Cho, SY, Bhuiyan, MJH, Kim, KH, and Lee, SJ. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr. (2010) 104:180–8. doi: 10.1017/S0007114510001765
56. Weidner, C, Wowro, SJ, Freiwald, A, Kodelja, V, Abdel-Aziz, H, Kelber, O, et al. Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol Nutr Food Res. (2014) 58:903–7. doi: 10.1002/mnfr.201300477
57. Dias, MC, Pinto, DCGA, and Silva, AMS. Plant flavonoids: chemical characteristics and biological activity. Molecules. (2021) 26:5377. doi: 10.3390/MOLECULES26175377
58. Boronat, A, Rodriguez-Morató, J, Serreli, G, Fitó, M, Tyndale, RF, Deiana, M, et al. Contribution of biotransformations carried out by the microbiota, drug-metabolizing enzymes, and transport proteins to the biological activities of phytochemicals found in the diet. Adv Nutr. (2021) 12:2172–89. doi: 10.1093/ADVANCES/NMAB085
59. Scalbert, A, and Williamson, G. Dietary intake and bioavailability of polyphenols. J Nutr. (2000) 130:2073S–85S. doi: 10.1093/JN/130.8.2073S
60. Balta, V, Đikić, D, Landeka Jurčević, I, Odeh, D, Oršolić, N, Ferara, N, et al. The effect of a high-protein diet supplemented with blackthorn flower extract on polyphenol bioavailability and antioxidant status in the organs of C57BL/6 mice. Nutrients. (2023) 15:4066. doi: 10.3390/NU15184066
61. Sęczyk, Ł, Gawlik-Dziki, U, and Świeca, M. Influence of phenolic-food matrix interactions on in vitro bioaccessibility of selected phenolic compounds and nutrients digestibility in fortified white bean paste. Antioxidants. (2021) 10:1825. doi: 10.3390/ANTIOX10111825
Keywords: polyphenols, antioxidants, type 2 diabetes, obesity, lipid
Citation: Alqarni S, Alsebai M, Alsaigh BA, Alrashedy AS, Albahrani IT, Aljohar AY and Alazmi AO (2024) Do polyphenols affect body fat and/or glucose metabolism? Front. Nutr. 11:1376508. doi: 10.3389/fnut.2024.1376508
Edited by:
Alessandra Durazzo, Council for Agricultural Research and Economics, ItalyReviewed by:
Claudia Ivette Gamboa, Mexican Social Security Institute, MexicoYongsheng Chen, Jinan University, China
Copyright © 2024 Alqarni, Alsebai, Alsaigh, Alrashedy, Albahrani, Aljohar and Alazmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saleha Alqarni, c2FsZWhhLmFscWFybmlAb3V0bG9vay5zYQ==
 Saleha Alqarni
Saleha Alqarni Mashael Alsebai
Mashael Alsebai Batool Adal Alsaigh1
Batool Adal Alsaigh1 Abeer Sayer Alrashedy
Abeer Sayer Alrashedy Israa Talal Albahrani
Israa Talal Albahrani