- 1Department of Zoology, Kohat University of Science and Technology, Kohat, Khyber Pakhtunkhwa, Pakistan
- 2Department of Plant Protection, Ministry of National Food Security and Research, Karachi, Pakistan
- 3Department of Biological Sciences, University of Baltistan, Skardu, Pakistan
- 4Department of Zoology, Abdul Wali Khan University, Mardan, Khyber Pakhtunkhwa, Pakistan
- 5Department of Pathology, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan
- 6Department of Cosmetic Science, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
- 7Ph.D. Program in Translational Medicine, Rong Hsing Research Center for Translational Medicine, National Chung Hsing University, Taichung, Taiwan
- 8Department of Biotechnology and Bioindustry Sciences, College of Bioscience and Biotechnology, National Cheng Kung University, Tainan, Taiwan
- 9Biotechnology Center, National Chung Hsing University, Taichung, Taiwan
- 10Department for Biology and Pathology of Fish and Bees, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia
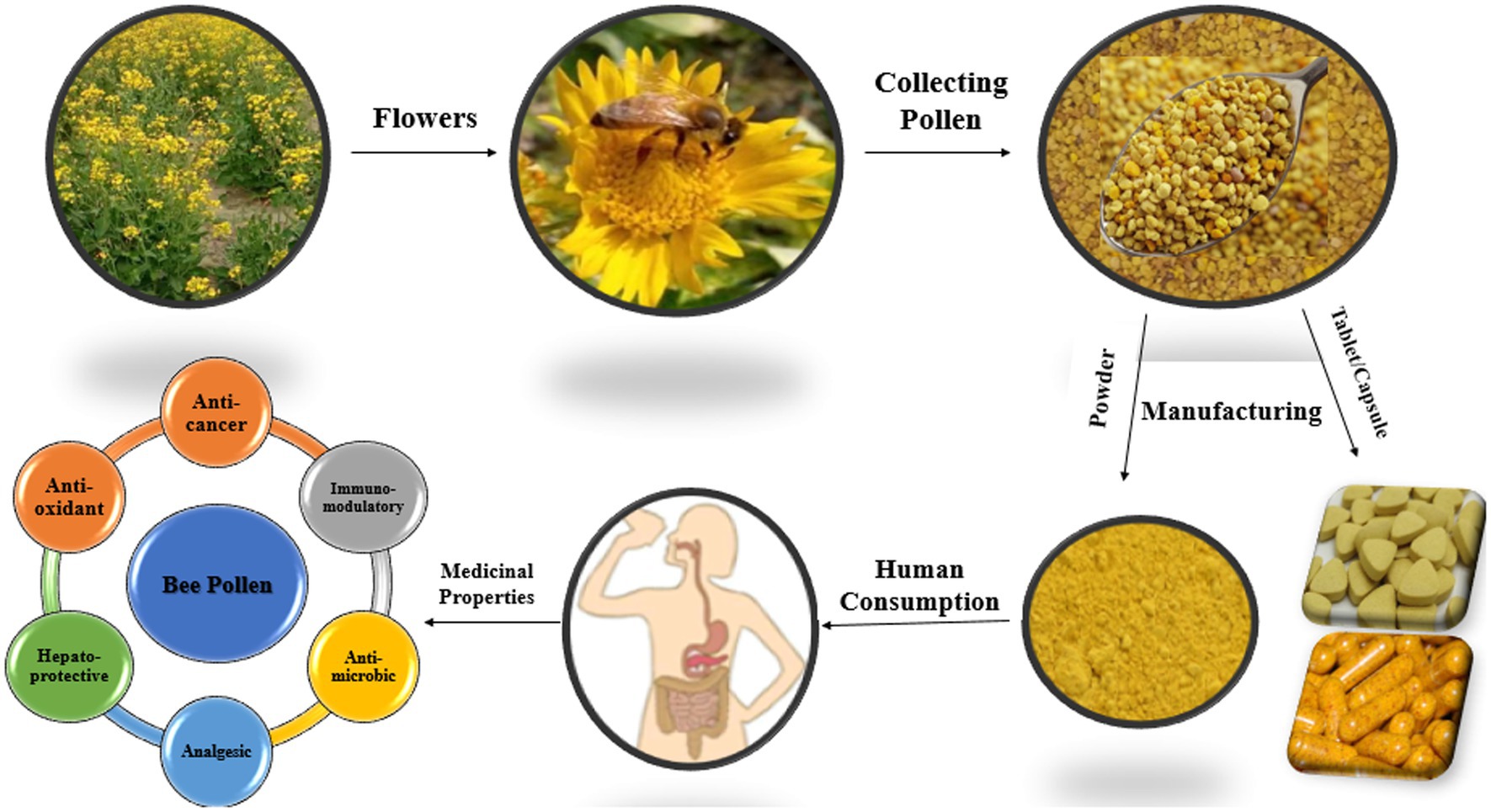
Pollen grains are the male reproductive part of the flowering plants. It is collected by forager honey bees and mixed with their salivary secretions, enzymes, and nectar, which form fermented pollen or “bee bread” which is stored in cells of wax honeycombs. Bee pollen (BP) is a valuable apitherapeutic product and is considered a nutritional healthy food appreciated by natural medicine from ancient times. Recently, BP has been considered a beneficial food supplement and a value-added product that contains approximately 250 different bioactive components. It contains numerous beneficial elements such as Mg, Ca, Mn, K, and phenolic compounds. BP possesses strong antioxidant, anti-inflammatory, antimicrobial, antiviral, analgesic, immunostimulant, neuroprotective, anti-cancer, and hepatoprotective properties. It is used for different purposes for the welfare of mankind. Additionally, there is a growing interest in honey bee products harvesting and utilizing for many purposes as a natural remedy and nutritive function. In this review, the impacts of BP on different organisms in different ways by highlighting its apitherapeutic efficacy are described.
1 Introduction
Bee pollen (BP) is a raw flower plant reproductive material. In the 17th century, the term pollen was established from a Latin word which means fine powder or flour. For centuries, this plant component has been known as a “food” with biological functions (1). Honey bees rely on pollen for proteins, lipids, fatty acids, sterols, carbohydrates, and vitamins (2, 3). Honey bee foragers shape field-gathered pollen in pellets and transport it to a hive that produces a high-quality product called bee bread (4–6). Pollen stored in honey bee wax combs after lactic fermentation in a hive is referred to as bee bread or fermented pollen (7, 8). It is obtained as a result of lactic acid fermentation of pollens by some bacteria and fungi (4–6). In addition, corbiculate bees add regurgitated fluid with the grains to facilitate pollen adherence to the pollen baskets and pollen grains (8–10). BP is collected by adult forager bees and transported to the hive. The flightless house bees fragment the mixture of collected pollen, saliva, honey, digestive enzymes, and lactic acid, which is then stored in wax honeycomb cells. After collecting pollen, it is fermented, stored, and covered with a thin layer of wax and honey (11). The bee bread undergoes anaerobic lactic fermentation during the ripening process, which is caused by bacteria (e.g., Lactobacillus spp., Pseudomonas spp.) and yeasts (Saccharomyces spp.). Bee bread is the primary source of protein for honey bee colonies and also provides minerals, vitamins, and other nutrients needed for the production of royal jelly (11–13).
Approximately 50 to 250 grams of pollen can be collected by forager bees of one colony per day, while 14 to 40 kilograms of pollen collected by forager bees each year (4, 14). BP preservation can be achieved by drying for a longer period at room temperature in special driers. This helps to prevent microbial spoilage and rapid fermentation, resulting in easy marketability and increased revenue for beekeepers (2, 15). Furthermore, BP with high moisture content easily spoils in a short period after harvesting because it is highly vulnerable to microbial attacks (2, 16). Two pollen pellets have a combined total mass near 20 mg (17), which is more than 25% of the average body mass of the honey bee (17, 18). Bees that collect pollen do not consume pollen from the flower but they take it for the consumption of their offspring in the hive and behave as social bees (9).
Honey bee products are highly recommended and used worldwide for various purposes, such as natural food, medicine, and cosmetics, and their importance was revealed in the Holy books (19). BP is a nutritional and functional bee product that is used as an apitherapeutic product and food supplement. The composition of BP depends on flower source, season, geographical origin, and plant species. BP contains carbohydrates, proteins, lipids, phenolics, vitamins, and minerals in its composition. BP has many pharmacological properties and can be used to treat metabolic abnormalities such as hyper-dyslipidemia, obesity, diabetes, and cardiovascular diseases (20, 21). BP can be found in the form of pills, granules, oral liquids, tonics, and candy bars (22). Moreover, BP can be used as a food supplement due to its antioxidant, hepatoprotective, antimicrobial, cardioprotective, and immunomodulatory properties (23, 24). The main aim of the study was to highlight the benefits of BP regarding health and its efficacy toward different human and animal health problems.
2 Composition and morphology of bee pollen
The BP composition generally depends on plant species, geographical origin, and seasonal conditions. Generally, BP consists of carbohydrates (40–85%), proteins (14–30%), lipids (1–10%), vitamins, minerals, carotenoids, phenolics, and other trace elements (14, 20, 25, 26) (Table 1). A total of 250 bioactive compounds in BP have been known so far (44–47). The characterization of the BP according to the parameters of antioxidant activity, botanical origin, and phenolic profile was previously described (48). The chemical composition of BP is known to vary with the used methods of extraction and storage, the floral origin, and environmental conditions (5, 49, 50). Pollen comes in different colors depending on botanical origins, such as green, black, grey, purple, orange, white, and red (44, 51, 52). Pollen grains collected of the same color are said to be monochromatic while having different colors called polychromatic BP (53). Colored pollen (anthers) might act as an additional visual indication, helping the foraging insect pollinators to detect the flower over a small distance (52, 54). BP collected from different flowers is called multifloral while collection that takes place from a single flower or plant is said to be monofloral (55).
In closely related plant species, the amino acid composition of their pollen is similar, while in distantly related plant species, the amino acid composition differs (56, 57). Pollen mixing may dilute the potentially toxic compounds originating from toxic plants (58). In the BP, the predominant minerals are calcium, magnesium, manganese, and potassium. The BP is considered a rich source of various vitamins, including B1, B2, and B6 (3, 59). The composition and quantity of amino acids in pollen proteins depend on the plant species visited by honey bees (60).
3 Factors affecting bee pollen
3.1 Plant species effect
The dietary component of pollen grains differs strongly among various plant species and is directly related to honey bee health (61). Honey bees and bumble bees detect and make decisions about foraging based on the nutritional content of floral resources (62). Bees often grasp flowers with their antennae while sometimes grasping and scraping pollen from the anthers with the help of their mandibles (63). Even some species have mouthparts with specialized hairs intended for pollen collection from flowers (63, 64). If honey bees collect pollen from a single plant species, it is called monofloral (at least 80%) (45, 65, 66); if they collect from multiple species, it is called multifloral (less than 80%) (4, 53, 65, 66). According to the research, honey bees have a preference to collect pollen from 2 to 8 plants every single month (67). BP with small amount of protein content can have adverse effects on health of bees, such as reducing hypopharyngeal gland size in adult honey bees (68, 69), low larval weight in bumblebees (Bombus terrestris) (69, 70), sweat bees (Lasioglossum zephyrum) offspring weight (69, 71), and immune function in honey bee colonies (69, 72, 73).
3.2 Geographical effects
Climatic conditions and the pollen ripening period influenced the quality of pollen and the vitality of pollen during the years of cryopreservation. On the vitality of pollen cryopreservation differences between the genetic variability of trees (inter-individual variability) were very pronounced and enabled the selection of trees that are to be pollinated by insects and which keep their initial vitality (74). The composition of pollen may vary in different annual seasons, years, and different geographical locations (49). BP has an extensive chemical nutritive composition but the composition has variation in the maximum and minimum range of values, especially because of different botanical origins, edaphoclimatic, and geographical conditions (75). However, because of geographic and botanical origin and other factors such as beekeepers’ practices, weather conditions, and soil type, there is a variety in BP composition (76).
3.3 Temperature effects
The viability of pollen can decrease by 30–70% under heat stress. Pollen wall thickness increases under heat stress (77). In vitro germination was used to assess the viability of pollen and it was measured at intervals up to 2 h with the removal of pollen grains from the anthers. It was quantified for various alfalfa plant (Medicago sativa) varieties at three temperatures including both, Roundup Ready (RR) and conventional varieties. The most prevalent factor affecting the viability of pollen in alfalfa was the time since the removal from the anthers. The viability of pollen declines with increasing time for all tested varieties at all three temperatures. Additionally, the viability of pollen is not affected by the temperatures ranging between 25 and 37°C and does not vary among varieties of plants, including RR and conventional varieties (78).
4 Apitherapy
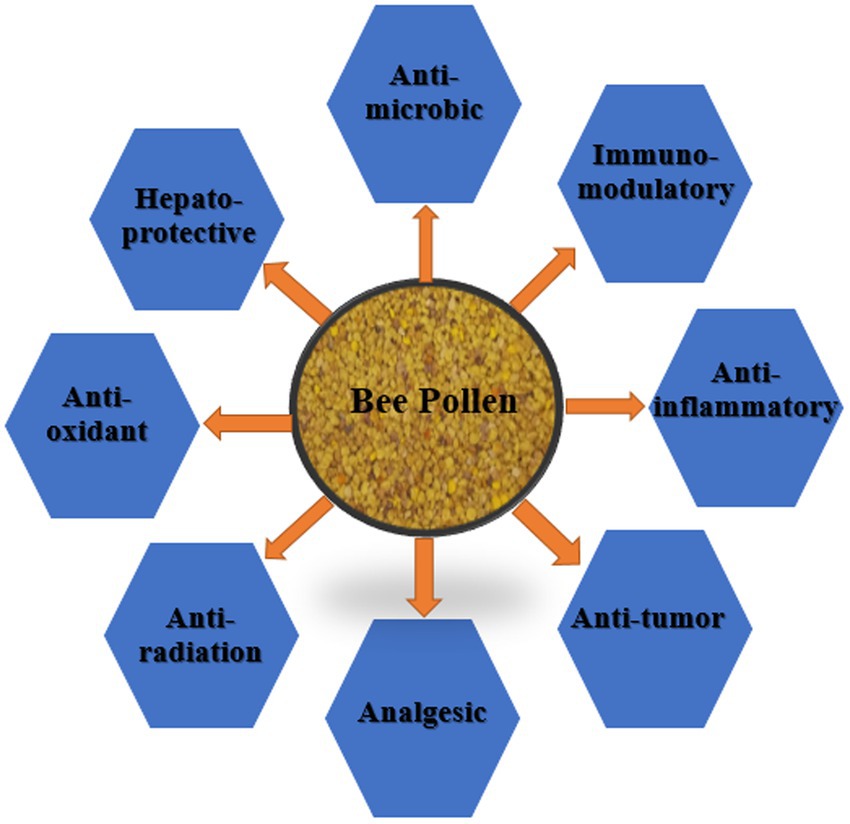
A zootherapy type called “apitherapy” is based on the bioactivity of several chemical compounds from honey bee products (79, 80). Apitherapy contains a number of chemical compounds which are natural agents with an approved range of activities and actions (11, 81). BP, a commonly used apitherapeutic, varies in chemical composition depending on geographic origin, plant source, soil type, bee race, activities, and climatic conditions (8, 11, 30, 46, 81). BP shows abundant therapeutic and nutritional properties because of the richness of its bioactive and nutritive components (82). Pharmacopoeia Committee of the People’s Republic of China officially recognized BP as a healthy food. It has been concluded that BP can delay aging, improve the cardiovascular system, maintain the digestive system, prevent prostate degeneration, and enhance immunity according to a previously published article (76, 83). In the apitherapeutic treatment, BP is used because it shows medicinal activities such as antimicrobial, antibiotic, anti-inflammatory, antiviral, analgesic, immunostimulant (84–86), anti-radiation, anti-tyrosinase, hepatoprotective, and antioxidant (22, 87–89) (Figures 1, 2).
Pollen consumption can lower blood pressure and increase hemoglobin and red blood cells. It may benefit those with sterility, hypertension, and pernicious anemia, as well as the endocrine system and nervous system (90–92). BP is rich in protein, fat, and minerals (particularly Ca, Mg, Fe, and P) and gives a nutritional value to pollen similar to or greater than dry legumes and is used as a food supplement (93, 94). In other studies, restorative, positive, and protective effects of pollen addition in feed have been reported (95). The pollen proteins improve regeneration of liver tissue and increase levels of serum albumin and state of malnutrition in cirrhotic rats when compared with the untreated group (95, 96). In diabetic rat, bee bread beneficially affects the intake of water and metabolism of glucose and is helpful for the cure of other diseases in diabetes and hyperglycemia (97). It has been studied that the use of BP extract considerably reduces the level of phosphate and calcium in urine which avoids kidney stone formation in fed rats (98).
4.1 Antioxidant activity of bee pollen
BP contains antioxidants with low molecular weight compounds. Among them, the most important compounds are ascorbic acid (vitamin C) and phenolics which are associated with the hydrophobic class of antioxidants (99, 100), whereas carotenoids and tocopherol (vitamin E) belong to hydrophobic antioxidants. Additionally, vitamin C reacts with hydroxyl radicals as it is a water-phase antioxidant. It helps in retaining the NO (nitric oxide) level in oxidative stress, and the compound can help in the relaxation of smooth muscles of arteries (100–102). Additionally, it was found that the fraction of ethyl acetate of camellia (flowering plants) BP possesses higher anti-tyrosinase and antioxidant potential than other pollen such as rose BP, lotus BP, and rape BP (87). It has been noted that the fermentation of BP can increase its phenolic compound contents and promote its antioxidant capability (21). Furthermore, BP was added to beef burgers and examined its oxidative stability, chemical composition, and antioxidant properties. Using TBARS (thiobarbituric acid reactive substances) assay method, it was noticed that the malondialdehyde value per kg of beef burger enhanced up to 2.09 mg at −12°C after 42 days of storage. In addition, BP extract repressed 31.78% of lipid oxidation of beef burgers at the end of the analyses (59). Therefore, due to its strong antioxidant activity and high nutritive value, BP may be used as a natural anti-oxidant in meat products (59, 103). Similarly, lyophilized BP also has strong antioxidant capability in pork sausage (103). Another study concluded that the addition of BP to broiler meat can increase water decrease fat contents and ameliorate meat quality which is functional for human health (104). Anjos et al. noted that BP inhibited lipid oxidation in black pudding and enhanced product quality in Portugal (48, 59). The addition of BP to meat products can increase their preservation time due to the presence of quercetin and other derivatives in BP which acts as an antimicrobial agent. Furthermore, the usage of BP (high amount) in frankfurters can increase its oxidative stability and shelf life during chilled storage which is attractive to potential market consumers (105). It has been found that BP decreases the toxic effect of fluorine (due to its antioxidant activity) via the reduction of MDA levels in albino male rats. In addition, it also enhanced the total protein level, phosphorous, calcium, magnesium, and serum level than the fluoride-treated control group. The mixing of 0.5 to 3% BP (ground form) with yogurt (made from sheep, goat, or cow milk) significantly increases its antioxidant capability and phenolic compounds and also ameliorates its smell, taste, and appearance (20). Furthermore, the addition of BP to malt beverages significantly enhances the antioxidant action because of the presence of a greater quantity of phenolic contents, especially Papaver somniferum (poppy) pollen than the control group (106).
The ratio of polysaturated fatty acid in pollen is higher than the saturated fatty acid and monosaturated fatty acid (107, 108), mainly depending on plant species and storage conditions (109, 110). Dietary supplementation with BP expresses a positive effect on stress, reducing lipid peroxidation due to the presence of antioxidants in its composition (107, 108) and increasing polyunsaturated fatty acid in some tissues due to higher polyunsaturated fatty acid content. The polyunsaturated fatty acid dietary supplements have a beneficial impact on the antioxidant status of consumers (108, 111) and change in the health and performance of Japanese quails (Coturnix coturnix japonica) (108). It has been stated that the gastrointestinal infection of broiler chickens was cured through BP (45). The addition of 1.5% BP to the feed of broiler chicken significantly increased immunoglobulin M (IgM) concentration and weight of thymus after 21 and 42 days, respectively, and enhanced the immune system of chicken (at 21 days of age). In another study, it was shown that the presence of antioxidants such as luteolin, apigenin, and quercetin in BP considerably improved intellectual activities and functions (20).
4.2 Anti-inflammatory activity of bee pollen
The components of BP include proteins, minerals, lipids, vitamins, carbohydrates, and some other components in a small amount. Previous studies have revealed that BP has antibacterial, antioxidant, anti-carcinogenic, anti-allergic, and anti-inflammatory properties (25, 36, 46). Both BP and propolis are rich in trace elements, flavonoids, and other healthy components and have been shown to have many health benefits, such as anti-allergic and anti-inflammatory properties (112). In addition, BP is also rich in quercetin, which has been found to affect the metabolism of arachidonic acid and decrease the level of pro-inflammatory prostaglandins (22, 113). Due to its anti-inflammatory properties, quercetin also targets cyclooxygenase 1 and cyclooxygenase 2, following the obstruction of NFκB processes (114). Related to inflammation and oxidative stress, phenolic compounds present in many medicinal plants play an important role in treatment. Therefore, antioxidant activity may be one of the possible mechanisms of the anti-inflammatory effect of BP (115). Moreover, BP suppresses and downregulates the expression of NF-κB (induced nuclear factor) and tumor necrosis factor (TNF) due to its anti-inflammatory activity (84). Yeast-fermented BP possesses hepatoprotective efficacy via reducing malondialdehyde (MDA) levels while boosting the amount of catalase (CAT) and glutathione S-transferase (GST) in the mice liver (116). Both BP and bee bread possess a flavonoid called isorhamnetin which has anti-cancer and anti-inflammatory properties. Kaempferol is another phenolic compound which is known for its therapeutic potential and used for the additional therapy of hepatic fibrosis (4).
Furthermore, the component of BP called rutin has been used in preparation that acts as anti-inflammatory, neuroprotective, and antispasmodic agents (89). The presence of glucoside and flavone in BP makes it a nutraceutical agent which has therapeutic efficacy against various diseases such as amnesia, Alzheimer’s disease, and diabetes (117). Treatment with BP significantly diminished inflammation and TNF-α levels in polycystic ovary syndrome in mice model (118). Tyrosol is another BP (Rhododendron ponticum) phenolic component that possesses anti-cholinesterase and anti-hypoglycemic function and also restrains Parkinson’s and Alzheimer’s diseases (119). It was investigated that the component of BP called linalool holds anti-inflammatory characteristics that are correlated with the suppression of pro-inflammatory variation of mitogen-activated protein kinases (MAPKs) and NF-kB pathways (6). The BP fatty acids called linoleic and linolenic combine with histamine H1 receptor while flavonoids decrease the production of nitric oxide and both act synergistically by diminishing the inflammatory responses (120). Furthermore, BP significantly suppresses oxidative stress and lipid peroxidation and neuroinflammation in the hippocampus tissues of mice brain (121). Moreover, the chemical composition of BP and other bee products is complicated and diverse and depends on many biological factors. While its clinical applications are still limited, ongoing future research will validate and consider BP hopefully as a clinical therapeutic remedy with promising healthy outcomes.
4.3 Anti-microbial activity of bee pollen
The extracts of both BP and bee bread possess anti-bacterial activity toward gram-positive bacteria and gram-negative bacteria. However, the extract of bee bread (minimum inhibitory concentration of 2.5 to 10% v/v) has higher antibiotic potential against Staphylococci species than bee pollen extract (minimum inhibitory concentration of 5% to >20% v/v). It was further studied that gram-positive bacteria were more susceptible to both extracts as compared with gram-negative bacteria (122). BP ethanol extracts show quite strong antibiotic properties against pathogenic fungi, gram-negative bacteria, and gram-positive bacteria because it contains phenolic acids. Flavonoid effects on bacteria are related to their metabolic disruption. The mechanism involves the formation of complexes with the cell wall of bacteria by surface-exposed polypeptides or cell membrane enzymes and adhesion, which blocks ion channels and inhibits electron flow in the electron transport chain which determines adenosine triphosphates (ATP) production by electron scavenging and also the disruption of cell wall integrity (100). By the use of a suitable extraction solvent, the bioactive properties of the honey BP can be increased (123). Additionally, both BP and bee bread contain beneficial bacterial and fungal species that have antimicrobial potential against some human clinical bacterial isolates such as E. coli, Staphylococci spp., and P. aeruginosa (124). Ethanol and methanol extracts of BP have similar antibacterial action against described bacteria. In addition, they show antifungal activity against Aspergillus flavus, A niger, and A fumigatus, and yeasts such as Rhodotorula mucilaginous, Candida krusi, C glabrata, and C albicans (100, 125). It has been stated that the number of Lactococcus species raised in the oral microbe’s community in the mice model (BP group) has antimicrobial activity against P. gingivalis and S. mutans (biofilm-forming bacteria) species. Thus, BP can protect and have an expedient impact on the oral cavity and the gastrointestinal tract (84). Recent studies reported that the BP from Wadi Al-Nahil showed antibacterial activity (in vitro) toward Clostridium perfringens (a pathogenic bacteria present in autism patients higher than in healthy individuals) (36).
It was examined that BP collected from Attalea funifera can have leishmanicidal activity against amastigote and promastigote types of Leishmania amazonensis due to various active compounds (126). The addition of BP to meat products can increase its shelf life because of its natural antimicrobial and antioxidant activities (4). The component of BP called rutin has been used in drug that acts as antibacterial, antiviral, and cardio-protective agents (89). In a recent study, five types of Portuguese BP were used against both types of bacteria (gram-positive bacteria and gram-negative bacteria) and yeasts which revealed growth inhibitory and antimicrobial action (50). In contrast, some BP extracts were examined against gram-negative bacteria which exhibited antibiotic action such as Acinetobacter baumannii and E. coli, while K. pneumoniae and P. aeruginosa showed resistance (127). It was also noted that the BP from Cistus and Castanea plants possesses higher antimicrobial activity as compared with Rubus (flowering plants), which may correlated with the high content of phenolics such as hydroxycinnamic acid (50). It has been stated that the fermented BP possesses strong antifungal action toward Penicillium roqueforti (128). A few studies revealed that the phenolic content of BP has a positive impact on the growth of useful gut microbiota while inhibiting the development of pathogenic microbes (45). Due to BP’s antimicrobial activity, it is also used in toothpaste, which inhibits germ proliferation and reduces oral inflammation (22).
In the current era, an excessive number of antibiotic drugs have been used against various diseases and pathogens which created an alarming situation because several pathogens showed resistance toward these antibiotics. Researchers throughout the world trying to investigate natural products (nutraceuticals) that are non-resistible and environmentally friendly (129–131). Like other beneficial products of animals and plants, bee hive products such as propolis, royal jelly, bee venom, and BP are well recognized as a natural remedy and considered healthy and supplemental food products (132–134).
4.4 Impact of bee pollen on skin health
From ancient times, beneficial effects of BP on skin conditions have been known. Sun et al. also studied the effect of free phenolic extract of BP on melanogenesis using B16 mouse melanoma cells (76). The melanin relative content and intracellular tyrosinase activity decreased in a very distinct and dose-dependent way by the studied substance. Free phenolic extract of BP expresses great effectiveness by increasing the reducing power that indirectly contributes to the reduced synthesis of melanin, which shows the usefulness of BP by protecting the cell from abnormal production of melanin (76, 135). Furthermore, Korean scientists also confirmed BP’s usefulness in the manufacturing of cosmetics that protect the skin from oxidative stress and hyperpigmentation (135, 136). BP is used in the form of lipid, aqueous, and lyophilized extracts. Furthermore, active substances can be extracted with oils, glycerin, water, and propylene glycol. BP extracts are used in cosmetics in a concentration of 0.5–5%. The high content of flavonoids has a significant effect on skin tissues which allocates the BP to seal and strengthen the capillaries caused by higher vitamin C content. In addition, BP stimulates mitotic division, enhances cell metabolism, and improves regeneration. Due to the occurrence of phospholipids, zinc, and methionine, BP normalizes the action of sebaceous glands. Moreover, BP strengthens the hair shaft because of sulfur-containing amino acids (137, 138).
BP has conditioning, regenerating, and moisturizing properties, stops the scalp itching and limits the growth of fungus, and is also added to the anti-dandruff shampoo (22, 138). Researchers studied that the best solution would be to mix the BP with ethyl esters of essential unsaturated fatty acids from flaxseeds. Additionally, BP may effectively increase safe mechanisms against skin dryness, oxidative damage caused by ultraviolet radiation, melanogenesis and inflammation have a role in a great variety of negative effects on human skin (139). Studies have shown that 1% BP can decrease lactate dehydrogenase release by 18.73% of dermal fibroblast. A clinical study revealed that BP had useful effects on wrinkles around the eyes, skin roughness, transparency, and hydration. Furthermore, there are no adverse effects on the skin by the BP (140). In addition, BP ointment was applied in the topical burn treatment for the first time (141). On two white domestic pigs, burn wounds were imposed and treated with bee pollen or silver sulfadiazine ointment. The comparative material was constituted by either tissue which was treated from a wound or tissue obtained from wounds treated with physiological saline. Histopathological and clinical evaluation revealed that applied apitherapeutic product decreases the burn wound healing time and positively affects the general condition of an animal. Moreover, the BP ointment may have a positive impact on the process of healing burn wounds, avoiding newly developed tissues from infection (142).
Rape BP phenolic extracts showed strong anti-tyrosinase activity in vitro (76). Studies have shown that phenolics are found in free and bound form (esterified and insoluble bound form). Both phenolics are produced in the endoplasmic reticulum and transported to the vacuole (free form) and cell wall matrix (bound or insoluble form) (143, 144) where they attached (via covalent, ester, and ether bonds with a cell wall) with arabinoxylans, pectin, cellulose, structural proteins, and hemicellulose of the plant cell (143, 145). Phenolic compounds have a major role in plant cells, i.e., protecting cells from UV rays of sunlight and insect predators (producing annoying/toxic substances) and penetration of pathogens (fungi) in addition to attracting plant pollinators (143, 146). Free-form phenolic activities were greater than the bound form. Free phenolic extracts of rape BP can be used as a natural anti-melanogenesis composition source (76). Furthermore, the components of BP such as kaempferol, levulinic acid, 5-hydroxyfurfural, phenolamides, sterols, p-coumaric acid ethyl ester, and caffeine exhibit anti-tyrosinase activity. However, phenylamides were regarded as a more powerful anti-tyrosinase agent than the other pollen components (147). It has been documented that BP from a corn tree (Quercus acutissima) is reported as an excellent antioxidant and anti-melanogenesis agent due to the presence of high amount of phenolic acids, and its possible mode of action is the obstruction of melanin-producing enzyme (tyrosinase) (22).
On the other hand, free phenolics are digestible in the small intestine while bound phenolic compounds are not absorbed (in the small intestine) and reached in the large intestine where they pass the fermentation process (by colon microbiota) and liberate bound phenolic compounds. Additionally, these phenolics have a great role in the colon by inhibiting cancer-causing microorganism growth via reducing pH in the fermentation environment (143, 148). Moreover, free phenolic extract from rape BP possesses higher capabilities to defend body cells from aberrant melanogenesis than bound phenolic extract via the suppression of microphthalmia-associated transcription factor (MITF) and downregulation of cyclic adenosine monophosphate (cyclic adenosine monophosphate) while preventing the anti-tyrosinase and antioxidant pathways in vitro (22). The methyl gallate and gallic acid (phenolic acids) obtained from Givotia rottleriformis diminish the growth of skin cancer cells (human epidermoid carcinoma) (149) and restrain hepatitis C virus (HCV) which causes hepatocellular carcinoma and liver cirrhosis (143, 150). In addition, a flavonoid called kaempferol possesses many biological activities such as cardio-protective, anti-inflammatory, anti-allergic, antidiabetic, osteoporotic, and analgesic (143, 151). The valuable effects of BP on human skin are well recognized so far. In addition, the negative impact of chemical cosmetics on human skin health warned people to use natural products that have both properties of chemo-protective and healthy dermal maintenance.
4.5 Effect of bee pollen on animal immunity
BP comprises over 50% more protein than beef; however, its lipid content is very low. To reduce the radiation effect and retard aging and also improve the immune response, pollen may be used because of its flavonoid and antioxidant components (152). The addition of BP in the diet of freshwater fish has led to improved immune status and growth, reduction of the mortality caused by Aeromonas hydrophila, and increase in the number of phagocytic cells (i.e., neutrophils and monocytes) in Nile tilapia (Oreochromis niloticus) (153). In the Nile tilapia, the therapeutic mode of action of BP was detected on pathogen A. hydrophila, where all treated fish showed significant protection. In detail, the experimental group fed with 2.5% (w/v) BP for 20 and 30 days showed the greatest protection against the described pathogen. In addition, BP increased significantly the growth performance parameters [length, average daily weight (ADG), body weight, feed efficiency ratio (FER), and specific growth rate (SGR)], as well as biochemical (albumin, globulin, and serum total protein ratios), immunological (serum bactericidal activity), nitro blue tetrazolium assay (NBT), and hematological parameters [hematocrit (Hct), leucrit (Lct)] as the number of lymphocytes, neutrophils, monophils, and phagocytic activity (154). Pollen and propolis extracts used for rainbow trout (Oncorhynchus mykiss) as a food additive had a positive effect on its final consumption weight and growth performance (155). Moreover, BP polysaccharides from corn enhance the activity of immunocytes in the body such as lymph nodes, spleen, thymus gland, and bone while strengthening immunity of the body against pathogenic microbes (22).
The fish diet added with the mixture of pollen and propolis (0.5%) was more effective with an increase in weight and a decrease in feed consumption as compared with the fish fed with pure pollen or propolis. This growth performance resulted from the mixture of propolis and pollen could be due to their components such as minerals, enzymes, or coenzymes as well as vitamins which enhance food digestion (156, 157). The diet enriched with a mixture of 0.5% propolis or 0.5% pollen is more effective than 1% propolis or 1% pollen alone on hematological parameters, plasma, and growth performance of rainbow trout (157). During the summer season under climatic conditions with very high air temperatures, the BP treatment impacts the hematological, physiological traits, and productive performance of rabbit bucks (158). An obvious improvement in immune status parameters was recorded after the supplementation of BP in diabetic rats as an antioxidant-rich food supplement (159). BP consumption significantly enhances the phagocytic capacity and index in the granulocytes of rabbits (160, 161). Previous studies revealed that the polysaccharides of BP (Chinese wolfberry) have immunomodulatory activity in vitro against RAW 264.7 cells via downregulating the level of tumor necrosis factor-alpha (TNF-α), interlukin-1 (IL-1), interlukin-6 (IL-6), and nitric oxide (NO) secretion (162).
Here, the consumption of BP led to the greatest increase in ex vivo proliferation of lymphocytes, while these results could simply be related to the presence of a great concentration of vitamins, trace elements, and amino acids that stimulate differentiation and proliferation of immune cells (161, 163). It is also possible that T lymphocyte formation is stimulated by the polysaccharide constituent in the BP (161). Various research studies have been done regarding pollen as a rich source of food for animals. Researchers concluded that pollen is a complete and functional food source because it contains important nutrients such as phenolics, vitamins, minerals, and other metabolites which are immune boosters, and food supplements have a positive impact on physiological health and possess promising therapeutic capabilities.
4.6 Effect of bee pollen on the animal reproductive system
The BP supplementation with 0.2 g per kg of body weight in the rabbit diet improved fertility (86.9%) of the control group (69.5%). By using BP mixed with propolis (100 + 100 mg/kg body weight), the experimental group showed increased body weight, the number of live kits (baby rabbits) at birth (p < 0.01) than the other groups, and greater litter size, but the number of stillborn kits did not differ significantly among all groups (164, 165). The complementary result of the supplementation of BP diet for rabbits on productive traits might be accredited to the high amount of macronutrients and micronutrients, e.g., minerals, in addition to phytosterols (165, 166). The enhancement of reproductive properties in rabbits might be related to the ability of BP to enhance estrogen levels appropriately and balance the hormones needed for conceiving. In addition, BP boosts the ability of eggs (in female rabbits) to withstand and survive during the period of incubation (165). The antigenotoxicity and genotoxicity as well as antiestrogenic and estrogenic activity of Cystus incanus L. and Salix alba L. of BP and its processed extracts in human and yeast cells were tested, and it was found that they were neither estrogenic nor genotoxic and able to reduce the damage of chromosome induced by the used cancer drugs, thus supporting their use as a chemoprotective agent (95, 96). BP and mannan oligosaccharides improved the antioxidant status and semen quality of rabbit bucks during the Egyptian summer season (167). Studies have shown that BP individually or coupled with metformin effectively suppressed the levels of TNF-α, Ki67, and NO while boosting mature follicles and P53 in polycystic ovary syndrome-treated rats (118).
Rabbit bucks raised under high temperature and treated with 1,000 mg and 500 mg of BP per buck, significantly ameliorating blood parameters, testosterone hormone, antioxidant activity, and semen characteristics (168). Ensuring animal welfare, increasing productivity, and improving product quality are common demands of animal breeders. Pollen and propolis can be added to the diet of laying quail to improve egg quality and enhance blood protein, egg production, lipid, and immunological responses (169). In the diet of Sinai chicken, BP could be used at 1000 mg/kg to ameliorate the live sperms, while the sperm aberrations were considerably decreased to approximately 36.28% than the control group (170). The BP (collected from date palm) was given orally to male Wister rats (having induced diabetes via streptozotocin) with a dosage of 100 mg/kg (daily up to 4 weeks), and considerable enhancements were detected in malondialdehyde (MDA), testicular nitric oxide, and blood glucose levels. As a result, significant development was observed in follicle-stimulating hormone (FSH), luteinizing hormone (LH), pancreas weight, serum insulin levels, body weight, testosterone, sperm viability, and motility than the diabetic control group. The administration of BP regulates the apoptotic function and secretion from ovaries of Wistar rats (40-days-old females who are clinically normal) (20, 22, 171).
It has been documented that the dose of 60 mg/day per animal of BP to a mice model (Turkish origin) significantly enhanced the sperm counts and testosterone levels for 1 month. Similarly, the treatment of lead-induced mice model with BP (Algerian origin) (100 mg/kg) noticeably improves the spermatogenesis process while demolishing sertoli cells (171). The presence of gonadotropin hormone in the date palm pollen surprisingly raised the possibility of the treatment of sterility. Many animal studies revealed that BP enhances semen quality and quantity as well as improves physiological health and other chemical substances that take part in the reproduction process of an individual organism. In addition, the human population also has some fertility and sexual problems, which can be treated using BP as a natural reproductive medicine and innovative food supplement.
4.7 Anticancer properties of bee pollen
Cancer is one of the known diseases worldwide and a major cause of human disease. It has been stated that the cytotoxicity effect of BP is mainly linked with flavonoids and phenolic compounds present in its chemical composition. The mechanism of anti-carcinogenic activity of these components is due to the secretion of TNF-α and stimulation of apoptosis, while the suppression of reactive oxygen species (ROS) inhibits the proliferation of cells to prevent DNA damage. Furthermore, Amalia et al. reported that a BP sample from Indonesia (Trigona spp) had an antiproliferative effect on human cancer cell lines with an IC50 (half-maximal inhibitory concentration) value (18.6 ± 0.03 mg/mL) in a time and dose-dependent manner after 24 h. Additionally, BP possesses very low toxicity rather than water-soluble propolis toward normal cells (120, 172). Ravishankar et al. studied that the BP component called quercetin may have anticancer activity by upregulating tumor suppressor genes while disrupting oncogene expression. BP samples (Olea europaea and Coriandrum spp.) from Morocco have been used as an anticancer agent against HeLa (cervical carcinoma) and MCF-7 (breast adenocarcinoma) cell lines, respectively, and their anticancer activity may be associated with the presence of flavonoids, i.e., kaempferol-3-O-rhamnoside and quercetin-O- di-glucoside (173). Studies revealed that the BP sample (collected from South Korea) was used against various human cancer cell lines such as NCI-H727 (human lung carcinoma), PC-3 (human prostate adenocarcinoma), AGS (human gastric adenocarcinoma), MCF-7 (breast adenocarcinoma), and A549 (human lung carcinoma) which showed IC50 value ranging from 0.9 to >25 mg/mL (174, 175).
The BP protein called hydrolysate (enzymatically cleaved BP protein) can have strong anticancer activity by inhibiting human bronchogenic carcinoma (ChaGo-K1 cells), resulting in IC50 value of 1.37 μg/mL (174, 176). In another study, BP along with doxorubicin (DOX) was tested synergistically on mice models having induced breast tumor (4 T1 tumor cells) for 35 days. The blood was taken on 36th day in order to quantify the level of nitric oxide, cytokines, progesterone, estrogen, and testosterone. In addition, it was noted that both BP and DOX work synergistically and upregulate the expression of apoptotic genes and proteins and eventually decrease the volume of tumor cells while decreasing the level of nitric oxide and estrogen and downregulating the level of pro-inflammatory cytokines in mice models with induced 4 T1 breast tumors. BP isoflavones have been used as anticancer agent against breast cancer cells such as triple-negative breast cancer (TNBC) and restrain the activity of endogenous estrogen (177). Research has shown that isoflavones and flavonoids of BP restrain the cell cycle at G1 and or G2/M stages and upregulate the expression of pro-apoptotic genes and dysfunction of the capability of enzymes associated with the growth and development of tumor cells while hindering the activity of intermediates such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) and also inhibiting the production of ROS (177, 178). Wang et al. concluded that BP polysaccharides (extracted from Rosa rugosa) exhibited anticancer activity in vitro against HCT116 (human colon carcinoma) and HT-29 (colon cancer) cells in a dose-dependent way (179, 180). Furthermore, BP samples of three stingless bees, i.e., Heterotrigona itama, Tetrigona apicalis, and Geniotrigona thoracica (from Malaysia) were tested in vitro upon MCF-7 (breast adenocarcinoma human cell lines) and MCF-10A (mammary epithelial human cell lines), which inhibits the proliferation of both tumor cell lines in a dose-dependent way (181).
5 Bee pollen and honey bee health
There are 20 biogenic amino acids, but honey bee requires only 10 amino acids that are essential to their diet. Different plant origins of pollen grains contain all essential amino acids in fewer amount (89, 182). Except for nutrition, honey bees use those amino acids for maintaining individual immunity and social behavioral patterns. The queen royal jelly as a protein-rich diet can enhance ovarian development and egg production in honey bees (A. mellifera) and worker bees since pollen is their main protein source, and its digestibility and quality might be important nutritive factors that determine reproductive capability (183). The most important elements of honey bee nutrition are carbohydrates, proteins, amino acids, and lipids, all of which have a significant impact on individual and colony health (184, 185). The nutrition value of these (carbohydrates, proteins, amino acids, and lipids) contents of some pollen types varies between species (186, 187). In A. mellifera scutellata workers, the effect of two types of pollen was examined: aloe (Aloe greatheadii var. davyana) and sunflower (Helianthus annuus) were compared in the field conditions with the queen, and when feeding on aloe pollen, the workers have exhibited greater ovarian development (183). Maize pollen is thought to be lacking essential amino acids and proteins and is believed to be a minor bee feed source (188).
A healthy honey bee colony can consume up to 7 kg of pollen per year. During times of scarcity, it is crucial for a bee colony to have supply of pollen as it greatly impacts their survival and reproductive abilities, such as activating ovarian function of the queen bee. Fermented bee pollen also has the added benefit of inhibiting the growth of harmful bacteria such as Melissococcus plutonius (European foulbrood) and Paenibacillus larvae (American foulbrood) due to the presence of dodecanoic acid, linoleic acid, myristic acid, and linolenic acids (110). There is a correlation between the reproduction of honey bee colonies and the protein content found in pollen. Honey bees that collect pollen with high protein content tend to have higher colony reproduction rates and bee brood production. Pollen collected from plants that bloom during the spring season contains a higher protein content (average of 24.2%) compared with those that bloom during the summer and autumn seasons (average of 19.3 and 20.5%, respectively). During the spring season, pollen containing protein content of more than 27% enables honey bee colonies to enhance their reproduction (189). Beekeepers need to provide pollen patties as a supplement to bee colonies during pollen deficient season because pollen is the main protein source for honey bees. Additionally, it is also recommended that beekeeper should move their colonies to pollen-rich areas because brood production and development and also the reproductive capability of bees are solely dependent on pollen availability of different plants.
6 Bee pollen availability and its consumption
Nowadays, it is a developing business for the beekeeping industry, as BP is regarded as a functional food for human health. To collect pollen from bees, various types of pollen-collecting traps are designed (with bee size small holes) and transplanted with the hive entrance gates for the purpose of detaching pollen from the legs of bees when they enter the hive (190, 191). It is consumed after drying to guarantee long-term safety and stability and is mostly commercialized. If pollen is dried upon heat (more than 40°C to 50°C), it affects the phenolic contents and pollen organoleptic features (191, 192). An alternative way to preserve its nutritional content and organoleptic properties might be freezing (191). BP is a nutrient-rich supplement and has a beneficial impact on health (193). It was reported that the consumption of pollen by women can be considered a source of good health, a strong body, and beauty in old history. Routine use of BP prevents the body from harmful radiotherapy and chemotherapy reactions. It is also useful for people doing hard physical work and children with appetite loss and patients who are starving. Various pollen products can be found in the form of oral liquids, candy bars, tonics, pellets, tablets capsules, powders, and granules in the market (25).
BP can be mixed with biscuits, which increases its protein, sugar, phenolics, and fiber content and also enhances the antioxidant efficacy of biscuits. As a supplemental food, BP added wheat flour in bakeries to produce various food items (4, 14). The recommended dose of pollen consumption for an adult human should range from 20 to 40 g daily (3, 11, 20, 24, 194). Athlete players use BP as a highly nutritive food to increase their athletic ability (47). However, dried or fresh pollen grains frequently have a hard shell that can affect significantly the digestive enzyme penetration into pellets of pollen (194). Bee bread (fermented BP) is said to be more nutritive as compared with BP because the content of some of their valuable components increases, which are highly digestible and penetrable through the gastrointestinal tract (4). Furthermore, the consumption of BP also has significant effect on the beneficial microbiota of animal gut which further helps in food digestion and assimilation and strengthens immunity (46). Thus, it is recommended to use fermented pollen which is more acceptable for nutrient absorption by the digestive tract of humans (194, 195) because microbes degrade the external pollen walls during fermentation, and its internal nutrients can easily be consumed (8).
7 Bee pollen and human health risk
In additiob to its beneficial nutrients, some harmful substances such as allergens and other toxic (mycotoxin) compounds are also found in BP. The allergic substances present in pollen are highly hazardous for sensitive people if they consume or inhale pollen. Special care must be taken during pollen consumption and can be utilized according to the physician’s suggestions. BP is an important apicultural product, which is used as a nutritive ingredient and mixed with other products for therapeutic purposes. Alongside its beneficial properties, BP also possesses environmentally hazardous contaminants such as pesticides, toxic elements, and other health risk factors, which can be dangerous both for bee’s life and indirectly for public health (82, 85, 196). During the collection of pollen, environmental contaminants are also attached to the forager body and are transferred to their hives, so beehive products can be used as an environmental contaminants’ indicator (196–199). A total of 189 samples of BP were examined for pesticide adulteration in China, which showed the detection of insecticides such as chlorpyrifos, fenpropathrin, thiamethoxam, imidacloprid, and bifenthrin and fungicides such as triadimefon and carbendazim while acaricides such as fluvalinate and coumaphos were also found in BP samples (85). In a recent study, the researcher examined 45 different samples of BP from various regions of Greece, which exhibited the presence of harmful elements such as arsenic, cadmium, mercury, and lead while some pesticides such as coumaphos (22%) cypermethrin, propargite, dimethoate, and azoxystrobin were also detected (82). The researcher collected BP samples from 13 sites in northern Italy (during the flowering season 2019 to 2020) and examined for various pesticide compounds (insecticides, fungicides, herbicides, and acaricides). They found 97 different pesticides, i.e., mostly fungicides but highly toxic acaricides and insecticides (organophosphates and neonicotinoids), but the concentration of herbicides was low in the tested BP samples. The fungicide called zoxamide affects the motor and cytoskeleton proteins while penconazon and spiroxamine (fungicides) also have a negative impact on the synthesis of sterol in membranes. Another fungicide of most concern is called carbendazim which has been officially prohibited in EU countries since 2014 but is still found in BP samples from Italy (200).
It was reported that the acaricides (mostly used by beekeepers for the controlling of varroa mites) called tau-fluvalinate and coumaphos were found in BP samples of 5.22–85.22 μg/kg and 5.13–39.81 μg/kg, respectively (201). Additionally, 145 BP samples were collected from 10 honey bee colonies in Brazil (São Paulo State) and were analyzed for pesticide residues. The experts identified two pesticides, namely, pendimethalin and bioallethrin in the tested BP samples (199). Furthermore, the consumption of BP also creates some clinical problems such as allergic reactions in pollen-allergic sensitive individuals. However, the allergenicity can be overcome due to the treatment of BP with specific enzymes such as pectinase, papain, and cellulase. Additionally, enzyme-treated BP can mitigate the allergenicity of BP and harmonize the composition of gut microbe (23). BP also contains some fungal species such as Cladosporium, Alternaria, and Aspergillus, which cause allergic responses (85, 162). Other mycotoxin-producing fungal species were also detected in BP samples such as Fusarium spp. and Penicillium spp. Studies revealed that there are different types of mycotoxin which are found in pollen samples such as AFB1 (aflatoxin B1), ZEN (zearalenone), OTA (ochratoxin A), T-2, and DON (deoxynivalenol) (202), but the most important and harmful mycotoxin is AFB1 both for animal and human health causing DNA and chromosomal damage (202, 203).
Similarly, mycotoxin T2 and DON have detrimental effects on neurotransmitter and metabolic activities while ZEN and OTA mycotoxins possess neurotoxicity, hepatotoxicity, and immunotoxin effects. In addition, multifloral BP showed a higher concentration of mycotoxin than monofloral BP, whereas bee bread exhibited maximum amount of mycotoxins in analyzed samples (202). Another study from Lithuania stated that different fungi genera such as Yeast, Cladosporium, Alternaria, and Fusarium spp. were found in 45 samples of BP after 3 days of storage condition along with the presence of mycotoxin DON (120 μg/kg) and ZEN (280 μg/kg) (204). Researchers also detected various molds in BP samples from Ukraine Serbia, and Slovakia, such as Rhizopus, Aspergillus, Alternaria, Fusarium, Penicillium. and Mucor (196). In the same way, along with fungi, other allergens such as pyrrolizidine alkaloids and toxic elements are also present in BP. Mostly, pollen-allergic people show more clinical symptoms when they inhale airborne pollen, such as hay fever, infection of oral mucosa, skin, and cardiovascular and respiratory tract septicity. But allergic reactions after the consumption of pollen are rare. The allergenicity of pollen depends on the temperature and pH value because the main allergens present in pollen are glycoproteins (water-soluble proteins), which can easily diffuse after contact with airways mucosa (1).
The pollen allergic individuals should follow physician precaution measures while using other bee products (205). The presence of pyrrolizidine alkaloids in BP can create severe conditions of lung cancer and hepatotoxicity, which are strongly related to the botanical origin of BP. Additionally, pyrrolizidine alkaloid-producing plants belong to three botanical families, namely, Fabaceae, Boraginaceae, and Asteraceae. The BP of the plant species called Echium vulgare possesses high pyrrolizidine alkaloid contents in most European countries (1, 206), while the toxic elements include aluminum, lead, strontium, cadmium, arsenic, chromium, nickel, and mercury, which can cause neurotoxicity and other abnormalities (1, 207). The occurrence and bioaccumulation of heavy metals in the human physique create rigorous problems, such as intellectual disability, carcinogenesis, and other body abnormalities, such as disruption of metabolism and growth and nervous disabilities (85). The use of BP in food products and pharmaceutical and cosmetics industries considered as a vital bee product worldwide. Further research and clinical trials of BP in vitro and in vivo are needed to overcome the adverse effects of BP and eliminate its undesirable consequences on human health.
8 Honey bee products and nanotechnology/nanoparticles
Honey bee products have been used as a natural medicine and food supplement since the ancient era. These valuable products were used not only by humans but also utilized for the benefit of other animals. The way of its use is different, depending on the type of bee product and treatment procedure or consumption, such as injection, tablets, syrup, balm, or in conjugation with other products. Like other medicine or pharmaceutical products, api-products also have some side effects or are dangerous for allergic individuals. To overcome these difficulties, scientists are trying to use bee products in nanotechnology, which are safe, effective, and target-specific. Nanotechnology or nanoparticles (NPs) is a modern technique, where a specific carrier or vector (containing medicine) is used to target a specific site within the body of a patient. Different bee products such as bee venom, BP, propolis, royal jelly, and honey were incorporated into nanoparticles, which showed effective results and safe deliveries in many trial studies. Soman et al. used melittin (bee venom component) in perfluorocarbon nanoparticles against tumor cells and exhibited successful results (208).
Additionally, the main advantage of nanoparticles is that it protects the holding product from degrading enzymes in bloodstream, enhances bio-dispersal, and is target specific. Huang et al. administered alpha-melittin nanoparticles (α-MEL-NPs) and injected intravenously with a concentration of 20 mg/kg which showed the growth inhibition of B16F10 (human melanoma cells) and have no toxic effects on normal cells (209). Furthermore, the hemolytic activity of melittin in nanoparticles reduces by approximately 90% to direct utilization, enhances circulation duration, and gradually releases in a target site (210). The fungal chitosan (an amino polysaccharide derived from Fusarium oxysporum) loaded with bee venom nanoparticles was an effective anti-tumor agent for cervix carcinoma (HeLa cells) (211). In addition, chitosan nanoparticles containing BV possess inhibitory activity toward many clinical fungal strains, such as Kodamaea ohmeri, Cryptococcus neoformans, and Candida albicans (212). In another study, Saber et al. successfully administered chitosan nanoparticles, holding bee venom for the treatment of diseases caused by amoeba in the mice model (174).
Similarly, BP nanoparticles showed anti-cancer activity against A549 cancer cells (lung cancer cell lines) (213). It was documented that BP silver-based nanoparticles (AgNPs) have anti-diabetic properties and inhibit α-glucosidase and α-amylase enzymes more effectively than treatment with BP extract (214). Additionally, AgNPs prepared with honey bee extract (12–18 nm size) were used against colon cancer cells, which revealed anti-cancer activity (215). AgNPs containing an aqueous solution of rapeseed pollen exhibited anti-cancer capability against MCF-7 and MDA-MB-231 cancer cell lines (216). It has been studied that the magnetite nanoparticles (Fe3O4/PABA/MNPs) coated with BP extract could have anti-microbial activity toward gram-positive bacteria and gram-negative bacteria and some fungal species (217). Moreover, ethanolic extract of propolis-containing polymeric nanoparticles has anti-fungal activity against Candida albicans (174). Do Nascimento et al. concluded that ethanolic extract of Brazilian red propolis loaded with polymeric nanoparticles possesses anti-leishmanicidal capability (174, 218). Studies revealed that selenium nanoparticles (SeNPs) conjugated with ethanolic extract of propolis (Indian origin) possess antioxidant and anti-biotic and anti-fungal properties (219).
Furthermore, AgNPs loaded with propolis of stingless bees showed anti-cancer action against A549 cell lines (human lung cancer cells) with an inhibitory concentration (IC50) value of 38 μg/mL (216). It has been stated that zinc oxide (ZnO) nanoparticles in conjugation with bacterial cellulose and propolis extract revealed combined anti-microbial activity toward Candida albicans, Bacillus subtilis, and Escherichia coli (220). In another study, royal jelly-loaded AgNPs showed antibacterial activity against S. aureus (gram-positive bacteria) and S. typhimurium (gram-negative bacteria). The author further stated that the inhibitory effect of royal jelly-based AgNPs was more on S. typhimurium than on S. aureus (221). Similarly, AgNPs containing royal jelly also showed stronger antibiotic activity against E. coli than B. subtilis (222). Research has shown that nano-silver with royal jelly can have anti-inflammatory action, which enhances the activity of immune cells in mice models (223).
9 Conclusion and future remarks
BP is a nutritional product and an excellent apitherapeutic agent for different health problems. It contains many beneficial components which are necessary for health maintenance and can be used in different recopies because of its antioxidant capacity for long-lasting preservation of fats. BP is also used as an immunity booster, and its beneficial effects on reproduction. Its quality varies with botanical origin and geographic area conditions. BP shows significant therapeutic effects when experimented on different organisms against different health issues. It is a natural food and medicine, and it needs much attention as a health-oriented product. Future recommendations suggest its use in different types of apitherapy for the treatment of different human and animal diseases and physiological alterations. Few countries have regulation strategies for the usage of honey bee products such as China, Argentina, Brazil, Switzerland, and Poland. There must be legislative principles to standardize honey bee products as supplemental food and minimize the contaminants for safe and healthy use. It is important to prioritize the health and safety of honey bees by protecting their foraging areas and habitats and minimizing human impact.
Author contributions
SA: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. AU: Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing. FG: Conceptualization, Methodology, Project administration, Writing – original draft. GR: Software, Supervision, Visualization, Writing – review & editing. MK: Conceptualization, Data curation, Formal analysis, Writing – review & editing. MH: Conceptualization, Methodology, Project administration, Writing – review & editing. AA: Writing – review & editing, Supervision, Validation, Funding acquisition. C-CC: Writing – review & editing, Supervision, Funding acquisition. IT: Conceptualization, Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research work was financially supported by C-CC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kostić, AŽ, Milinčić, DD, Barać, MB, Ali Shariati, M, Tešić, ŽL, and Pešić, MB. The application of pollen as a functional food and feed ingredient—the present and perspectives. Biomol Ther. (2020) 10:84. doi: 10.3390/biom10010084
2. Isik, A, Ozdemir, M, and Doymaz, I. Effect of hot air drying on quality characteristics and physicochemical properties of bee pollen. Food Sci Technol. (2019) 39:224–31. doi: 10.1590/fst.02818
3. Erdoğan, A, Şeker, ME, and Kahraman, SD. Evaluation of environmental and nutritional aspects of bee pollen samples collected from East Black Sea region, Turkey, via elemental analysis by ICP-MS. Biol Trace Elem Res. (2022) 201:1488–502. doi: 10.1007/s12011-022-03217-3
4. Aylanc, V, Falcão, SI, Ertosun, S, and Vilas-Boas, M. From the hive to the table: nutrition value, digestibility and bioavailability of the dietary phytochemicals present in the bee pollen and bee bread. Trends Food Sci Technol. (2021) 109:464–81. doi: 10.1016/j.tifs.2021.01.042
5. Aylanc, V, Tomás, A, Russo-Almeida, P, Falcão, SI, and Vilas-Boas, M. Assessment of bioactive compounds under simulated gastrointestinal digestion of bee pollen and bee bread: bioaccessibility and antioxidant activity. Antioxidants. (2021) 10:651. doi: 10.3390/antiox10050651
6. Filannino, P, Di Cagno, R, Gambacorta, G, Tlais, AZA, Cantatore, V, and Gobbetti, M. Volatilome and bioaccessible phenolics profiles in lab-scale fermented bee pollen. Food Secur. (2021) 10:286. doi: 10.3390/foods10020286
7. Loper, G, Standifer, L, Thompson, M, and Gilliam, M. Biochemistry and microbiology of bee-collected almond (prunus dulcis) pollen and bee bread. I-fatty acids, sterols, vitamins and minerals. Apidologie. (1980) 11:63–73. doi: 10.1051/apido:19800108
8. Zhang, H, Lu, Q, and Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. (2022) 375:131908. doi: 10.1016/j.foodchem.2021.131908
9. Ruedenauer, FA, Wöhrle, C, Spaethe, J, and Leonhardt, SD. Do honeybees (Apis mellifera) differentiate between different pollen types? PLoS One. (2018) 13:e0205821. doi: 10.1371/journal.pone.0205821
10. Hegedüs, K, Fehér, C, Jalsovszky, I, Kristóf, Z, Rohonczy, J, Vass, E, et al. Facile isolation and analysis of sporopollenin exine from bee pollen. Sci Rep. (2021) 11:1–16. doi: 10.1038/s41598-021-87619-8
11. Komosinska-Vassev, K, Olczyk, P, Kaźmierczak, J, Mencner, L, and Olczyk, K. Bee pollen: chemical composition and therapeutic application. Evid Based Complement Alternat Med. (2015) 2015:1–6. doi: 10.1155/2015/297425
12. Couto, RHN, and Couto, LA. Apicultura: manejo e produtos: Funep Jaboticabal. Brazil, 3rd edition, (2006).
13. Pereira, FM, Freitas, BM, Vieira Neto, JM, Lopes, MTR, Barbosa, AL, and Camargo, RCR. Desenvolvimento de colônias de abelhas com diferentes alimentos protéicos. Pesq Agrop Brasileira. (2006) 41:1–7. doi: 10.1590/S0100-204X2006000100001
14. Thakur, M, and Nanda, V. Composition and functionality of bee pollen: a review. Trends Food Sci Technol. (2020) 98:82–106. doi: 10.1016/j.tifs.2020.02.001
15. Barajas, J, Cortes-Rodriguez, M, and Rodríguez-Sandoval, E. Effect of temperature on the drying process of bee pollen from two zones of Colombia. J Food Process Eng. (2012) 35:134–48. doi: 10.1111/j.1745-4530.2010.00577.x
16. De-Melo, AAM, Estevinho, MLMF, Sattler, JAG, Souza, BR, da Silva, FA, Barth, OM, et al. Effect of processing conditions on characteristics of dehydrated bee-pollen and correlation between quality parameters. LWT-Food Sci Technol. (2016) 65:808–15. doi: 10.1016/j.lwt.2015.09.014
17. Shin, D, Choi, WT, Lin, H, Qu, Z, Breedveld, V, and Meredith, JC. Humidity-tolerant rate-dependent capillary viscous adhesion of bee-collected pollen fluids. Nat Commun. (2019) 10:1–9. doi: 10.1038/s41467-019-09372-x
18. Mares, S, Ash, L, and Gronenberg, W. Brain allometry in bumblebee and honey bee workers. Brain Behav Evol. (2005) 66:50–61. doi: 10.1159/000085047
19. Ullah, A, Aldakheel, FM, Anjum, SI, Raza, G, Khan, SA, and Gajger, IT. Pharmacological properties and therapeutic potential of honey bee venom. Saudi Pharmaceutical Journal. (2023) 31:96–109. doi: 10.1016/j.jsps.2022.11.008
20. Khalifa, SA, Elashal, MH, Yosri, N, Du, M, Musharraf, SG, Nahar, L, et al. Bee pollen: current status and therapeutic potential. Nutrients. (2021) 13:1876. doi: 10.3390/nu13061876
21. Adaškevičiūtė, V, Kaškonienė, V, Barčauskaitė, K, Kaškonas, P, and Maruška, A. The impact of fermentation on bee pollen polyphenolic compounds composition. Antioxidants. (2022) 11:645. doi: 10.3390/antiox11040645
22. Algethami, JS, El-Wahed, AAA, Elashal, MH, Ahmed, HR, Elshafiey, EH, Omar, EM, et al. Bee pollen: clinical trials and patent applications. Nutrients. (2022) 14:2858. doi: 10.3390/nu14142858
23. Tao, Y, Zhou, E, Li, F, Meng, L, Li, Q, and Wu, L. Allergenicity alleviation of bee pollen by enzymatic hydrolysis: regulation in mice allergic mediators, metabolism, and gut microbiota. Food Secur. (2022) 11:3454. doi: 10.3390/foods11213454
24. Oroian, M, Dranca, F, and Ursachi, F. Characterization of Romanian bee pollen—An important nutritional source. Food Secur. (2022) 11:2633. doi: 10.3390/foods11172633
25. Li, Q-Q, Wang, K, Marcucci, MC, Sawaya, ACHF, Hu, L, Xue, X-F, et al. Nutrient-rich bee pollen: a treasure trove of active natural metabolites. J Funct Foods. (2018) 49:472–84. doi: 10.1016/j.jff.2018.09.008
26. Karabagias, IK, Karabagias, VK, Gatzias, I, and Riganakos, KA. Bio-functional properties of bee pollen: the case of “bee pollen yoghurt”. Coatings. (2018) 8:423. doi: 10.3390/coatings8120423
27. Chantarudee, A, Phuwapraisirisan, P, Kimura, K, Okuyama, M, Mori, H, Kimura, A, et al. Chemical constituents and free radical scavenging activity of corn pollen collected from Apis mellifera hives compared to floral corn pollen at Nan, Thailand. BMC Complement Altern Med. (2012) 12:1–12. doi: 10.1186/1472-6882-12-45
28. Liolios, V, Tananaki, C, Dimou, M, Kanelis, D, Rodopoulou, M-A, and Thrasyvoulou, A. Exploring the sugar profile of unifloral bee pollen using high performance liquid chromatography. J Food Nutr Res. (2018) 57
29. Bertoncelj, J, Polak, T, Pucihar, T, Lilek, N, Kandolf Borovšak, A, and Korošec, M. Carbohydrate composition of Slovenian bee pollens. Int J Food Sci Technol. (2018) 53:1880–8. doi: 10.1111/ijfs.13773
30. Da Silva, GR, da Natividade, TB, Camara, CA, da Silva, EMS, dos Santos, FAR, and Silva, TMS. Identification of sugar, amino acids and minerals from the pollen of Jandaíra stingless bees (Melipona subnitida). Food Nutr Sci. (2014) 5:1015–21. doi: 10.4236/fns.2014.511112
31. Yang, K, Wu, D, Ye, X, Liu, D, Chen, J, and Sun, P. Characterization of chemical composition of bee pollen in China. J Agric Food Chem. (2013) 61:708–18. doi: 10.1021/jf304056b
32. Paramás, AMG, Bárez, JAG, Marcos, CC, García-Villanova, RJ, and Sánchez, JS. HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem. (2006) 95:148–56. doi: 10.1016/j.foodchem.2005.02.008
33. Margaoan, R, Mărghitaş, LA, Dezmirean, DS, Dulf, FV, Bunea, A, Socaci, SA, et al. Predominant and secondary pollen botanical origins influence the carotenoid and fatty acid profile in fresh honeybee-collected pollen. J Agric Food Chem. (2014) 62:6306–16. doi: 10.1021/jf5020318
34. Kostić, AŽ, Pešić, MB, Trbović, D, Petronijević, R, Dramićanin, AM, Milojković-Opsenica, DM, et al. The fatty acid profile of Serbian bee-collected pollen–a chemotaxonomic and nutritional approach. J Apic Res. (2017) 56:533–42. doi: 10.1080/00218839.2017.1356206
35. De Arruda, VAS, Pereira, AAS, de Freitas, AS, Barth, OM, and de Almeida-Muradian, LB. Dried bee pollen: B complex vitamins, physicochemical and botanical composition. J Food Compos Anal. (2013) 29:100–5. doi: 10.1016/j.jfca.2012.11.004
36. Alfawaz, HA, El-Ansary, A, Al-Ayadhi, L, Bhat, RS, and Hassan, WM. Protective effects of bee pollen on multiple propionic acid-induced biochemical autistic features in a rat model. Meta. (2022) 12:571. doi: 10.3390/metabo12070571
37. Taha, E-KA. Chemical composition and amounts of mineral elements in honeybee-collected pollen in relation to botanical origin. J Apicult Sci. (2015) 59:75–81. doi: 10.1515/jas-2015-0008
38. Freire, KR, Lins, AC, Dórea, MC, Santos, FA, Camara, CA, and Silva, TM. Palynological origin, phenolic content, and antioxidant properties of honeybee-collected pollen from Bahia, Brazil. Molecules. (2012) 17:1652–64. doi: 10.3390/molecules17021652
39. Zhang, Y, Yang, F, Jamali, MA, and Peng, Z. Antioxidant enzyme activities and lipid oxidation in rape (Brassica campestris L.) bee pollen added to salami during processing. Molecules. (2016) 21:1439. doi: 10.3390/molecules21111439
40. Sattler, JAG, de Melo, ILP, Granato, D, Araújo, E, de Freitas, AS, Barth, OM, et al. Impact of origin on bioactive compounds and nutritional composition of bee pollen from southern Brazil: a screening study. Food Res Int. (2015) 77:82–91. doi: 10.1016/j.foodres.2015.09.013
41. Abd Alla, AE, and Salem, RA. Impact of storage period on different types of bee pollen pigments. J Plant Protect Pathol. (2020) 11:9–13. doi: 10.21608/jppp.2020.68178
42. Salazar-González, CY, Stinco, CM, Rodríguez-Pulido, FJ, Díaz-Moreno, C, Fuenmayor, C, Heredia, FJ, et al. Characterization of carotenoid profile and α-tocopherol content in Andean bee pollen influenced by harvest time and particle size. LWT. (2022) 170:114065. doi: 10.1016/j.lwt.2022.114065
43. Karkar, B, Şahin, S, and Güneş, ME. Evaluation of antioxidant properties and determination of phenolic and carotenoid profiles of chestnut bee pollen collected from Turkey. J Apic Res. (2021) 60:765–74. doi: 10.1080/00218839.2020.1844462
44. Mazurek, S, Szostak, R, Kondratowicz, M, Węglińska, M, Kita, A, and Nemś, A. Modeling of antioxidant activity, polyphenols and macronutrients content of bee pollen applying solid-state 13C NMR spectra. Antioxidants. (2021) 10:1123. doi: 10.3390/antiox10071123
45. Ilie, C-I, Oprea, E, Geana, E-I, Spoiala, A, Buleandra, M, Gradisteanu Pircalabioru, G, et al. Bee pollen extracts: chemical composition, antioxidant properties, and effect on the growth of selected probiotic and pathogenic Bacteria. Antioxidants. (2022) 11:959. doi: 10.3390/antiox11050959
46. Di Chiacchio, IM, Gómez-Abenza, E, Paiva, IM, de Abreu, DJ, Rodríguez-Vidal, JF, Carvalho, EE, et al. Bee pollen in zebrafish diet affects intestinal microbiota composition and skin cutaneous melanoma development. Sci Rep. (2022) 12:1–18. doi: 10.1038/s41598-022-14245-3
47. Lu, P, Takiguchi, S, Honda, Y, Lu, Y, Mitsui, T, Kato, S, et al. NMR and HPLC profiling of bee pollen products from different countries. Food Chem Mol Sci. (2022) 5:100119. doi: 10.1016/j.fochms.2022.100119
48. Anjos, O, Fernandes, R, Cardoso, SM, Delgado, T, Farinha, N, Paula, V, et al. Bee pollen as a natural antioxidant source to prevent lipid oxidation in black pudding. LWT. (2019) 111:869–75. doi: 10.1016/j.lwt.2019.05.105
49. Morgano, MA, Martins, MCT, Rabonato, LC, Milani, RF, Yotsuyanagi, K, and Rodriguez-Amaya, DB. A comprehensive investigation of the mineral composition of Brazilian bee pollen: geographic and seasonal variations and contribution to human diet. J Braz Chem Soc. (2012) 23:727–36. doi: 10.1590/S0103-50532012000400019
50. Gabriele, M, Frassinetti, S, and Pucci, L. Antimicrobial activity and protective effect of Tuscan bee pollens on oxidative and endoplasmic reticulum stress in different cell-based models. Food Secur. (2021) 10:1422. doi: 10.3390/foods10061422
51. Wang, XY, Quan, QM, Wang, B, Li, YX, and Huang, SQ. Pollen competition between morphs in a pollen-color dimorphic herb and the loss of phenotypic polymorphism within populations. Evolution. (2018) 72:785–97. doi: 10.1111/evo.13445
52. Xiong, YZ, Jia, LB, Zhang, C, and Huang, SQ. Color-matching between pollen and corolla: hiding pollen via visual crypsis? New Phytol. (2019) 224:1142–50. doi: 10.1111/nph.16012
53. Bleha, R, Shevtsova, TV, Živčáková, M, Korbářová, A, Ježková, M, Saloň, I, et al. Spectroscopic discrimination of bee pollen by composition, color, and botanical origin. Food Secur. (2021) 10:1682. doi: 10.3390/foods10081682
54. Lunau, K. The ecology and evolution of visual pollen signals. Plant Syst Evol. (2000) 222:89–111. doi: 10.1007/BF00984097
55. Tao, Y, Yin, S, Fu, L, Wang, M, Meng, L, Li, F, et al. Identification of allergens and allergen hydrolysates by proteomics and metabolomics: a comparative study of natural and enzymolytic bee pollen. Food Res Int. (2022) 158:111572. doi: 10.1016/j.foodres.2022.111572
56. Somme, L, Moquet, L, Quinet, M, Vanderplanck, M, Michez, D, Lognay, G, et al. Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst. (2016) 19:1149–61. doi: 10.1007/s11252-016-0555-z
57. Weiner, CN, Hilpert, A, Werner, M, Linsenmair, KE, and Blüthgen, N. Pollen amino acids and flower specialisation in solitary bees. Apidologie. (2010) 41:476–87. doi: 10.1051/apido/2009083
58. Kriesell, L, Hilpert, A, and Leonhardt, SD. Different but the same: bumblebee species collect pollen of different plant sources but similar amino acid profiles. Apidologie. (2017) 48:102–16. doi: 10.1007/s13592-016-0454-6
59. Heldt, LFS, Pereira, D, Souza, BR, Almeida-Muradian, LB, and Carpes, ST. Fortification of beef burger with the addition of bee pollen from Apis mellifera L. Emir J Food Agric. (2019):895–901. doi: 10.9755/ejfa.2019.v31.i11.2025
60. Şahin, S, and Karkar, B. The antioxidant properties of the chestnut bee pollen extract and its preventive action against oxidatively induced damage in DNA bases. J Food Biochem. (2019) 43:e12888. doi: 10.1111/jfbc.12888
61. Nürnberger, F, Keller, A, Härtel, S, and Steffan-Dewenter, I. Honey bee waggle dance communication increases diversity of pollen diets in intensively managed agricultural landscapes. Mol Ecol. (2019) 28:3602–11. doi: 10.1111/mec.15156
62. McFrederick, QS, and Rehan, SM. Wild bee pollen usage and microbial communities co-vary across landscapes. Microb Ecol. (2019) 77:513–22. doi: 10.1007/s00248-018-1232-y
63. Nicholls, E, and Hempel de Ibarra, N. Assessment of pollen rewards by foraging bees. Funct Ecol. (2017) 31:76–87. doi: 10.1111/1365-2435.12778
64. Müller, A. Morphological specializations in central European bees for the uptake of pollen from flowers with anthers hidden in narrow corolla tubes (Hymenoptera: Apoidea). Entomol Gen. (1995) 20:43–57. doi: 10.1127/entom.gen/20/1995/43
65. Campos, MG, Anjos, O, Chica, M, Campoy, P, Nozkova, J, Almaraz-Abarca, N, et al. Standard methods for pollen research. J Apic Res. (2021) 60:1–109. doi: 10.1080/00218839.2021.1948240
66. Campos, MG, Bogdanov, S, de Almeida-Muradian, LB, Szczesna, T, Mancebo, Y, Frigerio, C, et al. Pollen composition and standardisation of analytical methods. J Apic Res. (2008) 47:154–61. doi: 10.1080/00218839.2008.11101443
67. Radev, Z. Collected pollen by the honey bee (Apis Mellifera L.) according to its protein content. New knowl J Sci. (2019) 2:99–109. doi: 10.32474/OAJESS.2019.02.000144
68. Pernal, SF, and Currie, RW. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie. (2000) 31:387–409. doi: 10.1051/apido:2000130
69. LoCascio, GM, Aguirre, L, Irwin, RE, and Adler, LS. Pollen from multiple sunflower cultivars and species reduces a common bumblebee gut pathogen. R Soc Open Sci. (2019) 6:190279. doi: 10.1098/rsos.190279
70. Tasei, J-N, and Aupinel, P. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie. (2008) 39:397–409. doi: 10.1051/apido:2008017
71. T'ai, HR, and Cane, JH. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol Ecol. (2002) 16:49–65. doi: 10.1023/A:1016048526475
72. Rasmont, P, Regali, A, Ings, TC, Lognay, G, Baudart, E, Marlier, M, et al. Analysis of pollen and nectar of Arbutus unedo as a food source for Bombus terrestris (Hymenoptera: Apidae). J Econ Entomol. (2005) 98:656–63. doi: 10.1603/0022-0493-98.3.656
73. Brunner, FS, Schmid-Hempel, P, and Barribeau, SM. Protein-poor diet reduces host-specific immune gene expression in Bombus terrestris. Proc R Soc B Biol Sci. (2014) 281:20140128. doi: 10.1098/rspb.2014.0128
74. Batos, B, and Miljković, D. The vitality of the Serbian spruce (Picea omorika) pollen during the long-term cryopreservation. Grana. (2019) 58:433–46. doi: 10.1080/00173134.2019.1668053
75. Araújo, JS, Chambó, ED, Costa, MAPC, Cavalcante da Silva, SMP, Lopes de Carvalho, CA, Estevinho, M, et al. Chemical composition and biological activities of mono-and heterofloral bee pollen of different geographical origins. Int J Mol Sci. (2017) 18:921. doi: 10.3390/ijms18050921
76. Sun, L, Guo, Y, Zhang, Y, and Zhuang, Y. Antioxidant and anti-tyrosinase activities of phenolic extracts from rape bee pollen and inhibitory melanogenesis by cAMP/MITF/TYR pathway in B16 mouse melanoma cells. Front Pharmacol. (2017) 8:104. doi: 10.3389/fphar.2017.00104
77. Hinojosa, L, Matanguihan, JB, and Murphy, KM. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). J Agron Crop Sci. (2019) 205:33–45. doi: 10.1111/jac.12302
78. Brunet, J, Ziobro, R, Osvatic, J, and Clayton, MK. The effects of time, temperature and plant variety on pollen viability and its implications for gene flow risk. Plant Biol. (2019) 21:715–22. doi: 10.1111/plb.12959
79. Easton-Calabria, A, Demary, KC, and Oner, NJ. Beyond pollination: honey bees (Apis mellifera) as zootherapy keystone species. Front Ecol Evol. (2019) 6:161. doi: 10.3389/fevo.2018.00161
80. Ünal, M, and Öztürk, O. Knowledge and opinions about apitherapy among the term 1 and term 6 medical students. J Apic Res. (2020) 59:956–9. doi: 10.1080/00218839.2019.1665248
81. Nogueira, C, Iglesias, A, Feás, X, and Estevinho, LM. Commercial bee pollen with different geographical origins: a comprehensive approach. Int J Mol Sci. (2012) 13:11173–87. doi: 10.3390/ijms130911173
82. Zafeiraki, E, Kasiotis, KM, Nisianakis, P, Manea-Karga, E, and Machera, K. Occurrence and human health risk assessment of mineral elements and pesticides residues in bee pollen. Food Chem Toxicol. (2022) 161:112826. doi: 10.1016/j.fct.2022.112826
83. Dong, J, Gao, K, Wang, K, Xu, X, and Zhang, H. Cell wall disruption of rape bee pollen treated with combination of protamex hydrolysis and ultrasonication. Food Res Int. (2015) 75:123–30. doi: 10.1016/j.foodres.2015.05.039
84. Khurelchuluun, A, Uehara, O, Paudel, D, Morikawa, T, Kawano, Y, Sakata, M, et al. Bee pollen diet alters the bacterial Flora and Antimicrobial peptides in the Oral cavities of mice. Food Secur. (2021) 10:1282. doi: 10.3390/foods10061282
85. Végh, R, Csóka, M, Sörös, C, and Sipos, L. Food safety hazards of bee pollen–a review. Trends Food Sci Technol. (2021) 114:490–509. doi: 10.1016/j.tifs.2021.06.016
86. Adaškevičiūtė, V, Kaškonienė, V, Kaškonas, P, Barčauskaitė, K, and Maruška, A. Comparison of physicochemical properties of bee pollen with other bee products. Biomol Ther. (2019) 9:819. doi: 10.3390/biom9120819
87. Su, J, Yang, X, Lu, Q, and Liu, R. Antioxidant and anti-tyrosinase activities of bee pollen and identification of active components. J Apic Res. (2021) 60:297–307. doi: 10.1080/00218839.2020.1722356
88. Filannino, P, Di Cagno, R, Vincentini, O, Pinto, D, Polo, A, Maialetti, F, et al. Nutrients bioaccessibility and anti-inflammatory features of fermented bee pollen: a comprehensive investigation. Front Microbiol. (2021) 12:7. doi: 10.3389/fmicb.2021.622091
89. Bayram, NE, Gercek, YC, Çelik, S, Mayda, N, Kostić, AŽ, Dramićanin, AM, et al. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin–similarities and differences. Arab J Chem. (2021) 14:103004. doi: 10.1016/j.arabjc.2021.103004
90. Waykar, B, and Alqadhi, YA. Beekeeping and bee products; boon for human health and wealth. Int J Pharm Biol res. (2016) 4:20–7. doi: 10.30750/ijpbr.4.3.4
91. Munstedt, K, and Franke, FE. Pollen therapy and its scientific evidence. Am Bee J. (2005) 145:511–3.
92. Elist, J. Effects of pollen extract preparation Prostat/Poltit on lower urinary tract symptoms in patients with chronic nonbacterial prostatitis/chronic pelvic pain syndrome: a randomized, double-blind, placebo-controlled study. Urology. (2006) 67:60–3. doi: 10.1016/j.urology.2005.07.035
93. Cornara, L, Biagi, M, Xiao, J, and Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol. (2017) 8:412. doi: 10.3389/fphar.2017.00412
94. Llnskens, H, and Jorde, W. Pollen as food and medicine—a review. Econ Bot. (1997) 51:78–86. doi: 10.1007/BF02910407
95. Shi, HB, Kong, M, Chen, G, Zhao, J, Shi, HL, Chen, Y, et al. Compound pollen protein nutrient increases serum albumin in cirrhotic rats. Gastroenterol Res. (2010) 3:253. doi: 10.4021/gr240e
96. Hajková, Z, Toman, R, Hluchý, S, Gálik, B, Bíro, D, Martiniaková, M, et al. The effect of pollen on the structure of the small intestine in rats after an experimental addition in diet. Sci Papers Anim Sci Biotechnol. (2013) 46:232–7.
97. Capcarova, M, Kalafova, A, Schwarzova, M, Schneidgenova, M, Prnova, MS, Svik, K, et al. Consumption of bee bread influences glycaemia and development of diabetes in obese spontaneous diabetic rats. Biologia. (2019) 75:705–11. doi: 10.2478/s11756-019-00337-5
98. Elghouizi, A, Al-Waili, N, Elmenyiy, N, Elfetri, S, Aboulghazi, A, Al-Waili, A, et al. Protective effect of bee pollen in acute kidney injury, proteinuria, and crystalluria induced by ethylene glycol ingestion in rats. Sci Rep. (2022) 12:1–11. doi: 10.1038/s41598-022-12086-8
99. Rice-Evans, CA, Miller, NJ, and Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Bio Med. (1996) 20:933–56. doi: 10.1016/0891-5849(95)02227-9
100. Rzepecka-Stojko, A, Stojko, J, Kurek-Górecka, A, Górecki, M, Kabała-Dzik, A, Kubina, R, et al. Polyphenols from bee pollen: structure, absorption, metabolism and biological activity. Molecules. (2015) 20:21732–49. doi: 10.3390/molecules201219800
101. Zabłocka, A, and Janusz, M. Dwa oblicza wolnych rodników tlenowych the two faces of reactive oxygen species. Postepy Hig Med Dosw. (2008) 62:118–24.
102. Kuźnicki, D. Antioxidants and cholesterol-reducing agents with antiatherogenic activity contained in plant raw materials. Postępy Fitoterapii. (2006)
103. de Florio, AJ, dos Reis, AS, Heldt, LFS, Pereira, D, Bianchin, M, de Moura, C, et al. Lyophilized bee pollen extract: a natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT-Food Science and Technology. (2017) 76:299–305. doi: 10.1016/j.lwt.2016.06.017
104. Elimam, I, and Mohammed, HA. The influence of bee pollen on the meat chemical composition for broiler ย ด s ross 308 muscles. J Microbiol Biotechnol Food Sci. (2013) 2:1128–37.
105. Novaković, S, Djekic, I, Pešić, M, Kostić, A, Milinčić, D, Stanisavljević, N, et al. Bee pollen powder as a functional ingredient in frankfurters. Meat Sci. (2021) 182:108621. doi: 10.1016/j.meatsci.2021.108621
106. Solgajová, M, Ivanišová, E, Nôžková, J, Frančáková, H, Tóth, Ž, and Dráb, Š. Antioxidant activity and polyphenol content of malt beverages enriched with bee pollen. J Microbiol Biotechnol Food Sci. (2021) 2021:281–4.
107. Campos, MG, Webby, RF, Markham, KR, Mitchell, KA, and Da Cunha, AP. Age-induced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. J Agric Food Chem. (2003) 51:742–5. doi: 10.1021/jf0206466
108. Tatlı Seven, P, Sur Arslan, A, Seven, İ, and Gökçe, Z. The effects of dietary bee pollen on lipid peroxidation and fatty acids composition of Japanese quails (Coturnix coturnix japonica) meat under different stocking densities. J Appl Anim Res. (2016) 44:487–91. doi: 10.1080/09712119.2015.1091339
109. Avni, D, Hendriksma, HP, Dag, A, Uni, Z, and Shafir, S. Nutritional aspects of honey bee-collected pollen and constraints on colony development in the eastern Mediterranean. J Insect Physiol. (2014) 69:65–73. doi: 10.1016/j.jinsphys.2014.07.001
110. Al-Kahtani, SN, Taha, E-KA, Farag, SA, Taha, RA, Abdou, EA, and Mahfouz, HM. Harvest season significantly influences the fatty acid composition of bee pollen. Biology. (2021) 10:495. doi: 10.3390/biology10060495
111. Chen, W, Wang, J, and Huang, Y. Effects of dietary n-6: n-3 polyunsaturated fatty acid ratio on cardiac antioxidative status, T-cell and cytokine mRNA expression in the thymus, and blood T lymphocyte subsets of broilers. Livest Sci. (2012) 150:114–20. doi: 10.1016/j.livsci.2012.08.008
112. Aabed, K, Bhat, RS, Al-Dbass, A, Moubayed, N, Algahtani, N, Merghani, NM, et al. Bee pollen and propolis improve neuroinflammation and dysbiosis induced by propionic acid, a short chain fatty acid in a rodent model of autism. Lipids Health Dis. (2019) 18:200. doi: 10.1186/s12944-019-1150-0
113. Denisow, B, and Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: a review. J Sci Food Agric. (2016) 96:4303–9. doi: 10.1002/jsfa.7729
114. Mohamed, AE, El-Magd, MA, El-Said, KS, El-Sharnouby, M, Tousson, EM, and Salama, AF. Potential therapeutic effect of thymoquinone and/or bee pollen on fluvastatin-induced hepatitis in rats. Sci Rep. (2021) 11:15688. doi: 10.1038/s41598-021-95342-7
115. Eteraf-Oskouei, T, Shafiee-Khamneh, A, Heshmati-Afshar, F, and Delazar, A. Anti-inflammatory and anti-angiogenesis effect of bee pollen methanolic extract using air pouch model of inflammation. Res Pharm Sci. (2020) 15:66–75. doi: 10.4103/1735-5362.278716
116. Yan, S, Wang, K, Wang, X, Ou, A, Wang, F, Wu, L, et al. Effect of fermented bee pollen on metabolic syndrome in high-fat diet-induced mice. Food Sci Human Wellness. (2021) 10:345–55. doi: 10.1016/j.fshw.2021.02.026
117. Kostić, AŽ, Milinčić, DD, Nedić, N, Gašić, UM, Špirović Trifunović, B, Vojt, D, et al. Phytochemical profile and antioxidant properties of bee-collected artichoke (Cynara scolymus) pollen. Antioxidants. (2021) 10:1091. doi: 10.3390/antiox10071091
118. Naseri, L, Khazaei, MR, and Khazaei, M. Synergic effect of bee pollen and metformin on proliferation and apoptosis of granulosa cells: rat model of polycystic ovary syndrome. J Food Biochem. (2021) 46:e13635. doi: 10.1111/jfbc.13635
119. Ecem, BN. Vitamin, mineral, polyphenol, amino acid profile of bee pollen from Rhododendron ponticum (source of “mad honey”): nutritional and palynological approach. Food Measure. (2021) 15:2659–66. doi: 10.1007/s11694-021-00854-5
120. Rodríguez-Pólit, C, Gonzalez-Pastor, R, Heredia-Moya, J, Carrera-Pacheco, SE, Castillo-Solis, F, Vallejo-Imbaquingo, R, et al. Chemical properties and biological activity of bee pollen. Molecules. (2023) 28:7768. doi: 10.3390/molecules28237768
121. Saral, Ö, Şahin, H, Saral, S, Alkanat, M, Akyıldız, K, Topçu, A, et al. Bee pollen increases hippocampal brain-derived neurotrophic factor and suppresses neuroinflammation in adult rats with chronic immobilization stress. Neurosci Lett. (2022) 766:136342. doi: 10.1016/j.neulet.2021.136342
122. Pełka, K, Otłowska, O, Worobo, RW, and Szweda, P. Bee bread exhibits higher antimicrobial potential compared to bee pollen. Antibiotics. (2021) 10:125. doi: 10.3390/antibiotics10020125
123. Carpes, ST, Begnini, R, Alencar, SM, and Masson, ML. Study of preparations of bee pollen extracts, antioxidant and antibacterial activity. Cienc e Agrotecnologia. (2007) 31:1818–25. doi: 10.1590/S1413-70542007000600032
124. Pełka, K, Worobo, RW, Walkusz, J, and Szweda, P. Bee pollen and bee bread as a source of Bacteria producing antimicrobials. Antibiotics. (2021) 10:713. doi: 10.3390/antibiotics10060713
125. Kacániová, M, Vuković, N, Chlebo, R, Haščík, P, Rovna, K, Cubon, J, et al. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch Biol Sci. (2012) 64:927–34. doi: 10.2298/ABS1203927K
126. Gomes, ANP, Camara, CA, dos Santos, SA, dos Santos, FAR, de Santana Filho, PC, Dorneles, GP, et al. Chemical composition of bee pollen and Leishmanicidal activity of Rhusflavone. Rev Bras. (2021) 31:176–83. doi: 10.1007/s43450-021-00130-z
127. Özkök, A, Koru, Ö, Bedir, O, Cetinkaya, S, Gençay Çelemlİ, Ö, Özenİrler, Ç, et al. Total bioactive compounds and antimicrobial capacities of bee pollen with different botanical origins. Buasvmcn-FST. (2021) 78:57–67. doi: 10.15835/buasvmcn-fst:2020.0061
128. Kaškonienė, V, Adaškevičiūtė, V, Kaškonas, P, Mickienė, R, and Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. (2020) 34:100532. doi: 10.1016/j.fbio.2020.100532
129. Tlak Gajger, I, and Sušec, P. Efficacy of varroacidal food additive appliance during summer treatment of honeybee colonies (Apis mellifera). Veterinarski arhiv. (2019) 89:87–96. doi: 10.24099/vet.arhiv.0441
130. Tlak Gajger, I, Vlainić, J, Šoštarić, P, Prešern, J, Bubnič, J, and Smodiš Škerl, MI. Effects on some therapeutical, biochemical, and immunological parameters of honey bee (Apis mellifera) exposed to probiotic treatments, in field and laboratory conditions. Insects. (2020) 11:638. doi: 10.3390/insects11090638
131. Tlak Gajger, I, Smodiš Škerl, MI, Šoštarić, P, Šuran, J, Sikirić, P, and Vlainić, J. Physiological and immunological status of adult honeybees (Apis mellifera) fed sugar syrup supplemented with pentadecapeptide BPC 157. Biology. (2021) 10:891. doi: 10.3390/biology10090891
132. Šuran, J, Cepanec, I, Mašek, T, Radić, B, Radić, S, Tlak Gajger, I, et al. Propolis extract and its bioactive compounds—from traditional to modern extraction technologies. Molecules. (2021) 26:2930. doi: 10.3390/molecules26102930
133. Šuran, J, Cepanec, I, Mašek, T, Starčević, K, Tlak Gajger, I, Vranješ, M, et al. Nonaqueous polyethylene glycol as a safer alternative to ethanolic propolis extracts with comparable antioxidant and antimicrobial activity. Antioxidants. (2021) 10:978. doi: 10.3390/antiox10060978
134. Tlak Gajger, I, Pavlović, I, Bojić, M, and Kosalec, I. The components responsible for the antimicrobial activity of Propolis from continental and Mediterranean regions in Croatia. (2017).
135. Kocot, J, Kiełczykowska, M, Luchowska-Kocot, D, Kurzepa, J, and Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxidative Med Cell Longev. (2018) 2018:1–29. doi: 10.1155/2018/7074209
136. Kim, SB, Jo, YH, Liu, Q, Ahn, JH, Hong, IP, Han, SM, et al. Optimization of extraction condition of bee pollen using response surface methodology: correlation between anti-melanogenesis, antioxidant activity, and phenolic content. Molecules. (2015) 20:19764–74. doi: 10.3390/molecules201119656
137. Kurek-Górecka, A, Górecki, M, Rzepecka-Stojko, A, Balwierz, R, and Stojko, J. Bee products in dermatology and skin care. Molecules. (2020) 25:556. doi: 10.3390/molecules25030556
138. Basista, K, and Sodzawiczny, K. Bee pollen—a new natural material, possibilities of use in medicine and cosmetology. Gaz Farm. (2011) 12:30–2.
139. Xi, X, Li, J, Guo, S, Li, Y, Xu, F, Zheng, M, et al. The potential of using bee pollen in cosmetics: a review. J Oleo Sci. (2018) 67:1071–82. doi: 10.5650/jos.ess18048
140. Pyeon, H-I, Bak, J, Seok, J-I, So, S, Suh, H-J, Oh, M, et al. Effects of nano-sized bee pollen as a new cosmetic ingredient. Asian J Beauty Cosmetol. (2017) 15:1–9. doi: 10.20402/ajbc.2016.0078
141. Souza, M. Recent patents on anti-infective drug discovery 1, 33; Souza, MVN (2005). Sci World J. (2006) 5:609.
142. Olczyk, P, Koprowski, R, Kaźmierczak, J, Mencner, L, Wojtyczka, R, Stojko, J, et al. Bee pollen as a promising agent in the burn wounds treatment. Evid Based Complement Alternat Med. (2016) 2016:1–12. doi: 10.1155/2016/8473937
143. Shahidi, F, and Yeo, J. Insoluble-bound phenolics in food. Molecules. (2016) 21:1216. doi: 10.3390/molecules21091216
144. Zhao, J, and Dixon, RA. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. (2010) 15:72–80. doi: 10.1016/j.tplants.2009.11.006
145. McLusky, SR, Bennett, MH, Beale, MH, Lewis, MJ, Gaskin, P, and Mansfield, JW. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J. (1999) 17:523–34. doi: 10.1046/j.1365-313X.1999.00403.x
146. Jansen, MA, van den Noort, RE, Tan, MA, Prinsen, E, Lagrimini, LM, and Thorneley, RN. Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol. (2001) 126:1012–23. doi: 10.1104/pp.126.3.1012
147. Zhang, X, Yu, M, Zhu, X, Liu, R, and Lu, Q. Metabolomics reveals that phenolamides are the main chemical components contributing to the anti-tyrosinase activity of bee pollen. Food Chem. (2022) 389:133071. doi: 10.1016/j.foodchem.2022.133071
148. Andreasen, MF, Kroon, PA, Williamson, G, and Garcia-Conesa, M-T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic Biol Med. (2001) 31:304–14. doi: 10.1016/S0891-5849(01)00585-8
149. Kamatham, S, Kumar, N, and Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. And their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol Rep. (2015) 2:520–9. doi: 10.1016/j.toxrep.2015.03.001
150. Hsu, W-C, Chang, S-P, Lin, L-C, Li, C-L, Richardson, CD, Lin, C-C, et al. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir Res. (2015) 118:139–47. doi: 10.1016/j.antiviral.2015.04.003
151. Calderon-Montano J, M, Burgos-Morón, E, Pérez-Guerrero, C, and López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. (2011) 11:298–344. doi: 10.2174/138955711795305335
152. Babaei, S, Rahimi, S, Torshizi, MAK, Tahmasebi, G, and Miran, SNK. Effects of propolis, royal jelly, honey and bee pollen on growth performance and immune system of Japanese quails. Vet Res Forum. (2016) 7:13–20.
153. Panettieri, V, Chatzifotis, S, Messina, CM, Olivotto, I, Manuguerra, S, Randazzo, B, et al. Honey bee pollen in meagre (Argyrosomus regius) juvenile diets: effects on growth, diet digestibility, intestinal traits, and biochemical markers related to health and stress. Animals. (2020) 10:231. doi: 10.3390/ani10020231
154. El-Asely, AM, Abbass, AA, and Austin, B. Honey bee pollen improves growth, immunity and protection of Nile tilapia (Oreochromis niloticus) against infection with Aeromonas hydrophila. Fish Shellfish Immunol. (2014) 40:500–6. doi: 10.1016/j.fsi.2014.07.017
155. Asgari, M, Abedian Kenari, A, Esmaeili, M, and Rombenso, A. Effects of hydroalcoholic extract of honeybee pollen on growth performance, flesh quality, and immune and stress response response of rainbow trout (Oncorhynchus mykiss). Aquac Nutr. (2020) 26:1505–19. doi: 10.1111/anu.13098
156. Xu, X, Sun, L, Dong, J, and Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov Food Sci Emerg Technol. (2009) 10:42–6. doi: 10.1016/j.ifset.2008.08.004
157. Choobkar, N, Kakoolaki, S, Mohammadi, F, and Rezaeimanesh, M. The effect of dietary propolis and pollen extracts on growth performance and haematological responses of rainbow trout (Onchorhynchus mykiss). Iran J Aquat Animal Health. (2017) 3:16–25. doi: 10.18869/acadpub.ijaah.3.1.16
158. Abuoghaba, A, El-Hammady, H, and El-Fattah, A. Productive performance, blood constituents and some physiological parameters of rabbit bucks administered with bee pollen under hot conditions prevalent in Assiut. EJRS. (2017) 27:23–41. doi: 10.21608/ejrs.2017.41822
159. Mohamed, A, Abou El Ella, GA, and Hayder, IA. Antioxidant effect of bee pollen on immune status of hyperglycemic rats. Assiut Vet Med J. (2013) 59:107–16. doi: 10.21608/avmj.2013.191997
160. Attia, Y, El-Hanoun, A, Bovera, F, Monastra, G, El-Tahawy, W, and Habiba, H. Growth performance, carcass quality, biochemical and haematological traits and immune response of growing rabbits as affected by different growth promoters. J Anim Physiol Anim Nutr (Berl). (2014) 98:128–39. doi: 10.1111/jpn.12056
161. El-Bialy, BE, Abdeen, EE, El-Borai, NB, and El-Diasty, EM. Experimental studies on some Immunotoxicological aspects of aflatoxins containing diet and protective effect of bee pollen dietary supplement. Pak J Biol Sci. (2016) 19:26–35. doi: 10.3923/pjbs.2016.26.35
162. El-Seedi, HR, Eid, N, Abd El-Wahed, AA, Rateb, ME, Afifi, HS, Algethami, AF, et al. Honey bee products: preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front Nutr. (2022) 8:761267. doi: 10.3389/fnut.2021.761267
163. Akter, S, Khan, M, Jahan, M, Karim, M, and Islam, M. Histomorphological study of the lymphoid tissues of broiler chickens. BJVM. (2006) 4:87–92. doi: 10.3329/bjvm.v4i2.1289
164. Attia, Y, Bovera, F, El-Tahawy, W, El-Hanoun, A, Al-Harthi, M, and Habiba, H. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. (2015) 23:273–82. doi: 10.4995/wrs.2015.3644
165. Abdelnour, SA, Abd El-Hack, ME, Alagawany, M, Farag, MR, and Elnesr, SS. Beneficial impacts of bee pollen in animal production, reproduction and health. J Anim Physiol Anim Nutr (Berl). (2019) 103:477–84. doi: 10.1111/jpn.13049
166. Šarić, A, Balog, T, Sobočanec, S, Kušić, B, Šverko, V, Rusak, G, et al. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem Toxicol. (2009) 47:547–54. doi: 10.1016/j.fct.2008.12.007
167. Zeweil, H, Zahran, S, Abd-El-Rahman, M, El-Gindy, YM, and Gebril, M. Effects of dietary bee pollen and mannan oligosaccharide on semen quality in rabbits under Egyptian summer conditions. Egypt Poult Sci J. (2016) 36:973–84. doi: 10.21608/epsj.2016.168816
168. El-Hammady, H, Abuoghaba, A, El-Fattah, A, and El-Rahman, A. Semen physical characteristics, blood parameters and some physiological estimates of rabbit bucks administered with bee pollen under upper Egypt climatic conditions. EJRS. (2017) 27:43–64. doi: 10.21608/ejrs.2017.41826
169. Desoky, A, and Kamel, NN. Egg production performance, blood biochemical and immunological response of laying Japanese quail fed diet supplemented with Propolis and bee pollen. Egypt J Nutr Health. (2018) 21:549–57. doi: 10.21608/ejnf.2018.75747
170. Abuoghaba, AA-K. Impact of bee pollen supplementation on productive performance, some hematological parameters, blood constituents and semen physical characteristics of Sinai chickens. Egypt Poult Sci J. (2018) 38:621–35.
171. Suleiman, JB, Bakar, ABA, and Mohamed, M. Review on bee products as potential protective and therapeutic agents in male reproductive impairment. Molecules. (2021) 26:3421. doi: 10.3390/molecules26113421
172. Amalia, E, Diantini, A, and Subarnas, A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol Lett. (2020) 20:1. doi: 10.3892/ol.2020.12137
173. Aylanc, V, Larbi, S, Calhelha, R, Barros, L, Rezouga, F, Rodríguez-Flores, MS, et al. Evaluation of antioxidant and anticancer activity of mono-and polyfloral Moroccan bee pollen by characterizing phenolic and volatile compounds. Molecules. (2023) 28:835. doi: 10.3390/molecules28020835
174. Nainu, F, Masyita, A, Bahar, MA, Raihan, M, Prova, SR, Mitra, S, et al. Pharmaceutical prospects of bee products: special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics. (2021) 10:822. doi: 10.3390/antibiotics10070822
175. Zou, Y, Hu, J, Huang, W, Zhu, L, Shao, M, Dordoe, C, et al. The botanical origin and antioxidant, anti-BACE1 and antiproliferative properties of bee pollen from different regions of South Korea. BMC Complement Med Therap. (2020) 20:1–14. doi: 10.1186/s12906-020-03023-1
176. Saisavoey, T, Sangtanoo, P, Srimongkol, P, Reamtong, O, and Karnchanatat, A. Hydrolysates from bee pollen could induced apoptosis in human bronchogenic carcinoma cells (ChaGo-K-1). J Food Sci Technol. (2021) 58:752–63. doi: 10.1007/s13197-020-04592-2
177. Li, J. Bee pollen and doxorubicin by synergistic effects inhibit the proliferation of breast tumors in 4T1 tumor-bearing BALB/c mice: a biochemical, Immunohistochemical, and molecular approach. Pharmacogn Mag. (2024) 20:159–78. doi: 10.1177/09731296231203809
178. Sánchez-Carranza, JN, Alvarez, L, Marquina-Bahena, S, Salas-Vidal, E, Cuevas, V, Jiménez, EW, et al. Phenolic compounds isolated from Caesalpinia coriaria induce S and G2/M phase cell cycle arrest differentially and trigger cell death by interfering with microtubule dynamics in cancer cell lines. Molecules. (2017) 22:666. doi: 10.3390/molecules22040666
179. Moskwa, J, Naliwajko, SK, Dobiecka, D, and Socha, K. Bee products and colorectal Cancer—active components and mechanism of action. Nutrients. (2023) 15:1614. doi: 10.3390/nu15071614
180. Wang, B, Diao, Q, Zhang, Z, Liu, Y, Gao, Q, Zhou, Y, et al. Antitumor activity of bee pollen polysaccharides from Rosa rugosa. Mol Med Rep. (2013) 7:1555–8. doi: 10.3892/mmr.2013.1382
181. Fadzilah, NH, and Wan Omar, WA. Therapeutic evaluation of Ethanolic bee pollen extract from Malaysian stingless bee in MCF-7 and MCF-10A cell lines. Pertanika J Trop Agric Sci. (2023) 46:37–48. doi: 10.47836/pjtas.46.1.03
182. Radev, Z. Variety in protein content of pollen from 50 plants from Bulgaria. Bee World. (2018) 95:81–3. doi: 10.1080/0005772X.2018.1486276
183. Human, H, Nicolson, S, Strauss, K, Pirk, C, and Dietemann, V. Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata). J Insect Physiol. (2007) 53:649–55. doi: 10.1016/j.jinsphys.2007.04.002
184. Paoli, PP, Donley, D, Stabler, D, Saseendranath, A, Nicolson, SW, Simpson, SJ, et al. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids. (2014) 46:1449–58. doi: 10.1007/s00726-014-1706-2
185. Vaudo, AD, Tooker, JF, Grozinger, CM, and Patch, HM. Bee nutrition and floral resource restoration. Curr Opin Insect Sci. (2015) 10:133–41. doi: 10.1016/j.cois.2015.05.008
186. Donkersley, P, Rhodes, G, Pickup, RW, Jones, KC, Power, EF, Wright, GA, et al. Nutritional composition of honey bee food stores vary with floral composition. Oecologia. (2017) 185:749–61. doi: 10.1007/s00442-017-3968-3
187. T’ai, HR, and Cane, JH. (2000). Pollen nutritional content and digestibility for animals. Pollen and pollination : Berlin: Springer. p. 187–209.
188. Höcherl, N, Siede, R, Illies, I, Gätschenberger, H, and Tautz, J. Evaluation of the nutritive value of maize for honey bees. J Insect Physiol. (2012) 58:278–85. doi: 10.1016/j.jinsphys.2011.12.001
189. Radev, Z. The impact of different protein content of pollen on honey bee (Apis mellifera L.) reproduction. New Knowl J Sci. (2019) 8:80–8.
190. Raja, S, Waghchoure, ES, Mahmood, RSG, Iftikhar, F, and Munawar, S. Comparative study on improvement in pollen collection technology. Halteres. (2010) 1:1–6.
191. Mauriello, G, De Prisco, A, Di Prisco, G, La Storia, A, and Caprio, E. Microbial characterization of bee pollen from the Vesuvius area collected by using three different traps. PLoS One. (2017) 12:e0183208. doi: 10.1371/journal.pone.0183208
192. Canale, A, Benelli, G, Castagna, A, Sgherri, C, Poli, P, Serra, A, et al. Microwave-assisted drying for the conservation of honeybee pollen. Materials. (2016) 9:363. doi: 10.3390/ma9050363
193. Straumite, E, Bartule, M, Valdovska, A, Kruma, Z, and Galoburda, R. Physical and microbiological characteristics and antioxidant activity of honey bee pollen. Appl Sci. (2022) 12:3039. doi: 10.3390/app12063039
194. Kieliszek, M, Piwowarek, K, Kot, AM, Błażejak, S, Chlebowska-Śmigiel, A, and Wolska, I. Pollen and bee bread as new health-oriented products: a review. Trends Food Sci Technol. (2018) 71:170–80. doi: 10.1016/j.tifs.2017.10.021
195. Dozuotų, BDTIJ, and Kūrimas, F. Investigation of bee bread and development of its dosage forms. Proteins. (2015) 24:70.
196. Ćirić, J, Haneklaus, N, Baltić, T, Simunović, S, Parunović, N, Trbović, D, et al. Honeybee pollen as a bioindicator of contamination: an overview. Sci J Meat Technol. (2023) 64:273–6. doi: 10.18485/meattech.2023.64.2.50
197. Martinello, M, Manzinello, C, Dainese, N, Giuliato, I, Gallina, A, and Mutinelli, F. The honey bee: an active biosampler of environmental pollution and a possible warning biomarker for human health. Appl Sci. (2021) 11:6481. doi: 10.3390/app11146481
198. Tutun, H, Aluç, Y, Kahraman, HA, Sevin, S, Yipel, M, and Ekici, H. The content and health risk assessment of selected elements in bee pollen and propolis from Turkey. J Food Compos Anal. (2022) 105:104234. doi: 10.1016/j.jfca.2021.104234
199. Rusnáková, M, Hrouzek, J, and Hrouzková, S. Present state and perspectives in analytical methods for pesticide residues analysis in bee pollen: an overview. J Apic Res. (2023) 62:76–96. doi: 10.1080/00218839.2022.2153485
200. Cappellari, A, Malagnini, V, Fontana, P, Zanotelli, L, Tonidandel, L, Angeli, G, et al. Impact of landscape composition on honey bee pollen contamination by pesticides: a multi-residue analysis. Chemosphere. (2024) 349:140829. doi: 10.1016/j.chemosphere.2023.140829
201. Carrera, MA, Sánchez, LM, Morales, MM, Fernández-Alba, AR, and Hernando, MD. Method optimisation for large scope pesticide multiresidue analysis in bee pollen: a pilot monitoring study. Food Chem. (2024) 436:137652. doi: 10.1016/j.foodchem.2023.137652
202. Carrera, MA, Miguel, E, Fernández-Alba, AR, and Hernando, MD. First survey on the presence of mycotoxins in commercial bee pollen sourced from 28 countries. Food Control. (2023) 152:109816. doi: 10.1016/j.foodcont.2023.109816
203. Schrenk, D, Bignami, M, Bodin, L, Chipman, JK, del Mazo, J, Grasl-Kraupp, B, et al. Risk assessment of aflatoxins in food. EFSA J. (2020) 18:e06040. doi: 10.2903/j.efsa.2020.6040
204. Sinkevičienė, J, Tarasevičienė, Ž, and Tamutis, V. Fusarium Fungi and mycotoxins in bee pollen collected in Lithuania. Appl Sci. (2023) 13:1571. doi: 10.3390/app13031571
205. Leang, ZX, Thalayasingam, M, and O’Sullivan, M. A paediatric case of exercise-augmented anaphylaxis following bee pollen ingestion in Western Australia. Asia Pac Allergy. (2022) 12:e23. doi: 10.5415/apallergy.2022.12.e23
206. Kast, C, Kilchenmann, V, Reinhard, H, Bieri, K, and Zoller, O. Pyrrolizidine alkaloids: the botanical origin of pollen collected during the flowering period of Echium vulgare and the stability of pyrrolizidine alkaloids in bee bread. Molecules. (2019) 24:2214. doi: 10.3390/molecules24122214
207. Shaw, C, and Tomljenovic, L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. (2013) 56:304–16. doi: 10.1007/s12026-013-8403-1
208. Soman, NR, Baldwin, SL, Hu, G, Marsh, JN, Lanza, GM, Heuser, JE, et al. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest. (2009) 119:2830–42. doi: 10.1172/JCI38842
209. Cui-Cui, L, Hao, D-j, Zhang, Q, An, J, Zhao, J-j, Chen, B, et al. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother Pharmacol. (2016) 78:1113–30. doi: 10.1007/s00280-016-3160-1
210. Abd El-Wahed, AA, Khalifa, SA, Sheikh, BY, Farag, MA, Saeed, A, Larik, FA, et al. Bee venom composition: from chemistry to biological activity. Stud Nat Prod Chem. (2019) 60:459–84. doi: 10.1016/B978-0-444-64181-6.00013-9
211. Alalawy, AI, El Rabey, HA, Almutairi, FM, Tayel, AA, Al-Duais, MA, Zidan, NS, et al. Effectual anticancer potentiality of loaded bee venom onto fungal chitosan nanoparticles. Int J Polymer Sci. (2020) 2020:1–9. doi: 10.1155/2020/2785304
212. El-Didamony, SE, Kalaba, MH, El-Fakharany, EM, Sultan, MH, and Sharaf, MH. Antifungal and antibiofilm activities of bee venom loaded on chitosan nanoparticles: a novel approach for combating fungal human pathogens. World J Microbiol Biotechnol. (2022) 38:244. doi: 10.1007/s11274-022-03425-y
213. Hanafy, NA, Salim, EI, Mahfouz, ME, Eltonouby, EA, and Hamed, IH. Fabrication and characterization of bee pollen extract nanoparticles: their potential in combination therapy against human A549 lung cancer cells. Food Hydrocolloids Health. (2023) 3:100110. doi: 10.1016/j.fhfh.2022.100110
214. Keskin, M. Synthesis, characterization and antidiabetic potential of bee pollen based silver nanoparticles. El-Cezeri. (2022) 9:266–75. doi: 10.31202/ecjse.963670
215. El-Deeb, NM, El-Sherbiny, IM, El-Aassara, MR, and Hafez, EE. Novel trend in colon cancer therapy using silver nanoparticles synthesized by honey bee. J Nanomed Nanotechnol. (2015) 6:265.
216. Al-Yousef, HM, Amina, M, Alqahtani, AS, Alqahtani, MS, Malik, A, Hatshan, MR, et al. Pollen bee aqueous extract-based synthesis of silver nanoparticles and evaluation of their anti-cancer and anti-bacterial activities. PRO. (2020) 8:524. doi: 10.3390/pr8050524
217. Spulber, R, Chifiriuc, C, Fleancu, M, Popa, O, and Băbeanu, N, editors. Antibacterial activity of magnetite nanoparticles coated with bee pollen extracts. Agriculture for life, life for agriculture” Conference Proceedings. (2018).
218. Do Nascimento, TG, Da Silva, PF, Azevedo, LF, Da Rocha, LG, de Moraes Porto, ICC, Moura TFA, LE, et al. Polymeric nanoparticles of Brazilian red propolis extract: preparation, characterization, antioxidant and leishmanicidal activity. Nanoscale Res Lett. (2016) 11:1–16. doi: 10.1186/s11671-016-1517-3
219. Shubharani, R, Mahesh, M, and Yogananda, MV. Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of bee Propolis. J Nanomed Nanotechnol. (2019) 10:1000522. doi: 10.4172/2157-7439.1000522
220. Mocanu, A, Isopencu, G, Busuioc, C, Popa, O-M, Dietrich, P, and Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: synergistic antimicrobial effect. Sci Rep. (2019) 9:1–10. doi: 10.1038/s41598-019-54118-w
221. Gevorgyan, S, Schubert, R, Yeranosyan, M, Gabrielyan, L, Trchounian, A, Lorenzen, K, et al. Antibacterial activity of royal jelly-mediated green synthesized silver nanoparticles. AMB Express. (2021) 11:1–8. doi: 10.1186/s13568-021-01213-9
222. Gevorgyan, S, Schubert, R, Falke, S, Lorenzen, K, Trchounian, K, and Betzel, C. Structural characterization and antibacterial activity of silver nanoparticles synthesized using a low-molecular-weight Royal Jelly extract. Sci Rep. (2022) 12:14077. doi: 10.1038/s41598-022-17929-y
Keywords: bee pollen, composition, bee pollen vitality, apitherapeutic, bee pollen consumption and nanotechnology
Citation: Anjum SI, Ullah A, Gohar F, Raza G, Khan MI, Hameed M, Ali A, Chen C-C and Tlak Gajger I (2024) Bee pollen as a food and feed supplement and a therapeutic remedy: recent trends in nanotechnology. Front. Nutr. 11:1371672. doi: 10.3389/fnut.2024.1371672
Edited by:
Özlem Emir Çoban, Firat University, TürkiyeReviewed by:
Aleksandar Ž. Kostic, University of Belgrade, SerbiaSerap Saler, Firat University, Türkiye
Copyright © 2024 Anjum, Ullah, Gohar, Raza, Khan, Hameed, Ali, Chen and Tlak Gajger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Ishtiaq Anjum, aXNodGlhcTExQGdtYWlsLmNvbQ==; aXNodGlhcUBrdXN0LmVkdS5waw==; Chien-Chin Chen, aGxtYXJrY0BnbWFpbC5jb20=
†These authors share first authorship
 Syed Ishtiaq Anjum
Syed Ishtiaq Anjum Amjad Ullah
Amjad Ullah Faryal Gohar1
Faryal Gohar1 Muhammad Ilyas Khan
Muhammad Ilyas Khan Mehwish Hameed
Mehwish Hameed Ivana Tlak Gajger
Ivana Tlak Gajger