- 1Department of Genomic Medicine in Hepatology, Civil Hospital of Guadalajara, Fray Antonio Alcalde, Guadalajara, Jalisco, Mexico
- 2Health Sciences Center, University of Guadalajara, Guadalajara, Jalisco, Mexico
- 3Doctoral Program in Molecular Biology in Medicine, Health Sciences Center, University of Guadalajara, Guadalajara, Jalisco, Mexico
Personalized Nutrition emerged as a new trend for providing nutritional and food advice based on the individual’s genetic composition, a field driven by the advancements in the multi-omic sciences throughout the last century. It intends not only to tailor the recommended daily allowances of nutrients and functional foods that a person may need but also to maintain the principles of sustainability and eco-friendliness. This principle implies the implementation of strategies within the healthcare system to advocate for the ending of the one-diet-fits-all paradigm by considering a personalized diet as an ally to prevent diet-related chronic diseases. In this Perspective, we highlight the potential benefits of such a paradigm within the region of Latin America, particularly Mexico, where the genetic admixture of the population, food biodiversity, and food culture provide unique opportunities to establish personalized nutrigenetic strategies. These strategies could play a crucial role in preventing chronic diseases and addressing the challenges confronted in the region.
1 Introduction
The field of Genomic Nutrition, also known as Nutritional Genomics, is rapidly advancing with the integration of multi-omic analyses in nutritional science (1, 2). This discipline mainly encompasses two subfields, Nutrigenomics and Nutrigenetics, which explore the bidirectional interactions between genes, diet (nutrients), and health outcomes (3, 4). Nutrigenetics focuses on how a specific polymorphism (allele/genotype) or genetic profile affects the body’s metabolic responsiveness to foods (nutrients) (5). Nutrigenomics studies the impact of nutrients and bioactive food compounds on gene expression, specifically in transcriptomics, proteomics, and metabolomics, regardless of the inherited genotype (6). In addition, the emerging fields of Nutri-epigenetics and Nutri-metagenomics widen our understanding of gene expression modulation at the chromatin level (7, 8) and the signaling between the gut microbiota, considered our “second genome,” and the host’s organs/tissues (9, 10), respectively. Other interacting environmental factors to consider are physical activity, psychosocial context (stress, emotions), and contaminants (11). Together, they provide the scientific basis for designing more effective dietary interventions that consider genetic diversity, gut microbiota, and lifestyle to design personalized nutrition strategies that align with an individual’s unique needs.
Research in Nutritional Genomics and its practical application in personalized nutrition have incited two interconnected aspects. On the one hand, much enthusiasm has risen due to the potential to predict nutritional recommendations based on individual genetic profiles, leading to improved health outcomes (12). In addition, understanding the genomic landscape contributing to the risk of chronic diseases in a specific population has advantages. It allows for proactive measures to prevent these diseases rather than relying solely on reactive healthcare (13, 14). On the other hand, the inception of personalized nutrition based on multi-omic data has sparked a significant debate among health experts regarding the interpretation of this data and ethical and privacy concerns surrounding it (15). These controversies have led to the establishment of standardized definitions, regulations, and ethical delivery of nutritional care (16). Although crucial for ensuring professional clinical practices, these aspects go beyond the scope of this Perspective. Nonetheless, it is undeniable that personalized nutrition, indicating the right food for all, is an ongoing trend in our society (17, 18) that will inherently lead to the end of the universal approach of the “one-diet-fits-all era”.
Herein, we examine our rationale and the implications of implementing personalized nutrition in Latin America, particularly Mexico. The population’s varying genetic backgrounds, food diversity, and cultural traditions present a potential for tailoring regionalized nutritional genomic strategies to move away from a “one-diet-fits-all” to fight chronic illnesses.
2 Evolution of the human diet and mismatch between genes and nutrients
Gene-nutrient interactions are highly dynamic. Firstly, there have been significant evolutionary changes in how humans obtain nutrients from the environment and how these needs are regulated by genes (19). Dietary patterns have substantially changed across different regions throughout history (20). Currently, numerous societies are amid a shift from pre- and post-globalization movements. This transition has resulted in detrimental effects on food quality and quantity, as well as an increase in chronic diseases due to greater food processing and accessibility (21, 22). Likewise, humans have experienced different stages of evolutionary adaptation through exposure to various environments and selective pressures. The genome of modern humans has been shaped by the availability of nutrients in different environments, leading to multiple locally positively selected gene variations (23). While advantageous in one setting, these adaptations can become problematic when faced with a changing nutritional landscape, such as in the current epidemiological transition (24–26). The significant shifts in lifestyle factors, such as reduced physical activity, increased stress levels, and exposure to environmental pollutants, are causing an evolutionary mismatch that contributes to the development of chronic illnesses (26).
From a nutritional standpoint, some societies maintain a historically traditional diet, while others have adopted either an imported traditional diet or a more modern diet (27). Unfortunately, this does not suggest that our overall health surpasses that of our ancestors or previous generations. The prevalence of obesity-related chronic diseases has increased globally, affecting individuals of all economic backgrounds. This trend is particularly evident among children, adolescents, and adults residing in urban areas where ultra-processed foods have surged alongside the acculturation process (28, 29).
In contrast, sustaining or reintroducing indigenous food knowledge and protecting traditional cultural food practices worldwide positively impacts social-cultural well-being and substantially reduces the likelihood of developing chronic diseases (30). The composition of traditional diets, which refers to the predominant diet consumed for many generations and comprises a higher share of natural staple foods, reflects earlier phases of food evolution before industrialization (31). Hence, personalized nutrition should focus on reintroducing the main staple foods that have influenced human DNA in the past. However, these foods are not universal and will vary significantly according to geography, the population’s ethnicity, and cultural practices.
3 Personalized nutrition to prevent diet-related chronic diseases
In the early days before the genomics era, Dr. Richard O. Brennan introduced the concept of nutrigenetics in 1977. He used this term to describe the notion that diet could alleviate hypoglycemia, which is linked to genetics (32). In addition, newborns that inherit single-gene inborn metabolism errors and diseases require personalized nutritional support to address these diseases at early stages (33). However, the boom of personalized nutrition, as we recognize it today, occurred during the post-genomic era (6). The Human Genome Project gained significant attention because it became clear that most common chronic disease phenotypes derive from the complex interplay between multiple genetic variations, (single nucleotide polymorphisms (SNPs), number copy variations and insertion-deletion polymorphisms) and environmental factors such as diet, containing nutrients and bioactive compounds (34, 35). The Human Microbiome Project was subsequently established to disseminate knowledge, resources, and discoveries that connect human-microbiome interactions and health-related outcomes (36).
Personalized nutrition or precision nutrition multi-omic technologies have ultimately revolutionized healthcare strategies by recognizing the unique nature of individuals and the need for tailored dietary recommendations. Nevertheless, it is crucial not to disregard a fundamental principle. Personalized nutrition should consider the occurrence of the diseases it aims to prevent, which are, in turn, shaped by genetic and cultural factors specific to the target population (37, 38). Hence, it is not only the inheritance of “risk alleles” that is important, but also the traditions and history (food culture) that shaped that genome, so diets should not be recommended indiscriminately. Developing personalized nutrition plans is and will be a complex task, but it is feasible due to the growing scientific research, societal acceptance, political backing, and health and economic policies (39, 40).
4 The one-diet-fits-all saga
Good nutrition is fundamental in sustaining a healthy lifestyle across all stages of human development (41). Preventing chronic diseases is crucial because life expectancy has increased, and living disease-free enhances the quality of the aging process (42–44). In the past, mothers had an important role in feeding, and nourishment was delivered by moms’ dietary approaches, which influenced the food environment and shaped our eating patterns within the family (45, 46). Family recipes, influenced by the geographic availability of food resources, transmit wisdom to the younger generations by guiding the consumption of essential foods and nutrients and cooking practices (food culture).
Maintaining good habits throughout life can contribute to one’s overall health. It is worth noting that prior to industrialization, most individuals’ primary cause of death was infectious diseases rather than nutrition-related chronic diseases (47). While there may be some overlap between undernutrition and overnutrition, human dietary habits have culturally shifted regarding the types, locations, and quantities of food we consume, which differs from the biological needs encoded in our genes. Following “mom’s advice” has become increasingly difficult due to shifts in the food system, insufficient personalization of dietary recommendations, and the promotion of non-regional cuisines.
The convergence of multiple tendencies may have endorsed the one-diet-fits-all regimen. First, globalization of the food supply facilitated the importation of highly or ultra-processed products into underdeveloped countries or their targeted marketing to underprivileged sectors, chiefly because of their affordability and widespread availability (48). Globalization has increased the likelihood of societies to prefer “globalized” food that may differ in quality from locally produced foods. Secondly, several health organizations in the United States reached a consensus on the recommended guidelines for essential nutrients such as carbohydrates, proteins, fats, vitamins, antioxidants, minerals, and fiber to prevent atherosclerosis, cancer, diabetes, and obesity (49). As previously stated, the need to reverse the growing prevalence of chronic diseases led to the unification of standard dietary guidelines for the clinical management of chronic-diseased patients without considering genetic or cultural factors, endorsing a one-diet-fits-all approach (50). Thirdly, the recommendation to adopt traditional diets such as the “Mediterranean diet,” “Japanese diet,” or “Nordic diet” (51–53) to lower the onset of chronic disease outside their region of origin overlooks the equally beneficial nutritional and economic advantages of local diets (54). All things considered, one type of local diet is not healthier or better than any other, and trying to adapt specific diets to resemble, for example, the Mediterranean diet as a universal diet to treat chronic disease (55, 56) is against the required food system, food culture, or a personalized nutrition approach.
Furthermore, when incorporated into national clinical practice guidelines, these exotic dietary programs may not be practical for most people or suitable for their genetic composition (57). Furthermore, parallel to the criticism against the one-diet-fits-all trend, there is a need for tailored “normal” cut-off values of health indicators regarding body mass index, glucose levels, liver function tests, or fat percentage to account for the variability in human body measurements. Thus, in a broader sense, the personalized nutrition movement is an opportunity to provide real-life population-based or regionalized recommendations for societies seeking to prevent chronic diseases based on their characteristics.
5 Shaping the basis of personalized nutrition in Mexico
Research has revealed that human populations possess genetic variations that enable them to withstand better extreme climates, high altitudes, infectious diseases, or varying levels of nutrient availability. As mentioned before, these adaptations are specific to local environments (23). However, there is limited knowledge regarding the nutritional adaptations of Latin American populations, including Mexico, and the mechanisms behind their development. Thus, our research has focused on developing a comprehensive biocultural model of genome-based nutrition integrating the individual’s or population’s genetic background with their food culture to create personalized nutritional recommendations or interventions (58, 59).
The genetic history of Mexico traces back to the arrival of the Amerindians or First Nations People, who exploited the rich ambient biodiversity of the region. The early events after the Spanish conquest involved the introduction of European and African genes, which now form the mixed genetic pool of the present population. Additionally, there was an exchange of Old and New World foods, leading to the development of a distinct regional food culture heritage and subsequent food preferences. However, before European colonialism, the Amerindian tribes inhabited distinct ecological regions. Aridoamerica, located in the northern part of Mexico, was the land of nomadic hunter-gatherers, and Mesoamerica, spanning from central Mexico to part of Nicaragua, was home to sedentary/agricultural ethnic groups (60). Recent studies reveal that the Mexican Indigenous people exhibit signatures of local adaptations resulting from their specific dietary and cultural practices. These adaptations have influenced their biological networks, substrate metabolism, and disease susceptibility (61, 62).
Figure 1 illustrates the transformative changes in Mexico’s genetics and food culture throughout the last five centuries. These transitions have played a crucial role in shaping dietary preferences and impacting the population’s vulnerability to infectious and chronic diseases. The period of Spanish rule led to the admixture of the European, Amerindian, and African lineages with a gradient shift in the ancestral components from north to south (63). This feature translates into the fact that North Mexicans with mixed ancestry could benefit from tailored recommendations based on their higher European heritage, contrasting with individuals from the South with Amerindian ancestry, as discussed later (64–66).
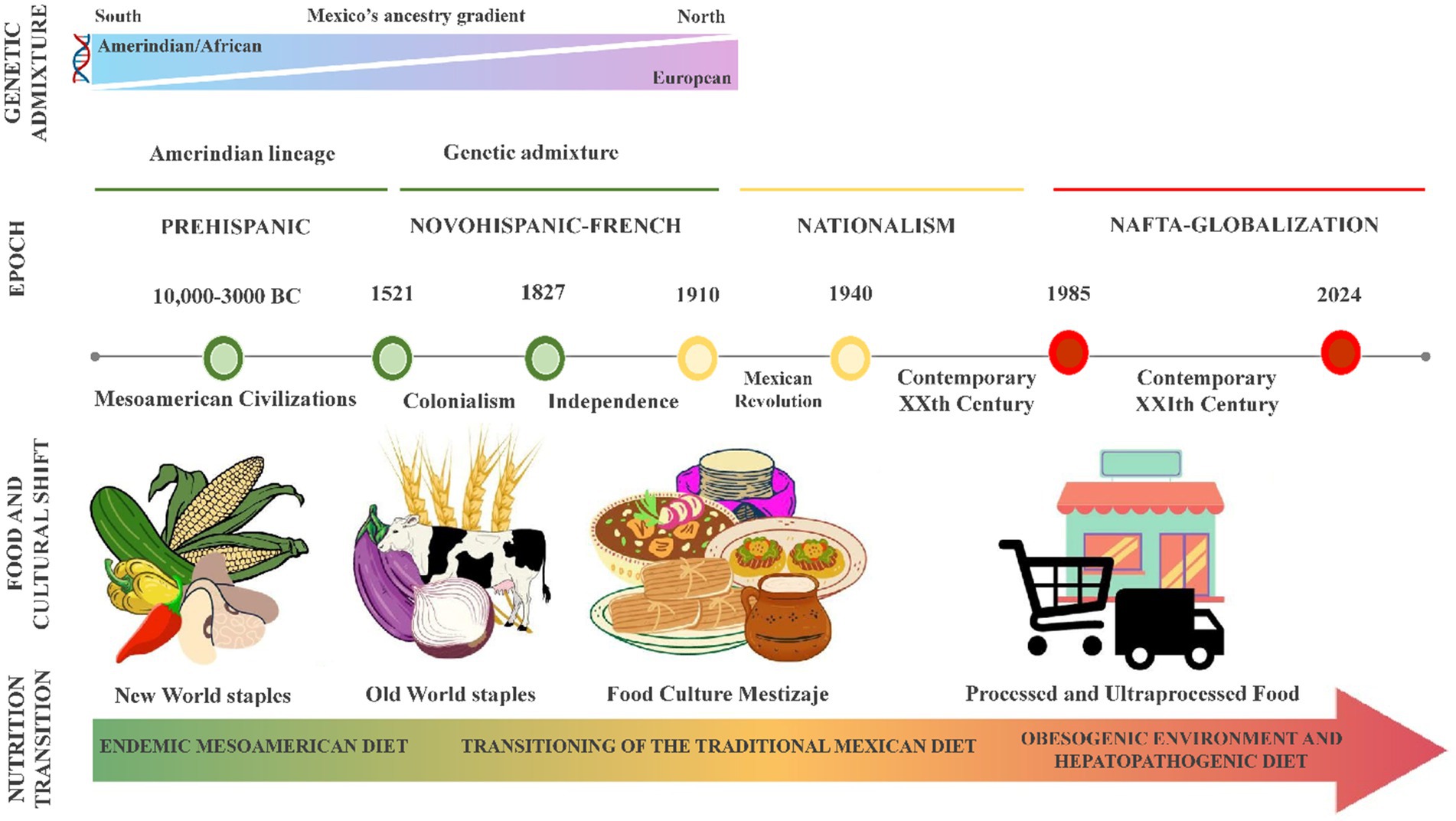
Figure 1. Genetic and alimentary evolution of the Mexican population. Mexico’s population genetics and food culture have undergone significant shifts with each economic/historical period (herein marked illustratively), shaping dietary preferences and influencing susceptibility to infectious and chronic diseases. This timeline highlights the shift from harmony with the environment to a growing mismatch between genes and the modern diet. The endemic Mesoamerican diet, rich in plant-based staples like maize, beans, and chili, was historically linked to lower metabolic risk factors. However, this has been replaced by a highly palatable, energy-dense diet dominated by processed and ultra-processed foods, poor nutritional quality, and promoting metabolic diseases. In this obesogenic environment, Mexico faces an obesity epidemic among its young and adult population, fueled by dietary patterns that result in metabolic disturbances like dyslipidemia, insulin resistance, diabetes, and metabolic-associated steatotic liver disease (MASLD). NAFTA, North American Free Trade.
The Mesoamerican food system constitutes the foundation of the traditional Mexican diet, primarily consisting of plant-based foods such as maize, beans, chili, squash, tomato, chia, pumpkin seeds, amaranth, prickly pears, and cacao. Research has shown that this diet is associated with a reduced risk of metabolic disorders and is nutritionally well-balanced and culturally acceptable (67). Nevertheless, the Mexican population is immersed in an obesogenic environment, consuming a hepatopathogenic diet that causes dyslipidemias, insulin resistance, steatosis, and metabolic-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD) (65, 68–70). As shown in Figure 1, the transition from a state of evolutionary concordance to a mismatch between the nature of our ancestral genes and the environment is part of the current health problem. Based on this evidence, a comprehensive approach was developed to address the prevalent health issues within the Mexican population.
6 The Genomex diet: benefits and challenges
The evidence mentioned above is substantial enough to establish a framework for implementing a public health policy that considers the balance between ancestral genes and our food options. Paradoxically, the battle against the prevailing Westernized diet and its detrimental effects is fought by advocating for the “gold standard” Mediterranean diet as if it were the silver bullet. Almost all major clinical practice guidelines in Mexico, endorsed by the medical associations, recommend adopting the Mediterranean diet to prevent chronic diseases and overlook the fundamental national dietary recommendations (71). With this attitude, we fail to seize the valuable opportunity to offer nutritious, environmentally friendly, and sustainable food choices to a population immersed in an obesity epidemic and with financial constraints that prevent them from purchasing expensive non-local ingredients regularly.
Even though the traditional Mediterranean and Mexican diets are culturally inherited eating patterns that consist of distinct recipes made using locally sourced ingredients and are highly valued in each society, they are not head-to-head comparable (72). Established initially on earlier agricultural and rural models, the Mediterranean diet reflects the illustrious history of culinary and cultural exchanges that have occurred in the countries surrounding the Mediterranean Basin for millennia (73). This diet contains wheat-based (bread, pasta, or couscous) foods, a wide range of plant-based foods, and virgin olive oil as the primary source of fat. It also includes a moderate intake of red wine, seafood, fermented dairy products, poultry, and eggs and a low consumption of red and processed meat and sweets (74, 75). On the contrary, the traditional Mexican dietary regimen predominantly comprises Mesoamerican staples cultivated in this area. These include maize and its by-products, such as “tortillas,” legumes (beans), high amounts of vegetables (e.g., dark-green leafy vegetables named “quelites,” squash, tomato, chile, and prickly pears), and plant-based fats obtained from avocado, chia, pumpkin seeds, and amaranth. Complementary foods include fruits, beverages (e.g., “cacao,” “pulque,” and “tesgüino” fermented beverages), fish and seafood, small wild animal meat, herbs, and condiments (76). Therefore, it is worth noting that Mexico can potentially promote the components of the Mesoamerican diet and the traditional postcolonial dishes as healthy dietary options (67), whereas the Mediterranean diet is not entirely feasible.
Furthermore, the traditional Mexican diet received the prestigious recognition of the Intangible Cultural Heritage in 2010 (77). This distinction is valuable for educating the public about this cultural legacy’s significance rather than merely being displayed on a wall. The healthcare community frequently stigmatizes the typical Mexican diet as being high in fat and contributing to weight gain. However, the root issue lies in the overconsumption of modern, non-native, calorie-dense processed foods and the lack of nutrition education.
Genomic analyses conducted on Mexicans have provided valuable insights into the influence of ancestry on the population’s health (62, 66, 78) and the potential consequences of not implementing preventive measures to mitigate disease risks. In light of the significant rise in unhealthy eating habits in Mexico and the growing prevalence of obesity and associated co-morbidities, we set forth to develop a strategy to prevent and address the metabolic alterations driven by the gen-environment mismatch.
To this end, the next step was to create a genome-based nutrition program aligning with the Mexican population’s genetic background and food culture, denoted as the Genomex diet. Despite its trendy name, the diet is not meant to be a fad diet because it adheres to the principles of a correct diet, and it seeks to promote the consumption of nutritious staples that are culturally accepted while considering regional genetic and culinary variations. The Genomex diet combines the nutritional advantages of traditional local dishes, which contain Mesoamerican staple foods rich in nutrients and bioactive components that are prepared in a healthy manner and align with the Mexican population’s functional nutrigenetic and nutrigenomic characteristics (58).
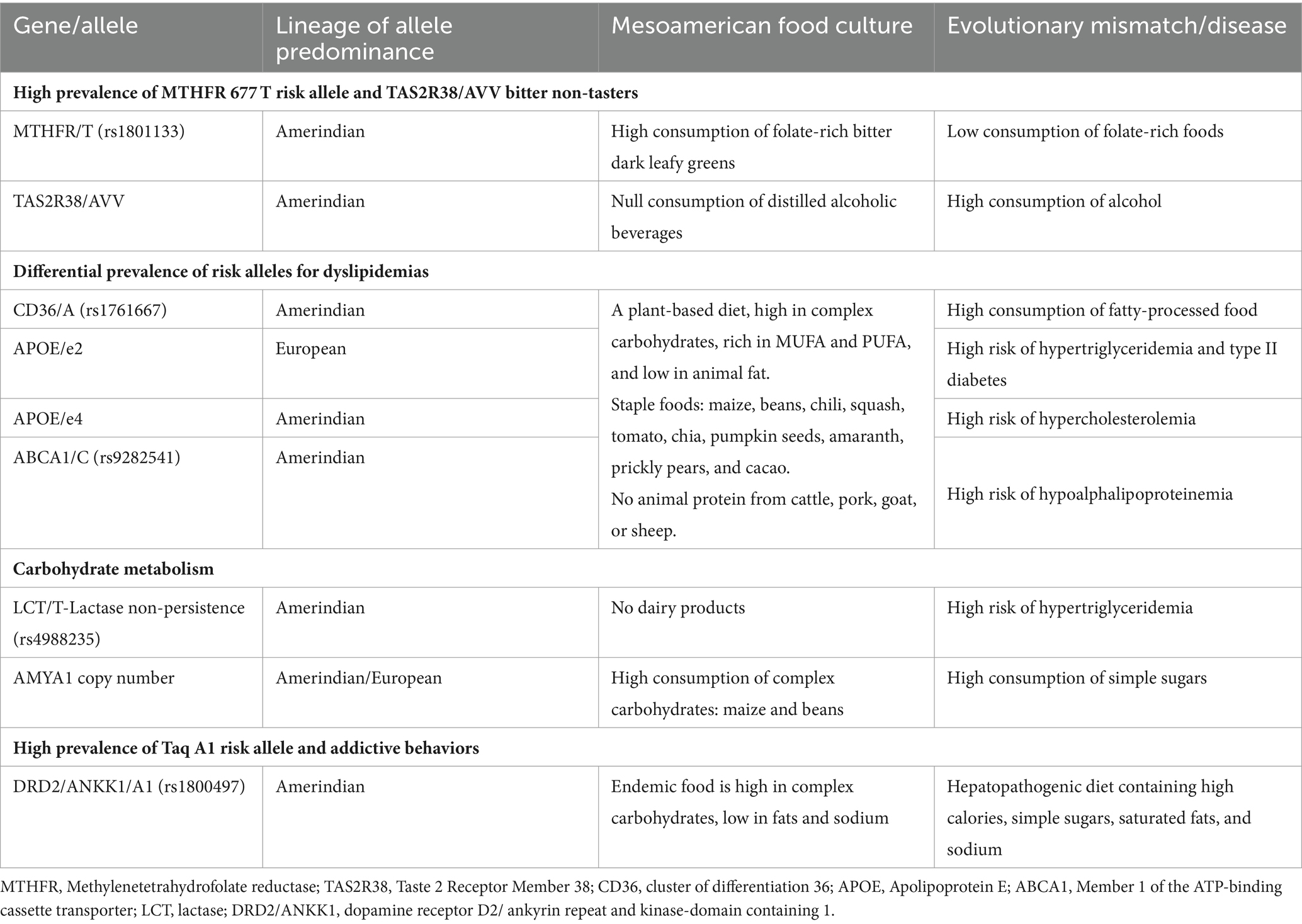
Table 1 provides an overview of the genetic polymorphisms and their evolutionary mismatch, which the Genomex diet intends to counteract, highlighting the adaptive alleles that become “risk alleles” due to unhealthy eating habits. For example, the high frequency of the MTHFR 677 T allele among the Amerindian population is consistent with their historical dietary habits of consuming folate-rich leafy greens (64). In addition, admixed subpopulations with a higher percentage of Amerindian ancestry are more likely to carry the 677 T allele than those with European ancestry, predominantly carriers of the 677C allele. Furthermore, a correlation has been observed between the 677 T risk allele and the presence of liver steatosis and other co-morbidities (79). Therefore, encouraging the inclusion of sufficient quantities of green leafy foods in a balanced diet aligns with the genetic background of most individuals while preserving the traditional intake of these foods (80) and minimizing the risk of chronic disease.
Another interesting example is the highly prevalent TAS2R38 AVV haplotype that encodes the non-taster phenotype (81). This genetic trait aligns with the ability to tolerate bitter taste tolerances and the abundance of endemic bitter leafy vegetables mentioned before. Conversely, this haplotype is a risk factor for a higher consumption of alcohol among the Mexican population in modern times, which culturally has revolutionized over the centuries.
Similarly, when studying lipid-transporter genes, it is important to consider the differential allele distribution, within the admixed population (Table 1) (64). For example, the ApoE e2 and e4 alleles (65, 69), have been associated with hyperglyceridemia and hypercholesteremia, respectively. Thus, consuming chia, pumpkin seeds, and amaranth can offer a plant-based supply of mono-unsaturated fatty acids (MUFA) and poly-unsaturated fatty acids (PUFA) while inhibiting anti-inflammatory cell-signaling pathways that are frequently triggered in various chronic diseases (58). In line with these features, a recent study assessed the impact of specific nutrigenetic recommendations for 11 genetic variants associated with dyslipidemias, reducing blood lipids and low-grade inflammation in adults with excess weight (82).
Regarding carbohydrate metabolism, most Mexicans have inherited the lactose-intolerant trait, which is consistent with dairy animals not being part of the Mesoamerican ambient until after Spanish colonialization. Likewise, regulating the intake of complex carbohydrates from maize-based foods and legumes supports glucose homeostasis through enhanced insulin sensitivity based on the population’s average number of six AMY1 copies. Also, the starch-resistant properties of these foods contribute to maintaining a healthy gut microbiota (64). Finally, the high prevalence of the DRD2/ANKK1 A1 allele has been associated with unhealthy food choices among the population (83). In conjunction, this information can help tailor nutrigenetic recommendations to address the prevalent dyslipidemias among Mexicans based on their unique genetic profiles.
Recently, the Genomex diet, containing most of the food staples of Mexico, was tested during a 6-month intervention nutrigenetic study, normalizing the anthropometric and biochemical profile of the study group (84) and improving auto-efficacy to maintain adherence to the nutritional plan (85). One significant effect was the 50% reduction of the HOMA-IR value after 3 months, which can be attributed to the components of the above dietary pattern. These results suggest that adherence to the Genomex diet decreases the risk of nutrition-related chronic diseases based on the millenary genetic adaptations that the Mexicans have inherited. Finally, an important feature of the Genomex diet is that the population culturally recognizes the recipes used to support this dietary program. Thus, nutritional recommendations based on the genetic profile of the population can be endorsed by “mom’s advice” by asking family members about the recipes of the food dishes comprising the staple indigenous foods.
In the same line of thought, studies carried out in East Asian populations where wild rice or other millet were consumed before their cultivation have revealed biological adaptations against the detrimental side effects of consuming high amounts of polished rise compared to other Asian populations (86). Thus, maintaining legendary dietary patterns may be the answer to preventing the risk of chronic diseases while conserving the local culinary culture.
Developing the genome-based nutrition strategy in Mexico has been challenging. The road to incorporating the principles of the Genomex diet into the Mexican clinical practice guidelines has faced skepticism from medical and nutrition associations. Another challenge is the food industry’s marketing consistently advocating for high-calorie ultra-processed foods and external dietary alternatives that impede food sovereignty and are not aligned with the Mexican population’s genetic makeup and food traditions. A marked rise in the consumption of ultra-processed foods in Mexico has been reported in the last three decades. Concurrently, there has been a decrease in the acquisition of unprocessed or minimally processed foods and processed culinary ingredients (87). Reversing this situation will require training and education in Genomic Nutrition to adequately prepare frontline clinicians, nutritionists, and other medical specialists to fight against these external factors and promote better eating patterns (88–92). In addition, research in complementary fields, including population genetics, food anthropology, social sciences, and evolutionary medicine studies, is crucial to provide an integrated framework defining the biological and cultural basis of a personalized or regionalized nutrition approach.
7 Conclusion
Will there be a time for the era of one-diet-fits-all to come to an end? We believe that the principles of the Genomex diet can aid in recovering and eating the traditional Mexican dietary ingredients while providing the nutrients that keep our adaptive genes healthy. Our mission is to educate health professionals and the general population on how to keep themselves nutritionally healthy, given our genetic legacy, regional food biodiversity, and food culture compatible with the Mexican population. Nonetheless, extrapolating the results throughout Mexico will require adjustments due to intraregional genetic and cultural differences, as mentioned before. In addition, implementing this approach in other Latin American countries will also require analyzing the regional prevalence of chronic diseases, prevailing risk alleles/traits, food culture, and other lifestyle factors (93–97) despite sharing a similar historical background of colonialism and globalization as Mexico. In this “weakness” lies the strength of eluding the one-diet-fits-all scheme and avoiding foreign alternatives while promoting entrepreneurism toward national eco-friendly agricultural and food industries aiming to contribute toward the world’s Sustainable Development Goals (98). Further research, training, teaching, and advocacy activities are required to advance toward a compelling personalized nutrition approach in Mexico to reclaim the benefits of a healthy diet.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SR: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. LC-M: Investigation, Writing – review & editing, Visualization. LL-M: Investigation, Writing – review & editing, Visualization.
Funding
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
To the members of the Nutrigenetic team for their dedication and willingness to bring alive the Genomex Diet project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ordovas, JM, and Corella, D. Nutritional genomics. Annu Rev Genomics Hum Genet. (2004) 5:71–118. doi: 10.1146/annurev.genom.5.061903.180008
2. Fenech, M, El-Sohemy, A, Cahill, L, Ferguson, LR, French, T-AC, Tai, ES, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. (2011) 4:69–89. doi: 10.1159/000327772
3. Mutch, DM, Wahli, W, and Williamson, G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J. (2005) 19:1602–16. doi: 10.1096/fj.05-3911rev
4. Kussmann, M, and Fay, LB. Nutrigenomics and personalized nutrition: science and concept. Pers Med. (2008) 5:447–55. doi: 10.2217/17410541.5.5.447
6. Simopoulos, AP . Nutrigenetics/nutrigenomics. Annu Rev Public Health. (2010) 31:53–68. doi: 10.1146/annurev.publhealth.031809.130844
7. Feinberg, AP . The key role of epigenetics in human disease prevention and mitigation. N Engl J Med. (2018) 378:1323–34. doi: 10.1056/NEJMra1402513
8. Huo, M, Zhang, J, Huang, W, and Wang, Y. Interplay among metabolism, epigenetic modifications, and gene expression in Cancer. Front Cell Dev Biol. (2021) 9:793428. doi: 10.3389/fcell.2021.793428
9. Wang, W-L, Xu, S-Y, Ren, Z-G, Tao, L, Jiang, J-W, and Zheng, S-S. Application of metagenomics in the human gut microbiome. World J Gastroenterol. (2015) 21:803–14. doi: 10.3748/wjg.v21.i3.803
10. Schroeder, BO, and Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. (2016) 22:1079–89. doi: 10.1038/nm.4185
11. Shyam, S, Lee, KX, Tan, ASW, Khoo, TA, Harikrishnan, S, Lalani, SA, et al. Effect of personalized nutrition on dietary, physical activity, and health outcomes: a systematic review of randomized trials. Nutrients. (2022) 14:4104. doi: 10.3390/nu14194104
12. Van Karnebeek, CDM, Wortmann, SB, Tarailo-Graovac, M, Langeveld, M, Ferreira, CR, Van De Kamp, JM, et al. The role of the clinician in the multi-omics era: are you ready? J Inherit Metab Dis. (2018) 41:571–82. doi: 10.1007/s10545-017-0128-1
13. Bloss, CS, Jeste, DV, and Schork, NJ. Genomics for disease treatment and prevention. Psychiatr Clin North Am. (2011) 34:147–66. doi: 10.1016/j.psc.2010.11.005
14. Waldman, SA, and Terzic, A. Healthcare evolves from reactive to proactive. Clin Pharmacol Ther. (2019) 105:10–3. doi: 10.1002/cpt.1295
15. Derecho, CMP . Regulations and ethical considerations in nutrigenomics research In: G Dable-Tupas and C Egbuna, editors. Role of nutrigenomics in modern-day healthcare and drug discovery. Drug discovery update. Netherlands: Elsevier (2023). 557–65.
16. Camp, KM, and Trujillo, E. Position of the academy of nutrition and dietetics: nutritional genomics. J Acad Nutr Diet. (2014) 114:299–312. doi: 10.1016/j.jand.2013.12.001
17. Sales, NMR, Pelegrini, PB, and Goersch, MC. Nutrigenomics: definitions and advances of this new science. J Nutr Metab. (2014) 2014:202759:1–6. doi: 10.1155/2014/202759
18. Wang, F, Zheng, J, Cheng, J, Zou, H, Li, M, Deng, B, et al. Personalized nutrition: a review of genotype-based nutritional supplementation. Front Nutr. (2022) 9:992986. doi: 10.3389/fnut.2022.992986
19. Soloway, PD . Gene nutrient interactions and evolution. Nutr Rev. (2006) 64:S52–4. doi: 10.1301/nr.2006.may.s52-s54
20. Jew, S, AbuMweis, SS, and Jones, PJH. Evolution of the human diet: linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J Med Food. (2009) 12:925–34. doi: 10.1089/jmf.2008.0268
21. Alt, KW, Al-Ahmad, A, and Woelber, JP. Nutrition and health in human evolution-past to present. Nutrients. (2022) 14:3594. doi: 10.3390/nu14173594
22. Münster, A, Knipper, C, Oelze, VM, Nicklisch, N, Stecher, M, Schlenker, B, et al. 4000 years of human dietary evolution in Central Germany, from the first farmers to the first elites. PLoS One. (2018) 13:e0194862. doi: 10.1371/journal.pone.0194862
23. Rees, JS, Castellano, S, and Andrés, AM. The genomics of human local adaptation. Trends Genet. (2020) 36:415–28. doi: 10.1016/j.tig.2020.03.006
24. Benton, ML, Abraham, A, LaBella, AL, Abbot, P, Rokas, A, and Capra, JA. The influence of evolutionary history on human health and disease. Nat Rev Genet. (2021) 22:269–83. doi: 10.1038/s41576-020-00305-9
25. Kennedy, ET . The global face of nutrition: what can governments and industry do? J Nutr. (2005) 135:913–5. doi: 10.1093/jn/135.4.913
26. Corbett, S, Courtiol, A, Lummaa, V, Moorad, J, and Stearns, S. The transition to modernity and chronic disease: mismatch and natural selection. Nat Rev Genet. (2018) 19:419–30. doi: 10.1038/s41576-018-0012-3
27. Sproesser, G, Ruby, MB, Arbit, N, Akotia, CS, Alvarenga Dos, S, Bhangaokar, R, et al. Understanding traditional and modern eating: the TEP10 framework. BMC Public Health. (2019) 19:1606. doi: 10.1186/s12889-019-7844-4
28. Templin, T, Hashiguchi, TCO, Thomson, B, Dieleman, J, and Bendavid, E. The overweight and obesity transition from the wealthy to the poor in low- and middle-income countries: a survey of household data from 103 countries. PLoS Med. (2019) 16:e1002968. doi: 10.1371/journal.pmed.1002968
29. Popkin, BM, and Ng, SW. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev. (2022) 23:e13366. doi: 10.1111/obr.13366
30. Olstad, DL, Nejatinamini, S, Blanchet, R, Moubarac, J-C, Polsky, J, Vanderlee, L, et al. Protecting traditional cultural food practices: trends in diet quality and intake of ultra-processed foods by indigenous status and race/ethnicity among a nationally representative sample of adults in Canada. SSM Popul Health. (2023) 24:101496. doi: 10.1016/j.ssmph.2023.101496
31. Trichopoulou, A, Soukara, S, and Vasilopoulou, E. Traditional foods: a science and society perspective. Trends Food Sci Technol. (2007) 18:420–7. doi: 10.1016/j.tifs.2007.03.007
32. Brennan, RO, and Mulligan, WC. Nutrigenetics: new concepts for relieving hypoglycemia. New York, NY: Evans. (1975). Available at: https://www.ncbi.nlm.nih.gov/nlmcatalog?cmd=PureSearch&term=7603392%5Bnlmid%5D (Accessed November 18, 2023).
33. Bernstein, LE, Rohr, F, and Helm, JR Nutrition management of inherited metabolic diseases. Lessons from Metabolic University | Springer: Cham. Available at: https://link.springer.com/book/10.1007/978-3-030-94510-7 (Accessed November 18, 2023).
34. Davies, K . The era of genomic medicine. Clin Med (Lond). (2013) 13:594–601. doi: 10.7861/clinmedicine.13-6-594
35. Auffray, C, Griffin, JL, Khoury, MJ, Lupski, JR, and Schwab, M. Ten years of genome medicine. Genome Med. (2019) 11:7. doi: 10.1186/s13073-019-0618-x
36. Proctor, LM, Creasy, HH, Fettweis, JM, Lloyd-Price, J, Mahurkar, A, Zhou, W, et al. The integrative human microbiome project. Nature. (2019) 569:641–8. doi: 10.1038/s41586-019-1238-8
37. Gurdasani, D, Barroso, I, Zeggini, E, and Sandhu, MS. Genomics of disease risk in globally diverse populations. Nat Rev Genet. (2019) 20:520–35. doi: 10.1038/s41576-019-0144-0
38. Dries, DL . Genetic ancestry, population admixture, and the genetic epidemiology of complex disease. Circ Cardiovasc Genet. (2009) 2:540–3. doi: 10.1161/CIRCGENETICS.109.922898
39. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Food Forum. Challenges and opportunities for precision and personalized nutrition: Proceedings of a workshop—In brief. AE Callahan, editor. Washington (DC): National Academies Press (US). (2021). Available at: http://www.ncbi.nlm.nih.gov/books/NBK575794/ (Accessed November 18, 2023).
40. Ordovas, JM, Ferguson, LR, Tai, ES, and Mathers, JC. Personalised nutrition and health. The. BMJ. (2018) 361:bmj.k2173. doi: 10.1136/bmj.k2173
41. Kimokoti, RW, and Millen, BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. (2016) 100:1185–98. doi: 10.1016/j.mcna.2016.06.003
42. Willett, WC, Koplan, JP, Nugent, R, Dusenbury, C, Puska, P, and Gaziano, TA. “Prevention of chronic disease by means of diet and lifestyle changes.,” In: DT Jamison, JG Breman, AR Measham, G Alleyne, M Claeson, and DB Evans, et al. editors. Disease control priorities in developing countries. Washington (DC): The International Bank for Reconstruction and Development / The World Bank (2006) Available at: http://www.ncbi.nlm.nih.gov/books/NBK11795/ (Accessed November 18, 2023).
43. Gropper, SS . The role of nutrition in chronic disease. Nutrients. (2023) 15:664. doi: 10.3390/nu15030664
44. Li, Y, Schoufour, J, Wang, DD, Dhana, K, Pan, A, Liu, X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. (2020) 368:l6669. doi: 10.1136/bmj.l6669
45. Dietert, RR . Dietary approaches from moms, farms, and nature to overcome chronic diseases and the Pharmacracy. Nutrients. (2023) 15:3965. doi: 10.3390/nu15183965
46. Laila, A, Leme, AC, Hou, S, Ma, DWL, and Haines, J. Perceived challenges and strategies to achieve Canada’s food guide recommendation to “cook more often”: findings from parents of young children. Appetite. (2023) 182:106413. doi: 10.1016/j.appet.2022.106413
47. Shaw-Taylor, L . An introduction to the history of infectious diseases, epidemics and the early phases of the long-run decline in mortality. Econ Hist Rev. (2020) 73:E1–E19. doi: 10.1111/ehr.13019
48. Barquera, S . Intersection of public health, nutrition, and mental health: challenges to progress. Salud Ment. (2022) 45:211–2. doi: 10.17711/sm.0185-3325.2022.027
49. Deckelbaum, RJ, Fisher, EA, Winston, M, Kumanyika, S, Lauer, RM, Pi-Sunyer, FX, et al. Summary of a scientific conference on preventive nutrition: pediatrics to geriatrics. Circulation. (1999) 100:450–6. doi: 10.1161/01.CIR.100.4.450
51. D'Innocenzo, S, Biagi, C, and Lanari, M. Obesity and the Mediterranean diet: a review of evidence of the role and sustainability of the Mediterranean diet. Nutrients. (2019) 11:1306. doi: 10.3390/nu11061306
52. Abe, S, Zhang, S, Tomata, Y, Tsuduki, T, Sugawara, Y, and Tsuji, I. Japanese diet and survival time: the Ohsaki cohort 1994 study. Clin Nutr. (2020) 39:298–303. doi: 10.1016/j.clnu.2019.02.010
53. Olsen, A, Egeberg, R, Halkjær, J, Christensen, J, Overvad, K, and Tjønneland, A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr. (2011) 141:639–44. doi: 10.3945/jn.110.131375
54. Burt, KG, and College, L. The whiteness of the Mediterranean diet: a historical, sociopolitical, and dietary analysis using critical race theory. J Crit Diet. (2021) 5:41–52. doi: 10.32920/cd.v5i2.1329
55. Phokaewvarangkul, O, Kantachadvanich, N, Buranasrikul, V, Phoumindr, A, Phumphid, S, Jagota, P, et al. From evidence to the dish: a viewpoint of implementing a Thai-style Mediterranean diet for Parkinson’s disease. J Mov Disord. (2023) 16:279–84. doi: 10.14802/jmd.23021
56. Figueroa, C, Echeverría, G, Villarreal, G, Martínez, X, Ferreccio, C, and Rigotti, A. Introducing plant-based Mediterranean diet as a lifestyle medicine approach in Latin America: opportunities within the Chilean context. Front Nutr. (2021) 8:680452. doi: 10.3389/fnut.2021.680452
57. Ojeda-Granados, C, and Roman, S. Mediterranean diet or genome-based nutrition diets in Latin America’s clinical practice guidelines for managing chronic liver diseases? Ann Hepatol. (2021) 20:100291. doi: 10.1016/j.aohep.2020.100291
58. Roman, S, Ojeda-Granados, C, Ramos-Lopez, O, and Panduro, A. Genome-based nutrition: an intervention strategy for the prevention and treatment of obesity and nonalcoholic steatohepatitis. World J Gastroenterol. (2015) 21:3449–61. doi: 10.3748/wjg.v21.i12.3449
59. Panduro, A, and Roman, S. Personalized medicine in Latin America. Pers Med. (2020) 17:339–43. doi: 10.2217/pme-2020-0049
60. García-Ortiz, H, Barajas-Olmos, F, Contreras-Cubas, C, Cid-Soto, MÁ, Córdova, EJ, Centeno-Cruz, F, et al. The genomic landscape of Mexican indigenous populations brings insights into the peopling of the Americas. Nat Commun. (2021) 12:5942. doi: 10.1038/s41467-021-26188-w
61. Ojeda-Granados, C, Abondio, P, Setti, A, Sarno, S, Gnecchi-Ruscone, GA, González-Orozco, E, et al. Dietary, cultural, and pathogens-related selective pressures shaped differential adaptive evolution among native Mexican populations. Mol Biol Evol. (2021) 39:msab290. doi: 10.1093/molbev/msab290
62. García-Ortiz, H, Barajas-Olmos, F, Contreras-Cubas, C, Reynolds, AW, Flores-Huacuja, M, Snow, M, et al. Unraveling signatures of local adaptation among indigenous groups from Mexico. Genes. (2022) 13:2251. doi: 10.3390/genes13122251
63. Ongaro, L, Scliar, MO, Flores, R, Raveane, A, Marnetto, D, Sarno, S, et al. The genomic impact of European colonization of the Americas. Curr Biol. (2019) 29:3974–3986.e4. doi: 10.1016/j.cub.2019.09.076
64. Ojeda-Granados, C, Panduro, A, Gonzalez-Aldaco, K, Sepulveda-Villegas, M, Rivera-Iñiguez, I, and Roman, S. Tailoring nutritional advice for Mexicans based on prevalence profiles of diet-related adaptive gene polymorphisms. J Pers Med. (2017) 7:16. doi: 10.3390/jpm7040016
65. Torres-Valadez, R, Roman, S, Ojeda-Granados, C, Gonzalez-Aldaco, K, and Panduro, A. Differential distribution of gene polymorphisms associated with hypercholesterolemia, hypertriglyceridemia, and hypoalphalipoproteinemia among native American and mestizo Mexicans. World J Hepatol. (2022) 14:1408–20. doi: 10.4254/wjh.v14.i7.1408
66. Moreno-Estrada, A, Gignoux, CR, Fernández-López, JC, Zakharia, F, Sikora, M, Contreras, AV, et al. Human genetics. The genetics of Mexico recapitulates native American substructure and affects biomedical traits. Science. (2014) 344:1280–5. doi: 10.1126/science.1251688
67. Valerino-Perea, S, Armstrong, MEG, and Papadaki, A. Adherence to a traditional Mexican diet and non-communicable disease-related outcomes: secondary data analysis of the cross-sectional Mexican National Health and nutrition survey. Br J Nutr. (2022) 129:1266–79. doi: 10.1017/S0007114522002331
68. Sepulveda-Villegas, M, Roman, S, Rivera-Iñiguez, I, Ojeda-Granados, C, Gonzalez-Aldaco, K, Torres-Reyes, LA, et al. High prevalence of nonalcoholic steatohepatitis and abnormal liver stiffness in a young and obese Mexican population. PLoS One. (2019) 14:e0208926. doi: 10.1371/journal.pone.0208926
69. Gonzalez-Aldaco, K, Roman, S, Torres-Reyes, LA, and Panduro, A. Association of Apolipoprotein e2 allele with insulin resistance and risk of type 2 diabetes mellitus among an admixed population of Mexico. Diabetes Metab Syndr Obes. (2020) 13:3527–34. doi: 10.2147/DMSO.S268329
70. Moreno-Altamirano, L, García-García, JJ, Salvatore, P, Soto-Estrada, G, and Hernández-Montoya, D. Metabolic syndrome: changes in mediterranean and mesoamerican diet due to socioeconomic factors in Mexico and Italy. Mediterr J Nutr Metab. (2017) 10:49–59. doi: 10.3233/MNM-16124
71. SSA, INSP, GISAMAC, UNICEF. Guias alimentarias saludables y sostenibles para la población mexicana 2023. Mexico. Available at: http://www.gob.mx/promosalud/articulos/que-son-las-guias-alimentarias?idiom=es (Accessed January 8, 2024).
72. Ojeda-Granados, C, Barchitta, M, La Rosa, MC, La Mastra, C, Roman, S, Panduro, A, et al. Evaluating dietary patterns in women from southern Italy and Western Mexico. Nutrients. (2022) 14:1603. doi: 10.3390/nu14081603
73. Bach-Faig, A, Berry, EM, Lairon, D, Reguant, J, Trichopoulou, A, Dernini, S, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. (2011) 14:2274–84. doi: 10.1017/S1368980011002515
74. Royo, C, Soriano, JM, and Alvaro, F. Wheat: a crop in the bottom of the Mediterranean diet pyramid | IntechOpen. (2017). Available at: https://www.intechopen.com/chapters/55480 (Accessed May 8, 2024).
75. Donini, LM, Serra-Majem, L, Bulló, M, Gil, Á, and Salas-Salvadó, J. The Mediterranean diet: culture, health and science. Br J Nutr. (2015) 113:S1–3. doi: 10.1017/S0007114515001087
76. Valerino-Perea, S, Lara-Castor, L, Armstrong, MEG, and Papadaki, A. Definition of the traditional Mexican diet and its role in health: a systematic review. Nutrients. (2019) 11:2803. doi: 10.3390/nu11112803
77. UNESCO – Traditional Mexican cuisine – ancestral, ongoing community culture, the Michoacán paradigm. Available at: https://ich.unesco.org/en/RL/traditional-mexican-cuisine-ancestral-ongoing-community-culture-the-michoacan-paradigm-00400 (Accessed January 8, 2024).
78. Sohail, M, Palma-Martínez, MJ, Chong, AY, Quinto-Cortés, CD, Barberena-Jonas, C, Medina-Muñoz, SG, et al. Mexican biobank advances population and medical genomics of diverse ancestries. Nature. (2023) 622:775–83. doi: 10.1038/s41586-023-06560-0
79. Raghubeer, S, and Matsha, TE. Methylenetetrahydrofolate (MTHFR), the one-carbon cycle, and cardiovascular risks. Nutrients. (2021) 13:4562. doi: 10.3390/nu13124562
80. Díaz-José, J, Guevara-Hernández, F, Morales-Ríos, V, and López-Ayala, J. Traditional knowledge of edible wild plants used by indigenous communities in Zongolica, Mexico. Ecol Food Nutr. (2019) 58:511–26. doi: 10.1080/03670244.2019.1604340
81. Panduro, A, Rivera-Iñiguez, I, and Ramos-Lopez, O. Roman S. Chapter 50 – genes and alcoholism: taste, addiction, and metabolism In: VR Preedy , editor. Neuroscience of alcohol. Academic Press (2019). 483–91.
82. Pérez-Beltrán, YE, González-Becerra, K, Rivera-Iñiguez, I, Martínez-López, E, Ramos-Lopez, O, Alcaraz-Mejía, M, et al. A Nutrigenetic strategy for reducing blood lipids and low-grade inflammation in adults with obesity and overweight. Nutrients. (2023) 15:4324. doi: 10.3390/nu15204324
83. Rivera-Iñiguez, I, Panduro, A, Ramos-Lopez, O, Villaseñor-Bayardo, SJ, and Roman, S. DRD2/ANKK1 TaqI A1 polymorphism associates with overconsumption of unhealthy foods and biochemical abnormalities in a Mexican population. Eat Weight Disord. (2019) 24:835–44. doi: 10.1007/s40519-018-0596-9
84. Ojeda-Granados, C, Panduro, A, Rivera-Iñiguez, I, Sepúlveda-Villegas, M, and Roman, S. A regionalized genome-based Mexican diet improves anthropometric and metabolic parameters in subjects at risk for obesity-related chronic diseases. Nutrients. (2020) 12:645. doi: 10.3390/nu12030645
85. Rivera-Iñiguez, I, Panduro, A, Villaseñor-Bayardo, SJ, Sepulveda-Villegas, M, Ojeda-Granados, C, and Roman, S. Influence of a Nutrigenetic intervention on self-efficacy, emotions, and rewarding behaviors in unhealthy eating among Mexicans: an exploratory pilot study. Nutrients. (2022) 14:213. doi: 10.3390/nu14010213
86. Landini, A, Yu, S, Gnecchi-Ruscone, GA, Abondio, P, Ojeda-Granados, C, Sarno, S, et al. Genomic adaptations to cereal-based diets contribute to mitigate metabolic risk in some human populations of east Asian ancestry. Evol Appl. (2020) 14:297–313. doi: 10.1111/eva.13090
87. Marrón-Ponce, JA, Tolentino-Mayo, L, Hernández-F, M, and Batis, C. Trends in ultra-processed food purchases from 1984 to 2016 in Mexican households. Nutrients. (2018) 11:45. doi: 10.3390/nu11010045
88. Roman, S, and Panduro, A. Genomic medicine in gastroenterology: a new approach or a new specialty? World J Gastroenterol. (2015) 21:8227–37. doi: 10.3748/wjg.v21.i27.8227
89. Roman, S . Genome-based nutritional strategies to prevent chronic liver disease. Ann Hepatol. (2019) 18:537–8. doi: 10.1016/j.aohep.2019.05.005
90. Panduro, A, and Roman, S. Advancements in genomic medicine and the need for updated regional clinical practice guidelines in the field of hepatology. Ann Hepatol. (2020) 19:1–2. doi: 10.1016/j.aohep.2019.12.002
91. Roman, S, Ramos-Lopez, O, and Panduro, A. Genomic medicine in hepatology: towards personalized medicine in obesity and chronic liver disease. Ann Hepatol. (2023) 28:100875. doi: 10.1016/j.aohep.2022.100875
92. Panduro, A, Roman, S, Mariscal-Martinez, IM, Jose-Abrego, A, Gonzalez-Aldaco, K, Ojeda-Granados, C, et al. Personalized medicine and nutrition in hepatology for preventing chronic liver disease in Mexico. Front Nutr. (2024) 11:1379364. doi: 10.3389/fnut.2024.1379364
93. Elena, R-IM, Gabriela, G-D, Arnulfo, G-C, and Enrique, CA. Studying the gut microbiome of Latin America and Hispanic/Latino populations. Insight into obesity and diabetes: systematic review. Curr Diabetes Rev. (2019) 15:294–301. doi: 10.2174/1573399814666180730124817
94. Biruete, A, Leal-Escobar, G, Espinosa-Cuevas, Á, Mojica, L, and Kistler, BM. Dieta de la Milpa: a culturally-concordant plant-based dietary pattern for Hispanic/Latine people with chronic kidney disease. Nutrients. (2024) 16:574. doi: 10.3390/nu16050574
95. Ruz, M, and Solomons, NW. A vision for nutritional research for the Latin American region. Food Nutr Bull. (2019) 40:14–25. doi: 10.1177/0379572119832780
96. Zambrano, AK, Cadena-Ullauri, S, Guevara-Ramírez, P, Ruiz-Pozo, VA, Tamayo-Trujillo, R, Paz-Cruz, E, et al. Genetic diet interactions of ACE: the increased hypertension predisposition in the Latin American population. Front Nutr. (2023) 10:1241017. doi: 10.3389/fnut.2023.1241017
97. Fernández-Rhodes, L, Graff, M, Buchanan, VL, Justice, AE, Highland, HM, Guo, X, et al. Ancestral diversity improves discovery and fine-mapping of genetic loci for anthropometric traits—the Hispanic/Latino anthropometry consortium. HGG Adv. (2022) 4:100149. doi: 10.1016/j.xhgg.2022.100149
98. THE 17 GOALS | sustainable development. Available at: https://sdgs.un.org/goals (Accessed November 20, 2023).
Keywords: genes, polymorphisms, ancestry, Genomex diet, hepatopathogenic diet, food culture, Latin America, Mexico
Citation: Roman S, Campos-Medina L and Leal-Mercado L (2024) Personalized nutrition: the end of the one-diet-fits-all era. Front. Nutr. 11:1370595. doi: 10.3389/fnut.2024.1370595
Edited by:
Claudia Ojeda-Granados, University of Catania, ItalyReviewed by:
Venkata Saroja Voruganti, University of North Carolina at Chapel Hill, United StatesCopyright © 2024 Roman, Campos-Medina and Leal-Mercado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonia Roman, c29uaWEucm9tYW5AYWNhZGVtaWNvcy51ZGcubXg=
 Sonia Roman
Sonia Roman Liliana Campos-Medina
Liliana Campos-Medina Leonardo Leal-Mercado
Leonardo Leal-Mercado