- 1Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Department of Community Nutrition, School of Nutrition and Food Science, Nutrition and Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 3Department of Kinesiology and Health Promotion, University of Kentucky, Lexington, KY, United States
- 4Department of Clinical Nutrition & Dietetics, Faculty of Nutrition and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Food Safety Research Center (Salt), Semnan University of Medical Sciences, Semnan, Iran
- 6Division of Sleep Medicine, Harvard Medical School, Boston, MA, United States
- 7Medical Chronobiology Program, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women's Hospital, Boston, MA, United States
Aims: The present study was conducted to examine the association between dietary acid load (DAL) and markers of inflammation, oxidative stress, and malnutrition in a group of Iranian hemodialysis (HD) patients.
Methods: This cross-sectional study was performed on individuals aged ≥18 years who were on HD at least 6 months before their enrollment in the study. A 4-day dietary recall was used for the evaluation of dietary intake. DAL was calculated using two methods including potential renal acid load (PRAL) and net endogenous acid production (NEAP). For assessing the malnutrition status, we used the subjective global assessment (SGA), dialysis malnutrition score (DMS), and malnutrition inflammation score (MIS). Fasting blood samples were collected from each participant to assess serum levels of high-sensitivity C-reactive protein (hs-CRP), soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular adhesion molecule-1 (sVCAM-1), sE-selectin, malondialdehyde (MDA), nitric oxide (NO), and endothelin-1.
Results: In total, 291 patients with a mean age of 57.73 ± 0.88 years and HD vintage of 4.27 ± 0.25 months were enrolled in the current study. Significant positive associations were observed between PRAL and hs-CRP (β = 1.77, 95% CI: 0.88, 2.65), sICAM-1 (β = 83.21, 95% CI: 10.39, 156.04), sVCAM-1 (β = 194.63, 95% CI: 74.68, 314.58), and sE-selectin (β = 6.66, 95% CI: 1.81, 11.50) among participants with the highest PRAL scores, compared to those with the lowest PRAL scores. NEAP was positively correlated with hs-CRP (β = 1.34, 95% CI: 0.46, 2.22), sICAM-1 (β = 88.83, 95% CI: 16.99, 160.67), and MDA (β = 0.35, 95% CI: 0.005, 0.71). Additionally, marginally significant higher odds of SGA (OR = 1.98, 95% CI: 0.95, 4.11) and DMS (OR = 1.94, 95% CI: 0.92, 4.05) were observed in individuals in the third tertile of PRAL vs. the first tertile of PRAL. NEAP had also a marginally significant positive correlation with DMS (OR = 2.01, 95% CI: 0.93, 4.31).
Conclusion: This study illustrates that higher consumption of acidic foods is correlated with markers of inflammation, oxidative stress, and malnutrition in HD patients.
Introduction
End-stage renal disease (ESRD) is a major public health concern with a global prevalence of 11–13% of the population (1, 2). The ESRD incidence rate has been increasing in recent years, and it is associated with a significant economic burden for the affected individuals and healthcare systems (1). A high prevalence of protein-energy malnutrition (PEM) and inflammation have been observed in ESRD patients undergoing hemodialysis (HD) (3). PEM and inflammation are believed to be intertwined and are correlated with high morbidity, cardiovascular mortality, and all-cause mortality in maintenance HD patients (3). The strong association between PEM and inflammation has given rise to the medical phenomena known as the malnutrition inflammation atherosclerosis (MIA) syndrome and malnutrition-inflammation complex syndrome (MICS) (3). Thus, it is of great importance to seek useful strategies for the management of malnutrition and inflammation in HD patients.
Possible causes of MICS include but not limited to comorbid illnesses, uremia, low clearance of inflammatory factors, anorexia, and loss of soluble nutrients during HD (4). Moreover, researchers believe that diet may be a significant contributor to MICS (5). Previous studies indicate that the intake of acidic foods, particularly animal proteins, in individuals whose endogenous acid–base balance is disturbed was associated with metabolic acidosis and subsequent malnutrition and inflammation (6). To explore the contribution of acidic dietary compounds to various health markers, dietary acid load (DAL) has been defined to quantify the acidic potential of the diet.
In epidemiological studies, two methods have been used for the evaluation of DAL including potential renal acid load (PRAL) (7) and net endogenous acid production (NEAP) (8). Both PRAL and NEAP are validated approaches for assessing DAL in renal transplant recipients due to these methods having a significant correlation with 24-h urinary net acid excretion (NAE) (9). Therefore, they are speculated to be useful indicators of DAL status in patients with HD.
Previous studies have found a correlation between DAL and diseases such as diabetic nephropathy (10), kidney stones (11), and kidney cancer (12). However, to our knowledge, no study has previously investigated the predictive role of DAL in HD patients. Therefore, in the present cross-sectional study, we intended to investigate the association between DAL and markers of inflammation, oxidative stress, and malnutrition in HD patients among a sample of Iranian adults. We anticipated that higher DAL is associated with greater inflammation and oxidative stress levels and unfavorable malnutrition status in HD patients.
Methods
Study population and design
In the current cross-sectional study, 2,302 HD patients were selected from August 2019 to June 2020 from multiple HD centers in Tehran, Iran. To identify appropriate participants, the list of all the HD centers in Tehran was obtained from the Iran Dialysis Center, where the names of all the HD patients were taken from each of the 50 HD centers, and then the names of the patients who met the eligibility criteria to be enrolled in this study were recorded (n = 2,302). Next, the names of HD centers in Tehran were sorted alphabetically, and then the names of the patients in these centers were listed. Finally, 291 out of 2,302 subjects were selected using the systematic sampling method. Prior to enrollment in the study, written consent was obtained from all patients. The present study was conducted based on the Declaration of Helsinki, and the study protocol was also approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Iran (IR.SBMU.NNFTRI.REC.1387.319). HD patients aged ≥18 years who were on hemodialysis at least 6 months prior to enrollment were included in the study. However, patients with a history of liver disease, inflammatory diseases, malignancies, chronic or acute pancreatitis, and HIV infection were excluded. All patients underwent HD three times a week for 4 h using polysulfone capillary dialyzers and bicarbonate dialysate.
Assessment of dietary intakes
The dietary intakes of participants were evaluated by an expert dietitian using a 4-day, 24-h dietary recall, including 2 dialysis days and 2 non-dialysis days through face-to-face interviews. For the examination of the daily intakes of energy, macronutrients, and micronutrients, Nutritionist IV software (First Databank, Hearst Corp., San Bruno, CA, United States), modified for Iranian foods, was used. Since dietary intakes of patients may be different on dialysis vs. non-dialysis days, both days were selected to capture day-to-day variations in diet (13). Participants were asked to recall all the drinks and food items consumed within 24 h. Portion size models were used to help people estimate portion size and improve accuracy.
DAL calculation
DAL was calculated using 2 surrogate measures, PRAL and NEAP, which have both been routinely used in epidemiological studies. The PRAL score was calculated using the following equation by Remer et al. (7): PRAL (mEq/d) = [0.49 × protein intake (g/d) + 0.037× dietary phosphorous (mg/d) – 0.021 × dietary potassium (mg/d) – 0.013 × calcium (mg/d) – 0.026 × magnesium (mg/d)]. Moreover, the NEAP score was estimated using the following algorithm of Frasseto et al. (8): NEAP (mEq/d) = [54.4 × protein intake (g/d) ÷ potassium intake (mEq/d) − 10.2]. Negative values of PRAL and NEAP indicate an alkaline potential of a diet; however, positive PRAL and NEAP scores illustrate an acidic potential of a diet (12, 14).
We used both PRAL and NEAP scores for acid load assessment due to their differences in nutritional aspects and biological mechanisms. In the PRAL method, the intestinal absorption rates of protein, potassium, calcium, magnesium, and phosphate were considered, and this method has been validated against urine pH in healthy populations. Moreover, the NEAP score considers sulfuric acid generation rate from the metabolism of protein and bicarbonate production from the metabolism of potassium salts. In addition, a previous study found a positive correlation between PRAL and NEAP (correlation coefficient = 0.84, p < 0.001) (12, 14).
Assessment of malnutrition
We used the subjective global assessment (SGA), dialysis malnutrition score (DMS), and malnutrition inflammation score (MIS) to determine the malnutrition status of subjects. The SGA, which is a validated tool for the assessment of malnutrition status in HD patients, consists of two criteria: (1) medical history (i.e., dietary intake, functional capacity, weight change, and gastrointestinal symptoms) and (2) clinical assessment (i.e., physician’s grading of the loss of subcutaneous fat, presence of edema, and muscle wasting). Each component had three levels of severity, including normal nutritional status (grade A), mild to moderately affected (grade B), and severely affected (grade C). Accordingly, participants with grades of A, B, and C were placed in the corresponding well-nourished, mild to moderately malnourished, and severely malnourished groups (15, 16). The DMS, a modified quantitative version of SGA, includes 7 variables (i.e., muscle wasting, functional capacity, loss of subcutaneous fat, gastrointestinal symptoms, weight change, dietary intake, and comorbidity) that each score from 1 (normal) to 5 (very severe), resulting in a total score of 7–35 (17). Patients were classified into three categories based on DMS: (1) normal nutrition status (score of 7–13), (2) mild to moderately malnourished (score of 14–23), and (3) severely malnourished (score of 24–35). The MIS was generated by adding the total iron-binding capacity (TIBC) (mg/dL), serum albumin (g/dL), and body mass index (BMI) (kg/m2) to DMS, including four levels of severity from 0 (normal) to 3 (severely abnormal). The sum score of MIS ranges from 0 to 30 and is categorized into three groups: 0–7 (well-nourished), 8–18 (mild to moderately malnourished), and 19–30 (severely malnourished) (17).
Biochemical assessment
The serum concentrations of high-sensitivity C-reactive protein (hs-CRP) were determined using ELISA kits (Diagnostics Biochem Canada, London, Canada) with an intra-and inter-assay CV of 4.6%. The serum levels of soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular adhesion molecule-1 (sVCAM-1), and sE-selectin were also evaluated by Diaclone ELISA kits (Diaclone, Besancon, France). Intra-and inter-assay CVs for sICAM-1, sVCAM-1, and sE-selectin were 3.5, 6.3, and 6.7%, respectively. Endothelin-1 was evaluated with Biomedica kits (Biomedica, Vienna, Austria) with an intra-and inter-assay CV of 8.5%. The colorimetric method was used to assess serum nitric oxide (NO) and serum malondialdehyde (MDA) concentrations with an intra-and inter-assay CV of 7.8% for NO and 4.6% for MDA (Cayman Chemical, Ann Arbor, MI, United States).
Assessment of other variables
The measurement of dry weight was conducted with a digital scale (Omron BF511, Omron Corp., Kyoto, Japan) to the nearest 100 g at the end of a dialysis session. For the assessment of height, an upright measuring tape was used, with measurements taken to the nearest 1 mm. To calculate BMI, weight in kg was divided by squared height in meters (m2). To evaluate the dialysis adequacy based on the Kt/V index, post-dialysis weight, duration of dialysis, ultrafiltration volume, and pre-and post-dialysis serum urea levels were used (17). Dialysis vintage is presented as the time in months that each patient has spent on HD. Blood samples of 8 milliliters were collected from participants after a 10–12 h fast and immediately centrifuged at 2500 rpm for 15 min to isolate serum. Serum specimens were stored at −70°C for later analyzes. Serum concentrations were measured using a colorimetric method for creatinine and calcium, a photometric method for urea and phosphorous, a Bromocresol green approach for albumin, and a flame photometric approach for serum potassium. All biochemical analyzes were conducted using Pars Azmoon (Tehran, Iran) commercial kits.
Statistical analysis
All analyzes were conducted using SPSS version 26 (IBM Corp., Armonk, NY, United States). Prior to data analysis, the normality of variables was explored via the skewness statistic, Q-Q plot, and Kolmogorov–Smirnov test. The subjects were first categorized into tertiles according to PRAL and NEAP scores. Tertile categorization was chosen because it is more practical for nutritional interpretation as subjects classify in three qualitative groups (low/intermediate/high). Quantitative and qualitative variables were expressed as mean ± standard deviation (SD) and frequency (percentage), respectively. The difference between continuous variables across tertiles of PRAL and NEAP was assessed via one-way analysis of variance (ANOVA). The distribution of categorical variables across tertiles of PRAL and NEAP was examined using the Chi-squared test. The association between DAL and the biomarkers of oxidative stress and inflammation was investigated using multiple linear regression analysis, and beta (β) estimates with 95% confidence intervals (CIs) were reported for three different models. The first model was controlled for age (continuous), sex, and total energy intake (continuous). The next model was additionally adjusted for dialysis adequacy (continuous), dialysis vintage (continuous), serum potassium (continuous), serum calcium (continuous), serum phosphorous (continuous), serum urea (continuous), and albumin (continuous). Further adjustment was made for BMI (continuous) in the last model. Multiple logistic regression analysis was implemented to assess the link between DAL and malnutrition in the three adjusted models, and odds ratios (OR) with corresponding 95% CIs were reported. The first model was adjusted for age (continuous), sex, and total energy intake (continuous). The next model was additionally adjusted for dialysis adequacy (continuous), dialysis vintage (continuous), and serum urea (continuous). The last model was also adjusted for serum potassium (continuous), serum calcium (continuous), and serum phosphorous (continuous). p-values <0.05 were considered statistically significant, and all analyzes were two-tailed.
Results
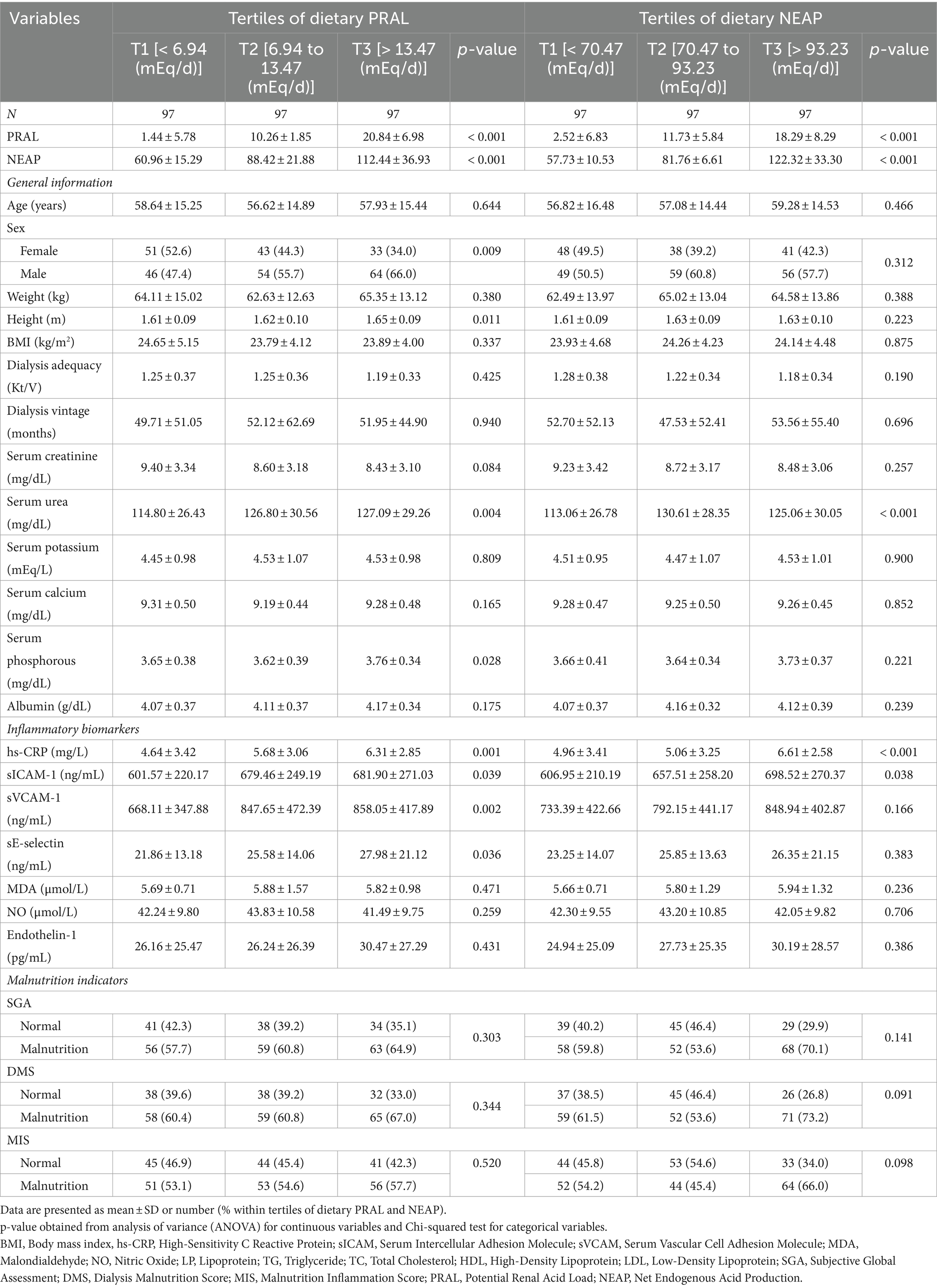
In total, 291 HD patients, including 127 women and 164 men were included in the current study with a mean (SD) age of 57.73 ± 15.17 years, BMI of 24.11 ± 4.45 kg/m2, dialysis vintage of 4.27 ± 4.43 months, and dialysis adequacy of 1.23 ± 0.35 Kt/V. The general characteristics of participants across tertiles of PRAL and NEAP are presented in Table 1. As can be seen, height, serum urea, serum phosphorous, hs-CRP, sICAM-1, sVCAM-1, and sE-selectin were significantly higher in patients with the highest scores of PRAL compared to those with the lowest scores. Moreover, participants in the highest tertile of PRAL were more likely to be male. In terms of NEAP tertiles, the mean serum urea, hs-CRP, and sICAM-1 were significantly higher in patients in the highest tertile of NEAP compared to patients in the lowest tertile of NEAP. No other significant differences were seen across PRAL and NEAP tertiles regarding other variables (Table 1).
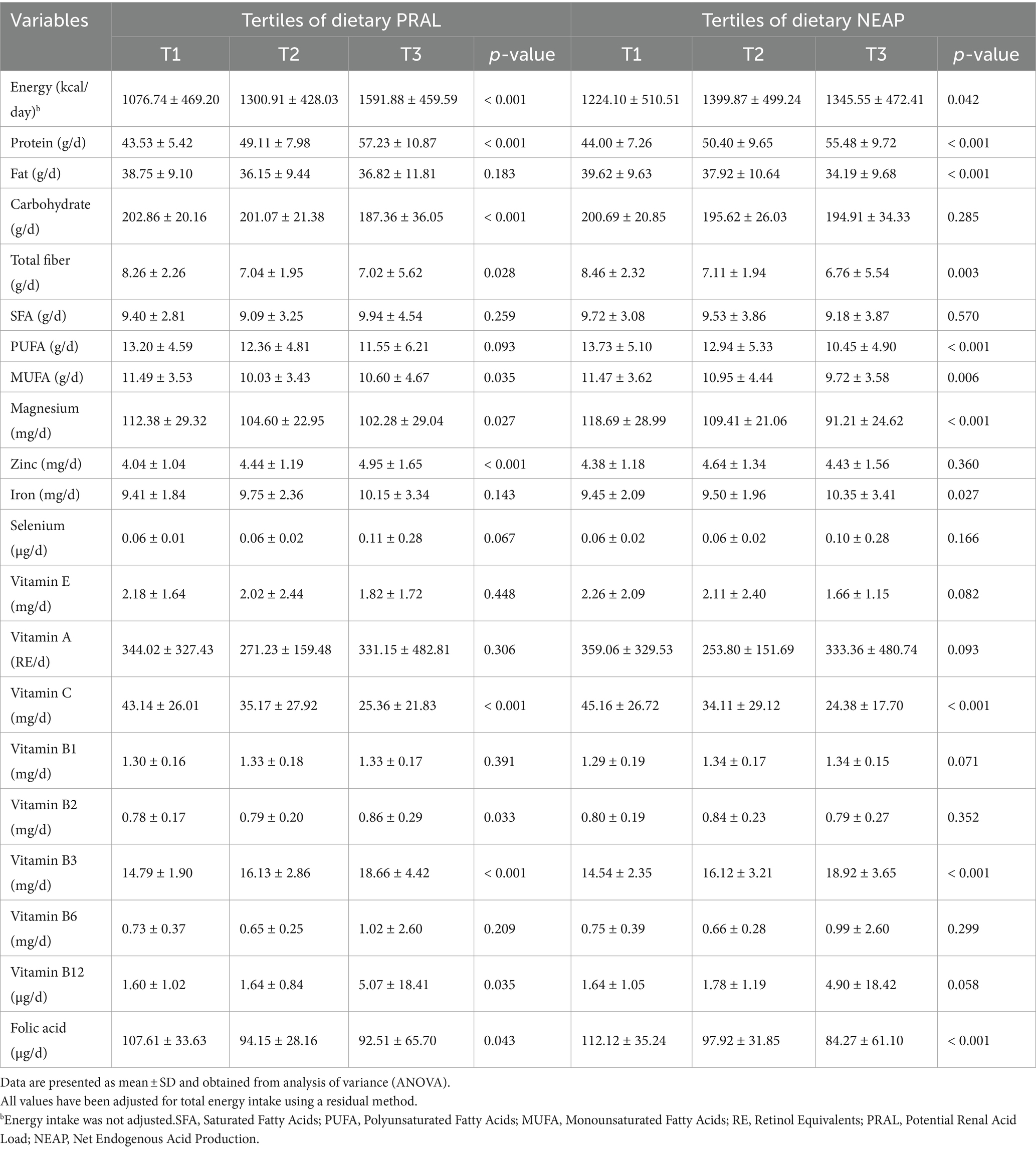
The dietary intake of macro- and micro-nutrients of participants across tertiles of PRAL and NEAP are described in Table 2. Patients in the highest tertile of PRAL had significantly higher intakes of total energy, protein, zinc, and vitamins B2, B3, and B12 than those in the lowest tertile of PRAL. Moreover, lower intakes of carbohydrates, total fiber, mono-unsaturated fatty acids (MUFA), magnesium, vitamin C, and folic acid were observed in patients in the third tertile of PRAL than patients in the first tertile of PRAL. Participants in the third tertile of NEAP had significantly higher intakes of total energy, protein, iron, and vitamin B3. However, intakes of total fat, total fiber, polyunsaturated fatty acids (PUFA), MUFA, magnesium, vitamin C, and folic acid were significantly lower in patients in the highest tertile of NEAP in comparison to patients in the lowest tertile of NEAP.
Beta (β) estimates and 95% CIs for biomarkers of inflammation and oxidative stress across tertiles of PRAL and NEAP are presented in Table 3. Serum levels of hs-CRP were positively correlated with a higher PRAL score in patients within the last tertile of PRAL compared to those in the lowest tertile, both before (β = 1.67, 95%CI: 0.79, 2.54; Ptrend < 0.001) and after adjustment for potential confounders (β = 1.77, 95%CI: 0.88, 2.65; Ptrend < 0.001). In the crude model, positive associations were observed between PRAL values and serum concentrations of sICAM-1 (β = 80.33, 95%CI: 10.78, 149.88; Ptrend = 0.024), sVCAM-1 (β = 189.93, 95%CI: 73.14, 306.73; Ptrend = 0.002), and sE-selectin (β = 6.12, 95% CI: 1.48, 10.76; Ptrend = 0.010) for individuals in the third tertile of PRAL compared to those in the first tertile of PRAL. These associations persisted after controlling for age, sex, energy intake, dialysis adequacy, dialysis vintage, serum potassium, serum calcium, serum phosphorous, serum urea, albumin, and BMI. In the crude model, serum levels of hs-CRP (β = 1.64, 95%CI: 0.77, 2.51; Ptrend < 0.001) and sICAM-1 (β = 91.56, 95%CI: 22.02, 161.11; Ptrend = 0.010) tended to be positively associated with NEAP scores in participants of the last tertile of NEAP compared to the first tertile of NEAP. These findings remained significant in the fully adjusted models. A non-significant positive association was found between NEAP and MDA concentrations in the crude model (β = 0.28, 95%CI: −0.04, 0.60; Ptrend = 0.087). However, the association became significant after adjusting for age, sex, energy intake, dialysis adequacy, dialysis vintage, serum potassium, serum calcium, serum phosphorous, serum urea, albumin, and BMI (β = 0.35, 95%CI: 0.005, 0.71; Ptrend = 0.048). No further correlations were seen across PRAL or NEAP tertiles and other variables in the crude and fully adjusted models (Table 3).
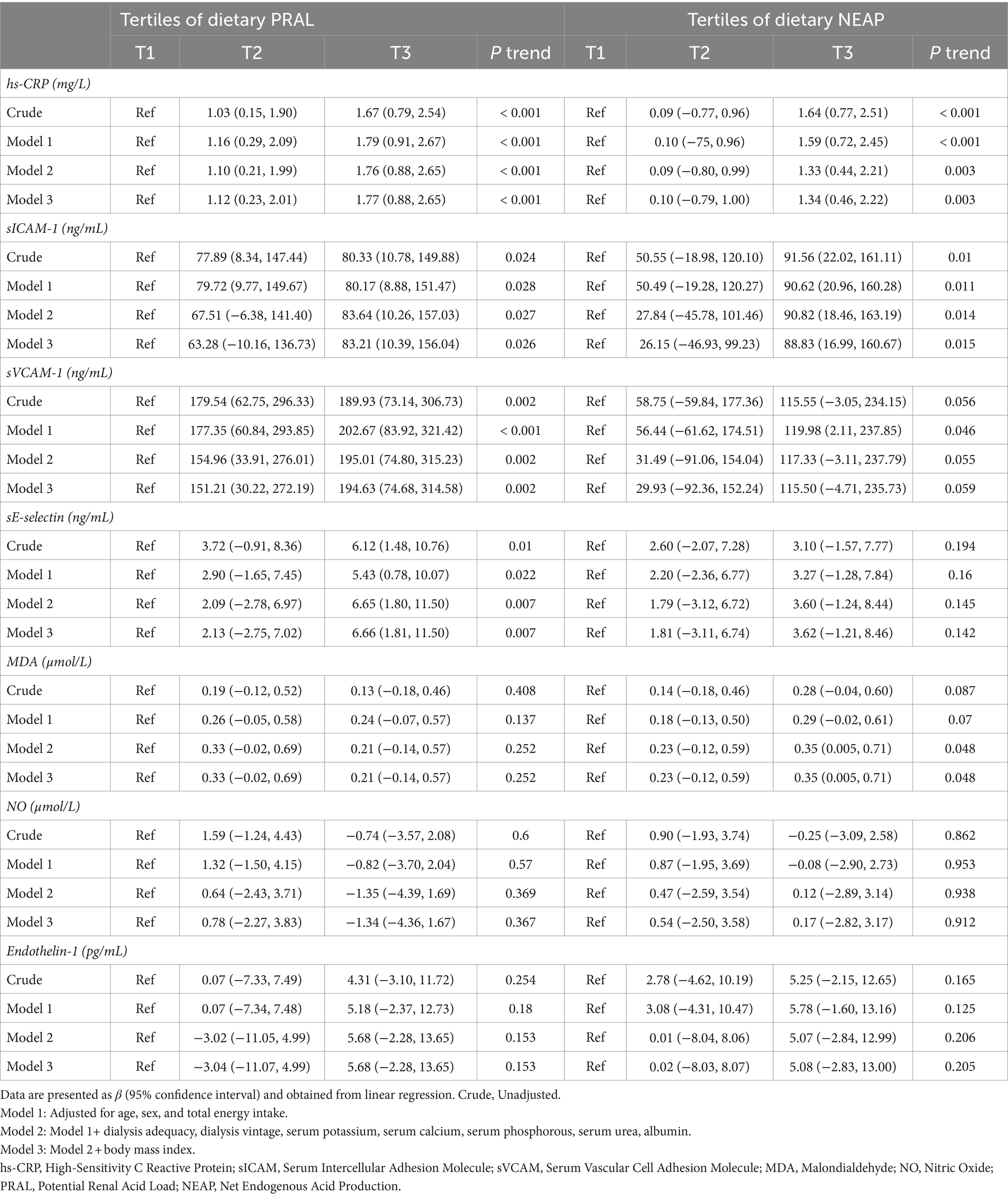
Table 3. Beta (β) and 95% confidence intervals for biomarkers of inflammation and oxidative stress across tertiles of dietary PRAL and NEAP.
OR and 95% CIs for parameters of malnutrition across tertiles of PRAL and NEAP are summarized in Table 4. Although no significant association was found between PRAL and SGA (OR = 1.35, 95%CI: 0.76, 2.42; Ptrend = 0.303) in the crude model, this association became marginally significant after controlling for age, sex, energy intake, dialysis adequacy, dialysis vintage, serum potassium, serum calcium, serum phosphorous, and serum urea (OR = 1.98, 95%CI: 0.95, 4.11; Ptrend = 0.065). Moreover, DMS had a marginally significant positive relationship with PRAL (OR = 1.94, 95%CI: 0.92, 4.05; Ptrend = 0.077) and NEAP (OR = 2.01, 95%CI: 0.93, 4.31; Ptrend = 0.072) in fully adjusted models. No significant associations were found between dietary PRAL or NEAP and MIS either before or after adjustment for confounders.
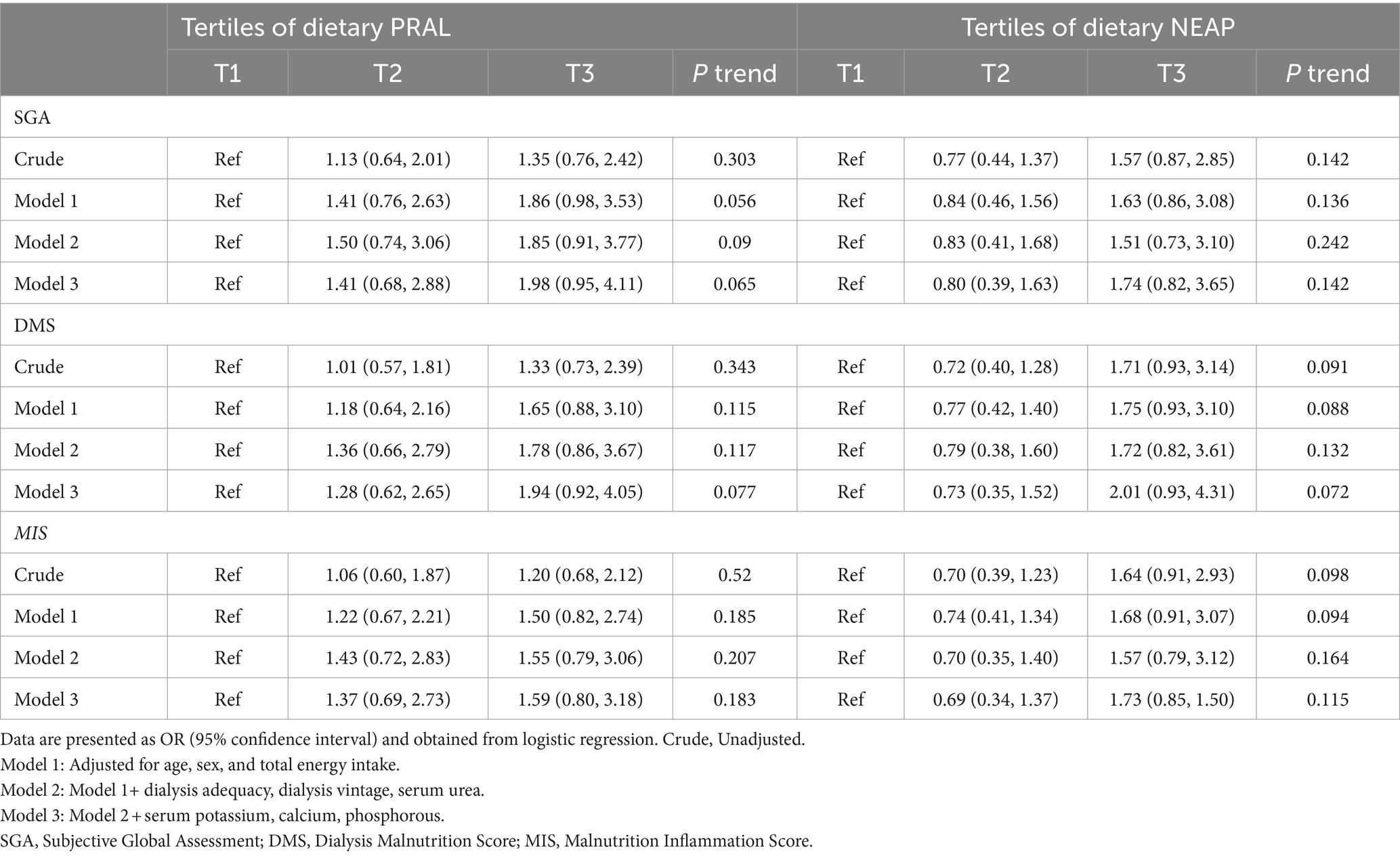
Table 4. Odds ratios (OR) and 95% confidence intervals for parameters of malnutrition across tertiles of dietary PRAL and NEAP.
Discussion
To the best of our knowledge, this is the first study examining the association between DAL and inflammatory and malnutrition markers in maintenance HD patients. The results of the current study revealed that hs-CRP, sICAM-1, sVCAM-1, and sE-selectin were positively associated with PRAL in subjects with higher scores of PRAL than lower scores of PRAL. These associations remained significant after controlling potential confounders. Additionally, each unit increase in NEAP score was correlated with 1.34, 88.83, and 0.35 times higher serum levels of hs-CRP, sICAM-1, and MDA, respectively. Therefore, this study supports the idea that DAL is independently associated with inflammation. Greater PRAL scores were also correlated with higher odds of SGA and DMS. Moreover, NEAP was linked with a 2.01 times higher chance of DMS.
A potential issue with our findings is the reverse causation hypothesis, meaning the association we studied could exist in the opposite direction. It is worth considering that HD patients should intake 1–1.2 g/kg/day of high biological value proteins such as meat, eggs, and fish (acidic sources) and also consume fewer fruits and vegetables (alkaline foods), which could lead to high DAL scores in these patients; thus, a bidirectional association may exist between DAL and HD outcomes. However, this is only a possibility, and these patients should not be advised to drastically reduce protein intake in order to reduce DAL. Future longitudinal and prospective studies are warranted to investigate the aforementioned correlation.
Previous studies investigated the association between different dietary factors and inflammation and oxidative stress in HD patients (18, 19). A growing body of evidence suggests that DAL is associated with inflammation in other populations (20, 21). Confirming the hypothesis that DAL and inflammation are correlated in HD patients, the present study indicates that patients with the highest DAL scores consumed significantly higher amounts of protein and had greater increases in their levels of inflammatory biomarkers than those with the lowest DAL scores. A limited number of studies have investigated the effects of acidic foods on inflammation and oxidative stress status in HD patients. A clinical trial of 92 HD patients who took 45 grams of whey protein per week observed a significant reduction in hs-CRP and MDA levels after an 8-week intervention (22). Whey protein increases anti-inflammatory and antioxidant activity by scavenging free radicals and improving inflammatory pathways. But, when consumed in high quantities, whey protein can induce inflammatory processes (22). Thus, in the present study, higher protein intakes of participants with the highest DAL scores may also be due to higher intakes of whey protein, which could result in higher level of inflammatory biomarkers. However, a pilot study on 14 chronic HD patients aged 16–71 years showed that red meat snacks (27 grams of protein per day) for 30 days did not affect CRP levels in these patients (23). The discrepancy found with the current study could be related to the differences in the study design, study population, and various potential covariates. Moreover, in a cross-sectional study by Wu et al. conducted on 104 HD patients, there was no association between serum CRP levels and higher intakes of red and processed meats (24). The null findings may be clarified by two potential explanations. One possibility is the mild inflammatory status of HD patients since only 33% of them had CRP concentrations of >5 mg/L. Another potential explanation is their small sample size.
Previous studies found a significant association between inflammation and malnutrition in HD patients (25–27). In this study, a higher predisposition to malnutrition was observed in participants with higher levels of inflammatory biomarkers (T3 vs. T1 of PRAL and NEAP). Accordingly, our findings could also support the positive link between DAL and malnutrition in HD patients. Although these associations were marginally significant, it is possible that larger sample sizes would result in a stronger statistically significant relationship. Despite higher dietary energy and protein intake, subjects in the third tertile of PRAL and NEAP had poor nutritional status when compared to those in the first tertile. Moreover, in this study, lower intakes of total fiber, MUFA, magnesium, vitamin C, and folic acid were observed in participants in the top tertiles of PRAL and NEAP. Taken together, lower intakes of these nutrients could worsen malnutrition status in patients with the highest DAL score. Thus, the contribution of both micro- and macronutrients to nutritional status is important for the management of malnutrition in HD patients. Previous surveys on the association between protein intake and malnutrition status in HD patients reported conflicting results. In a case–control study of 94 stable HD patients compared to 52 healthy individuals, there was no evidence of poor nutritional status despite low protein intake (28). However, Akhlaghi et al. observed a mild-to-moderate malnutrition status in HD patients with lower intakes of protein (29). Moreover, another observational study indicated an inverse association between lower protein intake and malnutrition status in HD patients (30). This inconsistency may be associated with differences in eating habits, statistical methods, and outcome and exposure evaluation measures.
Previous studies demonstrated that the total energy and protein intakes of HD patients were noticeably low compared to recommendations (31, 32). Similarly, in this study, intakes of energy (24.9 ± 10.1 kcal/kg/day) and protein (0.64 ± 0.4 g/kg/day) among participants were noticeably low. It is worth considering that subjects may have underreported energy intake due to the use of a recall-based questionnaire for the assessment of dietary intake. Moreover, we observed that participants in the third tertile of DAL consumed higher amounts of energy compared to the first tertile. There are several explanations regarding this finding. First, diets with higher acid loads are often associated with specific dietary patterns, such as higher intake of animal protein and lower intake of fruits and vegetables. These dietary patterns may contribute to higher overall energy intake. Second, the metabolic breakdown of certain nutrients, such as proteins and sulfur-containing amino acids, can contribute to an acidic environment. Foods that are rich in these nutrients might also be energy-dense, further contributing to a higher energy intake. Additionally, the overall impact of dietary acid load on health is a complex and debated topic within the scientific community. It’s advisable to consider various factors, including overall dietary patterns and lifestyle, rather than focusing solely on a single aspect like dietary acid load.
The exact mechanism for the association between DAL and HD outcomes is still unclear. However, some previous animal and experimental investigations may help to explain our findings. These previous studies observed that acidosis causing tissue damage can induce the expression of inflammatory molecules (e.g., nitric oxide synthases), elevate the activity of inflammatory enzymes (e.g., myeloperoxidase), and increase the level of inflammatory cytokines, like tumor necrosis factor α (TNF-α) (21, 33). Additionally, a higher secretion of pro-inflammatory cytokines, such as interleukin 6, and a lower secretion of anti-inflammatory cytokines, such as interleukin 10, were observed in HD patients with metabolic acidosis (34). Acidosis can affect oxidative stress through incremental free radical formation by H(+)-dependent reactions, and iron released from the binding of protein following decrements in pH catalyzes these reactions (35). Furthermore, acidosis diminishes the level of some antioxidants such as glutathione (35, 36) as well as the activity of antioxidant enzymes (37). Previous reports suggest that acidosis is linked with malnutrition in HD patients through an increase in protein catabolism and a decrease in protein synthesis, causing inflammation and insulin resistance, and also diminishing leptin levels (5, 38).
While the current study is novel in exploring the association between DAL and inflammation, oxidative stress, and malnutrition indices in maintenance HD patients as well as considering a comprehensive profile of inflammation and malnutrition concerning DAL, the results should be interpreted with caution due to some limitations. First, due to the cross-sectional design of the present study, no causal relationship can be inferred. Second, the true habitual food consumption of participants was likely underestimated with the 4-day dietary recall. Third, since the present study was conducted on a small sample of HD patients, its generalizability to all patients should be interpreted with caution. Fourth, although several potential confounders were controlled for in our analyzes, the effects of residual confounders cannot be excluded from our results. Finally, the high prevalence of malnutrition and inflammation in this population may lead to an overestimation of the effect size.
We recommend further large-scale studies, especially prospective clinical trials, to validate and explore potential causal relationships. It is crucial to address the following considerations: firstly, employing a 7-day food diary or extending the recall period to assess patients’ typical dietary intake; secondly, accounting for potential confounders such as medications and other dietary factors in the analysis; and thirdly, assessing the body composition and muscle mass of hemodialysis (HD) patients to provide a comprehensive understanding of the observed associations.
Conclusion
In the current study, high DAL according to PRAL and NEAP scores had a positive significant association with hs-CRP and sICAM-1. Moreover, PRAL scores were positively associated with sVCAM-1 and sE-selectin. NEAP scores also had a positive correlation with MDA. Additionally, high PRAL scores were associated with a higher likelihood of SGA and DMS. A positive correlation was also observed between high NEAP scores and DMS. However, it should not be inferred from our results that HD patients should be recommended to significantly reduce protein intake or increase fruit/vegetable consumption in order to alleviate DAL score and subsequent inflammation and malnutrition. There is a need for longitudinal studies to be conducted to further understand our cross-sectional results and to elucidate potential underlying mechanisms.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to YWFyYWIxQGJ3aC5oYXJ2YXJkLmVkdQ==.
Ethics statement
The studies involving humans were approved by local Ethics Committee of Shahid Beheshti University of Medical Sciences and Health Services. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AB: Writing – original draft, Writing – review & editing. MN: Writing – review & editing. HT: Conceptualization, Data curation, Methodology, Writing – review & editing. AtA: Conceptualization, Methodology, Writing – review & editing. ArA: Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rezende, LR, Souza, PBD, Pereira, GRM, and Lugon, JR. Metabolic acidosis in hemodialysis patients: a review. Braz J Nephrol. (2017) 39:305–11. doi: 10.5935/0101-2800.20170053
2. Thurlow, JS, Joshi, M, Yan, G, Norris, KC, Agodoa, LY, Yuan, CM, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. (2021) 52:98–107. doi: 10.1159/000514550
3. Kalantar-Zadeh, K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: what is next? Semin Dial. (2005) 18:365–9. doi: 10.1111/j.1525-139X.2005.00074.x
4. Kalantar-Zadeh, K, Ikizler, TA, Block, G, Avram, MM, and Kopple, JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. (2003) 42:864–81. doi: 10.1016/j.ajkd.2003.07.016
5. Kalantar-Zadeh, K, Mehrotra, R, Fouque, D, and Kopple, JD. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. (2004) 17:455–65. doi: 10.1111/j.0894-0959.2004.17606.x
6. Noce, A, Marrone, G, Wilson Jones, G, Di Lauro, M, Pietroboni Zaitseva, A, Ramadori, L, et al. Nutritional approaches for the management of metabolic acidosis in chronic kidney disease. Nutrients. (2021) 13:2534. doi: 10.3390/nu13082534
7. Remer, T, Dimitriou, T, and Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. (2003) 77:1255–60. doi: 10.1093/ajcn/77.5.1255
8. Frassetto, LA, Todd, KM, Morris, RC Jr, and Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. (1998) 68:576–83. doi: 10.1093/ajcn/68.3.576
9. Van Den Berg, E, Engberink, MF, Brink, EJ, Van Baak, MA, Joosten, MM, Gans, RO, et al. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol. (2012) 7:1811–8. doi: 10.2215/CJN.04590512
10. Van Den Berg, E, Hospers, F, Navis, G, Engberink, MF, Brink, EJ, Geleijnse, JM, et al. Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in westernized south Asian people. Group. (2011) 24:11–7. doi: 10.5301/JN.2010.5711
11. Haghighatdoost, F, Sadeghian, R, Clark, CC, and Abbasi, B. Higher dietary acid load is associated with an increased risk of calcium oxalate kidney stones. J Ren Nutr. (2021) 31:467–74. doi: 10.1053/j.jrn.2020.08.012
12. Ronco, A, Storz, M, Martinez-Lopez-W, CJ, and Golomar, W. Dietary acid load and risk of kidney cancer: an epidemiologic case-control study. World Cancer Res J. (2021) 8:e2096 doi: 10.32113/wcrj_20219_2096
13. Therrien, M, Byham-Gray, L, and Beto, J. A review of dietary intake studies in maintenance dialysis patients. J Ren Nutr. (2015) 25:329–38. doi: 10.1053/j.jrn.2014.11.001
14. Ronco, AL, Martínez-López, W, Calderón, JM, and Golomar, W. Dietary acid load and lung cancer risk: a case-control study in men. Cancer Treat Res Commun. (2021) 28:100382. doi: 10.1016/j.ctarc.2021.100382
15. Desbrow, B, Bauer, J, Blum, C, Kandasamy, A, Mcdonald, A, and Montgomery, K. Assessment of nutritional status in hemodialysis patients using patient-generated subjective global assessment. J Ren Nutr. (2005) 15:211–6. doi: 10.1053/j.jrn.2004.10.005
16. Steiber, A, Leon, JB, Secker, D, Mccarthy, M, Mccann, L, Serra, M, et al. Multicenter study of the validity and reliability of subjective global assessment in the hemodialysis population. J Ren Nutr. (2007) 17:336–42. doi: 10.1053/j.jrn.2007.05.004
17. Harvinder, GS, Swee, WCS, Karupaiah, T, Sahathevan, S, Chinna, K, Ahmad, G, et al. Dialysis malnutrition and malnutrition inflammation scores: screening tools for prediction of dialysis-related protein-energy wasting in Malaysia. Asia Pac J Clin Nutr. (2016) 25:26–33. doi: 10.6133/apjcn.2016.25.1.01
18. Nguyen, TTU, Kim, HW, and Kim, W. Effects of probiotics, prebiotics, and synbiotics on uremic toxins, inflammation, and oxidative stress in hemodialysis patients: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. (2021) 10:4456. doi: 10.3390/jcm10194456
19. Stockler-Pinto, MB, Mafra, D, Moraes, C, Lobo, J, Boaventura, GT, Farage, NE, et al. Brazil nut (Bertholletia excelsa, HBK) improves oxidative stress and inflammation biomarkers in hemodialysis patients. Biol Trace Elem Res. (2014) 158:105–12. doi: 10.1007/s12011-014-9904-z
20. Mousavi, M, Jahromi, SR, Togha, M, Ghorbani, Z, Hekmatdoost, A, Rafiee, P, et al. The association between dietary acid load and odds of migraine: A case–control survey. Neurol Therap. (2021) 10:335–48. doi: 10.1007/s40120-021-00247-2
21. Wu, T, Seaver, P, Lemus, H, Hollenbach, K, Wang, E, and Pierce, JP. Associations between dietary acid load and biomarkers of inflammation and hyperglycemia in breast cancer survivors. Nutrients. (2019) 11:1913. doi: 10.3390/nu11081913
22. Sohrabi, Z, Eftekhari, MH, Eskandari, MH, Rezaianzadeh, A, and Sagheb, MM. Intradialytic oral protein supplementation and nutritional and inflammation outcomes in hemodialysis: a randomized controlled trial. Am J Kidney Dis. (2016) 68:122–30. doi: 10.1053/j.ajkd.2016.02.050
23. Maduro, IPDNN, Nonino, CB, Sakamoto, LM, Meirelles, MG, Cardeal Da Costa, JA, and Marchini, JS. Red meat snacks for chronic hemodialysis patients: effect on inflammatory activity (a pilot study). Ren Fail. (2013) 35:830–4. doi: 10.3109/0886022X.2013.794659
24. Wu, P-Y, Yang, S-H, Wong, T-C, Chen, T-W, Chen, H-H, Chen, T-H, et al. Association of processed meat intake with hypertension risk in hemodialysis patients: a cross-sectional study. PLoS One. (2015) 10:e0141917. doi: 10.1371/journal.pone.0141917
25. Arab, A, Golpour-Hamedani, S, Tabibi, H, and As’habi, A. Association between inflammatory potential of diet and markers of malnutrition in haemodialysis patients. Br J Nutr. (2023) 129:1820–6. doi: 10.1017/S0007114522002574
26. Arab, A, Karimi, E, Nazari, M, Tabibi, H, and As’habi, A. Association between the dietary inflammatory index and markers of endothelial and systemic inflammation in hemodialysis patients. Front Nutr. (2023) 10:1230747. doi: 10.3389/fnut.2023.1230747
27. Yao, Q, Lindholm, B, and Stenvinkel, P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodial Int. (2004) 8:118–29. doi: 10.1111/j.1492-7535.2004.01085.x
28. Cupisti, A, D'alessandro, C, Valeri, A, Capitanini, A, Meola, M, Betti, G, et al. Food intake and nutritional status in stable hemodialysis patients. Ren Fail. (2010) 32:47–54. doi: 10.3109/08860220903391234
29. Akhlaghi, Z, Sharifipour, F, Nematy, M, Safarian, M, Malekahmadi, M, Barkhidarian, B, et al. Assessment of nutritional status in maintenance hemodialysis patients: A multicenter cross-sectional study in Iran. Semin Dial. (2021) 34:77–82. doi: 10.1111/sdi.12917
30. Maigoda, TC, Maisyorah, E, Krisnasary, A, and Ardiansyah, S. Energy, protein, and potassium intake with nutritional status among chronic renal failure patients undergoing hemodialysis in hospital Dr. M. Yunus, Bengkulu, Indonesia. Ann Trop Med Public Health. (2020) 23:1292–300. doi: 10.36295/ASRO.2020.23816
31. Kalantar-Zadeh, K, Kopple, JD, Deepak, S, Block, D, and Block, G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. (2002) 12:17–31. doi: 10.1053/jren.2002.29598
32. Kim, H, Lim, H, and Choue, R. A better diet quality is attributable to adequate energy intake in hemodialysis patients. Clin Nutr Res. (2015) 4:46–55. doi: 10.7762/cnr.2015.4.1.46
33. Giugliano, D, Ceriello, A, and Esposito, K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. (2006) 48:677–85. doi: 10.1016/j.jacc.2006.03.052
34. Kraut, JA, and Madias, NE. Adverse effects of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis. (2017) 24:289–97. doi: 10.1053/j.ackd.2017.06.005
35. Rustom, R, Wang, B, Mcardle, F, Shalamanova, L, Alexander, J, Mcardle, A, et al. Oxidative stress in a novel model of chronic acidosis in LLC-PK1 cells. Nephron Exp Nephrol. (2003) 95:e13–23. doi: 10.1159/000073019
36. Lewerenz, J, Dargusch, R, and Maher, P. Lactacidosis modulates glutathione metabolism and oxidative glutamate toxicity. J Neurochem. (2010) 113:502–14. doi: 10.1111/j.1471-4159.2010.06621.x
37. Ying, W, Han, SK, Miller, JW, and Swanson, RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. (1999) 73:1549–56. doi: 10.1046/j.1471-4159.1999.0731549.x
38. Sajgure, AD, Dighe, TA, Korpe, JS, Bale, CB, Sharma, AO, Shinde, NS, et al. The relationship between metabolic acidosis and nutritional parameters in patients on hemodialysis. Indian J Nephrol. (2017) 27:190–4. doi: 10.4103/0971-4065.202404
Glossary
Keywords: acid load, nutrition, hemodialysis, malnutrition, inflammation
Citation: Balali A, Nehls MS, Tabibi H, As’habi A and Arab A (2024) Dietary acid load and markers of malnutrition, inflammation, and oxidative stress in hemodialysis patients. Front. Nutr. 11:1369206. doi: 10.3389/fnut.2024.1369206
Edited by:
Roberta Zupo, University of Bari Aldo Moro, ItalyReviewed by:
Antonietta Gigante, Sapienza University of Rome, ItalyRongshao Tan, Guangzhou Red Cross Hospital, China
Ines Panjkota Krbavcic, University of Zagreb, Croatia
Copyright © 2024 Balali, Nehls, Tabibi, As’habi and Arab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atefeh As’habi, YXNoYWJpX251dHJpdGlvbkB5YWhvby5jb20=; Arman Arab, YWFyYWIxQGJ3aC5oYXJ2YXJkLmVkdQ==
 Arghavan Balali
Arghavan Balali Marilyn S. Nehls3
Marilyn S. Nehls3 Hadi Tabibi
Hadi Tabibi Atefeh As’habi
Atefeh As’habi Arman Arab
Arman Arab