- 1Department of Diagnostics and Public Health, University of Verona, Verona, Italy
- 2Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy
- 3Scuola Provinciale Superiore di Sanità, Bolzano, Italy
- 4Department of General and Pancreatic Surgery, University of Verona, Verona, Italy
Background and aims: Postoperative ileus is a frequent condition, leading to complications and a longer hospital stay. Few studies have demonstrated the benefit of early oral feeding in preventing ileus after gastrointestinal surgery. This study aims to evaluate the efficacy of early versus delayed oral feeding on the recovery of intestinal motility, length of hospital stay, and complications.
Methods: We conducted a systematic review and meta-analysis of randomized control trials, searching PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, and the ClincalTrials.gov until 31 December 2022. We evaluated the first passage of the stool, the first flatus, complications, length of postoperative stay, and vomiting. We assessed the risk of bias using the Cochrane risk of bias tool (version 2) for randomized trials and the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation methodology.
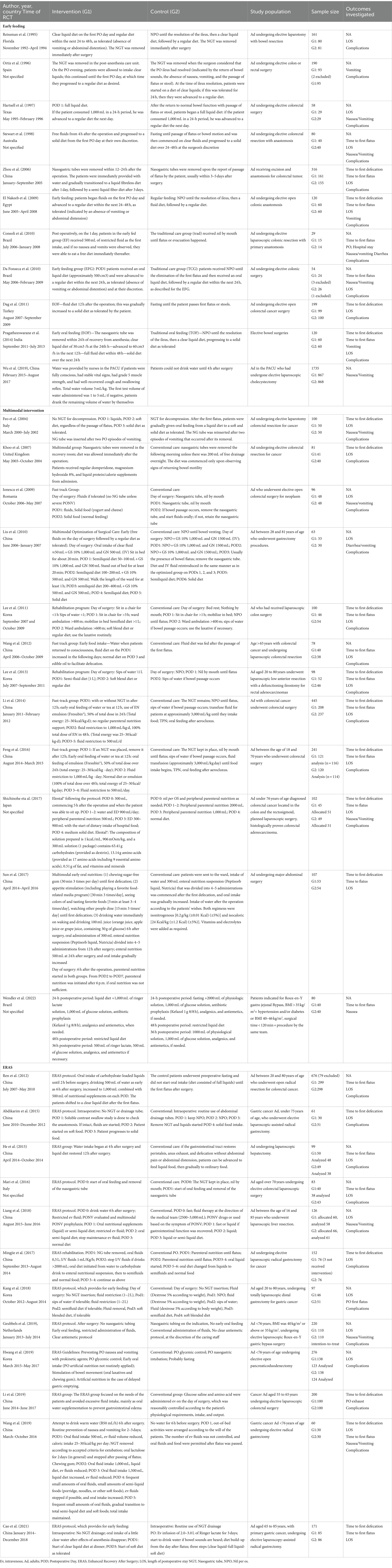
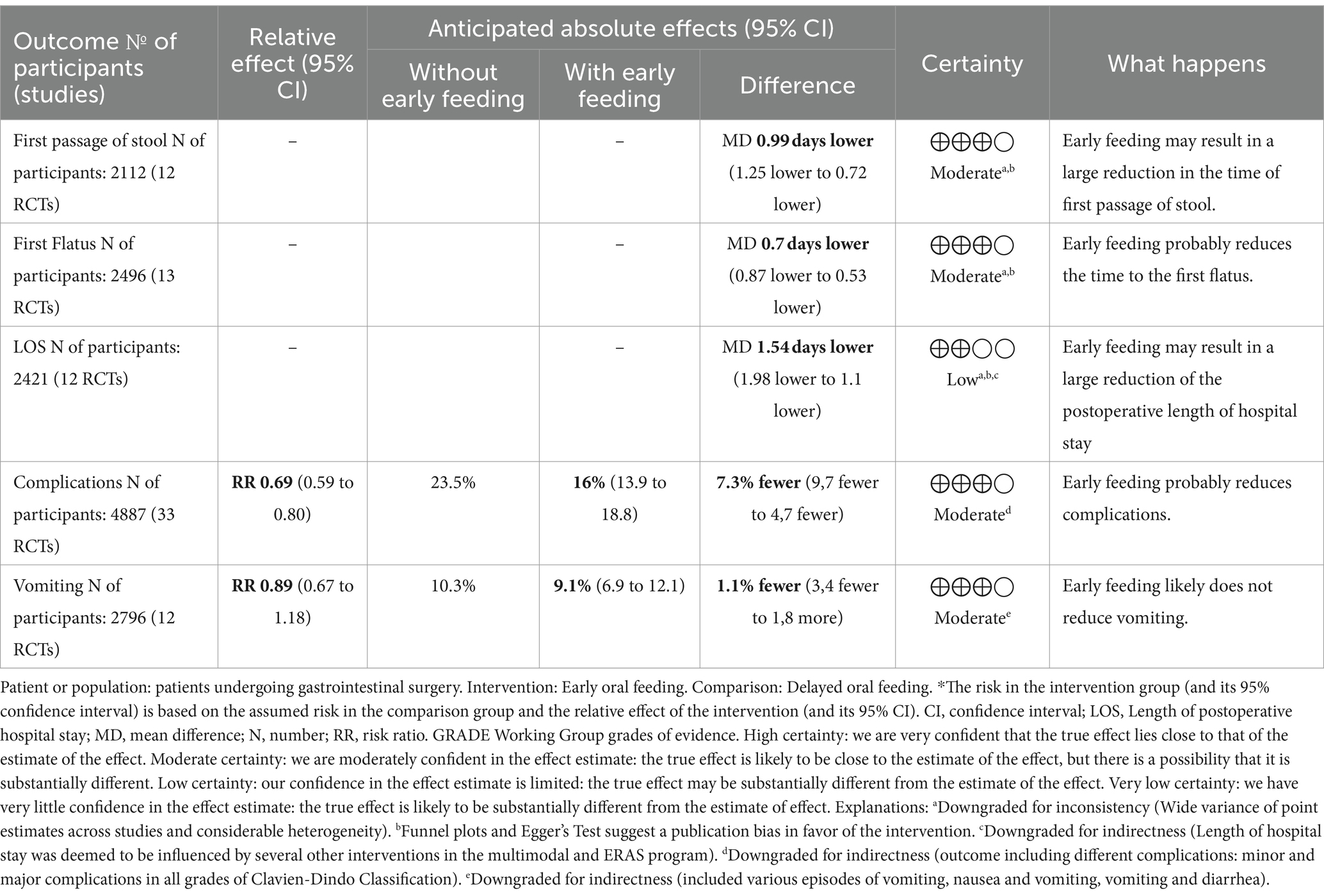
Results: We included 34 studies with a median sample size of 102 participants. With a moderate certainty of the evidence, the early oral feeding may reduce the time taken for the first passage of the stool (MD −0.99 days; CI 95% −1.25, −0.72), the first flatus (MD −0.70 days; CI 95% -0.87, −0.53), and the risk of complications (RR 0.69; CI 95% 0.59–0.80), while with a low certainty of evidence, it may reduce the length of stay (MD −1.31 days; CI 95% −1.59, −1.03). However, early feeding likely does not affect the risk of vomiting (RR 0.90; CI 95% 0.68, 1.18).
Conclusion: This review suggests that early oral feeding after gastrointestinal surgery may lead to a faster intestinal recovery, shorter postoperative stays, and fewer complications. However, careful interpretation is needed due to high heterogeneity and the moderate-to-low quality of evidence. Future studies should focus on the type and starting time of early oral feeding.
1 Introduction
Postoperative ileus (POI) is an iatrogenic condition after gastrointestinal surgery, defined as transient deceleration or cessation of intestine motility due to a chain reaction caused by the surgical intervention and the manipulation of the digestive tract (1, 2). Indeed, POI results from a pathophysiological process mainly distinguished in an early neurogenic phase that suppresses enteric neural reflex pathways and a second immunological and inflammatory phase that usually leads to prolonged POI (2, 3).
This condition can manifest with multiple symptoms, such as intolerance to oral intake, nausea, vomiting, and failure to pass flatus or stool (4, 5). No consensus is currently available on the time of physiological restoration of normal intestinal motility (4, 6). However, some studies report that the reappearance of bowel sounds, passing of gas or stool, and tolerance to food and fluids indicate POI resolution (5, 7).
Despite several pathophysiological and treatment studies available in the literature, POI is still a common condition after gastrointestinal surgery, with an estimated prevalence ranging from 17 to 80% (8). Different studies have demonstrated the association between POI and increased health complications, such as nutritional requirements and protein deficiency, pneumonia, anastomotic failure, renal and hepatic failure, delayed autonomy recovery, and mortality (6, 9–11), leading to prolonged hospital stay and readmission (6). This results in high healthcare costs in radiology, laboratory, staffing, and medication costs (12).
Different interventions have been developed and tested over the years to reduce this phenomenon, focusing on every phase of the surgical intervention: preoperative, perioperative, and postoperative periods. The interventions refer to the use of minimally invasive procedures (13), along with proper perioperative fluid management based on “Goal-Directed Therapy” (14), and the use of opioid-free local epidural anesthetics (15), with a demonstrated effectiveness for POI prevention. In addition, the “enhanced recovery after surgery” (ERAS) program, which has been intensively studied and globally recognized for its positive effects in accelerating postoperative recovery (16–18), strongly recommends early feeding strongly during the postoperative phase (19). Early feeding by mouth prevents significant metabolic changes such as insulin resistance (20) and facilitates surgical wound healing. Moreover, compared to parenteral nutrition, it enables the gastrointestinal system to regain its functions faster by stimulating motility and accelerating the first passage of flatus and stool (21). Although early oral feeding might be considered a safe intervention for POI prevention, there is no conclusive evidence of its safety for gastrointestinal function and postoperative complications due to different aspects (9, 22). For instance, a standard definition of “early feeding” is currently missing, and the type of feeding reported in the studies has been poorly described. For example, it is unclear whether the feeding involves liquids or solid food. Consequently, the guidelines do not provide clear indications regarding when and what to administer in the early feeding phase. As a result, the indications may vary widely across surgical settings and cultures. In addition, the direct association between early feeding and POI has been partially investigated. Available reviews have considered feeding as a component of ERAS and multimodal programs (23) or as a single intervention (22, 24–30). Therefore, no single review has yet determined the impact of early oral feeding as a single or a combined intervention.
Moreover, most previous systematic reviews considered only colorectal (23, 26), lower (22), upper gastrointestinal surgery (24, 25), or cancer indication to surgery (24) and assessed the impact of early feeding on outcome such as the length of stay (LOS), complications (22, 25), or nutritional status (24). In two reviews with POI as the primary outcome (26, 27), the POI was measured only by time to first flatus or bowel movement, the intervention was focused only on diet as a single intervention (26) or fluids (27), and the last search on databases was in June 2019 (26) and September 2020 (27).
Therefore, this study aims to evaluate the efficacy of early versus delayed oral feeding on the recovery of intestinal motility as the primary outcome to fill the gaps described above.
2 Materials and methods
We performed a systematic review and meta-analysis according to the Cochrane guidelines (31) and reported it following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (32). We searched PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials (CENTRAL), and the ClincalTrials.gov register from inception to 31 December 2022. We also searched System for Information on Grey Literature (SIGLE) to identify further studies or papers that were not published, checked the references of articles included and relevant reviews on the topic, and contacted the corresponding authors to clarify doubts and consider unpublished data. The search in the databases and registries was conducted using both free texts and MeSH and EMTREE terms by adopting the search strings reported in Supplementary File S1. We prospectively registered the protocol in the International Prospective Register of Systematic Reviews (CRD42022298777) and published it (with references blinded for the reviewer).
2.1 Inclusion and exclusion criteria
We included randomized clinical trials (RCTs) that met the following inclusion criteria: (i) aimed at comparing the effect of early postoperative (fluids and food by mouth within 24 h) versus delayed oral feeding; (ii) treated patients >18 years of age undergoing both elective and emergency gastrointestinal surgery; (iii) assessed intestinal recovery outcomes, complications, and LOS after gastrointestinal surgery; (iv) published in English, Italian, and German. The primary outcome is the time to the first passage of stool, while secondary outcomes include the time to first flatus, LOS, and any negative effects, such as nausea, vomiting, infection, organ failure, and major complications, as classified according to the Clavien-Dindo Classification (33). Studies were excluded if the intervention involved the exclusive use of the nasogastric tube. Moreover, studies referring to patients treated for bariatric surgery, appendectomy, and hemorrhoid surgery were excluded. Studies involving gynecological procedures were also excluded.
2.2 Selection process
The records identified through the search methods were transferred to Excel® (Microsoft Corporation, Redmond, WA) spreadsheets and then uploaded to Covidence. First, two review authors (blinded for review) independently screened titles and abstracts and then performed full-text revision. A third review author (blinded for review) resolved any disagreements.
2.3 Data extraction and management
For each study, data were extracted by two independent authors using electronic data collection forms in Covidence. The extracted data included article references (first author, journal, and year), setting, research methods (study design, total duration of the study, and washout period), type of surgery (emergency or elective surgery); participant characteristics (age and sex), intervention (experimental and control), study’s primary and secondary outcomes, main results, and free notes. We dealt with missing data by contacting the authors of the trials to retrieve relevant information.
2.4 Risk of bias assessment
Two independent reviewers performed the quality and risk of bias assessment of the included studies using the revised Cochrane risk of bias tool for randomized trials (RoB2) (34), and a third reviewer solved any disagreements. The risk of bias in each study was classified as high, low, or moderate according to the overall grade agreed upon by the reviewers (34).
2.5 Data analysis
The mean difference (MD) with 95% confidence intervals (CIs) was calculated to estimate the effect size of the continuous variables, including the first passage of the stool, first flatus, and LOS. The risk ratio with 95% CI was calculated to estimate the risk likelihood of incurring postoperative complications and vomiting episodes. The risk of complication was defined based on the number of patients who underwent at least one postoperative complication, since it was not possible to classify the complications according to the Clavien-Dindo Classification due to missing information in most of the articles.
A random-effect meta-analysis was conducted for all outcomes, considering the differences in intervention characteristics identified during the data collection and extraction process.
The statistical heterogeneity was assessed by visual inspection of the forest plot and applying the I2 statistic with the Q statistic test. Values greater than 75% were considered as expressing considerable heterogeneity (31).
We performed two subgroup analyses to explore the source of considerable heterogeneity according to the types of interventions and the surgery site. Types of interventions were classified into two categories, namely “early feeding” and “multimodal interventions or ERAS interventions.” The category of multimodal interventions includes studies that investigated programs composed of early feeding and other elements, such as fast-track, preoperative routine changes in feeding, and early mobilization. Regarding the surgery sites, we grouped studies into two categories, one targeting only patients undergoing colon and rectal surgery, while the other including a broader site definition (bowel and abdominal surgeries) or different sites (gastric surgery).
To corroborate the results of the overall analysis, a sensitivity analysis was performed by removing studies at high risk of bias and studies with a sample smaller than 100 participants.
We assessed the publication bias through the funnel plot inspections, and for continuous outcomes, we assessed using Egger’s test. The analysis was performed with RevMan 5.4 (35) and R software (36). The results reported in the included studies as median and interquartile ranges were described narratively.
2.6 Summary of evidence
The quality of evidence was evaluated for all outcomes by adopting the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group methodology (37). The level of evidence certainly was considered ‘high’, ‘moderate’, ‘low’, or ‘very low’ based on the risk of bias, inconsistency, indirectness, imprecision, publication bias, and additional domains.
3 Results
3.1 Characteristics of studies
After removing duplicates, we retrieved 6,490 records, of which 35 (38–72) were included (Figure 1; Supplementary File S2: List of excluded studies). The majority of RCTs were conducted in China (15, 44.1%), followed by Korea (4, 11.8%) (Table 1). The median sample size of the studies was 101 participants (IQR, 80–185, min =29, max = 1735). Furthermore, 12 studies evaluated the ERAS protocol, 11 studies evaluated the effectiveness of early feeding interventions, and the remaining 9 studies evaluated multimodal interventions (Table 1). The multimodal interventions included components similar to the ERAS protocol, for example, diet changes in the preoperative phase (53), the use of chewing gum and appetite stimulation programs (56), and early mobilization (48) (Table 1). Nineteen studies included patients undergoing colon and rectal surgery, while another other eight included patients undergoing gastric surgery. The remaining studies referred to the bowel (50, 71) or abdominal (56) surgery, including hepatectomy (51), liver resection (57), cholecystectomy (51), and pancreaticoduodenectomy (61).
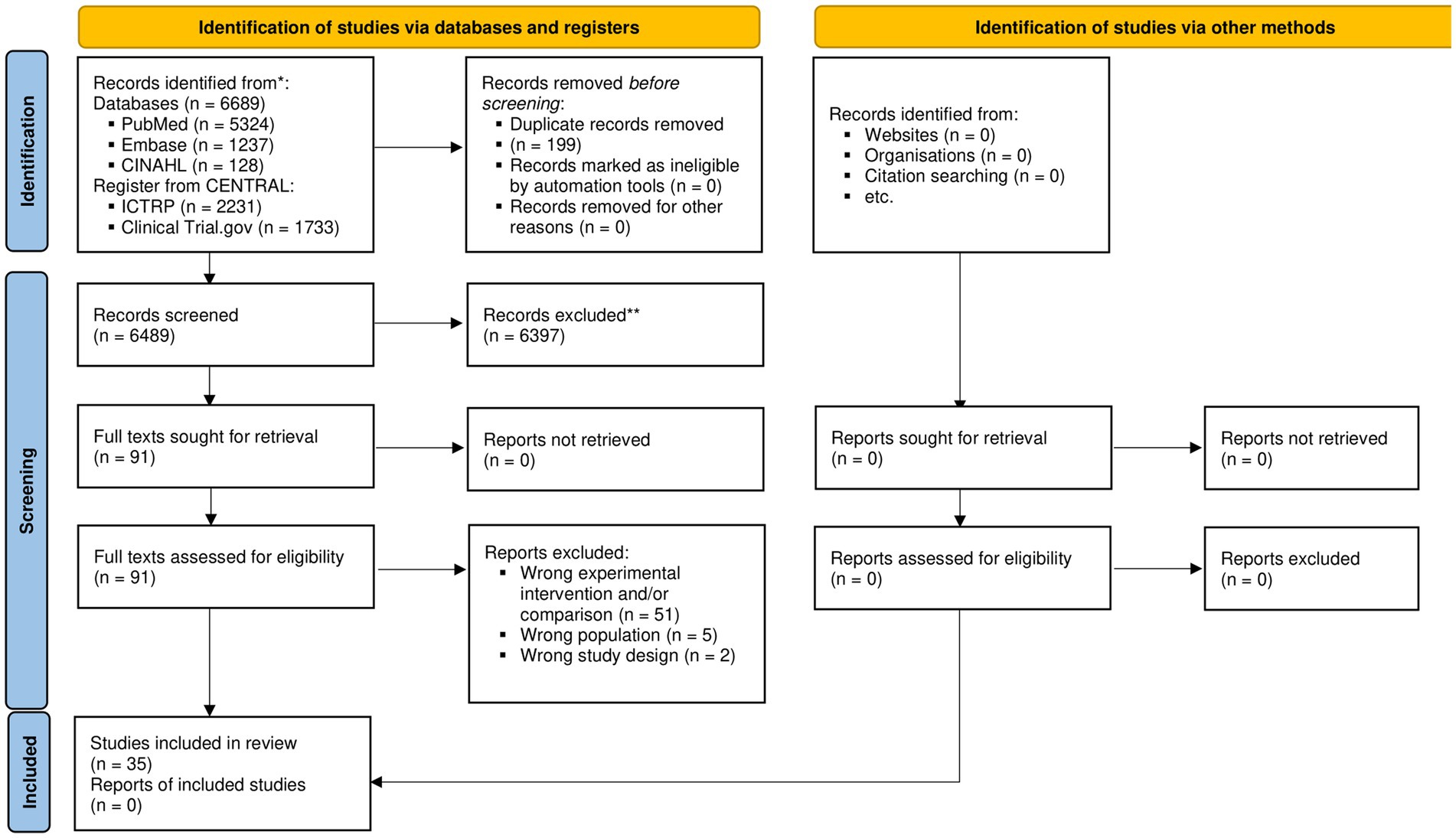
Figure 1. PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases, registers, and other sources. CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; ICTRP, International Clinical Trials Registry Platform.
3.2 Risk of bias and publication bias
Twenty-two studies resulted in an unclear risk of bias due to different reasons: 16 had an unclear randomization process, 13 had unclear information concerning the deviations from the intended interventions, 13 regarding the selection of the results, mainly due to a prespecified protocol not available, and nine regarding the measurement of the outcome (Supplementary File S3). Regarding the remaining articles, seven were judged at low risk of bias, while the other six were at high risk of bias. A high risk of bias was detected in three studies due to issues related to the outcome measurement; in one study, it was due to the randomization process, and in another study, it was due to outcome measurement process deviations from the intended protocol.
The funnel plot (Supplementary File S4) and Egger’s test revealed a strongly suspected publication bias in favor of the intervention for the outcomes “First passage of the stool,” “First flatus,” and LOS, but not for “Complications” and “Vomiting.”
3.3 Outcomes
We have reported the results according to the five outcomes investigated, which are the first passage of stool, the first passage of flatus, LOS, complications, and vomiting.
Furthermore, 17 trials (50%) reported the first passage of stool, 33 studies (94.3%) reported LOS and postoperative complications, 23 studies (65.7%) investigated the first passage of flatus, and 15 studies (47.1%) reported vomiting (Table 1). Meta-analyses were conducted, including 12 studies for the first passage of stool, 13 studies for the first flatus, 12 studies for LOS, 31 studies for complications, and 12 studies for vomiting (Table 2).
3.3.1 First passage of stool
We pooled data from 12 studies out of 17 investigating the first passage of stool, four of which were evaluated as interventions for early oral feeding, while eight were the ERAS program or other multimodal programs. With a moderate certainty of evidence (Table 2), early feeding, whether standalone or within a wider program, may reduce the time to first passage of stool compared to delayed feeding (2,112 patients; MD −0.99 days; CI95% −1.25, −0.72; I2 88%, Supplementary File S5).
Out of the five studies investigating multimodal (40, 48) or ERAS (63) interventions not pooled in the meta-analysis, three reported a statistically significant reduction in the time to first defecation in favor of the intervention (Supplementary File S6).
3.3.2 First passage of flatus
Out of 22 studies (64.7%) evaluating the time to first passage of flatus, 13 provided useful data to be pooled in the meta-analysis. There is moderate certainty of evidence that early feeding, either alone or as part of a larger program, may reduce the time to the first flatus among the intervention group compared to delayed feeding (2,496 patients; MD −0.70 days; CI 95% −0.87, −0.53; I2 85%, Table 2; Supplementary File S7). Seven out of eight studies, which were not pooled in the meta-analysis, showed a statistically significant difference in the time to the first flatus in favor of the intervention (Supplementary File S6). Two of these studies involved early feeding (65, 68), while the remaining used the ERAS program (51, 57, 58, 63) or multimodal interventions (45, 48). One study investigating a multimodal intervention reported no differences between the groups (72).
3.3.3 Los
All but two of the 34 included studies evaluated LOS. Pooled data from 16 studies with low certainty of evidence showed that early feeding, either alone or as part of a larger program, may lead to a reduced postoperative hospital stay (2,819 patients; MD −1.31 days; CI 95% −1.59, −1.03 days; I2 83%, Table 2; Supplementary File S8).
Sixteen studies reported median values, and in half of these studies, early feeding favored the intervention group with statistically significant results (Supplementary File S6), both as a single intervention (45, 65) and when embedded in a multimodal (40, 56) or ERAS program (51, 57, 58, 63).
3.3.4 Complications
Thirty-three studies (94.2%) investigated the occurrence of postoperative complications and were pooled in the meta-analysis to assess the risk likelihood of incurring at least one complication. The most common complications were anastomotic leakage and wound infection (Supplementary File S9). With moderate certainty of the evidence, early feeding, either alone or as part of a larger program, may reduce the risk of incurring at least one complication by 31%, with the risk reduction ranging from 41 to 20% compared to delayed feeding (4,887 participants; RR 0.69; CI 95% 0.59, 0.80; I2 34%, Supplementary File S10A).
3.3.5 Vomiting
Vomiting was reported in 15 studies. Based on a meta-analysis of 13 studies, there is moderate certainty in the evidence that early feeding, either alone or as part of a larger program, has no overall effect on vomiting compared to delayed feeding (2,856 patients; RR 0.90; CI 95% 0.68, 1.18; I2 32%, Supplementary File S10B).
3.4 Subgroup and sensitivity analyses
The subgroup analysis was performed for the first passage of the stool, the first flatus, and LOS due to the significant heterogeneity detected in the overall analysis. The subgroup analysis based the type of the intervention (early oral feeding vs. multimodal/ERAS interventions) revealed no differences in the time to the first passage of the stool (Supplementary Files S11A, S12 studies) and the first flatus (Supplementary Files S12A, S13 studies). However, multimodal or ERAS programs led to a greater reduction in LOS (p = 0.02, Supplementary Files S13A, S16 studies). However, heterogeneity remained high among subgroups for all the outcomes. The subgroup analysis based on the site of intervention (colon and rectal surgery vs. bowel/abdominal/gastric surgery) showed no differences in the outcomes (Supplementary Files S11B–S13B). However, a reduction from an overall considerable (I2 = 85%) to substantial (I2 = 52%) heterogeneity was observed among studies targeting colon and rectal surgery for the outcome “First flatus” (Supplementary File S12B).
All the sensitivity analyses confirmed the results obtained from the overall analysis (Supplementary Files S14–S18).
3.5 Effects on outcomes by the type of oral feeding
A meta-analysis according to the type of oral feeding or start of the oral diet was not possible due to the high heterogeneity among studies. However, a visual representation shows that there is no hypothetical association between the type of oral feeding on the first postoperative day and differences in outcomes (Supplementary File S19).
4 Discussion
4.1 Main findings
To the best of our knowledge, this is the first review assessing the effectiveness of early oral feeding, both alone and within a wider peri-operative program, on the recovery of intestinal motility among patients undergoing gastrointestinal surgery. In 34 trials, our findings suggest that early oral feeding may reduce the time to the first defecation and flatus while reducing the length of hospital stay (LOS) and postoperative complications without increasing the risk of vomiting. Studies support the safety of early oral feeding, whether standalone or within broader programs. The subgroup analysis indicates that the intervention type and the surgery site do not impact its effectiveness in reducing time to first defecation, flatus, and LOS. However, multimodal and ERAS programs show a greater LOS reduction compared to studies on early oral feeding alone, possibly due to additional components such as early mobilization and diverse pain management strategies.
The explanation for the considerable statistical heterogeneity among studies may have been missed due to differences in intervention components, which made it difficult to group the studies. For example, Sun et al. (56) investigated multiple oral feeding strategies, including appetite stimulation programs, drinking juice, enteral nutrition suspension, and the use of chewing gum, all of which demonstrated efficacy in reducing the likelihood of postoperative ileus (POI) (73). Relevant differences in nasogastric tube management were also detected; for example, in some studies, the tube was not positioned (52), removed according to accepted criteria for extubating (60), or removed within 12–24 h after surgery (38, 50). Different types of diet and start timing of fluids and solid food were detected, with or without parenteral nutrition (53) or oral supplements (56, 57). The administration of fluids alone (38) compared with a soft diet on the first postoperative day, as well as administering lactulose (60) or laxatives (61), could also affect the recovery of intestinal motility.
We could not categorize articles by feeding modality due to missing information, terminology variations, and differences in the timing and type of diets. Consequently, recommendations on the most beneficial early oral feeding type for investigated outcomes are unavailable. Despite diverse concepts and timings of “early oral feeding,” all effect estimates for the primary endpoint, first stool passage, consistently favor significance.
In addition, differences in the type of intervention and the underlying pathology could differently impact the incidence and length of POI, LOS, and complications and, therefore, increase the statistical heterogeneity among studies. However, analyzing the first stool passage as a proxy for postoperative ileus (POI), we conducted a subgroup analysis comparing colon-rectal interventions to bowel, abdominal, and gastric surgeries. No outcome differences were observed, affirming the reliability of the results.
Moreover, differences between countries might be relevant in terms of progress in surgery techniques and perioperative management protocols.
Despite the significant heterogeneity among the studies, results support the safe and beneficial transferability of early oral feeding in clinical practice. However, publication bias in favor of early feeding for the time to first defecation, first flatus, and LOS among studies included in the meta-analysis should be considered in interpreting results since it might result in overestimating effects. However, among the studies not included in the meta-analysis, two, one, and eight studies with no statistically significant results were found for the time to the first defecation, first stool, and LOS, respectively. This balances the publication bias, further mitigated by the negative rating assigned by applying the GRADE approach, thus reducing the quality of the evidence. According to the GRADE approach, the results are based on a moderate quality of evidence for the first passage of stool, the first flatus, complications, and vomiting, meaning that we are moderately confident that the true effect is likely to be close to the estimate of the effect. The evidence was rated low quality for the LOS, reducing our confidence in the effect estimate because the true effect may be substantially different.
4.2 Comparison with previous evidence
Our findings were mostly consistent with the evidence available on the time to first stool passage, LOS, and vomiting, while discordant results emerged on complications. We compared our findings with those of five recently published reviews on the effectiveness of early feeding in gastrointestinal surgery (28) (14 studies), digestive tract surgery (29) (11 studies), lower gastrointestinal surgery (22) (17 studies), and colorectal surgery [7 studies (30), plus 8 of only fluids (27)].
We found a similar reduction in the time to the first defecation (28) (−0.99 vs. −1 day) in the group receiving early nutritional support compared to those receiving delayed feeding (28).
According to previous reviews, early feeding reduces LOS (22, 28, 30); however, we found a smaller reduction of −1.31 days compared to −1.59 days (30), −1.95 days (22), and − 2.29 days (28).
By comparing the findings on complication prevention, discordant results emerged. Specifically, we found a significant reduction in the likelihood of incurring a postoperative complication, according to two previous reviews with almost similar statistically significant results [RR 0.70 compared to 0.70 (30), 0.72 (29), and 0.61 (28)]. In addition, our positive results on complications are further supported by the review of Shu and colleagues (29), which showed a reduction in infectious complication rates (RR 0.50, CI95% 0.38, 0.67). However, two reviews reported no effects on complication prevention by early feeding (22, 27), but Herbert and colleagues’ review was not entirely comparable with our results, as the authors analyzed the risk of mortality, anastomotic leakage, wound infection, abdominal abscess, and pneumonia separately. Regarding nausea and vomiting, this review found no evidence of the beneficial effect of early feeding, which is consistent with the previous literature (22, 27, 30).
Therefore, future research should focus on the effect of early oral feeding on LOS to confirm the consistency of our positive findings. Furthermore, despite the moderate confidence in the effect estimate, more studies are needed to investigate the effect of early feeding on several types of complications since we investigated the risk of at least one complication without specifying it.
Other suggestions for future research, gathered by comparing with previous research studies, encompass the need for a standardized definition of early oral feeding and the need to investigate the relationship between different modalities of early oral feeding with components of the multimodal program recommended by the ERAS guidelines (19). The lack of clarity on the type of food, start timing, and food consistency could threaten the reliability of the comparison between studies, and further efforts should be devoted to solving this issue by academics and clinicians. This could be helped by a more detailed reporting of interventions in the published studies. According to the ERAS guidelines, early oral feeding is considered safe in patients with a new non-diverted colorectal anastomosis, starting 4 h post-surgery. Furthermore, adopting a low residue diet and incorporating oral nutritional supplements might better improve outcomes (19). However, we were not able to confirm these results or provide further recommendations due to heterogeneity among studies. Therefore, we suggest additional research to determine the best type of early diet and its most effective combination with other perioperative interventions. Furthermore, differences in surgery sites and techniques should be further investigated as confounders of the effect of early oral feeding on POI and LOS.
4.3 Strengths and limitations
This review has some strengths and limitations. The inclusion of studies assessing the multimodal or ERAS program was considered both a weakness and a strength point. Specifically, our findings might have been biased by other interventions, including the use of opioids, vomiting prevention protocol, parenteral nutrition, and early mobilization. However, the subgroup analysis confirmed the benefit of early feeding alone and provided evidence for the effectiveness of multimodal and ERAS interventions in promoting recovery of intestinal motility and LOS. This subgroup analysis was possible since we included early oral feeding both as a single intervention or a component of complex interventions, which is different from previous reviews. Indeed, available reviews included studies only on oral feeding or multimodal interventions, with a range of 7 (26, 27, 30) to 17 (22) studies, while we gathered 34 studies.
Combining complications into a single outcome poses a limitation in assessing the postoperative risk, potentially yielding biased results due to variations in severity. Early feeding may not be directly linked to many detected complications, and outcomes could be influenced by perioperative patient management in studies incorporating multimodal or ERAS programs. If statistically significant results favored the intervention, confidence in establishing a direct association between early feeding and mortality, bleeding, anastomotic leakage, and infections would be uncertain. Vomiting was the only directly associable complication, and we performed a separate analysis for it.
Additionally, our study did not specify a publication time frame, encompassing studies from 1995 to 2021. This lack of temporal specificity could have introduced potential influences from advancements in surgical techniques and LOS reduction. Nevertheless, upon scrutinizing the extracted data, we found no linear improvement in LOS or other outcomes based on the publication year or the study’s country.
Finally, there was substantial heterogeneity in the surgeries included in terms of (i) the type (upper and lower gastrointestinal surgeries and hepatobiliopancreatic procedures); (ii) the underlying disease (benign diseases and malignant tumors); (iii) the complexity (laparoscopic cholecystectomy and some bariatric surgeries have a lower risk of POI, compared to pancreaticoduodenectomy or colorectal surgeries); and (iv) the surgical approach (both open and minimally invasive surgical approaches were gathered). This merger reasonably may have impacted the results. However, we still consider that the results are reliable and generalizable.
5 Conclusion
Our study supports the practice of postoperative early oral feeding as a standalone intervention or within a multi-component program, including the ERAS protocol, after gastrointestinal surgery, especially referring to colorectal, bowel, abdominal, and gastric surgeries. We showed that postoperative early oral feeding may shorten the time of the first passage of the stool by 1 day on average, thereby reducing POI by fastening intestinal mobility. This could help to improve the nutritional status and autonomy recovery and prevent complications and prolonged LOS (6, 9–11). Indeed, our results support moderate confidence to a 30% reduction in the risk complications and a decrease of 1.3 days in LOS, even though the effect on LOS is of lower confidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FC: Conceptualization, Methodology, Project administration, Writing – review & editing. JL: Formal analysis, Investigation, Visualization, Writing – original draft. AC: Investigation, Methodology, Writing – review & editing. MC: Conceptualization, Methodology, Writing – review & editing. EM: Investigation, Methodology, Writing – review & editing. SP: Conceptualization, Methodology, Writing – review & editing. EA: Data curation, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The University of Verona is acknowledged for the support provided through the extraordinary university fund for Open Access publications.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1369141/full#supplementary-material
References
1. Venara, A, Neunlist, M, Slim, K, Barbieux, J, Colas, PA, Hamy, A, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg. (2016) 153:439–46. doi: 10.1016/j.jviscsurg.2016.08.010
2. Buscail, E, and Deraison, C. Postoperative ileus: a pharmacological perspective. Br J Pharmacol. (2022) 179:3283–305. doi: 10.1111/bph.15800
3. Sui, C, Tao, L, Bai, C, Shao, L, Miao, J, Chen, K, et al. Molecular and cellular mechanisms underlying postoperative paralytic ileus by various immune cell types. Front Pharmacol. (2022) 13:929901. doi: 10.3389/fphar.2022.929901
4. Bragg, D, El-Sharkawy, AM, Psaltis, E, Maxwell-Armstrong, CA, and Lobo, DN. Postoperative ileus: recent developments in pathophysiology and management. Clin Nutr. (2015) 34:367–76. doi: 10.1016/j.clnu.2015.01.016
5. Hedrick, TL, McEvoy, MD, Mythen, MMG, Bergamaschi, R, Gupta, R, Holubar, SD, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on postoperative gastrointestinal dysfunction within an enhanced recovery pathway for elective colorectal surgery. Anesth Analg. (2018) 126:1896–907. doi: 10.1213/ANE.0000000000002742
6. Smeets, BJJ, and Luyer, MDP. Nutritional interventions to improve recovery from postoperative ileus. Curr Opin Clin Nutr Metab Care. (2018) 21:394–8. doi: 10.1097/MCO.0000000000000494
7. Chapman, SJ, Thorpe, G, Vallance, AE, Harji, DP, Lee, MJ, and Fearnhead, NS. Systematic review of definitions and outcome measures for return of bowel function after gastrointestinal surgery. BJS Open. (2019) 3:1–10. doi: 10.1002/bjs5.102
8. Bugaev, N, Bhattacharya, B, Chiu, WC, Como, JJ, Cripps, MW, Ferrada, P, et al. Promotility agents for the treatment of ileus in adult surgical patients: a practice management guideline from the eastern Association for the Surgery of trauma. J Trauma Acute Care Surg. (2019) 87:922–34. doi: 10.1097/TA.0000000000002381
9. Chapman, SJ, Pericleous, A, Downey, C, and Jayne, DG. Postoperative ileus following major colorectal surgery. Br J Surg. (2018) 105:797–810. doi: 10.1002/bjs.10781
10. Peters, EG, Pattamatta, M, Smeets, BJJ, Brinkman, DJ, Evers, S, de Jonge, WJ, et al. The clinical and economical impact of postoperative ileus in patients undergoing colorectal surgery. Neurogastroenterol Motil. (2020) 32:e13862. doi: 10.1111/nmo.13862
11. Scarborough, JE, Schumacher, J, Kent, KC, Heise, CP, and Greenberg, CC. Associations of specific postoperative complications with outcomes after elective Colon resection: a procedure-targeted approach toward surgical quality improvement. JAMA Surg. (2017) 152:e164681. doi: 10.1001/jamasurg.2016.4681
12. Traeger, L, Koullouros, M, Bedrikovetski, S, Kroon, HM, Moore, JW, and Sammour, T. Global cost of postoperative ileus following abdominal surgery: meta-analysis. BJS Open. (2023) 7:zrad054. doi: 10.1093/bjsopen/zrad054
13. Schwenk, W, Haase, O, Neudecker, J, and Müller, JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. (2005) 2008:CD003145. doi: 10.1002/14651858.CD003145.pub2
14. Corcoran, T, Rhodes, JE, Clarke, S, Myles, PS, and Ho, KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. (2012) 114:640–51. doi: 10.1213/ANE.0b013e318240d6eb
15. Guay, J, Nishimori, M, and Kopp, S. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev. (2016) 2017:CD001893. doi: 10.1002/14651858.CD001893.pub2
16. Huang, ZD, Gu, HY, Zhu, J, Luo, J, Shen, XF, Deng, QF, et al. The application of enhanced recovery after surgery for upper gastrointestinal surgery: Meta-analysis. BMC Surg. (2020) 20:3. doi: 10.1186/s12893-019-0669-3
17. Hajibandeh, S, Hajibandeh, S, Bill, V, and Satyadas, T. Meta-analysis of enhanced recovery after surgery (ERAS) protocols in emergency abdominal surgery. World J Surg. (2020) 44:1336–48. doi: 10.1007/s00268-019-05357-5
18. Ni, X, Jia, D, Guo, Y, Sun, X, and Suo, J. The efficacy and safety of enhanced recovery after surgery (ERAS) program in laparoscopic digestive system surgery: a meta-analysis of randomized controlled trials. Int J Surg. (2019) 69:108–15. doi: 10.1016/j.ijsu.2019.07.034
19. ERAS Society . Enhanced recovery after surgery Available at: https://erassociety.org
20. Fukuzawa, J, Terashima, H, and Ohkohchi, N. Early postoperative oral feeding accelerates upper gastrointestinal anastomotic healing in the rat model. World J Surg. (2007) 31:1234–9. doi: 10.1007/s00268-007-9003-9
21. Boelens, PG, Heesakkers, FF, Luyer, MD, van Barneveld, KW, de Hingh, IH, Nieuwenhuijzen, GA, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg. (2014) 259:649–55. doi: 10.1097/SLA.0000000000000288
22. Herbert, G, Perry, R, Andersen, HK, Atkinson, C, Penfold, C, Lewis, SJ, et al. Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications. Cochrane Database Syst Rev. (2019) 2019:CD004080. doi: 10.1002/14651858.CD004080.pub4
23. Ashcroft, J, Singh, AA, Ramachandran, B, Habeeb, A, Hudson, V, Meyer, J, et al. Reducing ileus after colorectal surgery: a network meta-analysis of therapeutic interventions. Clin Nutr. (2021) 40:4772–82. doi: 10.1016/j.clnu.2021.05.030
24. Carmichael, L, Rocca, R, Laing, E, Ashford, P, Collins, J, Jackson, L, et al. Early postoperative feeding following surgery for upper gastrointestinal cancer: a systematic review. J Hum Nutr Diet. (2022) 35:33–48. doi: 10.1111/jhn.12930
25. Willcutts, KF, Chung, MC, Erenberg, CL, Finn, KL, Schirmer, BD, and Byham-Gray, LD. Early Oral feeding as compared with traditional timing of Oral feeding after upper gastrointestinal surgery: a systematic review and Meta-analysis. Ann Surg. (2016) 264:54–63. doi: 10.1097/SLA.0000000000001644
26. Hogan, S, Steffens, D, Rangan, A, Solomon, M, and Carey, S. The effect of diets delivered into the gastrointestinal tract on gut motility after colorectal surgery-a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. (2019) 73:1331–42. doi: 10.1038/s41430-019-0474-1
27. MacVicar, E, Cullen, F, Kastora, SL, Parnaby, C, Mackay, C, and Ramsay, G. A systematic review of the impact of post-operative oral fluid intake on ileus following elective colorectal surgery. Int J Surg. (2022) 103:106651. doi: 10.1016/j.ijsu.2022.106651
28. He, LB, Liu, MY, He, Y, and Guo, AL. Nutritional status efficacy of early nutritional support in gastrointestinal care: a systematic review and meta-analysis. World J Gastrointest Surg. (2023) 15:953–64. doi: 10.4240/wjgs.v15.i5.953
29. Shu, XL, Kang, K, Gu, LJ, and Zhang, YS. Effect of early enteral nutrition on patients with digestive tract surgery: a meta-analysis of randomized controlled trials. Exp Ther Med. (2016) 12:2136–44. doi: 10.3892/etm.2016.3559
30. Zhuang, CL, Ye, XZ, Zhang, CJ, Dong, QT, Chen, BC, and Yu, Z. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg. (2013) 30:225–32. doi: 10.1159/000353136
31. Higgins, J, Thomas, J, Chandler, JC M, Li, T, Page, M, and Welch, V. Cochrane handbook for systematic reviews of interventions version 6.3 (updated 2022). In: Cochrane, editor. Available at: www.training.cochrane.org/handbook.2022
32. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
33. Dindo, D, Demartines, N, and Clavien, PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
34. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
35. Collaboration TC . Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, (2020).
36. R Core Team . R: a language and environment for statistical computing In: R foundation for statistical computing. Vienna, Austria: (2022)
37. Schünemann, H, Brożek, J, Guyatt, G, and Oxman, A. GRADE handbook for grading quality of evidence and strength of recommendations Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group Available at: https://gdt.gradepro.org/app/handbook/handbook.html (2013).
38. Zhou, T, Wu, XT, Zhou, YJ, Huang, X, Fan, W, and Li, YC. Early removing gastrointestinal decompression and early oral feeding improve patients' rehabilitation after colorectostomy. World J Gastroenterol. (2006) 12:2459–63. doi: 10.3748/wjg.v12.i15.2459
39. Mingjie, X, Luyao, Z, Ze, T, YinQuan, Z, and Quan, W. Laparoscopic radical gastrectomy for Resectable advanced gastric Cancer within enhanced recovery programs: a prospective randomized controlled trial. J Laparoendosc Adv Surg Tech A. (2017) 27:959–64. doi: 10.1089/lap.2016.0057
40. Khoo, CK, Vickery, CJ, Forsyth, N, Vinall, NS, and Eyre-Brook, IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg. (2007) 245:867–72. doi: 10.1097/01.sla.0000259219.08209.36
41. El Nakeeb, A, Fikry, A, El Metwally, T, Fouda, E, Youssef, M, Ghazy, H, et al. Early oral feeding in patients undergoing elective colonic anastomosis. Int J Surg. (2009) 7:206–9. doi: 10.1016/j.ijsu.2009.03.003
42. Ionescu, D, Iancu, C, Ion, D, Al-Hajjar, N, Margarit, S, Mocan, L, et al. Implementing fast-track protocol for colorectal surgery: a prospective randomized clinical trial. World J Surg. (2009) 33:2433–8. doi: 10.1007/s00268-009-0197-x
43. da Fonseca, LM, Profeta da Luz, MM, Lacerda-Filho, A, Correia, MI, and Gomes da Silva, R. A simplified rehabilitation program for patients undergoing elective colonic surgery—randomized controlled clinical trial. Int J Color Dis. (2011) 26:609–16. doi: 10.1007/s00384-010-1089-0
44. Lee, TG, Kang, SB, Kim, DW, Hong, S, Heo, SC, and Park, KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: a prospective randomized controlled trial. Dis Colon Rectum. (2011) 54:21–8. doi: 10.1007/DCR.0b013e3181fcdb3e
45. Wang, Q, Suo, J, Jiang, J, Wang, C, Zhao, YQ, and Cao, X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Color Dis. (2012) 14:1009–13. doi: 10.1111/j.1463-1318.2011.02855.x
46. Ren, L, Zhu, D, Wei, Y, Pan, X, Liang, L, Xu, J, et al. Enhanced recovery after surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. (2012) 36:407–14. doi: 10.1007/s00268-011-1348-4
47. Dag, A, Colak, T, Turkmenoglu, O, Gundogdu, R, and Aydin, S. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (São Paulo). (2011) 66:2001–5. doi: 10.1590/S1807-59322011001200001
48. Lee, SM, Kang, SB, Jang, JH, Park, JS, Hong, S, Lee, TG, et al. Early rehabilitation versus conventional care after laparoscopic rectal surgery: a prospective, randomized, controlled trial. Surg Endosc. (2013) 27:3902–9. doi: 10.1007/s00464-013-3006-4
49. Li, K, Li, JP, Peng, NH, Jiang, LL, Hu, YJ, and Huang, MJ. Fast-track improves post-operative nutrition and outcomes of colorectal surgery: a single-center prospective trial in China. Asia Pac J Clin Nutr. (2014) 23:41–7. doi: 10.6133/apjcn.2014.23.1.09
50. Pragatheeswarane, M, Muthukumarassamy, R, Kadambari, D, and Kate, V. Early oral feeding vs. traditional feeding in patients undergoing elective open bowel surgery-a randomized controlled trial. J Gastrointest Surg. (2014) 18:1017–23. doi: 10.1007/s11605-014-2489-1
51. He, F, Lin, X, Xie, F, Huang, Y, and Yuan, R. The effect of enhanced recovery program for patients undergoing partial laparoscopic hepatectomy of liver cancer. Clin Transl Oncol. (2015) 17:694–701. doi: 10.1007/s12094-015-1296-9
52. Abdikarim, I, Cao, XY, Li, SZ, Zhao, YQ, Taupyk, Y, and Wang, Q. Enhanced recovery after surgery with laparoscopic radical gastrectomy for stomach carcinomas. World J Gastroenterol. (2015) 21:13339–44. doi: 10.3748/wjg.v21.i47.13339
53. Shichinohe, T, Sasaki, T, Kitashiro, S, Morita, T, Ono, K, Senmaru, N, et al. Impact of elemental diet on early recovery after laparoscopic colectomy: findings of a randomized controlled trial. Surg Today. (2017) 47:166–73. doi: 10.1007/s00595-016-1365-x
54. Feng, J, Li, K, Li, L, Wang, X, Huang, M, Yang, J, et al. The effects of fast-track surgery on inflammation and immunity in patients undergoing colorectal surgery. Int J Color Dis. (2016) 31:1675–82. doi: 10.1007/s00384-016-2630-6
55. Mari, G, Costanzi, A, Crippa, J, Falbo, R, Miranda, A, Rossi, M, et al. Surgical stress reduction in elderly patients undergoing elective colorectal laparoscopic surgery within an ERAS protocol. Chirurgia (Bucur). (2016) 111:476–80. doi: 10.21614/chirurgia.111.6.476
56. Sun, DL, Li, WM, Li, SM, Cen, YY, Xu, QW, Li, YJ, et al. Comparison of multi-modal early oral nutrition for the tolerance of oral nutrition with conventional care after major abdominal surgery: a prospective, randomized, single-blind trial. Nutr J. (2017) 16:11. doi: 10.1186/s12937-017-0228-7
57. Liang, X, Ying, H, Wang, H, Xu, H, Liu, M, Zhou, H, et al. Enhanced recovery care versus traditional care after laparoscopic liver resections: a randomized controlled trial. Surg Endosc. (2018) 32:2746–57. doi: 10.1007/s00464-017-5973-3
58. Kang, SH, Lee, Y, Min, SH, Park, YS, Ahn, SH, Park, DJ, et al. Multimodal enhanced recovery after surgery (ERAS) program is the optimal perioperative Care in Patients Undergoing Totally Laparoscopic Distal Gastrectomy for gastric Cancer: a prospective, randomized. Clinical Trial Ann Surg Oncol. (2018) 25:3231–8. doi: 10.1245/s10434-018-6625-0
59. Wu, M, Yang, L, Zeng, X, Wang, T, Jia, A, Zuo, Y, et al. Safety and feasibility of early Oral hydration in the Postanesthesia care unit after laparoscopic cholecystectomy: a prospective, randomized, and controlled study. J Perianesth Nurs. (2019) 34:425–30. doi: 10.1016/j.jopan.2018.06.093
60. Wang, WK, Tu, CY, Shao, CX, Chen, W, Zhou, QY, Zhu, JD, et al. Impact of enhanced recovery after surgery on postoperative rehabilitation, inflammation, and immunity in gastric carcinoma patients: a randomized clinical trial. Braz J Med Biol Res. (2019) 52:e8265. doi: 10.1590/1414-431x20198265
61. Hwang, DW, Kim, HJ, Lee, JH, Song, KB, Kim, MH, Lee, SK, et al. Effect of enhanced recovery after surgery program on pancreaticoduodenectomy: a randomized controlled trial. J Hepatobiliary Pancreat Sci. (2019) 26:360–9. doi: 10.1002/jhbp.641
62. Geubbels, N, Evren, I, Acherman, YIZ, Bruin, SC, van de Laar, A, Hoen, MB, et al. Randomized clinical trial of an enhanced recovery after surgery programme versus conventional care in laparoscopic roux-en-Y gastric bypass surgery. BJS Open. (2019) 3:274–81. doi: 10.1002/bjs5.50143
63. Cao, S, Zheng, T, Wang, H, Niu, Z, Chen, D, Zhang, J, et al. Enhanced recovery after surgery in elderly gastric Cancer patients undergoing laparoscopic Total gastrectomy. J Surg Res. (2021) 257:579–86. doi: 10.1016/j.jss.2020.07.037
64. Li, Q, Du, L, Lu, L, Tong, Y, Wu, S, Yang, Y, et al. Clinical application of enhanced recovery after surgery in perioperative period of laparoscopic colorectal Cancer surgery. J Laparoendosc Adv Surg Tech A. (2019) 29:178–83. doi: 10.1089/lap.2018.0708
65. Consoli, M, Fonseca, L, Silva, R, and Correia, M. Early postoperative oral feeding impacts positively in patients undergoing colonic resection: results of a pilot study. Nutrición Hospitalaria. (2010) 25:806–9. doi: 10.3305/nh.2010.25.5.4777
66. Liu, XX, Jiang, ZW, Wang, ZM, and Li, JS. Multimodal optimization of surgical care shows beneficial outcome in gastrectomy surgery. JPEN J Parenter Enteral Nutr. (2010) 34:313–21. doi: 10.1177/0148607110362583
67. Feo, CV, Romanini, B, Sortini, D, Ragazzi, R, Zamboni, P, Pansini, GC, et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. (2004) 74:298–301. doi: 10.1111/j.1445-1433.2004.02985.x
68. Stewart, BT, Woods, RJ, Collopy, BT, Fink, RJ, Mackay, JR, and Keck, JO. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust N Z J Surg. (1998) 68:125–8. doi: 10.1111/j.1445-2197.1998.tb04721.x
69. Hartsell, PA, Frazee, RC, Harrison, JB, and Smith, RW. Early postoperative feeding after elective colorectal surgery. Arch Surg. (1997) 132:518. doi: 10.1001/archsurg.1997.01430290064011
70. Ortiz, H, Armendariz, P, and Yarnoz, C. Is early postoperative feeding feasible in elective colon and rectal surgery? Int J Color Dis. (1996) 11:119–21. doi: 10.1007/s003840050032
71. Reissman, P, Teoh, TA, Cohen, SM, Weiss, EG, Nogueras, JJ, and Wexner, SD. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Ann Surg. (1995) 222:73–7. doi: 10.1097/00000658-199507000-00012
72. Wendler, E, Nassif, PAN, Malafaia, O, Brites Neto, JL, Ribeiro, JGA, Proença, LB, et al. Shorten preoperative fasting and introducing early eating assistance in recovery after gastrojejunal bypass? Arq Bras Cir Dig. (2022) 34:e1606. doi: 10.1590/0102-672020210003e1606
Keywords: early feeding, oral feeding, gastrointestinal surgery, ileus, length of hospital stay
Citation: Canzan F, Longhini J, Caliaro A, Cavada ML, Mezzalira E, Paiella S and Ambrosi E (2024) The effect of early oral postoperative feeding on the recovery of intestinal motility after gastrointestinal surgery: a systematic review and meta-analysis of randomized clinical trials. Front. Nutr. 11:1369141. doi: 10.3389/fnut.2024.1369141
Edited by:
Alessandra Milani, European Institute of Oncology (IEO), ItalyReviewed by:
Xuejin Gao, Nanjing University, ChinaQian Xu, Shandong Provincial Qianfoshan Hospital, China
Copyright © 2024 Canzan, Longhini, Caliaro, Cavada, Mezzalira, Paiella and Ambrosi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Canzan, ZmVkZXJpY2EuY2FuemFuQHVuaXZyLml0; Salvatore Paiella, c2FsdmF0b3JlLnBhaWVsbGFAdW5pdnIuaXQ=
 Federica Canzan1*
Federica Canzan1* Jessica Longhini
Jessica Longhini Salvatore Paiella
Salvatore Paiella