
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 05 April 2024
Sec. Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1362226
This article is part of the Research Topic The Model of Ramadan Diurnal Intermittent Fasting: Unraveling the Health Implications - Volume 3 View all 14 articles
Background and objective: Polycystic ovary syndrome (PCOS) is a complex hormonal disorder that leads to ovarian cysts, irregular ovulation, and hormonal swings in women. It is a complex and heterogeneous condition that affects 4 to 20% of women of reproductive age worldwide and relates to reproductive, metabolic, and psychosocial dysfunction. Dietary and lifestyle modifications have been proposed to play a central role in the management of PCOS. This study aimed to provide a comprehensive systemic overview of the existing literature on the effects of intermittent fasting (IF) and calorie restriction (CR) regimens on disease markers of PCOS.
Designs and methods: Several databases, such as CINAHL, Cochrane, EBSCOhost, EMBASE, Google Scholar, ProQuest Medical, PubMed/MEDLINE, ScienceDirect, Scopus, and Web of Science databases were searched for clinical trials and observational studies examined the effects of IF regimens such as time-restricted eating and Ramadan model of IF (RIF) on glucose homeostasis, lipid profile, inflammatory and hormonal markers in patients with PCOS.
Results: This systematic review solicited three articles, comprising a collective sample size of 75 females diagnosed with PCOS. The studies were published between 2015 to 2023 and were undertaken in three countries: China, Turkey, and Iran. The research articles examined the effects of intervention with IF and CR on PCOS-related parameters such as anthropometric measures and biochemical tests which included enzymes, glycemic control, lipid profile, hormonal, and oxidative stress, and inflammatory markers. The articles yielded mixed results, with two of them showing significant changes across all tested parameters. One of the three studies did not exhibit any significant changes.
Conclusion: Very limited studies examined the relationship between IR and CR with markers of PCOS. Further well-controlled studies need to be undertaken the combined results from the limited studies illustrate the intricate and diverse nature of IF, including the RIF, and its influence on measurements of body composition and biochemical markers related to PCOS.
Polycystic ovary syndrome (PCOS) is a prevalent endocrine condition distinguished by persistent anovulation, biochemical and/or clinical hyperandrogenism, and the presence of polycystic ovary morphology (1). The World Health Organization (WHO) estimates that PCOS affects more than 116 million women worldwide (1). This disease has significant clinical implications and can lead to health issues related to the accumulation of adipose tissue, including obesity, insulin resistance (IR), metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM) (2). Insulin resistance can be elucidated by the necessity of elevated insulin levels to support metabolic processes, as well as its involvement in mitogenic and reproductive functions (3). Previous studies have indicated that a substantial proportion of women with PCOS experience impaired glucose tolerance and IR and are at increased risk for developing T2DM (3). In general, the presence of abdominal obesity or visceral adiposity in individuals with PCOS may contribute to IR, potentially triggered by subclinical, systemic low-grade inflammation. Recent research has determined that obesity is the primary risk factor for IR in persons diagnosed with PCOS (3). Another significant issue related to PCOS is hyperandrogenism, a condition that is also associated with IR. The premature secretions of androgen during early stages are commonly regarded as a characteristic feature of PCOS and are believed to contribute to the development of IR in preceding stages (3).
Sarahian et al. (4) propose that PCOS can arise from a combination of lifestyle, genetic, and prenatal influences, hence giving rise to a range of effects with differing magnitudes. The risk of PCOS is heightened by an unhealthy lifestyle and dietary choices, or exposure to infectious agents (5, 6). Environmental factors, such as physical exercise, dietary and lifestyle behaviors, and food choices exhibit significant variability among different populations.
Frequently, PCOS treatment focuses on the management of underlying symptoms, typically involving progestin therapy and a combination of birth control medications (7). Furthermore, the implementation of lifestyle modifications, specifically the adoption of healthful dietary practices and nutritious food choices, is highlighted as a viable approach for effectively managing the condition (5). The overall condition is improved by the combination of pharmacological treatments and dietary and lifestyle modifications. According to Xu and Qiao (3), lifestyle therapies, including exercise, weight loss, and nutrition therapy, have demonstrated favorable results in individuals with PCOS.
The implementation of nutrition therapy for weight reduction in women diagnosed with PCOS has been found to have a substantial influence on metabolic conditions. Previous research has demonstrated that patients with obesity who lose between 5 and 10 percent of their body weight (BW) experience significant health benefits (8). In recent decades, fasting regimens, especially what is commonly known as intermittent fasting (IF), have emerged as a non-pharmaceutical lifestyle approach in integrative medicine and a means to reduce and control weight to enhance health (9). The implementation of IF has been shown to have the potential to reduce adiposity and improve IR through decreased calorie intake and metabolic reprogramming. It can result in several positive health outcomes, such as enhanced metabolic efficiency, enhanced cognitive acuity, and an extended life span (10, 11).
Ramadan intermittent fasting (RIF), is another type of IF regimen. During Ramadan, adult Muslims are mandated to refrain from food and drink for 12–22 h during the day, depending on the season and geographical location. This fasting pattern is followed consistently for 29–30 days. Multiple studies, including original research, systematic reviews, and meta-analyses, have provided evidence that RIF is linked to decreased BW, body fat mass especially visceral fat, serum lipids, and other cardiometabolic risk factors, inflammatory and oxidative stress markers, with slight improvements in glucometabolic regulation and liver function tests (12–19). Most, if not all, of these aforementioned factors, improved upon RIF have been implicated in the etiopathogenesis of PCOS in variable degrees and dimensions (20–22).
Based on the above literature, and previous research examining the impact of different models of IF on various metabolic, hormonal, and inflammatory markers and health indicators, as well as their reported protective effect, it is hypothesized that CR and IF will improve metabolic parameters in PCOS patients. Therefore, this review aims to provide a comprehensive summary of the existing literature on the effects of Ramadan and non-Ramadan IF and CR regimens on PCOS markers.
This systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to guide the reporting of the findings (23).
The PICO model was employed to expand the return from the review and applicability of the data collected (Table 1).
An electronic search of the databases was conducted of the Google Scholar, PubMed/MEDLINE, EBSCO-host, CINAHL, ScienceDirect, Cochrane, ProQuest Medical, Web of Science, and Scopus databases for targeted studies published from 1970 to the end of November 2023. The keywords were taken from a bibliometric analysis paper by Obaideen et al. (24). Accordingly, the following search criteria were applied: TITLE-ABS-KEY (“intermittent fasting”) OR (“Ramadan”) OR (Ramadhan) OR (Ramazan) OR (“Islamic fasting”) OR (“diurnal fasting”) OR (“Ramadan intermittent fasting”) OR (“Ramadan diurnal intermittent fasting”) OR (“consecutive 30 days of fasting”) OR (“religious fasting”) AND (“PCOS” OR “polycystic ovary syndrome” or “polycystic ovarian syndrome”).
Both observational and experimental studies investigating the impact of CR, IF including RIF on markers related to PCOS were included. The inclusion criteria for research articles were as follows: (1) experimental and observational studies; (2) adult female participants (>18 years) diagnosed with PCOS; (3) endpoints that included changes in at least one PCOS diagnostic biomarker before and after following the CR or IF regimen.
Specific exclusion criteria were applied to eliminate any potential quality or methodological issues: (1) studies involving patients with any other disease apart from PCOS; (2) lacking full-text; (3) non-English language; (4) lack of clear and pre and post data; (5) editorials, abstracts case reports, and review articles; and (6) non-peer-reviewed and unpublished data. The steps for study selection are summarized in the PRISMA flow diagram (Figure 1).
The main outcome was to report the effect of practicing CR and IF regimens including RIF-induced changes in PCOS-related parameters, namely anthropometric, glycemic control, lipid profile, hormonal, oxidative, and inflammatory parameters. The first step of screening was examining all titles and abstracts to exclude irrelevant publications.
Two authors (AK and DA) independently screened the titles and abstracts of identified studies and assessed them for eligibility against the inclusion criteria. Discrepancies during screening were resolved by the principal investigator (MF). All titles and abstracts were screened to exclude irrelevant publications. To standardize data collection, the researchers reported and tabulated the study characteristics, including the main author’s name, study country, year of publication, sample size, type of fasting, study design, and main findings for the examined outcomes.
Three studies with a total of 75 participants were included in this systematic review. Details of the study authors, year of publication, country, design and type of IF regimen, sample size, age, and biomarkers tested, and the effects of CR and IF regimens are shown in Table 2. The included studies were conducted in 3 different countries, i.e., China, Turkey, and Iran. The parameters included in these studies were as follows: anthropometrics and biochemical tests including enzymes, glycemic control, lipid profile, hormonal parameters, oxidative stress, and inflammatory markers.
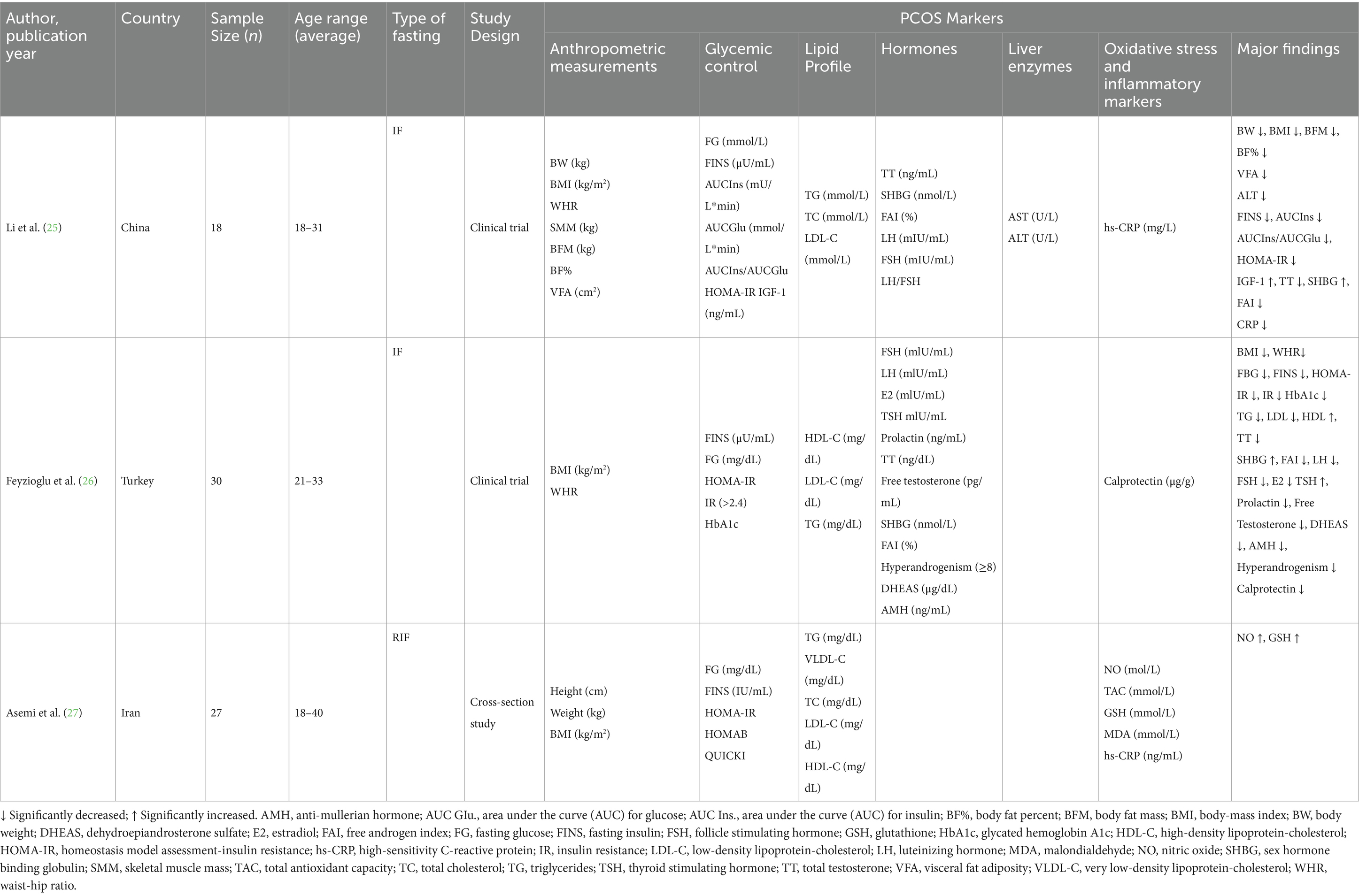
Table 2. Characteristics and major findings of the included studies on the effect of intermittent fasting on patients with PCOS.
In terms of the results obtained for the anthropometric parameters, Li et al. (25) in their study, reported a significant decrease in all anthropometric parameters, i.e., BMI, BW, BFM, BF%, and VFA (p ≤ 0.001), with the exception of WHR and SMM, which showed no significant association with IF. Feyzioglu et al. (26) reported significant reductions in anthropometric parameters, namely BMI and WHR. Asemi et al. (27) did not find any significant effect of RIF on anthropometric parameters.
In terms of enzymes, Li et al. (25) reported no significant effect of IF on enzymes such as UA and AST, while there was a significant decrease in ALT (p = 0.027). In relation to the glycemic control parameters, Li et al. (25) reported significant decreases in all glycemic control parameters, i.e., FINS, HOMA-IR, and HbA1c, with the exception of FBG. Similarly, Feyzioglu et al. (26) reported significant decreases in all glycemic control parameters, i.e., FINS, FBG, HOMA-IR, and HbA1c. However, Asemi et al. (27) did not find any significant effect of RIF on glycemic control parameters.
Additionally, all the 3 included studies reported on the effect of IF and RIF on the lipid profile. In their study, Li et al. (25) did not find any significant effect of IF on the serum lipids. Similarly, Asemi et al. (27), in their study, did not find any significant effect of RIF on the lipid profile parameters. Conversely, Feyzioglu et al. (26) reported a positive effect of IF on serum lipids, with significant decreases in LDL and TG and a significant increase in HDL.
Li et al. (25) reported no significant effects of IF on hormonal parameters such as LH and FSH, while a significant increase in IGF-1 and SHBG was observed after IF. Alternatively, they found significant reductions in TT and FAI. Feyzioglu et al. (26) reported significant effects of IF on all hormonal parameters, with decreases reported in FSH, LH, E2, Prolactin, TT, FT, FAI, DHEAS, and AMH, while reported significant increases in TSH and SHBG. The study by Asemi et al. (27) did not report any hormonal markers in their study.
Li et al. (25) found a significant decrease in inflammatory markers, hs-CRP. Feyzioglu et al. (26) found a significant decrease in the oxidative stress marker, calprotectin. Asemi et al. (27) found no significant effect of RIF on inflammatory and oxidative stress markers such as TAC, MDA, and hs-CRP. On the other hand, the authors reported significant increases in NO and GSH.
The current study tried to systematically elaborate on the effect of following CR and IF regimens on the disease parameters of PCOS. Only three studies were selected after applying the inclusion/exclusion criteria, a matter that denotes the relatively emerging topic we are currently addressing and the mass need for further studies to be conducted in this regard. The scarcity of studies reviewed is consistent with the finding of Floyd et al. (28), who examined the effect of practicing TRE on insulin levels and insulin sensitivity in patients diagnosed with PCOS. After screening 2,662 studies and assessing 37 eligible studies, only one study was found by Floyd and colleagues.
Across different studies, the effect of IF on anthropometric measurements has been considered. For instance, Li et al. (25) and Feyzioglu et al. (26) reported remarkable decreases in various anthropometric markers. Whilst, the study by Asemi et al. (27) which studied RIF in particular, revealed no significant relationship between RIF and these anthropometric markers. However, previous studies by Faris et al. (16) and Hooshiar et al. (29) state that RIF lowers visceral adiposity hence it can reduce BW and other parameters in individuals with PCOS. Additionally, Madkour et al. (30), in their study found that RIF resulted in weight loss, and reduced BMI, BF%, and waist circumference. In their study, the beneficial effects of RIF were observed across all subgroups in the study, regardless of age, sex, and fasting duration. Another meta-analysis was conducted by Jahrami et al. (17) to investigate the impact of RIF on BW. It was found that RIF can cause variable changes in BW, body composition, and fat mass. The reduction in BW and visceral adiposity could be attributed to the metabolic shift to ketogenesis and fatty acid oxidation during fasting (15). However, weight changes induced by RIF were found to be mostly reversed post-Ramadan, indicating that weight loss during this period is transient. This difference in findings represents a split landscape; some studies show that IF positively influences body composition, while others fail to back it up.
In terms of enzymes, the included studies reported minimal effect on the liver enzymes. Regarding the glycemic control parameters, two of the included reported a significant reduction in all glycemic control parameters (23, 26). However, Asemi et al. (27) did not find any significant effect of RIF on the glycemic control parameters. Previous studies have also reported such contradictory findings. A systematic review and meta-analysis by Faris et al. (14) examined the effects of RIF on glucometabolic markers in healthy people. The study found that RIF had a minimal impact on these markers and highlighted the influence of various factors such as sex, age, fasting duration, and country on glucometabolic changes. Conversely, Faris et al. (14) reported studies conducted on rodents, which showed that IF improved insulin sensitivity and glucose tolerance, and preserved β-cell mass in obesity-induced diabetes. Likewise, in a study by Carter et al. (31), the researchers conducted a large trial to evaluate the impact of IF versus a continuous energy-restricted diet on glycemic control in individuals with type T2DM. After a year of intervention, both groups showed comparable decreases in HbA1c levels. However, in a study by Faris et al. (16) despite a significant drop in plasma adiponectin levels, no significant change was found in insulin sensitivity or glucose homeostasis markers. Additionally, the study also found a significant increase in apelin levels, which could be responsible for the lack of significant change in insulin resistance, despite an increase in total sugar intake during Ramadan. The study also noted that the level of IGF-1 significantly decreased by the end of RIF, which may be another reason for the non-significant changes in markers of glucose homeostasis.
Regarding the lipid markers, 2 of the included studies did not find any significant effect of IF on the lipid profile parameters (23, 27) Conversely, Feyzioglu et al. (26) reported a positive effect of IF on serum lipids, with a significant reduction in LDL and TG and an increase in HDL. Similar findings have been reported by Jahrami et al. (18) in their meta-analysis, they reported that RIF improved lipid profile and coagulation parameters, and these improvements persisted for 4 weeks after fasting. The effects of RIF on the lipid markers are consistent with the impacts of other forms of IF and energy-restricted diets. Additionally, the study also found that IF has cardioprotective effects, possibly due to increased cellular stress resistance, reduced oxidative damage, and changes in the brain-derived neurotrophic factor signaling in the brain.
In terms of the hormonal marker, following IF, Li et al. (25) observed no significant effects of IF on hormonal parameters such as LH and FSH, while significant increases in IGF-1 and SHBG. Alternatively, they found significant reductions in TT and FAI. Likewise, Feyzioglu et al. (26) reported significant effects of IF on all hormonal parameters, with decreases reported in FSH, LH, E2, Prolactin, TT, FT, FAI, DHEAS, and AMH, while reported significant increases in TSH and SHBG. Similar findings have been reported by Cienfuegos et al. (32) in their study which suggested that IF can potentially lower androgen levels, specifically TT and FAI, and increase SHBG in obese premenopausal women, offering a potential treatment for hyperandrogenic conditions like PCOS. Additionally, Han et al. (33) in their study investigated the effects of time-restricted feeding (TRF) on a mouse model of PCOS. The study observed that TRF treatment significantly lowered plasma androgen levels and the LH/FSH ratio in PCOS mice, consistent with other dietary interventions. This suggests that TRF may regulate gonadotropin-releasing hormone secretion, influencing the synthesis of steroid hormones. During fasting, certain gut microbes can use host substrates to produce beneficial metabolites like butyrate, acetate, and mucin stimulants. This suggests that IF’s influence on reproductive hormones may be mediated by alterations in the gut microbiome (32).
Among the three included studies, following IF, Li et al. (25) and Feyzioglu et al. (26) found a significant decrease in the inflammatory and oxidative stress markers, hs-CRP and calprotectin, respectively. Similar findings have been reported in previous studies. For instance, Faris et al. (16) in their review, reported the effect of RIF on proinflammatory cytokines and oxidative stress markers in both obese and non-obese individuals. The studies included in the review reported a slight decrease in these markers after Ramadan, suggesting short-term protection against low-grade systemic inflammation and oxidative stress. This reduction could be due to weight loss during Ramadan or the lowering of the IGF-1 which is associated with inflammation and oxidative stress. Further, RIF has been linked with reduced serum glucose, insulin, and IR levels in obese individuals with metabolic syndrome. Additionally, RIF may also increase the expression of certain antioxidant genes, providing another potential mechanism for its health benefits (15). A study conducted by Madkour et al. (30) provides the first evidence of a link between RIF and fat mass and obesity-associated (FTO) gene expression in overweight/obese individuals. The authors found an association between reduced FTO expression and favorable effects such as suppression of pro-inflammatory markers and improved lipid profile. FTO is broadly distributed in many organs, and its expression may be influenced by dietary conditions. The study also found a reduction in pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor- α (TNF-α), and an increase in IL-10, an anti-inflammatory cytokine, during RIF. These findings are consistent with previous research showing significant reductions in pro-inflammatory cytokines and improvements in cardiometabolic risk factors during RIF (12, 34). The study by Madkour et al. (30) found no correlation between FTO expression and high-energy intake, waist circumference, or obesity, suggesting that RIF’s beneficial effects occur independently of dietary and anthropometric factors. The study also found that RIF upregulates several key regulatory proteins involved in tumor suppression, DNA repair, insulin signaling, glucose and lipid metabolism, circadian clock regulation, immune system, and cognitive function. Similarly, another study by Madkour et al. (35) investigated the impact of RIF on the genetic expression of metabolic and cellular regulator genes (SIRT1 and SIRT3) and antioxidant defense enzyme system genes (TFAM, SOD2, and Nrf2). The research revealed that the expression of SIRT1 shows a minor reduction at the end of RIF, while SIRT3 shows a significant reduction, which could be due to the lack of significant changes in total energy and fat intake during Ramadan. The study further revealed that the expression of antioxidant defense genes (SOD2, TFAM, and Nrf2) increases, suggesting their role in counteracting the increased oxidative stress during fasting. The research also notes that the significant reduction of IGF-1 reflects the significant activation of antioxidative stress genes, providing a positive transient protective impact against oxidative stress and subsequent pathological conditions.
Studies on the gut microbiota may be useful in interpreting the impact of IF, which may be mediated by alterations brought about by IF on the gut microbiota, and in explaining the plausible effect of IF on PCOS. Recent research by Dong and Rees (36) suggests a link between the gut microbiome and the development of PCOS, wherein they reported that women with PCOS exhibit higher levels of Bacteroides vulgatus (B. vulgatus) and lower levels of certain bile acids in their intestines. Experiments with mice showed that introducing these gut bacteria from PCOS patients or pure B. vulgatus led to insulin resistance, altered bile acid metabolism, and disrupted ovarian function. However, administration of IL-22 or glycodeoxycholic acid improved these symptoms, suggesting potential therapeutic strategies. Furthermore, genome-wide association studies have identified various susceptibility loci for PCOS, particularly in metabolic and neuroendocrine pathways. These include loci near genes such as the insulin receptor, follicle-stimulating hormone receptor, and others (36). Additionally, Cienfuegos et al. (32), in their study reported that IF has been found to positively affect the gut microflora’s composition and diversity, and reduce gut permeability, thereby diminishing obesity-linked postprandial endotoxemia and systemic inflammation.
The strength of our review is that is the first comprehensive systematic review that tackles the effect of different forms of IF and CR regimens on the different aspects related to PCOS, including anthropometrics and biochemical parameters including enzymes, glycemic control, lipid profile, hormonal parameters, oxidative stress, and inflammatory markers. However, the study also had some limitations that should be considered when interpreting the findings. Limited research and scarcity of works render the generalizability of the results unattainable. Additionally, the inclusion of an observational study as part of the reviewed articles is another weakness of the study as causality cannot be inferred in such a study design.
The following review thus presents IF and CR as a subject of much interest and promise in modulating different physiological responses in individuals with PCOS. A scarce of studies have examined the effect of IF and CR on markers of PCOS. Even so, such limited findings demonstrate the complicated and multifaceted nature of the IF regimen and its impact on body composition, metabolic, hormonal, and other biochemical markers related to PCOS. This diversity in outcomes, however, highlights the complex interaction between IF and responses at an individual level. Therefore, the current findings must be interpreted with caution due to these inconsistencies and different outcomes in various studies, alongside the very limited number. Like many developing fields of study, the research on IF highlights the need for more rigorous testing presented in terms of more well-controlled clinical trials. In the future, such efforts should focus on developing comprehensive and well-standardized research protocols; multifaceted populations of women diagnosed with PCOS must be selected for assessment. Longer-term assessments are needed, with more mechanisms revealed. Intermittent fasting may be a modulatory tool for many health parameters, but we must understand this more holistically and nuancedly.
The data that support the findings of this study are available from the corresponding author, (MF), upon reasonable request.
AK: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. DA: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. MF: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bharathi, R, Swetha, S, Neerajaa, J, Varsha Madhavica, J, Janani, DM, Rekha, SN, et al. An epidemiological survey: effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertil Soc J. (2017) 22:313–6. doi: 10.1016/j.mefs.2017.05.007
2. Rudnicka, E, Suchta, K, Grymowicz, M, Calik-Ksepka, A, Smolarczyk, K, Duszewska, AM, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. (2021) 22:3789. doi: 10.3390/ijms22073789
3. Xu, Y, and Qiao, J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J Healthc Eng. (2022) 2022:9240569. doi: 10.1155/2022/9240569
4. Sarahian, N, Sarvazad, H, Sajadi, E, Rahnejat, N, and Eskandari Roozbahani, N. Investigation of common risk factors between polycystic ovary syndrome and Alzheimer’s disease: a narrative review. Reprod Health. (2021) 18:156. doi: 10.1186/s12978-021-01203-x
5. Bulsara, J, Patel, P, Soni, A, and Acharya, S. A review: brief insight into polycystic ovarian syndrome. Endocr Metab Sci. (2021) 3:100085. doi: 10.1016/j.endmts.2021.100085
6. Nautiyal, H, Imam, SS, Alshehri, S, Ghoneim, MM, Afzal, M, Alzarea, SI, et al. Polycystic ovarian syndrome: a complex disease with a genetics approach. Biomedicines. (2022) 10:540. doi: 10.3390/biomedicines10030540
7. Wang, L, Liang, R, Tang, Q, and Zhu, L. An overview of systematic reviews of using Chinese medicine to treat polycystic ovary syndrome. Evid Based Complement Alternat Med. (2021) 2021:9935536. doi: 10.1155/2021/9935536
8. Ojo, TK, Joshua, OO, Ogedegbe, OJ, Oluwole, O, Ademidun, A, and Jesuyajolu, D. Role of intermittent fasting in the management of prediabetes and type 2 diabetes mellitus. Cureus. (2022) 14:e28800. doi: 10.7759/cureus.28800
9. Al-Jafar, R, Wahyuni, NS, Belhaj, K, Ersi, MH, Boroghani, Z, Alreshidi, A, et al. The impact of Ramadan intermittent fasting on anthropometric measurements and body composition: evidence from LORANS study and a meta-analysis. Front Nutr. (2023) 10:1082217. doi: 10.3389/fnut.2023.1082217
10. Correia, JM, Santos, I, Pezarat-Correia, P, Silva, AM, and Mendonca, GV. Effects of Ramadan and non-Ramadan intermittent fasting on body composition: a systematic review and meta-analysis. Front Nutr. (2020) 7:625240. doi: 10.3389/fnut.2020.625240
11. Kumar, S, and Diamond, T. Ramadan fasting and maternal and fetal outcomes in pregnant women with diabetes mellitus: literature review. Front Endocrinol. (2022) 13:900153. doi: 10.3389/fendo.2022.900153
12. Faris, ME, Hussein, RN, Al-Kurd, RA, Al-Fararjeh, MA, Bustanji, YK, and Mohammad, MK. Impact of Ramadan intermittent fasting on oxidative stress measured by urinary 15-f(2t)-isoprostane. J Nutr Metab. (2012) 2012:802924. doi: 10.1155/2012/802924
13. Faris, ME, Jahrami, H, Abdelrahim, D, Bragazzi, N, and BaHammam, A. The effects of Ramadan intermittent fasting on liver function in healthy adults: a systematic review, meta-analysis, and meta-regression. Diabetes Res Clin Pract. (2021) 178:108951. doi: 10.1016/j.diabres.2021.108951
14. Faris, ME, Jahrami, H, BaHammam, A, Kalaji, Z, Madkour, M, and Hassanein, M. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on glucometabolic markers in healthy subjects. Diabetes Res Clin Pract. (2020) 165:108226. doi: 10.1016/j.diabres.2020.108226
15. Faris, ME, Jahrami, H, Obaideen, AA, and Madkour, MI. Impact of diurnal intermittent fasting during Ramadan on inflammatory and oxidative stress markers in healthy people: systematic review and meta-analysis. J Nutr Intermed Metab. (2019) 15:18–26. doi: 10.1016/j.jnim.2018.11.005
16. Faris, ME, Madkour, M, Obaideen, AK, Dalah, EZ, Hasan, HA, Radwan, H, et al. Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res Clin Pract. (2019) 153:166–75. doi: 10.1016/j.diabres.2019.05.023
17. Jahrami, HA, Alsibai, J, Clark, CCT, and Faris, ME. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur J Nutr. (2020) 59:2291–316. doi: 10.1007/s00394-020-02216-1
18. Jahrami, HA, Faris, ME, Janahi, AI, Janahi, MI, Abdelrahim, DN, Madkour, MI, et al. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis. (2021) 31:2273–301. doi: 10.1016/j.numecd.2021.05.002
19. Madkour, MI, Islam, MT, Tippetts, TS, Chowdhury, KH, Lesniewski, LA, Summers, SA, et al. Ramadan intermittent fasting is associated with ameliorated inflammatory markers and improved plasma sphingolipids/ceramides in subjects with obesity: lipidomics analysis. Sci Rep. (2023) 13:17322. doi: 10.1038/s41598-023-43862-9
20. Singh, S, Pal, N, Shubham, S, Sarma, DK, Verma, V, Marotta, F, et al. Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med. (2023) 12:1454. doi: 10.3390/jcm12041454
21. Di Lorenzo, M, Cacciapuoti, N, Lonardo, MS, Nasti, G, Gautiero, C, Belfiore, A, et al. Pathophysiology and nutritional approaches in polycystic ovary syndrome (PCOS): a comprehensive review. Curr Nutr Rep. (2023) 12:527–44. doi: 10.1007/s13668-023-00479-8
22. Sanchez-Garrido, MA, and Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. (2020) 35:100937. doi: 10.1016/j.molmet.2020.01.001
23. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Obaideen, K, Abu Shihab, KH, Madkour, MI, and Faris, ME. Seven decades of Ramadan intermittent fasting research: bibliometrics analysis, global trends, and future directions. Diabetes Metab Syndr. (2022) 16:102566. doi: 10.1016/j.dsx.2022.102566
25. Li, C, Xing, C, Zhang, J, Zhao, H, Shi, W, and He, B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. (2021) 19:148. doi: 10.1186/s12967-021-02817-2
26. Feyzioglu, BS, Guven, CM, and Avul, Z. Eight-hour time-restricted feeding: a strong candidate diet protocol for first-line therapy in polycystic ovary syndrome. Nutrients. (2023) 15:2260. doi: 10.3390/nu15102260
27. Asemi, Z, Samimi, M, Taghizadeh, M, Esmaillzadeh, A, and Alhasan, F. Effects of Ramadan fasting on glucose homeostasis, lipid profiles, inflammation and oxidative stress in women with polycystic ovary syndrome in Kashan, Iran. Arch Iran Med. (2015) 18:806–10.
28. Floyd, R, Gryson, R, Mockler, D, Gibney, J, Duggan, SN, and Behan, LA. The effect of time-restricted eating on insulin levels and insulin sensitivity in patients with polycystic ovarian syndrome: a systematic review. Int J Endocrinol. (2022) 2022:2830545. doi: 10.1155/2022/2830545
29. Hooshiar, SH, Yazdani, A, and Jafarnejad, S. Comparison of the effect of modified intermittent fasting and daily calorie restriction on sleep quality, anthropometric data, and body composition in women with obesity or overweight: study protocol of a randomized controlled trial. Trials. (2023) 24:30. doi: 10.1186/s13063-023-07070-0
30. Madkour, MI, Malhab, LJB, Abdel-Rahman, WM, Abdelrahim, DN, Saber-Ayad, M, and Faris, ME. Ramadan diurnal intermittent fasting is associated with attenuated FTO gene expression in subjects with overweight and obesity: a prospective cohort study. Front Nutr. (2022) 8:741811. doi: 10.3389/fnut.2021.741811
31. Carter, S, Clifton, PM, and Keogh, JB. Effect of intermittent compared with continuous energy restricted diet on Glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. (2018) 1:e180756. doi: 10.1001/jamanetworkopen.2018.0756
32. Cienfuegos, S, Corapi, S, Gabel, K, Ezpeleta, M, Kalam, F, Lin, S, et al. Effect of intermittent fasting on reproductive hormone levels in females and males: a review of human trials. Nutrients. (2022) 14:2343. doi: 10.3390/nu14112343
33. Han, Y, Lin, B, Lu, W, Wang, X, Tang, W, Tao, X, et al. Time-restricted feeding improves metabolic and endocrine profiles in mice with polycystic ovary syndrome. Front Endocrinol. (2022) 13:1057376. doi: 10.3389/fendo.2022.1057376
34. Faris, ME, Kacimi, S, Al-Kurd, RA, Fararjeh, MA, Bustanji, YK, Mohammad, MK, et al. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. (2012) 32:947–55. doi: 10.1016/j.nutres.2012.06.021
35. Madkour, MI, El-Serafi, AT, Jahrami, HA, Sherif, NM, Hassan, RE, Awadallah, S, et al. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res Clin Pract. (2019) 155:107801. doi: 10.1016/j.diabres.2019.107801
Keywords: time-restricted eating, alternate-day fasting, Ramadan fasting, diurnal fasting, gynecology, infertility
Citation: Kalsekar AS, Abdelrahim DN and Faris ME (2024) Effect of calorie restriction and intermittent fasting on glucose homeostasis, lipid profile, inflammatory, and hormonal markers in patients with polycystic ovary syndrome: a systematic review. Front. Nutr. 11:1362226. doi: 10.3389/fnut.2024.1362226
Received: 27 December 2023; Accepted: 13 March 2024;
Published: 05 April 2024.
Edited by:
Domenico Sergi, University of Ferrara, ItalyReviewed by:
Pradeep M. K. Nair, Mirakle Integrated Health Centre, IndiaCopyright © 2024 Kalsekar, Abdelrahim and Faris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: MoezAlIslam E. Faris, bWZhcmlzQHNoYXJqYWguYWMuYWU=; bW9lemZhcmlzQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.