- 1Department of Endocrinology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China
- 2Center for Mitochondrial Biology and Medicine, The Key Laboratory of Biomedical Information Engineering of Ministry of Education, School of Life Science and Technology, Xi’an Jiao tong University, Xi’an, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Jilin University, Changchun, Jilin, China
- 4Department of Internal Medicine, University of Manitoba, Winnipeg, MB, Canada
- 5Department of Community Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 6Department of Pharmacy, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China
- 7Department of Clinical Laboratory, The Second Hospital of Jilin University, Changchun, Jilin, China
Context: Osteoporotic fracture is a major public health issue globally. Human research on the association between amino acids (AAs) and fracture is still lacking.
Objective: To examine the association between AAs and recent osteoporotic fractures.
Methods: This age and sex matched incident case-control study identified 44 recent x-ray confirmed fracture cases in the Second Hospital of Jilin University and 88 community-based healthy controls aged 50+ years. Plasma AAs were measured by high performance liquid chromatography coupled with mass spectrometry. After adjusting for covariates (i.e., body mass index, milk intake >1 time/week, falls and physical activity), we conducted conditional logistical regression models to test the association between AAs and fracture.
Results: Among cases there were 23 (52.3%) hip fractures and 21 (47.7%) non-hip fractures. Total, essential, and non-essential AAs were significantly lower in cases than in controls. In the multivariable conditional logistic regression models, after adjusting for covariates, each standard deviation increase in the total (odds ratio [OR]: 0.304; 95% confidence interval [CI]: 0.117–0.794), essential (OR: 0.408; 95% CI: 0.181–0.923) and non-essential AAs (OR: 0.290; 95%CI: 0.107–0.782) was negatively associated with recent fracture. These inverse associations were mainly found for hip fracture, rather than non-hip fractures. Among these AAs, lysine, alanine, arginine, glutamine, histidine and piperamide showed the significantly negative associations with fracture.
Conclusion: There was a negative relationship between AAs and recent osteoporotic fracture; such relationship appeared to be more obvious for hip fracture.
1 Introduction
Osteoporotic fracture is a major public health problem in China. The prevalence of vertebral fractures in men and women aged 40 years or above was 10.5 and 9.7%, respectively (1). Complications such as constipation, stroke, pneumonia and arrhythmias increase after an osteoporotic fracture (2). The total 5-year mortality rates in fracture patients are 39% for women and 51% for men (3). In western China, the mean total costs for hip, vertebral and wrist fractures of the first year were estimated at RMB 57,585 per patient (approximately 9,140 US dollars) (4).
Fracture is a major clinical consequence of osteoporosis, a skeletal disease characterized by decreased bone mass and deteriorated bone microstructure (5). In both animal and human studies, AAs are linked with bone health. After 9 weeks of feeding arginine supplements, the femur bone mineral density (BMD) in experimental rats was significantly higher than in a control group that did not receive the supplements (6). In humans, higher levels of valine, leucine, isoleucine and tryptophan were associated with decreased hip BMD decline and higher BMD (7, 8). Valine, alanine, histidine and tryptophan were inversely associated with osteoporosis risk (9, 10).
Although there is evidence suggesting a link between AAs and BMD, few studies have examined the relationship between AAs and osteoporotic fracture. Lower plasma levels of ornithine, taurine, and aspartic acid were found in fracture patients when compared with controls (11). A previous case-control study also showed that the majority of essential and non-essential amino acids in fracture patients were significantly lower compared with healthy controls (12). Further, compared with healed-fracture patients, hypertrophic and atrophic nonunion patients had significantly lower levels of arginine and citrulline, respectively (13).
Thus, the aim of the present study was to examine the association between AAs and recent osteoporotic fractures. Previous research has shown the beneficial role of AAs for maintaining muscle and kidney health (14, 15). This research may help to extend our understanding about the relationship between AAs and bone health including bone healing.
2 Materials and methods
2.1 Study setting and subjects
Similar to our previous study (16), incident cases were confirmed to be new fractures in the Second Hospital of Jilin University in 2020. Using the survivor sampling methods, we selected controls from a community-based generally healthy population who had no history of fracture in Changchun, Jilin in 2020. Individuals who were 50 years or older and had never used osteoporosis-related medications were enrolled. We excluded controls with secondary osteoporosis. Cases with pathological fractures or incomplete fracture information were excluded. Cases were matched with controls by age (±4 years) and sex in a ratio of 1:2. Based on a pilot study of 4 fracture cases and 8 controls, and their corresponding total AA levels (1226.68 ± 301.00 μmol/L in cases and 1446.97 ± 94.57 μmol/L in controls), to achieve study power > 0.80 with a = 0.05, we estimated minimum sample sizes for cases and controls of 11 and 22, respectively. Written informed consent was obtained from all individual participants. The study protocol was approved by the institutional review boards (IRBs) of the School of Public Health, Jilin University (Project #: 2022-02-02).
2.2 Blood collection and measurement of amino acids
The blood collection method was described in a previous study (16). For cases, we collected blood samples within 2 days after hospitalization for fracture, but before any treatment (i.e., fracture fixation, hip replacement and medications). Blood samples for controls were collected at the time of interview. The blood samples were processed and refrigerated at −80°C until tested.
Circular pieces of plasma filter paper were created with a diameter of 3 mm. The metabolites were extracted with ethanol and the supernatant was extracted after centrifugation. After filtration, the supernatant was transferred to a 96-well plate. AA metabolite standards (Cambridge Isotope Laboratory, Tewksbury, MA, United States) and AA quality control solution were likewise transferred to the 96-well plate. The 96-well plate was first dried with nitrogen, then cultured with a 1-butanol acetyl chloride mixture and then dried again with nitrogen. The test sample was mixed with mobile phase solution (80% acetonitrile aqueous solution) and detected by high performance liquid chromatography coupled with mass spectrometry.
All AAs were classified as essential or non-essential. Essential AAs included leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine; non-essential AAs were alanine, asparagine, aspartic acid, arginine, citrulline, cysteine, glutamine, glutamic acid, glycine, homocysteine, histidine, ornithine, piperamide, proline, serine and tyrosine. Aromatic AAs such as phenylalanine, tyrosine, tryptophan and branched-chain AAs such as leucine and valine were also considered. Consistent with a previous study (12), essential, non-essential, aromatic, branched-chain, and total AAs were summed together for testing their overall effects. We also calculated AA ratios such as glycine to alanine, methionine to phenylalanine and valine to phenylalanine to determine the effects of specific AA catabolism.
2.3 Scertainment of covariates
Consistent with a previous study (16), we included the following covariates in this study: demographics (sex and age), lifestyle factors (e.g., physical activity, smoking status, milk intake >1 time/week and calcium supplement), postmenopausal status in females, disease history (e.g., coronary heart disease, type 2 diabetes, stroke), height loss of more than 3 cm after age 40 years, falls from standing height or less within the last 12 months, family history of osteoporosis and fractures, and body mass index (BMI). These factors were included because they were major risk factors for fractures (17, 18). Except for the measured body weight and height in controls, body weight and height in cases and all the other data were collected via face-to-face methods using a structured questionnaire. BMI was computed as weight divided by height2 (kg/m2).
2.4 Statistical analysis
We descriptively analyzed the baseline characteristics and AA levels by fracture status using frequencies, percentages, means and standard deviations (SD). We conducted multivariable conditional logistical regression models, which account for matching pairs of cases and controls, to test the association between AAs and fracture. AA values that followed a normal distribution, as assessed by skewness and kurtosis, were scaled per 1-SD increase. For AA values that were not normally distributed, values were expressed per 1-SD increase on the logarithmic scale. We adjusted for BMI, physical activity, milk intake >1 time/week and falls in the models, because they showed bivariate associations with fracture at alpha = 0.1. Model fit was assessed by examining R2 of AAs associated with fracture (0.392). Multiple testing was addressed using false discovery rate (FDR) analysis, which means the proportion of false discoveries (19). Since homocysteine is suggested to have negative impact on fracture risk (20), we estimated the association between AAs and fracture after excluding homocysteine from the analysis. Subgroup analyses by hip and non-hip fracture were also performed; we used all controls to increase the study power and adjusted for age, sex, body mass index, physical activity, smoking, milk intake >1 time/week, calcium supplement, history of coronary heart disease, type 2 diabetes and stroke, height loss >3 cm, falls, family history of osteoporosis and fractures in the unconditional logistic regression models. Lastly, we tested the association of aromatic and branched-chain AAs and AA ratios with fracture risk in the conditional logistic regression models. All conditional regression models were further adjusted for age and sex to avoid potential collider bias. Descriptive and logistic regression analyses were performed using the SPSS software (version 24.0; SPSS, Chicago, IL). We used the “MatchIt” and “fdrtool” packages in the R language (R 4.1.2) to conduct case-control matching and FDR analyses, respectively.
3 Results
3.1 Descriptive data
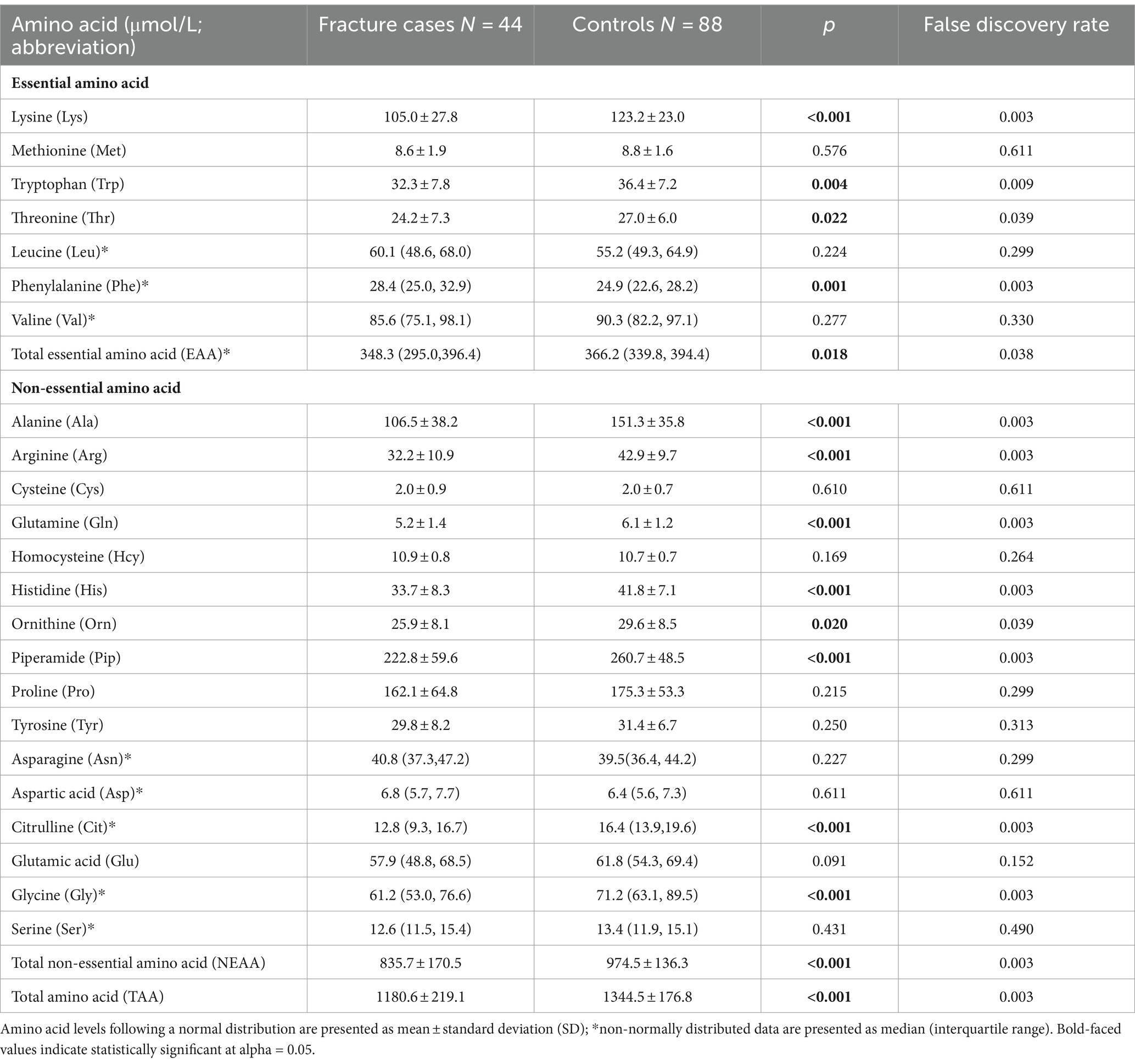
In this matched case-control study, we included 88 non-fracture controls and 44 fracture cases, which involved 23 (52.3%) hip fracture patients and 21 (47.7%) non-hip fracture patients (Table 1). Most fractures were due to falls (38.6%) or low-trauma sports injury (38.6%). As compared with controls, cases had significantly lower physical activity and milk intake >1 time/week, and a higher prevalence of falls; other characteristics including age, sex, BMI, smoking, calcium supplement, history of coronary heart disease, type 2 diabetes and stroke, height loss >3 cm, family history of osteoporosis and fractures, were not significantly different between cases and controls. All descriptive data by fracture status have been published (16).
3.2 Association between AAs and fracture
As shown in Table 1, total, essential and non-essential AAs were significantly lower in cases than controls. In the multivariable conditional logistic regression analysis, after adjusting for BMI, physical activity, milk intake >1 time/week and falls, total (odds ratio [OR]: 0.304; 95% confidence interval [CI]: 0.117–0.794; Figure 1), essential (OR: 0.408; 95% CI: 0.181–0.923) and non-essential AAs (OR: 0.290; 95% CI: 0.107–0.782) were negatively associated with fracture. Similar results were noted when we further adjusted for age and sex (Supplementary Figure S1). The FDRs for total, essential and non-essential AAs were 0.049, 0.09, and 0.049, respectively. After excluding homocysteine from analysis, the non-essential (OR: 0.289; 95%CI: 0.107–0.780) and total AAs (OR: 0.304; 95%CI: 0.117–0.793) also showed negative association with fracture. For the essential AAs, lysine showed the strongest association with fractures. Among the non-essential AAs, alanine, arginine, glutamine, histidine and piperamide levels were negatively associated with fracture. The negative relationship between AAs and fractures was mainly observed for hip fracture (Figure 2). Although aromatic and branched-chain AAs showed negative trends with fracture risk, ORs did not reach statistical significance (Supplementary Figure S2). The ratio of glycine to alanine was positively associated with fracture (Figure 3). In contrast, the ratios of methionine to phenylalanine and valine to phenylalanine were negatively associated with fracture. Again, these AA ratios had identical associations with fractures after further adjusting for age and sex (Supplementary Figure S3).
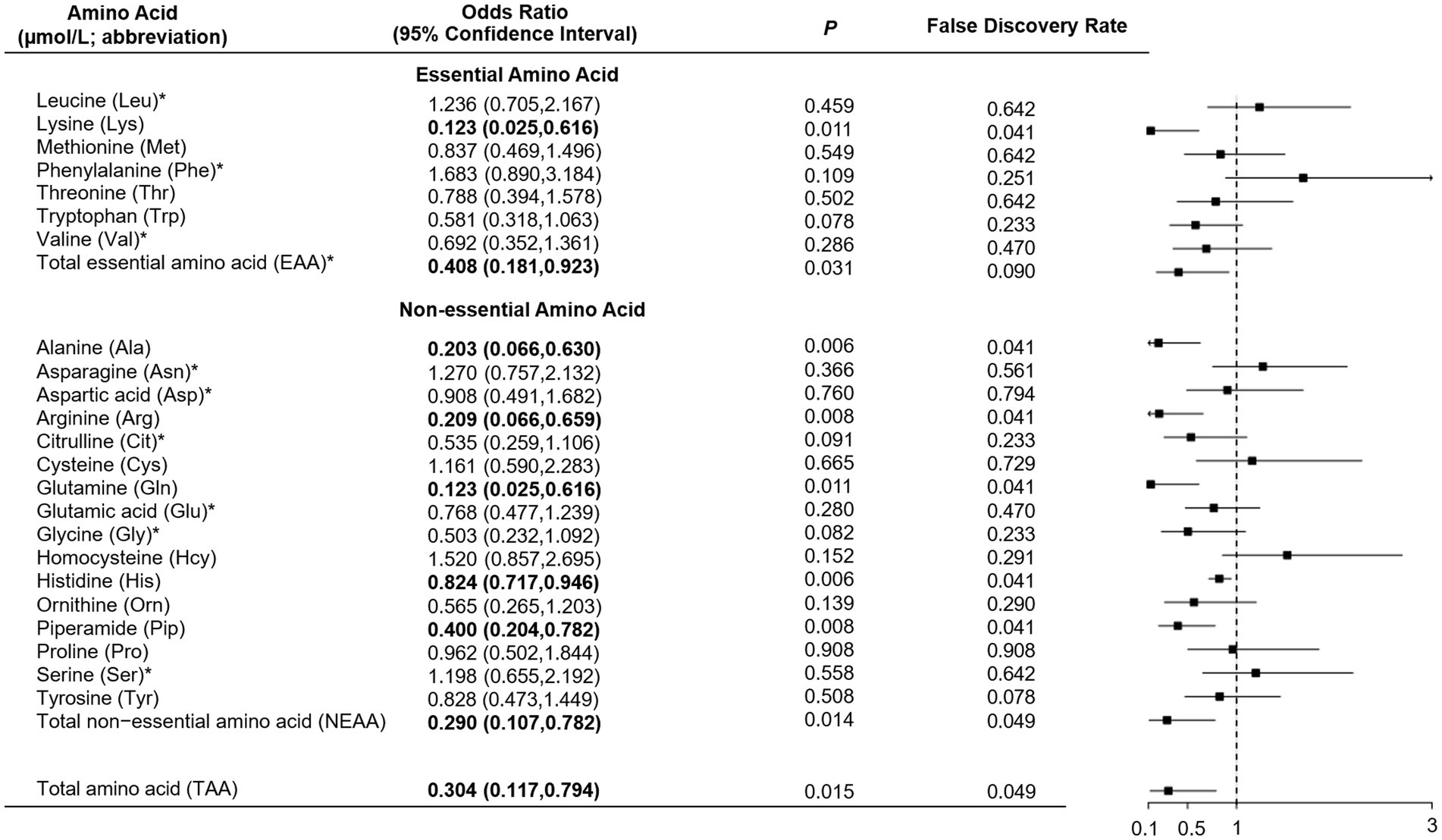
Figure 1. Multivariable conditional logistic regression analysis of the association between amino acid levels and fracture. *Odds ratio presented per 1-SD increase on the logarithmic scale; all the other odds ratios are presented per 1-SD increase on the linear scale. Odds ratios were adjusted for body mass index, physical activity, milk intake >1 time/week and falls.
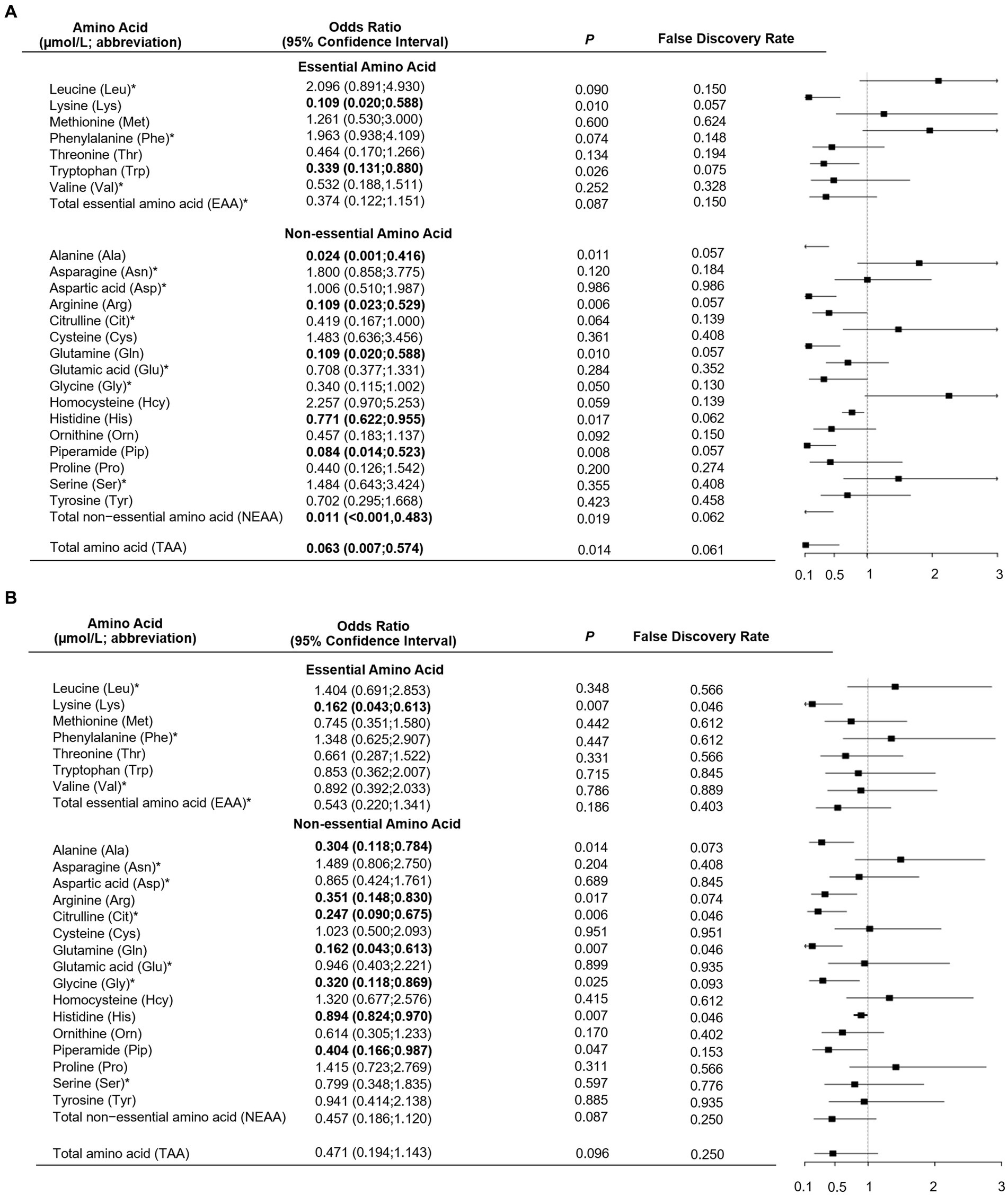
Figure 2. Multivariable unconditional logistic regression analysis of the association of amino acid levels with hip fracture (A) and non-hip fracture (B). *Odds ratio presented per 1-SD increase on the logarithmic scale; all the other odds ratios are presented per 1-SD increase on the linear scale. Odds ratios were adjusted for age, sex, body mass index, physical activity, smoking, milk intake >1 time/week, calcium supplement, history of coronary heart disease, type 2 diabetes and stroke, height loss >3 cm, falls, family history of osteoporosis and fractures.
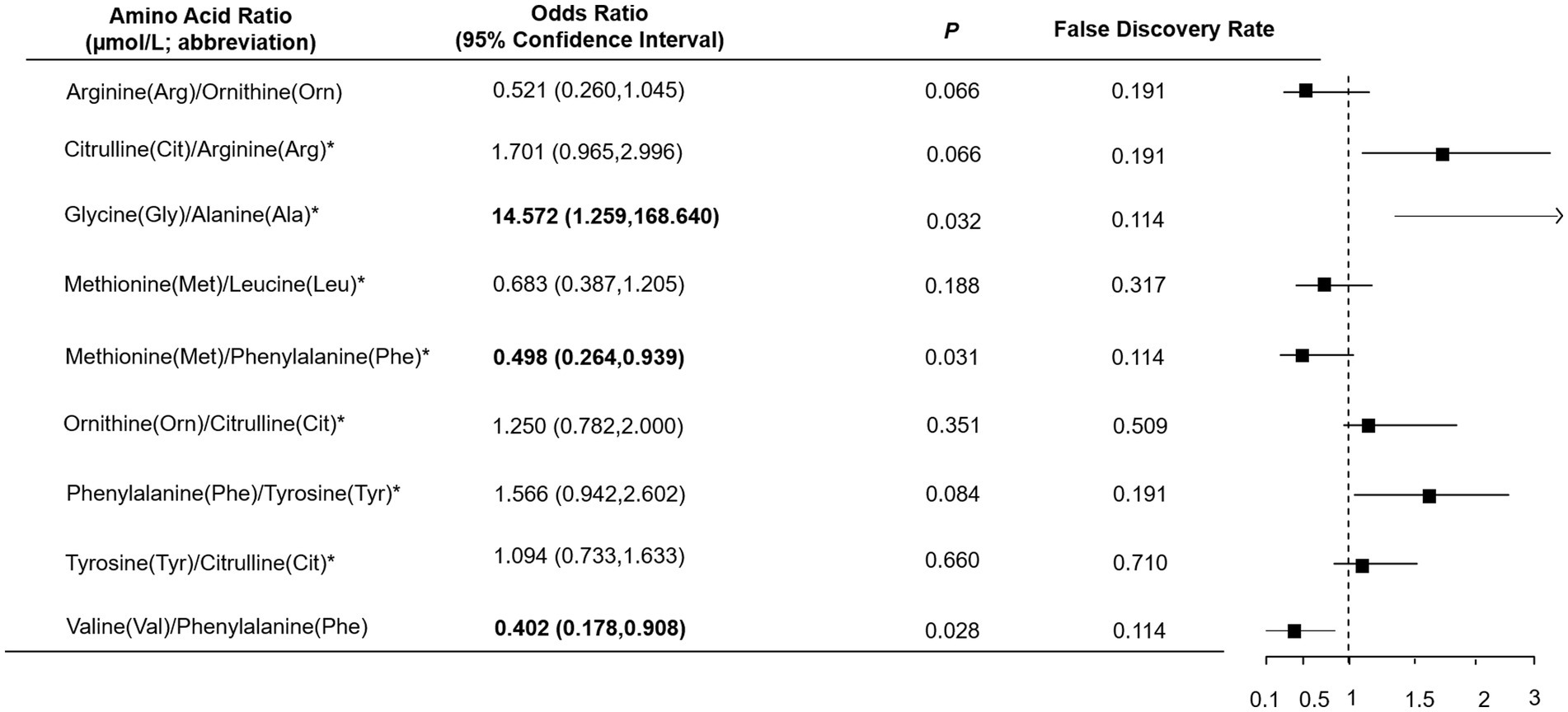
Figure 3. Multivariable logistic regression analysis of the association between amino acid ratio and fracture. *Odds ratio presented per 1-SD increase on the logarithmic scale; all the other odds ratios are presented per 1-SD increase on the linear scale. Odds ratios were adjusted for body mass index, physical activity, milk intake >1 time/week and falls.
4 Discussion
In this 1:2 individually matched case-control study, we found that higher levels of total, essential and non-essential AAs were negatively associated with osteoporotic fracture. The association was mainly found for hip fracture, rather than non-hip fracture. Among these AAs, lysine, alanine, arginine, glutamine, histidine and piperamide showed the strongest negative associations with fracture. The ratio of glycine to alanine was positively associated with fracture, while ratios of methionine to phenylalanine and valine to phenylalanine were negatively associated with fracture.
There is scant research to date investigating the association between AAs and osteoporotic fracture. Our study findings partially agree with a previous study, which found that levels of arginine and glutamine in men with normal BMD compared to those with osteopenia/osteoporosis were higher (21). Lysine may promote bone health through its ability to decrease urinary calcium excretion and enhance calcium absorption (22). Valine, tyrosine, and tryptophan levels have positive associations with BMD (10, 23). Consistent with this, we observed a non-significant inverse relationship between these AAs and osteoporotic fracture. Higher serum homocysteine was suggested to be a risk factor for fracture in postmenopausal women (20); a similar trend was observed in our study.
Our findings that higher valine to phenylalanine and lower glycine to alanine ratios were inversely associated with fracture are supported by prior research (9) which found that higher plasma levels of valine and alanine were negatively associated with osteoporosis in women. Similarly, in the female population, higher glycine levels were associated with lower BMD (24). Methionine was demonstrated to enhance osteoblast proliferation, activation, differentiation and BMD (25, 26), which is in line with our study finding that the ratio of methionine to phenylalanine is negatively associated with fracture.
Fracture remains a major public health issue. The negative association between AAs and fractures could have clinical implications for fracture healing. Significant catabolic response after fracture surgery has been found in a study, which showed that the levels of total AAs were lower in new fracture patients as compared with controls (12). Similarly, compared with healthy population and healed-fracture patients, the fracture and hypertrophic/atrophic nonunion patients exhibited lower levels of certain AAs (11, 13). A two-month human study showed that essential amino acid supplements could significantly increase the concentration of serum album (27). Among elderly fracture patients, higher albumin levels predict greater discharge Functional Independence Measurement scores (28), which is a functional status instrument for rehabilitation inpatients (29). Lower levels of serum albumin were significantly associated with greater length of stay and in-hospital mortality in institutionalized patients with hip fracture (30). A randomized controlled trial demonstrated that after two-months of essential amino acid supplements, fracture patients with sarcopenia showed significant improvements in appendicular muscle strength and physical performance (31). During 20 weeks of feeding foods rich in arginine and lysine, rats in the test group exhibited better fracture healing as compared with the control group (32). Several mechanisms may account for this beneficial effect. Nitric oxide is derived from l-arginine (33). Supplements of arginine increase the synthesis of nitric oxide, which could increase vascularity and angiogenesis (32). Lastly, l-lysine could stimulate intestinal absorption and renal conservation of calcium, which is significantly associated with increased BMD (34, 35).
Some study limitations are acknowledged. Although we found fracture patients had lower AAs than controls, the specific impact of fracture and its related factors (i.e., hospitalization, non-weight bearing and fasting) on AAs are still unclear. Further studies are warranted to examine this. Although AA profiles may have predicted reduced bone loss and lower risk of incident fracture (8), this cannot be tested in our case-control study as we collected blood samples after fracture occurrence and we cannot make causal inferences. Another limitation is that our study had a small sample size. However, based on our sample size calculation, the sample size is likely to be sufficient for testing the relationship between AAs and fracture. BMD of participants was not measured; protein and vitamin D intake levels were also not collected. However, a study suggested that the relationship between AAs and fracture risk was independent of diet and lifestyle factors (8). We did not have data on educational level and marital status in our study; these two factors are associated with sex and many conditions, including fractures (36–38). Data on body weight and height were self-reported. We cannot exclude the potential of confounding and information bias. Cases were identified from a hospital and controls were from a community-based population. Cases and controls were only matched on age and sex. Population selection and matching on limited factors may bias the reported results. Lastly, due to small sample size, subgroup results by fracture site and AA type lack reliability. Future validations are warranted.
5 Conclusion
In this matched case-control study, higher levels of AAs were negatively associated with osteoporotic fracture. This association appeared to be stronger for hip fracture than non-hip fracture. These findings, if confirmed by larger prospective studies, may have clinical implications for fracture healing. These findings extend our understandings about the beneficial effects of AAs on human health (i.e., muscle and kidney health) (14, 15).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review boards (IRBs) of the School of Public Health, Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BL: Conceptualization, Formal analysis, Methodology, Writing – review & editing. XinS: Conceptualization, Formal analysis, Methodology, Writing – review & editing. XW: Writing – original draft. CM: Writing – review & editing. WL: Writing – review & editing. LL: Writing – review & editing. XiaS: Writing – review & editing. BK: Funding acquisition, Writing – review & editing. SY: Conceptualization, Formal analysis, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported in part by Seed Funding from The First Affiliated Hospital of Jinzhou Medical University and the Norman Bethune Program, Jilin University (Grant Number: 2023B11). LL is supported by a Tier 1 Canada Research Chair.
Acknowledgments
We are especially grateful to the participants involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1360959/full#supplementary-material
References
1. Wang, L, Yu, W, Yin, X, Cui, L, Tang, S, Jiang, N, et al. Prevalence of osteoporosis and fracture in China: the China osteoporosis prevalence study. JAMA Netw Open. (2021) 4:e2121106. doi: 10.1001/jamanetworkopen.2021.21106
2. Liu, R, Chao, A, Wang, K, and Wu, J. Incidence and risk factors of medical complications and direct medical costs after osteoporotic fracture among patients in China. Arch Osteoporos. (2018) 13:12. doi: 10.1007/s11657-018-0429-5
3. Bliuc, D, Nguyen, ND, Nguyen, TV, Eisman, JA, and Center, JR. Compound risk of high mortality following osteoporotic fracture and Refracture in elderly women and men. J Bone Miner Res. (2013) 28:2317–24. doi: 10.1002/jbmr.1968
4. Qu, B, Ma, Y, Yan, M, Wu, HH, Fan, L, Liao, DF, et al. The economic burden of fracture patients with osteoporosis in Western China. Osteoporos Int. (2014) 25:1853–60. doi: 10.1007/s00198-014-2699-0
5. Compston, JE, McClung, MR, and Leslie, WD. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/s0140-6736(18)32112-3
6. Choi, MJ, and Chang, KJ. Effect of dietary taurine and arginine supplementation on bone mineral density in growing female rats. Adv Exp Med Biol. (2013) 776:335–45. doi: 10.1007/978-1-4614-6093-0_31
7. Carbone, L, Bůžková, P, Fink, HA, Robbins, JA, Barzilay, JI, Elam, RE, et al. Plasma levels of branched chain amino acids, incident hip fractures, and bone mineral density of the hip and spine. J Clin Endocrinol Metab. (2023) 108:e1358–64. doi: 10.1210/clinem/dgad275
8. Su, Y, Elshorbagy, A, Turner, C, Refsum, H, Chan, R, and Kwok, T. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone. (2019) 129:115082. doi: 10.1016/j.bone.2019.115082
9. Panahi, N, Fahimfar, N, Roshani, S, Arjmand, B, Gharibzadeh, S, Shafiee, G, et al. Association of Amino Acid Metabolites with osteoporosis, a Metabolomic approach: Bushehr elderly health program. Metabolomics. (2022) 18:63. doi: 10.1007/s11306-022-01919-2
10. Pernow, Y, Thorén, M, Sääf, M, Fernholm, R, Anderstam, B, Hauge, EM, et al. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone. (2010) 47:959–65. doi: 10.1016/j.bone.2010.08.017
11. Woolf, LI, Grovers, AC, Moore, JP, Duff, JH, Finley, RJ, and Loomer, RL. Arterial plasma amino acids in patients with serious postoperative infection and in patients with major fractures. Surgery. (1976) 79:283–92.
12. Long, CL, Geiger, JW, Richards, EW, Akin, JM, and Blakemore, WS. Plasma amino acid concentrations in geriatric control and hip-fracture patients. Am J Clin Nutr. (1992) 55:1135–41. doi: 10.1093/ajcn/55.6.1135
13. Wijnands, KA, Brink, PR, Weijers, PH, Dejong, CH, and Poeze, M. Impaired fracture healing associated with amino acid disturbances. Am J Clin Nutr. (2012) 95:1270–7. doi: 10.3945/ajcn.110.009209
14. Li, XY, Zheng, SX, and Wu, GY. Amino acid metabolism in the kidneys: nutritional and physiological significance. Adv Exp Med Biol. (2020) 1265:71–95. doi: 10.1007/978-3-030-45328-2_5
15. Posey, EA, Bazer, FW, and Wu, GY. Amino acids and their metabolites for improving human exercising performance. Adv Exp Med Biol. (2021) 1332:151–66. doi: 10.1007/978-3-030-74180-8_9
16. Yang, S, Feng, L, Lix, LM, Leslie, WD, Guo, D, Shi, X, et al. Global biomarkers of oxidative stress and fractures: a matched case-control study. Front Endocrinol (Lausanne). (2023) 14:1179521. doi: 10.3389/fendo.2023.1179521
17. Kanis, JA, Oden, A, Johansson, H, Borgström, F, Ström, O, and McCloskey, E. Frax and its applications to clinical practice. Bone. (2009) 44:734–43. doi: 10.1016/j.bone.2009.01.373
18. Zhang, HM, Liu, HL, Wang, X, Chen, W, Chen, D, Zhang, ZZ, et al. Clinical value of self-assessment risk of osteoporosis in Chinese. Open Med (Wars). (2016) 11:190–5. doi: 10.1515/med-2016-0036
19. Chen, X, Robinson, DG, and Storey, JD. The functional false discovery rate with applications to genomics. Biostatistics. (2021) 22:68–81. doi: 10.1093/biostatistics/kxz010
20. Kuroda, T, Tanaka, S, Saito, M, Shiraki, Y, and Shiraki, M. Plasma level of homocysteine associated with severe vertebral fracture in postmenopausal women. Calcif Tissue Int. (2013) 93:269–75. doi: 10.1007/s00223-013-9754-2
21. Wang, J, Yan, D, Zhao, A, Hou, X, Zheng, X, Chen, P, et al. Discovery of potential biomarkers for osteoporosis using Lc-Ms/Ms Metabolomic methods. Osteoporos Int. (2019) 30:1491–9. doi: 10.1007/s00198-019-04892-0
22. Aggarwal, R, and Bains, K. Protein, lysine and vitamin D: critical role in muscle and bone health. Crit Rev Food Sci Nutr. (2022) 62:2548–59. doi: 10.1080/10408398.2020.1855101
23. Palacios-González, B, Ramírez-Salazar, EG, Rivera-Paredez, B, Quiterio, M, Flores, YN, Macias-Kauffer, L, et al. A multi-Omic analysis for low bone mineral density in postmenopausal women suggests a relationship between diet, metabolites, and microbiota. Microorganisms. (2020) 8:1630. doi: 10.3390/microorganisms8111630
24. Palacios-González, B, León-Reyes, G, Rivera-Paredez, B, Ibarra-González, I, Vela-Amieva, M, Flores, YN, et al. Serum metabolite profile associated with sex-dependent visceral adiposity index and low bone mineral density in a Mexican population. Meta. (2021) 11:604. doi: 10.3390/metabo11090604
25. Vijayan, V, Khandelwal, M, Manglani, K, Gupta, S, and Surolia, A. Methionine Down-regulates Tlr4/Myd88/Nf-Κb Signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br J Pharmacol. (2014) 171:107–21. doi: 10.1111/bph.12434
26. Lv, Z, Shi, W, and Zhang, Q. Role of essential amino acids in age-induced bone loss. Int J Mol Sci. (2022) 23:11281. doi: 10.3390/ijms231911281
27. Aquilani, R, Zuccarelli, GC, Condino, AM, Catani, M, Rutili, C, Del Vecchio, C, et al. Despite inflammation, supplemented essential amino acids may improve circulating levels of albumin and Haemoglobin in patients after hip fractures. Nutrients. (2017) 9:637. doi: 10.3390/nu9060637
28. Mizrahi, EH, Fleissig, Y, Arad, M, Blumstein, T, and Adunsky, A. Admission albumin levels and functional outcome of elderly hip fracture patients: is it that important? Aging Clin Exp Res. (2007) 19:284–9. doi: 10.1007/bf03324703
29. Dodds, TA, Martin, DP, Stolov, WC, and Deyo, RA. A validation of the functional Independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. (1993) 74:531–6. doi: 10.1016/0003-9993(93)90119-u
30. Koval, KJ, Maurer, SG, Su, ET, Aharonoff, GB, and Zuckerman, JD. The effects of nutritional status on outcome after hip fracture. J Orthop Trauma. (1999) 13:164–9. doi: 10.1097/00005131-199903000-00003
31. Invernizzi, M, de Sire, A, D'Andrea, F, Carrera, D, Renò, F, Migliaccio, S, et al. Effects of essential amino acid supplementation and rehabilitation on functioning in hip fracture patients: a pilot randomized controlled trial. Aging Clin Exp Res. (2019) 31:1517–24. doi: 10.1007/s40520-018-1090-y
32. Sinha, S, and Goel, SC. Effect of amino acids lysine and arginine on fracture healing in rabbits: a radiological and Histomorphological analysis. Indian J Orthop. (2009) 43:328–34. doi: 10.4103/0019-5413.55972
33. Diwan, AD, Wang, MX, Jang, D, Zhu, W, and Murrell, GA. Nitric oxide modulates fracture healing. J Bone Miner Res. (2000) 15:342–51. doi: 10.1359/jbmr.2000.15.2.342
34. Civitelli, R, Villareal, DT, Agnusdei, D, Nardi, P, Avioli, LV, and Gennari, C. Dietary L-lysine and calcium metabolism in humans. Nutrition. (1992) 8:400–5.
35. Reid, IR, Mason, B, Horne, A, Ames, R, Reid, HE, Bava, U, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. (2006) 119:777–85. doi: 10.1016/j.amjmed.2006.02.038
36. Giannico, OV, Ambrosino, I, Patano, F, Germinario, C, Quarto, M, and Moretti, AM. Educational level, marital status and sex as social gender discharge determinants in chronic obstructive pulmonary disease exacerbations: a time-to-event analysis. Monaldi Arch Chest Dis. (2019) 89:171–8. doi: 10.4081/monaldi.2019.1017
37. Mincuzzi, A, Carone, S, Galluzzo, C, Tanzarella, M, Lagravinese, GM, Bruni, A, et al. Gender differences, environmental pressures, tumor characteristics, and death rate in a lung Cancer cohort: a seven-years Bayesian survival analysis using Cancer registry data from a contaminated area in Italy. Front Public Health. (2024) 11:11. doi: 10.3389/fpubh.2023.1278416
Keywords: fracture, amino acids, osteoporosis, metabolomics, bone health
Citation: Liang B, Shi X, Wang X, Ma C, Leslie WD, Lix LM, Shi X, Kan B and Yang S (2024) Association between amino acids and recent osteoporotic fracture: a matched incident case-control study. Front. Nutr. 11:1360959. doi: 10.3389/fnut.2024.1360959
Edited by:
Roberta Zupo, University of Bari Aldo Moro, ItalyReviewed by:
Zitian Zheng, Peking University, ChinaOrazio Valerio Giannico, Local Health Authority of Taranto, Italy
Copyright © 2024 Liang, Shi, Wang, Ma, Leslie, Lix, Shi, Kan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuman Yang, c2h1bWFueWFuZ0BqbHUuZWR1LmNu
†ORCID: Shuman Yang, https://orcid.org/0000-0002-9169-5850
 Bing Liang1,2
Bing Liang1,2 William D. Leslie
William D. Leslie Lisa M. Lix
Lisa M. Lix Bo Kan
Bo Kan Shuman Yang
Shuman Yang