- 1Postgraduate College, Córdoba Campus, Amatlán de los Reyes, Mexico
- 2Veracruzana University, Faculty of Chemical Sciences, Orizaba, Mexico
Consumer demand for healthier confectionery products has prompted the confectionery industry to create products that are reduced in sugar content and supplemented with vitamins, antioxidants or biological elements beneficial to health. The aim of this study was to develop marshmallows enriched with Apis mellifera honey and Lactobacillus rhamnosus and to evaluate the effect of honey concentration and gelatin bloom degrees on marshmallow properties. A completely randomized design with a factorial structure was applied with different honey concentrations (0, 50 and 75%) and at different gelatin bloom degrees (265, 300 and 315 bloom degrees); moreover, the physicochemical properties, total phenol content and antioxidant activity of the marshmallow were studied, as well as the viability of the probiotic. The physicochemical properties of the marshmallows were found to be adequate and showed good stability over time. The concentration of honey and gelatin bloom degrees did not significantly affect probiotic viability. The density of the marshmallows decreased as the percentage of honey increased. Additionally, the pH was lower at higher honey concentrations. The marshmallow with 75% honey and 265 bloom degrees had a higher °Brix value. The honey treatments exhibited higher levels of total antioxidant activity and total phenolic compounds than the sugar-only marshmallows. However, the bloom degrees did not have a significant impact on the antioxidant activity and total phenolic compound content. Although the probiotics did not reach the minimum viability needed, their use as paraprobiotics can be considered.
1 Introduction
Confectionery products are classified as sugar confections and bakers’ confections; the former includes hard candies, soft candies and aerated candies (1). Among the aerated candies are marshmallows, which are masses of sugar, glucose or other sweeteners that are stabilized with protein substances such as albumins and gelatin (2).
The global confectionery market, valued in 2019 at US$201.3 billion, is growing at an annual rate of 3.6% and is projected to reach US$270.5 billion by 2027 (3); from a health point of view, this increase in the candy market is worrying, since the intake of added sugars is one of the main causes of obesity and diabetes in the world, so the rates of diseases related to sugar consumption may increase accordingly. However, the medicated confectionery segment offers functional products that in turn meet consumer demands for sweet products. In this regard, an annual growth rate of 4.1% is forecast for this segment during 2019–2027 (3).
Functional foods contain bioactive compounds that bring benefits beyond their nutritional properties. These contribute to improving physiological functions, preventing and/or mitigating the incidence of chronic diseases such as obesity, hypertension, diabetes and cancer (4). Within the classification of functional foods are foods or beverages to which a component beneficial to health has been added, such as omega-3, fiber, biological components, and antioxidants.
Among the beneficial components that can be added to functional foods is honey. Honey is a natural sweetener with various health benefits, including hepatoprotective, antioxidant, anti-obesity, hypolipidemic, and hypoglycemic properties. Honey also has anti-atherosclerotic, anti-cancer, hypotensive, immunomodulatory and neuroprotective effects (5). In addition, honey has a greater sweetening power than sugar, so it can partially or totally replace the use of sugar in confectionery products.
Other components that a functional food can include are probiotics, which are live microorganisms that, when administered in adequate amounts, generate a health benefit. Probiotics are often considered a safe and low-cost alternative for treating a variety of chronic diseases and improving overall human health. Probiotics are recognized for their ability to modulate host immunity and protect against several infectious and non-infectious pathologies. Some important probiotic attributes include pathogen killing, colonization, and host cell induction, which affect various host functions (6). To ensure that probiotics added to functional foods remain physiologically active at the time of consumption, it is necessary to maintain their stability and viability through technologies such as encapsulation techniques. These techniques protect probiotics from heat treatments, storage, and gastrointestinal conditions (7).
Lactobacillus rhamnosus GG (LGG) is a widely used probiotic strain with well-documented health effects. These include preventing and treating gastrointestinal infections and diarrhea, stimulating immune responses that promote vaccination, and potentially preventing certain allergic symptoms (8). Additionally, Szajewska and Hojsak (9) provided evidence that LGG can prevent antibiotic- or healthcare-associated diarrhea and reduce symptoms of acute gastroenteritis, mainly among children in Europe. Therefore, a marshmallow containing L. rhamnosus could be an attractive way for people of all ages to consume probiotics, particularly for children.
A few studies have been reported on the development of marshmallows with functional properties, all with the addition of extracts or compounds with antioxidant properties, but not with probiotics. Periche et al. (10) developed a marshmallow using stevia extracts, oligofructose, and isomaltulose as sugar replacers. They found that the sucrose and glucose syrup in commercial marshmallows could be replaced by a mixture of these replacers. Artamonova et al. (11) produced marshmallows using natural anthocyanin dyes derived from Sudanese rose and black chokeberry, resulting in high-quality characteristics. Santoso et al. (12) developed marshmallows with added kinang (chew of betel) extract, which exhibited antibacterial and antioxidant activity, as well as caries inhibition. Milea et al. (13) incorporated anthocyanins from sweet cherry skins in the development of marshmallows, resulting in increased anthocyanin content and antioxidant activity over time.
Based on the above, the aim of this work was to develop a marshmallow enriched with Apis mellifera bee honey and encapsulated Lactobacillus rhamnosus, as well as to evaluate its physicochemical properties, antioxidant capacity, phenolic compounds and probiotic viability.
2 Materials and methods
2.1 Materials used
We used the microorganism Lactobacillus rhamnosus obtained from a lyophilized strain (Vivolac, United States), broth culture medium and Man Rogosa Sharpe (MRS) agar (BD Difco, Mexico and Sigma Aldrich, United States), sodium alginate (Sigma Aldrich, USA), calcium chloride (CaCl2) (Química Mercurio, Mexico), multifloral honey from the Apis mellifera bee from the community of Zimatlán de Álvarez, gelatin at 265, 300 and 315 °Bloom (Diamante, Progel mexicana, S.A. de C. V), and standard sugar purchased at a supermarket.
2.2 Characterization of bee honey
Honey was harvested in accordance with AOAC 920.180 (2019). For the following analyses, the crystallized honey was placed in a water bath at a maximum temperature of 60°C until the crystals dissolved. These analyses determined color (14) with a photometer (HANNA, Model 96,785, Italy), pH with a potentiometer (Oakton Eco Testr pH 2, WD-35423-10, United States), total acidity (AOAC 962.19), ash (AOAC 920.181), electrical conductivity with a conductivity meter (HANNA, DiST3, Italy), moisture (AOAC 969.38 B) with a honey refractometer (Olimpo, RH-5890Be, China), reducing sugars (AOAC 920.183b), total soluble solids (AOAC 932.12) with a honey refractometer (Olimpo, RH-5890Be, China) and viscosity (Vibro Viscomer, model SV-10/SV-100, Japan).
2.2.1 Hydroxymethylfurfural (HMF)
HMF is a yellow solid compound with great solubility in water, and its structure is a six-carbon heterocyclic structure with two functional groups: aldehyde and alcohol (hydroxymethyl). The amount of HMF present in honey is indicative of its freshness, and reflects its and storage length and conditions (15). The honey was heated in a water bath to 40°C to remove impurities and filtered. Five grams of sample was homogenized in 20 mL of distilled water, and HMF was measured at 25°C with a reflectometer (HANNA, RQflex 10, Italy).
2.2.2 Extraction of bioactive compounds from honey
The amount of honey used for the extraction of bioactive compounds varied depending on the analysis to be performed. Two grams were used for the determination of antioxidant activity, and 1 g was used for the quantification of total phenolic compounds. Ten milliliters of acidified water (adjusted to pH 2 with 2 N HCl) was added to the sample (16), homogenized for 1 min using a vortex device and subjected to an ultrasonic bath (Auto science, Serial Ultrasonic Cleaner, 110 V/60 Hz, China) for 30 min at 25°C. Subsequently, the sample was left to macerate for 24 h in the dark at a temperature of 25°C. Afterward, the extract was centrifuged at 18.0 x g for 15 min at a temperature of 27°C, the supernatant was filtered, and the resulting product was preserved in amber tubes.
2.2.3 Determination of antioxidant capacity (AC)
Antioxidant activity was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) synthetic free radical method (16). Absorbance was measured at a wavelength of 517 nm in a UV spectrophotometer (Thermo Fisher Scientific, Genesys 10-S, United States). A calibration curve was performed using Trolox at concentrations of 0–100 μg/mL in methanol. From the absorbance readings obtained, the antioxidant activity was calculated, and the results were expressed as mg Trolox/kg honey.
2.2.4 Determination of total phenolic compounds (TPC)
The concentration of total phenolic compounds in honey was determined by the Folin–Ciocalteu method (16). Absorbance was measured in a UV spectrophotometer (Thermo Fisher Scientific, Genesys 10-S, United States) at a wavelength of 760 nm. A calibration curve was performed using gallic acid as a standard at concentrations of 0–100 mg/mL, and the results were expressed as mg gallic acid/kg honey.
2.3 Growth kinetics of Lactobacillus rhamnosus
The growth kinetics of Lactobacillus were obtained with the objective of determining the moment at which the largest number of viable cells was obtained to carry out the encapsulation of the microorganism. To determine the growth kinetics, 1% (v/v) L. rhamnosus inoculum was added to 100 mL of MRS broth previously sterilized (121°C for 15 min) and incubated (Riossa, ECF-82, Mexico) under anaerobic conditions at 32°C for 24 h, and samples were taken every 4 h. The pour plate method was applied using serial dilutions of each sample in triplicate. Colony count sensitivity ranged from 30 to 300 colonies. The Gompertz model equation was used to determine the kinetic parameters using SAS version 9.4 software.
2.4 Activation of Lactobacillus rhamnosus
Culture inoculum suspended in MRS broth with glycerol (1:1), which was stored at −20°C, was used. It was employed at a concentration of 1% (v/v) in MRS broth and incubated (Riossa, ECF-82. D, Mexico) under anaerobic conditions at a temperature of 32°C for 18 h (17). Subsequently, it was centrifuged (Eppendorf, Centrifuge 5,810 R, Germany) at 18 x g at 4°C for 20 min. The supernatant was filtered, and the cell concentrate was washed with sterile peptone water (0.1% w/v) several times to discard any residue from the culture medium (18).
2.5 Encapsulation of Lactobacillus rhamnosus
To prepare 100 g of encapsulation solution, 2% (w/w) sodium alginate, 0.2% gelatin, 5% nopal mucilage (1:2 w/v) and 92.8% distilled water were mixed (19). The solution was placed in an autoclave at 108°C for 5 min for sterilization. For encapsulation, work was carried out under sterile conditions; 9 mL of polymer mixture previously heated to 37°C was taken, and 1 g of lactobacillus cell concentrate was added. Then, the mixture was homogenized on a stirring plate at 400 rpm for 15 min and kept at rest for 1 h to achieve cross-linking of the capsules. Subsequently, the solution was filtered, and the collected capsules were washed with sterile distilled water, placed in a 0.1% sterile peptone water solution and stored at 4°C.
2.6 Making the marshmallows
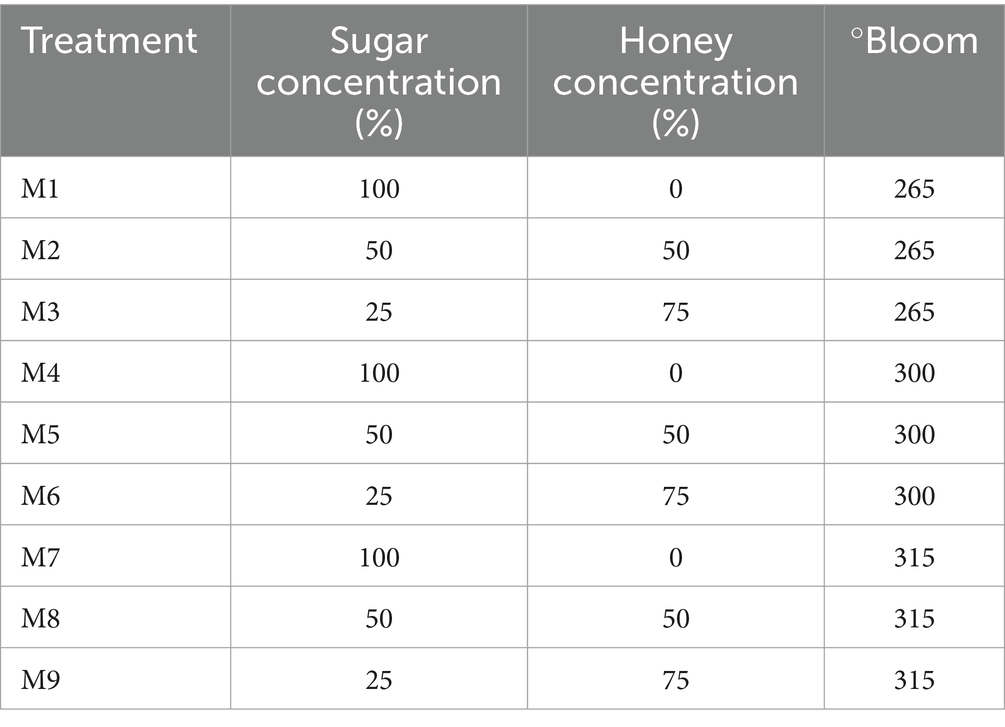
The marshmallows were made following the methodology of the Federal Consumer Protection Agency (20) with some modifications. Table 1 shows the concentrations of sugar (100% = 47 g) and honey, as well as the bloom degrees of the gelatins used.
To prepare the caramel, 5 g of glucose, sugar (depending on the treatment) and water in proportion to the amount of sugar (12 mL for 100% sugar) were heated to 121°C. On the other hand, to make the marshmallows, 5 mL of egg albumin was beaten at 280 rpm for 2 min using a mixer (KitchenAid, Artisan KSM150PSER, United States) until a shiny foam formed that adhered to the container. The speed was lowered to 180 rpm, and the previously prepared caramel was added. Once the caramel had been incorporated, the beating speed was increased to 280 rpm and maintained for another 2 min. Subsequently, gelatin (previously hydrated with water 1:5 w/v at 20°C for 10 min and heated to 80°C in a water bath) was added, beating at 225 rpm, and once incorporated, it was beaten at 280 rpm until the mixture cooled to a temperature of 40°C. Immediately afterward, honey preheated to 40°C was added, maintaining a beating speed of 280 rpm for 3 min; then, the beating speed was reduced to 180 rpm, and 10 g of probiotic capsules was added without stopping beating for another 2 min. The foam was then immediately poured into molds with corn starch, and a layer of starch was placed to cover the foam, which was left to dry for 24 h at 20°C. After this time, the excess cornstarch was removed from the marshmallows, and they were stored individually in polypropylene bags. The marshmallows were stored in a dry place at 25°C.
2.7 Physicochemical analyses of the marshmallows
Ash (AOAC 900.02 B), pH with a potentiometer (Oakton Eco Testr pH 2, WD-35423-10, United States), degrees Brix (AOAC 932.12) using a refractometer (Olimpo, RH-5890Be, China) and moisture (AOAC 925.45A) were determined.
2.7.1 Density
The density of the marshmallows was determined in a cylindrical container with a known volume and weight. The foam was uniformly poured into the container and weighed to calculate its mass by weight difference and subsequently its density in g/mL.
2.7.2 Extraction of bioactive compounds in marshmallow
For the determination of phenolic compounds and antioxidant capacity, extracts of the different marshmallow treatments were prepared. For this, freeze-dried samples were used at a vacuum pressure of 9.5 × 10 mm Hg for 24 h (10). Each extract was prepared by mixing 2 g of freeze-dried sample with acidified water (pH 2) at a 1:2 w/v ratio. This solution was homogenized in a vortex and subjected to ultrasonication for 30 min. It was then macerated in the dark for 24 h at a temperature of 25°C. After this time, the solution was centrifuged at 18.0 x g at 27°C for 20 min and finally filtered to remove the supernatant. The extracts were kept in amber tubes prior to use in the corresponding analyses.
2.7.3 Determination of antioxidant capacity (AC)
To determine the antioxidant capacity in marshmallows, the same methodology used for honey was implemented (16), with modification of the resting conditions; in this case, they were in a water bath at 37°C for 30 min.
2.7.4 Determination of total phenolic compounds (TPC)
The determination of total phenolic compounds was carried out using the Folin Ciocalteu method, according to the methodology of Cedeño-Pinos et al. (21), which was slightly modified. First, 0.25 mL of marshmallow extract was mixed with 7.75 mL of distilled water and 0.8 mL of 10% Folin–Ciocalteu reagent. This solution was left to stand for 8 min, and then 7.5% (w/v) Na2CO3 was added and homogenized. This mixture was left to stand in the dark at 25°C for 2 h. Finally, absorbance was determined at 760 nm in a spectrophotometer (Thermo Fisher Scientific, Genesys 10-S, USA).
2.7.5 Viability of Lactobacillus rhamnosus encapsulated in marshmallow
To determine the viability of the microorganism, 1 g of marshmallow was added to 9 mL of 5% (w/v) sodium citrate, and then the mixture was stirred for 15 min to disintegrate the capsules and release the cells. The first dilution was performed with 1 mL of the solution in 9 mL of 0.1% peptone water. It was sown in Petri dishes by the pour plate technique from the 10−5 to 10−8 dilution in triplicate using MRS agar as culture medium. The dishes were incubated at 32°C for 48 h, and then cell counts were performed. The viability of the microorganisms was expressed as CFU/g marshmallow.
2.8 Statistical analysis
A completely randomized factorial design was applied for the preparation of the marshmallows, where Factor A was the honey concentration at 0, 50 and 75% and Factor B was the gel strength of the gelatin expressed in bloom degrees, which was 265, 300 and 315° bloom. To all treatments, 10% probiotic capsules (w/w) were added, corresponding to 3.17 × 109 CFU/g marshmallow. To determine the influence of the factors on the physicochemical properties, total phenol content, antioxidant activity and viability of the probiotic (L. rhamnosus), ANOVA and a comparison of means with a 95% confidence level were performed using the GLIMMIX procedure of the SAS 9.4.3 statistical package.
3 Results and discussion
3.1 Physicochemical characteristics of bee honey
All the results obtained in the characterization of bee honey were within the values established in NOM-004-SAG/GAN-2018 (14). In relation to color, the honey presented a score of 9 on the Pfund scale, which corresponds to an “extra white” hue. Color in honey is defined by pigments such as phenolics, flavonoids, carotenoids, minerals and pollen. In this regard, it has been determined that darker honeys have a higher content of phenolic compounds (22).
On the other hand, the honey pH was 4.34, and its total acidity was 19.82 mEq acid/kg honey; when these quality parameters are outside standard values, they usually indicate sanitary deterioration (23). The ash content was 0.09%, which is explained by the fact that clear honeys have a low mineral content, while dark honeys contain higher mineral levels (24). The electrical conductivity of the honey was 0.23 mS/cm; this property is related to the soluble mineral content (25), since it is a technique for measuring the ability of a body or medium to conduct electric current.
On the other hand, honey moisture content was 17% and is related to the viscosity and crystallization properties, color, organoleptic properties and shelf life of honey (26). The reducing sugars in honey were 75.12%, while other honeys were in the range of 65–75% (27). The honey studied presented 81.5 °Brix, which is consistent with honeys from the state of Zacatecas, Mexico, ranging from 79.46 to 83.53 °Brix (28).
Additionally, the honey presented a viscosity of 26.1 Pa∙s; this parameter is affected by temperature, floral origin, water content and composition (29), while the HMF content was 5.57 mg/kg, which is an indicator of freshness since this compound is not present in fresh honey. Improper handling and storage conditions can also lead to its production (30); likewise, it increases when honey is heated to a high temperature (>100°C) in short periods of time (31).
Finally, the analyzed honey had an antioxidant capacity of 177.94 mg Trolox/kg. According to a study (32), the antioxidant content of honey produced by Apis mellifera in Central Serbia was 8.36 mg trolox/kg for acacia honey, 11.97 mg trolox/kg for polyfloral honey and 260.77 mg trolox/kg for forest honey. Furthermore, the total phenol content of the honey in the present study was 227.02 mg GA/kg; these results are somewhat low compared to other studies that have found phenol contents of 231 to 1,580 mg GA/100 g in polyfloral honeys (33), and 166.1 to 1019.2 mg GA/kg honey in honeys from the state of Hidalgo, Mexico (34).
3.2 Growth kinetics of Lactobacillus rhamnosus
Figure 1 shows the growth stages of the Lactobacillus L. rhamnosus. The beginning of the stationary phase is located at approximately 18 h of incubation, indicating the end of the exponential phase, so it was incubated at this time to prepare the inoculum to be encapsulated. In other studies, Lactobacillus rhamnosus B-445 inoculated at 37°C presented a higher bacterial population at 18 h (35), while at 22 h, there was a decrease in the rate of cell division (36), which is similar to what was observed in this study. On the other hand, the decrease in pH (6.1–3.6) is because L. rhamnosus is a lactic acid bacterium, so it can ferment carbohydrates present in the culture medium, resulting in the production of acids, mainly lactic acid (37).

Figure 1. Growth kinetics of L. rhamnosus and pH variation in MRS culture medium at 32°C. CFU = Colony Forming Units.
3.3 Viability of encapsulated Lactobacillus rhamnosus
Oxygen has been found to be toxic to probiotics (38), so the encapsulation process may be useful to protect them, especially from the addition of air that is typical of the marshmallow making process. The L. rhamnosus inoculum was added to the encapsulating solution at a cell concentration of 3.5 × 1010 CFU/g beads, while the capsules finally showed a viability of 3.17 × 109 CFU/g beads, so the encapsulation process did not affect the viability of the probiotic.
3.4 Marshmallow characterization
3.4.1 Physicochemical characteristics
Table 2 shows the results of the physicochemical analyses of the different marshmallow treatments. In general, the marshmallows had ash contents of 0.43–0.57% and pH values of 3.50–5.05 and 60.00–65.50 °Brix. In particular, the honey concentration had no effect on the ash content of the marshmallows, so it is likely that the ash came from the probiotic capsules added to the marshmallows, which were made with alginate, gelatin and mucilage, in addition to having been subjected to a CaCl2 solution. The pH was lower at higher honey concentrations (p < 0.0001), with pH values ranging from 4.67 to 5.05 for marshmallows without honey, 3.75 to 3.8 for marshmallows with 50% honey, and 3.5 to 3.7 for marshmallows with 75% honey. These pH variations may be related to the content of organic acids present in the honey, so that the higher the honey concentration is, the higher the organic acid content. On the other hand, the bloom degrees of gelatin had an inversely proportional effect on the °Brix of the marshmallows, while the addition of honey increased the °Brix proportionally.

Table 2. Results of proximate analyses of marshmallow treatments expressed as means ± standard error.
The marshmallows had a density of 0.35–0.44 g/mL. The density decreased as the percentage of honey increased and that of sugar decreased and was higher in marshmallows with 265 °bloom gelatin. Sugar likely limits the amount of air entering the foam during the whipping process, so honey may be useful in providing desirable characteristics in marshmallows. In general, this property is important for foamy foods because it can define softness, lightness or porosity (39). Other studies have shown a similar density in marshmallows: 0.474 g/cm3 (40) and 0.41 g/mL (41).
Figure 2 shows the moisture content of the marshmallow treatments on Days 1, 7 and 14 of storage. In general, moisture loss during storage was minimal and decreased with storage time in all samples, indicating the stability of the confection. Both physical and chemical aspects of freshness and stability during long-term storage can be affected by the moisture content. Even minor deviations from defined standards can have adverse effects on the physical properties of food (42). After 14 days of storage in a polypropylene wrapper, the moisture content of the marshmallows without honey was 25 to 35%, and those with the highest amount of honey (75%) had 32 to 36% moisture. Other authors have reported different moisture values in confectionery products with functional properties: 19 to 21.5% in marshmallows with anthocyanins (11); 16.7 to 22.9% in marshmallows with Stevia rebaudiana, oligofructose and isomaltose as sugar substitutes (10); 22.06 to 22.55% in jelly candies with rosemary extract (21); and 20.05 to 25.3% in jelly candies with honey and fruit juices (43). The marshmallows with probiotics had a higher moisture content than the aforementioned confections, probably due to the content of the probiotic beads.

Figure 2. Moisture content of the different marshmallow treatments during storage time. M1, 0% honey-265 °bloom; M2, 50% honey-265 °bloom; M3, 75% honey-265 °bloom; M4, 0% honey-300 °bloom; M5, 50% honey-300 °bloom; M6, 75% honey-300 °bloom; M7, 0% honey-315 °bloom; M8, 50% honey-315 °bloom; M9, 75% honey-315 °bloom.
3.4.2 Antioxidant capacity (AC)
Figure 3 shows the AC of the marshmallow treatments during storage. Antioxidant activity was not affected by the gelatin bloom degree; however, honey concentration had a significant effect on the treatments (p < 0.0001), as well as the interaction between the two factors (p < 0.0001). The marshmallows presented AC values from 61.87 to 100.81 mg Trolox/kg marshmallow. In general, it can be observed that marshmallows with honey presented more AC than those without honey, being less noticeable on Day 14, which may be due to a slight survival of probiotics, since strains of lactobacilli and some bididobacteria possess AC and are able to decrease the risk of free radical accumulation (44). Furthermore, Sharma et al. (45) found that L. rhamnosus GG had an inhibition percentage of 59%. Therefore, AC in marshmallows is likely influenced by probiotic content (21).

Figure 3. Antioxidant activity of marshmallow treatments on different days of storage. Means with the same letter do not present a significant statistical difference (α = 0.05). M1, 0% honey-265 °bloom; M2, 50% honey-265 °bloom; M3, 75% honey-265 °bloom; M4, 0% honey-300 °bloom; M5, 50% honey-300 °bloom; M6, 75% honey-300 °bloom; M7, 0% honey-315 °bloom; M8, 50% honey-315 °bloom; M9, 75% honey-315 °bloom.
At the end of the experiment (Day 14), treatment M7 (0% honey-315 bloom) presented the lowest AC with 58.58 mg Trolox/kg, while M3 (75% honey-265 bloom) had the highest AC with 82.02 mg Trolox/kg marshmallow. Periche et al. (10) made marshmallows with an aqueous extract of Stevia that had an AC of 117 mg Trolox/100 g aqueous extract, while Santoso et al. (12) prepared marshmallows with Kinang extract, and the treatment with 80% extract presented 2.78 mg/mL antioxidant activity.
3.4.3 Total phenolic compounds (TPC)
The honey concentration in the marshmallows presented a significant effect (p < 0.0001) on the total phenol content. Although bloom degrees did not present a significant effect, the interaction of the two factors had a significant influence (p < 0.0001). Figure 4 shows that the honey treatments had a higher phenol content, which is explained by the TPC of the honey added to the marshmallows (386 GA/kg). In general, the concentration of phenolic compounds increased with storage time.

Figure 4. Total phenol content of marshmallow treatments. Means with the same letter do not show a significant statistical difference (α = 0.05). M1, 0% honey-265 °bloom; M2, 50% honey-265 °bloom; M3, 75% honey-265 °bloom; M4, 0% honey-300 °bloom; M5, 50% honey-300 °bloom; M6, 75% honey-300 °bloom; M7, 0% honey-315 °bloom; M8, 50% honey-315 °bloom; M9, 75% honey-315 °bloom.
On Day 1, treatment M7 (0% honey-315°bloom) showed the lowest phenol content, with 54.45 mg GA/kg marshmallow, while M6 (75% honey-300°bloom) had a content of 100.55 mg GA/kg marshmallow. At the end of the experiment (Day 14), M7 (0% honey-315°bloom) again showed the lowest phenol content, with 95.33 mg GA/kg marshmallow, and the treatment with the highest concentration was M5 (50% honey-300°bloom) with 130.4 mg GA/kg marshmallow. Cedeño-Pinos et al. (21) analyzed jelly candies enriched with a rosemary extract with 73.9 mg polyphenols/g; however, the candies had polyphenol contents from 0.19 to 0.41 mg/g; this indicates that the phenol content can be easily lost over storage time, so it is important that the phenol source has a significant amount for the best results.
3.4.4 Viability of Lactobacillus rhamnosus
Figure 5 presents the results of the viability analysis of the probiotic L. rhamnosus incorporated in the marshmallows. On the first day of storage, the viability of the lactobacilli remained above the minimum recommended limit (106 CFU/g) (46) in all formulations. However, by Day 7, viability was reduced from 4.8 to 6.0% in marshmallows without honey, which presented 1.23 × 107, 1.58 × 107 and 1.76 × 107 CFU/g marshmallow for treatments with 265, 300 and 315°bloom, respectively, while marshmallows with 50 and 75% honey had a drastic reduction in CFU/g marshmallow on this same day. Finally, by Day 14, none of the treatments reached minimum viability.

Figure 5. Viability of L. rhamnosus in marshmallow formulations during the storage period. Means with the same letter do not show a statistically significant difference (α = 0.05). CFU=Colony Forming Units. M1, 0% honey-265 °bloom; M2, 50% honey-265 °bloom; M3, 75% honey-265 °bloom; M4, 0% honey-300 °bloom; M5, 50% honey-300 °bloom; M6, 75% honey-300 °bloom; M7, 0% honey-315 °bloom; M8, 50% honey-315 °bloom; M9, 75% honey-315 °bloom.
Lactobacillus survival can be influenced by several factors, such as storage temperature, water activity (aw), titratable acidity, presence of sugars, and processing conditions (heat treatment, cooling rate, packaging material, etc.) (47). On the other hand, pH limits the growth and stability of probiotic bacteria (47). The acidification of the medium due to lactic-acid bacteria growth poses a challenge for industrial production. The accumulation of lactic acid may affect cell physiology, leading to growth interruption or reduction. Therefore, maintaining a pH close to neutrality can promote a higher growth rate (48); however, the pH of the marshmallows varied from 3.50 to 5.05, being lower in the treatments with honey, since it showed a pH of 4.34, so that on Day 7, the viability of the marshmallows with honey was lower than that of the treatments without it.
On the other hand, another important factor in the viability of probiotics is acidity. This characteristic is due to the presence of organic acids, among which gluconic acid (~0.5% w/v) is important in honey; it is generated from the oxidation of glucose by the endogenous glucose oxidase enzyme and is a very powerful antibacterial agent. In addition, when honey is diluted, glucose oxidase is activated and acts on endogenous glucose to produce hydrogen peroxide (H2O2), which has antibacterial properties (49, 50). The antimicrobial activity of honey derived from low water activity (0.56 to 0.62) and its high viscosity, among other characteristics, can also be considered (51).
High concentrations of sugars in marshmallows could trigger the osmosis process in probiotics, causing water loss from bacterial cells and affecting bacterial growth (52). Additionally, the survival of probiotics can be affected by the characteristics of the packaging material, such as thickness and gas permeability (53).
According to the viability results of the marshmallows, the applied encapsulation was not able to protect the microorganisms from the mentioned factors. However, these probiotics fall within the classification of paraprobiotics, also called inactivated probiotics or ghost probiotics, which are inactivated cells or fractions of microbial cells that provide a benefit to the host at appropriate doses (54). Therefore, they could be used in more vulnerable patients without risk (55).
Shigwedha et al. (56) showed that the administration of inactivated L. rhamnosus V. probiotics is useful in the prevention and treatment of infectious diseases, allergies, fatigue and fibromyalgia, whereas Good et al. (57) demonstrated that oral administration of UV-inactivated L. rhamnosus HN001 attenuates the severity of necrotizing enterocolitis in neonatal mice and preterm piglets. Although the project aimed to preserve the viability of probiotics in marshmallows, more time is required to test other encapsulation methods and materials, so there are opportunities for future research. However, based on the above, it is possible that the marshmallows produced in this study could be studied by researchers in the area of health as a supplement with potential for the prevention of cancer and other gastrointestinal system conditions. On the other hand, the project made it possible to obtain marshmallows with antioxidant activity and phenolic compounds derived from Apis mellifera honey, thus obtaining a product with functional properties.
4 Conclusion
Marshmallows enriched with honey and L. rhamnosus probiotics were prepared, and their physicochemical properties, antioxidants and probiotic viability were studied; their physicochemical properties were adequate and showed good stability over time. The antioxidant capacity and total phenol content of the marshmallows were increased by the addition of honey, creating a functional food; furthermore, the bloom degrees did not affect the antioxidant capacity or the amount of total phenols in the marshmallows. Although the antioxidant activity and phenolic compounds were preserved during the storage time, the viability of the probiotics was not maintained despite being encapsulated; however, it is possible that the dead probiotics may still exert a beneficial effect on the consumer by acting as paraprobiotics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SC-C: Writing – original draft, Visualization, Investigation. AC-O: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Conceptualization. JS-R: Writing – review & editing, Formal analysis. JH-C: Writing – review & editing, Formal analysis. EF-A: Writing – review & editing, Supervision. EG-R: Writing – review & editing, Methodology, Investigation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the CONACYT for the support grant awarded to SC-C.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bedolla Bernal, S, Dueñas Gallegos, C, Esquivel Ibarra, I, Favela Torres, T, Guerrero Huerta, R, Mendoza Madrid, E, et al. Elaboración de confites con agentes gelificantes [confectionery production with gelling agents] In: Introducción a la tecnología de alimentos. Mexico: Limusa (2004). 141–8.
2. Gálvez Mariscal, A, Flores Argüello, I, and Farrés González Saravia, A. Proteínas [Proteins] In: S Badui Dergal , editor. Química de los alimentos. 4th ed. Mexico: Pearson educación (2006). 119–244.
3. Thorat, B, and Deshmukh, R. Confectionery market by product type (hard-boiled sweets, mints, gums & jellies, chocolate, caramels & toffees, medicated confectionery, fine bakery wares, and others), age group (children, adult, and geriatric), price point (economy, mid-range, and luxury), and distribution channel (supermarket/hypermarket, convenience stores, pharmaceutical & drug stores, food services, duty-free outlets, E-commerce, and others): Global opportunity analysis and industry forecast, 2021–2027. US: Allied Market Research (2020) ID: C779E53E0DB5EN.
4. Pérez-Marroquín, XA, Estrada-Fernández, AG, García-Ceja, A, Aguirre-Álvarez, G, and León-López, A. Agro-food waste as an ingredient in functional beverage processing: sources, functionality, market and regulation. Food Secur. (2023) 12:1583. doi: 10.3390/foods12081583
5. Wang, H, Li, L, Lin, X, Bai, W, Xiao, G, and Liu, G. Composition, functional properties and safety of honey: a review. JFSA. (2023) 103:6767–79. doi: 10.1002/JSFA.12720
6. Kumar Yadav, M, Kumari, I, Singh, B, Kant Sharma, K, and Kumar, TS. MINI-REVIEW probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol. (2022) 106:505–21. doi: 10.1007/s00253-021-11646-8
7. Rodríguez, YA, Rojas, AF, and Rodríguez-Barona, S. Encapsulación de probióticos Para aplicaciones alimenticias [encapsulation of probiotics for food applications]. Biosalud. (2016) 15:106–15. doi: 10.17151/biosa.2016.15.2.10
8. Segers, ME, and Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb Cell Factories. (2014) 13:S7. doi: 10.1186/1475-2859-13-S1-S7
9. Szajewska, H, and Hojsak, I. Health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 in children. Postgrad Med. (2020) 132:441–51. doi: 10.1080/00325481.2020.1731214
10. Periche, A, Castelló, ML, Heredia, A, and Escriche, I. Stevia rebaudiana, oligofructose and isomaltulose as sugar replacers in marshmallows: stability and antioxidant properties. J Food Process Preserv. (2015) 40:724–32. doi: 10.1111/jfpp.12653
11. Artamonova, M, Piliugina, I, Samokhvalova, O, Murlykina, N, Kravchenko, O, Fomina, I, et al. A study of properties of marshmallow with natural anthocyanin dyes during storage. Technol Equip Food Prod. (2017) 3:23–30. doi: 10.15587/1729-4061.2017.103857
12. Santoso, B, Verawati, E, Wardani Widowati, T, Puspa Dewi, SR, and Pambayun, R. Functional characteristics of marshmallow with Kinang (chew of betel) extract addition. J Food Nutr Res. (2019) 7:1–5. doi: 10.12691/jfnr-7-1-1
13. Milea, A, Vasile, A, Cîrciumaru, A, Dumitrascu, L, Barbu, V, Râpeanu, G, et al. Valorizations of sweet cherries skins phytochemicals by extraction, microencapsulation and development of value-added food products. Food Secur. (2019) 8:188. doi: 10.3390/foods8060188
14. Diario Oficial de la Federación . NOM-004-SAG/GAN-2018, Producción de miel y especificaciones [Honey production and specifications]. México: Diario Oficial de la Federación (2020).
15. Shapla, UM, Solayman, M, Alam, N, Khalil, MI, and Gan, SH. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chem Cent J. (2018) 12:35. doi: 10.1186/s13065-018-0408-3
16. Dżugan, M, Tomczyk, M, Sowa, P, and Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules. (2018) 23:269. doi: 10.3390/molecules23082069
17. Huerta, VK . Impregnación de probióticos y compuestos fenólicos de miel de abeja sin aguijón en Placas de plátano (Musa cavendishii) utilizando una emulsión múltiple [impregnation of probiotics and phenolic compounds from stingless honey on plantain (Musa cavendishii) slabs using a multiple emulsion]. [doctoral thesis]. Mexico: Colegio de Postgraduados (2015).
18. Noori Dokoohaki, Z, Saeed Sekhavatizadeh, S, and Hosseinzadeh, S. Dairy dessert containing microencapsulated Lactobacillus rhamnosus (ATCC 53103) with quince seed mucilage as a coating material. LWT. (2019) 115:108429–8. doi: 10.1016/j.lwt.2019.108429
19. Jiménez-Hernández, M, Del Ángel-Coronel, OA, Espejo-Bayona, DL, and Lugo-Damian, HN. Efecto de pH y la temperatura sobe la liberación de la bacteriocina producida por Pediococcus acidilactici ITV26 encapsulada en una matriz de alginato-almidón [Effect of pH and temperature on the release of bacteriocin produced by Pediococcus acidilactici ITV26 encapsulated in a starch-alginate matrix]. Revista de la Invención Técnica. (2017) 1:22–9.
20. Federal Consumer Protection Agency . Tecnologías Domésticas Profeco [Profeco domestic technologies]. Mexican government: (2018).
21. Cedeño-Pinos, C, Martínez-Tomé, M, Murcia, MA, Jordán, MJ, and Bañón, S. Assessment of rosemary (Rosmarinus officinalis L.) extract as antioxidant in jelly candies made with fructan fibres and stevia. Antioxidants. (2020) 9:1289. doi: 10.3390/antiox9121289
22. Becerril-Sánchez, AL, Quintero-Salazar, B, Dublán-García, O, and Escalona-Buendía, HB. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants. (2021) 10:1700. doi: 10.3390/antiox10111700
23. Federal Consumer Protection Agency . Estudio de calidad: Miel de abeja [Quality study: Bee honey]. Revista del consumidor. Febrero (2015): 36–47.
24. Cabrera, M, and Santander, E. Physicochemical and sensory analysis of honeys from eastern Formosa province (Argentina) and its relationship with their botanical origin. Food Chem Adv. (2022) 1:100026. doi: 10.1016/J.FOCHA.2022.100026
25. Terrab, A, Recamales, AF, Hernanz, D, and Heredia, FJ. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral content. Food Chem. (2004) 88:537–42. doi: 10.1016/j.foodchem.2004.01.068
26. Gürbüz, S, Çakici, N, Mehmetoǧlu, S, Atmaca, H, Demir, T, Arigül Apan, M, et al. Physicochemical quality characteristics of southeastern Anatolia honey, Turkey. Int J Anal Chem. (2020) 2020:8810029. doi: 10.1155/2020/8810029
27. Velásquez, D, and Goetschel, L. Determination of the physicochemical quality of bee honey sold in Quito and comparison with artificial honey. Enfoque UTE. (2019) 10:52–62. doi: 10.29019/enfoque.v10n2.406
28. Contreras-Martínez, CS, Macías-Nieves, JP, García-González, JM, Trejo-Guardado, VI, and Carranza-Concha, J. Antioxidant capacity and phenolic content of bee honey produced in Zacatecas. Mexico Rev Fitotec. (2020) 43:453–60. doi: 10.35196/rfm.2020.4.453
29. Katsara, K, Kenanakis, G, Alissandrakis, E, and Papadakis, V. Honey quality and microplastic migration from food packaging: A potential threat for consumer health? Microplastics. (2022) 1:406–27. doi: 10.3390/microplastics1030030
30. Gebeyehu, HR, and Jalata, DD. Physicochemical and mineral contents of honey from Fitche and Addis Ababa districts in Ethiopia. Food Chem Adv. (2023) 2:100177. doi: 10.1016/j.focha.2022.100177
31. Mondragón-Cortez, PM, Guatemala-Morales, GM, and Arriola-Guevara, E. Properties of some commercial honeys available in mexican market: effect of overheating on quality of the packaged honey. J Food Qual Hazards Control. (2019) 6:93–100. doi: 10.18502/jfqhc.6.3.1382
32. Srećković, NZ, Mihailović, VB, and Katanić Stanković, JS. Physico-chemical, antioxidant and antimicrobial properties of three different types of honey from Central Serbia. Kragujevac J Sci. (2019) 41:53–68. doi: 10.5937/KgJSci1941053S
33. Combarros-Fuertes, P, Estevinho, LM, Dias, LG, Castro, JM, Tomas-Barberán, FA, Tornadijo, ME, et al. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: A screening prior to clinical application. J Agric Food Chem. (2019) 67:688–98. doi: 10.1021/acs.jafc.8b05436
34. Quintero-Lira, A, López-Oliveira, A, Martínez-Juárez, V, Del Razo-Rodríguez, O, Jiménez-Alvarado, R, and Campos, MR. Bioactive compounds and selenium content determination in Hidalgo state honey. JEEOS. (2019) 3:1–18. doi: 10.19136/jeeos.a3n2.3405
35. Abdel-Rahman, MA, Sadek, ZI, Azab, MS, Darwesh, OM, and Hassan, MS. Incorporation of microencapsulated Lactobacillus rhamnosus into infant-foods inhibit proliferation of toxicogenic Bacillus cereus strains. Biocatal Agric Biotechnol. (2019) 18:101013. doi: 10.1016/j.bcab.2019.01.051
36. Castillo Salinas, ADD . Elaboración de un restaurador de microbiota urogenital con actividad antagónica contra cepas bacterianas aisladas de procesos de infección urogenital [development of a urogenital microbiota restorer with antagonistic activity against bacterial strains isolated from urogenital infection processes]. Bolivia: Universidad Major Pacensis Divi Andre AE (2017).
37. Jafarpour, D, and Hashemi, SMB. Pure and co-fermentation of quinoa seeds by Limosilactobacillus fermentum and Lacticaseibacillus rhamnosus: bioactive content, antidiabetic and antioxidant activities. Fermentation. (2023) 9:80. doi: 10.3390/fermentation9020080
38. Sharma, R, Rashidinejad, A, and Jafari, SM. Application of spray dried encapsulated probiotics in functional food formulations. Food Bioprocess Technol. (2022) 15:2135–54. doi: 10.1007/s11947-022-02803-6
39. Islas, JG, and Vera, NG. Efecto del proceso en la elaboración de merengues [effect of the process on the production of meringues]. Boletín de Ciencias Agropecuarias del ICAP. (2017) 3. doi: 10.29057/icap.v3i6.2451
40. Çelekli, A, and Maraşlı, S. Effect of Arthrospira platensis on physicochemical, texture, and microstructure properties of low-density marshmallows. Food Humanity. (2024) 2:100196. doi: 10.1016/j.foohum.2023.12.004
41. Mardani, M, Yeganehzad, S, Ptichkina, N, Kodatsky, Y, Kliukina, O, Nepovinnykh, N, et al. Study on foaming, rheological and thermal properties of gelatin-free marshmallow. Food Hydrocoll. (2019) 93:335–41. doi: 10.1016/j.foodhyd.2019.02.033
42. Gerardi, C, Durante, M, Tufariello, M, Grieco, F, and Giovinazzo, G. Effects of time and temperature on stability of bioactive molecules, color and volatile compounds during storage of grape pomace flour. Appl Sci (Switzerland). (2022) 12:3956. doi: 10.3390/app12083956
43. Mutlu, C, Tontul, SA, and Erbaş, M. Production of a minimally processed jelly candy for children using honey instead of sugar. LWT. (2018) 93:499–505. doi: 10.1016/j.lwt.2018.03.064
44. Misha, V, Shah, C, Mokashe, N, Chavan, R, Yadav, H, and Prajapati, J. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. (2015) 63:3615–26. doi: 10.1021/jf506326t
45. Sharma, K, Mahajan, R, Attri, S, and Goel, G. Selection of indigenous Lactobacillus paracasei CD4 and Lactobacillus gastricus BTM 7 as probiotic: assessment of traits combined with principal component analysis. J Appl Microbiol. (2017) 122:1310–20. doi: 10.1111/jam.13426
46. Hernández Hernández, A, Coronel Rodríguez, C, Monge Zamorano, M, and Quintana, HC. Microbiota, Probióticos, Prebióticos y Simbióticos [Microbiota, Probiotics, Prebiotics and Symbiotics]. Pediatría Integral. (2015) 19:337–54.
47. Tripathi, M, and Giri, S. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. (2014) 9:225–41. doi: 10.1016/j.jff.2014.04.030
48. Aristimuño Ficoseco, C, Mansilla, FI, Vignolo, GM, and Nader-Macías, MEF. Optimization of probiotic lactobacilli production for in-feed supplementation to feedlot cattle. Appl Microbiol. (2023) 3:339–57. doi: 10.3390/applmicrobiol3020024
49. Ndife, J, Abioye, M, and Dandago, M. Locations, quality assessment of Nigerian honey sourced from different floral. NIFOJ. (2014) 32:48–55. doi: 10.1016/S0189-7241(15)30117-X
50. Masoura, M, Passaretti, P, Overton, TW, Lund, PA, and Gkatzionis, K. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci Rep. (2020) 10:17692. doi: 10.1038/s41598-020-74937-6
51. Almasaudi, S . The antibacterial activities of honey. Saudi J Biol Sci. (2021) 28:2188–96. doi: 10.1016/j.sjbs.2020.10.017
52. Fan, X, Bao, T, Yi, H, Zhang, Z, Zhang, K, Liu, X, et al. Ribosome profiling and RNA sequencing reveal genome-wide cellular translation and transcription regulation under osmotic stress in Lactobacillus rhamnosus ATCC 53103. Front Microbiol. (2021) 12:781454. doi: 10.3389/fmicb.2021.781454
53. Chua, JCL, Hale, JDF, Silcock, P, and Bremer, PJ. Bacterial survival and adhesion for formulating new oral probiotic foods. Crit Rev Food Sci Nutr. (2020) 60:2926–37. doi: 10.1080/10408398.2019.1669528
54. Arrioja-Bretón, D, and López-Malo, A. Paraprobióticos y su aplicación en alimentos [Paraprobiotics and their application in food], vol. 2018 TSIA (2018) Available at: https://tsia.udlap.mx/paraprobioticos-y-su-aplicacion-en-alimentos/.
55. Hernández Hernández, A, Coronel Rodríguez, C, and Gil Vázquez, JM. Novedades en probióticos: evidencias, indicaciones y seguridad [novelties in probiotics: evidence, indications and safety]. Pediatr Integral. (2020) 24:151–65.
56. Shigwedha, N, Zhang, L, Sichel, L, Jia, L, Gong, P, Liu, W, et al. More than a few LAB alleviate common allergies: impact of Paraprobiotics in comparison to Probiotical live cells. J Biosci Med. (2014) 2:56–64. doi: 10.4236/jbm.2014.23008
57. Good, M, Sodhi, CP, Ozolek, JA, Buck, RH, Goehring, KC, Thomas, DL, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol. (2014) 306:G1021–32. doi: 10.1152/ajpgi.00452.2013
Keywords: antioxidants, bioactive compounds, coacervation, functional food, microencapsulation
Citation: Colmenares-Cuevas SI, Contreras-Oliva A, Salinas-Ruiz J, Hidalgo-Contreras JV, Flores-Andrade E and García-Ramírez EJ (2024) Development and study of the functional properties of marshmallow enriched with bee (Apis mellifera) honey and encapsulated probiotics (Lactobacillus rhamnosus). Front. Nutr. 11:1353530. doi: 10.3389/fnut.2024.1353530
Edited by:
Lesław Juszczak, University of Agriculture in Krakow, PolandReviewed by:
Elisa Julianti, University of North Sumatra, IndonesiaNasim Khorshidian, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2024 Colmenares-Cuevas, Contreras-Oliva, Salinas-Ruiz, Hidalgo-Contreras, Flores-Andrade and García-Ramírez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Contreras-Oliva, YWRyaWNvbkBjb2xwb3MubXg=
 Saira Itzel Colmenares-Cuevas1
Saira Itzel Colmenares-Cuevas1 Adriana Contreras-Oliva
Adriana Contreras-Oliva Juan Valente Hidalgo-Contreras
Juan Valente Hidalgo-Contreras