
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr., 20 March 2024
Sec. Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1348328
A correction has been applied to this article in:
Corrigendum: A natural sustained-intestinal release formulation of red chili pepper extracted capsaicinoids (Capsifen®) safely modulates energy balance and endurance performance: a randomized, double-blind, placebo-controlled study
Introduction: Overweight and obesity are major public health concerns, with a sharp increase in prevalence over the last few decades. The primary cause is an imbalance between calorie intake and expenditure due to a rise in calorie-rich processed food and reduced physical activity. Energy balance in humans involves complex processes including thermogenesis, a crucial factor in regulating energy expenditure.
Methods: In this randomized, double-blinded, placebo-controlled three-arm three-sequence study, we investigated the efficacy of Capsifen® (CapF), a pungency-masked sustained-intestinal release formulation of red chili extract, on energy expenditure, fat oxidation, and endurance using the Quark C-PET system in healthy overweight participants, with and without exercise. In the study, 105 healthy participants were randomized to receive either placebo, CapF 100 mg/day, or CapF 200 mg/day for 28 days.
Results: CapF demonstrated a dose-dependent response to increased energy expenditure and fatty acid oxidation with a concomitant reduction in body weight. Both CapF 100 and CapF 200 also increased the time to exhaustion.
Discussion: These results demonstrate the plausible efficacy of CapF in energy expenditure and physical performance in otherwise healthy adults who have a high body mass index.
Clinical trial registration: https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=MjQzNTg=&Enc=&userName=CTRI/2018/04/013157 dated 04 October 2018.
Obesity is a rapidly growing public health problem of significant concern. Over the past three decades, countries worldwide have experienced a 2- to 3-fold increase in the prevalence of obesity (1). In 2019, an estimated 5 million deaths worldwide were linked to obesity (2), and approximately one in three adults and one in six children in the United States were reported as obese (1). The primary cause of overweight and obesity is the imbalance between energy/calorie intake and expenditure. Increased consumption of calorie-dense, processed foods, and lack of sufficient physical activity have contributed to this imbalance (3). Modest adjustments to diet and physical activity, specifically reducing calorie intake and enhancing calorie expenditure, can effectively reduce obesity. Such minor changes are more feasible and sustainable in the long term than radical dietary or behavioral modifications (4, 5).
Maintaining a balance between energy intake and energy expenditure is a complex process. This balance is crucial for keeping the energy stores stable over time, following the principle of energy conservation (6). Energy expenditure consists of three components: resting energy expenditure (energy needed for basic bodily functions at rest), activity-induced energy expenditure (energy associated with physical activity), and diet-induced energy expenditure (energy used in digestion and metabolism). Diet-induced energy expenditure is approximately 10% of the total energy expenditure in normally active individuals (7), and it involves thermogenesis, a key factor in regulating temperature and energy balance (8, 9). Thermogenesis can be adaptive, responding to environmental changes, or facultative, triggered by eating (10, 11). Under obesity, around half of the weight loss was identified to be due to adaptive thermogenesis, which can be activated by specific food ingredients (12, 13). The major sites of thermogenesis are brown adipose tissues (BAT) and skeletal muscles (14). The average amount of BAT usually ranges from 0.02 to 300 g, which constitutes less than 0.5% of the total human body mass (15). Currently, there is a growing interest in exploring ways to modulate diet-induced thermogenesis to facilitate weight loss or maintaining a healthy body weight.
Several dietary spices and herbs, including red chili pepper, ginger, black pepper, long pepper, cinnamon, garcinia cambogia, yerba mate, green coffee, green tea, bitter orange, and guarana, have been shown to possess thermogenic effects or promote EE (8). Among different botanicals, red chili pepper stands out due to its remarkable spiciness (pungency). Furthermore, its medicinal properties are highly regarded, encompassing anti-inflammatory, anti-analgesic, anti-diabetic, anti-lipidemic, and anti-cancer effects in addition to the anti-obesity effects (16, 17). Several studies have demonstrated thermogenic, lipolytic, appetite-suppressing, and weight loss properties of red chilies (18, 19). However, the sensory burn and gastrointestinal side effects associated with red chili use remain a major challenge for its clinical use.
We have recently developed a taste-masked, sustained-intestinal release beadlet formulation of red chili pepper extract containing 2% capsaicinoids [a sum of capsaicin, dihydrocapsaicin (DC), and nordihydrocapsaicin (NDC)]. This was achieved using a proprietary hydrogel technology known as FenuMat® (20). The microbeadlets known as Capsifen® (CapF) are bioavailable and safe at dosages of 200 mg/day in individuals with obesity and helped to reduce body weight following 28 days of supplementation (21). However, it is unknown whether lower dosages are efficacious for supporting weight management. Therefore, the primary objective of this study was to examine the effects of a low dose (100 mg/day) and a high dose (200 mg/day) of CapF over a period of 28 days on energy expenditure, fat oxidation, and endurance. These parameters were evaluated using the Quark C-PET system, which served as the validated assessment tool during cardiopulmonary exercise tests (CPET) (22). The evaluation was conducted on healthy subjects, both with and without exercise.
The study involved a randomized placebo-controlled, three-arm, three-sequence, comparative as summarized in Figure 1 and conducted at Sri Rama Hospital, Bangalore, India, under the supervision of a registered medical practitioner in accordance with the Declaration of Helsinki. The institutional ethical committee reviewed and approved the protocol and the study was registered with the Clinical Trial Registry of India (CTRI/2018/04/013157). A written informed consent ensuring their awareness of the study details and voluntary participation was also obtained from all the participants prior to the commencement of the study.
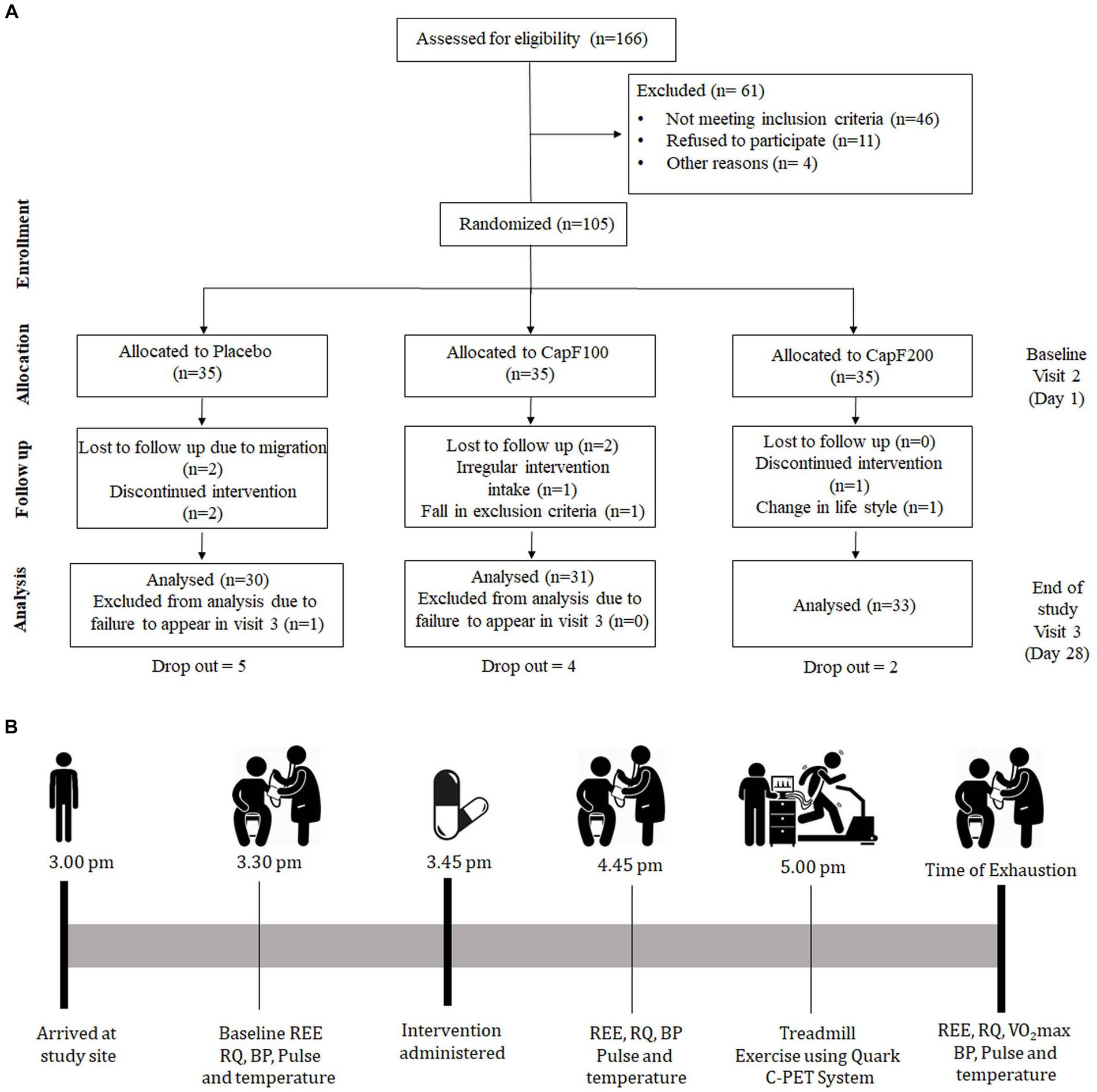
Figure 1. (A) CONSORT diagram illustrating the study design; (B) evaluation scheme on visit 2 and visit 3.
Healthy adults (males and females) aged 21–45 years with a body mass index (BMI) ≥ 25 kg/m2 were recruited for this study. A total of 166 healthy volunteers were screened, and 105 participants meeting the inclusion and exclusion criteria were enrolled in the study. The major inclusion/exclusion criteria are provided in Table 1. Those who expressed interest were provided with comprehensive information about the trial and an online screening questionnaire. This questionnaire assessed general health, medication usage, alcohol, and drug consumption (including nicotine), supplement and vitamin intake, and pregnancy or breastfeeding status. Individuals who were considered eligible based on the screening were invited to the study site for further screening.
Capsicum extracts infused in fenugreek galactomannan (mucilage) beadlets, known as Capsifen® (CapF), were standardized to contain 2.1% total capsaicinoids [comprising capsaicin, dihydrocapsaicin (DC), and nordihydrocapsaicin (NDC)]. Capsaicinoid content was estimated by a validated HPLC method (23), utilizing a Shimadzu LC 20 AT instrument equipped with an M20A photodiode array detector (PDA; Shimadzu Analytical Private Limited, Mumbai, India). In this study, identical hard shell gelatin capsules containing placebo (microcrystalline cellulose) 100 mg × 1 capsule/day, CapF 100 mg × 1 capsule/day, and CapF 200 mg × 1 capsule/ day were provided in airtight amber-colored bottles of similar size and shape. Each bottle contained 35 capsules, and the subjects were advised to consume the respective supplement after their major meal.
Subjects were randomized during visit 1, using computer-generated randomization codes, into one of three arms to receive a placebo, CapF 100, or CapF 200 for 28 days. All capsules were identical in size, shape, and appearance. The master randomization list prepared by an independent statistician was handed over to the pharmacist for the purpose of double blinding. The investigator and other faculties involved in the study were not aware of allocation or intervention details.
The primary objective of the study involved measuring EE and respiratory quotient (RQ) upon the supplementation of CapF. The REE is the collective energy required by the body cells to maintain post-absorptive homeostatic functions at rest (24). The RQ, also known as the respiratory ratio, is defined as the volume of carbon dioxide released over the volume of oxygen inhaled during respiration (25).
We conducted a cardiopulmonary exercise test (C-PET) using a metabolic cart (Quark C-PET Systems, COSMED, Italy) and measured EE and RQ for 10 min. Quark C-PET system is a validated system used for evaluating energy metabolism (22, 26). To measure these parameters, subjects wore a headgear attached to a mask which allowed metabolic assessment by measuring the volume of O2 inhaled and CO2 exhaled during exercise. This protocol required the participant to begin exercise at a self-selected speed between 3 and 6 km/h. The self-selected speed was maintained, while the treadmill elevation increased by 2% every 2 min during the test. Time to exhaustion was determined as the time that the subject could no longer maintain exercise intensity and/or reached volitional exhaustion. Data were acquired continuously during the exercise protocol, and the maximal RQ was obtained upon volitional exhaustion. Resting energy expenditure (REE—i.e., energy expenditure under resting conditions) was also obtained from the Quark C-PET System.
In a typical protocol, there were a total of three visits: visit 1, screening and randomization; visit 2, day 1, baseline; visit 3, day 28 or the end of study (Figures 1A,B). At visit 2, the baseline characteristics of the participants [demographic characteristics, health conditions, physical examination, anthropometric measurements, blood pressure, measurements of energy expenditure (EE) and respiratory quotient (RQ)] were measured. On visit 3, all the above-mentioned parameters were analyzed the same as on visit 2.
All participants were instructed to arrive at the study site on day 1 (visit 2) at approximately 3 pm and were allowed to rest for 15 to 20 min at their convenience. They were provided with a bottle containing 35 capsules and were instructed to take the assigned intervention (placebo, CapF 100, or CapF 200) daily for 28 days with 200 ± 20 mL water. Prior to supplementation and 1 h post-supplementation, EE, RQ, blood pressure, and heart rate were measured. On the completion of resting measures, a maximal cardiopulmonary exercise test (CPET) was performed to determine oxygen utilization. All participants were advised to maintain their regular physical activities. Adherence to the study protocol was ensured with weekly telephonic conversation, and the binding to the protocol was estimated by count-pill strategy. On day 28 (visit 3), the subjects returned to the study site and repeated the aforementioned measurements.
Participants were placed on a standardized Indian food diet (containing carbohydrates, proteins, and fat) comprising of rice, vegetables, and eggs for breakfast (protein: 27–30%; fat 20–24%; carbohydrate 35%–40%; energy 400–500 calories); rice, chicken, and vegetables for lunch (protein 27–30%; fat 20%–24%; carbohydrate 35%–40%; energy 400–500 calories); wheat, chicken, and vegetables for dinner (protein 27%–30%; fat 20%–24%; carbohydrate 25%–35%; energy 400–500 calories) during the duration of the study. There was no restriction on drinking water.
All measurements were performed at baseline (day 1) and at the end of the study (day 28). Anthropometric indexes, including weight and height, were assessed with light clothing and barefoot to the nearest 0.1 kg and 0.5 cm, respectively, using a scale with a stadiometer. Demographic characteristics, health history, and physical examination were performed in the presence of a registered medical practitioner. Blood pressure was measured using a digital device (IntelliSense®, Omron, Japan).
Based on a previous study, the sample size was calculated using G-Power (20). The sample size was estimated as 35 in each arm while having 80% power with an alpha of 0.05 and a dropout rate of 20%. All the statistical analyses were performed using SPSS software version 28.0 (SPSS Inc., Chicago, IL, United States). Data are represented as mean ± standard deviation (SD). A 2 × 3 repeated-measures ANOVA with post-hoc analysis was used to evaluate the statistical significance between placebo and CapF groups. A p-value < 0.05 was considered statistically significant.
Baseline demographic data of the participants demonstrate no intra- or inter-group differences in heart rate and systolic and diastolic blood pressure (p > 0.05; Table 2). Body weight was significantly reduced in the CapF 100 and CapF 200 groups, but not in the placebo group. The reduction in BMI in the CapF 100 and CapF 200 groups was also statistically significant (p < 0.05).
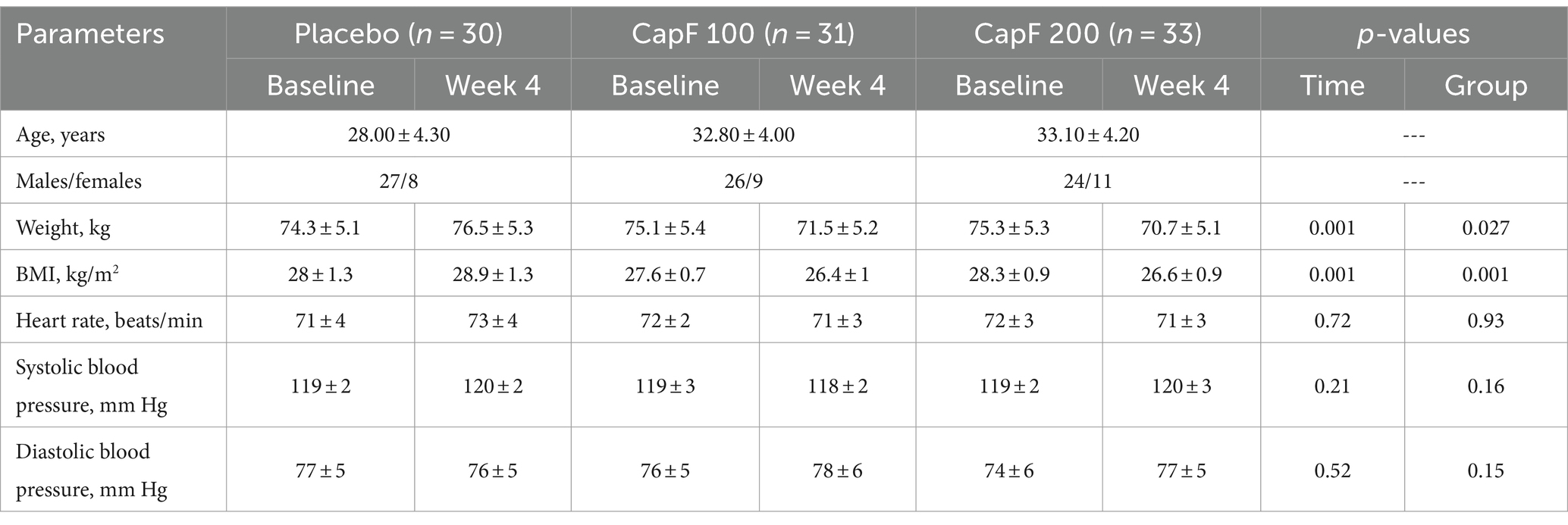
Table 2. Changes in subjective parameters upon supplementation of CapF at 100 and 200 mg/day with respect to placebo and baseline.
A 2 × 3 repeated-measures ANOVA was performed to compare the effect of CapF 100 and CapF 200 on EE-R. There was a statistically significant difference in EE-R between the placebo and treated groups [F(1,2) = 122.90, p = 0.026].
Analysis of within-subject effect showed a significant increase in EE-R for both CapF 100 (p < 0.001) and CapF 200 (p < 0.001) compared to baseline, but the placebo did not show any change (Figure 2A). This corresponded to a 195-kcal increase for CapF 100, 300 kcal for CapF 200, and − 18.2 kcal for placebo. The observed changes in EE-R (∆EE-R) for the CapF 100 and CapF 200 groups were 11-fold and 16-fold increase compared to placebo.
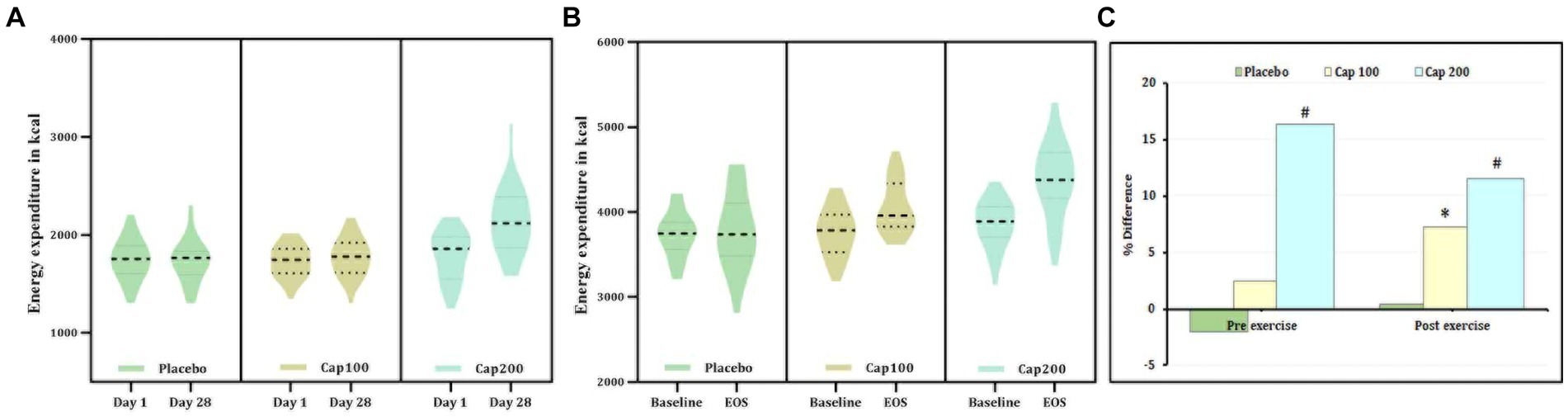
Figure 2. Violin plot showing the acute and chronic effects of CapF on energy expenditure at rest and at 1 h following the maximal exercise for three groups (placebo, CapF 100, and CapF 200). The violin plot outlines demonstrate kernel probability density. The width of the shaded area shows the distribution of the data. The thick dotted line shows the median, and the thin dotted lines represent quartiles. (A) Energy expenditure without exercise. The enhancement in energy expenditure under resting conditions (ΔEE-R) was >11-fold in CapF 100 mg/day compared to placebo, whereas CapF 200 showed 300 kcal ΔEE-R, which was 16-fold higher than placebo. (B) Energy expenditure 1 h after maximal exercise. The enhancement in exercise-induced energy expenditure (ΔEEE) was 11.08 kcal in the placebo group and 277.14 kcal in the 100 mg/day group, respectively, which was ~25-fold higher than the placebo. CapF 200 supplementation resulted in 330.8 kcal in ΔEEE, which was 30-fold higher than placebo. The values are expressed as mean ± SD. (C) Percentage difference based on baseline values for Placebo, CapF 100, and CapF 200. The symbols “*” and “#” in the bar diagram indicate significant differences at p < 0.05 compared to placebo.
The results of ANOVA revealed that there was a statistically significant interaction between the effects of placebo, CapF groups, and EEE [F(1, 2) = 7.12, p < 0.001].
The observed energy expenditure after exercise (on resting) is mentioned as exercise-induced energy expenditure. In both CapF 100 (p = 0.001) and CapF 200 groups (p < 0.001), the within-group effect of EEE demonstrated a significant rise compared to baseline, while the placebo group exhibited no significant change (Figure 2B). The relative change in the outcome (ΔEEE) representing the difference between the baseline and the end of the study was 277.14 kcal for CapF 100 (p = 0.001) and 330.8 kcal (p < 0.001) for the CapF 200 group, which were 25-fold and 30-fold higher, respectively, than the ΔEEE for the placebo (Figure 2C).
The respiratory quotient was measured before and after exercise. In this study, the simple main-effect analysis of resting respiratory quotient (RRQ) for the CapF 100 showed 4.2% (p < 0.001) and the CapF 200 showed a 11.45% reduction (p < 0.001) at the end of the study when compared to baseline, while the placebo showed no significant change [p > 0.05; Figure 3A; F(2,2) = 11.06; p < 0.001].
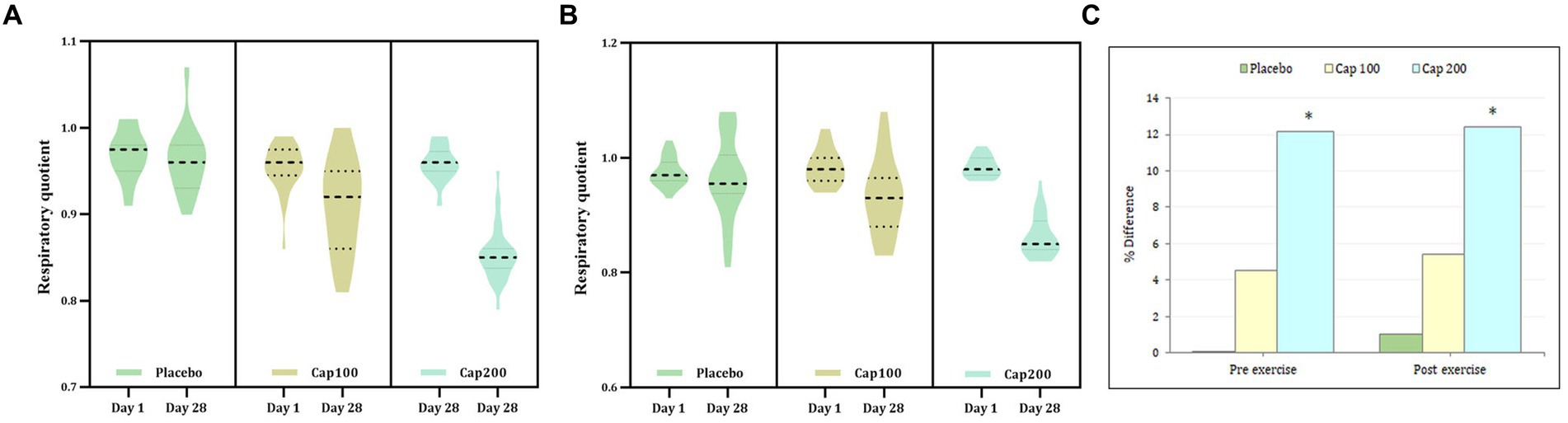
Figure 3. Violin plot showing the acute and chronic effects of CapF on respiratory quotient (RQ) at rest and at 1 h following the maximal exercise for three groups (placebo, CapF 100 and CapF 200). The violin plot outlines demonstrate kernel probability density. The width of the shaded area shows the distribution of the data. The thick dotted line shows the median, and thin dotted lines represent quartiles. (A) Treatment vs. time of RQ before exercise showed significant reduction at doses of 100 mg/day (p < 0.001) and 200 mg/day (p < 0.001) respectively at the end of study. The percentage of reduction were 4.2 and 11.45% respectively; (B) Treatment vs. time of RQ after exercise showed significant reduction at doses of 100 mg/day (p < 0.001) and 200 mg/day (p = 0.001) respectively at the end of study. The percentage of reduction were 5.10 and 12.24% respectively. The values are expressed as mean ± SD. (C) Percentage difference based on baseline values for placebo, CapF 100 and CapF 200. The symbol “*” in the bar diagram indicate significant difference at p < 0.05 compared to placebo.
Pairwise comparison of between-group effects at the end of the study showed a significant reduction in RRQ for CapF 100 (4.16%; p = 0.038) and CapF 200 (11.4%; p < 0.001) respectively compared to placebo. The CapF 200 showed a significant reduction (p < 0.001) when compared with the CapF 100.
Within-subject main-effect comparison of exercise-induced respiratory quotient (ERQ) showed a significant reduction for the CapF 100 (5.1%; p = 0.001) and a 12.24% reduction in the CapF 200 (p < 0.001), respectively, compared to baseline (Figure 3B). However, there was no significant change (p > 0.05) for the placebo [F(1,1) = 52.70; p = 0.001].
Between-group effect (the CapF 100 group vs. the placebo group) showed no significant reduction (3.12%; p > 0.05) for the CapF 100. However, the CapF 200 showed a significant reduction (10.41%; p < 0.001) compared with the placebo. The CapF 200 showed more reduction than the CapF 100 in ERQ, and the percentage of reduction was statistically significant (p < 0.001; Figure 3C).
Fat oxidation was calculated from RQ under conditions of rest, 1 h post-administration following exercise. The percentage increase in fat oxidation was 74.84% with the CapF 100 and 292.25% with the CapF 200, indicating a 3-fold increase in fat oxidation when the dosage was doubled (Figure 4A). This increase was statistically significant [F(1,2) = 37.69; p < 0.001]. At the end of the study, between-group effects resulted in a 77.25% increase in fat oxidation for the CapF 100 (p = 0.038) and a 231.7% increase for the CapF 200 (p < 0.001) compared to the placebo. Additionally, the comparison between the CapF 100 vs. the CapF 200 revealed a significant increase (p < 0.001) for the CapF 200 in RFO.
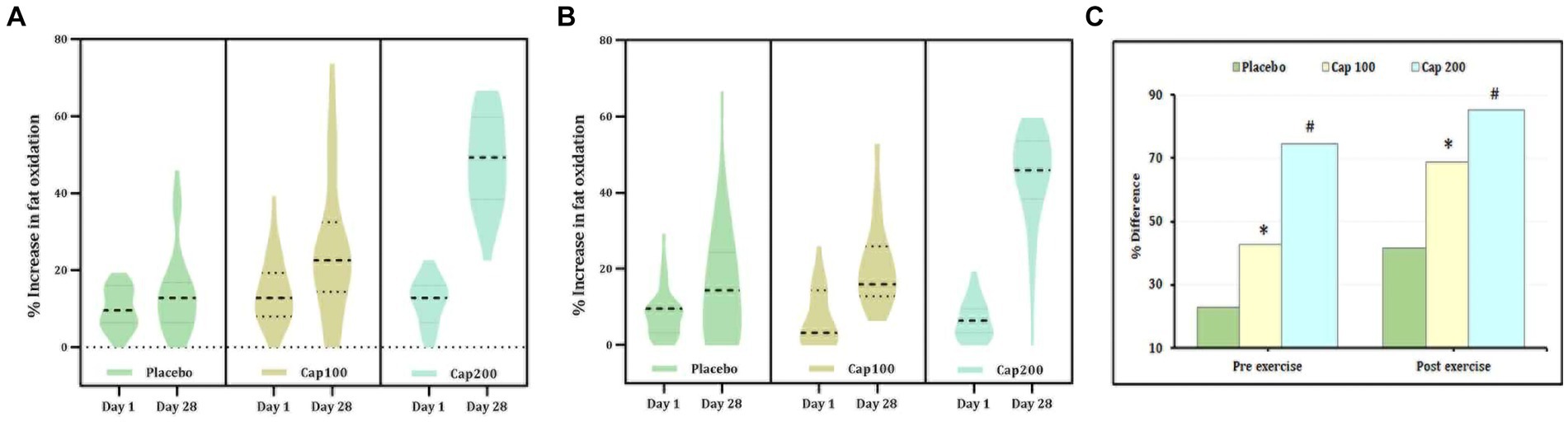
Figure 4. Violin plot showing the acute and chronic effects of CapF on fat oxidation (FO) at rest and at 1 h following the maximal exercise for three groups (placebo, CapF 100, and CapF 200). The violin plot outlines demonstrate kernel probability density. The width of the shaded area shows the distribution of the data. The thick dotted line shows the median, and the thin dotted lines represent quartiles. (A) Fat oxidation without exercise. The enhancement in fat oxidation was 77.25% in CapF 100 compared to placebo. However, the 200 mg/day supplemented group showed 292.25%, which was 10-fold higher than in placebo. Statistical analysis showed a significant increase in % fat oxidation (p < 0.001) in both 100 mg and 200 mg dosages. (B) Fat oxidation following maximal exercise. Statistical analysis showed a significant increase in % fat oxidation (p < 0.001) in both 100 mg and 200 mg dosages. (C) Percentage difference based on baseline values for placebo, CapF 100, and CapF 200. The symbols “*” and “#” in the bar diagram indicate significant differences at p < 0.05 compared to placebo.
The main effect observed in fat oxidation after exercise did not show a significant difference between the placebo and the CapF 100 or CapF 200 on day 1. However, supplementation with CapF 100 and CapF 200 resulted in a significant increase in fat oxidation by the end of the study [Figure 4B; F(1,2) = 28.78; p < 0.001].
The relative percentage increase upon between-group effects was 220% compared to baseline and 36% compared to the placebo. The CapF 200 group showed a 591% increase compared to baseline values (Figures 4B,C) and 185% compared to the placebo. The CapF 100 group vs. the CapF 200 group showed a significant difference (p = 0.001) in EFO, and the increase is high in the CapF 200.
The results of 2 × 3 repeated-measures ANOVA revealed a significant increase in time of exhaustion compared to baseline upon supplementation with CapF 100 and CapF 200 [F(1,2) = 21.41; p < 0.001]. The increase in CapF 100 is 68.52% (p < 0.001) and that of CapF 200 is 56.13% (p < 0.001; Figure 5). No significant change was observed in the placebo (p > 0.05).
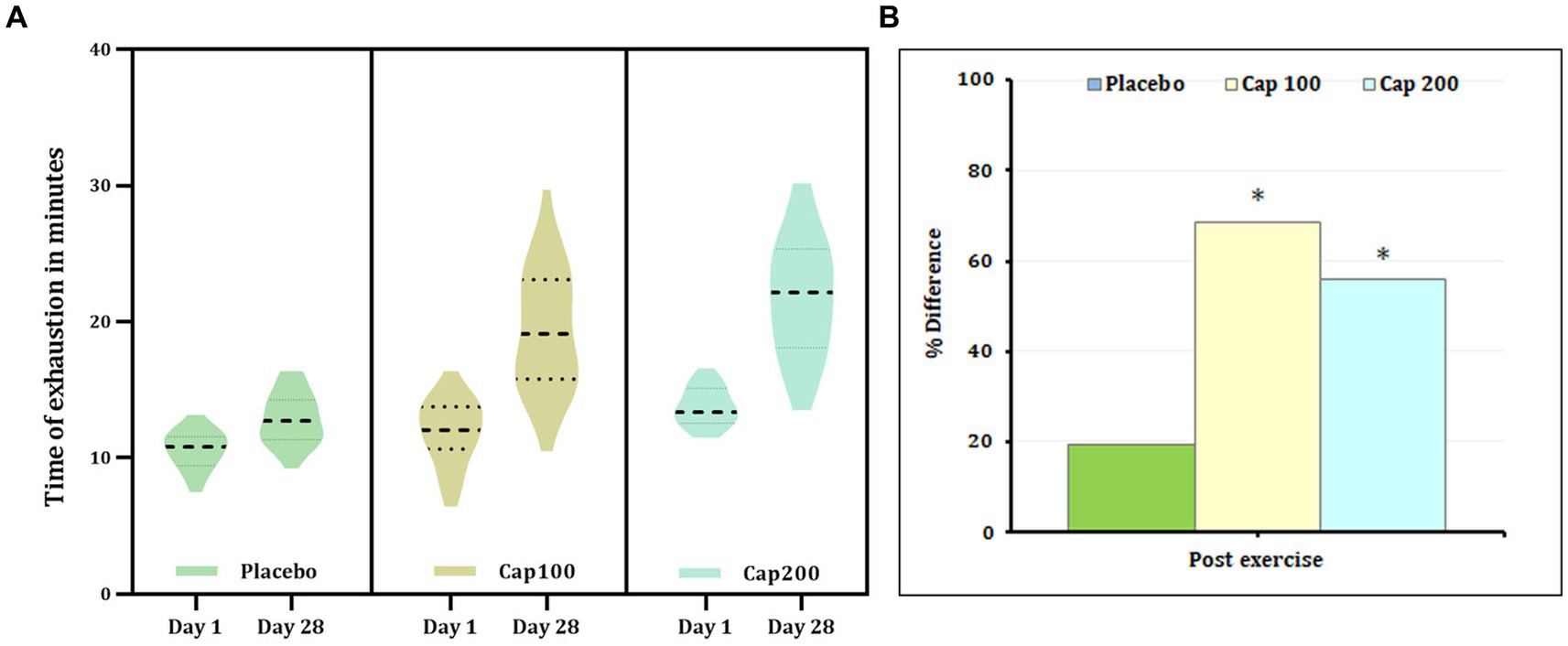
Figure 5. (A) Violin plot showing the acute and chronic effects of CapF on the time to exhaustion at rest and at 1 h following the maximal exercise for three groups (placebo, CapF 100, and CapF 200). The violin plot outlines demonstrate kernel probability density. The width of the shaded area shows the distribution of the data. The thick dotted line shows the median, and thin dotted lines represent quartiles. The results revealed significant increase in time of exhaustion compared to baseline upon supplementation with CapF 100 and CapF 200 (p < 0.001). The increase in CapF 100 is 68.52% (p < 0.001) and that of CapF 200 is 56.13% (p < 0.001). No significant change was observed in placebo (p > 0.05). (B) Percentage difference based on baseline values for placebo, CapF 100 and CapF 200. The symbol “*” in the bar diagram indicate significant difference at p < 0.05 compared to placebo.
Analysis of between-group effects at the end of the study revealed a 48.98% increase in the CapF 100 compared to the placebo, demonstrating statistical significance (p < 0.001). Additionally, the comparison between the placebo and the CapF 200 groups showed a significant 74.41% increase. Moreover, there was a significant increase in endurance for CapF 200 (p < 0.001) compared to CapF 100.
In total, four subjects in the CapF 200 group reported abdominal pain in the first 3 days, after intervention started; 2 subjects had diarrhea for the initial days and were able to control it in 1 week. Cold milk was administered to resolve the symptoms. No other adverse events were reported in either the CapF 100 or the CapF 200 group.
The present study demonstrates notable dose-dependent enhancements of energy expenditure and improvements in exercise performance with CapF, a pungency-masked sustained-intestinal release beadlets of pungent chili extract with fenugreek mucilage. Specifically, energy expenditure (both resting and exercise-induced), fat oxidation, and endurance were enhanced, without significant adverse events such as heart palpitations, abdominal pain, or nausea. Moreover, the respiratory quotient, a measure of substrate oxidation, was also found to be decreased in the CapF groups compared to placebo. Thus, our results indicate the safety and efficacy of CapF-red chili pepper extract formulation. A previous pilot study on overweight subjects had also reported the safety and efficacy of CapF on body weight, BMI, and waist/hip ratio when supplemented at 200 mg/day for 28 days (21). Moreover, animal studies have established the sustained-intestinal delivery, systemic absorption, and safety (acute and subchronic toxicity) of CapF (27).
Herein, we report the first randomized, double-blind, placebo-controlled study to investigate the influence of CapF on energy expenditure and respiratory quotient. High-calorie intake and low energy expenditure are the most common characteristics of a sedentary lifestyle leading to obesity (28). Dietary components are key to support low-calorie intake and enhanced energy expenditure. Red chili pepper consumption has been shown to have a positive influence on food intake and energy expenditure (29). Preclinical studies have substantiated the thermogenic and lipolytic effects of red chili pepper (30). Despite these encouraging effects, clinical studies have very often provided mixed results. The main challenge in clinical settings was the consumption of a physiologically relevant dosage of pungent red chili pepper extract for the systemic absorption of its bioactive capsaicinoids. We hypothesized that CapF would provide significant functional benefits due to its sustained intestinal release and systemic absorption as reported previously (20, 27). Moreover, 2 and 4 mg capsaicinoids per day, the dosage used in this study, has also been reported to have thermogenic effect in humans (31).
Our study indicates a substantial increase in both resting and exercise-induced energy expenditure (EE-R and EEE) when CapF was consumed at 100 and 200 mg/day (2 and 4 mg capsaicinoids) for 28 days. While the CapF 100 group showed an 11-fold increase in EE-R and a 25-fold increase in EEE, the CapF 200 group showed a 16-fold and 30-fold increase, respectively, compared to their baseline values. The relative change in the mean difference of outcomes observed in the CapF 200 group was significantly higher than the CapF 100 demonstrating a dose-dependent response. Previous human interventions with capsaicinoids have also reported enhanced energy expenditure (31, 32), supporting the current findings.
Thermogenic effects, elevation in energy expenditure and core body and skin temperature, of pungent chilies have been reported (33–37). It is known that a 10% to 13% increase in metabolic rate can increase the core body temperature by 1° centigrade, which would lead to an increase in caloric expenditure of 100 to 130 Kcal/day (38). We observed that the consumption of CapF is increasing the body temperature by approximately 1 to 2°C when measured in a temperature- and humidity-controlled environment.
A significant reduction of the respiratory quotient (RQ) in the CapF groups, under both resting and exercise conditions, indicates enhanced fatty acid metabolism (31). Furthermore, our findings demonstrate a dose-dependent response in fatty acid metabolism with higher CapF doses. Fat oxidation can be assessed clinically from the RQ; higher RQ values are indicative of low-fat oxidation and high carbohydrate oxidation (39). The observed reduction in RQ indicates increased fatty acid oxidation compared to placebo. Fatty acid oxidation can decrease plasma free fatty acids (FFAs), which otherwise can have various negative effects, including elevation in insulin resistance, non-alcoholic fatty liver disease (NAFLD), development of type II diabetes, and related comorbidities such as cardiovascular disease (CVD) (40). Thus, enhancing fatty acid oxidation with CapF may provide for a novel dietary approach to aid with weight management and other comorbidity as mentioned above.
Yet another important parameter measured in the present study was the time to exhaustion, which showed a significant increase for CapF groups indicating enhanced endurance performance (26). Recently, de Freitas et al. showed performance improvements in a 1,500-m running time trial, high-intensity intermittent exercise, and resistance training when supplemented with a higher dose (12 mg) of capsaicinoids (41–43). The influence of capsaicin on endurance performance may be attributed to its ability to activate the transient receptor potential vanilloid 1 (TRPV1) channel on sensory neurons, thereby improving ATP production, vascular function, and fatigue resistance (44). Additional studies are needed to elucidate the mechanisms for the performance-enhancing effects of CapF.
Increase in heart rate, palpitations, sweating, and abdominal pain are generally considered as the major side effects of capsaicin, whereas nausea, vomiting, dizziness, dysgeusia, headaches, and hypoesthesia are minor adverse events found to be associated with pungent red chili peppers or its extracts (45). We observed no significant change in heart rate or blood pressure after 28 days of treatment with both CapF 100 and CapF 200 indicating its primary safety. A few volunteers did experience abdominal pain, burning sensations, and diarrhea in the initial 4 to 5 days while consuming the supplement with an empty stomach. It is advised to avoid red chili pepper extract consumption with an empty stomach. No volunteers reported heart palpitations indicating its slow release.
Nevertheless, the study has certain limitations. Lack of detailed information on day-to-day food intake and physical activities, the lack of baseline resting energy expenditure data measured under fasting conditions, and the use of a self-selected treadmill speed include the major limitations physical activities, the lack of baseline resting energy expenditure data measured under fasting conditions, and the use of a self-selected treadmill speed include the major limitations. Additionally, measurement of thermogenesis and respective molecular markers would have added further value to the study. Future research addressing these factors, with a larger population, is recommended.
Pungent red chili pepper extracts and their principal bioactive, capsaicinoids, have been found to offer significant benefits for body physiology, especially under conditions of overweight and obesity by increasing thermogenesis via stimulating various physiological responses. However, consuming capsaicinoids at physiologically relevant dosages with systemic absorption is a primary challenge, due to the extreme pungency of chili peppers. This study demonstrates the efficacy and tolerability of the sustained-intestinal release of CapF at both 100 and 200 mg/day doses (2.1 mg and 4.2 mg capsaicinoids/day) to enhance energy expenditure (resting and exercise-induced), respiratory quotient, fat oxidation, and endurance performance. These findings support the potential use of CapF as an effective and safe supplement for weight maintenance and as an ergogenic aid.
The significance of the study also comes from the observation that CapF at the tested dose was tolerated and did not produce adverse events such as increased heart rate, pulse rate, palpitation, sweating, and abdominal pain despite its efficacy. The slow-release mechanism protected the capsaicinoids from the stomach environment, thereby preventing gastrointestinal irritation commonly associated with the consumption of capsaicin or pungent red chili extracts.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Sri Rama Hospital, Bangalore, India. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
NR: Investigation, Writing – review & editing. DS: Data curation, Writing – original draft. IK: Conceptualization, Formal analysis, Methodology, Writing – review & editing. KM: Investigation, Writing – review & editing. BF: Writing – review & editing. JT: Investigation, Project administration, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to express their sincere gratitude to the Sri Rama Hospital, Bangalore, and Lincoln Memorial University, Cumberland Gap Parkway, Harrogate, USA, for the support and advice during the course of this study.
CapF used in this study is a proprietary formulation of pungent red chili extract developed by Akay Natural Ingredients, Cochin, India, using their patented FenuMat® technology and registered as Capsifen®. IK and DS were employed by Akay Natural Ingredients. JT was employed by Leads Clinical Research and Bio Services Private Limited who conducted the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tiwari, A, and Balasundaram, P. Public health considerations regarding obesity. StatPearls (2023); Available at: https://www.ncbi.nlm.nih.gov/books/NBK572122/
2. Chong, B, Jayabaskaran, J, Kong, G, Chan, YH, Chin, YH, Goh, R, et al. Trends and predictions of malnutrition and obesity in 204 countries and territories: an analysis of the global burden of disease study 2019. EClinicalMedicine. (2023) 57:101850. doi: 10.1016/j.eclinm.2023.101850
3. Obesity and Overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2021).
4. Hill, JO, Wyatt, HR, Reed, GW, and Peters, JC. Obesity and the environment: where do we go from here? Science. (2003) 299:853–5. doi: 10.1126/science.1079857
5. Veerman, JL, Barendregt, JJ, Van Beeck, EF, Seidell, JC, and Mackenbach, JP. Stemming the obesity epidemic: a tantalizing prospect. Obesity. (2007) 15:2365–70. doi: 10.1038/oby.2007.280
6. Hall, KD, Heymsfield, SB, Kemnitz, JW, Klein, S, Schoeller, DA, and Speakman, JR. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. (2012) 95:989. doi: 10.3945/ajcn.112.036350
7. Lawrence, CB . The contribution of raised metabolic rate in the weight loss associated with Alzheimer’s disease. Diet Nutr Dement Cogn Decline. (2015) 2015:479–86. doi: 10.1016/B978-0-12-407824-6.00043-4
8. Bo, S, Fadda, M, Fedele, D, Pellegrini, M, Ghigo, E, and Pellegrini, N. A critical review on the role of food and nutrition in the energy balance. Nutrients. (2020) 12:1161. doi: 10.3390/nu12041161
9. Busbridge, NJ, and Rothwell, NJ. Thermogenic effects of cytokines: methods and mechanisms. Methods Neurosci. (1993) 17:96–110. doi: 10.1016/S1043-9471(13)70011-9
10. Joosen, AMCP, and Westerterp, KR. Energy expenditure during overfeeding. Nutr Metab. (2006) 3:25. doi: 10.1186/1743-7075-3-25
11. von Essen, G, Lindsund, E, Cannon, B, and Nedergaard, J. Adaptive facultative diet-induced thermogenesis in wild-type but not in UCP1-ablated mice. Am J Physiol Endocrinol Metab. (2017) 313:E515–27. doi: 10.1152/ajpendo.00097.2017
12. Saito, M, Yoneshiro, T, and Matsushita, M. Activation and recruitment of brown adipose tissue by cold exposure and food ingredients in humans. Best Pract Res Clin Endocrinol Metab. (2016) 30:537–47. doi: 10.1016/j.beem.2016.08.003
13. Mele, L, Bidault, G, Mena, P, Crozier, A, Brighenti, F, Vidal-Puig, A, et al. Dietary (poly)phenols, Brown adipose tissue activation, and energy expenditure: a narrative review. Adv Nutr. (2017) 8:694–704. doi: 10.3945/an.117.015792
14. Nalivaiko, E . Thermoregulation and nausea. Handb Clin Neurol. (2018) 156:445–56. doi: 10.1016/B978-0-444-63912-7.00027-8
15. Hachemi, I, and U-Din, M. Brown adipose tissue: activation and metabolism in humans. Endocrinol Metab. (2023) 38:214. doi: 10.3803/EnM.2023.1659
16. Lu, M, Chen, C, Lan, Y, Xiao, J, Li, R, Huang, J, et al. Capsaicin-the major bioactive ingredient of chili peppers: bio-efficacy and delivery systems. Food Funct. (2020) 11:2848–60. doi: 10.1039/D0FO00351D
17. Srinivasan, K . Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr. (2016) 56:1488–500. doi: 10.1080/10408398.2013.772090
18. Varghese, S, Kubatka, P, Rodrigo, L, Gazdikova, K, Caprnda, M, Fedotova, J, et al. Chili pepper as a body weight-loss food. Int J Food Sci Nutr. (2017) 68:392–401. doi: 10.1080/09637486.2016.1258044
19. Ludy, MJ, Moore, GE, and Mattes, RD. The effects of capsaicin and Capsiate on energy balance: critical review and Meta-analyses of studies in humans. Chem Senses. (2012) 37:103. doi: 10.1093/chemse/bjr100
20. Joseph, A, Maliakkal Balakrishnan, A, Natinga Mulakal, J, Das Sivadasan, S, Mohan, R, Maliakel, B, et al. A green approach for the sustained-intestinal delivery of red chili (Capsicum annum L) extracted capsaicinoids with enhanced bioavailability. J Funct Foods. (2021) 85:104658. doi: 10.1016/j.jff.2021.104658
21. Joseph MSc, A, John PhD, F, Thomas MSc, JV, Sivadasan, SDP, Maliakel PhD, B, Mohan PhD, R, et al. Influence of a novel food-grade formulation of red chili extract (Capsicum annum) on overweight subjects: randomized, double-blinded, placebo-controlled study. J Herb Pharmacother. (2021) 18:387–405. doi: 10.1080/19390211.2020.1780363
22. Nieman, DC, Austin, MD, Dew, D, and Utter, AC. Validity of COSMED’s quark CPET mixing chamber system in evaluating energy metabolism during aerobic exercise in healthy male adults. Res Sports Med. (2013) 21:136–45. doi: 10.1080/15438627.2012.757227
23. Hoffman, PG, Lego, MC, and Galetto, WG. Separation and quantitation of red pepper major heat principles by reverse-phase high-pressure liquid chromatography. J Agric Food Chem. (1983) 31:1326–30. doi: 10.1021/jf00120a044
24. Frings-Meuthen, P, Henkel, S, Boschmann, M, Chilibeck, PD, Alvero Cruz, JR, Hoffmann, F, et al. Resting energy expenditure of master athletes: accuracy of predictive equations and primary determinants. Front Physiol. (2021) 12:1455. doi: 10.3389/fphys.2021.641455
25. Patel, H, Kerndt, CC, and Bhardwaj, A. Physiology, Respiratory Quotient. StatPearls. (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK531494/
26. Ibrahim, A, Mat Ludin, AF, Shahar, S, Hamzah, NH, Chin, AV, and Singh, DKA. Association between maximal oxygen consumption and physical performance tests among older adults with cognitive frailty. Exp Gerontol. (2023) 184:112326. doi: 10.1016/j.exger.2023.112326
27. Joseph, A, Nm, J, Kumar, S, Maliakel, B, and Im, K. Safety assessment of a fenugreek dietary fiber-based formulation of capsaicinoids-rich red chili (Capsicum annum) extract (Capsifen®): acute and sub-chronic studies. Toxicol Rep. (2020) 7:602–9. doi: 10.1016/j.toxrep.2020.04.014
28. Park, JH, Moon, JH, Kim, HJ, Kong, MH, and Oh, YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean. J Fam Med. (2020) 41:365–73. doi: 10.4082/kjfm.20.0165
29. Siebert, E, Lee, SY, and Prescott, MP. Chili pepper preference development and its impact on dietary intake: a narrative review. Front Nutr. (2022) 9:9207. doi: 10.3389/fnut.2022.1039207
30. Zheng, J, Zheng, S, Feng, Q, Zhang, Q, and Xiao, X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep. (2017) 37:286. doi: 10.1042/BSR20170286
31. Janssens, PLHR, Hursel, R, Martens, EAP, and Westerterp-Plantenga, MS. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS One. (2013) 8:e67786. doi: 10.1371/journal.pone.0067786
32. Yoshioka, M, Lim, K, Kiyonaga, A, Tanaka, H, Shindo, M, and Suzuki, M. Effects of red-pepper diet on the energy metabolism in men. J Nutr Sci Vitaminol. (1995) 41:647–56. doi: 10.3177/jnsv.41.647
33. Galgani, JE, and Ravussin, E. Effect of dihydrocapsiate on resting metabolic rate in humans. Am J Clin Nutr. (2010) 92:1089–93. doi: 10.3945/ajcn.2010.30036
34. Josse, AR, Sherriffs, SS, Holwerda, AM, Andrews, R, Staples, AW, and Phillips, SM. Effects of capsinoid ingestion on energy expenditure and lipid oxidation at rest and during exercise. Nutr Metab. (2010) 7:65. doi: 10.1186/1743-7075-7-65
35. Ohnuki, K, Moritani, T, Ishihara, K, and Fushiki, T. Capsaicin increases modulation of sympathetic nerve activity in rats: measurement using power spectral analysis of heart rate fluctuations. Biosci Biotechnol Biochem. (2001) 65:638–43. doi: 10.1271/bbb.65.638
36. Hachiya, S, Kawabata, F, Ohnuki, K, Inoue, N, Yoneda, H, Yazawa, S, et al. Effects of CH-19 sweet, a non-pungent cultivar of red pepper, on sympathetic nervous activity, body temperature, heart rate, and blood pressure in humans. Biosci Biotechnol Biochem. (2007) 71:671–6. doi: 10.1271/bbb.60359
37. Ludy, MJ, and Mattes, RD. The effects of hedonically acceptable red pepper doses on thermogenesis and appetite. Physiol Behav. (2011) 102:251–8. doi: 10.1016/j.physbeh.2010.11.018
38. Landsberg, L, Young, JB, Leonard, WR, Linsenmeier, RA, and Turek, FW. Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Trans Am Clin Climatol Assoc. (2009) 120:287–95.
39. Schutz, Y . Abnormalities of fuel utilization as predisposing to the development of obesity in humans. Obes Res. (1995) 3:173s–8s. doi: 10.1002/j.1550-8528.1995.tb00460.x
40. Pujia, A, Mazza, E, Ferro, Y, Gazzaruso, C, Coppola, A, Doldo, P, et al. Lipid oxidation assessed by indirect calorimetry predicts metabolic syndrome and type 2 diabetes. Front Endocrinol. (2019) 9:10. doi: 10.3389/fendo.2018.00806
41. de Freitas, MC, Billaut, F, Panissa, VLG, Rossi, FE, Figueiredo, C, Caperuto, EC, et al. Capsaicin supplementation increases time to exhaustion in high-intensity intermittent exercise without modifying metabolic responses in physically active men. Eur J Appl Physiol. (2019) 119:971–9. doi: 10.1007/s00421-019-04086-w
42. de Freitas, MC, Cholewa, JM, Gobbo, LA, de Oliveira, JVNS, Lira, FS, and Rossi, FE. Acute capsaicin supplementation improves 1,500-m running time-trial performance and rate of perceived exertion in physically active adults. J Strength Cond Res. (2018) 32:572–7. doi: 10.1519/JSC.0000000000002329
43. de Freitas, MC, Cholewa, JM, Freire, RV, Carmo, BA, Bottan, J, Bratfich, M, et al. Acute capsaicin supplementation improves resistance training performance in trained men. J Strength Cond Res. (2018) 32:2227–32. doi: 10.1519/JSC.0000000000002109
44. Giuriato, G, Venturelli, M, Matias, A, Soares, EMKVK, Gaetgens, J, Frederick, KA, et al. Capsaicin and its effect on exercise performance, fatigue and inflammation after exercise. Nutrients. (2022) 14:232. doi: 10.3390/nu14020232
Keywords: capsaicin, thermogenesis, Capsifen, endurance, energy expenditure, FenuMat, respiratory quotient
Citation: Roopashree N, Syam DS, Krishnakumar IM, Mala KN, Fleenor BS and Thomas J (2024) A natural sustained-intestinal release formulation of red chili pepper extracted capsaicinoids (Capsifen®) safely modulates energy balance and endurance performance: a randomized, double-blind, placebo-controlled study. Front. Nutr. 11:1348328. doi: 10.3389/fnut.2024.1348328
Received: 04 December 2023; Accepted: 27 February 2024;
Published: 20 March 2024.
Edited by:
Eric Gumpricht, Isagenix International, LLC, United StatesReviewed by:
Abhirup Shaw, McGill University, CanadaCopyright © 2024 Roopashree, Syam, Krishnakumar, Mala, Fleenor and Thomas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:Jestin Thomas, amVzdGluQGxlYWRzYmlvLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.