- School of Economics and Management, Jiangsu University of Science and Technology, Zhenjiang, China
Background: While cataracts, the vision-clouding eye disease associated with aging, have long presumed dietary underpinnings, the relationship between dietary variety and cataract risk in developing nations has been nebulous. This research aims to investigate the association between dietary diversity scores (DDS) and the risk of cataracts, while considering various dietary diversity patterns.
Methods: This research utilized cross-sectional data from 2008 to 2018 extracted from the Chinese Longitudinal Healthy Longevity Survey (CLHLS), implementing the Visual Function Index-14 (VF-14) to gauge cataract probability. The researchers captured participants' diet diversity by using the DDS metric and categorized it into total, animal-based, and plant-based diet patterns. To explore associations between dietary variety and cataract potential, a generalized estimating equation (GEE) was statistically modeled using the data, with adjustments made to account for potentially confounding factors. Additionally, sensitivity analyses were conducted, excluding individuals with assorted eye conditions, to isolate cataract relationships.
Results: The study sample comprised 47,395 participants with a mean age of 86.1 years. The study found that a lower likelihood of developing cataract was correlated with both total diet (OR = 0.74; 95% CI: 0.69–0.79) and plant-based diet (OR = 0.65; 95% CI: 0.61–0.71), whereas a slightly higher risk was associated with animal-based diet (OR = 0.90; 95% CI = 0.84–0.96). The results remained unchanged in the sensitivity analysis.
Conclusion: The diversified diets are linked to a decreased likelihood of developing cataracts, but animal-based diet faced heightened cataract odds. The implementation of a varied dietary regimen has the potential to serve as a cost-effective and efficient intervention strategy for the prevention of cataracts.
1 Introduction
Cataract is a prevalent ocular disease caused by the opacification of the transparent lens within the ocular structure, resulting in visual impairment and blindness among elderly individuals (1). According to the World Health Organization (WHO), cataract contributes to 47.9% of reported cases of worldwide blindness and visual impairment (2). While the occurrence of cataracts varies across regions and age groups, the majority of cases are recorded among individuals aged 60 years and older (reported prevalence: 1% in the under-40 age group and 88.17% in the over-60 age group) (3). Although cataracts may not be serious in the early stages, they will develop into blindness without early intervention. Research indicates that delayed treatment is the primary cause of visual impairment for patients with congenital or pediatric cataracts (4). Therefore, early diagnosis and treatment of cataracts are crucial to prevent further visual impairment and improve wellness of the older adults (4, 5).
Cataract development is influenced by a range of factors, including age, heredity, and external risk factors. Smoking, diabetes, and UVB exposure are consistently identified as risk factors (6, 7). Other potential risk factors include plasma constituents, steroids, alcohol, hypertension, hyperlipidemia, and obesity (8). Certain drugs, such as steroids, nifedipine, and heavy smoking, are associated with a higher risk, while aspirin-like analgesics and cyclopenthiazide may offer protection (9). However, the role of dietary factors in prevention remains unclear (8). Further research is needed to fully understand the complex interplay of these factors in cataract development (6).
Cataracts have substantial effects on various aspects of health and wellbeing, including but not limited to reading ability, driving proficiency, and self-care capabilities (10). Furthermore, empirical evidence also suggests that the presence of cataracts may potentially increase the likelihood of experiencing falls, fractures, depressive symptoms, and social isolation (11). Traditional diagnostic methods rely on fundus exams and visual acuity tests, diagnosing only once vision dips below 20/40 (12). However, the expert equipment and skills required make such evaluations impractical for widespread community screening. Additionally, subclinical cataracts that conventional methods fail to identify may have impact on visual function and quality of life (13). The Visual Function Index-14 (VF-14) as a perceived visual function measure holds great potential to address the issue of subclinical cataracts (14). The VF-14 is a simple, user-friendly, and compliant measure of perceived visual function, developed by the US National Institute of Ophthalmology. It is widely used to evaluate the QoL, cost-effectiveness, and outcomes of cataract patients' interventions (15). Cataract prevalence according to the VF-14 criteria varies from 0.42% to 2.05% in low-income countries and from 0.63% to 13.6% in high-income countries (16). This research uses part of the VF-14 questionnaire to identify elderly Chinese subclinical cataracts as “possible cataracts.” This study aims to promote early adjustment of harmful dietary habits, which may contribute to cataract prevention and wellness improvement.
Dietary factors may affect the development of cataract by influencing the oxidative stress in the lens. And it is widely believed that antioxidant nutrients are the key factors (17). For example, the connection between specific antioxidant nutrients (such as vitamin B1, vitamin A, lutein, etc.) and cataract risk has been proven (18). The results claim that higher intake of these antioxidant nutrients is associated with lower incidence and severity of cataract (19). Therefore, researchers now aim to unlock the protective powers of antioxidant-rich foods, believing these supercharged ingredients may safeguard the lens from oxidative damage (20). But previous research on the connection between dietary variables and the development of cataracts has been hampered by a few key issues (21–29). First, most research has concentrated on single nutrients or individual meals, neglecting potential interactions and synergy between dietary components (20–24). Second, owing to data restrictions, self-reported food consumption data are commonly employed. Thus, measurement mistakes and memory bias are widespread (24, 26). Third, most research has been done in wealthy nations, which may have different diets and cataract rates than developing nations (21–29).
To fully expose the antioxidant nutrient's protective effects on the lens from oxidative damage, it is necessary to investigate the overall diet or dietary diversity. The prospective Dietary Diversity Score (DDS) measures dietary diversification. The level of dietary diversity is determined by combining DDS scores for different foods. Higher DDS indicates a more varied and balanced diet (19, 28–31). DDS is reliable for assessing dietary nutritional adequacy and quality, according to researchers. DDS, as a simple tally of consumed food groups, has emerged as a powerful tool for unraveling connections between nutrition and illness. Researchers have utilized DDS to illuminate diet's impacts on a sprawling spectrum of conditions: obesity, diabetes, hypertension, cognitive decline, and even mortality (25, 32, 33). Studies show inverse relationships between DDS and chronic diseases: as diet diversity decreases, disease risk increases. Each added food group may help prevent conditions like CVD, cancer, and diabetes, where mechanisms remain uncertain. While DDS reveals nutrition variety's broad impact on health, further research on diet nuances could offer targeted nutritional remedies based on individual risk factors. DDS also improves health and longevity (29, 31). Previous studies have examined the association between adherence to the Dietary Guidelines for Americans (measured by HEI-2015 scores) and risks of gout and hyperuricemia using data from the National Health and Nutrition Examination Survey (NHANES). These studies have found that higher HEI-2015 scores are associated with decreased risks of these diseases. The HEI-2015 score considers multiple aspects of diet quality, including adequacy and moderation of food components. In this regard, it is similar to the dietary diversity score (DDS) which also focuses on diet diversity. Therefore, the application of HEI-2015 in previous studies demonstrates that using scoring methods that capture multiple dimensions of diet quality such as DDS to assess diet-health outcome relationships is reasonable. This helps to support the utility of diversity-focused dietary scores like DDS (34). In addition, empirical evidence also links DDS to improved cognitive performance, fewer physical limitations, and lower psychological stress in elderly adults (35, 36).
Despite cataracts' toll on global aging populations, few studies have probed associations between DDS and cataract risk, particularly in developing nations (19, 37, 38). This research gap proves troubling given the afflictions posed to China's massive elderly community. Though this demographic faces amplified cataract susceptibility, scarce data has explored the phenomenon among Chinese seniors (38–40). Therefore, this pioneering study seeks to illuminate the intricacies between cataracts and different kinds of diet diversities in China's vulnerable older populations. By unraveling relationships between nutrition variety, gender, and cataract prevalence, findings could unveil dietary remedies to empower China's elderly to proactively protect their vision and wellness. While research overall remains limited, this investigation aims to catalyze further scholarship on targeted nutritional interventions against cataracts across high-risk demographics worldwide.
2 Materials and methods
2.1 Study population
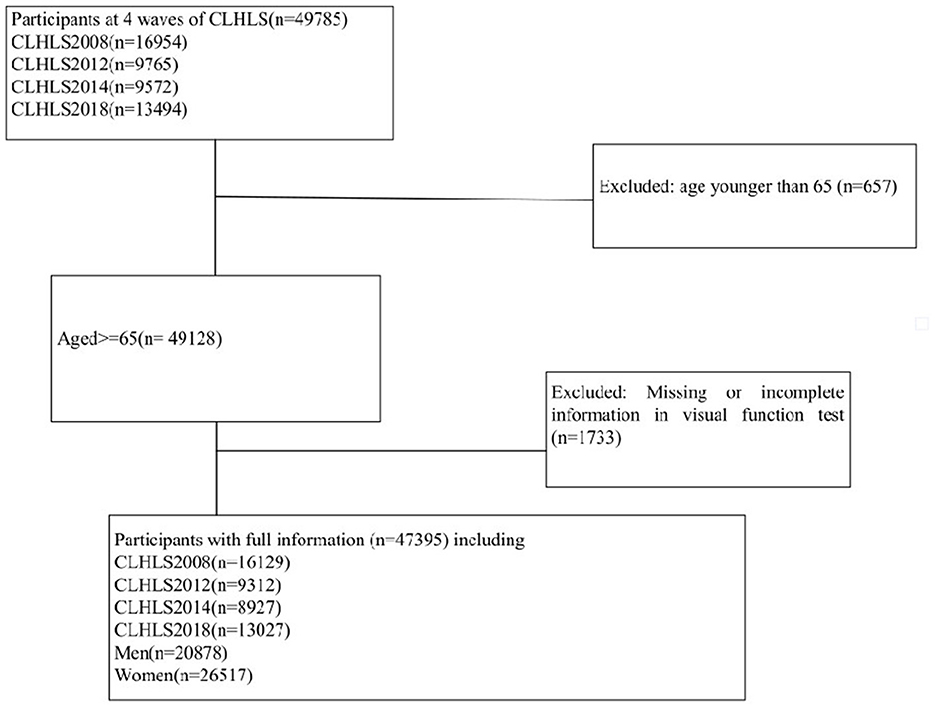
This study utilized the data from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) to examine the relationship between dietary diversity and cataract risk in the elderly population in China. The CLHLS is a nationwide survey that covers the majority of regions in China. As shown in Figure 1, this expansive investigation drew from a robust sample of 49,785 participants surveyed over the decade spanning 2008 to 2018. In chronological order, 16,954 subjects completed assessments in the 2008 cohort, followed by 9,765 in 2012, 9,572 in 2014, and culminating with 13,494 seniors surveyed in 2018. Following comprehensive vision evaluations, we excluded 657 participants under 65 years old, as well as 1,733 individuals with incomplete or missing data. This exhaustive filtering process ensured an optimized final sample of the target elderly demographic, enabling more authoritative analyses of associations between nutrition, age, and cataract prevalence. Finally, with quality data from 47,395 seniors, we could shed light on dietary strategies to safeguard vision among China's most vulnerable populations. Informed consent was obtained from all participants and/or their families, and the study was approved by the Ethics Committee of Peking University (IRB00001052-13074).
2.2 Assessment of dietary diversity
To quantify nutritional diversity, we developed a 10-point DDS aligned with national Chinese dietary guidelines recommending intake from 10 food groups (29, 41, 42). Utilizing food frequency questionnaires covering cereals, vegetables, fruits, legumes, nuts, meat, eggs, fish, dairy, and fungi, we assigned 1 point for “regular” or “almost daily” consumption of each food category. This scoring system enabled total DDS calculations for each participant on a 0–10 scale. Beyond the total DDS, we also evaluated animal-based and plant-based DDS (43). Animal-based DDS tracked meat, fish, eggs, and dairy, with consumption frequency scored from 0–4. Plant-based DDS spanned six categories—grains, vegetables, fruits, legumes, nuts, and fungi—rated on 0–6 scales based on intake regularity. With these multi-faceted DDS techniques, we could illuminate the nuanced contributions of diverse food groups, from animal vs. plant sources to overall nutritional variety. These insights into the unique dietary diversity patterns could help inform targeted nutritional interventions to avoid age-related vision loss. To enhance the survey methodology, validated attention-recall questions were incorporated alongside traditional questionnaires. This addition aimed to stimulate precise recollections of dietary habits. For instance, participants were tasked with tasks such as reproducing a figure displayed on a card or recalling a set of three words provided to them earlier. From the research methods and results, there exists certain similarity between the HEI-2015 scores adopted by previous studies and the DDS scores. Specifically, HEI-2015 contains 9 adequacy components and 4 moderation components, which can evaluate the overall dietary quality and diversity. Similarly, DDS usually considers the number of food groups or categories to assess diet diversity. Previous studies have found that the high score groups of HEI-2015 and DDS are both associated with improvement in various health outcomes, such as reduced risk of sleep disorders, just like the high score groups of DDS, which are beneficial to cure chronic diseases like diabetes. Therefore, although previous studies did not explicitly examine DDS, their methods and conclusions provide evidence that HEI-2015 and DDS scores have certain comparability in reflecting dietary quality and the association with health outcomes (34, 44).
2.3 Assessment of possible cataract
To identify potential cataract cases, we deployed a self-reported visual function assessment adapted from the validated VF-14 questionnaire on cataracts' daily impacts (15). This simple yet insightful screening technique presented participants with a circle containing a break, then asked them to rate their ability to locate the break on a 4-point scale. Those rating their vision as a 1 (“can see and distinguish”) were classified as cataract-free, while scores of 2 (“can see only”) or 3 (“cannot see”) indicated potential cataracts. And we excluded individuals rating themselves as 4 (“blind”) (45). By relying on participants' perceptions of their own functional vision rather than clinical testing, this questionnaire-based approach enabled efficient, large-scale screening for cataract prevalence. The nuanced 4-point scale also illuminated gradations in impairment, differentiating total blindness from mild or severe cataract symptoms. With a validated technique tailored to elderly self-reporting, investigators could rapidly identify participants potentially afflicted by cataracts for further analysis of associations with nutrition.
2.4 Covariates
In order to facilitate a more authoritative examination of the link between different diets and cataract prevalence, researchers adjusted for a diverse spectrum of co-varying participant traits that could confound analyses of the isolated diet-cataract relationship. Analyzed participant traits ranged from gender (male or female), segmented age groups (65–79 years, 80–99 years, 100 years or older), education level (educated or uneducated), marital status (married or unmarried/divorced/widowed), pre-retirement occupation (peasant or non-peasant), and household income (<100,000 yuan or ≥100,000 yuan) to lifestyle factors like smoking status (never, previous or current), alcohol consumption patterns (never, previous or current), and physical activity levels (never, previous or current). Adjusting for this extensive set of co-varying factors enabled the isolation of the diet-cataract link for more authoritative analysis of how nutritional diversity alone relates to age-linked visual changes.
2.5 Statistical analysis
The data, presented as mean ± standard deviation or percentages, underwent chi-square testing to analyze categorical variable relationships across groups and independent t-tests to evaluate numerical variable differences. Utilizing Generalized Estimating Equation (GEE) models incorporating auto regressive (AR) working correlation structures, we calculated cataract development odds ratios (OR) across DDS quartiles. Model 1 was the unadjusted model. Building on the initial model, subsequent models integrated additional controls. Model 2 adjusted for demographic variables including gender, age, and household income to address confounding. Then, Model 3 incorporated further factors like education level, marital status, and pre-retirement occupation. Finally, Model 4 also accounted for lifestyle elements, including physical activity, smoking, and drinking patterns. A sensitivity analysis was performed to assess the reliability of the estimates after removing older individuals with eye conditions that affect vision. The data analysis was conducted using STATA statistical software version 17.0, which was specifically developed for the Windows operating system. The statistical significance was assessed using a two-tailed p-value of 0.05.
3 Results
3.1 Descriptive characteristics
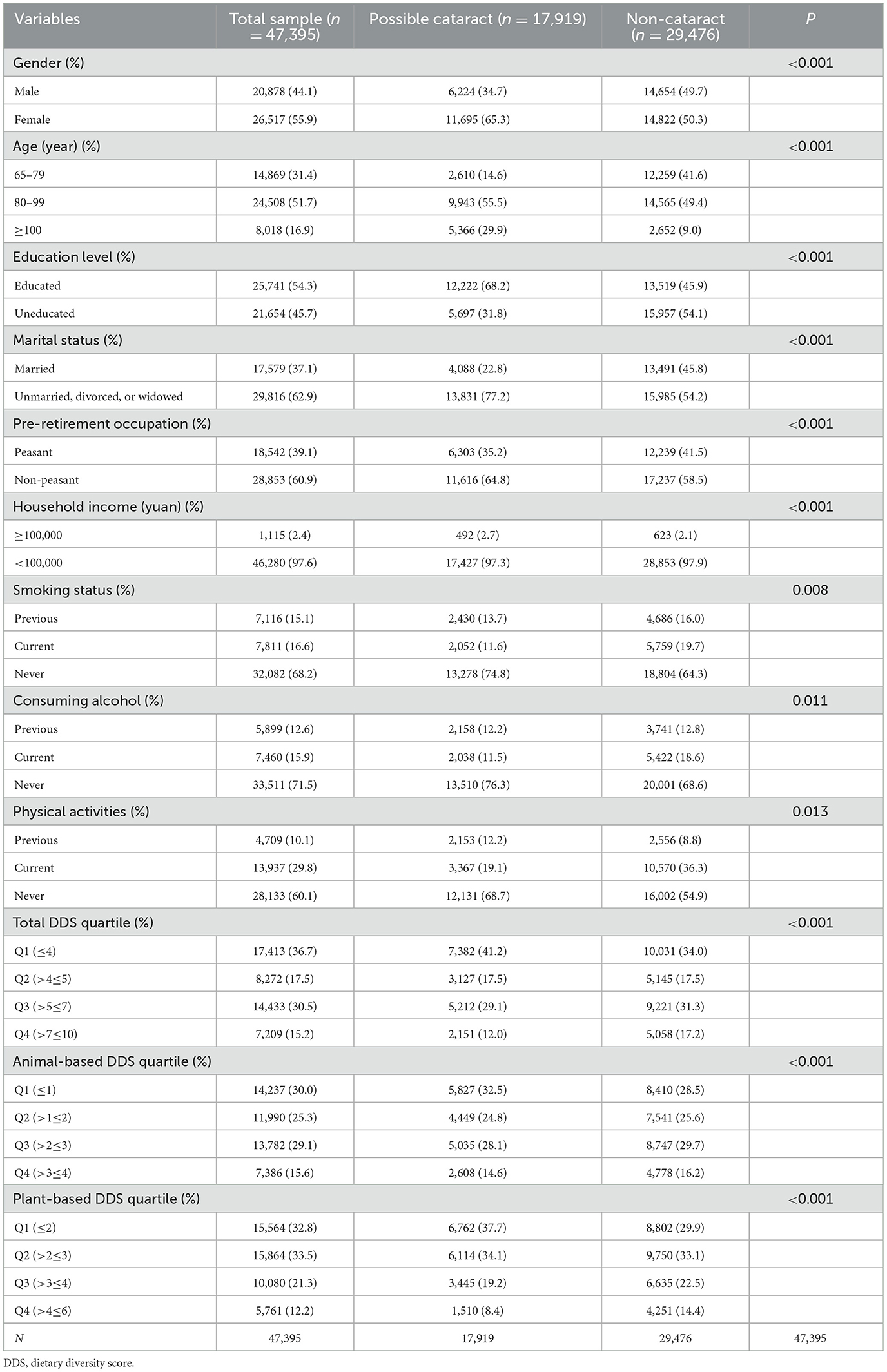
The 47,395 participants exhibited 17,919 participants (37.8%) possible cataract cases, with a gender breakdown of 20,878 (44.1%) men and 26,517 (55.9%) women, as is shown in Table 1. Marital status included 17,579 (37.1%) married individuals. A vast array of 14,869 participants ranging from 65 to 79 years old are involved; an even more substantial cohort of 24,508 aged individuals between 80 and 99 provided their information; additionally, a particularly remarkable group of 8,018 extraordinarily aged folks 100 years of age or older offered their information. Education levels proved relatively low, with 45.7% of the participants uneducated. As for pre-retirement occupation, 28,853 people (60.9%) resided in urban areas. In addition, 46,280 participants (97.6% of the total), reported a household income <100,000 yuan. Regarding lifestyle factors, 16.6% were smokers, 15.9% drank alcohol, and 29.8% performed physical activities. As demonstrated in Table 1, the highest quartile (Q4) of the animal-based DDS contained not only the greatest absolute number but also the highest percentage of possible cataract cases at 2,608 and 14.6% respectively; in contrast, Q4 of the plant-based DDS Quartile contained the fewest number and lowest percentage of possible cataracts cases at 1,510 and 8.4% respectively, while total DDS Quartile Q4 displayed moderate numbers and percentages of possible cataracts at 2,151 and 12.0%.
3.2 DDS and possible cataract
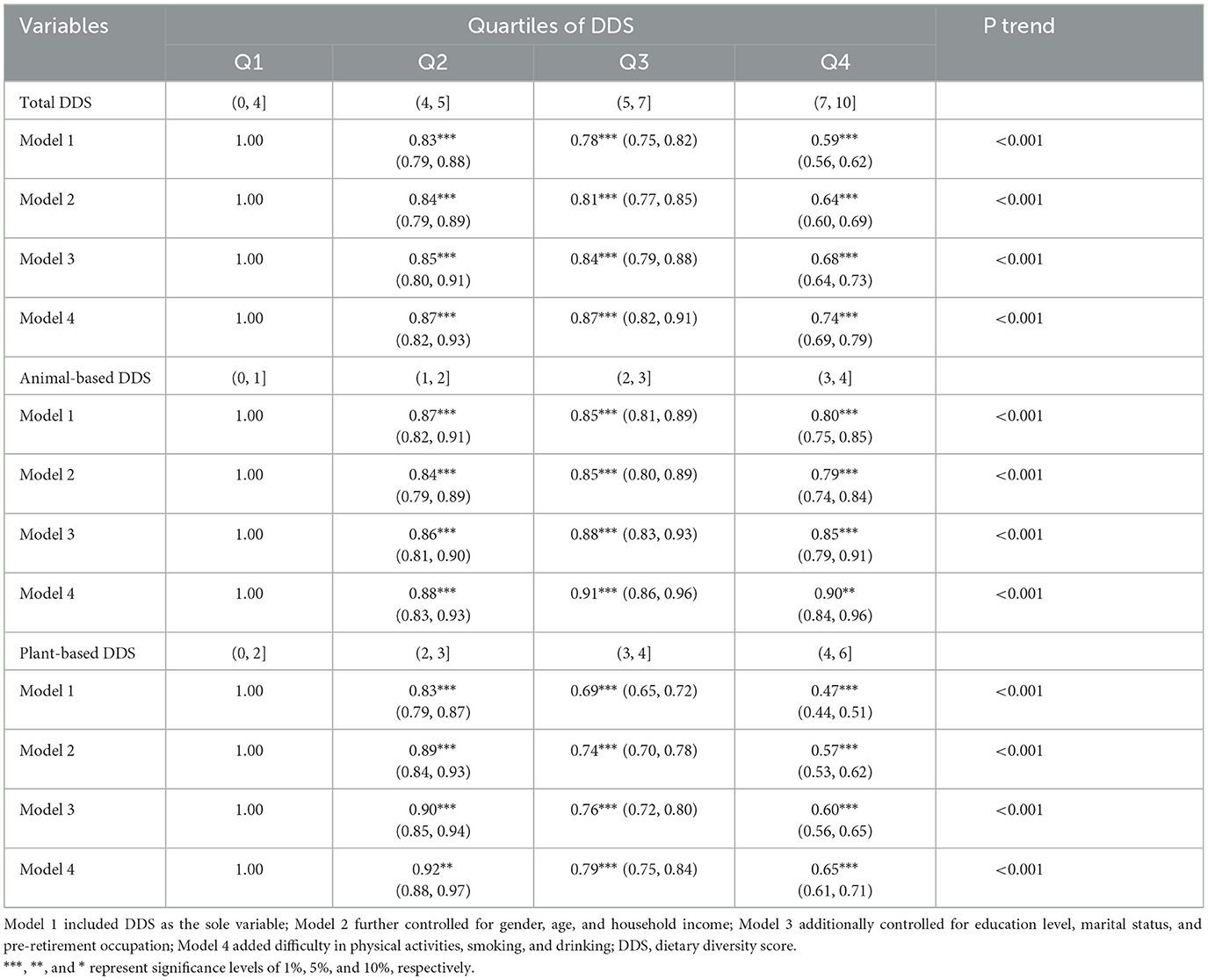
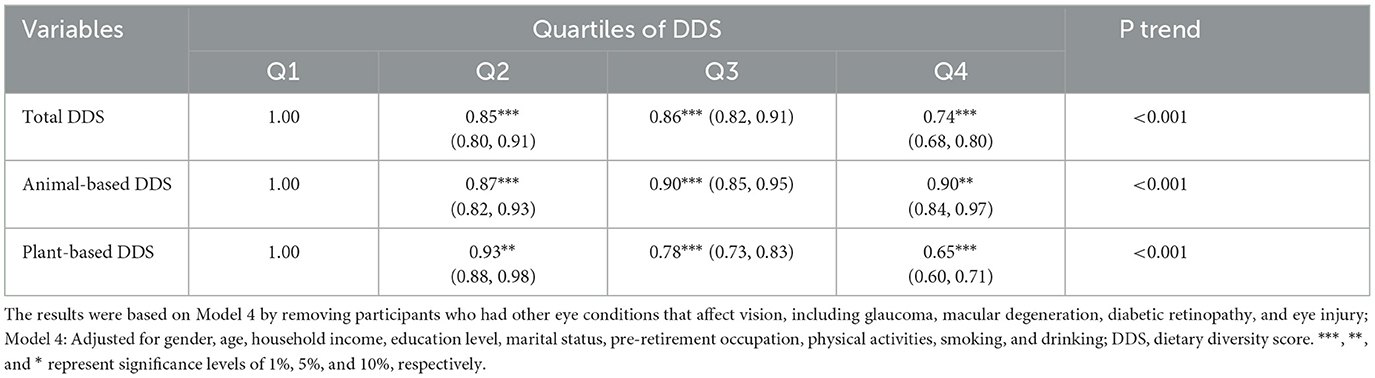
The odds of possible cataract were calculated using GEE models, considering both the overall DDS and the type of nutrition sources. The initial analysis showed that those in the highest quartile of DDS had a lower probability of having possible cataract compared to those in the lowest quartile. Specifically, total diet (OR = 0.59; 95% CI: 0.56–0.62), animal-based diet (OR = 0.80; 95% CI = 0.75–0.85), and plant-based diet (OR = 0.47; 95% CI = 0.44–0.51) showed significant associations with lower odds of potential cataract. In the subsequent model (Model 2 and Model 3), when we adjusted for more covariates including gender, age, household income, education level, marital status, and pre-retirement occupation, all associations and signs of coefficients remained unchanged. Final exhaustive adjustments for smoking, alcohol, and activity habits (Model 4) lead total diet (OR = 0.74; 95% CI: 0.69–0.79) and plant-based diet (OR = 0.65; 95% CI: 0.61–0.71) to retain their marked shielding effects. However, alarmingly, the animal-based diet relationship reversed to show heightened cataract odds (OR = 0.90; 95% CI: 0.84–0.96) in Table 2, marking a concerning departure from initial trends. Generally, ORs below 1 confirm animal-based diet associated with reduced cataract odds overall. But, from Q2 to Q4, animal-based diet ORs increased from 0.88 to 0.90, implying rising cataract risk as diversity grew. Moreover, animal-based diet ORs exceeded plant-based at all quartiles. For instance, Q4 animal-based diet showed higher cataract odds (OR = 0.90) than Q4 plant-based (OR = 0.65). These trends imply animal foods uniquely lack the pronounced cataract protection seen with plant-based diet. The results capture nuanced synergies, suggesting animal-based diets may increase cataract risk through inflammation or glycation despite general benefits of diet variety.
3.3 Subgroup analyses
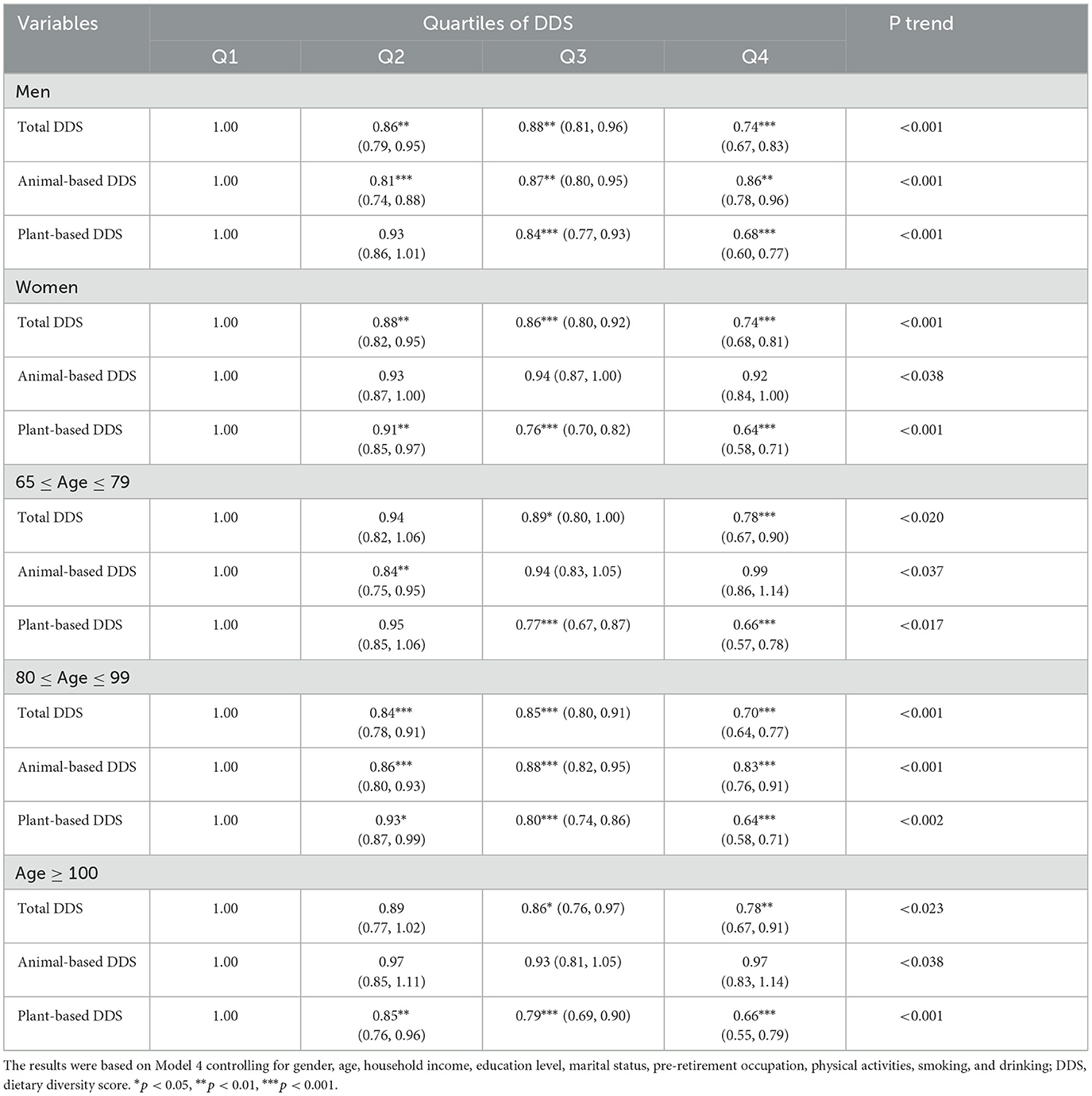
Delving into gender-stratified analyses through Model 4 in Table 3 reveals intriguing insights: total diet traced strong shielding effects both among men (OR = 0.74; 95% CI: 0.67–0.83) and women (OR = 0.74; 95% CI: 0.68–0.81) to the risk of getting cataracts. Plant-based diets followed suit, with matched robust benefits for men (OR = 0.68; 95% CI: 0.60–0.77) and women (OR = 0.64, 95% CI: 0.58–0.71). However, a concerning divergence emerged for animal-based diets. While women saw no significant links, men faced heightened cataract odds (OR = 0.86; 95% CI: 0.78–0.96), marking an alarming departure from initial trends. This gender-specific reversal introduces nuance, suggesting animal-based diets uniquely threaten men despite general protective effects across both genders for total and plant-based diets. Similar patterns of divergence between animal-based and plant-based diets were also evident in the results across various age groups.
3.4 Sensitivity analyses
As shown in Table 4, the same results were seen when Model 4 was used in a sensitivity analysis. After removing people with eye conditions that affect vision like glaucoma, macular degeneration, diabetic retinopathy, or eye injuries (n = 7,995), the associations between DDS (the total diet, the animal-based diet, and the plant-based diet) and possible cataracts were similar to the findings in Model 4 of Table 2.
4 Discussion
In this study, we investigated the association between dietary diversity and the prevalence of possible cataracts among the Chinese elderly population. We also explored the different patterns of dietary diversity in relation to possible cataracts. It was found that a higher DDS was associated with lower odds of possible cataract, but animal-based diet faced heightened cataract odds which marking an alarming departure from initial trends. Based on subgroup analysis, the animal-based diet showed a significant relationship between DDS and possible cataracts for men, while this association was not significant for women.
Our findings are in line with previous studies that have suggested a link between diet and cataract risk. Several epidemiological studies have reported protective effects of certain dietary components on cataract development (46, 47). However, single nutrients or foods may not reflect the overall quality and adequacy of a diet and may ignore the potential interactions and synergies among different dietary components (48, 49). We discovered associations between heightened dietary diversity and reduced cataract likelihood, even adjusting for confounders. This suggests that savoring an array of foods could be the recipe for robust lens protection. A potential explanation is that dietary diversity may increase antioxidants and nutrients that protect the lens from oxidative damage and inflammation. Oxidative stress has been proved that it can induce cataracts by warping lens protein architecture and function (50). Thus, antioxidants can scavenge free radicals and repair oxidative damage to the lens (17). In addition, inflammatory pathways and cytokines disrupting lens clarity can also catalyze cataracts (51). Therefore, anti-inflammatory nutrients may modulate inflammatory responses and attenuate lens injury (52). The impacts may stem from constituents like phytochemicals and antioxidants which mitigate oxidative stress and inflammation linked to cataract genesis. Abundant bioactive compounds in diverse diets can directly neutralize free radicals, boost endogenous antioxidant systems, and beneficially modulate signaling cascades driving ocular damage. Through attenuating these insults via numerous mechanisms, the synergistic actions of varied nutrition may preserve crystalline lenses. In summary, myriad constituents within diverse foods could synergistically attenuate molecular injuries underlying vision loss.
To capture nutrient interplays and synergies, we examined the different patterns of dietary diversity in relation to possible cataracts. DDS was classified into three categories: total diet, animal-based diet, plant-based diet. We found that a total diet and a plant-based diet can help reduce the risk of cataracts, but an animal-based diet increases the likelihood of cataracts. The development trend of DDS in animal-based diets is exactly opposite to the development trend of the two DDS mentioned above. This suggests that different types of dietary diversity may have different effects on the risk of cataracts. Previous studies have shown that an animal-based diet may increase the risk of cataracts by inducing inflammation or glycation (19, 28–31, 35). Animal-based foods are rich in saturated fat, cholesterol, heme iron, and advanced glycation end products (AGEs), which can increase oxidative stress or inflammation in the lens (17, 50). It is known that AGEs can accumulate in the lens and cause cross-linking or modification of lens proteins, leading to lens opacity (53, 54). Therefore, consuming a high amount of animal-based foods may not be beneficial for preventing cataract development. On the other hand, the plant-based diet may help reduce the risk of cataracts by providing antioxidants or anti-inflammatory nutrients (18, 19, 31). Their phytochemicals, vitamins C and E, carotenoids, flavonoids, polyphenols, and other plant nutrients can mitigate inflammatory responses and oxidative harm (48, 49). Additionally, plant fibers, omega-3s, and other nutrients modulate inflammation and attenuate lens injury (18, 27, 48). Thus, DDS analysis uncovers how food synergies and interactions influence cataract outcomes. Outcomes indicate dietary diversity helps safeguard aging vision, underscoring public health implications. Initiatives which increase affordable produce access may curb projected vision impairment among seniors. Animal foods distinctly lacked pronounced cataract protection seen with fruits/vegetables, although global meat intake rises. Thus diverse traditional cuisines centered on varied plants optimally support ocular health. Integrating these cultural patterns into lifestyle guidelines and interventions could promote elderly vision. In summary, promoting nutritional variety in aging populations carries significant implications for preventing vision loss via clinical recommendations and policy-level action.
A striking finding was the sex divergence in DDS-cataract links. Whereas higher animal-based DDS correlated with heightened cataract risks in men, no such significant association emerged for women (55). One study noted gender discrepancies in cataract prevalence, implicating hormones, genes, lifestyles, and, crucially, diets (17). Among these factors, diet may play an important role in modulating the gender differences in cataract risk. For example, males tend to consume more animal-based foods, while females tend to consume more plant-based foods (56). Males also tend to consume more alcohol and tobacco, while females tend to consume more tea and coffee (57). Such dietary variations could impact antioxidant and nutrient intake relevant to lens health. Moreover, dietary diversity may interact with gender-specific hormones, metabolism, and other physiological factors to influence cataract risk (58). Depending on the levels of oxidative stress markers and inflammatory cytokines involved in lens damage, males and females' oxidative stress and inflammation may be affected differently by dietary diversity (59).
According to the results of this study, it is important to promote a diversified diet to prevent the development of cataract in the Chinese elder group. The plant-based diet that provides antioxidants or anti-inflammatory nutrients is the key to reducing the odds of cataract. The results also point out the significant differences in the association of various diet patterns with possible cataract between men and women. While some studies claim that the animal-based diet causes lens damage, the effect of the animal-based diet on women remains questionable. Although the effect of the animal-based diet on possible cataract of women was not marked in this study, it is likely due to the relatively lower consumption of animal-based foods by women. The interaction between the animal-based diet and the odds of cataract in females requires further research.
The present study has some weaknesses. The cataracts may not match clinical diagnosis since they were identified by self-reported visual function assessment. Thus, we use “possible cataracts” to reflect the subjective nature of this screening approach. As our study lacks long-term, sustained follow-up surveys, we are unable to determine a causal relationship between nutritional diversity and cataract likelihood. Nonetheless, we argue that the discovered results demonstrate robustness, evidenced by multivariate analysis across various demographic groups. Additionally, the voluntary participation design carries risks of selection bias if systematic differences exist between surveyed groups and the broader elderly population that may impact nutrition and cataract development. However, our diverse, cross-regional sample mitigates potential sampling bias. While our study exclusively focuses on elderly Chinese, constraining generalization presently, we advocate for analogous multi-year studies in other ethnic groups to definitively assess nutritional impacts on vision-impairing lens changes. Furthermore, interpreting the divergence in cataract associations between animal and plant diversity requires prudent qualification, given inherent analytic challenges in nutrition epidemiology. Specifically, our food group-based diversity scores may fail to capture complexities within expansive food categories. We encourage future studies to pay closer attention to the intrinsic differences between diverse nutritional sources.
The present study also has multiple strengths. First, a substantial sample of elderly individuals from China was used, which was obtained through a nationwide survey with a commendable response rate. This enhances the generalizability and representativeness of our findings. Second, we used a straightforward and unbiased measure of dietary diversity by assessing the frequency of consumption of ten food groups that match the dietary standards established for Chinese populations. This approach helps us reduce the potential inaccuracies in measurements and the influence of recall bias commonly observed in self-reported dietary consumption data. Third, we adjusted for several potential confounding variables that could affect the relationship between DDS and the risk of developing cataracts. However, this study cannot conclude causality due to the cross-sectional study design and other residual and unmeasured confounding.
Further robust trials are required to confirm the suggested causal links, including cluster studies assigning seniors to interventions targeting total, animal, or vegetable dietary diversity, evaluating resultant ophthalmic changes. Additionally, quantitative intake diaries and repeated clinical eye checks in long-running elderly cohorts could correlate nutrition with lens function over time. Utilizing these prospective methodologies is vital for validating the diet-cataract connections identified here. In summary, randomized controlled trials and prospective cohorts tracking nutritional diversity and quantified vision changes longitudinally are essential next phases.
5 Conclusion
In summary, higher DDS was associated with reduced risk of cataract among elderly people in China. This implies that promoting a diversified diet may provide an economical and effective intervention for preventing cataract in the elder group. However, the causal relationship between dietary diversity and cataract risk requires further research.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: publicly available datasets were analyzed in this study. This data can be found here: https://opendata.pku.edu.cn/dataverse/CHADS.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University, Peking University, Beijing, China (IRB00001052-13074). The patients/participants provided their written informed consent to participate in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HZ: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – review & editing. JZha: Formal analysis, Visualization, Writing – original draft, Validation. JZho: Formal analysis, Visualization, Writing – original draft, Validation. YM: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We received funding from the Jiangsu Provincial Department of Education General Project of Philosophy and Social Sciences in Colleges and Universities (1042922307).
Acknowledgments
The authors are grateful to the CLHLS research team, the field team, and every respondent for the time and efforts that they have devoted to the CLHLS project, which provided the data for this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gupta VB, Rajagopala M, Ravishankar B. Etiopathogenesis of cataract: an appraisal. Indian J Ophthalmol. (2014) 62:103. doi: 10.4103/0301-4738.121141
2. Cataract - Middle East/North Africa,. American Academy of Ophthalmology. (2013). Available online at: https://www.aao.org/education/topic-detail/cataract–middle-eastnorth-africa (accessed October 12, 2023).
3. Hashemi H, Pakzad R, Yekta A, Aghamirsalim M, Pakbin M, Ramin S, et al. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye. (2020) 34:1357–70. doi: 10.1038/s41433-020-0806-3
4. You C, Wu X, Zhang Y, Dai Y, Huang Y, Xie L. Visual impairment and delay in presentation for surgery in Chinese pediatric patients with cataract. Ophthalmology. (2011) 118:17–23. doi: 10.1016/j.ophtha.2010.04.014
5. Murphy G, Owasil R, Kanavati S, Ashena Z, Nanavaty MA. Preoperative fundoscopy versus optical coherence tomography to detect occult maculopathy during cataract surgery preassessment. Eye. (2023) 37:665–9. doi: 10.1038/s41433-022-02027-0
6. Robman L, Taylor H. External factors in the development of cataract. Eye. (2005) 19:1074–82. doi: 10.1038/sj.eye.6701964
7. Abraham AG, Condon NG, West Gower E. The new epidemiology of cataract. Ophthalmol Clin North Am. (2006) 19:415–25. doi: 10.1016/j.ohc.2006.07.008
8. Harding JJ. Recent studies of risk factors and protective factors for cataract. Curr Opin Ophthalmol. (1997) 8:46–9. doi: 10.1097/00055735-199702000-00010
9. Harding JJ, Van Heyningen R. Drugs, including alcohol, that act as risk factors for cataract, and possible protection against cataract by aspirin-like analgesics and cyclopenthiazide. Br J Ophthalmol. (1988) 72:809–14. doi: 10.1136/bjo.72.11.809
10. Nuthethi R. Structural equation modelling and its application to quality of life of visually impaired people. [Thesis] UNSW Sydney. (2008).
12. Foster RE, Lowder CY, Meisler DM, Zakov ZN. Extracapsular cataract extraction and posterior chamber intraocular lens implantation in uveitis patients. Ophthalmology. (1992) 99:1234–41. doi: 10.1016/S0161-6420(92)31818-4
13. Jindra LF, Zemon V. Contrast sensitivity testing: A more complete assessment of vision. J Cataract Refract Surg. (1989) 15:141–8. doi: 10.1016/S0886-3350(89)80002-1
14. Valderas JM, Rue M, Guyatt G, Alonso J. Systematic Use of Quality of Life Measures in the Clinical Practice Working Group. The impact of the VF-14 index, a perceived visual function measure, in the routine management of cataract patients. Qual Life Res. (2005) 14:1743–53. doi: 10.1007/s11136-005-1745-y
15. Alonso J, Espallargues M, Andersen TF, Cassard SD, Dunn E, Bernth-Petersen P, et al. International applicability of the VF-14: an index of visual function in patients with cataracts. Ophthalmology. (1997) 104:799–807. doi: 10.1016/S0161-6420(97)30230-9
16. Sheeladevi S, Lawrenson JG, Fielder AR, Suttle CM. Global prevalence of childhood cataract: a systematic review. Eye. (2016) 30:1160–9. doi: 10.1038/eye.2016.156
17. Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. (2020) 11:694. doi: 10.3389/fphys.2020.00694
18. Lee S, Lee S, Jeong M, Jung S, Lee M, Yoo S. The relationship between nutrient intake and cataracts in the older adult population of Korea. Nutrients. (2022) 14:4962. doi: 10.3390/nu14234962
19. Barker FM. Dietary supplementation: effects on visual performance and occurrence of AMD and cataracts. Curr Med Res Opin. (2010) 26:2011–23. doi: 10.1185/03007995.2010.494549
20. Gammone MA, Riccioni G, D'Orazio N. Marine carotenoids against oxidative stress: effects on human health. Mar Drugs. (2015) 13:6226–46. doi: 10.3390/md13106226
21. Morris D, Fraser SG, Gray C. Cataract surgery and quality of life implications. Clin Interv Aging. (2007) 2:105–8. doi: 10.2147/ciia.2007.2.1.105
22. Kaur J, Kukreja S, Kaur A, Malhotra N, Kaur R. The oxidative stress in cataract patients. J Clin Diagn Res. (2012) 6:1629–32. doi: 10.7860/JCDR/2012/4856.2626
23. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. (2021) 20:689–709. doi: 10.1038/s41573-021-00233-1
24. Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. (2016) 2016:5276130. doi: 10.1155/2016/5276130
25. Liu D, Zhang X-R, Li Z-H, Zhang Y-J, Lv Y-B, Wang Z-H, et al. Association of dietary diversity changes and mortality among older people: a prospective cohort study. Clin Nutr. (2021) 40:2620–9. doi: 10.1016/j.clnu.2021.04.012
26. Zhang Y, Chen H, Carrillo-Larco RM, Lim CCW, Mishra SR, Yuan C, et al. Association of dietary patterns and food groups intake with multimorbidity: a prospective cohort study. Clin Nutr ESPEN. (2022) 51:359–66. doi: 10.1016/j.clnesp.2022.07.019
27. Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. (2021) 143:1–9. doi: 10.1016/j.maturitas.2020.08.003
28. Nachvak SM, Abdollahzad H, Mostafai R, Moradi S, Pasdar Y, Rezaei M, et al. Dietary diversity score and its related factors among employees of Kermanshah University of medical sciences. Clin Nutr Res. (2017) 6:247–55. doi: 10.7762/cnr.2017.6.4.247
29. Zhang J, Zhao A. Dietary diversity and healthy aging: a prospective study. Nutrients. (2021) 13:1787. doi: 10.3390/nu13061787
30. Lachat C, Raneri JE, Smith KW, Kolsteren P, Van Damme P, Verzelen K, et al. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc Nat Acad Sci. (2018) 115:127–32. doi: 10.1073/pnas.1709194115
31. Vadiveloo M, Dixon LB, Mijanovich T, Elbel B, Parekh N. Dietary variety is inversely associated with body adiposity among US adults using a novel food diversity index. J Nutr. (2015) 145:555–63. doi: 10.3945/jn.114.199067
32. Molani-Gol R, Kheirouri S, Alizadeh M. Does the high dietary diversity score predict dietary micronutrients adequacy in children under 5 years old? A systematic review. J Health, Popul Nutr. (2023) 42:2. doi: 10.1186/s41043-022-00337-3
33. Kruger R, Hepburn AJ, Beck KL, McNaughton S, Stonehouse W. Evaluating a novel dietary diversity questionnaire to assess dietary diversity and adequacy of New Zealand women. Nutrition. (2021) 91:111468. doi: 10.1016/j.nut.2021.111468
34. Nie J, Deng M-G, Wang K, Liu F, Xu H, Feng Q, et al. Higher HEI-2015 scores are associated with lower risk of gout and hyperuricemia: Results from the national health and nutrition examination survey 2007–2016. Front Nutr. (2022) 9:921550. doi: 10.3389/fnut.2022.921550
35. Zhang J, Zhao A, Wu W, Yang C, Ren Z, Wang M, et al. Dietary diversity is associated with memory status in chinese adults: a prospective study. Front Aging Neurosci. (2020) 12:580760. doi: 10.3389/fnagi.2020.580760
36. Yokoyama Y, Nishi M, Murayama H, Amano H, Taniguchi Y, Nofuji Y, et al. Association of dietary variety with body composition and physical function in community-dwelling elderly Japanese. J Nutr Health Aging. (2016) 20:691–6. doi: 10.1007/s12603-015-0632-7
37. McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. (2000) 11:3. doi: 10.1097/00055735-200002000-00002
38. Chang DF, Garcia IH, Hunkeler JD, Minas T. Phase II results of an intraocular steroid delivery system for cataract surgery. Ophthalmology. (1999) 106:1172–7. doi: 10.1016/S0161-6420(99)90262-2
39. Eperon S, Rodriguez-Aller M, Balaskas K, Gurny R, Guex-Crosier Y. A new drug delivery system inhibits uveitis in an animal model after cataract surgery. Int J Pharm. (2013) 443:254–61. doi: 10.1016/j.ijpharm.2012.12.033
40. Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or irvine-gass syndrome. Am J Ophthalmol. (2009) 147:1048–1054.e2. doi: 10.1016/j.ajo.2008.12.033
41. Meng L, Wang Y, Li T, Loo-Bouwman CA, van Zhang Y, Man-Yau Szeto I. Dietary diversity and food variety in chinese children aged 3–17 years: are they negatively associated with dietary micronutrient inadequacy? Nutrients. (2018) 10:1674. doi: 10.3390/nu10111674
42. Bahrami A, Shirani P, Sohouli M, Nasab SJ, Rafiee P, Naja F, et al. Dietary diversity score (DDS) and odds of colorectal cancer and adenoma: a case-control study. J Nutr Sci. (2022) 11:e34. doi: 10.1017/jns.2022.30
43. Doustmohammadian A, Amirkalali B, Gholizadeh E, Khoonsari M, Faraji AH, Nikkhah M, et al. Mediators of dietary diversity score (DDS) on NAFLD in Iranian adults: a structural equation modeling study. Eur J Clin Nutr. (2023) 77:370–9. doi: 10.1038/s41430-022-01240-0
44. Deng M-G, Nie J-Q, Li Y-Y, Yu X, Zhang Z-J. Higher HEI-2015 scores are associated with lower risk of sleep disorder: results from a nationally representative survey of United States adults. Nutrients. (2022) 14:873. doi: 10.3390/nu14040873
45. Rongrong G, Yan G, Haisi C, Sifang Z, Qinmei W, Ayong Y. Revision and application of Chinese version of visual function index-14 in the evaluation of life quality in cataract patients. Chin J Exper Ophthalmol. (2016) 34:823–8. doi: 10.3760/cma.j.issn.2095-0160.2016.09.011
46. Ma L, Dou H-L, Wu Y-Q, Huang Y-M, Huang Y-B, Xu X-R, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. (2012) 107:350–9. doi: 10.1017/S0007114511004260
47. Sedaghat F, Ghanavati M, Nezhad Hajian P, Hajishirazi S, Ehteshami M, Rashidkhani B. Nutrient patterns and risk of cataract: a case-control study. Int J Ophthalmol. (2017) 10:586–92. doi: 10.18240/ijo.2017.04.14
48. Wei L, Liang G, Cai C, Lv J. Association of vitamin C with the risk of age-related cataract: a meta-analysis. Acta Ophthalmol. (2016) 94:e170–176. doi: 10.1111/aos.12688
49. Zhang Y, Jiang W, Xie Z, Wu W, Zhang D. Vitamin E and risk of age-related cataract: a meta-analysis. Public Health Nutr. (2015) 18:2804–14. doi: 10.1017/S1368980014003115
50. Vinson JA. Oxidative stress in cataracts. Pathophysiology. (2006) 13:151–62. doi: 10.1016/j.pathophys.2006.05.006
51. Shaw E, Patel BC. “Complicated Cataract,” StatPearls. Treasure Island (FL): StatPearls Publishing (2023). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK572139/ (accessed October 22, 2023).
52. Grimble RF. Nutritional antioxidants and the modulation of inflammation: theory and practice. New Horiz. (1994) 2:175–85.
53. Nandi SK, Nahomi RB, Rankenberg J, Glomb MA, Nagaraj RH. Glycation-mediated inter-protein cross-linking is promoted by chaperone–client complexes of α-crystallin: implications for lens aging and presbyopia. J Biol Chem. (2020) 295:5701–16. doi: 10.1074/jbc.RA120.012604
54. Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced glycation end-products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells. (2022) 11:1312. doi: 10.3390/cells11081312
55. Chua J, Lim B, Fenwick EK, Gan ATL, Tan AG, Lamoureux E, et al. Prevalence, risk factors, and impact of undiagnosed visually significant cataract: the singapore epidemiology of eye diseases study. PLoS ONE. (2017) 12:e0170804. doi: 10.1371/journal.pone.0170804
56. Zhao J, Sun J, Su C. Gender differences in the relationship between dietary energy and macronutrients intake and body weight outcomes in Chinese adults. Nutr J. (2020) 19:45. doi: 10.1186/s12937-020-00564-6
57. White AM. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol Res. (2020) 40:01. doi: 10.35946/arcr.v40.2.01
58. Domínguez-López I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: a review. Nutrients. (2020) 12:2456. doi: 10.3390/nu12082456
Keywords: dietary diversity, cataract, plant-based diet, older adults, China
Citation: Zhao H, Zhang J, Zhou J and Ma Y (2024) Dietary diversity and possible cataract among Chinese elderly population. Front. Nutr. 11:1342190. doi: 10.3389/fnut.2024.1342190
Received: 21 November 2023; Accepted: 15 January 2024;
Published: 26 January 2024.
Edited by:
Jin Yang, Fudan University, ChinaReviewed by:
Shi Song Rong, Massachusetts Eye & Ear Infirmary and Harvard Medical School, United StatesMing-Gang Deng, Wuhan Mental Health Center, China
Copyright © 2024 Zhao, Zhang, Zhou and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghui Ma, bXloQGp1c3QuZWR1LmNu
 HaiYue Zhao
HaiYue Zhao Junyang Zhang
Junyang Zhang Yinghui Ma
Yinghui Ma