
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 07 May 2024
Sec. Nutritional Epidemiology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1341625
This article is part of the Research Topic Dietary Diversity Indicators: Cultural Preferences and Health Outcomes View all 5 articles
 Vanessa Tita Jugha1*
Vanessa Tita Jugha1* Juliana Adjem Anchang2
Juliana Adjem Anchang2 David Denis Sofeu-Feugaing3
David Denis Sofeu-Feugaing3 Germain Sotoing Taiwe1
Germain Sotoing Taiwe1 Helen Kuokuo Kimbi1,4,5
Helen Kuokuo Kimbi1,4,5 Judith Kuoh Anchang-Kimbi1
Judith Kuoh Anchang-Kimbi1Background: Nutritional deficiencies and its consequences such as anaemia are frequent among pregnant women residing in under resource settings. Hence, this study sought to investigate specific dietary micronutrient inadequacy and its effect on maternal haemoglobin levels.
Methods: This institution based cross-sectional survey enrolled 1,014 consenting pregnant women consecutively. Data on socio-demographic, economic and antenatal characteristics were recorded using a structured questionnaire. Minimum dietary diversity for women (MDD-W) was assessed using the 24-h recall method and haemoglobin (Hb) concentration (g/dL) determined using a portable Hb metre. Significant levels between associations was set at p < 0.05.
Results: Among those enrolled, 40.9% were anaemic while 89.6% had inadequate dietary nutrient intake. In addition, uptake of blood supplements, haem iron, plant and animal-based foods rich in vitamin A were 71.5, 86.2, 35.5 and 12.6%, respectively. Moreover, anaemia prevalence was significantly (p < 0.05) lower in women who took iron-folic acid along with food groups rich in haem iron (38.5%) or both plant and animal vitamin A (29.0%). Besides, mean maternal Hb levels was significantly (p < 0.001) higher in women who consumed haem iron (11.08 ± 1.35) and vitamin A food groups (11.34 ± 1.30) when compared with their counterparts who did not consume haem iron (10.54 ± 1.19) and vitamin A food groups (10.74 ± 1.31).
Conclusion: Dietary uptake of foods rich in haem-iron and vitamin A significantly improves Hb levels in Cameroonian pregnant women. Our findings underscore the importance of improving maternal nutritional awareness and counselling during antenatal period to reduce the anaemia burden.
Micronutrients are vital to health as they ensure normal growth, metabolism and physical wellbeing (1, 2). Although required in small amounts, the impact of their deficiency is severe (3). Globally, more than 2 billion people suffer from micronutrient deficiencies, with the main being iron, zinc, iodine, vitamins A and B (4, 5). During pregnancy, these deficiencies which results from; lack of consumption of nutrient-dense food groups, poor understanding of the importance of a diverse diet and inefficient utilisation of available micronutrients (6, 7) can lead to a myriad of adverse maternal and perinatal outcomes including; anaemia, increased susceptibility to infectious diseases, low birth weight, preterm birth, increased risk of maternal and neonatal mortality as well as cognitive deficit in the baby later in life (2, 8).
Anaemia is a widespread public health problem that has significant consequence for human health, social development, and economic growth (9–11). According to the World Health Organization (WHO), anaemia is a condition in which the haemoglobin concentration within the red blood cells are lower than normal and consequently their oxygen carrying capacity is insufficient to meet the physiological demands of the body (12, 13). This results in symptoms such as; body weakness, fatigue, dizziness, palpitations and shortness of breath (13, 14). In 2019, the prevalence rates of anaemia was estimated at 29.9% among women of reproductive ages (WRA) and 36.5% in pregnant women (15). Though preventable, in pregnancy it is still one of the leading causes of maternal and neonatal morbidity and mortality (16, 17). Apart from nutritional deficiencies of which iron deficiency is the most prevalent cause of anaemia, other conditions such as folate, zinc, vitamin A and B deficiencies, chronic inflammation, infectious diseases and inherited haemoglobin disorders can as well lead to anaemia (12, 18, 19).
Over the past decade, awareness for anaemia and its consequences for maternal and infant health has increased. For instance, in 2012, the 65th World Health Assembly (WHA) approved global targets for maternal, infant and young child nutrition with a commitment to reduce to half the prevalence of anaemia among WRA (15–49 years) by 2025 (20, 21). Ensuing this, the WHO and United Nations Children’s Fund (UNICEF) proposed extending this target to 2030 to align with the United Nations (UN) Sustainable Development Goals (21, 22). With this in mind, Cameroon has been committed to curb the burden of maternal anaemia through malaria prophylaxis and haematinic supplementation (16). Despite efforts, anaemia prevalence rates have not changed over the years as it is still a severe (≥ 40%) health problem in WRA (23, 24). An explanation to this high prevalence rates could be an underestimation of the role of dietary micronutrient inadequacy on anaemia. Besides, data on micronutrients are limited in the study area and are thus needed, to design and implement public health programmes targeted at reducing anaemia. Hence, this study aimed to investigate intake of dietary nutrients and its effect on maternal haemoglobin levels in the Mount Cameroon health area.
This study was conducted at the antenatal care units of various health facilities located in the Buea and Tiko Health Districts of the Mount Cameroon area. The characteristic of the study settings has been described in detail by Jugha et al. (25). More so, the different health facilities in these health districts were chosen based on their accessibility as well as the localities they serve (25, 26).
The tropical equatorial climate of the Mount Cameroon region is made up of a long rainy season accompanied by high rainfall (2,000–10,000 mm) and average temperatures conducive for agriculture, the principal economic activity in the region (27, 28). Irrespective of the agricultural biodiversity, starchy staple is the most commonly consumed food group (25). In addition, malaria is endemic in the area and transmission is perennial (29) with Plasmodium falciparum accounting for over 90% of malaria parasite infection (30). Also, anaemia prevalence among pregnant women (≥ 40%) over the years in the area has not changed (16, 25, 31).
This cross-sectional survey enrolled consenting pregnant women in any trimester of gestation consecutively. Study sample size was estimated using the Cochrane formulae for cross-sectional studies based on the prevalence of anaemia (40%) in the study area (25, 32). After adding for a 10% non-response rate (NRR) the overall number of women to be enrolled from both health district was 1,014.
A structured questionnaire (pre-tested) through a face-to-face interview was used to obtain maternal socio-demographic data (setting, age, marital status), educational level, household number, and antenatal clinic data (number of antenatal care visits, gestational age, parity, IPTp-SP and iron-folic acid uptake). Information relating to household wealth that is; housing type, house ownership, toilet type, possession of basic amenities (radio, car, bicycle, television, motorcycle and mobile phone) and source of drinking water were also documented. These indicators of household wealth were subjected to principal component analysis (PCA) in order to determine maternal wealth status (33).
The minimum dietary diversity for women (MDD-W) questionnaire, a proxy indicator of micronutrient adequacy was used to determine maternal dietary nutrient intake (34, 35). During questionnaire survey, each study respondent was requested to describe all food groups and drinks consumed day and/or night 24-h before the survey. These food groups (FGs) included: starchy staples; pulses; nuts and seeds; dairy; meat, poultry and fish; eggs; dark green leafy vegetables; vitamin A-rich fruits and vegetables; other vegetables and other fruits (25, 34). A score of 1 was attributed to the consumption of any food item within any food group as per the FAO guidelines (34). Dietary diversity score was obtained by summing up the FGs consumed among the 10 required FGs (34). Participants were then categorised as having adequate dietary nutrient intake if they consumed at least 5 of more food groups a day prior to the study (25, 34).
Moreover, the FGs; dark green leafy vegetables, vitamin A-rich fruits and vegetables, Meat (including organ meat), poultry, fish, eggs and milk products were further reclassified as vitamin A-rich plant foods (dark green leafy vegetables, vitamin A-rich fruits and vegetables), vitamin A-rich animal foods (organ meat, eggs and milk products) and foods rich in haem iron (meat, poultry and fish) as per the FAO guidelines (36).
Venous blood (2 mL) was collected from each pregnant woman using sterile techniques. Maternal Hb concentration (g/dL) was determined in the field using a portable URIT-12 Hb metre (URIT Medical Electronics Co., Ltd. Guangxi, China). In this study, anaemia status was defined as Hb < 11 g/dL for gravid women in the first and third trimester and Hb < 10.5 g/dL for those in the second trimester of gestation (25, 37).
Ethical clearance (Ref No: 2019/967-05/UB/SG/IRB/FHS) was obtained from the Faculty of Health Science Institutional Review Board (IRB), University of Buea whereas administrative authorization was gotten from the South West Regional Delegation of Public Health, District Medical and Chief Medical Officers in charge of the health districts and medical facilities, respectively. After sensitising the women on the study objectives, potential risks and benefits, those who gave their consent signed a written informed consent form and were thus included into the study whereas, those presenting with complicated pregnancy or a history of diabetes, hypertensive disorders or pre-eclampsia were not eligible to partake in the study and were therefore, excluded. In addition, participation in the study was voluntary.
Data was analysed using the IBM-Statistical Package for Social Sciences (SPSS) version 23. Continuous data were checked for normality and expressed as means and standard deviation (SD). Descriptive statistics such as mean, SD, frequency and percentages were used to describe data. Furthermore, the Pearson Chi-square test (χ2) was used to evaluate the differences in proportions between uptake of iron-folic acid (IFA), haem iron, vitamin-A food groups and maternal anaemia status. In addition, comparison between the continuous variable (Hb levels) and group parameters (intake of haem iron and vitamin A food groups) was done using the student’s paired t-test. Statistical test was two-tailed and the level of significance set at p < 0.05.
As shown in Table 1, mean maternal age (± SD) and household size (± SD) of those enrolled was 26.72 (± 5.48) years and 4.44 (± 2.20) persons. Besides, over 50% of the women were married and had a household size of at least four and more members. Furthermore, most (33.9%) of the study participants were within the age group 25–29 years followed by those aged 19–24 years (30.4%; Table 1).
Of those enrolled, mean gestational age (± SD) was 27.60 (± 7.61) weeks. In addition, gravid women with parity 1–2 constituted 43.3% of the study population. Besides, over 70% of the women had received blood supplements in the form of iron-folic acid. Moreover, 35.5, 12.6 and 86.2% of the women had consumed plant foods rich in vitamin A, animal foods rich in vitamin A and haem iron, respectively (Table 2).
As shown in Table 3, anaemia prevalence rates were lowest in women who took blood supplements (iron-folic acid) alongside food groups rich in haem iron (38.5%, p = 0.031) as well as both plant and animal vitamin A (29.0%, p < 0.001) when compared with their respective contemporaries who relied on IFA only (Table 3).
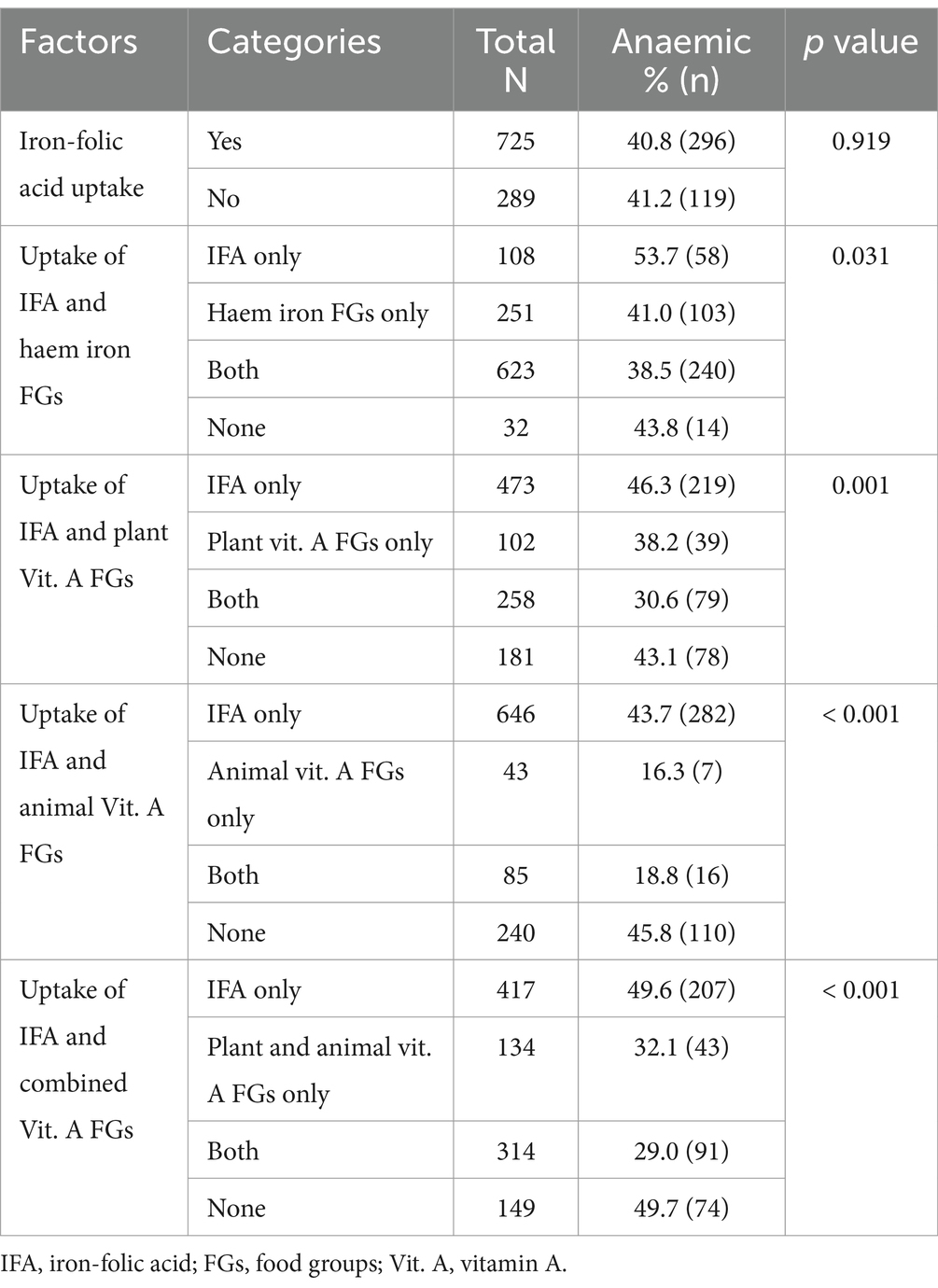
Table 3. Association between uptake of iron-folic acid, haem iron, vitamin A foods and maternal anaemia.
As illustrated on Figure 1, mean maternal haemoglobin (Hb) levels was significantly (p < 0.001) high in women who consumed haem iron (11.08 ± 1.35), plant (11.25 ± 1.29) and animal foods rich in vitamin A (11.82 ± 1.30) when compared with their counterparts who did not consume haem iron (10.54 ± 1.19), plant (10.87 ± 1.34) and animal foods rich in vitamin A (10.88 ± 1.30; Figure 1).
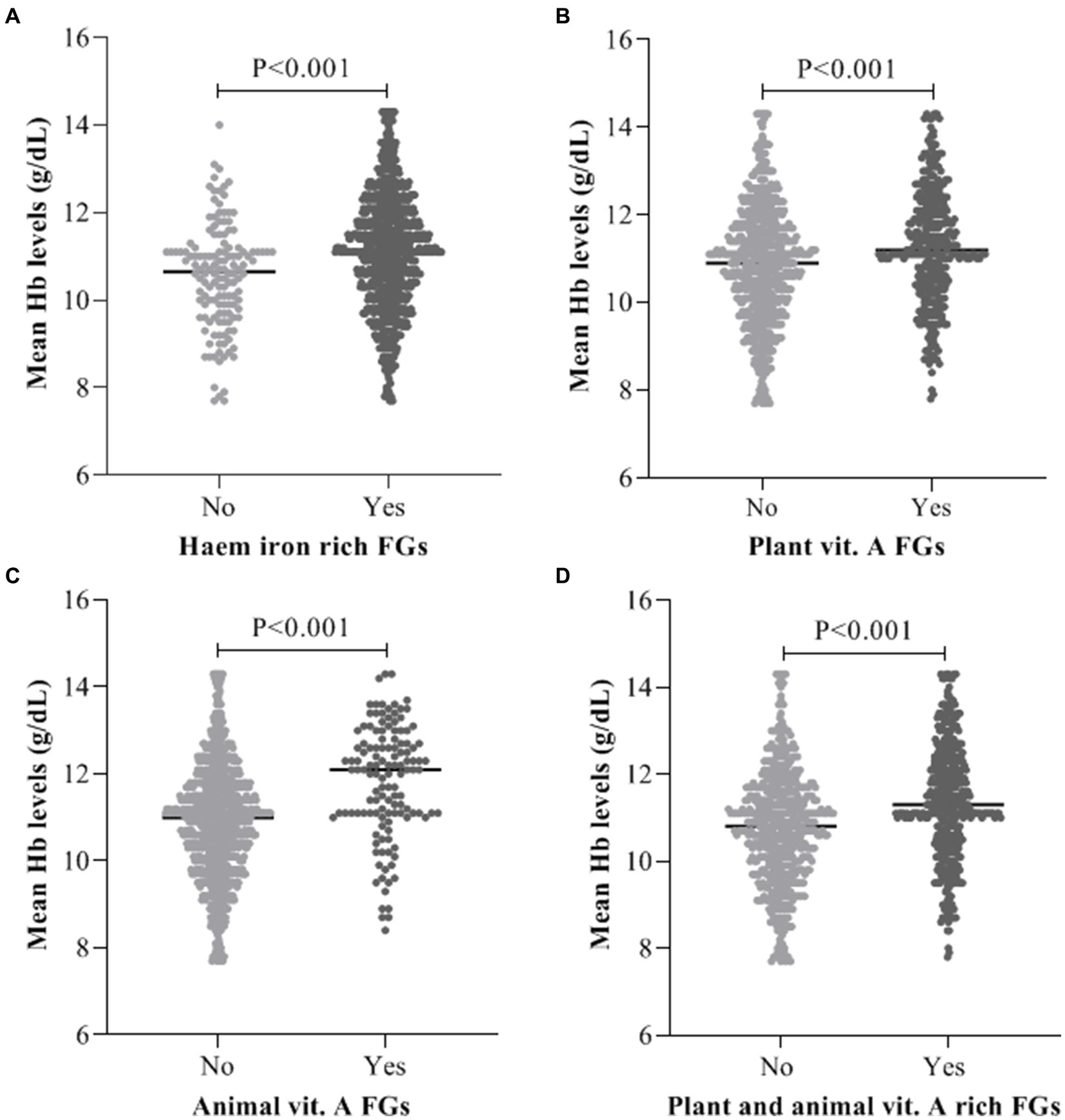
Figure 1. Average maternal Hb levels Vs intake of (A) Haem iron food groups, (B) Plant vitamin A food groups, (C) Animal vitamin A food groups, (D) Combine plant and animal vitamin A food groups.
In Cameroon, anaemia prevalence among women is still severe (≥ 40%) (16, 24, 25). This high prevalence rate may represent significant constraint for achieving the Global Nutrition Target endorsed by the World Health Assembly of halving anaemia prevalence among WRA by 2025 (20). This study therefore aimed to evaluate dietary micronutrient intake and their effect on haemoglobin levels of pregnant Cameroonian women.
In order to reduce the risk of anaemia during pregnancy, the WHO recommends a daily oral dose of 60 mg of iron along with 400 μg of folic acid throughout pregnancy and as part of the routine antenatal care services (37). In Cameroon, iron supplementation is the main strategy for anaemia control and prevention (16, 38). In addition, several studies have shown that iron-folic acid uptake during this critical period prevents maternal anaemia while reducing the risk of preterm labour, low birthweight, premature delivery, postpartum haemorrhage (39–41). The observed anaemia prevalence rate (40.9%) among study respondents in the study area despite uptake of iron-folic acid (71.5%) might be due to poor adherence, an aspect this study did not assess. Poor adherence to iron supplements may be as a result of inadequate supply of iron tablets, poor utilisation of prenatal health-care services, gastrointestinal discomfort accompanied with the drug, inability to purchase the tablet, forgetfulness, poor counselling by health care providers regarding the usefulness of the tablet as well as maternal knowledge and beliefs surrounding the tablet (42–44). Besides, this study further showed that combine uptake of iron-folic acid with a diet rich in haem iron or vitamin A food groups is more efficient in reducing the burden of anaemia than iron-folic acid taken alone.
Although diet holds great importance for maternal and neonatal health, inadequate proportions are often consumed most especially by women residing in low-and-middle income countries and study participants in the Mount Cameroon area were no exception (89.6%) (25, 45, 46). According to the WHO, the most common micronutrient deficiencies are; iron, vitamin A and iodine deficiencies (2, 47). In this study, 86.2% of the women consumed foods rich in iron specifically haem iron a day before the survey. Dietary iron is present in two forms that is haem iron, which is obtained from animal products such as meat, fish and poultry whereas non-haem iron is obtained from cereals, fruits and vegetables (36, 48, 49). Furthermore, it was observed in this study that consumption of haem iron was associated with increased haemoglobin levels of pregnant women. This finding is in line with observations from Jakarta (50), Ethiopia (51) and Pakistan (52). The increased haemoglobin levels among women who consumed meat, fish and poultry might be due to the fact that, foods rich in haem iron are absorbed from the gut with greater efficiency thus, making their iron content (the main component of haemoglobin) readily available for red blood cell production (51, 53).
Adequate vitamin A during pregnancy is essential for maternal and infant health (54, 55). Dietary vitamin A is available from two main sources that is, plants (provitamin A) and animals (preformed vitamin A) (55). Animal foods rich in vitamin A include; eggs, organ meat and dairy products while dark green leafy vegetables, vitamin A rich fruits and vegetables are plant foods rich in vitamin A (36, 56, 57). In this survey, 35.5 and 12.6% of the respondents enrolled consumed plant and animal food groups rich in vitamin A, respectively. The observed low intake of vitamin A animal food groups among study respondents might be due to the inability of the women to purchase eggs, organ meat and milk products. Furthermore, intake of foods rich in vitamin A was associated with maternal haemoglobin levels. Similar correlations have been described elsewhere (18, 58–60). Inadequate vitamin A intake is thought to cause anaemia through; reduction of the body’s immune response to infectious diseases which in turn leads to anaemia of infection, modulation of erythropoiesis and iron metabolism (6, 58, 61). Besides, vitamin A deficiency is known to increase the risk of iron deficient erythropoiesis and subsequently anaemia by altering absorption, storage, release and transport of iron to the bone marrow (62). This phenomenon might explain the low Hb levels observed among those who did not consume foods rich in vitamin A.
The current study had some limitations. Firstly, its cross-sectional nature could not establish the cause-and-effect relationship between dietary components and anaemia. In addition, this study did not measure biomarkers of micronutrient deficiency and other indicators of anaemia such as; mean corpuscular haemoglobin concentration (MCHC), mean corpuscular volume (MCV), reticulocyte count. In contrast, this study has as strength in its sample size as well as minimised recall bias by employing the use of the 24-h recall method to assess dietary diversity. Moreover, this study further demonstrates the effect haem iron and vitamin A rich food groups has on haemoglobin levels. Besides, this study sets the basis for future works determining the association and comparative influence of iron and vitamin A on Hb levels.
Overall, the prevalence of anaemia (40.9%) was high despite adequate uptake of iron supplement (71.5%). Moreover, dietary diversity was inadequate (89.6%). In addition, anaemia prevalence rate was significantly (p < 0.05) lower in women who took IFA coupled with a diet rich in haem iron (38.5%) and vitamin A (29.0%). Furthermore, mean haemoglobin levels were significantly (< 0.001) higher in women who consumed haem iron (11.08 ± 1.35) and vitamin-A (11.34 ± 1.30) rich foods a day before the survey when compared with their respective contemporaries who did not. Thus, apart from focusing on iron-folic acid supplementation alone to curb the burden of maternal anaemia, public health authorities and health care givers should improve maternal nutritional awareness on the importance of a diversified diet as this would in turn enhance uptake of foods rich in haematopoietic nutrients thereby reducing anaemia prevalence rate.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Institutional Review Board (IRB), Faculty of Health Science, University of Buea, Cameroon. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
VJ: Formal analysis, Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. JA: Formal analysis, Validation, Writing – review & editing. DS-F: Formal analysis, Validation, Writing – review & editing. GT: Formal analysis, Validation, Writing – review & editing. HK: Conceptualization, Supervision, Validation, Writing – review & editing. JA-K: Conceptualization, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors are grateful to all the pregnant women who gave their consent to partake in the study. We are equally thankful to the administrative staffs, midwives, nurses and laboratory technicians of the different health facilities where this study was conducted for their collaboration and assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Baker, BC, Hayes, DJ, and Jones, RL. Effects of micronutrients on placental function: evidence from clinical studies to animal models. Reproduction. (2018) 156:R69–82. doi: 10.1530/REP-18-0130
2. Fite, MB, Tura, AK, Yadeta, TA, Oljira, L, Wilfong, T, Mamme, NY, et al. Co-occurrence of iron, folate, and vitamin a deficiency among pregnant women in eastern Ethiopia: a community-based study. BMC Nutrition. (2023) 9:1–8. doi: 10.1186/s40795-023-00724-x
3. Organization WH. WHO antenatal care recommendations for a positive pregnancy experience: Nutritional interventions update: Multiple micronutrient supplements during pregnancy. Geneva, Switzerland. (2020).
4. Bailey, RL, West, KP Jr, and Black, RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. (2015) 66:22–33. doi: 10.1159/000371618
5. Littlejohn, PT, Bar-Yoseph, H, Edwards, K, Li, H, Ramirez-Contreras, CY, Holani, R, et al. Multiple micronutrient deficiencies alter energy metabolism in host and gut microbiome in an early-life murine model. Front Nutr. (2023) 10:670. doi: 10.3389/fnut.2023.1151670
6. Abizari, A-R, Azupogo, F, and Brouwer, ID. Subclinical inflammation influences the association between vitamin A-and iron status among schoolchildren in Ghana. PloS one. (2017) 12:e0170747. doi: 10.1371/journal.pone.0170747
7. Afata, TN, Mekonen, S, and Tucho, GT. Serum concentration of zinc, copper, iron, and its associated factors among pregnant women of small-scale farming in western Ethiopia. Sci Rep. (2023) 13:4197. doi: 10.1038/s41598-023-30284-w
8. Glosz, CM, Schaffner, AA, Reaves, SK, Manary, MJ, and Papathakis, PC. Effect of nutritional interventions on micronutrient status in pregnant malawian women with moderate malnutrition: a randomized, controlled trial. Nutrients. (2018) 10:879. doi: 10.3390/nu10070879
9. Organization WH. The world health report 2002: Reducing risks, promoting healthy life. Geneva, Switzerland: World Health Organization (2002).
10. Ntenda, PAM, Chilumpha, S, Mwenyenkulu, ET, Kazambwe, JF, and El-Meidany, W. Clinical malaria and the potential risk of anaemia among preschool-aged children: a population-based study of the 2015–2016 Malawi micronutrient survey. Infect Dis Poverty. (2019) 8:95–11. doi: 10.1186/s40249-019-0607-8
11. Kare, AP, and Gujo, AB. Anemia among pregnant women attending ante natal care clinic in Adare general hospital, southern Ethiopia: prevalence and associated factors. Health Services Insights. (2021) 14:11786329211036303. doi: 10.1177/11786329211036303
12. Organization WH. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity World Health Organization (2011).
13. Chaparro, CM, and Suchdev, PS. Anemia epidemiology, pathophysiology, and etiology in low-and middle-income countries. Ann N Y Acad Sci. (2019) 1450:15–31. doi: 10.1111/nyas.14092
14. Lema, EJ, and Seif, SA. Prevalence of anemia and its associated factors among pregnant women in Ilala municipality-Tanzania: analytical cross-sectional study. Medicine. (2023):102. doi: 10.1097/MD.0000000000033944
15. World Health Organization (WHO). Anaemia in women and children: who global anaemia estimates. Geneva, Switzerland: World Health Organization. (2001). Available at: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children
16. Anchang-Kimbi, JK, Nkweti, VN, Ntonifor, HN, Apinjoh TOChi, HF, Tata, RB, et al. Profile of red blood cell morphologies and causes of anaemia among pregnant women at first clinic visit in the Mount Cameroon area: a prospective cross sectional study. BMC Res Notes. (2017) 10:1–7. doi: 10.1186/s13104-017-2961-6
17. Bwana, VM, Rumisha, SF, Mremi, IR, Lyimo, EP, and Mboera, LE. Patterns and causes of hospital maternal mortality in Tanzania: a 10-year retrospective analysis. PloS one. (2019) 14:e0214807. doi: 10.1371/journal.pone.0214807
18. Wirth, JP, Rohner, F, Woodruff, BA, Chiwile, F, Yankson, H, Koroma, AS, et al. Anemia, micronutrient deficiencies, and malaria in children and women in Sierra Leone prior to the Ebola outbreak-findings of a cross-sectional study. PLoS One. (2016) 11:e0155031. doi: 10.1371/journal.pone.0155031
19. McGann, PT, Williams, AM, Ellis, G, McElhinney, KE, Romano, L, Woodall, J, et al. Prevalence of inherited blood disorders and associations with malaria and anemia in Malawian children. Blood Adv. (2018) 2:3035–44. doi: 10.1182/bloodadvances.2018023069
21. Sappani, M, Mani, T, Asirvatham, ES, Joy, M, Babu, M, and Jeyaseelan, L. Trends in prevalence and determinants of severe and moderate anaemia among women of reproductive age during the last 15 years in India. PLoS One. (2023) 18:e0286464. doi: 10.1371/journal.pone.0286464
22. Branca, F, Grummer-Strawn, L, Borghi, E, Blössner, M, and Onis, M. Extension of the WHO maternal, infant and young child nutrition targets to 2030. SCN News. (2015) 41:55–8.
24. National Institute of StatisticsICF. Cameroon Demographic and Health Survey, Yaoundé, Cameroon and Rockville. Maryland, USA: NIS and ICF. (2018).
25. Jugha, VT, Anchang-Kimbi, JK, Anchang, JA, Mbeng, KA, and Kimbi, HK. Dietary diversity and its contribution in the etiology of maternal anemia in conflict hit Mount Cameroon area: a cross-sectional study. Front Nutr. (2021) 7:625178. doi: 10.3389/fnut.2020.625178
26. Anchang-Kimbi, JK, Nkweti, VN, Ntonifor, HN, Apinjoh TOTata, RB, Chi, HF, et al. Plasmodium falciparum parasitaemia and malaria among pregnant women at first clinic visit in the Mount Cameroon area. BMC Infect Dis. (2015) 15:1–10. doi: 10.1186/s12879-015-1211-6
27. Wanji, S, Tanke, T, Atanga, SN, Ajonina, C, Nicholas, T, and Fontenille, D. Anopheles species of the Mount Cameroon region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Int Health. (2003) 8:643–9. doi: 10.1046/j.1365-3156.2003.01070.x
28. Wanji, S, Kengne-Ouafo, AJ, Eyong, EEJ, Kimbi, HK, Tendongfor, N, Ndamukong-Nyanga, JL, et al. Genetic diversity of plasmodium falciparum merozoite surface protein-1 block 2 in sites of contrasting altitudes and malaria endemicities in the Mount Cameroon region. American J Tropical Med Hygiene. (2012) 86:764–74. doi: 10.4269/ajtmh.2012.11-0433
29. Anchang-Kimbi, JK, Kalaji, LN, Mbacham, HF, Wepnje, GB, Apinjoh TONgole Sumbele, IU, et al. Coverage and effectiveness of intermittent preventive treatment in pregnancy with sulfadoxine–pyrimethamine (IPTp-SP) on adverse pregnancy outcomes in the Mount Cameroon area, South West Cameroon. Malaria J. (2020) 19:1–12. doi: 10.1186/s12936-020-03155-2
30. Sumbele, IUN, Bopda, OSM, Kimbi, HK, Ning, TR, and Nkuo-Akenji, T. Nutritional status of children in a malaria meso endemic area: cross sectional study on prevalence, intensity, predictors, influence on malaria parasitaemia and anaemia severity. BMC Public Health. (2015) 15:1099–9. doi: 10.1186/s12889-015-2462-2
31. Fokam, EB, Ngimuh, L, Anchang-Kimbi, JK, and Wanji, S. Assessment of the usage and effectiveness of intermittent preventive treatment and insecticide-treated nets on the indicators of malaria among pregnant women attending antenatal care in the Buea Health District, Cameroon. Malar J. (2016) 15:1–7. doi: 10.1186/s12936-016-1228-3
33. Jugha, VT, Anchang, JA, Taiwe, GS, Kimbi, HK, and Anchang-Kimbi, JK. Association between malaria and undernutrition among pregnant women at presentation for antenatal care in health facilities in the Mount Cameroon region. PLoS One. (2023) 18:e0292550. doi: 10.1371/journal.pone.0292550
34. FAF. Minimum dietary diversity for women: a guide for measurement. Rome: Food and nutrition technical assistance (FANTA III) (2016). 82 p.
36. Kennedy, G, Ballard, T, and Dop, MC. Guidelines for measuring household and individual dietary diversity. US: Nutrition and Consumer Protection Division, Food and Agriculture Organization of the United Nations (2013).
37. WHO. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva. (2016). Available at: https://iris.who.int/bitstream/handle/10665/250796/9789241549912-eng.pdf?sequence=1
38. Fouelifack, FY, Sama, JD, and Sone, CE. Assessment of adherence to iron supplementation among pregnant women in the Yaounde gynaeco-obstetric and paediatric hospital. Pan Afr Med J. (2019) 34:211. doi: 10.11604/pamj.2019.34.211.16446
39. Bhutta, ZA, Darmstadt, GL, Hasan, BS, and Haws, RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. (2005) 115:519–617. doi: 10.1542/peds.2004-1441
40. Abu-Ouf, NM, and Jan, MM. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med J. (2015) 36:146–9. doi: 10.15537/smj.2015.2.10289
41. Malek, L, Umberger, W, Makrides, M, and Zhou, SJ. Poor adherence to folic acid and iodine supplement recommendations in preconception and pregnancy: a cross-sectional analysis. Aust N Z J Public Health. (2016) 40:424–9. doi: 10.1111/1753-6405.12552
42. Taye, B, Abeje, G, and Mekonen, A. Factors associated with compliance of prenatal iron folate supplementation among women in Mecha district, Western Amhara: a cross-sectional study. Pan Afr Med J. (2015) 20:43. doi: 10.11604/pamj.2015.20.43.4894
43. Titilayo, A, Palamuleni, M, and Omisakin, O. Sociodemographic factors influencing adherence to antenatal iron supplementation recommendations among pregnant women in Malawi: analysis of data from the 2010 Malawi demographic and health survey. Malawi Med J. (2016) 28:1–5. doi: 10.4314/mmj.v28i1.1
44. Moshi, FV, Millanzi, WC, and Mwampagatwa, I. Factors associated with uptake of iron supplement during pregnancy among women of reproductive age in Tanzania: an analysis of data from the 2015 to 2016 Tanzania demographic and health survey and malaria indicators survey. Front Public Health. (2021) 9:604058. doi: 10.3389/fpubh.2021.604058
45. Garcia-Casal, MN, Estevez, D, and De-Regil, LM. Multiple micronutrient supplements in pregnancy: implementation considerations for integration as part of quality services in routine antenatal care. Objectives, results, and conclusions of the meeting. Matern Child Nutr. (2018) 14:e12704. doi: 10.1111/mcn.12704
46. Victora, CG, Christian, P, Vidaletti, LP, Gatica-Domínguez, G, Menon, P, and Black, RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. (2021) 397:1388–99. doi: 10.1016/S0140-6736(21)00394-9
47. Allen, L, de Benoist, B, Dary, O, and Richard, H. Guidelines on food fortification with micronutrients Geneva, Switzerland: World Health Organization, Food and Agricultural Organization of the United Nations (2006).1–376. Available at: https://iris.who.int/bitstream/handle/10665/43412/9241594012_eng.pdf
48. Abbaspour, N, Hurrell, R, and Kelishadi, R. Review on iron and its importance for human health. J Res Medical Sci: Official J Isfahan University of Med Sci. (2014) 19:164–74.
49. Young, I, Parker, HM, Rangan, A, Prvan, T, Cook, RL, Donges, CE, et al. Association between haem and non-haem iron intake and serum ferritin in healthy young women. Nutrients. (2018) 10:81. doi: 10.3390/nu10010081
50. Ferdi, J, Bardosono, S, and Medise, BE. Iron intake and its correlation to ferritin and hemoglobin level among children aged 24–36 months in Jakarta in 2020. World Nutrition J. (2021) 5:106–12. doi: 10.25220/WNJ.V05.i1.0014
51. Hailu, T, Kassa, S, Abera, B, Mulu, W, and Genanew, A. Determinant factors of anaemia among pregnant women attending antenatal care clinic in Northwest Ethiopia. Tropical Dis, Travel Med Vaccines. (2019) 5:13–7. doi: 10.1186/s40794-019-0088-6
52. Baig-Ansari, N, Badruddin, SH, Karmaliani, R, Harris, H, Jehan, I, Pasha, O, et al. Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan. Food Nutr Bull. (2008) 29:132–9. doi: 10.1177/156482650802900207
53. Kumar, A, Sharma, E, Marley, A, Samaan, M, and Brookes, M. Iron deficiency Anaemia: pathophysiology, assessment, practical Management. BMJ Open Gastroenterol. (2022) 9:e000759. doi: 10.1136/bmjgast-2021-000759
54. Spiegler, E, Kim, Y-K, Wassef, L, Shete, V, and Quadro, L. Maternal–fetal transfer and metabolism of vitamin a and its precursor β-carotene in the developing tissues. Biochimica et Biophysica Acta (BBA)-molecular and cell biology of. Lipids. (2012) 1821:88–98. doi: 10.1016/j.bbalip.2011.05.003
55. Bastos Maia, S, Rolland Souza, AS, Costa Caminha, MF, Lins da Silva, S, Callou Cruz, RSBL, Carvalho dos Santos, C, et al. Vitamin a and pregnancy: a narrative review. Nutrients. (2019) 11:681. doi: 10.3390/nu11030681
56. FAO WA. Vitamin and mineral requirements in human nutrition. 2nd ed. Geneva, Switzerland: WHO (2004). 362 p.
57. Tanumihardjo, SA, Russell, RM, Stephensen, CB, Gannon, BM, Craft, NE, Haskell, MJ, et al. Biomarkers of nutrition for development (BOND)—vitamin a review. J Nutr. (2016) 146:1816S–48S. doi: 10.3945/jn.115.229708
58. Semba, R, and Bloem, M. The anemia of vitamin a deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. (2002) 56:271–81. doi: 10.1038/sj.ejcn.1601320
59. Engle-Stone, R, Aaron, GJ, Huang, J, Wirth, JP, Namaste, SM, Williams, AM, et al. Predictors of anemia in preschool children: biomarkers reflecting inflammation and nutritional determinants of Anemia (BRINDA) project. Am J Clin Nutr. (2017) 106:402S–15S. doi: 10.3945/ajcn.116.142323
60. Sunardi, D, Bardosono, S, Basrowi, RW, Wasito, E, and Vandenplas, Y. Dietary determinants of anemia in children aged 6–36 months: a cross-sectional study in Indonesia. Nutrients. (2021) 13:2397. doi: 10.3390/nu13072397
61. Fishman, SM, Christian, P, and West, KP. The role of vitamins in the prevention and control of anaemia. Public Health Nutr. (2000) 3:125–50. doi: 10.1017/S1368980000000173
Keywords: dietary diversity, micronutrients, haem iron, vitamin A, haemoglobin levels, pregnant women, Mt. Cameroon area, cross-sectional study
Citation: Jugha VT, Anchang JA, Sofeu-Feugaing DD, Taiwe GS, Kimbi HK and Anchang-Kimbi JK (2024) Dietary micronutrients intake and its effect on haemoglobin levels of pregnant women for clinic visit in the Mount Cameroon health area: a cross-sectional study. Front. Nutr. 11:1341625. doi: 10.3389/fnut.2024.1341625
Received: 20 November 2023; Accepted: 10 April 2024;
Published: 07 May 2024.
Edited by:
Minatsu Kobayashi, Otsuma Women's University, JapanReviewed by:
Hilali Abderraouf, Hassan Premier University, MoroccoCopyright © 2024 Jugha, Anchang, Sofeu-Feugaing, Taiwe, Kimbi and Anchang-Kimbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa Tita Jugha, anVnaGF2QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.