
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 26 March 2024
Sec. Nutrition and Food Science Technology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1341539
This article is part of the Research Topic Analysis of Innovations in Food Development: Improving Nutritional Value, Flavor and Texture in Food Products View all 4 articles
Quinoa is a gluten-free pseudocereal, with an excellent nutrient profile containing considerable amounts of fiber and minerals and rich in antioxidants such as polyphenols. The purpose of this research was to investigate the effects of quinoa bread on physical, chemical, bioactive components, glycaemic index (GI), and biochemical parameters. Human subjects aged between 20 and 50 years with the absence of morbid factors were fed daily with quinoa bread for 3 months in order to study its pre-and post-treatment effects on blood glucose, glycosylated haemoglobin, and lipid profile. The effort was made to incorporate the maximum amount of quinoa into the bread without compromising the acceptability of the bread. Of the 14 formulations, TQ13, containing 20% quinoa flour with 3% wheat bran, was selected for further analysis. The GI study revealed that quinoa bread peaked at 45 min with a gradual increase after ingestion of the bread and a steady decline thereafter. The observed value for blood glucose levels, before and after supplementation with quinoa-incorporated bread, was 86.96 ± 15.32 mg/dL and 84.25 ± 18.26 mg/dL, respectively. There was a statistically significant (p ≤ 0.05) decrease in levels of triglycerides, total cholesterol, low-density lipoprotein (LDL), and very-LDL (VLDL) level before and after supplementation. However, non-significant changes were observed for high-density lipoprotein levels from the pre- and post-treatment with the quinoa-incorporated bread. Quinoa-incorporated bread possessed low GI (42.00 ± 0.83) compared to control (69.20 ± 1.84) and long-term consumption proved to contain functional efficacies in terms of hypolipidemic effect.
The food industry is always in the process of developing new food products according to the demands of the consumer for products with improved quality. This is also a demand of the hour to produce food products with health benefits to counteract increased incidences of non-communicable diseases all over the world (1). Hence, the emphasis is on the incorporation of functional ingredients into new food products for better quality and benefits (2). Functional foods may be regarded as innovative, physiologically active foods, which can provide additional health benefits beyond basic nutrition. Fortification of food products is one of the major techniques used to create functional food products (2).
Formulation of functional foods with the inclusion of grains from the categories of cereals, millets, pseudocereals, legumes, and oilseeds enhances the nutritional qualities of foods. Furthermore, if these grains are unprocessed or minimally processed, the benefits of whole grains are further increased with probable capabilities of disease prevention (3). Consumption of whole-grain products is associated with reduced incidence of diseases, such as cancer (4), cardiovascular disease (5), high blood pressure (6), and diabetes (7).
Currently, composite or multigrain flours are increasingly being used to produce products such as cookies (8–10) breads (11–13), and cakes (14, 15). Pseudocereal grains such as quinoa, buckwheat, and amaranth are rich in a wide range of compounds such as flavonoids, phenolic acids, fatty acids, trace elements, and vitamins with known effects on human health (16). Quinoa, a native plant belonging to the Andean region, is considered gluten-free with an excellent nutrient profile (17). They contain considerable amounts of fiber (3.8 g/100 g) and minerals, such as calcium (1,487 mg kg−1 dry wt) and iron (132 mg kg−1 dry wt) (18). Quinoa is also rich in antioxidants such as polyphenols (19). Once known to the Incas as the “mother of all grains,” today quinoa is receiving increasing attention because of its high nutritional quality (20). Efforts to improve the quality of baked goods via substitution of cereal grains for quinoa flour have revealed that up to 10% quinoa flour in breads improves nutritional quality without negatively affecting loaf volume. Considering the lower nutritional value of most gluten-free products in the market, further study on the behavior of quinoa proteins and carbohydrates in bread is warranted (21).
Control bread was formulated to contain a maximum amount of whole wheat flour without compromising sensory quality. Based on preliminary studies (data not shown), it was found that a 60:40 ratio of refined wheat flour to whole wheat flour performed the best for making quality bread, as compared to that of 100% whole wheat flour bread and also without the use of bread improver and/or enzymes. Therefore, this ratio was used as a control (coded as T0) for subsequent studies. Thus, a control bread formulation of a 60:40 ratio of refined wheat flour to whole wheat flour was used as the base. All raw materials were sourced from the local market of Jorhat, Assam. A total of fourteen formulations were developed with 5, 10, 15, 20, 25 and 30 % quinoa flour with and without fenugreek flour and wheat bran (Table 1). Fenugreek seeds were added as it is a rich source of soluble dietary fiber; 100 g of seeds provides more than 65% of dietary fiber and contains saponins, hemicelluloses, mucilage, tannins, and pectin, which help to decrease the level of low-density lipoprotein cholesterol (LDL) in blood by decreasing the reabsorption of bile salts in the colon (22).
Sensory evaluation of the developed quinoa breads was performed by 15 trained and semi-trained panel members from the Department of Food Science and Nutrition, College of Community Science, Assam Agricultural University, Jorhat (23). The panellists were asked to score the products for every quality attribute such as color, texture, taste, flavor, appearance and overall acceptability, using a scorecard of a 9-point Hedonic Rating Scale. The bread was completely cooled and then stored for 24 h before sensory evaluation.
Loaf weight, loaf volume, specific volume, texture profile, and color were studied for the developed quinoa bread sample.
The bread is simply weighed in an electronic weighing balance to record the loaf weight (23).
The loaf volume of bread was measured using the rapeseed replacement method (24). Loaf volume (VL) was calculated according to the following formula:
VL (cm3) = VC – VR.
Specific volume is an important parameter in bread making and indicates the final gas retention in the bread and affects consumer preference. The specific volume (VS) of bread was measured by using the following expression:
VS (cm3/g) = VL/W.
Texture profile analysis (TPA) was carried out using a texture analyser (TA-XT Plus, Stable Micro Systems, United Kingdom) as adopted by the standard method by AACC (24). The sample was removed from its place of storage and placed centrally over the supports just prior to testing (25, 26). The texture analyser was equipped with a 36-mm-radius probe. The first and second compression cycles indicate the force vs. time data during the first and second compression of the product by the instrumental probe. A P0.5R cylindrical probe with 2 mm/s of pre-test and post-test speeds and 45% compression was taken for TPA. TPA is a two-bite test, which includes the first and second compression cycles. The first and second compression cycles indicate the force vs. time data during the first and second compression of the product by the instrumental probe. Three sets of measurements per loaf for replications were recorded.
Color analysis of multigrain breads was done by using a Hunter Lab colorimeter (model SM-3001476 microsensors). The instrument was calibrated with user-supplied black plate calibration standard that was used for zero setting, and white calibration plates were used for white calibration settings. The instrument was placed at three different exposures at different places. Readings were displayed as L*, a*, and b* color parameters according to the CIELAB system of color measurement. The value of a* ranged from −100 (redness) to +100 (greenness), the b* values ranged from −100 (blueness) to +100 (yellowness), while as L* value indicating the measure of lightness, ranged from 0 (indicating black) to 100 (indicating white) (27). The three values are required to completely describe the color of an object.
The analysis of moisture, crude fat, crude protein, crude fiber, and ash was carried out as described in AOAC, 2000. The carbohydrate content was calculated by the difference method. The energy value (kcal) of the bread sample was calculated by the method of Gopalan et al. (28). Total dietary fiber was also estimated as described by AOAC (25).
The minerals calcium and iron were determined by using an atomic absorption spectrophotometer according to the method of AACC (24).
In total, 2 g of dried sample was extracted with 20 mL of methanol (99.5%). The extraction was done twice each for hours in a shaking machine. The supernatant was filtered using Whatman no. 1 filter paper after centrifuging the suspension at 10,000 rpm for 15 min, the filtrate was stored at -20°C till analysis. A 100 μL of an aliquot of sample extract was taken in a test and add 2.9 mL of DPPH solution (0.005 mM solution of 2,2-diphenyl-1-picryl-hydrazyl prepared in 99.5% methanol); after this solution was added, it was vortex mixed vigorously. The test tube was incubated in the dark for half an hour. Discoloration of DPPH was measured against a blank at 517 nm. Methanol was used as blank, and DPPH methanolic extract was used as standard.
Total phenolics were determined spectrophotometrically using Folin–Ciocalteu reagent and expressed as gallic acid equivalent/g (mg of GAE/g of the sample) (28). A known aliquot (0.2 mL) of sample extract was taken, and the volume was made up to 1.5 mL with D/W. To the extract, 0.5 mL of Folin–Ciocalteu reagent was added by addition of 10 mL of 7.5% sodium carbonate solution and mixed well by shaking. Incubated at 37°C for 60 min and absorbance was measured at 750 nm in a spectrophotometer, concentration was calculated from a standard curve prepared from different concentrations of gallic acid (5–20 μg) and distilled water as the blank.
Total flavonoid content (TPC) was determined by using the method described by Zhishen et al. (29). A known aliquot of the sample was taken, and the volume was made up to 5 mL with distilled water; 0.3 mL of 5 % of NaNO2 was added. After 5 min, 0.6 mL of 10 % AlCl3 was added and mixed. After 6 min, 2 mL of 1 N NaOH was added and mixed. Then, 2.1 mL of distilled water was added to make the volume up to 10 mL. The absorbance of the resulting pink color was read at 510 nm against a blank (distilled water), and Rutin (50 μg to 200 μg) was taken as standard.
For the estimation of glycemic index, the procedure given by Wolever et al. (30) was followed.
Selection of subjects for the intervention was based on age (20–50 years) and in the absence of morbid factors. The subjects were asked to sign a consent form, and the ethical committee recommended the study vide authorization number AAU/CCS/FSN/IEC/241. Each subject was given 100 g of multigrain bread daily for 3 months in order to study pre- and post-treatment effects on blood glucose, glycosylated haemoglobin, and lipid profile.
Blood glucose was estimated by a commercial assay kit (Coral Glucose estimating kit). The blood samples were collected in heparinized sterile centrifuge tubes and were centrifuged at 1107 grams-force (3,000 rpm for 20 min). Serum was collected in a microcentrifuge and estimated by auto analyser using a commercial assay kit (Coral Glucose estimation kit using GOD/POD method). The standard laboratory method of blood glucose estimation was done through a spectrophotometer (V-730 UV–Visible Spectrophotometer).
The collected whole-blood samples were assayed for the measurement of glycosylated haemoglobin using high-performance liquid chromatography (LC-4000 Series HPLC model, with the use of the Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer method in 2003–2004 and the Tosoh G7 method in 2007–2008, Tosoh Corp).
Lipid profile analysis was studied for triglycerides, total cholesterol, HDL, LDL, and VLDL by the method described by Wagner et al. (31).
The Ethical Committee of the University approved the research study after a thorough discussion, and a certificate was issued for the same.
The results are expressed as mean ± SD. Data obtained were statistically analysed by using SPSS statistics (Ver. 20) software using one-way analysis of variance (ANOVA), and the significance of the difference between means of tested parameters was carried out using Duncan’s post-hoc test. Differences were considered statistically significant at the p ≤ 0.05 level at a 5% level of significance.
Of the 14 formulations, TQ1, TQ2, TQ3, TQ4, and TQ13 had similar (p ≥ 0.05) scores in all sensory parameters and scores were significantly (p ≤ 0.05) higher as compared to all other formulations. Although TQ1, TQ2, TQ3, TQ4, and TQ13 had statistically similar scores (p ≥ 0.05), TQ13 was expected to have better nutritional profiles as the quinoa incorporation was higher as compared to TQ1, TQ2, and TQ3. Furthermore, although TQ4 and TQ13 both contained 20% quinoa flour, TQ13 was further incorporated with 3% bran (Table 1) and was selected for further studies. The increase in the percentage of incorporation of quinoa and other ingredients led to the decrease in acceptability (Table 2) of the breads formulated from quinoa flour. In some of the formulations, sensory scores were even lower than 5.
The loaf weight, loaf volume, and specific volume of the quinoa-incorporated multigrain bread were 434.28 ± 0.56 g, 1300.32 ± 0.65 cm3, and 2.99 ± 0.60 cm3/g, respectively, whereas those for the control, it was 434.28 g ± 0.56, 1486.32 ± 0.64 cm3, and 3.42 ± 0.75 cm3/g, respectively. Compared to the control bread sample, quinoa bread had a lesser loaf volume and specific volume but their loaf weights were the same. White bread or refined wheat bread is the commonly consumed form of bread around the globe due to its good physical properties such as loaf volume, but it has less value in terms of nutrition (Table 3).
Commonly considered parameters used to determine bread texture is the study of hardness, cohesiveness, springiness, and chewiness of the bread. The values obtained for quinoa bread were 1.58 ± 1.19 kg (hardness), 0.95 ± 0.43 s (cohesiveness), 0.94 ± 1.45 (springiness), and 1.42 ± 3.22 kg-s (chewiness). The values for control bread were 0.33 ± 0.56 kg, 0.45 ± 1.22 s, 1.00 ± 0.26, and 0.06 ± 1.27 kg-s for hardness, cohesiveness, springiness, and chewiness, respectively. From Table 4, it can be inferred that the control bread possessed superior quality in terms of the texture of the bread.
The L*, a*, and b* values in regard to the crust color are presented in Table 5. The values obtained for L*, a*, and b* for quinoa bread were 56.64 ± 1.24, 3.30 ± 0.18, and 22.11 ± 0.19, respectively, and those for the control 67.50 ± 1.15, 2.09 ± 0.05, and 16.46 ± 0.58, respectively. The values for the quinoa bread are more intense compared to the control sample. The values for the crumb color of the quinoa bread were 53.64 ± 1.54 (L* value), 13.53 ± 0.58 (a* value), and 31.70 ± 0.19 (b* value) as shown in Table 6. In both the crust and crumb color of the quinoa bread, a* and b* color values that indicate redness and yellowness were more than those of control. The color value for L*, indicating lightness or whiteness was found to be lesser than that of the control bread.
The proximate analysis involves the determination of moisture, crude protein, crude fat, crude fiber, and ash of quinoa bread sample. The mean values of quinoa bread were 39.05 ± 0.67 g/100 g (moisture), 4.82 ± 0.41 g/100 g (crude fat), 14.28 ± 1.65 g/100 g (crude protein), 2.53 ± 0.55 g/100 g (crude fiber), 1.15 ± 0.88 g/100 g (total ash), 27.86 ± 0.23 g/100 g (carbohydrate), 302 ± 0.49 kcal (energy), 154.89 ± 0.48 mg/100 g (calcium), 8.49 ± 0.12 mg/100 g (iron), 13.75 ± 0.54 g/100 g (total dietary fiber), 10.56 ± 0.74 g/100 g (insoluble dietary fiber), and 3.19 ± 0.46 g/100 g (soluble dietary fiber). From Table 7, it is concluded that the chemical composition of quinoa bread was higher than the control values, which proved that quinoa is rich in many nutrients (Tables 8–10).
Antioxidant capacity of quinoa bread was measured by the ability of the test sample to scavenge DPPH radicals. Table 11 shows that quinoa bread had a mean antioxidant capacity of 33.26 ± 1.53 %. Phenolic compounds in cereals are found in free, soluble conjugated, and bound forms. The bound form represents the major proportion of phenolic acid in cereals. In this study, the total phenolic content of the quinoa bread was 2.31 ± 1.58 mg GAE/g. Flavonoids are a group of polyphenolic compounds that are widely distributed and possess health-related properties such as anticancer, antioxidant, anti-inflammatory, and antiviral properties, which are based on their antioxidant activity. The total flavonoid content of quinoa multigrain bread was 0.22 ± 0.46 mg QE/g. In all the three findings, the values were higher than those of the control sample.
The glycaemic index (GI) is a concept that allows the ranking of carbohydrate-rich foods in terms of their potential to raise blood glucose levels. White bread, which served as the control for determination of GI, peaked at 30 min and remained comparatively high over a 120-min period of investigation. Quinoa bread (TQ13) showed a slower peaking and decline. Quinoa bread was found to peak at 45 min, showing a slower, gradual increase after ingestion of the bread and then showed a steady decline. The observed GI of quinoa bread was 42.00 ± 0.83, which is considered as low-GI foods.
The effect of quinoa-incorporated MG bread having low GI (42.00 ± 0.82) on the blood profile was studied in terms of pre- and post-intervention on blood glucose levels, glycosylated haemoglobin, and lipid profile. A single meal of a low-fiber food like white bread may stimulate high postprandial blood glucose response and influence glucose and insulin metabolism. The observed blood glucose levels, as indicated in Table 12, showed that before supplementation with quinoa-incorporated multigrain bread, the value was 86.96 ± 15.32 mg/dL, and after supplementation, it reduced to 84.25 ± 18.26 mg/dL. Though there was a decrease in the blood glucose levels after supplementation, it was non-significant (p ≥ 0.05).
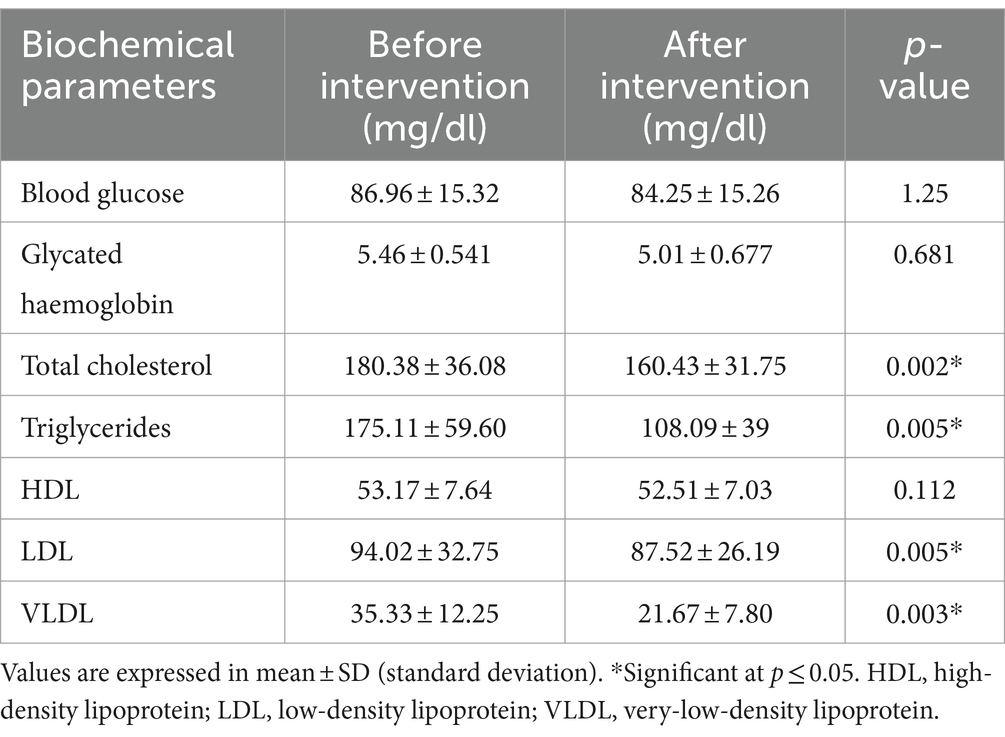
Table 12. Biochemical parameters (mg/dl) pre- and post-treatment with quinoa-incorporated multigrain bread.
Glycosylated haemoglobin is a form of haemoglobin (Hb) that is chemically linked to sugar. Most monosaccharides, including galactose and fructose, spontaneously bond with haemoglobin, when present in the bloodstream of humans. The test for glycosylated haemoglobin as presented in Table 12 showed a decrease in values, but the changes observed were non-significant (p ≥ 0.05). The mean value was 5.46 ± 0.541 mg/dL before supplementation, and it lowered slightly to 5.01 ± 0.677 mg/dL after supplementation. The value observed fell within the normal range (4 and 5.6%).
The lipid profile values observed before and after supplementation with quinoa bread are presented in Table 12. The values obtained before supplementation were 180.38 ± 36.08 mg/dL, 175.11 ± 59.60 mg/dL, 53.17 ± 7.64 mg/dL, 94.02 ± 32.75/dl, and 35.33 ± 12.25 for cholesterol, triglyceride, HDL, LDL, and VLDL, respectively, and those after supplementation were 160.43 ± 31.75 mg/dL, 108.09 ± 39 mg/dL, 52.51 ± 7.03 mg/dL, 87.52 ± 26.19 mg/dL, and 21.67 ± 7.80 mg/dL for cholesterol, triglyceride, HDL, LDL, and VLDL, respectively. A statistically significant (p ≤ 0.05) decrease in the levels of triglycerides, total cholesterol, LDL and VLDL was observed. However, there was no significant change observed for HDL levels from the pre- and post-treatment with the quinoa-incorporated multigrain bread (TQ13).
Refined wheat flour is not a good source of protein, minerals, and certain bioactive components as compared to whole quinoa flour; therefore, quinoa flour was incorporated into wheat-based bread to improve nutritional and bioactive properties. The addition of quinoa flour and wheat bran in appropriate proportions improved the chemical properties of wheat bread as well as its nutritional status. Wheat flour bread is deficient or poor in many nutrients. Development and study of the physical and chemical properties and bioactive components of quinoa bread have led to many positive effects on health. The addition of quinoa flour and wheat bran to wheat bread resulted in a nutrient-dense bread that with positive response in terms of decreasing the levels of triglycerides, total cholesterol, LDL, and VLDL significantly.
Some studies have suggested that 20% quinoa inclusion in white bread to have the highest acceptability (31) while some other studies suggest 15% of quinoa (32) and 10% (33) as the most acceptable. In this study, the selected formulation TQ13 had an incorporation of 20% quinoa flour as well as 3% wheat bran and was found to be satisfactory from both sensory and nutritional points of view. Increased bran content was assumed to add to the health benefits of the quinoa bread.
The loaf volume of quinoa bread was lower than that of the control bread. The addition of too much fiber was reported to affect the bread quality when it comes to texture, loaf volume, and appearance (34, 35). High levels of fiber dilute gluten and lowers gas retention causing a decrease in loaf volume. This might be the reason for obtaining a lesser loaf volume in the quinoa bread formulated in this study. Several other studies reported less bread volume due to the incorporation of ingredients, such as finger millet (36), barley (37, 38), and composite flour (39). A good loaf volume is obtained if the gas bubbles in the fermented dough expand with minimal rupturing of the gluten network during proofing and baking. The presence of ß-glucans-a fraction of total dietary fiber in high levels reduces the specific volume of the breads (40), which can be compared to the present study where fiber from quinoa and bran might have reduced the volume. The decrease in volume was proportional to the increase in non-cereal flour. The specific volume decreased with increased incorporation of composite flour (41). The specific volume of bread reveals the development of bread dough after baking. The greater the specific volume value, the more inflated and voluminous is the bread dough after baking.
The hardness of quinoa bread, as seen in Table 4, was higher than the control. The increase in hardness might be attributed to the higher water absorption of fiber-rich-incorporated dough. This can be explained by an interaction between water and hydroxyl groups of polysaccharides through hydrogen bonding (42). Higher hardness with increasing bran addition with regard to dough texture is attributed to the thickening of the walls surrounding the air bubbles in the crumb (34, 43). The cohesiveness of quinoa bread was higher than that of the control bread. Higher cohesiveness in composite breads may be due to higher moisture retention compared to control bread (44). Cohesiveness in composite bread may be attributed to the decreased aeration and compact texture (45). The results of the present investigation were similar to the findings of Abdelghafor et al., (46), Nasar-Abbas and Jayasena (47), and Chhavi and Sarita (48), who reported that chewiness increased progressively with an increase in the level of multigrain flour in the composite bread as compared to the control. This may be attributed to the dilution of wheat gluten with an increased proportion of other flours and added to the weakening of the strength of gluten.
The L*, a*, and b* values in regard to the crust color are presented in Table 5. The values obtained for L*, a* and b* for quinoa bread were 56.64 ± 1.24, 3.30 ± 0.18, and 22.11 ± 0.19 and those for the control 67.50 ± 1.15, 2.09 ± 0.05, and 16.46 ± 0.58. The values for the quinoa bread are more intense compared to the control sample. The values for crumb color (Table 6) of the quinoa bread were 53.64 ± 1.54 (L* value), 13.53 ± 0.58 (a* value), and 31.70 ± 0.19 (b* value) as shown in Table 6. In both the crust and crumb color of the quinoa bread, the a* and b* color values, which indicate redness and yellowness, were more than those of control (49–51). The color value for L*, indicating lightness or whiteness, was found to be lesser than that of the control bread. The increasing darkness and redness of composite breads might be due to the high content of protein in the case of quinoa flour, which resulted in the Maillard browning during baking (52).
The moisture content of quinoa bread (39.05 ± 0.67) is reported to contain a higher value than the control (34.79 ± 0.62). The results of the present investigation are well in accordance with those reported by Otegbayo et al. (53), Ngozi (54), Ameh et al. (55), and Rehman et al., (56), who reported higher moisture in wheat bran-incorporated breads and composite breads. The increased moisture content of composite breads may be a consequence of the increased water absorption capacity of dough (57, 58) and also an increase in fiber content (59). In the present study also, the crude fiber content of quinoa multigrain experimental bread was higher as compared to control bread, which could be the reason for the higher moisture levels of experimental breads in comparison with control breads. The results of the present study are in accordance with a study by Sharma et al. (59) and Sanz-Penella et al. (60) who also observed an increase in fat content in composite breads enriched with millets and pseudocereals. Quinoa has a fat content ranging from 5 to 7 %, thus contributing to the high fat content of quinoa-incorporated breads [65]. In the present study, quinoa MG breads had higher levels of crude fat as compared to control bread. Wright et al. (61) and Comai et al. (62) also reported that quinoa had higher total protein content (12.9–16.5%) compared to other grains and pseudocereals. Other studies have also reported a similar increase in protein content in quinoa composite breads (58, 63). The effects of the addition of whole-grain barley flour to wheat flour reported improved levels of β-glucan (64). Higher fiber content may be due to the fiber content of the individual grains and the wheat bran added to the quinoa bread. The carbohydrate content of quinoa bread was higher than that of the control bread. Composite breads are known to contain higher carbohydrate content compared to control bread (65). The results of the present study are also in agreement with studies by Olaoye et al. (66) and Ambreen et al. (67). The energy content of quinoa bread was 302.2 ± 0.49 kcal. Shehry (68) also reported a higher energy content of quinoa-incorporated breads compared to control. This may be due to the high fat content of quinoa. Graf et al. (21) and Lalit (69) also reported higher energy content in quinoa-based composite breads.
Demin et al. (32) reported that quinoa-supplemented bread had a 40% increase in calcium content compared to the control and the reason might be the calcium content of quinoa, which is 126.94 mg/100 g (70). Similar increasing trends were also observed in other studies (71, 72). Kumari (73) reported 8.60 mg/100 g iron for toast bread incorporated with full-fat rice bran (10%) and 9.20 mg/100 g iron for defatted rice bran (10%). The formulated toast bread was found to have significant results over control bread containing 7.90 mg/100 g iron. Young (74) reported bread prepared from rice bran had an iron content of 9.32–20.52 mg. Naikare (75) prepared bread from a 15% sorghum blend with 85% wheat flour, and the iron content was 3.4 mg/100 g more than that of the control.
Alvarez-Jubete et al. (76) documented the influence of amaranth, quinoa, and buckwheat on polyphenol profile and antioxidant capacity and revealed that buckwheat demonstrated the most antioxidant activity. Quinoa is known to contain phenolics as a major group of secondary metabolites (21), which may be the reason for high values in TQ13. The high TPC of whole grain and bran are due to the presence of pericarp and aleurone layers, which are rich in antioxidant compounds (59). Quinoa-incorporated breads contain higher flavonoids with possible nutraceutical benefits (68). Flavonoids are a group of polyphenolic compounds possessing health-related properties based on their antioxidant activity. Pandey et al. (77) also pointed out the protective role of flavonoids, specifically flavones and flavonols, from cardiovascular and cancer diseases.
As per the classification given by Augustin et al., (78), foods having less than 55 GI are considered as low-GI foods, 56–69 medium-GI foods, and above 70 high-GI foods. Based on the classification, quinoa-incorporated bread (42.00 ± 0.83) can be categorized under low-GI foods. Quinoa, known to contain good amounts of dietary fiber, modulates postprandial insulin response, promotes endogenous cholesterol conversion to bile acids and improves intestinal microbiota. Epidemiological studies have shown an inverse relationship between dietary fiber intake and the development of cardiovascular disease, obesity, and type 2 diabetes (79). The effects of various commercial whole-grain breads on postprandial blood glucose response and GI in healthy subjects reported that whole-grain oat bread exhibited the lowest peaking of blood glucose level and also reported the lowest GI (80). They stated that the oat bread was especially rich not only in total fat and protein but also in dietary fiber compared to other breads under study, which could be the reason for low blood glucose peak and GI. The management of the postprandial blood glucose response is crucial, as high blood glucose response may instigate the incidence of diabetes, obesity, coronary heart diseases, and some types of cancer (81). The observed blood glucose levels, as indicated in Table 12, which shows that before supplementation with quinoa-incorporated multigrain bread, the value was 86.96 ± 15.32 mg/dL, and after supplementation, it reduced to 84.25 ± 18.26 mg/dL. Although there was a decrease in the blood glucose levels after supplementation, it was not significant (p ≥ 0.05). This indicates that the usual trend of lowering blood glucose levels with the intervention of high-fiber supplements (82) was observed but not significant (p ≥ 0.05) in the present study. It could be due to the reason that post-prandial blood glucose response is dose dependent (83). The composition of the quinoa-incorporated MG bread of the present study was refined wheat flour (52%), whole wheat flour (25%), and quinoa flour (20%). Farinazzi-Machado et al. (84) showed that long-term consumption (30 days) of quinoa cereal bars led to a significant (p ≤ 0.05) reduction in blood glucose as quinoa bar was made with only quinoa and no other cereals.
Haemoglobin A1c levels between 5.7 and 6.4 % indicate pre-diabetes and a higher chance of getting diabetes. A person with levels of 6.5 % or higher indicates a person has diabetes (85). In the present case, subjects having HbA1c within the normal range both before and after supplementation were 5.46 ± 0.541 and 5.01 ± 0.677 mg/dL, respectively. Foods containing dietary fiber are associated with a reduction in the risk of diseases and can prevent hyperlipidemias, cardiovascular diseases, diabetes, and obesity (86–88). Carbohydrates from quinoa, including insoluble and soluble fiber, can be considered as nutraceuticals because they help in the reduction of blood glucose, triglyceride, total cholesterol, and free fatty acid levels in the blood (89). The results obtained in the present study are similar to the data reported in the literature, indicating that quinoa bread can be used to lower plasma lipids and glycemic control. Quinoa contains considerably high amounts of vitamin E, iron, zinc, and magnesium (90). These nutrients have shown hypocholesterolemic effects and increased postprandial sensitivity and release of plasma insulin (91–93). The presence of antioxidant capacity compounds, such as polyphenols, phytosterols, and flavonoids in grains of quinoa (94), might be the cause for the positive effects on reduction in plasma lipids such as total cholesterol, triglycerides, LDL and VLDL, and blood glucose levels in the subjects tested for biochemical parameters after the post-treatment with multigrain bread. Similarly, Farinazzi-Machado et al., (84) showed that after 30 days of treatment with a quinoa cereal bar, a significant reduction in blood glucose, cholesterol, triglycerides, and LDL and increased levels of HDL were observed. Increased consumption of phenolic compounds has been associated with a reduced risk of cardiovascular diseases and certain cancers (95, 96).
Based on the data obtained in this study, it can be concluded that the incorporation of quinoa into wheat bread results in essential health benefits. The incorporation level in the present study was dependent on the workability of the bread as well as acceptability, keeping the nutrient profile in mind. The increase in the percentage of incorporation of non-wheat flour decreased its acceptability. Of 14 formulations, TQ13 with an incorporation of 20% quinoa flour as well as 3% wheat bran was found to be satisfactory from both sensory and nutritional points of view. The GI of quinoa bread fell under the category of low-GI foods. Therefore, the maximum possible incorporation of quinoa in wheat-based bread helped in maintaining blood glucose levels with a non-significant reduction. Blood lipid profile of the individuals, especially LDL and VLDL, reduced significantly. There was no change observed for the values of HDL. These benefits have proved to be crucial in the dietary treatment of diabetes mellitus by resulting in improved glycaemic control as well as several metabolic parameters, such as improved blood lipid levels. High consumption of phenolic compounds has long been associated with reduced risk of cardiovascular diseases and certain cancers. Current trends in the enhancement of the antioxidant capacity of wheat bread by the addition of quinoa flour rich in phenolic compounds might play a beneficial role in the health status of a population. The results obtained in the present study corroborate the data reported in the literature, indicating that quinoa bread can be used in plasma lipids and glycemic control.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Assam Agricultural University Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
NM: Conceptualization, Investigation, Writing – original draft. PD: Supervision, Writing – review & editing. MD: Writing – review & editing. LB: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research study was carried out at Assam Agricultural University, Jorhat, Assam, as a part of doctoral research.
The author is grateful to the Assam Agricultural University, Jorhat and the Central Agricultural University, Imphal, for making this research study possible. The author is also thankful to the study subjects who volunteered to be a part of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Oomah, BD, and Mazza, G. Flaxseed Products for Disease Prevention in Functional Foods–Biochemical and Processing Aspects. Technomic Publishing Company Inc., Pennyslvania, USA, pp. (1998); 91–99.
2. Hasler, MC. Functional foods: benefits, concerns and challenges - a position paper from the American council on science and health. J Nutr. (2002) 132:3772–81. doi: 10.1093/jn/132.12.3772
3. Kulkarni, S. Current Scenario of Lifestyle Disorders. Manual of Winter School. Department of Food Science and Nutrition, College of Rural Home Science, Dharwad, (2009); 1–7.
5. Walsh, MPT, and Herrington, D. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. (2008) 18:283–90. doi: 10.1016/j.numecd.2006.12.008
6. Flint, AJ, Hu, FB, Glynn, RJ, Jensen, MK, Franz, M, and Sampson, W. Whole grains and incident hypertension in men. Am J Clin Nutr. (2009) 90:493–8. doi: 10.3945/ajcn2009.27460
7. Lutsey, PL, Jacobs, DRJ, Kori, S, Mayer-Davis, E, Shea, S, and Steffen, LM. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: the MESA study. Br J Nutr. (2007) 98:397–405. doi: 10.1017/S0007114507700715
8. Gupta, M, Singh, BA, and Dutt, SA. Effect of barley flour blending on functional, baking and organoleptic characteristics of high-fiber rusks. J Food Proc Preserv. (2011) 35:46–63. doi: 10.1111/j.1745-4549.2009.00446
9. Sharma, P, and Gujral, HS. Cookie making behavior of wheat-barley flour blends and effects on antioxidant properties. Food Sci Technol. (2014) 55:301–7. doi: 10.1016/j.lwt.2013.08.019
10. Arshad, MU, Anjum, FM, and Zahoor, T. Nutritional assessment of cookies supplemented with defatted wheat germ. Food Chem. (2007) 102:123–8. doi: 10.1016/j.foodchem.2006.04.040
11. Flander, L, Salmenkallio-Marttila, M, Suortti, T, and Autio, K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT. (2007) 40:860–70. doi: 10.1016/j.lwt.2006.05.004
13. Skrbic, B, Milovac, S, Dodig, D, and Filipcev, B. Effects of hull-less barley flour and flakes on bread nutritional composition and sensory properties. Food Chem. (2009) 115:982–8. doi: 10.1016/j.foodchem.2009.01.028
14. Moza, J, and Gujral, HS. Influence of barley non-starchy polysaccharides on selected quality attributes of sponge cakes. Food Sci Technol. (2017) 85:252–61. doi: 10.1016/j.lwt.2017.07.024
15. Avila, BP, Braganca, GCM, Rockenbach, R, Alves, GD, Monks, J, Gularte, MA, et al. Physical and sensory characteristics of cake prepared with six whole-grain flours. J Food Measur Charact. (2017) 11:1486–92. doi: 10.1007/s11694-017-9527-0
16. Gorinstein, S, Vargas, OJM, Jaramillo, NO, Salas, IA, Ayala, ALM, and Arancibia-Avila, P. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur Food Res Technol. (2008) 225:321–8. doi: 10.1007/s00217-006-0417-7
17. Alvarez-Jubete, L, Arendt, EK, and Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci Techno. (2010) 21:106–13. doi: 10.1016/j.tifs.2009.10.014
18. Gimenez, A, Varela, P, Salvador, A, Ares, G, Fiszman, S, and Garitta, L. Shelf life estimation of brown pan bread: a consumer approach. Food Qual Prefer. (2007) 18:196–204. doi: 10.1016/j.foodqual.2005.09.017
19. Schoenlechner, R, Siebenhand, S, and Berghofer, E. Pseudocereals. Gluten-Free Cereal Products and Beverages. San Diego: Academic Press (2008). 149 p.
20. Koziol, MJ. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J Food Comp Anal. (1992) 5:35–68. doi: 10.1016/0889-1575(92)90006-6
21. Graf, BL, Silva, PR, Rojo, LE, Delatorre-Herrera, J, Manuel, EB, and Raskin, I. Innovations in health value and functional food development of quinoa (Chenopodium quinoa Willd.). Compr Rev Food Sci Food Saf. (2015) 14:431–45. doi: 10.1111/1541-4337.12135
22. Nasim, K, Asli, MY, Arab, M, Mirzaie, AA, and Mortazavian, AM. Fenugreek: potential applications as a functional food and neutraceutical. Nutrition and food sciences. Research. (2016) 3:5 Formulation of quinoa flour-incorporated multigrain breads. doi: 10.18869/acadpub.nfsr.3.1.5
23. Devani, BM, Jani, BL, Kapopara, MB, Vyas, DM, and Ningthoujam, MD. Study on quality of white bread enriched with finger millet flour. Int J Agric Envt Biotechnol. (2016) 9:903–8. doi: 10.5958/2230-732X.2016.00116.9
24. AACC. Approved Methods of American Association of Cereal Chemists. St. Paul, Minnesota: American Association of Cereal Chemists Inc. (2000).
25. Malik, H, Nayik, GA, and Dar, BN. Optimisation of process for development of nutritionally enriched multigrain bread. J. Food Process Technol. (2015) 7:1.
26. AOAC. Official Method of Analysis. 17th ed. Washington, DC: Association of Official Analytical Chemist (2000).
27. Codina, GG, Geanina, FS, and Todosi, ES. Studies on the influence of quinoa flour addition on bread quality. J Fac Food Eng. (2016) 15:165–74.
28. Gopalan, C, Ramasastri, BV, and Balasubramanian, SC. Nutritive Value of Indian Foods. National Institute of Nutrition. Hyderabad, India: ICMR (2015).
29. Zhishen, J, Mengcheng, T, and Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. (1999) 64:555–9. doi: 10.1016/S0308-8146(98)00102-2
30. Wolever, TM, Jenkins, DJ, Jenkins, AL, and Josse, RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. (1991) 54:846–54. doi: 10.1093/acjn/54.5.864
31. Wagner, JFFR, Flavio, MAM, Adman, CSL, Christina, RPD, Garcia, AAMP, and Coelho, DMM. Study of lipid profile in a population of university students. Rev Latino Am Enfermagem. (2013) 21:1151–8. doi: 10.1590/S0104-11692013000500019
32. Demin, MA, Vucelic-Radovic, BV, Banjac, NR, Tipsina, NN, and Milovanovic, MM. Buckwheat and quinoa seeds as supplements in wheat bread production. Hem Ind. (2013) 67:115–21. doi: 10.2298/HEMIND120314048D
33. Chauhan, GS, Eskin, AM, and Tkachuk, R. Nutrients and antinutrients in quinoa seeds. Cereal Chem. (1992) 69:85–8.
34. Gomez, M, Ronda, F, Blanco, CA, Caballero, PA, and Apesteguia, A. Effect of dietary fibre on dough rheology and bread quality. Eur Food Res Technol. (2003) 216:51–6. doi: 10.1007/s00217-002-0632-9
35. Wang, J, Rosell, CM, and Barber, BC. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. (2002) 79:221–6. doi: 10.1016/S0308-8146(20)00135-8
36. Nalawade, RS. (2015) Preparation of Bread by Incorporation of Soybean and Ragi Flour. MSc. Thesis. Post Graduate Institute Mahatma Phule Krishi Vidyapeeth, Rahuri, Ahmednagar, India
37. Sullivan, P, Flaherty, O, Brunton, N, Arendt, E, and Gallagher, E. Fundamental rheological and textural properties of doughs and breads produced from milled pearled barley flour. Eur Food Res Technol. (2010) 231:441–53. doi: 10.1007/s00217-010-1297-4
38. Cleary, LJ, Andersson, R, and Brennan, CS. The behaviour and susceptibility to degradation of high and low molecular weight barley b-glucan in wheat bread during baking and in vitro digestion. Food Chem. (2007) 102:889–97. doi: 10.1016/j.foodchem.2006.06.027
39. Feili, R, Wahidu, ZW, Nadiah, WA, and Tajul, AY. Physical and sensory analysis of high fiber bread incorporated with jackfruit rind flour. Food Sci Technol. (2013) 1:30–6. doi: 10.13189/fst.2013.010203
40. Skendi, A, Papagroriou, M, and Biliaderi, CG. Effect of barley ß-glucan molecular size and level on wheat dough reological properties. J Food Eng. (2009) 91:594–601. doi: 10.1016/j.jfoodeng.2008.10.009
41. Maneju, H, Udobi, CE, and Ndife, J. Effect of added brewers dry grain on the physico-chemical, microbial and sensory quality of wheat bread. Am J Food Nutr. (2011) 1:39–43. doi: 10.5251/ajfn.2011.1.1.39.43
42. Mildner-Szkudlarz, S, Zawirska-Wojtasiak, R, Szwengiel, A, and Pacynski, M. Use of grape by-product as a source of dietary fibre and phenolic compounds in sourdough mixed rye bread. Intl J Food Sci Technol. (2011) 46:1485–93. doi: 10.1111/j.1365-2621.2011.02643.x
43. Curti, E, Carini, E, Bonacini, G, Tribuzio, G, and Vittadini, E. Effect of the addition of bran fractions on bread properties. J Cereal Sci. (2013) 57:325–32. doi: 10.1016/j.jcs.2012.12.003
44. Bhavsar, GJ, Sawate, AR, Kshirsagar, RB, and Vijaykumar, CM. Studies on physico-chemical characteristics of buckwheat and its exploration in bread as functional food. Intl J Eng Res Technol. (2013) 2:3971–80.
45. Goksen, G, and Ibrahim, EH. Effect of Prunus mahaleb seed powder on dough rheology and bread quality. J Food Qual. (2016) 39:436–44. doi: 10.1111/jfg.12220
46. Abdelghafor, RF, Mustafa, AI, Ibrahim, AMH, and Krishnan, PG. Quality of bread from composite flour of sorghum and hard white winter wheat. J Food Sci Technol. (2011) 3:9–5.
47. Nasar-Abbas, SM, and Jayasena, V. Effect of lupin flour incorporation on the physical and sensory properties of muffins. Q Assur Saf Crops Foods. (2012) 4:41–9. doi: 10.1111/j.1757-837X.2011.00122.x
48. Chhavi, A, and Sarita, S. Evaluation of composite millet breads for sensory and nutritional qualities and glycemic response. Mal J Nut. (2012) 18:89–101.
49. Tuncel, NB, Yilmaz, N, Kocabiyik, H, and Uygur, A. The effect of infrared stabilized rice bran substitution on B vitamins, minerals and phytic acid content of pan breads: part II. J Cereal Sci. (2014) 59:162–6. doi: 10.1016/j.jcs.2013.12.005
50. Salazar, DM, Naranjo, M, Perez, LV, Valencia, AF, Acurio, LP, Gallegos, LM, et al. Development of newly enriched bread with quinoa flour and whey. IOP Conf Ser Earth Environ Sci. (2017) 77:012018–7. doi: 10.1088/1755-1315/77/1/012018
51. Lorenz, K, and Coulter, L. Quinoa flour in baked products. Plant Foods Hum Nutr. (1991) 41:213–23. doi: 10.1007/BF02196389
52. Wang, S, Opassathavorn, A, and Zhu, F. Influence of quinoa flour on quality characteristics of cookie, bread and chinese steamed bread: quinoa bakery products. J Texture Stud. (2015) 46:121–8.
53. Otegbayo, BO, Adebiyi, OM, Bolaji, OA, and Olunlade, BA. Effect of soy enrichment on bread quality. Intl Food Res J. (2018) 25:1120–5.
54. Ngozi, AA. Effect of whole wheat flour on the quality of wheatbaked bread. Glob J Food Sci Technol. (2014) 2:123–7.
55. Ameh, D, Gernah, B, and Igbabul, D. Physico-chemical and sensory evaluation of wheat bread supplemented with stabilized undefatted rice bran. Food Nutri Sci. (2013) 4:43–8. doi: 10.4236/fns.2013.49A2007
56. Rehman, S, Paterson, A, Hussain, S, Murtaza, MA, and Mehmood, S. Influence of partial substitution of wheat flour with vetch (Lathyrus sativus L.) flour on quality characteristics of doughnuts. Leben Smitted Wissen Chaffund Technol. (2007) 40:73–2. doi: 10.1016/j.lwt.2005.09.015
58. Ndife, J, Abdulraheem, LO, and Zakari, UM. Evaluation of the nutritional and sensory quality of functional breads produced from whole wheat and soya bean flour blends. Afr J Food Sci. (2011) 5:466–2.
59. Sharma, S, Sharma, N, Sharma, R, and Handa, S. Formulation of functional multigrain bread and evaluation of their health potential. Int J Curr Microbiol App Sci. (2018) 7:4120–6. doi: 10.20546/ijcmas.2018.707.480
60. Sanz-Penella, JM, Wronkowska, M, Soral-Smietana, M, and Haros, M. Effect of whole amaranth flour on bread properties and nutritive value. Food Sci Technol. (2013) 50:679–85. doi: 10.1016/j.lwt.2012.07.031
61. Wright, KH, Pike, OA, Fairbanks, DJ, and Huber, CS. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. J Food Sci. (2002) 67:1383–5. doi: 10.1111/j.1365-2621.2002.tb10294.x
62. Comai, S, Bertazzo, A, Bailoni, L, Zancato, M, Costa, CVL, and Allegri, G. The content of proteic and nonproteic (free and proteinbound) tryptophan in quinoa and cereal flours. Food Chem. (2007) 100:1350–5. doi: 10.1016/j.foodchem.2005.10.072
63. Kumar, KA, Kumar, GS, Khan, MA, and Semwal, AD. Optimization of multigrain premix for high protein and dietary fibre biscuits using response surface methodology (RSM). Food Nutr Sci. (2015) 6:747–6. doi: 10.4236/fns.2015.69077
64. Koletta, P, Irakli, M, Papageorgiou, MM, and Skendi, A. Physicochemical and technological properties of highly enriched wheat breads with wholegrain non wheat flours. J Cereal Sci. (2014) 60:561–8. doi: 10.1016/j.jcs.2014.08.003
65. Eke-Ejiofor, J, and Kiin-Kabari, DB. Effect of substitution on the functional properties of flour, proximate and sensory properties of wheat/plantain composite bread. Int J Agri Sci. (2012) 2:281–4.
66. Olaoye, OA, Onilude, AA, and Idowu, OA. Quality characteristics of bread produced from composite flours of wheat, plantain and soybeans. African. J Biotechnol. (2006) 5:1102–6.
67. Ambreen, N, Hanif, NQ, and Khatoon, S. Chemical composition of rice polishing from different sources. Pak Vet J. (2006) 26:190–2.
68. AAG, S. Use of corn and quinoa flour to produce bakery products for celiac disease. Adv Envt Biol. (2016) 10:237–4.
69. Lalit, H. Development and Organoleptic Evaluation of Bakery Products Formulated by Using Wheat Flour, Barley Flour and Germinated Fenugreek Seed Powder for Diabetics. MSc. Thesis (Food and Nutrition). College of Home Science, Punjab Agricultural University Ludhiana, India, vol. 1. (2017).
70. Atef, A, Zaid, A, El-Faham, WSY, and Emam, H. Use of quinoa meal to produce bakery products to celiac and autism stuffs. Int J Sci Res. (2012) 3:1344–4.
71. Bojnanska, T, Chlebo, P, Gazar, R, and Horna, A. Buckwheat-enriched bread production and its nutritional benefits. Eur J Plant Sci Biotechnol. (2009) 3:49–5.
72. Wronkowska, M, Troszynka, A, Soral-Smietana, M, and Wolejszo, A. Effects of buckwheat flour (Fagopyrum esculentum Moench) on the quality of gluten-free. Pol J Food Nutri Sci. (2008) 58:211–6.
73. Kumari, N. Development Sensory and Nutritional Evaluation of Value Added Bread and Muffins Incorporating Rice Bran. MSc. Thesis (Foods and Nutrition). CCS Haryana Agricultural University, Hisar, Haryana, India. (2015).
74. Young, J. Functional bakery products: current directions and future opportunities. Food Ind J. (2001) 5:71–8. doi: 10.4236/fns.2014.51010
75. Naikare, SM. Processing of Millets for Value Addition and Development of Health Foods. Ganeshkhind, Pune: NATP Report Submitted by NARP, (MPKV) (2003).
76. Alvarez-Jubete, L, Wijngaard, HH, Arendt, EK, and Gallagher, E. Polyphenol composition and in-vitro antioxidant activity of amaranth, quinoa and buckwheat as affected by sprouting and bread baking. Food Chem. (2010) 119:770–8. doi: 10.1016/j.foodchem.2009.07.032
77. Pandey, KB, and Rizvi, SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev. (2009) 2:270–8. doi: 10.4161/oxim.2.5.9498
78. Augustin, LS, Kendall, CW, Jenkins, DJ, Willett, WC, Astrup, A, Barclay, AW, et al. Glycemic index, glycemic load and glycemic response: an international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutr Metab Cardiovasc Dis. (2015) 25:795. doi: 10.1016/j.numecd.2015.05.005
79. De Carvalho, FG, Ovidio, PP, Padovan, GJ, Junior, AAJ, Marchini, JS, and Navarro, AM. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake – a prospective and double-blind study. Int J Food Sci Nutr. (2014) 65:380–5. doi: 10.3109/09637486.2013.866637
80. Lanzerstorfer, P, Rechenmacher, E, Lugmayr, O, Stadlbauer, V, Hoglinger, O, Vollmar, A, et al. Effects of various commercial whole-grain breads on postprandial blood glucose response and glycemic index in healthy subjects. Austin. J Clin Med. (2018) 5:1–6.
81. Patrizia, G, Sara, G, Carlo, LV, and Patrick, M. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. (2008) 87:1793–801. doi: 10.1093/ajcn/87.6.1793
82. Weickert, MO, and Pfeiffer, AFH. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. (2008) 138:439–42. doi: 10.1093/jn/138.3.439
83. Brennan, CS, and Cleary, LJ. The potential use of cereal (1/3,1/4)-b- D-glucans as functional food ingredients. J Cereal Sci. (2005) 42:1–3. doi: 10.1016/j.jcs.2005.01.002
84. Farinazzi-Machado, FMV, Barbalho, SM, Oshiiwa, M, Goulart, R, and Pessan, O. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Cienc Tecnol Aliment Campinas. (2012) 32:239–44. doi: 10.1590/S0101-20612012005000040
85. McCowen, KC, and Smith, RJ. Diabetes mellitus: classification and chemical. Encyclopedia Hum Nutr. (2005):543–1. doi: 10.1016/B0-12-226694-3/00078-8
86. Jain, S, Malvi, R, and Purviya, KJ. Concept of self-medication: a review. Int J Pharm Biol Arch. (2011) 2:831–6.
87. Camilleri, M, Van Outryve, MJ, Beyens, G, Kerstens, R, Robinson, P, and Vandeplassche, L. Clinical trial: the efficacy of open-label prucalopride treatment in patients with chronic constipation – follow-up of patients from the pivotal studies. Aliment Pharmacol Ther. (2010) 32:1113–23. doi: 10.1111/j.1365-2036.2010.04455.x
88. Paiva, AP. Estudos Tecnologico, Quimico, Fisico-Quimico e Sensorial de Barras Alimenticias Elaboradas com Subprodutos e Residuos Agroindustriais. Dissertacao (Mestrado em Ciencia dos Alimentos)-Universidade Federal de Lavras Lavras. (2008).
89. Berti, C, Riso, P, Brusamolino, A, and Marisa Porrini, M. Effect on appetite control of minor cereal and pseudocereal products. Br J Nutr. (2005) 94:850–8. doi: 10.1079/BJN20051563
90. Ruales, J, and Nair, BM. Nutritional quality of the protein in quinoa (Chenopodium quinoa Willd.) seeds. Plant Food Hum Nutr. (1992) 42:1–11. doi: 10.1007/BF02196067
91. Kwon, DY, Kim, YS, Hong, SM, and Park, S. Long-term consumption of saponins derived from Platycodi radix (22 years old) enhances hepatic insulin sensitivity and glucose-stimulated insulin secretion in 90% pancreatectomized diabetic rats fed a high-fat diet. Br J Nutr. (2008) 101:358–66. doi: 10.1017/S000711450801218X
92. Zhang, L, Lucas, T, Doursat, C, Flick, D, and Wagner, M. Effects of crust constraints on bread expansion and CO2 release. J Food Eng. (2007) 80:1302–11. doi: 10.1016/j.jfoodeng.2006.10.008
93. Cicero, AF. Different effect of psyllium and guar dietary supplementation on blood pressure control in hypertensive overweight patients: a six-month, randomized clinical trial. Clin Expt Hyperten. (2007) 29:383–94. doi: 10.1080/10641960701578378
94. Abugoch, JLE. Quinoa (Chenopodium quinoa Willd.) composition, chemistry, nutritional, and functional properties. Adv Food Nutr Res. (2009) 58:1–31. doi: 10.1016/S1043-4526(09)58001-1
95. Liu, S, Manson, JE, Stampfer, MJ, Hu, FB, Giovannucci, E, and Colditz, GA. Perspective study of whole-grain intake and risk of type of type-2 diabetes mellitus in US women. Am J Public Health. (2000) 90:1409–15. doi: 10.2105/ajph.90.9.1409
Keywords: multigrain bread, phenolics, flavonoids, antioxidant, quinoa
Citation: Marak NR, Das P, Das Purkayastha M and Baruah LD (2024) Effect of quinoa (Chenopodium quinoa W.) flour supplementation in breads on the lipid profile and glycemic index: an in vivo study. Front. Nutr. 11:1341539. doi: 10.3389/fnut.2024.1341539
Received: 20 November 2023; Accepted: 01 March 2024;
Published: 26 March 2024.
Edited by:
José M. Alvarez-Suarez, Universidad San Francisco de Quito, EcuadorReviewed by:
Beatriz Andrea Acosta-Estrada, Monterrey Institute of Technology and Higher Education (ITESM), MexicoCopyright © 2024 Marak, Das, Das Purkayastha and Baruah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natasha R. Marak, bmF0YXNoYS5tYXJha0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.