- 1Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy
- 2Department of Pediatric, Buzzi Children's Hospital, Milan, Italy
- 3Department of Health Sciences, University of Milan, Milan, Italy
- 4Department of Health Sciences, University of Florence, Florence, Italy
- 5Meyer Children's Hospital Scientific Institute for Research, Hospitalization and Health Care (IRCCS), Florence, Italy
- 6Department of Biomedical and Clinical Science, University of Milan, Milan, Italy
Nutrition is emerging as a pivotal environmental factor shaping both the endocrinological landscape and reproductive capabilities (1–3).
Through their role as precursors to crucial molecules involved in bodily reactions, nutrients wield significant influence over physiological processes and biochemical pathways, including those governing hormones (4). Maintaining hormonal balance is crucial for sustaining reproductive functions and fertility.
Thus, the quantity, quality, and composition of foods wield substantial influence over our health span, particularly impacting endocrine and reproductive functions from intrauterine development through adolescence (5, 6).
In this opinion paper, we aim to underscore the profound interplay between food and hormones and its ramifications on endocrine and reproductive health, a relationship evident from fetal inception to adulthood. Sharing viewpoints fosters discourse and prompts reflection on strategies to safeguard reproductive wellbeing.
Nutrition during the periconceptional period and pregnancy emerges as a critical determinant for fetal development, growth, and overall wellbeing. Inadequate nutrition can disrupt the normal development and growth of organs, including the hypothalamic-pituitary-gonadal axis (HPA-axis), thereby potentially affecting endocrine, reproductive health, and fertility in adulthood (7).
The reproductive capability is influenced by maternal nutritional status through various pathways.
Firstly, nutrition during gestation can influence the development of the fetal endocrine and reproductive systems (8). The transportation of nutrients and metabolic processes within the placenta play a vital role in determining fetal weight, which represents a crucial factor in the programming of endocrine systems during critical phases of fetal development (6). As reported in the literature (6), restricted prenatal growth may lead to permanent alterations in endocrine axes, subsequently affecting sexual maturation and reproductive function. A deficiency in key nutrients during this critical period may lead to structural and functional changes in organs, including the hypothalamus, pituitary gland, thyroid and gonads; these changes can affect numerous aspects of growth and development, potentially causing hormonal imbalances and infertility (7). Additionally, the theory of “metabolic programming” suggests that specific nutritional conditions during fetal development can influence metabolism and health in adulthood, increasing the risk of developing metabolic diseases like type 2 diabetes and obesity (9), which, in turn, can affect endocrinological balance and fertility (Figure 1).
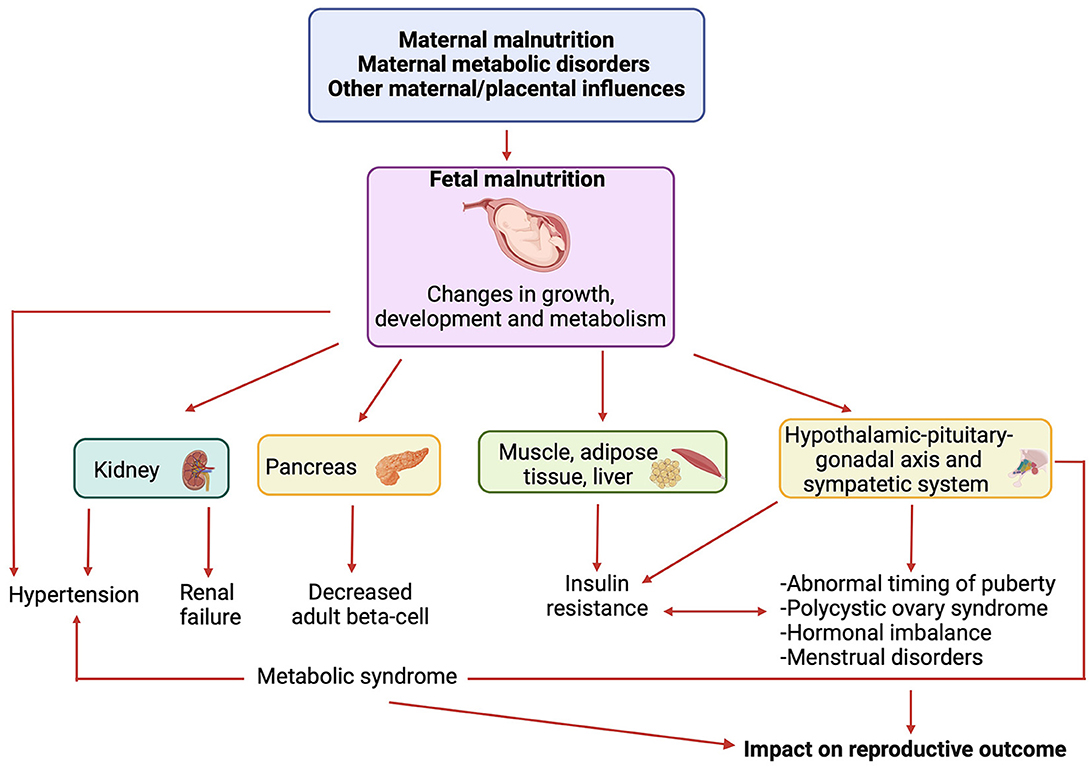
Figure 1. Link between nutritional conditions during fetal development, metabolic diseases and reproductive outcome. Created by Biorender.com.
In addition, nutrition profoundly impacts the quality of oocytes, with deficiencies in antioxidants, vitamins, and minerals during pregnancy potentially leading to transgenerational consequences (2, 10).
Reproductive outcomes are also influenced by maternal metabolic status during pregnancy, with gestational diabetes mellitus (GDM) associated not only with adverse fetal outcomes, such as death from birth, visceromegaly, and fetal macrosomia but also a heightened risk of metabolic disorders in children (11), such as hyperinsulinism and type 2 diabetes. These disorders may impact the timing of pubertal development and conditions such as polycystic ovary syndrome (PCOS), thereby influencing women's reproductive health (12) (Figure 1).
Childhood and adolescence are critical periods for protecting endocrine and reproductive health, with nutrition playing a significant role in all stages of growth, particularly during pubertal development (6).
Puberty represents a crucial phrase of physical maturation, marked by the activation of the hypothalamic-pituitary-gonadal (HPG) axis and the release of hormones like gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Both genetic and environmental factors are involved in the activation and maintenance of HPG integrity. Adequate nutrition is essential for the initiation and progression of puberty, as nutrients serve as cofactors, precursors, and regulators in the synthesis and function of reproductive hormones, such as gonadotropin and sex hormones such as estrogen, progesterone, and testosterone. Thus, proper nutrition is linked to consistent hormonal production, and consequently, plays a crucial role in the development of secondary sexual characteristics and menstrual cycles (6).
The onset of puberty requires a state of positive energy balance, with chronic malnutrition potentially delaying puberty (13) and overnutrition and obesity which can be associated with increased estrogen production and leptin levels, contributing to early puberty (14).
Different nutritional choices have been associated with distinct patterns of puberty, with diets high in energy, fat, and protein, and a high glycemic index linked to unbalanced micronutrient supplies involved in hormonal stimulation, leading to precocious puberty (15); proposed pathogenic mechanisms implicated in this early activation include the activation of GnRH via hypothalamic inflammation and microglial cell activation, dietary signals affecting the hypothalamus, alterations in gut microbiota influencing hormone secretion, and the overexpression of transcription factors (15).
Disruptions in normal pubertal development may have long term health effects including menstrual irregularities and infertility in adulthood (16). Early pubertal maturation, such as premature adrenarche and premature pubarche, represents childhood risk factors for PCOS, impacting reproductive function (12).
Nutritional imbalance and dietary patterns can disrupt the normal development and functioning of the reproductive system, affecting the regularity of ovulation and the quality of oocytes released during the menstrual cycle. Diets rich in fish and seafood, vegetables and fruit, cereals, and low-fat dairy products are positively correlated with the quality of ovulation (2). On the contrary, diets rich in processed meats, soy, potatoes, full-fat dairy products, sugary drinks, and sweets seem to negatively impact endocrine and reproductive health (2, 17).
Obesity, in particular, poses significant challenges to reproductive function, with obese adolescents and women at increased risk of menstrual dysfunction, anovulation, PCOS, subfecundity, and infertility (14, 18). Female obesity disrupts the HPG axis, leading to hormonal imbalances that impair reproductive control (14).
This arises from heightened peripheral conversion of androgens to estrogens, hyperandrogenism stemming from insulin resistance and hyperinsulinemia, thyroid irregularities, reduced levels of sex hormone-binding globulin, growth hormone, and insulin-like growth factor binding proteins, alongside elevated leptin levels. These collective hormonal disturbances contribute to impaired regulation of the reproductive system (14). Specifically, hyperandrogenism and insulin resistance are key factors in the etiology of PCOS (19), which often emerges during the early puberty and affects pregnancy outcomes, such as an increased risk of gestational diabetes mellitus (GDM), pregnancy-induced hypertension, and preeclampsia (19).
Chronic inflammation and oxidative stress associated with overnutrition can further negatively impact fertility (20, 21). Additionally, metabolic disorders linked to obesity, such as insulin resistance and hyperandrogenism, can disrupt hormonal balance and contribute to conditions like PCOS, thus impacting fertility (14).
Similar to obesity, underweight individuals may also face challenges associated with the regulation of the endocrine system, including the HPG- and growth hormone—insulin-like growth factor (IGF)-1 axis, thyroid and adrenal functions. These factors can influence pubertal development, sexual characteristics, and fertility (22, 23).
In conclusion, the intricate relationship between nutrition and hormonal balance profoundly impacts female endocrine and reproductive health. From fetal development onward, nutritional, and metabolic factors significantly influence reproductive outcomes. Recognizing nutrition as a modifiable factor in preventing adverse reproductive outcomes is crucial. A balanced diet rich in essential nutrients plays a pivotal role in maintaining hormonal equilibrium, preventing reproductive disorders, and safeguarding fertility. Embracing a holistic approach that integrates nutrition with healthy lifestyle practices is instrumental in promoting the reproductive wellbeing of adolescent girls.
Author contributions
VC: Conceptualization, Supervision, Writing—original draft, Writing—review & editing. EV: Conceptualization, Supervision, Writing—original draft, Writing—review & editing. SS: Conceptualization, Supervision, Writing—original draft, Writing—review & editing. GZ: Conceptualization, Supervision, Writing—original draft, Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organisation. Available online at: https://www.who.int/
2. Silvestris E, Lovero D, Palmirotta R. Nutrition and female fertility: an interdependent correlation. Front Endocrinol. (2019) 10:346. doi: 10.3389/fendo.2019.00346
3. Hally SS. Nutrition in reproductive health. J Nurse Midwifery. (1998) 43:459–70. doi: 10.1016/S0091-2182(98)00056-1
4. Pang G, Xie J, Chen Q, Hu Z. Energy intake, metabolic homeostasis, and human health. Food Sci Hum Wellness. (2014) 3:89–103 doi: 10.1016/j.fshw.2015.01.001
5. Wickramasinghe K, Mathers JC, Wopereis S, Marsman DS, Griffiths JC. From lifespan to healthspan: the role of nutrition in healthy ageing. J Nutr Sci. (2020) 9:e33. doi: 10.1017/jns.2020.26
6. Calcaterra V, Cena H, Regalbuto C, Vinci F, Porri D, Verduci E, et al. The role of fetal, infant, and childhood nutrition in the timing of sexual maturation. Nutrients. (2021) 13:419. doi: 10.3390/nu13020419
7. Montagnoli C, Santoro CB, Buzzi T, Bortolus R. Maternal periconceptional nutrition matters. A scoping review of the current literature. J Matern Fetal Neonatal Med. (2022) 35:8123–40. doi: 10.1080/14767058.2021.1962843
8. Che L, Yang Z, Xu M, Xu S, Che L, Lin Y, et al. Maternal nutrition modulates fetal development by inducing placental efficiency changes in gilts. BMC Genomics. (2017) 18:213. doi: 10.1186/s12864-017-3601-1
9. Hoffman DJ, Powell TL, Barrett ES, Hardy DB. Developmental origins of metabolic diseases. Physiol Rev. (2021) 101:739–95. doi: 10.1152/physrev.00002.2020
10. Ashworth CJ, Toma LM, Hunter MG. Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Philos Trans R Soc Lond B Biol Sci. (2009) 364:3351–61. doi: 10.1098/rstb.2009.0184
11. Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomed Pharmacother. (2021) 143:112183. doi: 10.1016/j.biopha.2021.112183
12. Rosenfield RL. Clinical review: identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. (2007) 92:787–96. doi: 10.1210/jc.2006-2012
13. Soliman A, De Sanctis V, Elalaily R. Nutrition and pubertal development. Indian J Endocrinol Metab. (2014) 18:S39–47. doi: 10.4103/2230-8210.145073
14. Bauman D. Impact of obesity on female puberty and pubertal disorders. Best Pract Res Clin Obstet Gynaecol. (2023) 91:102400. doi: 10.1016/j.bpobgyn.2023.102400
15. Calcaterra V, Magenes VC, Hruby C, Siccardo F, Mari A, Cordaro E, et al. Links between childhood obesity, high-fat diet, and central precocious puberty. Children. (2023) 10:241. doi: 10.3390/children10020241
16. Hill JW, Alreja M, Elias CF. From precocious puberty to infertility: metabolic control of the reproductive function. Front Endocrinol. (2013) 4:43. doi: 10.3389/fendo.2013.00043
17. Aoun A, Khoury VE, Malakieh R. Can nutrition help in the treatment of infertility? Prev Nutr Food Sci. (2021) 26:109–20. doi: 10.3746/pnf.2021.26.2.109
18. Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Women's Health. (2008) 4:183–94. doi: 10.2217/17455057.4.2.183
19. Choudhury AA, Rajeswari VD. Polycystic ovary syndrome (PCOS) increases the risk of subsequent gestational diabetes mellitus (GDM): a novel therapeutic perspective. Life Sci. (2022) 310:121069. doi: 10.1016/j.lfs.2022.121069
20. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. (2012) 10:49. doi: 10.1186/1477-7827-10-49
21. Zavatta A, Parisi F, Mandò C, Scaccabarozzi C, Savasi VM, Cetin I. Role of inflammaging on the reproductive function and pregnancy. Clin Rev Allergy Immunol. (2023) 64:145–60. doi: 10.1007/s12016-021-08907-9
22. Fricke C, Voderholzer U. Endocrinology of underweight and anorexia nervosa. Nutrients. (2023) 15:3509. doi: 10.3390/nu15163509
Keywords: nutrition, hormones, reproductive health, endocrine system, fertility, adolescents
Citation: Calcaterra V, Verduci E, Stagi S and Zuccotti G (2024) How the intricate relationship between nutrition and hormonal equilibrium significantly influences endocrine and reproductive health in adolescent girls. Front. Nutr. 11:1337328. doi: 10.3389/fnut.2024.1337328
Received: 12 November 2023; Accepted: 05 March 2024;
Published: 14 March 2024.
Edited by:
Demin Cai, Yangzhou University, ChinaCopyright © 2024 Calcaterra, Verduci, Stagi and Zuccotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Calcaterra, dmFsZXJpYS5jYWxjYXRlcnJhQHVuaXB2Lml0
 Valeria Calcaterra
Valeria Calcaterra Elvira Verduci
Elvira Verduci Stefano Stagi
Stefano Stagi Gianvincenzo Zuccotti
Gianvincenzo Zuccotti