- 1Second Clinical Medical College, Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 2Department of Rheumatology and Immunology, The Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, China
Objective: The causal relationship between saturated fatty acids (SFAs) and rheumatoid arthritis (RA) remains poorly understood. This study aimed to determine whether SFAs are causally related to RA using Mendelian randomisation (MR) analyses.
Methods: Genome-wide association study (GWAS) summary data for RA (ukb-d-M13_RHEUMA) and SFAs (met-d-SFA) were obtained from the Integrative Epidemiology Unit OpenGWAS database. A bidirectional MR analysis was performed using a suite of algorithms, namely the MR-Egger, weighted median, simple mode, weighted mode, and inverse-variance weighted (IVW) algorithms, all integrated using the “MR” function. The robustness of the MR findings was further evaluated through sensitivity analyses, including heterogeneity, horizontal pleiotropy, and leave-one-out tests.
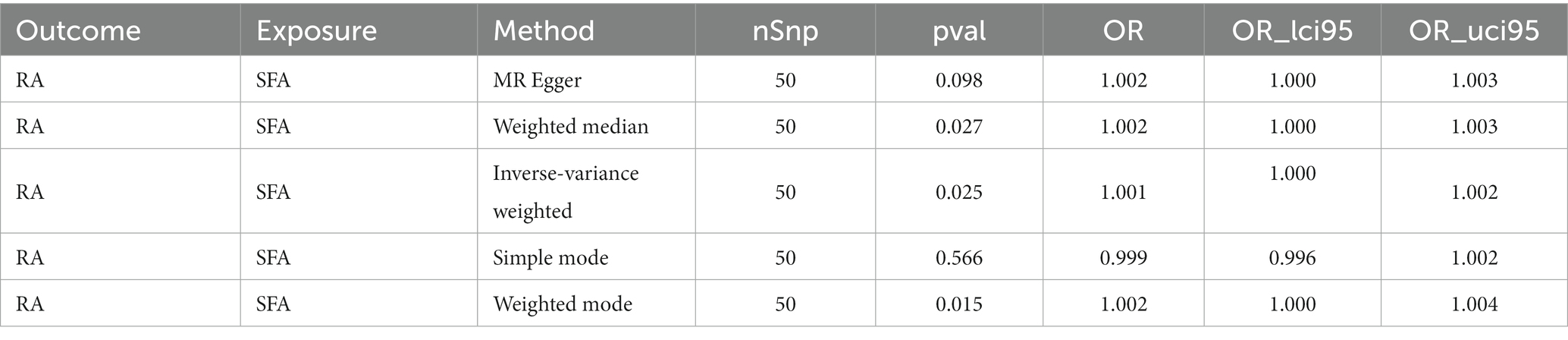
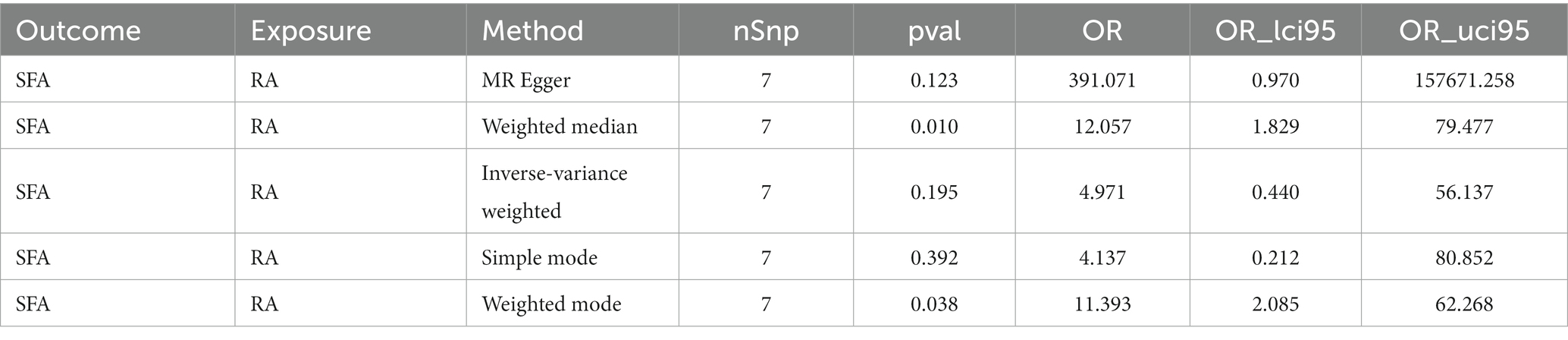
Results: The IVW algorithm in the forward MR analysis indicated a causal link between SFAs and RA (p = 0.025), identifying SFAs as a risk factor for RA (odds ratio = 1.001). Sensitivity analyses indicated no significant heterogeneity, horizontal pleiotropy, or severe bias, reinforcing the credibility of the forward MR results. However, the reverse MR analysis revealed that RA does not causally affect SFA levels (p = 0.195), and this finding was supported by corresponding sensitivity analyses.
Conclusion: The findings of this study substantiate the positive causal effect of SFAs on the incidence of RA through bidirectional MR analysis, thereby offering a consequential direction for future research on the diagnosis and treatment of RA.
Highlights
• The causal relationship between SFAs and RA was evaluated in a two-sample bidirectional MR analysis.
• SFA was causally related to RA as a risk factor, with an increase in SFA levels leading to an elevated risk of RA.
• RA was found to have no causal effect on SFA.
1 Introduction
Rheumatoid arthritis (RA) is a complex chronic autoimmune disorder with an uncertain aetiology and is characterised by persistent inflammation resulting in synovitis, pannus formation, and gradual degeneration of articular cartilage and bone. Symptoms typically include joint swelling, deformity, and muscle atrophy, leading to functional impairment (1, 2). The 2017 Global Burden of Disease study indicated rising age-standardised prevalence and incidence rates of RA from 1990 to 2017 (3). The lack of targeted therapeutic solutions for RA necessitates a deeper understanding of its aetiological factors to develop strategies for slowing its progression.
Various factors have been implicated in RA pathogenesis, such as genetic predisposition, environmental exposures, metabolic disturbances, autoimmune responses, and microbial influences (4–11). Fatty acids (FAs), which are ubiquitous in human tissues, play vital roles in tissue homeostasis, immune function modulation, and metabolic pathways (12, 13). Whilst the role of unsaturated fatty acids in RA has been the focus of recent research, the impact of saturated fatty acids (SFAs) remains relatively unexplored (14, 15). SFAs that lack double bonds in their carbon chains are essential lipid components (16). A recent observational study suggested that excessive SFA intake might trigger inflammation and muscle degradation in patients with RA, possibly leading to sarcopenia and inflammatory processes (17). The American College of Rheumatology dietary guidelines for RA recommend a Mediterranean diet with limited SFA intake (18). Nevertheless, given the extant controversies and inherent biases in observational research methodologies, it is imperative to rigorously assess the causative implications of SFAs for RA.
Observational studies have found an association between SFAs and RA. However, neither the direction nor the cause–effect chain is clear. By leveraging inherent genetic variations, Mendelian randomisation (MR) serves as a robust statistical approach to assess causality between SFA and RA (19, 20). MR is superior to traditional observational methods in controlling confounders and minimising potential biases from confounding and reverse causation, and it can thus provide more reliable evidence for establishing causality between SFA and RA (21). This study aimed to investigate the causal relationship between SFA and RA through a two-sample bidirectional MR analysis, contributing to future aetiological research on RA.
2 Materials and methods
2.1 Data sources and summary
The genome-wide association study (GWAS) summary data on RA and SFAs were retrieved from the Integrative Epidemiology Unit OpenGWAS database.1 The data on RA (ukb-d-M13_RHEUMA) were obtained from 1,605 cases and 359,589 controls and covered 10,079,899 single-nucleotide polymorphisms (SNPs). This dataset was sourced using the keyword “Rheumatoid arthritis” and selected from the UK Biobank database2 results. For SFAs (met-d-SFA), the sample and SNP counts were 114,999 and 12,321,875, respectively.
2.2 GWAS data pre-processing
The “extract_instruments” function of the R package “TwoSampleMR” was adopted to read the data on exposure factors and filter the instrumental variables (IVs) (22). SNPs significantly associated with exposure factors were selected as IVs (p < 5 × 10−8), and those in linkage disequilibrium were excluded (clump = TRUE, r2 = 0.001, kb = 10,000). Each SNP was also cross-referenced with the PhenoScanner GWAS database to check for associations with vitamin D, arthrosis, and pain as potential confounding factors. These SNPs were not associated with the confounding factors of RA. Simultaneously, the SNPs that were markedly related to the outcome, identified based on GWAS data on the outcome, were also removed. In the forward MR analysis, the exposure factor was SFA, and the outcome was RA. In the reverse MR analysis, SFAs and RA were interchanged as the outcome and exposure factors, respectively. The F-statistics for each genetic instrument were assessed to ensure method reliability. An F-value of >10 indicated a low likelihood of bias due to weak instruments.
2.3 Bidirectional MR analysis
The MR analysis was based on three core assumptions for the IVs: (1) genetic variation is associated with risk factors; (2) genetic variation is not associated with confounding factors; and (3) genetic variation affects the outcome solely through risk factors (23). First, the “harmonise_data” function was employed to harmonise the effect equipotential with the effect size. The “MR” function of the R package “TwoSampleMR” and five algorithms, namely MR-Egger, weighted median, inverse-variance weighted (IVW), simple mode, and weighted mode, were used to perform the bidirectional MR analysis. The MR results primarily relied on the IVW algorithm due to its high statistical efficiency and unbiased nature. The results were presented through scatter plots, forest plots, and a funnel plot. Finally, sensitivity analyses, including heterogeneity, horizontal pleiotropy, and leave-one-out (LOO) sensitivity tests, were conducted to ascertain the reliability of the MR findings (Supplementary Figure S1).
3 Results
3.1 Positive causal relationships between SFAs and the onset of RA
Following filtration, 52 independent SNPs of SFAs were identified as IVs (Supplementary Table S1). The forward MR results are presented in Table 1. A causal relationship was established between SFAs and RA (p = 0.025), with SFAs identified as a risk factor for RA (odds ratio [OR] = 1.001) according to the IVW method. The results of two other methods, weighted median and weighted mode, also supported this result (p < 0.05, OR > 1). The scatter plot indicated a minimal impact of confounding factors on the credibility of the forward MR analysis due to a negligible intercept, suggesting SFAs as a risk factor for RA with a positive slope based on the IVW method (Figure 1A). A forest plot was created to evaluate the diagnostic efficiency of each SNP of SFA for RA. Whilst the effects of individual SNPs of SFAs on RA were not significant, their collective impact in the IVW model was considerable, implying that SFAs are a potential risk factor for RA (Figure 1B). In addition, the forward MR analysis of the causal effect of SFAs on RA aligned with Mendel’s second law of random grouping (Figure 1C).
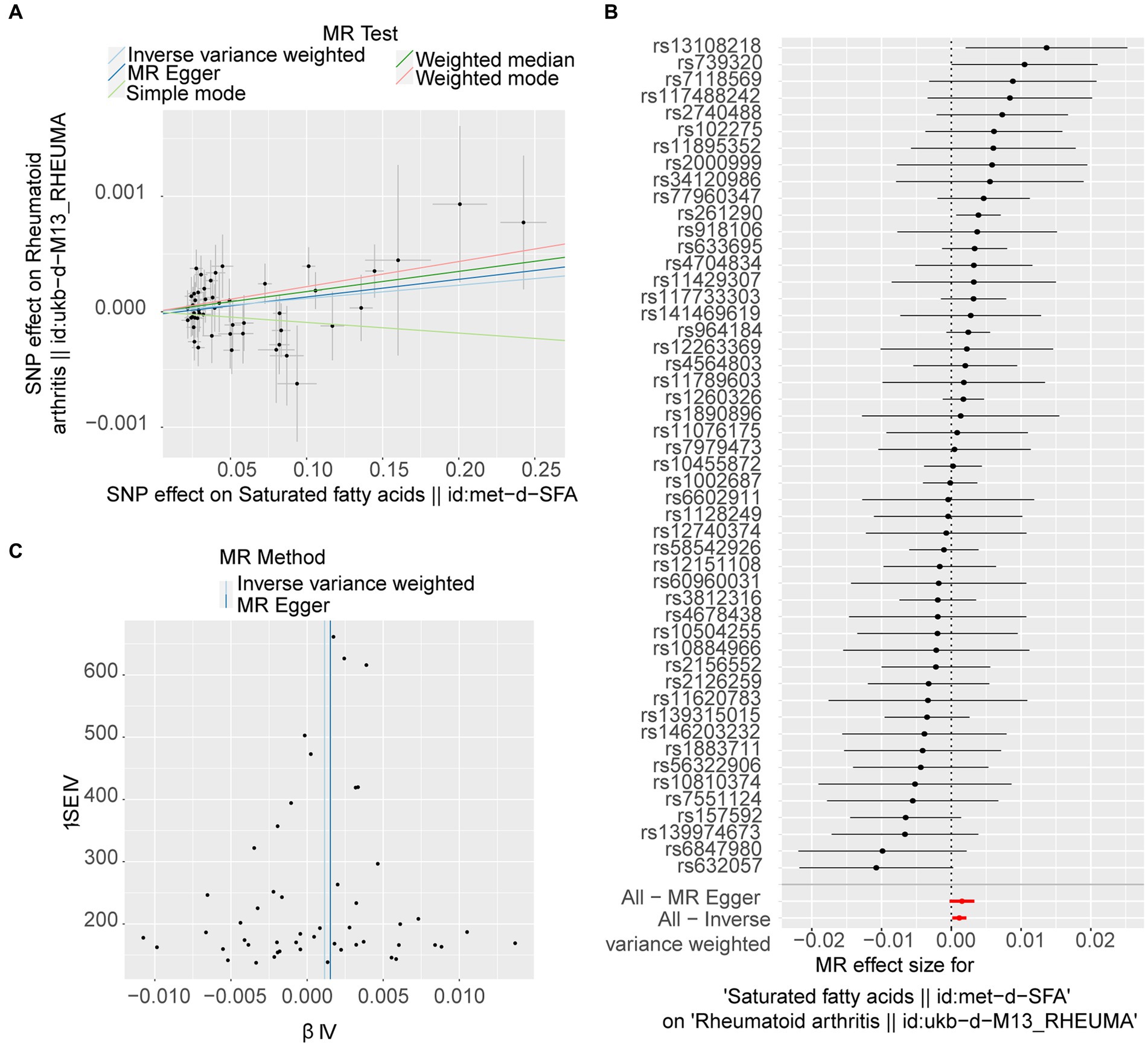
Figure 1. Forward Mendelian randomisation (MR) analysis of the causal effect of saturated fatty acids (SFAs) on rheumatoid arthritis (RA) occurrence. (A) The scatter plot of MR analysis. The X-axes show the SNP-exposure effect, and the Y-axes show the SNP-outcome effect. The positive slope reflects a positive causal effect of SFAs on RA. (B) Forest map of MR analysis, combining a Wald ratio method for each SNP effect (horizontal black solid line) and an inverse-variance weighted (IVW) method for fixed-effects (horizontal red solid line). The solid line completely on the right side of 0 indicates positive links between SFAs and the risk of RA. (C) Funnel plot of MR analysis. The SNPs are symmetrically distributed along both sides of the IVW line, indicating MR links to Mendel’s second law.
3.2 Sensitivity analysis illustrated the reliability of the forward MR results
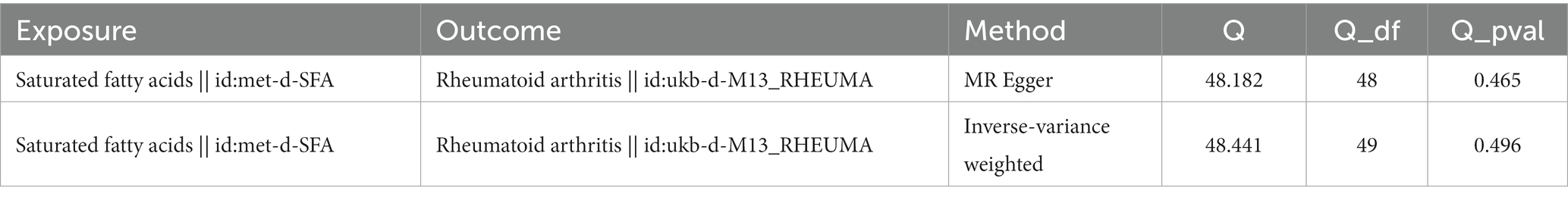
The sensitivity analysis further confirmed the reliability of the forward MR findings. There was no significant heterogeneity (Q_pval = 0.496) (Table 2) and no evidence of horizontal pleiotropy (p = 0.613) in relation to SFAs (Table 3). The LOO method was used to evaluate the influence of each SNP on the MR result, and the results revealed no instances of severe bias (Figure 2). Thus, SFAs were affirmed as a risk factor for the onset of RA with verified dependability.
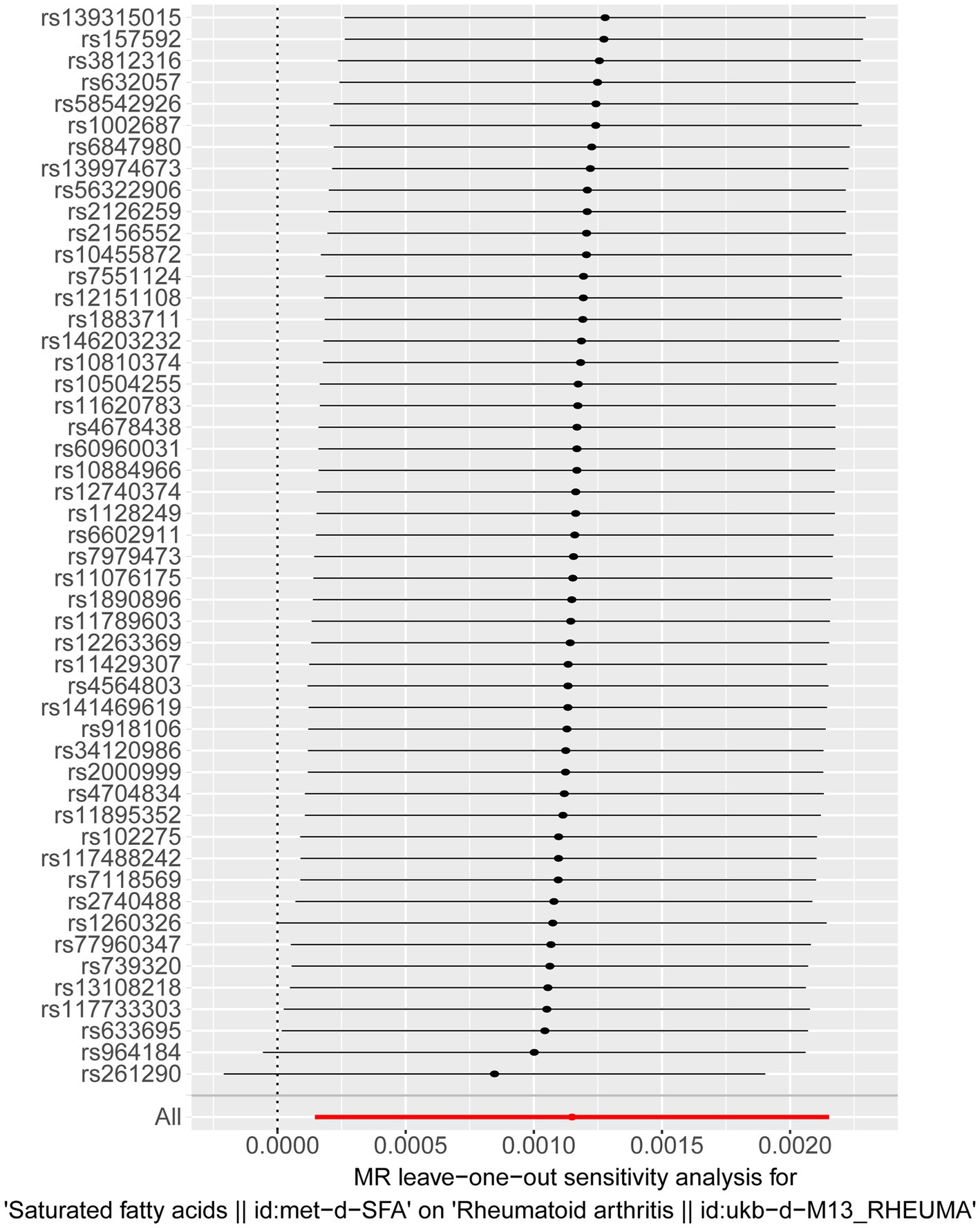
Figure 2. Results of a “leave-one-out” sensitivity analysis performed for SFAs on RA in the forward MR analysis. Calculate the MR results of the remaining SNPs after removing them one by one.
3.3 No causal effect of RA on SFA
For the reverse MR analysis, seven independent SNPs were selected as IVs following filtration (Supplementary Table S2). The reverse MR results are presented in Table 4. The p-values for SFAs as an outcome were greater than 0.05, indicating no statistical significance (p = 0.195). This suggests the absence of a causal relationship between RA as the exposure factor and SFAs as the outcome. Although heterogeneity existed (Q_pval = 0.035), it did not impact the IVW results, meaning that the conclusion of the reverse MR analysis was reliable. Additionally, no horizontal pleiotropy (p = 0.201) or points of severe bias were detected (Figure 3). In summary, no causal effect of RA on SFAs was found.
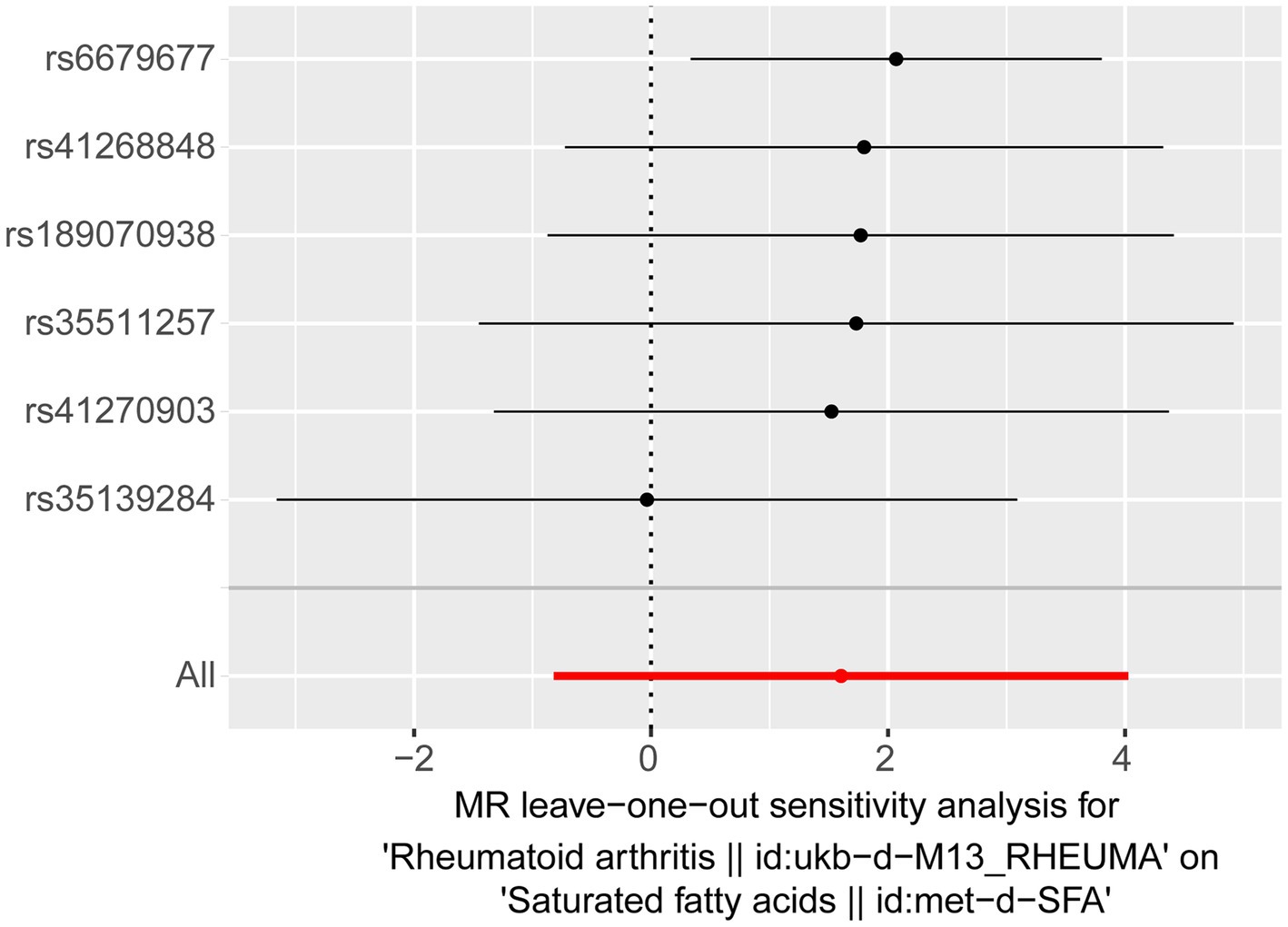
Figure 3. Results of a “leave-one-out” sensitivity analysis performed for RA on SFAs in the reverse MR analysis.
4 Discussion
The exact aetiology and underlying pathogenic mechanisms of RA are not fully understood. Dietary components, as potential environmental factors, might influence early autoimmune responses in individuals who are genetically susceptible to RA. These effects are mediated by regulating the intestinal microbiota composition and function, altering gut permeability, and inducing immunomodulation (24, 25). Beyond conventional pharmacological approaches, dietary intervention is emerging as a promising complementary therapy for RA management. This study evaluated GWAS data in a bidirectional two-sample MR framework, providing solid genetic evidence of a causal link between SFAs and the risk of RA. Importantly, our analysis confirmed a positive causal association between these factors.
The current body of research supports the link between SFAs and RA. Serum or plasma is often used to study the relationship between fatty acids and health or disease states (26). Analysing serum or plasma can reveal the fatty acid status, which can serve as an indicator of health status and disease risk in a clinical setting (27). Notably, elevated levels of total SFAs and hexadecanoic acid (C16:0) have been observed in the plasma/serum and synovial fluid of patients with RA relative to controls (28–30). A comprehensive cross-sectional analysis from the National Health and Nutrition Examination Survey, including 16,530 participants and 1,053 patients with RA, highlighted a significant correlation between C16:0 and an elevated risk of RA, along with increased levels of hypersensitive C-reactive protein (31). Similarly, a prospective cohort study by Sebe et al. focussing on 53 female patients with RA showed a strong association between SFA intake and a greater than 5% decrease in the skeletal muscle index over 1 year; similar results were also observed in murine RA models (17).
However, the relationship between SFAs and RA continues to be a topic of debate. Using gas chromatography–mass spectrometry, Yao et al. found that lower levels of acetic and propionic acids in patients with RA were correlated with a higher percentage of regulatory B cells in peripheral blood, suggesting an immunomodulatory effect via free fatty acid receptor 2 (32). Additionally, the microbiota-derived short-chain fatty acid (SCFA) butyrate is believed to induce the differentiation of B cells towards a regulatory phenotype, which may in turn reduce arthritis severity through increasing the serotonin-derived metabolite 5-hydroxyindole-3-acetic acid (33, 34). Because of the inconclusive nature of these observational studies, which are often affected by factors such as age, drug usage, and lifestyle, this study utilised MR as an IV to establish a causal link between SFA exposure and RA outcome. The inherent random distribution of genetic variations helps to minimise confounding factors and reverse causality, thereby reinforcing the causal evidence (20, 35). Further sensitivity analyses were conducted using various methods to eliminate bias from both correlated and uncorrelated pleiotropy. Consequently, this study provides preliminary evidence of a causal relationship between SFA and RA, enhancing our understanding of the aetiological and risk factors involved in RA progression.
The specific biochemical pathways through which SFAs increase the risk of RA are not fully understood, although current studies suggest a link between inflammation and gut dysbiosis (12, 36, 37). Diets high in SFAs are shown to promote T cell activation and their differentiation towards Th1 and Th17 cells (38, 39). Concurrently, elevated expression of inflammatory mediators such as C-X-C motif chemokine receptor 3 intensifies the inflammatory response (38, 39). Zhou et al. revealed that hexadecanoic acid (C16:0) activates the STAT5-PI3K/Akt signalling pathway in T cells, leading to a marked upregulation of signal lymphocyte-activating molecule family member 3 and proinflammatory cytokines such as tumour necrosis factor-α, interleukin (IL)-1β, IL − 2, and IL-6 (40). Moreover, SCFAs, the primary metabolic products of gut microorganisms, influence gut microbiota composition, alter intestinal microbial metabolites, maintain intestinal mucosal integrity, and regulate immune homeostasis (41). For example, Prevotella histicola in the gut has been shown to delay arthritis onset in murine models by increasing the expression of butyrate and normalising the composition of the gut microbiota (42). Additionally, SFAs in the intestine have been implicated in promoting IL-22 production by inhibiting G protein receptor 41 and histone deacetylase (43).
Nevertheless, this study has several limitations. First, MR cannot pinpoint the exact biological mechanisms underlying the causal link between SFAs and RA, highlighting the need for further research. Second, the lack of data on specific SFA subtypes in databases such as the GWAS database limits the ability to identify the SFAs that should be reduced or eliminated to manage RA. Third, the unavailability of information on lifestyle, age, and other factors precludes subgroup analyses and the investigation of inter-subgroup differences in the effects of SFAs on RA. This study merely underscores that the data available on this topic are limited, and more comprehensive studies are urgently required.
In conclusion, this study confirmed a causal relationship between SFAs and the onset of RA, identifying SFAs as a significant risk factor for RA. Our findings suggest that elevated SFA levels may increase the risk of RA. Interventions aimed at modifying SFA levels could help to reduce the burden of this chronic disease. This research offers new insights into the genetic links between SFAs and RA, setting the stage for future investigations to explore the underlying mechanisms and inform early intervention strategies for RA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XiY: Conceptualization, Methodology, Validation, Writing – original draft. YY: Formal analysis, Methodology, Validation, Writing – review & editing. ZJ: Data curation, Software, Validation, Writing – review & editing. WM: Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – review & editing. XuY: Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Guizhou Provincial Clinical Research Center for Rheumatism and Immunology (Qian Kehe Platform Talent No. 2202); National and Provincial Science and Technology Innovation Talent Team Cultivation Program of Guizhou University of Traditional Chinese Medicine (Guizhou TCM TD Hopes [2022]004), and National Natural Science Foundation of China (82274678).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1337256/full#supplementary-material
Abbreviations
SFA, saturated fatty acid; RA, rheumatoid arthritis; MR, Mendelian randomisation; GWAS, genome-wide association study; IVW, inverse-variance weighted; FAs, fatty acids; SNPs, single-nucleotide polymorphisms; IVs, instrumental variables; LOO, leave-one-out; OR, odds ratio; SCFA, short-chain fatty acid; IL, interleukin.
Footnotes
References
1. Boissier, MC, Semerano, L, Challal, S, Saidenberg-Kermanac’h, N, and Falgarone, G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. (2012) 39:222–8. doi: 10.1016/j.jaut.2012.05.021
2. Coutant, F, and Miossec, P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr Opin Rheumatol. (2020) 32:57–63. doi: 10.1097/BOR.0000000000000664
3. Finckh, A, Gilbert, B, Hodkinson, B, Bae, SC, Thomas, R, Deane, KD, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. (2022) 18:591–602. doi: 10.1038/s41584-022-00827-y
4. Okada, Y, Wu, D, Trynka, G, Raj, T, Terao, C, Ikari, K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. (2014) 506:376–81. doi: 10.1038/nature12873
5. Dedmon, LE. The genetics of rheumatoid arthritis. Rheumatology (Oxford). (2020) 59:2661–70. doi: 10.1093/rheumatology/keaa232
6. Kallberg, H, Padyukov, L, Plenge, RM, Ronnelid, J, Gregersen, PK, van der Helm-van Mil, AH, et al. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. (2007) 80:867–75. doi: 10.1086/516736
7. Ishikawa, Y, and Terao, C. The impact of cigarette smoking on risk of rheumatoid arthritis: a narrative review. Cell. (2020) 9:475. doi: 10.3390/cells9020475
8. Cutolo, M, and Nikiphorou, E. Nutrition and diet in rheumatoid arthritis. Nutrients. (2022) 14:888. doi: 10.3390/nu14040888
9. Jang, S, Kwon, EJ, and Lee, JJ. Rheumatoid arthritis: pathogenic roles of diverse immune cells. Int J Mol Sci. (2022) 23:905. doi: 10.3390/ijms23020905
10. Li, S, Yu, Y, Yue, Y, Zhang, Z, and Su, K. Microbial infection and rheumatoid arthritis. J Clin Cell Immunol. (2013) 4:174. doi: 10.4172/2155-9899.1000174
11. Petrovská, N, Prajzlerová, K, Vencovský, J, Šenolt, L, and Filková, M. The pre-clinical phase of rheumatoid arthritis: from risk factors to prevention of arthritis. Autoimmun Rev. (2021) 20:102797. doi: 10.1016/j.autrev.2021.102797
12. Radzikowska, U, Rinaldi, AO, Çelebi Sözener, Z, Karaguzel, D, Wojcik, M, Cypryk, K, et al. The influence of dietary fatty acids on immune responses. Nutrients. (2019) 11:2990. doi: 10.3390/nu11122990
13. Christ, A, Lauterbach, M, and Latz, E. Western diet and the immune system: an inflammatory connection. Immunity. (2019) 51:794–811. doi: 10.1016/j.immuni.2019.09.020
14. Sun, L, Zhu, J, Mi, S, Li, Y, Wang, T, and Li, Y. Causal association of monounsaturated fatty acids with rheumatoid arthritis but not osteoarthritis: a two-sample Mendelian randomization study. Nutrition. (2021) 91–92:111363. doi: 10.1016/j.nut.2021.111363
15. Tański, W, Świątoniowska-Lonc, N, Tabin, M, and Jankowska-Polańska, B. The relationship between fatty acids and the development, course and treatment of rheumatoid arthritis. Nutrients. (2022) 14:1030. doi: 10.3390/nu14051030
16. Burlingame, B, Nishida, C, Uauy, R, and Weisell, R. Fats and fatty acids in human nutrition: introduction. Ann Nutr Metab. (2009) 55:5–7. doi: 10.1159/000228993
17. Sebe, M, Tsutsumi, R, Senoura, S, Kishi, J, Iuchi, M, Mishima, Y, et al. Saturated fatty acids intake is associated with muscle atrophy in rheumatoid arthritis. JCSM Rapid Commun. (2022) 5:86–101. doi: 10.1002/rco2.53
18. Everett, S. 2022 American College of Rheumatology (ACR) guideline for exercise, rehabilitation, diet, and additional integrative interventions for rheumatoid arthritis. Arthritis Care Res. (2023) 75:1629–30. doi: 10.1002/acr.25119
19. Lawlor, DA, Harbord, RM, Sterne, JA, Timpson, N, and Davey, SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
20. Skrivankova, VW, Richmond, RC, Woolf, BAR, Davies, NM, Swanson, SA, VanderWeele, TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021) 375:n 2233. doi: 10.1136/bmj.n2233
21. Verduijn, M, Siegerink, B, Jager, KJ, Zoccali, C, and Dekker, FW. Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant. (2010) 25:1394–8. doi: 10.1093/ndt/gfq098
22. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. elife. (2018) 7:7. doi: 10.7554/eLife.34408
23. Skrivankova, VW, Richmond, RC, Woolf, BAR, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
24. Lin, L, Zhang, K, Xiong, Q, Zhang, J, Cai, B, Huang, Z, et al. Gut microbiota in pre-clinical rheumatoid arthritis: from pathogenesis to preventing progression. J Autoimmun. (2023) 141:103001. doi: 10.1016/j.jaut.2023.103001
25. Malesza, IJ, Malesza, M, Walkowiak, J, Mussin, N, Walkowiak, D, Aringazina, R, et al. High-fat, Western-style diet, systemic inflammation, and gut microbiota. Cell. (2021) 10:3164. doi: 10.3390/cells10113164
26. Liu, G, Makrides, M, Coates, P, Lam, K, Ranieri, E, Mas, E, et al. A rapid method for the screening of fatty acids in lipids in plasma or serum without prior extraction. Prostaglandins Leukot Essent Fatty Acids. (2022) 178:102416. doi: 10.1016/j.plefa.2022.102416
27. Buchanan, CDC, Lust, CAC, Burns, JL, Hillyer, LM, Martin, SA, Wittert, GA, et al. Analysis of major fatty acids from matched plasma and serum samples reveals highly comparable absolute and relative levels. Prostaglandins Leukot Essent Fatty Acids. (2021) 168:102268. doi: 10.1016/j.plefa.2021.102268
28. Mustonen, AM, Käkelä, R, Lehenkari, P, Huhtakangas, J, Turunen, S, Joukainen, A, et al. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res Ther. (2019) 21:124. doi: 10.1186/s13075-019-1914-y
29. Navarro, E, Esteve, M, Olivé, A, Klaassen, J, Cabré, E, Tena, X, et al. Abnormal fatty acid pattern in rheumatoid arthritis. A rationale for treatment with marine and botanical lipids. J Rheumatol. (2000) 27:298–303.
30. Jacobsson, L, Lindgärde, F, Manthorpe, R, and Akesson, B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann Rheum Dis. (1990) 49:901–5. doi: 10.1136/ard.49.11.901
31. Xie, R, and Zhang, Y. Association between 19 dietary fatty acids intake and rheumatoid arthritis: results of a nationwide survey. Prostaglandins Leukot Essent Fatty Acids. (2023) 188:102530. doi: 10.1016/j.plefa.2022.102530
32. Yao, Y, Cai, X, Zheng, Y, Zhang, M, Fei, W, Sun, D, et al. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br J Pharmacol. (2022) 179:4315–29. doi: 10.1111/bph.15852
33. Guilloteau, P, Martin, L, Eeckhaut, V, Ducatelle, R, Zabielski, R, and Van Immerseel, F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. (2010) 23:366–84. doi: 10.1017/S0954422410000247
34. Rosser, EC, Piper, CJM, Matei, DE, Blair, PA, Rendeiro, AF, Orford, M, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. (2020) 31:837–51.e10. doi: 10.1016/j.cmet.2020.03.003
35. Burgess, S, Davey Smith, G, Davies, NM, Dudbridge, F, Gill, D, Glymour, MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
36. Mustonen, AM, and Nieminen, P. Fatty acids and Oxylipins in osteoarthritis and rheumatoid arthritis-a complex field with significant potential for future treatments. Curr Rheumatol Rep. (2021) 23:41. doi: 10.1007/s11926-021-01007-9
37. Kim, CH. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol Immunol. (2023) 20:341–50. doi: 10.1038/s41423-023-00987-1
38. Mauro, C, Smith, J, Cucchi, D, Coe, D, Fu, H, Bonacina, F, et al. Obesity-induced metabolic stress leads to biased effector memory CD4(+) T cell differentiation via PI3K p110δ-Akt-mediated signals. Cell Metab. (2017) 25:593–609. doi: 10.1016/j.cmet.2017.01.008
39. Haghikia, A, Jörg, S, Duscha, A, Berg, J, Manzel, A, Waschbisch, A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. (2015) 43:817–29. doi: 10.1016/j.immuni.2015.09.007
40. Zhou, T, Wang, G, Lyu, Y, Wang, L, Zuo, S, Zou, J, et al. Upregulation of SLAMF3 on human T cells is induced by palmitic acid through the STAT5-PI3K/Akt pathway and features the chronic inflammatory profiles of type 2 diabetes. Cell Death Dis. (2019) 10:559. doi: 10.1038/s41419-019-1791-y
41. Xu, X, Wang, M, Wang, Z, Chen, Q, Chen, X, Xu, Y, et al. The bridge of the gut-joint axis: gut microbial metabolites in rheumatoid arthritis. Front Immunol. (2022) 13:1007610. doi: 10.3389/fimmu.2022.1007610
42. Balakrishnan, B, Luckey, D, Bodhke, R, Chen, J, Marietta, E, Jeraldo, P, et al. Prevotella histicola protects from arthritis by expansion of Allobaculum and augmenting butyrate production in humanized mice. Front Immunol. (2021) 12:609644. doi: 10.3389/fimmu.2021.609644
Keywords: rheumatoid arthritis, saturated fatty acids, mendelian randomisation, causality, bidirectional
Citation: Yao X, Yang Y, Jiang Z, Ma W and Yao X (2024) The causal impact of saturated fatty acids on rheumatoid arthritis: a bidirectional Mendelian randomisation study. Front. Nutr. 11:1337256. doi: 10.3389/fnut.2024.1337256
Edited by:
Ifigenia Kostoglou-Athanassiou, Asclepeion Hospital, GreeceCopyright © 2024 Yao, Yang, Jiang, Ma and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wukai Ma, d2Fsa2VyNTVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaoling Yao
Xiaoling Yao Yuzheng Yang1†
Yuzheng Yang1† Zong Jiang
Zong Jiang