
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 17 January 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1335074
This article is part of the Research Topic Personalized Nutrition in Chronic Kidney Disease View all 14 articles
Background: A link between food-induced inflammation and common chronic diseases has been identified in studies. However, there was uncertainty about the influence of dietary inflammatory potential on the risk of chronic kidney disease (CKD) among middle-aged and older groups. Our research aimed to examine the connection between dietary inflammatory index (DII) to CKD in people aged 40 years and older.
Methods: This study comprised ten cycles of the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018. Linear associations of DII with CKD, low-eGFR, and albuminuria were examined using multiple logistic regression, whereas non-linear associations were assessed by smoothed curve fitting. Besides, we conducted subgroup analyses and interaction tests.
Results: Of the 23,175 middle-aged and older individuals, a total of 5,847 suffered from CKD, making up 25.23% of all participants. After adjustment for all covariates, we found that increased DII scores were positive with an increased hazard of CKD (OR = 1.08, 95% CI: 1.05, 1.10, p < 0.0001), and the same was shown between DII and low-eGFR (OR = 1.16, 95% CI: 1.13, 1.19, p < 0.0001). After further converting DII into categorical variables, the above relationship still existed. These relations were consistent in different ages, genders, BMI, whether smoking, whether suffering from hypertension, and whether suffering from diabetes, with no significant stratification differences (all P for interaction >0.05). Surprisingly, we did not find a statistically significant correlation of DII to albuminuria after complete adjustment for covariates (OR = 1.02, 95% CI: 1.00, 1.05, p = 0.0742). Even when DII was considered as a categorical variable, this relation was still not statistically significant. Furthermore, we found an association in the shape of a U between DII and low-eGFR in the fully adjusted model, with a turning point at a DII of 1.6.
Conclusion: Our findings indicated that middle-aged and older persons with greater levels of DII had a significantly higher risk of CKD.
Chronic kidney disease (CKD) is known to be a common chronic disease, especially in the elderly population (1). Its most prominent feature is the damage to kidney structure and function caused by a variety of reasons (2). It has been shown that between 1990 and 2016, the global burden of CKD rose by 87%, and that CKD fatalities have climbed by 100%, becoming one of the top non-communicable causes of mortality globally (3–5). A growing number of older persons are suffering from CKD as the population ages (6). CKD is closely linked to appetite loss, falls and fractures, cognitive decline, diminished physical function, poor quality of life, and inflammation. In recent years, it has also steadily gained recognition as a factor in hastened aging (7, 8). For the prevention and treatment of CKD as well as to relieve the global healthcare burden, it is crucial to actively explore the causes underlying CKD in middle-aged and elder individuals.
Diet plays a central role in regulating inflammatory processes and is closely related to the occurrence and progression of various chronic diseases. The Dietary Inflammatory Index (DII) is an indicator that has been widely used in recent years for the assessment of the inflammatory potential of individual diets (9). The DII scoring system consists of 45 food nutrients. Each food parameter was scored according to its effect on pro-inflammatory markers interleukin-1β (IL-1β), IL-6, tumor necrosis factor α (TNFα), C-reactive protein (CRP) and anti-inflammatory markers (IL-4, IL-10): “-1” indicates an anti-inflammatory effect, “ + 1” indicates a pro-inflammatory effect and “0” indicates that the food parameter had no effect on inflammatory markers. After a series of calculations and weighting, a complete evaluation system for dietary inflammation was ultimately developed (10). Most importantly, the DII assesses the combined effects of multiple dietary components, not just one nutrient or food. Positive DII scores indicate pro-inflammatory diets, while negative DII scores indicate anti-inflammatory diets, with higher absolute values indicating greater anti-inflammatory/pro-inflammatory effects. It has been proposed that numerous inflammatory disorders, including obesity, diabetes, and cardiovascular disease, have a favorable correlation with DII scores (11–15). Systemic low-grade inflammation is one of the most important pathogenic processes in CKD, and it is also influenced by the dietary status of patients. However, there remained a dearth of relevant research on the connection of DII to CKD in the middle-aged and elderly population.
Therefore, we conducted this study to look into the connection of DII to CKD in people aged 40 years and older.
Data for our study came from the 1999 to 2018 National Health and Nutrition Examination Survey (NHANES). NHANES is a research project hosted by the National Centre for Health Statistics (NCHS). Updating of its data is still in progress. The sample utilized in this investigation, which employed stratified multistage random sampling, was well-represented. The study had the approval of the NCHS Ethical Review Committee, and written informed consent was obtained from all subjects. All information from our research is available on the NHANES website.1
Initially, there were 101,316 participants. After excluding those with incomplete or missing dietary information (n = 53,881), those under the age of 40 (n = 23,915), those who were pregnant or breastfeeding (n = 31), and those with missing information on CKD (n = 314), our final analysis comprised 23,175 participants (Figure 1).
In this study, we treated DII as an exposure variable and used two 24-h dietary reviews to obtain dietary information. There were 26 dietary parameters employed in calculating the DII, including carbohydrates, proteins, total fats, saturated fats, polyunsaturated fatty acid (PUFA), n-3 fatty acids, cholesterol, energy, alcohol, fiber, folate, iron, magnesium, zinc, selenium, monounsaturated fatty acid (MUFA), caffeine, niacin, riboflavin, thiamin, beta-carotene, Vitamins A/B6/B12/C/E. Initially, determine the mean intake level of each nutrient for the subjects, subtract the global average intake, and then divide the result by the standard deviation to compute the z-score. Subsequently, transform the z-scores into percentiles, and double the transformed values while subtracting “1” for data centering. The central percentile values for each food parameter are then multiplied by their respective inflammatory effect scores, resulting in a “food parameter-specific DII score.” Finally, each value was summed to obtain an individual “overall DII score” (10, 16).
The outcome variable was CKD, which was defined by albuminuria and/or low estimated glomerular filtration rate (low-eGFR) (17). Albumin-to-creatinine ratio (ACR) >30 mg/g was the diagnostic standard for albuminuria, and ACR was calculated using the urinary albumin/creatinine ratio. The diagnostic criteria for low-eGFR was eGFR < 60 ml/min/1.73 m2, and we calculated eGFR based on the Chronic Kidney Disease Epidemiology Collaborative equation (CKD-EPI Cr) (18).
Our study also incorporated a series of other factors as covariates, such as race, sex, age, educational level, smoking status, BMI, the poverty-to-income ratio (PIR), albumin, glucose, glycosylated hemoglobin, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), serum iron, potassium, blood urea nitrogen (BUN), uric acid, cholesterol, and triglycerides. Additionally, variations in health status for arthritis, cancer, hypertension, congestive heart failure, coronary heart disease, and diabetes were also enrolled as covariates.
In accordance with NHANES analytical guidelines, statistical analyses were conducted with appropriate sampling weights. Continuous data were given as medians [interquartile range (IQR)] and categorical variables as percentages. The effect of DII on three outcome variables, including CKD, albuminuria, and low-eGFR, was analyzed under three different models using multiple logistic regression. Model 1 did not incorporate any adjustment for covariates. Model 2 incorporated race, age, and sex. Model 3 incorporated more than just the three variables mentioned above, but also education level, smoking status, PIR, BMI, albumin, glucose, glycosylated hemoglobin, ALT, AST, serum iron, potassium, cholesterol, triglycerides, BUN, uric acid, hypertension, coronary heart disease, congestive heart failure, stroke, arthritis, cancer, and diabetes. Furthermore, smoothed curve fitting and threshold effect evaluation were used to evaluate the non-linear connection of DII with these three outcome variables. Subgroup analyses were carried out to further examine the correlation of DII with these outcome variables. P < 0.05 has been identified to be statistically significant in this study. R (version 4.2.3) and EmpowerStats (version 2.0) were used for statistical calculations and graphics.
This research included 23,175 individuals, with a mean age of 60. A total of 49.82% of them were men, and 50.18% were women. After treating the DII as a quantile variable, the basic information for all subjects was presented in Table 1. According to our statistics, those with greater DII tertiles were more likely to have hypertension, coronary heart disease, congestive heart failure, arthritis, cancer, stroke, diabetes, and CKD than people in other categories.
There were 5,847 persons with CKD among all participants, accounting for 25.23% of the total. Basic information on subjects grouped by CKD situation was shown in Table 2. The findings suggested that CKD was more prevalent in older, less educated, lower PIR, male, and non-Hispanic white groups. Those with CKD possessed higher levels of potassium, glucose, glycosylated hemoglobin, uric acid, BUN, triglycerides, BMI, and DII. In addition, these people had higher rates of hypertension, arthritis, cancer, stroke, congestive heart failure, coronary heart disease, and diabetes than non-CKD people. In contrast, serum iron, albumin, ALT, and cholesterol levels were lower in the CKD population.
The results of the multiple regression analysis were shown in Table 3, including the association of DII with CKD, low-eGFR, and albuminuria. Our results indicated that higher DII scores were connected with a higher risk of CKD. This relation was significant in all models (Model 1, OR = 1.11, 95% CI: 1.09, 1.13, p < 0.0001; Model 2, OR = 1.10, 95% CI: 1.08, 1.12, p < 0.0001; Model 3, OR = 1.08, 95% CI: 1.05, 1.10, p < 0.0001). In the unadjusted model (model 1), each one-unit increase in DII score increased the risk of CKD in subjects by 11%, whereas, in the fully adjusted model (model 3), this risk increased by 8%. After transforming DII into tertiles, this relation still maintained its statistical significance. In the unadjusted model, individuals in the highest tertile of DII scores had a significantly increased risk of CKD of 53% (OR = 1.53, 95% CI: 1.42, 1.65; p for trend <0.0001) compared with participants in the bottom tertile of DII scores, and this risk increased by 35% (OR = 1.35, 95% CI: 1.23, 1.48; p for trend <0.0001) after adjusting for all covariates.
A similar positive correlation was also observed for DII with low-eGFR. We noted a significant correlation between DII and low-eGFR in all models (Model 1, OR = 1.14, 95% CI: 1.12, 1.17, p < 0.0001; Model 2, OR = 1.15, 95% CI: 1.12, 1.18, p < 0.0001; Model 3, OR = 1.16, 95% CI: 1.13, 1.19, p < 0.0001). Without adjusting for any covariates (model 1), the risk of low-eGFR in subjects increased by 14% for each unit increase in DII, whereas this effect value further increased to 16% after adjusting for all covariates (model 3). This positive association persisted when DII was transformed into tertiles, too. Individuals in the highest tertile of DII were 75% more likely to have a low-eGFR than individuals in the lowest tertile in the unadjusted model (OR = 1.75, 95% CI: 1.59, 1.92; p for trend <0.0001), whereas in the fully adjusted model, this likelihood was 85% higher (OR = 1.85, 95% CI: 1.62, 2.10; p for trend <0.0001).
Our results showed a positive correlation between DII and albuminuria in all models, however, this correlation was not significant in the fully adjusted model (Table 3). The likelihood of albuminuria increased by 9% for each unit increase in DII in the unadjusted model (OR = 1.09, 95% CI: 1.06, 1.11, p < 0.0001) and by 7% in the minimally adjusted model (OR = 1.07, 95% CI: 1.05, 1.09, p < 0.0001). Both of these results were statistically significant. This correlation remained significant even when DII was converted to a tertile variable. However, the relation of DII to albuminuria was not statistically significant after adjusting for all covariates, regardless of whether DII was a continuous variable or a categorical variable.
Figures 2, 3 depicted the non-linear relationships in the fully adjusted model between DII and CKD, as well as between DII and low-eGFR, through smooth curve fitting. It was worth noting that we found a U-shaped relation between DII and low-eGFR with a turning point of 1.6 (Table 4).
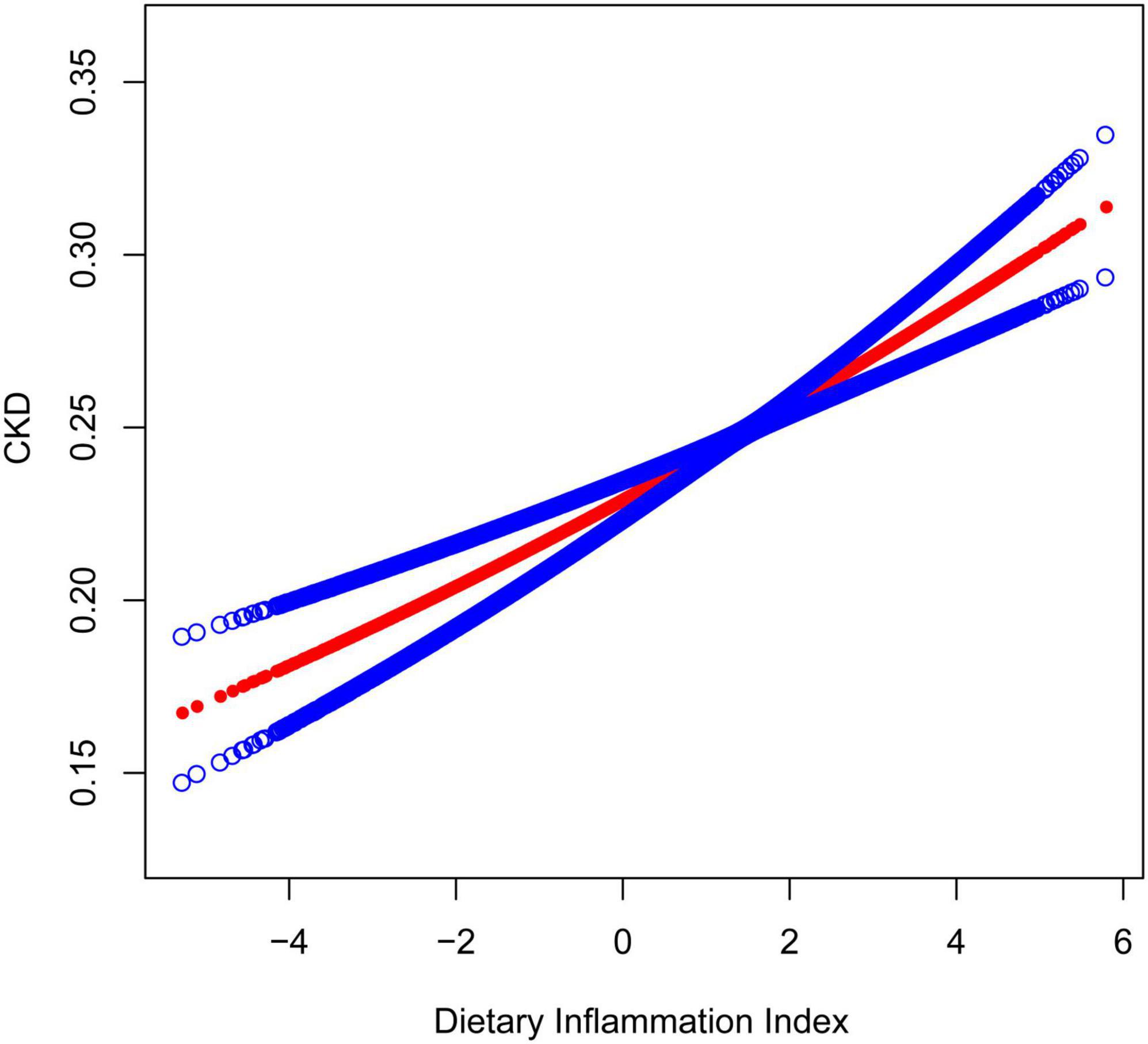
Figure 2. The association between dietary inflammatory index and CKD. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. The model adjusted for sex, age, race, education level, smoking status, PIR, BMI, albumin, glucose, glycosylated hemoglobin, ALT, AST, serum iron, potassium, cholesterol, triglycerides, BUN, uric acid, hypertension, coronary heart disease, congestive heart failure, stroke, arthritis, cancer, and diabetes.
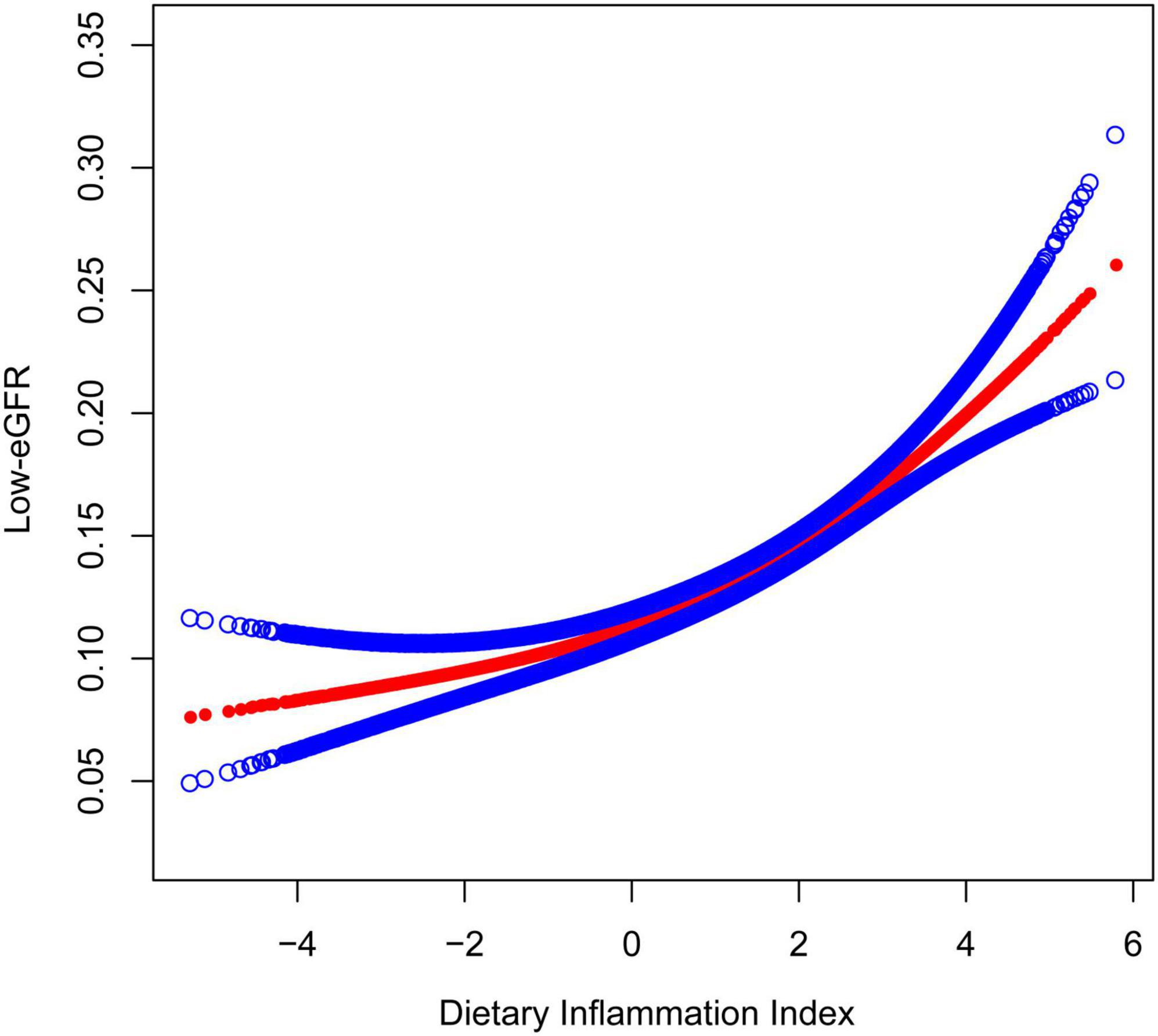
Figure 3. The Association between dietary inflammatory index and low-eGFR. The model adjusted for sex, age, race, education level, smoking status, PIR, BMI, albumin, glucose, glycosylated hemoglobin, ALT, AST, serum iron, potassium, cholesterol, triglycerides, BUN, uric acid, hypertension, coronary heart disease, congestive heart failure, stroke, arthritis, cancer, and diabetes.
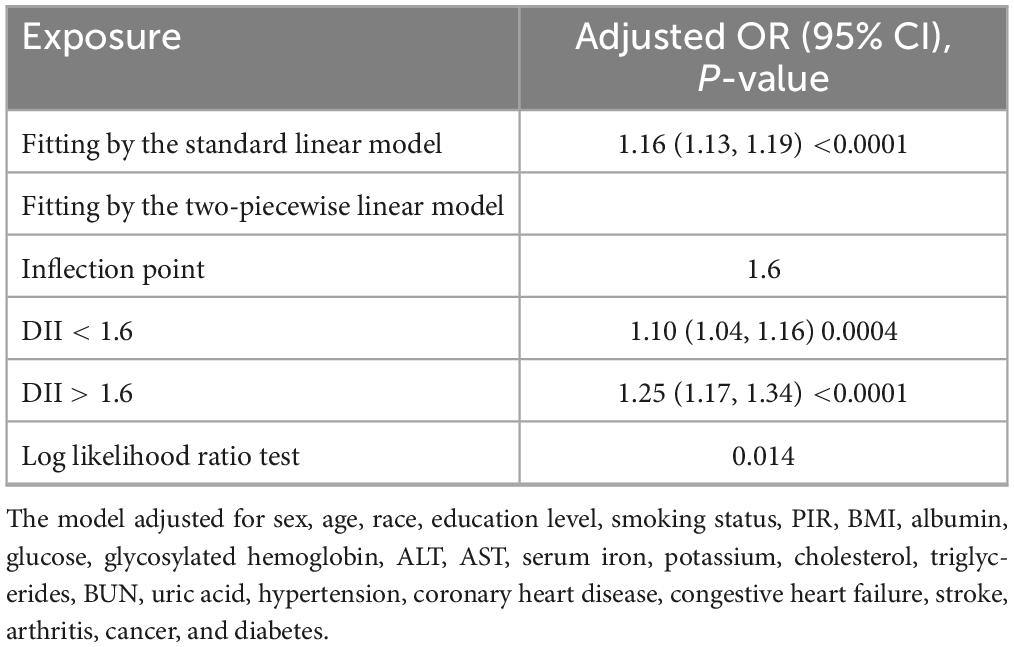
Table 4. Threshold effect analysis of dietary inflammatory index on low-eGFR using two-piecewise linear regression model.
For the associations of DII with CKD, DII with low-eGFR, and DII with albuminuria, we performed subgroup analyses including age, sex, BMI, smoking status, diabetes status, and hypertension status in the completely adjusted model (Table 5). Results indicated that the positive associations of DII with CKD and DII with low-eGFR remained stable and were not significantly different in all subgroups (all P for interaction >0.05). Consistent with prior results, we did not observe any statistically significant correlation between DII and albuminuria in all subgroups in the fully adjusted model, except in the non-hypertensive population (OR = 1.06, 95% CI: 1.02, 1.10). This group difference based on having or not having hypertension was statistically significant (P for interaction = 0.0095).
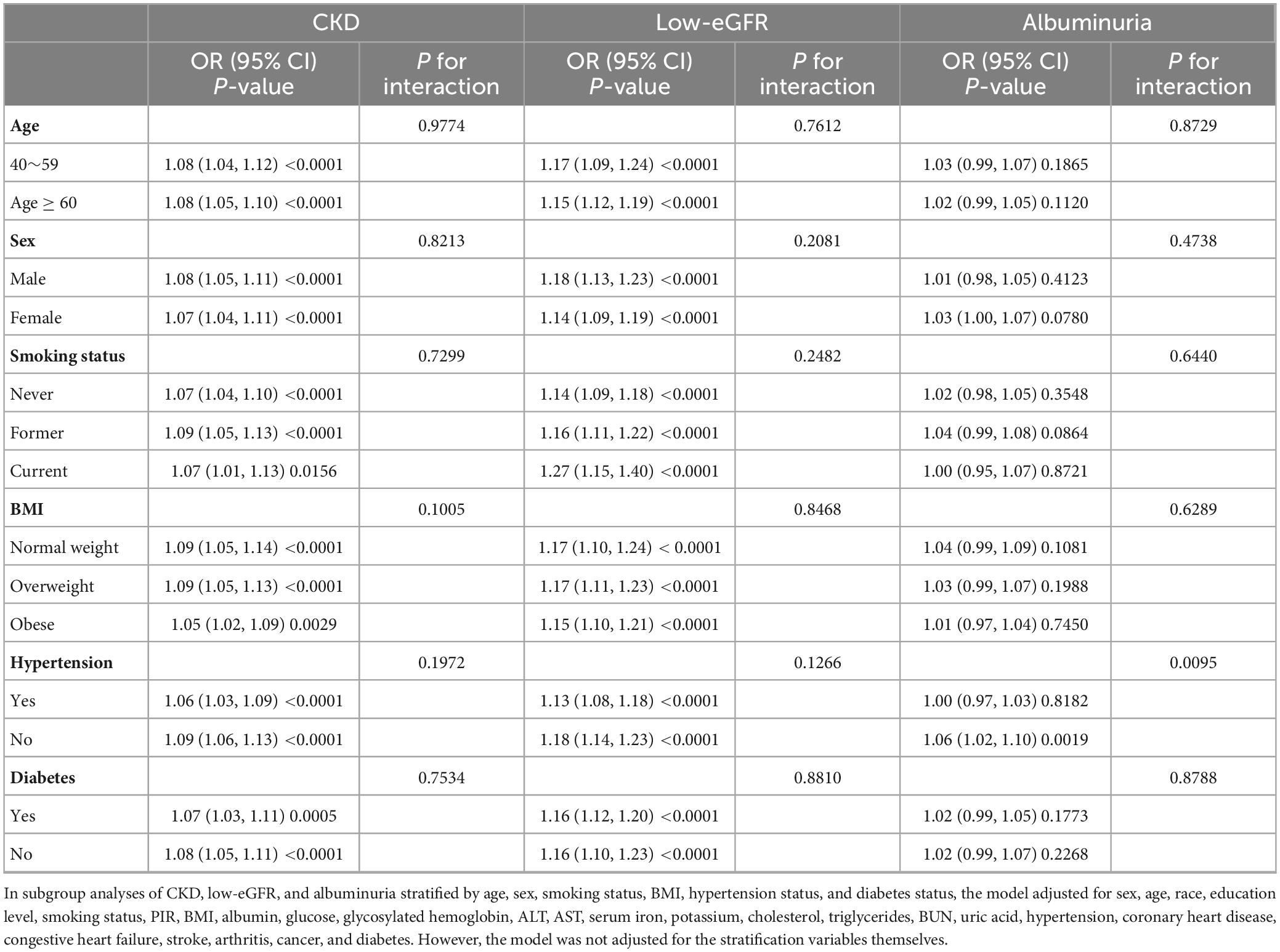
Table 5. Association between dietary inflammatory index and CKD, low e-GFR, and albuminuria in different subgroups.
In this research involving 23,175 participants, we assessed the relation between DII and CKD in individuals aged 40 years and older in the United States. We found that higher levels of DII were significantly and positively correlated with a higher risk of CKD and low-eGFR in these middle-aged and older adults. This association remained stable across strata of age, sex, BMI, smoking status, diabetes mellitus status, and hypertension status, and no significant stratification differences were seen. In addition, there was a U-shaped relation between DII and low-eGFR with a turning point of 1.6.
The link of diet-induced inflammation to prevalent chronic illnesses has been shown in an array of earlier research. Shate Xiang et al. discovered that DII was positively linked to rheumatoid arthritis risk and superimposed on other risk variables (19). Through meta-analysis, some researchers found that a greater DII score might raise the risk of colorectal cancer and was strongly associated with an elevated cancer risk of the ovaries among postmenopausal females (20, 21). It is essential to note that the greater the positive DII score, the greater the pro-inflammatory nature of the diet. Therefore, adhering to a balanced and anti-inflammatory diet may potentially lower the risk of cancer development. An increasing corpus of research has indicated that diet has a significant impact on the emergence of cardiovascular disease (CVD) (11). A cross-sectional study involving 48,733 participants indicated that a rise in DII could heighten the danger of CVD among individuals aged 18 and above in the United States. Furthermore, this occurrence showed a more significant effect on women. At the same time, there was continuing evidence of a link between DII and mortality in the population. For instance, it has been shown that DII has a positive association with the prevalence of diabetes and a diet with high levels of pro-inflammatory compounds could potentially contribute to higher mortality rates among people with diabetes (22).
In the past few years, numerous investigations have demonstrated there were certain links between DII and CKD. In a cross-sectional investigation, Ying Huang and colleagues discovered that a rise in DII might be contributory to the development of sarcopenia in individuals with CKD and that an anti-inflammatory diet might be protective against CKD-associated sarcopenia (23). It has also been suggested that treatments using an anti-inflammatory diet might lower rates to get hyperparathyroidism in patients with CKD (24). Moreover, Ying Huang et al. found that the association of elevated energy-adjusted dietary inflammatory index (E-DII) with increased risk of 5-year all-cause and cardiovascular mortality in individuals with CKD was not modified by other factors, while subgroup analyses showed that individuals with worsening renal function were more vulnerable to the effects of E-DII (25). With the aging of populations worldwide, researchers are increasingly concentrating on middle-aged and older populations, including DII-related studies. As it is widely acknowledged, there exists an anti-aging protein in plasma, soluble Klotho (S-Klotho). A research had indicated a negative association between S-Klotho and pro-inflammatory food patterns evaluated based on DII in people over 40 years old (26). Furthermore, these middle-aged and older people with high DII scores also had an increased likelihood of suffering from low HDL cholesterol, hyperglycemia, and metabolic syndrome (27). Epidemiologic studies of DII and CKD are emerging. Zeng’s team found that higher DII scores were independently associated with increased odds of CKD stages 3–5 in adults aged 50 years and older. And its linear association remained stable across stratified analyses of age, gender, and ethnicity (28). This is similar to our findings. And in a large retrospective cohort study with a mean follow-up of 132.03 months, Yan et al. observed an association between DII and the risk of all-cause mortality in a population of US adults with CKD. The results showed that the risk of death was more pronounced in those with higher DII scores, especially in those under 65 years of age (29). However, further research is needed to delve deeper into the relationship between DII and CKD.
There have been many studies on DII and CKD, but the exact mechanism was still unknown. Below are some potential pathological processes that could exist. Inflammation plays a key role in CKD progression, and poor dietary habits can contribute to this inflammatory response, impacting kidney health through diverse mechanisms. High-calorie and high-fat diets can lead to obesity, and the chronic low-grade inflammation that it triggers is one of the common initiators of CKD. Adipose tissue releases lipid mediators and cytokines such as TNF-α and IL-6, which can trigger systemic inflammation and cause direct damage to the kidneys (30). The hyperglycemic state resulting from a prolonged high-sugar diet triggers the production of glycosylation end-products (AGEs), which activate inflammatory pathways and lead to insulin resistance, further contributing to the development of inflammation (31). Micronutrients such as zinc, selenium and antioxidants such as vitamins C and E are essential for maintaining the health of living organisms (32). Inadequate diets may lead to deficiencies in these nutrients, potentially making the kidneys more susceptible to damage from oxidative stress, which can trigger an inflammatory response. Chronic high-salt diets can alter the structure and function of small renal arteries. In addition, it has been shown that high salt intake independently promotes immune activation of macrophages, exacerbating inflammation and accompanying renal deterioration (33). Previous studies have shown that there is an interaction between chronic inflammation and CKD (34). For one thing, chronic systemic inflammation could cause renal failure and speed up the development of renal fibrosis and CKD (35, 36). And for another, the uremic setting in the advanced stage of CKD would lead to uremic inflammation, which is a long-term, low-grade inflammation related to aging (37–39). Plenty of cytokines and inflammatory indicators, like TNF-α, CRP, IL-1, and IL-6, have been related to the development of CKD (40). These inflammatory factors are closely linked to the triggering of the nuclear factor-κB (NF-κB) cascade (41). Such a pathway would induce mitochondrial dysfunction and increased amounts of reactive oxygen species (ROS) (42, 43), and NF-κB may cause an inflammatory cytokine feedback loop that might maintain kidney inflammation (44). Thus, a more pro-inflammatory diet may enhance the expression of inflammatory factors, thereby promoting an inflammatory state in the body, leading to an increased risk of CKD.
This is the first study we know of to look at the relation of DII to CKD in individuals aged 40 years and older. Second, the participants in this study were selected from the national population through a stratified, multistage sampling procedure and were well represented, so our outcomes could apply to the whole group of middle-aged and older adults in this country. Most notably, the large nationwide sample size made it possible to perform subgroup analyses by age, sex, diabetes status, and hypertension status. The results revealed that the positive relation of DII to CKD remained consistent in all subgroups. However it is necessary to be aware of the shortcomings of our study. In the first instance, because of the cross-sectional nature of this study, we could not establish a clear causal connection of DII with CKD. Secondly, it is generally recognized that two consecutive 24-h dietary recall interviews might only be used as a reference, and there might be a degree of memory uncertainty that could not accurately reflect long-term dietary habits. Thirdly, whether or not to perform hemodialysis is crucial for the study of CKD patients, but we did not include it as a variable in our study due to the lack of relevant data in the NHANES database. Fourth, disparities exist among CKD patients at various stages, necessitating more comprehensive investigations in the future to ascertain the generalizability of our findings. Finally, we only used the American population as the research sample, and the research results cannot represent all populations. Further research is needed to demonstrate our findings in the future.
As a result, our findings have demonstrated that increased DII was substantially and positively correlated with elevated hazard of CKD in individuals of middle and older age. This highlighted the importance of an anti-inflammatory diet in reducing the risk of CKD in this group. But further extensive prospective studies are needed to test the validity of our findings.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MG: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review and editing. YL: Data curation, Writing – original draft. XuL: Data curation, Writing – original draft. XiL: Writing – review and editing. YX: Writing – review and editing. DZ: Conceptualization, Writing – review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Number: 82170757).
We appreciate all the participants in NHANES for their generous contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Heras Benito M, Fernández-Reyes Luis MJ. Shared decision-making in advanced chronic kidney disease in the elderly. Med Clin. (2019) 152:188–94.
3. Jha V, Modi GK. Getting to know the enemy better-the global burden of chronic kidney disease. Kidney Int. (2018) 94:462–4. doi: 10.1016/j.kint.2018.05.009
4. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. (2022) 12:7–11.
5. Tabatabaei-Malazy O, Saeedi Moghaddam S, Khashayar P, Keykhaei M, Tehrani YS, Malekpour MR, et al. Regional burden of chronic kidney disease in North Africa and Middle East during 1990-2019; Results from Global Burden of Disease study 2019. Front Public Health. (2022) 10:1015902. doi: 10.3389/fpubh.2022.1015902
6. Alfano G, Perrone R, Fontana F, Ligabue G, Giovanella S, Ferrari A, et al. Rethinking chronic kidney disease in the aging population. Life. (2022) 12:1724.
7. Merchant RA, Vathsala A. Healthy aging and chronic kidney disease. Kidney Res Clin Pract. (2022) 41:644–56.
8. Fletcher BR, Damery S, Aiyegbusi OL, Anderson N, Calvert M, Cockwell P, et al. Symptom burden and health-related quality of life in chronic kidney disease: a global systematic review and meta-analysis. PLoS Med. (2022) 19:e1003954. doi: 10.1371/journal.pmed.1003954
9. Kotemori A, Sawada N, Iwasaki M, Yamaji T, Shivappa N, Hebert JR, et al. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: a cross-sectional study of the JPHC-FFQ validation study. Nutrition. (2020) 69:110569. doi: 10.1016/j.nut.2019.110569
10. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96.
11. Zhang J, Jia J, Lai R, Wang X, Chen X, Tian W, et al. Association between dietary inflammatory index and atherosclerosis cardiovascular disease in U.S. adults. Front Nutr. (2022) 9:1044329. doi: 10.3389/fnut.2022.1044329
12. Ji M, Hong X, Chen M, Chen T, Wang J, Zhang N. Dietary inflammatory index and cardiovascular risk and mortality: a meta-analysis of cohort studies. Medicine. (2020) 99:e20303.
13. Motamedi A, Askari M, Mozaffari H, Homayounfrar R, Nikparast A, Ghazi ML, et al. Dietary inflammatory index in relation to type 2 diabetes: a meta-analysis. Int J Clin Pract. (2022) 2022:9953115.
14. de Mello RN, de Gois BP, Kravchychyn AC, Dâmaso AR, Horst MA, Lima GC, et al. Dietary inflammatory index and its relation to the pathophysiological aspects of obesity: a narrative review. Arch Endocrinol Metab. (2023) 67:e000631. doi: 10.20945/2359-3997000000631
15. Blaudt L, de Souza Lopes T, de Moura Souza A, Yokoo EM, da Rocha CM, Pereira RA. Association between dietary inflammatory index and anthropometric indicators of adiposity in Brazilian adolescents. Pediatr Obes. (2023) 18:e13011.
16. Xie R, Ning Z, Xiao M, Li L, Liu M, Zhang Y. Dietary inflammatory potential and biological aging among US adults: a population-based study. Aging Clin Exp Res. (2023) 35:1273–81. doi: 10.1007/s40520-023-02410-1
17. 2013 International Society of Nephrology. Chapter 1: definition and classification of CKD. Kidney Int Suppl. (2013) 3:19–62.
18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med. (2009) 150:604–12.
19. Xiang S, Wang Y, Qian S, Li J, Jin Y, Ding X, et al. The association between dietary inflammation index and the risk of rheumatoid arthritis in Americans. Clin Rheumatol. (2022) 41:2647–58.
20. Yang J, Ma J, Jin Y, Cheng S, Huang S, Wang Y. Dietary inflammatory index and ovarian cancer risk: a meta-analysis. Nutr Cancer. (2022) 74:796–805. doi: 10.1080/01635581.2021.1931366
21. Zhang C, Wang W, Zhang D. Association between dietary inflammation index and the risk of colorectal cancer: a meta-analysis. Nutr Cancer. (2018) 70:14–22.
22. Tan J, Liu N, Sun P, Tang Y, Qin W. A proinflammatory diet may increase mortality risk in patients with diabetes mellitus. Nutrients. (2022) 14:2011.
23. Huang Y, Zeng M, Zhang L, Shi J, Yang Y, Liu F, et al. Dietary inflammatory potential is associated with sarcopenia among chronic kidney disease population. Front Nutr. (2022) 9:856726. doi: 10.3389/fnut.2022.856726
24. Qin Z, Yang Q, Liao R, Su B. The association between dietary inflammatory index and parathyroid hormone in adults with/without chronic kidney disease. Front Nutr. (2021) 8:688369. doi: 10.3389/fnut.2021.688369
25. Huang Y, Zhang L, Zeng M, Liu F, Sun L, Liu Y, et al. Energy-adjusted dietary inflammatory index is associated with 5-year all cause and cardiovascular mortality among chronic kidney disease patients. Front Nutr. (2022) 9:899004. doi: 10.3389/fnut.2022.899004
26. Ma TC, Zhou J, Wang C, Fang M, Gao F. Association between dietary inflammatory index and S-klotho plasma levels in middle-aged and elderly people. Front Nutr. (2022) 9:853332. doi: 10.3389/fnut.2022.853332
27. Zhao Q, Tan X, Su Z, Manzi HP, Su L, Tang Z, et al. The relationship between the Dietary Inflammatory Index (DII) and Metabolic Syndrome (MetS) in middle-aged and elderly individuals in the United States. Nutrients. (2023) 15:1857. doi: 10.3390/nu15081857
28. Zeng S, Qi L, Sun Y, Zhuang G. Association of chronic kidney disease with dietary inflammatory index in adults aged 50 years and older: dose-response analysis of a nationally representative population-based study. J Ren Nutr. (2023). doi: 10.1053/j.jrn.2023.09.007 [Epub ahead of print].
29. Yan LJ, Zhang FR, Ma CS, Zheng Y. Higher dietary inflammatory index is associated with increased all-cause mortality in adults with chronic kidney disease. Front Nutr. (2022) 9:883838. doi: 10.3389/fnut.2022.883838
30. Kern L, Mittenbühler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers. (2018) 11:24.
31. Pal R, Bhadada SK. AGEs accumulation with vascular complications, glycemic control and metabolic syndrome: a narrative review. Bone. (2023) 176:116884. doi: 10.1016/j.bone.2023.116884
32. Kaźmierczak-Barańska J, Boguszewska K, Karwowski BT. Nutrition can help DNA repair in the case of aging. Nutrients. (2020) 12:3364. doi: 10.3390/nu12113364
33. Liu Y, Dai X, Yang S, Peng Y, Hou F, Zhou Q. High salt aggravates renal inflammation via promoting pro-inflammatory macrophage in 5/6-nephrectomized rat. Life Sci. (2021) 274:119109. doi: 10.1016/j.lfs.2021.119109
34. Podkowińska A, Formanowicz D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants. (2020) 9:752. doi: 10.3390/antiox9080752
35. Ortega-Hernández J, Springall R, Sánchez-Muñoz F, Arana-Martinez JC, González-Pacheco H, Bojalil R. Acute coronary syndrome and acute kidney injury: role of inflammation in worsening renal function. BMC Cardiovasc Disord. (2017) 17:202. doi: 10.1186/s12872-017-0640-0
36. Wang M, Lin X, Yang X, Yang Y. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren Fail. (2022) 44:615–24.
37. Ebert T, Neytchev O, Witasp A, Kublickiene K, Stenvinkel P, Shiels P. Inflammation and oxidative stress in chronic kidney disease and dialysis patients. Antioxid Redox Signal. (2021) 35:1426–48.
38. Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dialysis Transpl. (2018) 33(suppl_3):iii35–40.
39. Andrade LS, Dalboni MA, Carvalho JT, Grabulosa CC, Pereira NB, Aoike DT, et al. In vitro effect of uremic serum on barrier function and inflammation in human colonocytes. J Brasil Nefrol. (2018) 40:217–24. doi: 10.1590/2175-8239-jbn-3949
40. Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. (2018) 2018:2180373. doi: 10.1155/2018/2180373
41. Lacasse É, Gudimard L, Dubuc I, Gravel A, Allaeys I, Boilard É, et al. SARS-CoV-2 Nsp2 contributes to inflammation by activating NF-κB. Viruses. (2023) 15:334.
42. Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med. (2013) 65:162–74.
43. Zhang C, Jiang H, Wang P, Liu H, Sun X. Transcription factor NF-kappa B represses ANT1 transcription and leads to mitochondrial dysfunctions. Sci Rep. (2017) 7:44708. doi: 10.1038/srep44708
Keywords: dietary inflammatory index, NHANES, population aging, estimated glomerular filtration rate, albuminuria, chronic kidney disease
Citation: Guo M, Lei Y, Liu X, Li X, Xu Y and Zheng D (2024) Association between dietary inflammatory index and chronic kidney disease in middle-aged and elderly populations. Front. Nutr. 11:1335074. doi: 10.3389/fnut.2024.1335074
Received: 08 November 2023; Accepted: 02 January 2024;
Published: 17 January 2024.
Edited by:
Alice Sabatino, University of Parma, ItalyReviewed by:
Dorota Formanowicz, Poznan University of Medical Sciences, PolandCopyright © 2024 Guo, Lei, Liu, Li, Xu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donghui Zheng, aGFleXpkaEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.