- 1Unit of Nephrology, Dialysis and Renal Transplantation - Fondazione IRCCS Ca’Granda Ospedale Maggiore Policlinico di Milano, Milan, Italy
- 2Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
Background: Protein restriction has been extended to stage 3 chronic kidney disease (CKD) regardless of age in the latest K-DOQI guidelines for the dietary management of patients with CKD. However, in elderly CKD patients there is a tendency to a spontaneous reduction in protein and energy intake that may impair the overall nutritional status. The aim of our study is to assess whether there are differences in malnutrition, exercise capacity and inflammatory status in elderly CKD patients with spontaneously low protein intake (sLPI) compared with patients with normal protein intake (NPI).
Methods: We performed a cross-sectional analysis of 123 incident patients. Malnutrition was assessed using Malnutrition Inflammation Score (MIS) and serum markers; As for physical performance, we used Short Physical Performance Battery (SPPB) and handgrip strength.
Results: We found that in older patients with advanced CKD, as many as 68% had low spontaneous protein intake, and they were more malnourished evaluated with MIS (25% vs. 10%, p = 0.033), protein-energy wasting (PEW) (43% vs. 14%, p = 0.002) and nPCR (0.63[0.51–0.69] vs. 0.95[0.87–1.1], p < 0.0001). They also had worse body composition, in terms of lower mid-arm muscular circumference (MAMC), fat tissue index (FTI) and higher overhydration (OH). sLPI patients also had higher levels of IL6 (4.6[2.9–8.9] vs. 2.8[0.8–5.1], p = 0.002). Moreover, sLPI patients were frailer (33% vs. 24%, p = 0.037) and had poorer physical performance especially when assessed with (SPPB) (7[5–9] vs. 9[7–10], p = 0.004) and gait test time (6.08 + 2 vs. 7.22 + 2.7, p = 0.04). sLPI was associated with lower physical performance [SPPB OR, 0.79 (0.46–0.97), p = 0.046] and malnutrition [MIS 1.6 (1.05–3.5), p = 0.041] independently from patients’ age and eGFR.
Conclusion: We found that in older patients with advanced CKD, up to 68% had low spontaneous protein intake and were frailer, more malnourished and with lower physical performance. These findings emphasize the importance of assessing patients’ needs, and personalized approaches with individual risk–benefit assessments should be sought. To achieve the best possible outcomes, targeted interventions should use all available tools.
1 Introduction
Latest K-DOQI guidelines on dietary management of patients with chronic kidney disease (CKD) extend the range of dietary interventions, particularly protein restriction, to stage 3 CKD regardless of age, and emphasize that optimization of protein intake is associated with reduced mortality and morbidity (1). However, in elderly CKD patients there is a tendency to a spontaneous reduction in protein and energy intake (2–5) that may impair the overall nutritional status. Therefore, the necessity to maintain a balance between the preservation of renal function and the prevention of malnutrition, may lead in clinics to therapeutic minimalism. Thus, nutritional interventions in elderly patients with CKD are far from being straight forward and homogeneously applicated (6).
The indications to prescribe a low protein diet irrespectively of age, as reported in the recent K-DOQI guidelines, are in apparent contradiction with the dietary indications for the elderly that have been expressed by other scientific societies. In particular, the recent ESPEN guidelines suggest that high-protein diets may help to counteract sarcopenia and malnutrition in the elderly (7, 8).
The aim of our study was to assess the prevalence of elderly patients with advanced CKD with a spontaneous reduction of protein intake among those that are incident in an outpatient CKD clinic. Furthermore, we evaluated whether in patients with spontaneously reduced protein intake (sLPI) there was any difference in malnutrition, sarcopenia and systemic inflammation when they were compared to patients with normal protein intake (NPI). These data will possibly help to develop tailored nutritional approaches and educational interventions based on individual risk–benefit assessment in elderly patients with advanced CKD.
2 Materials and methods
2.1 Patients and study design
Between 9/2016 and 3/2018, 123 incident elderly CKD patients attending our outpatient clinic were evaluated in this cross-sectional study. Inclusion criteria were: age ≥ 65 years, CKD stages 3a to 5 on conservative therapy and relatively stable eGFR over the previous 6 months (with less than 2 mL/min/1.73/m2 variation). eGFR was estimated using the CKD-EPI formula. To eliminate potential confounders, we excluded patients with cancer, cirrhosis and/or ascites. We also excluded patients taking immunosuppressive drugs and those with severe heart failure (NYHA class III-IV), nephrotic syndrome, thyroid disease, inflammatory bowel disease, and inability to cooperate. Patients who had been hospitalized in the previous 3 months were also excluded. Biochemical and urinary parameters were collected on the morning of the index visit after an overnight fast of at least 12 h. Written informed consent was obtained from the individuals and the study was conducted in accordance with the ICP Good Clinical Practice Guidelines. The study was approved by the Ethics Committee of our institution (Milano 2 approval n. 347/2010).
2.2 Body composition and nutritional status
Anthropometric measures included: body weight, height, body mass index (BMI), waist circumference (WC), mid arm circumference (MAC), tricipital and bicipital skinfold thickness (TST, BST; measured with a Harpenden skinfold caliper). The mid-arm muscle circumference (MAMC) was calculated as follows: MAMC (cm) = MAC (cm) − (πxTST (cm)); these measurements were taken on the dominant arm as described elsewhere (9).
Nutritional status was assessed using the Malnutrition Inflammation Score (MIS) and Protein Energy Wastage (PEW) criteria.
The MIS is an adaptation of the SGA questionnaire specifically for hemodialysis (HD) patients proposed by Amparo et al. (10) and Kalantar-Zadeh et al. (11). By adding some objective clinical and laboratory markers relevant to CKD, MIS transforms SGA into a semiquantitative scoring system. MIS has been validated against other nutritional/inflammatory biomarkers and is associated with poorer prognosis in patients on HD (10–12), peritoneal dialysis (13, 14), kidney transplantation (15) and in non-dialyzed CKD patients (10). The MIS is a composite score made up of 10 components, each with four levels of severity: from 0 (normal) to 3 (severely abnormal). A total score of 4–7 indicates risk of malnutrition and a score of ≥8 indicates malnutrition (16).
The diagnosis of PEW was made using the ISRNM criteria, which are divided into 4 distinct domains: serum chemistry, body mass, muscle mass, and dietary intake, with different indicators for each domain. A positive indicator in at least 3 of the domains is sufficient for the diagnosis of PEW (17).
Patients were divided into sLPI and NPI groups based on nPCR values (respectively: nPCR ≤0.8 g/kg or > 8 g/kg), which was estimated using the Maroni and Mitch formula (18).
Caloric intake was estimated using 3-day food diaries (filled in during the 3 days prior to the visits -Sunday to Tuesday) and then calculated using the Winfood nutritional software (Medimatica Srl, Teramo, Italy).
2.3 Frailty assessment
The frailty phenotype (FP) proposed by Fried et al. (19) was used for the assessment of frailty. Five components were used to define frailty: (1) involuntary weight loss ≥4.5 kg in 12 months; (2) exhaustion as feeling fatigued ≥4 days per week for more than 3 months; (3) weakness as handgrip strength <16 kg in women and < 27 kg in men; (4) slow walking as a 4-meter walk test speed >0.8 m/s; reduced physical activity as a score < 7 on a physical activity scale described elsewhere (11, 20). Patients with a score of three or more of the impaired items were classified as frail.
2.4 Physical performance
The Short Physical Performance Battery (SPPB) and handgrip strength were used to assess physical performance.
The SPPB includes: standing balance test, walking 4 meters, and time to get up from a chair five times (21). Each SPPB component test is scored from 0 to 4, with higher scores indicating better physical performance (22). Hand grip strength was measured using the Jamar dynamometer. Values <16 kg in women and < 27 kg in men were considered impaired (23).
2.5 Biochemical parameters
On the same days of the visits, all biochemical analyses to evaluate renal function, metabolic and nutritional status were performed in the central laboratory of our institution.
2.6 Detection of IL-6 serum levels, MCP-1 and TNF-alpha
Serum samples were frozen and stored at −80°C at the laboratory of nephrology of our Institution. Values were evaluated in duplicate by using enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions. We used these specific kits: Quantikine ELISA Human CCL2/MCP-1 Immunoassay DCP00, Human TNF-alpha ELISA Kit (Thermo Fisher Scientific, Monza, Italy), Quantikine HS ELISA Human IL-6 Immunoassay HS600B (R&D Systems, Space, Milano, Italy). Zero was included in each resulting curve as the last standard value. Results were validated by using Quantikine Immunoassay Control Group 1–4 or 10 (R&D Systems, Space, Milano, Italy). Absorbance readings were measured at 450 nm by spectrophotometer (Xenius Safas, Monaco).
2.7 Statistical analysis
All data are expressed as mean ± SD or median ± IQR, as appropriate. Between-group comparisons of parametric variables were made using Student’s t-test, while between-group comparisons of non-parametric variables were made using the Mann–Whitney U test. Intra-group comparisons of parametric variables were made using the paired t-test, while intra-group comparisons of non-parametric variables were made using the Wilcoxon test. Proportions and categorical variables were compared using the independent chi-squared test (χ2). Pearson or Spearman tests were used for regression analyses, as appropriate. Statistical significance was set at p < 0.05. SPSS software version 5.0.1 was used for all analyses.
3 Results
3.1 General population characteristics
We evaluated cross-sectionally 123 patients, whose general characteristics are depicted in Table 1.
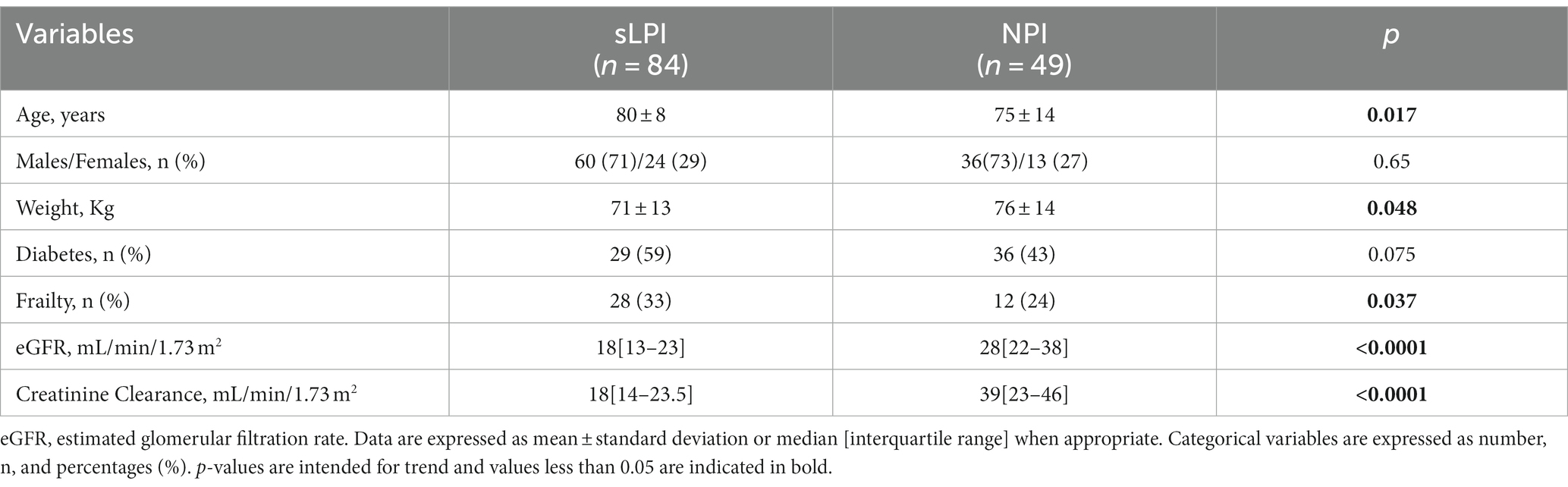
Table 1. Comparison of general characteristics between patients with spontaneous low proteins intake and normal protein intake.
sLPI patients, were generally older (80 ± 8 vs. 75 ± 14, p = 0.017) and has a higher prevalence of frailty [24, (33%) vs. 12, (24%), p = 0.037]. These patients also had markedly worse renal function parameters, such as lower eGFR (18[13–23] vs. 28[22–38], p < 0.0001), creatinine clearance (18[14–23.5] vs. 39[23–46], p < 0.0001) and higher serum urea (107[83–132] vs. 80[67–119], p = 0.04). No differences in terms of sex and diabetes prevalence were observed.
3.2 Correlations of protein intake with nutritional status, and physical performance
First of all, patients with sLPI had lower BMI when compared to NPI patients (25.9[24.2–29.6] vs. 27.7[25.0–32.2], p = 0.038).
sLPI were significantly more malnourished compared to NPI when malnutrition was assessed both with MIS (25% in sLPI vs. 10%in NPI, p = 0.033) or PEW (43% in sLPI vs. 14% in NPI, p = 0.002). Moreover, nPCR levels were significantly lower in sLPI patients, when compared to NPI ones (0.63[0.51–0.69] vs. 0.95[0.87–1.1]; p < 0.0001, respectively) (Table 2).
Concerning other biochemical nutritional markers, albumin (3.9[3.6–4.2] g/dL vs. 4.0[3.8–4.2] g/dL, p = 0.029), hemoglobin (11.6[10.8–12.8] vs. 13.0[12.1–13.8], p < 0.0001) and urinary urea excretion (13.1[11.0–15.6] vs. 21.7[18.4–24.3], p < 0.0001) were significantly lower in sLPI patients, when compared to NPI ones. No other differences were observed in terms of the remaining markers of iron status and regarding lipid profile (Table 2).
Important differences were also noticeable in terms of muscular mass and body composition between sLPI and NPI patients. Indeed, sLPI patients had lower MAMC (23.6[21.8–26.1] vs. 25.0[22.9–27.5], p = 0.049) and FTI (11.1[8.8–13.5] vs. 13.7[9.2–17.7], p = 0.049) and were more overhydrated (1.8[0.8–2.8] vs. 0.6[−0.1–1.4], p < 0.0001) when compared to NPI patients. No differences in terms of sarcopenia or LTI were noticed.
We also wanted to look for eventual differences in terms of physical performance among the patients of our cohort. sLPI had worse scores for physical performance compared to NPI patients evaluated as SPPB (7[5–9] vs. 9[7–10], p = 0.004) and gait test time (7.22 ± 2.7 vs. 6.08 ± 2, p = 0.04). Handgrip strength was not different among the two patients’ subgroups (Table 2).
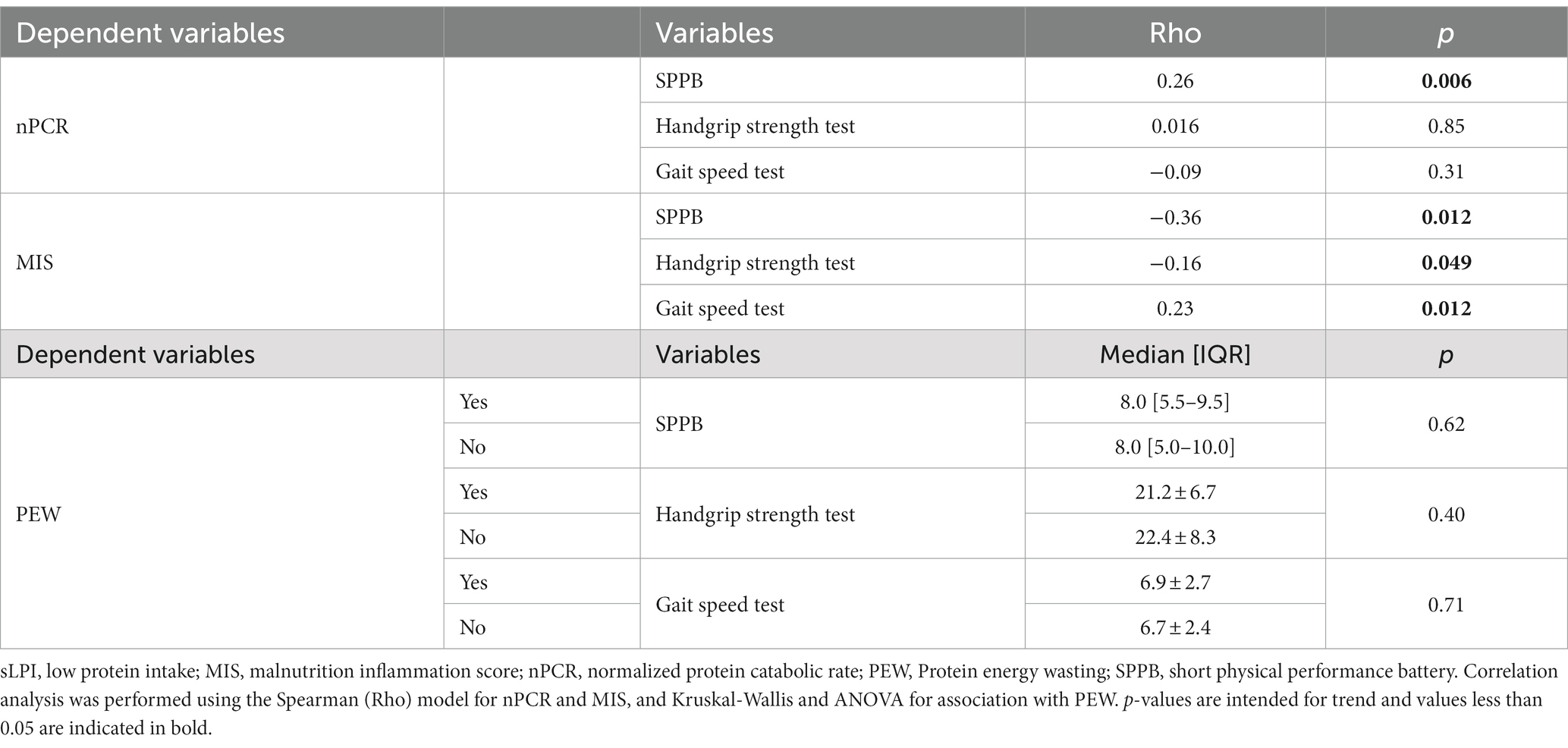
A worse overall nutritional status was correlated with impaired physical activity parameters. In particular, a higher MIS score strongly correlated with a worse overall physical performance, represented by lower SPPB (Rho −0.36, p < 0.0001), handgrip strength (Rho −0.16, p = 0.049) and longer gait speed test (Rho 0.23, p = 0.012). Regarding the other main indicators of nutritional status, lower nPCR significantly correlated with lower SPPB (Rho 0.26, p = 0.006) but not with other physical performance parameters, while PEW seemed not to be correlated with patients’ performance (Table 3).
3.3 Correlations of protein intake with overall inflammatory status
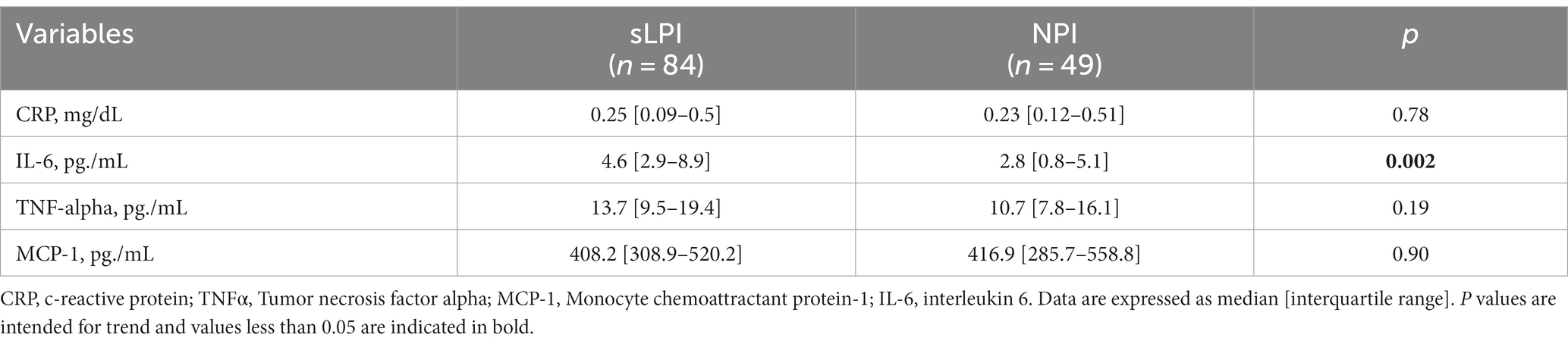
Among inflammatory markers IL-6 was markedly higher in sLPI patients (4.6[2.9–8.9] vs. 2.8[0.8–5.1], p = 0.002) while other markers were not different between the two groups (Table 4).
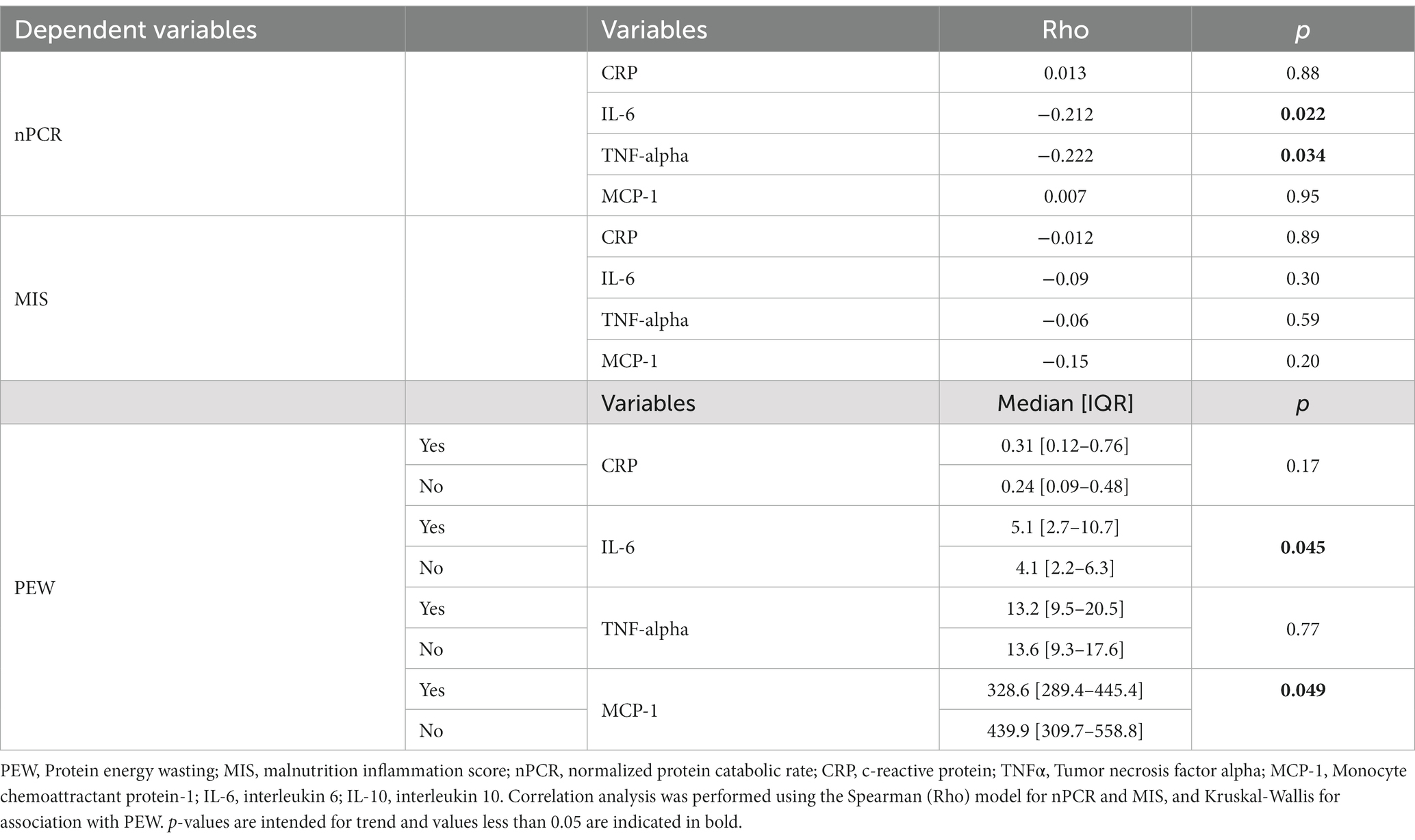
When we evaluated the correlation between inflammatory markers and nPCR (Table 5), we found an inverse correlation of protein intake (nPCR) with both IL-6 and TNF-alpha (Rho −0.212, p = 0.022 and Rho −0.222, p = 0.034 respectively). We also evaluated the correlation between inflammatory markers and other markers of nutritional status. Higher IL-6 and lower MCP-1 were associated with PEW (5.1[2.7–10.7] vs. 4.1[2.2–6.3], p = 0.045 and 328.6[289.4–445.4] vs. 439.9[309.7–558.8], p = 0.049 respectively), while we did not find any correlation with MIS.
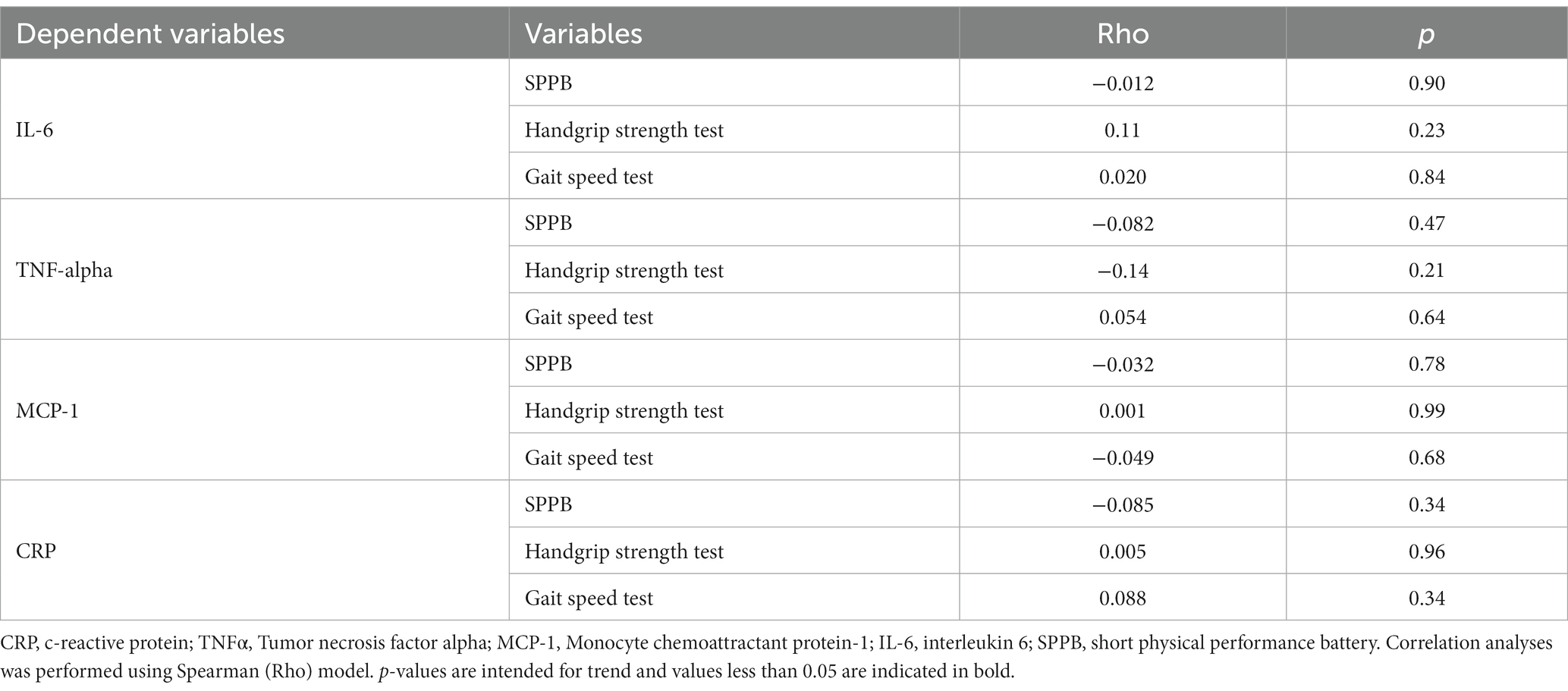
We also performed correlation between inflammatory markers and physical performance tests, but we did not find any correlation (Table 6).
3.3.1 sLPI is associated with malnutrition and worse physical performance
To confirm our findings, we performed a logistic regression evaluating the association between sLPI, malnutrition evaluated by MIS, frailty and physical performance parameters. Among the main risk factors for sLPI development, we inserted in this model eGFR and patients’ age, to correct for main confounders and to better understand the strength of eventual associations. sLPI was strongly associated with lower eGFR [OR, 0.9 (0.86–0.95), p < 0.0001]. However, sLPI remained associated with lower SPPB score [OR, 0.79 (0.46–0.97), p = 0.046] and malnutrition [1.6 (1.05–3.5), p = 0.041] independently from patients age and eGFR. The correlation between sLPI, frailty and handgrip was not confirmed in this analysis (Table 7).
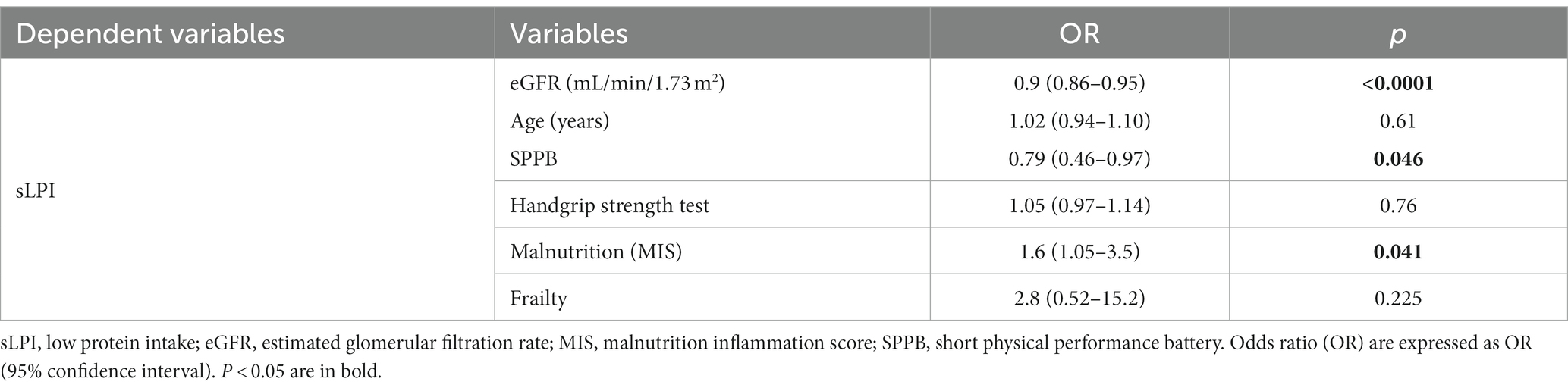
Table 7. Logistic regression analysis evaluating the associations of sLPI with MIS and eGFR and physical performance tests.
4 Discussion
Our study demonstrates that among elderly patients incident in an outpatients CKD clinic, almost two thirds have a spontaneous reduction in dietary protein intake. sLPI was also independently associated with malnutrition and worse performance status. In addition, sLPI was associated with systemic inflammation. Our findings support those of some other recent studies, which have highlighted the importance of personalizing the nutritional approach for elderly patients with CKD (24–29). The K-DOQI 2020 guidelines for nutrition in CKD recommend moderate to severe protein restriction from CKD stage 3 (1). Nutritional therapy based on reducing protein intake, together with a qualitative approach to nutrient selection, is currently the mainstay of nutritional management in CKD patients of all ages (1, 30). However, CKD is “per se” a major determinant of protein and energy wasting and it may synergistically act with other age-related risk factors and with the concurrent comorbidities to induce malnutrition in the elderly. A condition that is exacerbated when low protein consumption is accompanied by an inadvertent reduction in energy intake (31, 32). Therefore, these peculiarities may lead to a kind of trade-off in the elderly between the need to delay the progression of renal disease and the risk of malnutrition. In particular, the risk of malnutrition may become significant if the initial nutritional status and protein-energy requirements are not accurately assessed and adequate follow-up is not planned (33–36). However, despite these necessary cautions, the indication to reduce protein intake in patients with CKD is supported by clear pathophysiological considerations and reliable clinical evidence (30, 37–39).
The primary aim of our study was to investigate the clinical relevance of sLPI as a potential risk factor or surrogate for malnutrition. Strikingly, we documented that up to two-thirds of elderly patients with advanced CKD have sLPI and that this condition is associated with malnutrition, inflammation and reduced overall performance status. Therefore, the main concern arising from our findings is what dietary regimen should be used in elderly patients with sLPI. Previously, we showed in a pilot study that in elderly CKD patients at risk of malnutrition, prescribing a low-protein diet, if supported by adequate energy intake, is safe and may even lead to an overall improvement in nutritional status. However, in the current study, 25% of patients with sLPI were malnourished at MIS and 43% were affected by PEW, conditions in which LPD is contraindicated and indeed oral supplementation should be considered (1). Our data support the importance of carefully assessing the nutritional status of elderly CKD patients before prescribing a low protein diet, as recommended by the latest K-DIGO guidelines (1). However, we also recognize that a thorough nutritional assessment is time consuming and may be difficult to administer on a large scale. We propose the assessment of spontaneous protein intake derived from steady-state nPCR as a reliable and cost-effective marker to identify patients with sLPI, a condition that should prompt a more comprehensive nutritional assessment. As sLPI is often associated with malnutrition and PEW in elderly CKD patients, it may help to screen for patients who may benefit more from oral supplements rather than LPD (40–44). In these circumstances, supplementation with alpha-keto analogs and amino acid blends may also be a reasonable option for patients at risk or already affected by protein malnutrition (36, 40, 45).
Moreover, with regard to inflammatory markers, although CRP is the most commonly used marker in clinical practice due to its limited cost and ease of detection, part of the message of our article includes that other inflammatory markers could also be routinely included for the assessment of the nutritional and metabolic status of the CKD patient. Indeed, among patients with CKD, the coexistence of malnutrition and inflammation is a well-recognized condition (46–50).
Overall, our data support the evidence that the prescription of appropriate nutritional interventions in elderly patients with CKD should follow a stepwise approach based on individual assessment of renal inflammatory and nutritional status. Therefore, different combinations of renal and nutritional parameters may configure different scenarios of relative risks and benefits, and clinical decisions should prioritize renal or nutritional issues as needed. We are aware that there are several limitations to our study. Firstly, the associations of sLPI with malnutrition, inflammation and exercise capacity in elderly CKD patients cannot be attributed to causality because of the cross-sectional design. Second, our study is monocentric and our population is relatively small. However, by using a highly standardized protocol for patient selection, biochemical analyses and clinical observations, the monocentric nature of our study allowed us to reduce potential sources of bias. In particular, we excluded patients who might have spontaneously reduced their protein intake due to certain clinical conditions by using strict inclusion and exclusion criteria. To our knowledge, this is the first study to comprehensively assess the relationship among SLPI, nutritional status, inflammation, Physical performance and frailty in older non-dialyzed patients with advanced CKD.
5 Conclusion
In conclusion, we found that in older patients with advanced CKD, up to 68% had low spontaneous protein intake and were frailer, malnourished and had worse overall physical performance. These findings emphasize the importance of assessing patients’ needs, and personalized approaches with individual risk–benefit assessments should be sought. nPCR and MIS are simple and easy tools to assess protein intake and malnutrition that could be used by those who are not able to perform a refined assessment of patients’ nutritional status and protein and calorie intakes, and could ensure a sound nutritional approach in daily clinical practice for a large population of elderly CKD patients.
Data availability statement
The datasets analyzed for this study can be found in the OSF repository (https://osf.io/m8uyd/?view_only=45bae23631794e24a855ff3cebaccffc).
Ethics statement
The studies involving humans were approved by the Ethics Committee of our Institution (Milano 2- approval no. 347/2010). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
SV: Conceptualization, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. PM: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. SA: Methodology, Writing – original draft. GC: Funding acquisition, Resources, Writing – review & editing. LC: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Italian Ministry of Health—Current Research IRCCS.
Acknowledgments
We thank Daniela Rusconi and Lucia Baiguini for helping us with the collection of biological samples and Marina Balderacchi for organizing the logistics of the study.
Conflict of interest
SV served as consultant at advisory boards for Merk Sharp & Dohme and held a sponsorized lecture by Shär. LC worked as a consultant for Shär.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Erratum Regarding “KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update” (Am J Kidney Dis. 2020;76[3][suppl 1]:S1-S107). Am J Kidney Dis. (2021) 77:308. doi: 10.1053/j.ajkd.2020.11.004
2. Batsis, JA, and Villareal, DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
3. Cederholm, T, Barazzoni, R, Austin, P, Ballmer, P, Biolo, G, Bischoff, SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
4. Dent, E, Morley, JE, Cruz-Jentoft, AJ, Woodhouse, L, Rodríguez-Mañas, L, Fried, LP, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. (2019) 23:771–87. doi: 10.1007/s12603-019-1273-z
5. Cruz-Jentoft, AJ, and Sayer, AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
6. Kalinkovich, A, and Livshits, G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
7. Barazzoni, R, Bischoff, SC, Boirie, Y, Busetto, L, Cederholm, T, Dicker, D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. (2018) 37:1787–93. doi: 10.1016/j.clnu.2018.04.018
8. Norman, K, Haß, U, and Pirlich, M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. (2021) 13:2764. doi: 10.3390/nu13082764
9. Vettoretti, S, Caldiroli, L, Armelloni, S, Ferrari, C, Cesari, M, and Messa, P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD. Nutrients. (2019) 11:1378. doi: 10.3390/nu11061378
10. Amparo, FC, Kamimura, MA, Molnar, MZ, Cuppari, L, Lindholm, B, Amodeo, C, et al. Diagnostic validation and prognostic significance of the malnutrition-inflammation score in nondialyzed chronic kidney disease patients. Nephrol Dial Transplant. (2015) 30:821–8. doi: 10.1093/ndt/gfu380
11. Kalantar-Zadeh, K, Kopple, JD, Block, G, and Humphreys, MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2001) 38:1251–63. doi: 10.1053/ajkd.2001.29222
12. Rambod, M, Bross, R, Zitterkoph, J, Benner, D, Pithia, J, Colman, S, et al. Association of Malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. (2009) 53:298–309. doi: 10.1053/j.ajkd.2008.09.018
13. He, T, An, X, Mao, H-P, Wei, X, Chen, J-H, Guo, N, et al. Malnutrition-inflammation score predicts long-term mortality in Chinese PD patients. Clin Nephrol. (2013) 79:477–83. doi: 10.5414/CN107659
14. Ho, L-C, Wang, H-H, Chiang, C-K, Hung, K-Y, and Wu, K-D. Malnutrition-inflammation score independently determined cardiovascular and infection risk in peritoneal dialysis patients. Blood Purif. (2010) 30:16–24. doi: 10.1159/000316682
15. Molnar, MZ, Czira, ME, Rudas, A, Ujszaszi, A, Lindner, A, Fornadi, K, et al. Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis. (2011) 58:101–8. doi: 10.1053/j.ajkd.2010.11.027
16. Afşar, B, Sezer, S, Ozdemir, FN, Celik, H, Elsurer, R, and Haberal, M. Malnutrition-inflammation score is a useful tool in peritoneal dialysis patients. Perit Dial Int. (2006) 26:705–11. doi: 10.1177/089686080602600616
17. Fouque, D, Kalantar-Zadeh, K, Kopple, J, Cano, N, Chauveau, P, Cuppari, L, et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. (2008) 73:391–8. doi: 10.1038/sj.ki.5002585
18. Maroni, BJ, Steinman, TI, and Mitch, WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. (1985) 27:58–65. doi: 10.1038/ki.1985.10
19. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.M146
20. Vettoretti, S, Caldiroli, L, Porata, G, Vezza, C, Cesari, M, and Messa, P. Frailty phenotype and multi-domain impairments in older patients with chronic kidney disease. BMC Geriatr. (2020) 20:371. doi: 10.1186/S12877-020-01757-8
21. Legrand, D, Vaes, B, Matheï, C, Adriaensen, W, Van Pottelbergh, G, and Degryse, JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc. (2014) 62:1030–8. doi: 10.1111/jgs.12840
22. Treacy, D, and Hassett, L. The short physical performance battery. J Physiother. (2018) 64:61. doi: 10.1016/j.jphys.2017.04.002
23. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
24. Thilly, N . Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant. (2013) 28:2203–5. doi: 10.1093/ndt/gft235
25. Piccoli, GB, Nazha, M, Capizzi, I, Vigotti, FN, Scognamiglio, S, Consiglio, V, et al. Diet as a system: an observational study investigating a multi-choice system of moderately restricted low-protein diets. BMC Nephrol. (2016) 17:197. doi: 10.1186/s12882-016-0413-5
26. D’Alessandro, C, Piccoli, GB, Calella, P, Brunori, G, Pasticci, F, Egidi, MF, et al. “Dietaly”: practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. (2016) 17:102. doi: 10.1186/s12882-016-0296-5
27. Fois, A, Torreggiani, M, Trabace, T, Chatrenet, A, Longhitano, E, Mazé, B, et al. Quality of life in CKD patients on low-protein diets in a multiple-choice diet system. Comparison between a French and an Italian experience. Nutrients. (2021) 13:1354. doi: 10.3390/nu13041354
28. Piccoli, GB, Di Iorio, BR, Chatrenet, A, D’Alessandro, C, Nazha, M, Capizzi, I, et al. Dietary satisfaction and quality of life in chronic kidney disease patients on low-protein diets: a multicentre study with long-term outcome data (Torino-Pisa study). Nephrol Dial Transplant. (2020) 35:790–802. doi: 10.1093/ndt/gfz147
29. Piccoli, GB, Cederholm, T, Avesani, CM, Bakker, SJL, Bellizzi, V, Cuerda, C, et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease – implications for low protein intake and nutritional care: a critical review endorsed by ERN-ERA and ESPEN. Clin Nutr. (2023) 42:443–57. doi: 10.1016/j.clnu.2023.01.018
30. Kalantar-Zadeh, K, and Fouque, D. Nutritional management of chronic kidney disease. N Engl J Med. (2018) 378:583–5. doi: 10.1056/NEJMc1715765
31. Hanna, RM, Ghobry, L, Wassef, O, Rhee, CM, and Kalantar-Zadeh, K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and Cachexia in patients with chronic kidney disease. Blood Purif. (2020) 49:202–11. doi: 10.1159/000504240
32. Oliveira, EA, Zheng, R, Carter, CE, and Mak, RH. Cachexia/protein energy wasting syndrome in CKD: causation and treatment. Semin Dial. (2019) 32:493–9. doi: 10.1111/sdi.12832
33. Rosansky, SJ, Schell, J, Shega, J, Scherer, J, Jacobs, L, Couchoud, C, et al. Treatment decisions for older adults with advanced chronic kidney disease. BMC Nephrol. (2017) 18:200. doi: 10.1186/s12882-017-0617-3
34. Voorend, CGN, van Oevelen, M, Verberne, WR, van den Wittenboer, ID, Dekkers, OM, Dekker, F, et al. Survival of patients who opt for dialysis versus conservative care: a systematic review and meta-analysis. Nephrol Dial Transplant. (2022) 37:1529–44. doi: 10.1093/ndt/gfac010
35. Verberne, WR, van den Wittenboer, ID, Voorend, CGN, Abrahams, AC, van Buren, M, Dekker, FW, et al. Health-related quality of life and symptoms of conservative care versus dialysis in patients with end-stage kidney disease: a systematic review. Nephrol Dial Transplant. (2021) 36:1418–33. doi: 10.1093/ndt/gfaa078
36. Barrett, BJ, Parfrey, PS, Morgan, J, Barré, P, Fine, A, Goldstein, MB, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis. (1997) 29:214–22. doi: 10.1016/s0272-6386(97)90032-9
37. Hahn, D, Hodson, EM, and Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev. (2018) 10:CD001892. doi: 10.1002/14651858.CD001892.pub4
38. Marckmann, P, Osther, P, Pedersen, AN, and Jespersen, B. High-protein diets and renal health. J Ren Nutr. (2015) 25:1–5. doi: 10.1053/j.jrn.2014.06.002
39. Brenner, BM . Nephron adaptation to renal injury or ablation. Am J Phys. (1985) 249:F324–37. doi: 10.1152/ajprenal.1985.249.3.F324
40. Fois, A, Chatrenet, A, Cataldo, E, Lippi, F, Kaniassi, A, Vigreux, J, et al. Moderate protein restriction in advanced CKD: a feasible option in An elderly, high-comorbidity population. A stepwise multiple-choice system approach. Nutrients. (2018) 11:36. doi: 10.3390/nu11010036
41. Guo, Y, Zhang, M, Ye, T, Qian, K, Liang, W, Zuo, X, et al. Non-protein energy supplement for malnutrition treatment in patients with chronic kidney disease. Asia Pac J Clin Nutr. (2022) 31:504–11. doi: 10.6133/apjcn.202209_31(3).0017
42. Wong, MMY, Renouf, D, Zheng, Y, Sheriff, Z, and Levin, A. Nutritional status, nutritional phenotypes, and Oral nutritional supplement prescription patterns among patients with non-Dialysis chronic kidney disease in British Columbia. J Ren Nutr. (2022) 32:414–22. doi: 10.1053/j.jrn.2021.08.011
43. Yamasaki, T, and Kumagai, S. Nonwearable sensor-based in-home assessment of subtle daily behavioral changes as a candidate biomarker for mild cognitive impairment. J Pers Med. (2021) 12:11. doi: 10.3390/jpm12010011
44. Wong, MMY, Zheng, Y, Renouf, D, Sheriff, Z, and Levin, A. Trajectories of nutritional parameters before and after prescribed oral nutritional supplements: a longitudinal cohort study of patients with chronic kidney disease not requiring dialysis. Can J Kidney Health Dis. (2022) 9:205435812110690. doi: 10.1177/20543581211069008
45. Brown, EA, Blake, PG, Boudville, N, Davies, S, de Arteaga, J, Dong, J, et al. International society for peritoneal dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. (2020) 40:244–53. doi: 10.1177/0896860819895364
46. Flores, EA, Bistrian, BR, Pomposelli, JJ, Dinarello, CA, Blackburn, GL, and Istfan, NW. Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest. (1989) 83:1614–22. doi: 10.1172/JCI114059
47. Gabay, C, and Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54. doi: 10.1056/NEJM199902113400607
48. Ikizler, TA, Cano, NJ, Franch, H, Fouque, D, Himmelfarb, J, Kalantar-Zadeh, K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. (2013) 84:1096–107. doi: 10.1038/KI.2013.147
49. Kalantar-Zadeh, K, Ikizler, TA, Block, G, Avram, MM, and Kopple, JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. (2003) 42:864–81. doi: 10.1016/J.AJKD.2003.07.016
Keywords: protein intake, malnutrition, oral supplementation, chronic kidney disease (CKD), low protein diet
Citation: Vettoretti S, Molinari P, Armelloni S, Castellano G and Caldiroli L (2024) Spontaneous low-protein intake in older CKD patients: one diet may not fit all. Front. Nutr. 11:1328939. doi: 10.3389/fnut.2024.1328939
Edited by:
Refaat Hegazi, Abbott, United StatesReviewed by:
Adamasco Cupisti, University of Pisa, ItalyFrancesco Locatelli, Alessandro Manzoni Hospital, Italy
Copyright © 2024 Vettoretti, Molinari, Armelloni, Castellano and Caldiroli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simone Vettoretti, c2ltb25lLnZldHRvcmV0dGlAcG9saWNsaW5pY28ubWkuaXQ=
 Simone Vettoretti
Simone Vettoretti Paolo Molinari
Paolo Molinari Silvia Armelloni1
Silvia Armelloni1 Lara Caldiroli
Lara Caldiroli