
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 28 February 2024
Sec. Nutrition and Food Science Technology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1328620
 Gul Naz Saleem1,2
Gul Naz Saleem1,2 Ruixia Gu1,2*
Ruixia Gu1,2* Hengxian Qu1,2
Hengxian Qu1,2 Gul Bahar Khaskheli3
Gul Bahar Khaskheli3 Imran Rashid Rajput4
Imran Rashid Rajput4 Muhammad Qasim5
Muhammad Qasim5 Xia Chen1,2*
Xia Chen1,2*In the current arena of time, the transformation of society has improved the standard of living in terms of lifestyle and their nutritional demands and requirements. The microorganisms under controlled conditions and the enzymatic transformation of dietary components are the processes that resulted in fermented foods and beverages. Fermented dairy products with high nutritional value are “the pearls of the dairy industry.” During fermentation, fermented dairy products produce bioactive compounds and metabolites derived from bacteria. Research indicates the beneficial effects of probiotics found in dairy products on human health is making lightning-fast headway these days. The utilization of lactic acid bacteria as probiotics for the prevention or treatment of disease has been a driving force behind the discovery of novel potential probiotics found in naturally fermented milk. Probiotics such as lactic acid bacteria and bifidobacteria found in fermented dairy products have a variety of health benefits, including innate immune enhancement, diarrhea treatment, inflammatory bowel disease, diabetes, Tuberculosis, and obesity, relieving irritable bowel disease symptoms, preventing cancer, improving lactose tolerance, lowering cholesterol, enhancing antioxidant activity, and antimicrobial activity against pathogens. This review aims to evaluate the therapeutic efficacy and nutritional and microbiological properties of popular fermented dairy products and their health benefits.
Bioactive substances and vital nutrients abound in dairy products. The primary dairy product, milk, has lipids, sugar, and proteins including whey and casein (1). Yogurt is made by fermenting milk with certain bacteria and is renowned for its probiotics, which are good for the digestive system. Another derivative, cheese, varies in kind and aging procedure but delivers concentrated proteins, lipids, vitamins, and minerals (2). Fat-soluble vitamins and short-chain fatty acids are found in butter. The minerals calcium, phosphorus, and vitamins B12 and D included in these products are also vital for healthy bones. Dairy products also include bioactive peptides that have antioxidant and antibacterial qualities that may be beneficial to health (3). Moreover, dairy products such as milk can be fermented to produce yogurt and cheese etc. Fermentation is used for the conversion of carbohydrates into alcohol or acids via the action of microbes such as yeast and bacteria (4). This process improves the taste and shelf life of food as well as the availability of nutrients, adds beneficial microorganisms, facilitates digestion, and strengthens immunity (5). Yogurt, sauerkraut, kimchi, and kombucha are examples of fermented foods that have many health advantages such as they improve digestion, strengthening the immune system, improving gut health, and promoting nutrient absorption. They may also lower the risk of chronic diseases (2). Numerous nations utilize fermented milk products due to their health benefits. In emerging nations, particularly in Africa and Asia, most of their population uses fermented milk and products (6). Fermented foods can be described as food products or beverages produced by the controlled development of microorganisms and the enzymatic conversion of dietary constituents (7). The health advantages of fermented milk are contingent upon the functionality of living microbes, commonly known as starter cultures, in conjunction with the nutritional composition of the milk (8). These microbe’s presence enhances milk longevity by augmenting its acidity levels and facilitating the emergence of organoleptic characteristics, such as flavor and texture (9). Most of the microorganisms used in milk are probiotic and known as lactic acid bacteria (LAB) (6, 10). There is a possibility that certain LAB strains are employed as probiotics in the food industry. Lactic acid bacteria (LAB) play a vital role as dietary microorganisms, Lactic acid bacteria (LAB) are commonly obtained from diverse dietary sources, and strains exhibiting exceptional efficacy and strong competitive abilities are employed as probiotics. Recently, there has been a growing scholarly focus on extracting and analyzing lactic acid bacteria (LAB) from a diverse range of fermented food items and commodities.
The majority of microorganisms are “Generally Recognized as Safe (GRAS).” Globally consuming dairy products principally utilize the dietary sources for LAB to generate the milk into its unique and beneficial products (11, 12). In the past decade, the LAB has attracted the industry and frequently employed as probiotics, which offers health benefits to the host when taken in appropriate numbers (13). The utilization of LAB strains, namely Enterococcus spp., Lactococcus spp., and Lactobacillus spp., possessing antibacterial capabilities, has been employed in bio-control approaches aimed at diminishing mycotoxins and augmenting bioavailability (14, 15). Recent research has elucidated that probiotic lactic acid bacteria (LAB) strains had the potential to eliminate mycotoxins effectively. LAB has been shown to improve intestinal transit, keep intestinal flora in balance, and keep the acid–base balance in the colon. This helps to regulate the immune system and lower serum cholesterol levels. Improves the equilibrium of intestinal microorganisms to promote human health (16). Certain strains of lactic acid bacteria (LAB) have been observed to elevate the concentrations of pro-inflammatory markers such as TNF-α, IL-1β, and IL-6 while concurrently reducing the expression of anti-inflammatory markers (Arg 1, TGF-β, and CD206). This effect is achieved through the induction of macrophage polarization toward the M1 phenotype (17, 18). The worldwide interest in functional foods containing nutrients with potential health benefits in Europe, North America, and Asia accounts for up to 77% of the fermented milk and yoghurt business, which is presently worth €46 billion (19, 20). Among the fermented products, Yoghurt is also one of the world’s most widely consumed fermented dairy products owing to its health advantages beyond its essential nutritional value. Yoghurt is generally considered a nutrient-dense food due to its nutritional profile. A calcium-rich diet offers considerable quantities of calcium in the bioavailable form (21). Cheese is a globally recorded product made from the milk of ruminants via a mix of physical processes. Caseingniz and calcium are both essential for transforming milk into curd (22). In recent years, kefir’s significant health benefits have attracted the scientific community’s attention (23). In addition, Koumiss is a well-known dairy product made from fresh mare milk and contains a small amount of alcohol. It is naturally fermented with the original combination of yeasts and bacteria (lactic acid bacteria and yeast) (24). Mongolians use it to treat various diseases that have been globally well-known (25). Although numerous scientific studies have been reported on the therapeutic effects of dairy products on human health (Figure 1), this encompasses all the relevant data and mechanisms of dairy products on human health such as yoghurt, kefir, cheese, and koumiss due to their popularity and therapeutic effects. Also, this study seeks to describe and characterize popular fermented foods, their methods of action (including influence on microbiota), and their effects on human gastrointestinal health and illness.
The origins of yoghurt may be traced back to the Middle East and Western Asia, where it has since become a staple diet for many people (26). Yogurt is a rich source of several critical minerals, including calcium and protein. Various studies documented the therapeutic effects of yoghurt (Table 1) and it is considered a healthy food because it is easy to digest and the body absorbs nutrients easily. According to the committee on the medical aspects of food policy, one cup of yoghurt (245 g) provides 40% of the reference nutrient intakes (RNI) for calcium, 40% for phosphorus, 10% for potassium, 10% for vitamin A, 30% for vitamin B2, and 60% for vitamin B12 for males and females aged 19–50 (COMA) (27). Yoghurt consumers have been demonstrated to have much lower rates of riboflavin, vitamin B-12, calcium, magnesium, and zinc deficits than non-yoghurt consumers (28). However, most studies on the health advantages of yoghurt focus on its live bacterial content (2). Yogurt is a carrier of probiotics and can be divided into two categories: regular culture yoghurt and bio- or probiotic yoghurt. Standard yoghurt is manufactured using Lactobacillus delbrueckii subsp. bulgaricus and S. thermophilus bacteria. Bio yoghurts are tic strains (29). Eating yoghurts may also promote microbial diversity in the intestine (28). It is also indicated for gastrointestinal problems such as inflammatory bowel disease and irritable bowel illness, immunological function, lactose intolerance, and produced by cultivating extra helpful microbes, mainly Bifidobacterium and L. acidophilus (2, 29).
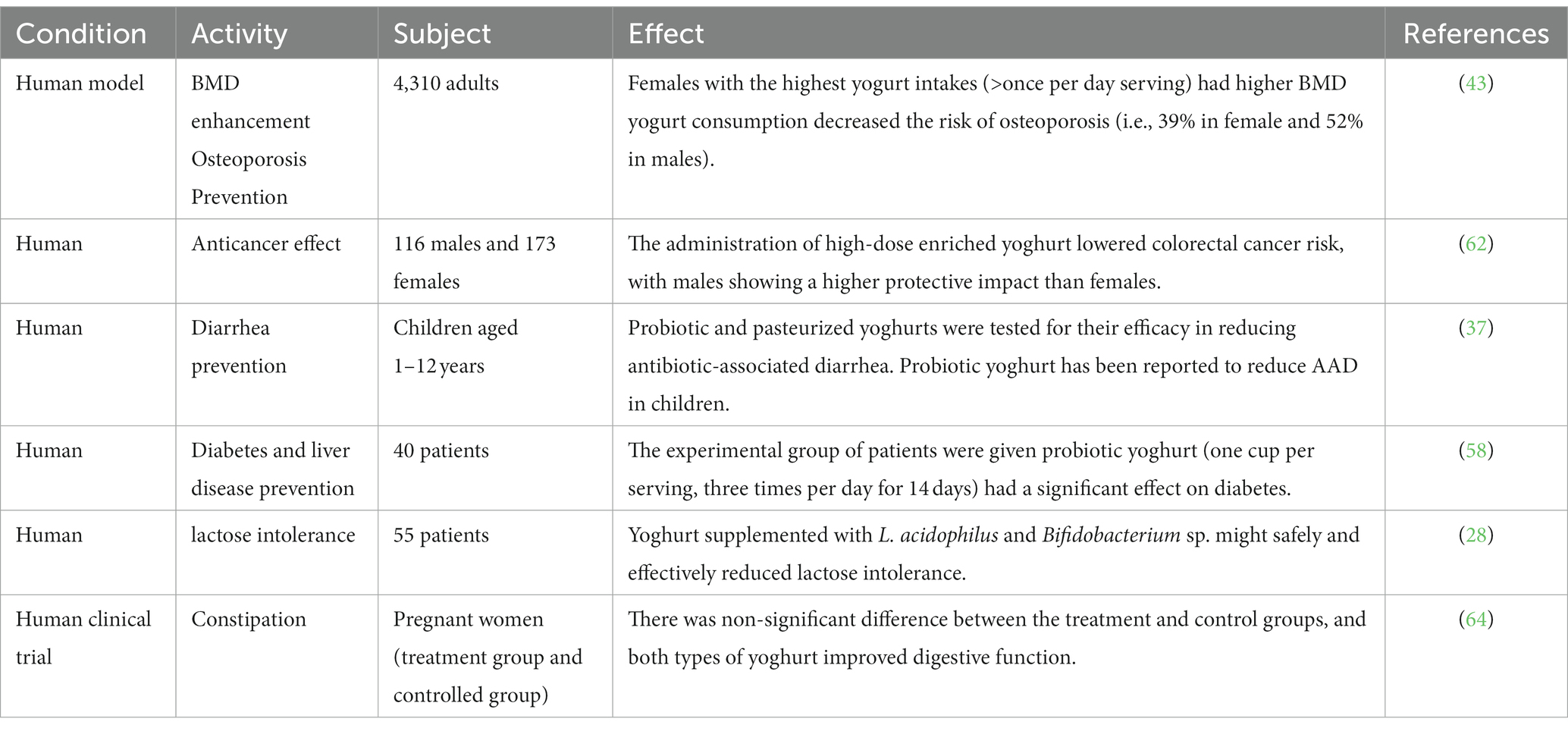
Table 1. Health promising therapeutic effects of yoghurt consumption documented in human against some major pathological disorders.
Obesity is an increased risk of chronic diseases and affects both industrialized and developing nations (30). According to the latest review and meta-analysis of 39 randomized controlled trials, probiotic fermented milk products might be used as adjuvant therapy to lower total cholesterol, LDL cholesterol, and triglycerides in the blood, particularly in men (31). Because of its probiotics, high protein content, low glycemic index, and calcium content, yogurt may help reduce weight. These elements support intestinal health, fullness, and effective metabolism, all of which lower caloric consumption frugis. Nevertheless, a person’s total diet and lifestyle determine how beneficial it is, thus going with basic, unsweetened versions is advised (32). Individuals who drank the most whole-fat yoghurt were substantially less likely to develop metabolic syndrome components such as abdominal obesity, hypertriglyceridemia, low HDL cholesterol, high blood pressure, and high fasting plasma glucose, according to Babio et al. (33). Yoghurt consumption was connected with a lower chance of gaining weight, according to a study that tracked three large cohorts of people for up to 20 years and included 120,877 obese men and women free of chronic conditions at the start of the trial. The authors postulated that shifts in the bacteria that live in the digestive tract could be responsible for observed variations in weight (34).
It is a frequent, typically brief ailment that many people experience yearly. Ingestion of probiotics in the weeks preceding a trip has been shown to lower the incidence of traveler’s diarrhea by up to 15% (35). A meta-analysis of 63 randomized controlled studies indicates that probiotics may reduce the duration of diarrhea caused by bacterial, viral, or parasite diseases by around 25 h (36). Fox et al. (37) investigated the efficacy of a probiotic yoghurt (200 g/day) containing Lacticaseibacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis, and Lactobacillus acidophilus in preventing antibiotic-associated diarrhea in children aged 1–12 with that of a pasteurized yoghurt. There is evidence that probiotic yoghurt decreases the prevalence of ADD in children. The effects of yoghurt for infants aged 6–24 months hospitalized with severe diarrhea were favorable. In addition to routine hospital treatment, at least 15 mL/kg/day of pasteurized cow milk yoghurt was administered orally to infants in the case group. According to the data, there were substantial improvements in the average number of hospitalized days, the frequency of diarrheal episodes, and weight gain (38). Probiotics, which restore gut flora balance, and lactic acid bacteria, which suppress pathogenic bacteria, are the main ways that yogurt helps manage diarrhea (39). While it is not a panacea for all diarrheas, it is simple to digest, helps with nutritional absorption, boosts immunity, and is appropriate for those with lactose sensitivity (40).
It is a serious disease caused by decreased bone mineral density (BMD) and is associated with a significantly increased fracture risk (41, 42). Evidence from the past suggests that consuming more calcium-rich dairy products may protect individuals against bone loss (21). Among the reviewed studies was an Irish study of over 4,000 individuals over the age of 60, in which a higher yoghurt intake was associated with a reduced risk of osteoporosis—a more significant effect than that observed with milk intake (43). Another researcher reported that fermented dairy products exhibit a notable impact on bone health in comparison to regular milk (44). In addition, probiotics have been found to impact the permeability of the intestinal wall. The gut microbiota plays a role in the breakdown of dietary minerals and can potentially enhance calcium absorption. The enhanced absorption of calcium may decrease the generation of parathyroid hormone, potentially causing a decline in bone reabsorption. The modulation of serotonin secretion may also increase bone growth (45). The major way that yogurt helps manage osteoporosis is because it contains a lot of calcium, which is good for strong bones. Enhanced with vitamin D, it also improves the absorption of calcium. Bone health is enhanced by the protein and other nutrients included in it. Yogurt is good, but it should only be used in conjunction with a whole osteoporosis treatment strategy that includes food, activity, and maybe medicine (46).
In the Framingham offspring study with 2,506 male and female participants, those who consumed a considerable amount of yoghurt (more than four servings per week) had a higher bone mineral density (BMD) at the trochanter than those who did not consume yoghurt (47). However, other locations in the skeleton showed no significant relationships. Additionally, research conducted on a cohort of 4,310 individuals found an association between the consumption of yoghurt and higher bone mineral density (BMD) and physical function ratings. Yoghurt was consumed by a significantly more significant proportion of females than men, and the average amount of yoghurt ingested by females daily was considerably more than that of males (0.42 vs. 0.32 servings per day, respectively). Consumption of yoghurt by females was an excellent predictor of bone mineral density across all areas, those females who consumed the most yoghurt (more than one serving per day) had higher total hip and femoral neck bone mineral density compared to those who consumed the least yoghurt (one serving per week or never). Men who did not consume yoghurt had a vertebral BMD that was 4.1% higher than those who consumed it but drank less of it. There was a correlation between increasing one’s consumption of yoghurt by one unit and a reduction of 31% in the incidence of osteopenia, 39% in the risk of osteoporosis in females, and 52% in the risk of osteoporosis in males (43).
People with lactose intolerance have stomach problems when they drink milk or eat milk products because they do not have enough lactase activity in their small intestines to digest the milk sugar lactose (48). Many studies have shown that lactose-intolerant can benefit from consuming fermented milk products because some lactic acid bacterial strain products secrete lactase into the digestive system (49). Recently in-depth research found that probiotics such as Lactobacillus spp., B. longum, and B. animalis had beneficial impacts, justifying the usage of probiotic yoghurt comprising L. acidophilus and Bifidobacterium spp. (50). Another study found that probiotic yoghurt supplemented with L. acidophilus and Bifidobacterium spp. may safely and successfully lower lactose intolerance symptoms and HBT. As a result of this discovery, our probiotic can be suggested as the therapy of choice for lactose intolerance in patients (28). Low levels of the enzyme lactase induce lactose intolerance, which makes it difficult to digest lactose in dairy products. Because yogurt has less lactose, bacteria assist in digestion, and tolerance levels vary, it might be beneficial (51). For those with severe intolerances, lactose-free alternatives like Greek yogurt are especially good. Moreover, supplements might help with digestion (52).
In 2015, 8.8% of the world’s adults, or 415 million people, had diabetes. By 2040, this number is expected to rise 10.4%, or 642 million people (53). Dairy products are an excellent source of vitamins, magnesium, vitamin D, and certain fatty acids. Moderate dairy consumption is associated with a reduced incidence of type 2 diabetes, according to research (54). The relationship between yoghurt intake and health advantages is more stable than those of other dairy products, for which results have been inconsistent. Daily yoghurt consumption may also lessen the incidence of cardiovascular disease and type 2 diabetes (55, 56). Two meta-analyses of prospective cohort studies found that daily intake of yoghurt decreased the risk of developing type 2 diabetes by 18 and 14% (54, 57). It is considered that beneficial bacteria in yoghurt may decrease inflammation or enhance the body’s natural insulin effectiveness. In addition to reducing the intestinal flora imbalance that patients with chronic liver disease experience, probiotic yoghurt significantly impacts persons with chronic liver disease. Forty patients in the experimental group were given probiotic yoghurt containing Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophilus three times daily for 14 days. This medication effectively decreased liver conditions, especially in diabetes (58). Because of its high nutritional content, low glycemic index, and probiotics for gut health, yogurt may help treat diabetes. It is a fantastic alternative to meals with a higher GI and may help lower inflammation. It’s preferable to choose low-sugar options, such as Greek yogurt. Nevertheless, it ought to support a well-balanced diet (59).
The World Health Organization ranks colorectal cancer (CRC) as the third most frequent form of the disease overall and the 2nd largest cause of cancer-related death (60). Yoghurt has an anti-cancer impact on the mucosa of the colon and rectum due to its microbial components (61). The 10 male participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort in Italy reaped the benefits of the protective effect of high-dose supplemented yoghurt to a greater extent than the nine female individuals (62). According to the findings of Margolis et al. (56), post-menopausal women who drink a greater quantity of yoghurt had a lower risk of acquiring diabetes. In a past study that included 1,183 men and women in Australia between the ages of 39 and 65, a cross-sectional examination revealed a significant connection between the use of low-fat yoghurt by males and self-reported measures of memory recall and social functioning (63), but it is still not fully understood that how the yoghurt acts as anti-cancerous effects.
A clinical investigation observed whether probiotic yoghurt may alleviate some pregnant women’s constipation problems. A randomization procedure and varied controls were utilized in this study. The experimental group drank 300 grams of Bifidobacterium and Lactobacillus-containing yoghurt, while the control group ate regular yoghurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus; each group carried out the experimental procedures in equal time, and neither group experienced any adverse effects. Regarding the enhancement of bowel function brought about by probiotics and plain yoghurt, there was no discernible difference between the treatment and control groups (64). Makino et al. (65) showed that regular ingestion of yoghurt containing live culture might increase the adult ‘s resistance to respiratory infections, especially in a cold atmosphere. Kefir is a little sour and alcoholic fermented milk product with a creamy consistency (66). In the experimental group, 40 individuals consumed probiotic yoghurt three times daily for 14 days. It contained Streptococcus thermophilus, Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus delbrueckii subsp. bulgaricus. This medication considerably improved hepatic conditions, especially diabetes (67).
As yogurt is rich in probiotics, it enhances the gut health and indirectly boosting the immune system. This phenomenon aids in controlling respiratory infections, highlighting the gut-lung axis interplay Daniel. The probiotics load in the yogurt can also directly inhibit the pathogens and reduce inflammation, thereby contributing to overall human health and respiratory infection resistance (68).
Kefir has a carbohydrate content of 3%, a lipid content of 3.5%, a protein content of 3%, and an ash content of 0.7%. When it is safe to consume, kefir has a wealth of vitamins (69). During fermentation, acid coagulation and proteolysis improve protein digestion. The amino acid profile of kefir is identical to the amino acid profile of fermented milk (70). Kefir has elevated ammonia, serine, lysine, alanine, and threonine (71), as well as tryptophan, valine, lysine, methionine, phenylalanine, and isoleucine (72). Kefir is a rich source of magnesium, calcium, and phosphorus. Milk kefir also contains essential minerals such as Zinc, Copper, Manganese, Iron, Cobalt, and Molybdenum (73). Kefir, and kefir-related strains, have been shown to have a significant impact on health as presented in Table 2.
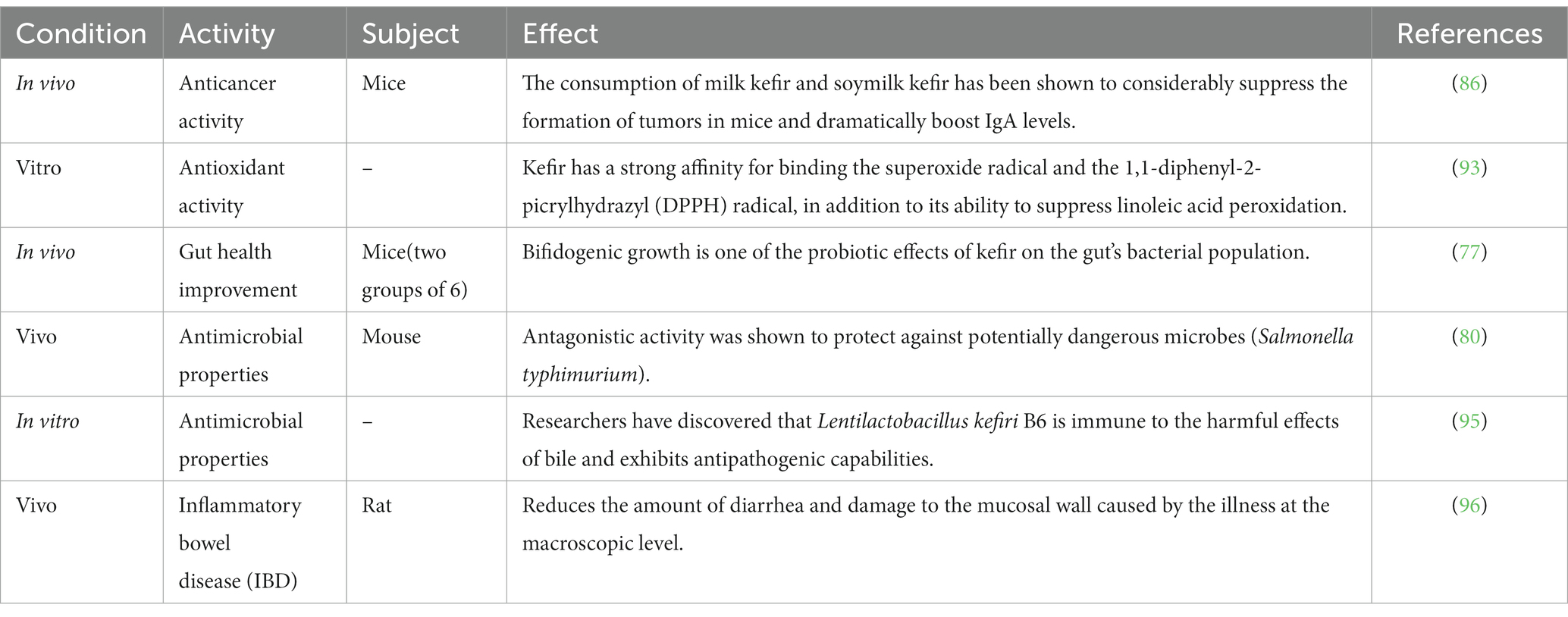
Table 2. Invitro and In vivo evidences of therapeutics benefits offered by kefir “a functional dairy foods.”
Kefir provides beneficial bacteria with probiotic effects. Several bacterial species isolated from kefir exhibit extraordinary resistance to the gastrointestinal system’s low pH and bile salts, as well as the capacity to adhere to intestinal mucus (74). Furthermore, the bacteria in kefir may produce antimicrobial substances such as organic acids and bacteriocins (75) and interfere with the adhesion of pathogenic bacteria in the intestinal mucosa, possibly contributing to gut health improvement (76). In the intervention group (n = 6), it was similarly found in vivo with a daily dose of 0.75–1 mg of dairy. The animal units were divided into two groups of six, Kefir a probiotic affects gut bacterial populations by boosting bifidogenic bacteria (77). Additionally, kefir regulates digestion, strengthens the immune system, and has anti-inflammatory properties. It helps in lactose digestion, rehabilitates the gut lining for better nutrient absorption, and reduces allergy and asthma risks, making it beneficial for overall gastrointestinal wellbeing (78).
According to research conducted in the early twentieth century, the good impact of regular yogurt eating, including lactic acid-producing microorganisms, on life expectancy was related to the struggle between LAB and dangerous pathogens. Kefir is said to be bactericidal in Gram-negative bacteria, although it is more effective against Gram-positive bacteria (79). A mouse model of Salmonella typhimurium infection control was used in research conducted by Cordeiro et al. (80). The results of that investigation have shown that fermented dairy beverages that are widely eaten protec against pathogenic bacteria. Oral kefir feeding to mice for 28 days resulted in increased levels of Lactobacillus and Bifidobacterium in the animal feces while simultaneously reducing the amount of Clostridium perfringens was seen (81). Studies have shown that Shigella, Staphylococcus, Helicobacter pylori, Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, Bacillus subtilis, and Micrococcus luteus all exhibit traits that are hostile to one another (79, 82, 83). Kefir was also shown to be antibacterial when tested against Candida albicans, Escherichia coli, Staphylococcus aureus, Salmonella typhi, and Shigella sonnei (75). It exhibits antimicrobial properties due to being loaded with probiotics, organic acids, and bioactive compounds which work together to inhibit harmful bacteria, fungi, and viruses, lower pH, and outcompete pathogens for nutrients and boost the immune system (84).
The second leading cause of mortality worldwide is cancer. However, Weir et al. (85) reported that a healthy diet might prevent up to 50% of cancers. Therefore, kefir’s probiotics are vital as a possible co adjuvant treatment or cancer prevention. Throughout the years, several in vitro and in vivo studies have demonstrated the anti-cancer potential of kefir. The mechanisms by which kefir exerts its anticancer effect is shown in Figure 2. Numerous cancer forms, including hematological malignancies, breast cancer, digestive tract tumors, and sarcoma, were examined for the anti-carcinogenic efficacy of kefir and kefir fractions. In 2002, Liu et al. reported that milk kefir and soymilk kefir intravenously to mice with sarcoma. After 30 days of ingestion, both forms of kefir effectively inhibited tumor development by inducing apoptotic cell death in tumor cells. They considerably boosted IgA levels in mice, suggesting that both forms of kefir possess anti-cancer capabilities and enhanced mucosal resistance to gastrointestinal infection (86). Kefir supernatant has been studied as a potential adjuvant for doxorubicin (DOX) therapy because of its chemo-sensitizing effects on multidrug-resistant (MDR) human colorectal cancer cells (HT-29) (87). Kefir and DOX contributed to an increase in intracellular ROS buildup in HT-29 MDR-developed cells, resulting in a down regulation of ABC transporters. Researchers observed that all of the bacterial strains that were isolated from kefir have a high potential to adhere to mutagens (>985%), which may then be eliminated via feces, therefore maintaining colonocytes (88). In addition, Khoury et al. (89) found that kefir induces apoptosis and inhibits the development of HT 29 and Caco 2 colorectal cancer cells. Kefir also suppresses the growth of colorectal cancer cells.
Several biochemical experiments have been used to determine that kefir has antioxidant capabilities (90, 91). These qualities changed during the fermentation and aging processes. Kefir exhibited more potent antioxidant effects than vitamin E when tested in a mouse toxicity study with carbon tetrachloride (CCl4) conducted by Güven et al. (92). Liu and colleagues investigated the antioxidant capacity of kefir produced from goat and cow milk. Many researchers found that kefir has the potential to bind DPPH and superoxide radicals as well as reduce the amount of linoleic acid that was peroxidized (93). Additionally, the DPPH radical scavenging ability and the inhibitory effects on linoleic acid autoxidation were improved. Consequently, the TPC and its ability to inhibit ascorbate autoxidation were found to be better upon kefir utilization (94).
Koumiss has long been considered a great meal and beverage with potent medicinal properties (97). Koumiss was traditionally being produced by inoculating raw, unpasteurized mare’s milk in tanks (98). The important microorganisms in Koumiss are lactic acid bacteria, which convert lactose into lactic acid, and yeast, contributing to the 3–8-h fermentation (24). Koumiss therapeutic services are typically supplied by small and medium-sized lodging businesses, especially in rural parts of Asian nations (99). Koumiss has many fatty and protein-containing nutrients, vitamins, amino acids, carbohydrates, and trace mineral elements (100). Due to its lower fat content, mare milk has lower calories (480 kcal kg/l) than human or bovine milk. Furthermore, it is abundant in vitamins C, A, E, D, B1, B2, and B12, trace minerals, and antibiotics (101, 102). Koumiss being a well-known probiotic beverage, contains all of the essential amino acids required by humans, including proline, lysine, tyrosine, valine, and leucine (102). Koumiss’s microflora consists primarily of LAB cultures, including Lactobacillus delbrueckii subsp. bulgaricus, Lactiplantibacillus plantarum, and Lactobacillus helveticus L. casei L. acidophilus and two species of yeast, Kluyveromyces marxianus and Saccharomyces cerevisiae (103). Koumiss’s nutritional and therapeutic characteristics qualify it as a functional food (104). Health promoting benefits of Koumiss are highlighted in Table 3, out of those some of them are due to its high probiotic content, antibacterial and antifungal properties, regulation of immunity, maintenance of a healthy gastrointestinal tract, regulation of cholesterol and blood sugar levels, regulation of blood pressure, and induction of several essential vitamins etc. As a functional food component, Koumiss has generated considerable industry interest due to its great potential in treating several health disorders (105). Recently, the intake of koumiss in meals has been regarded as a healthy eating trend in European countries (106).
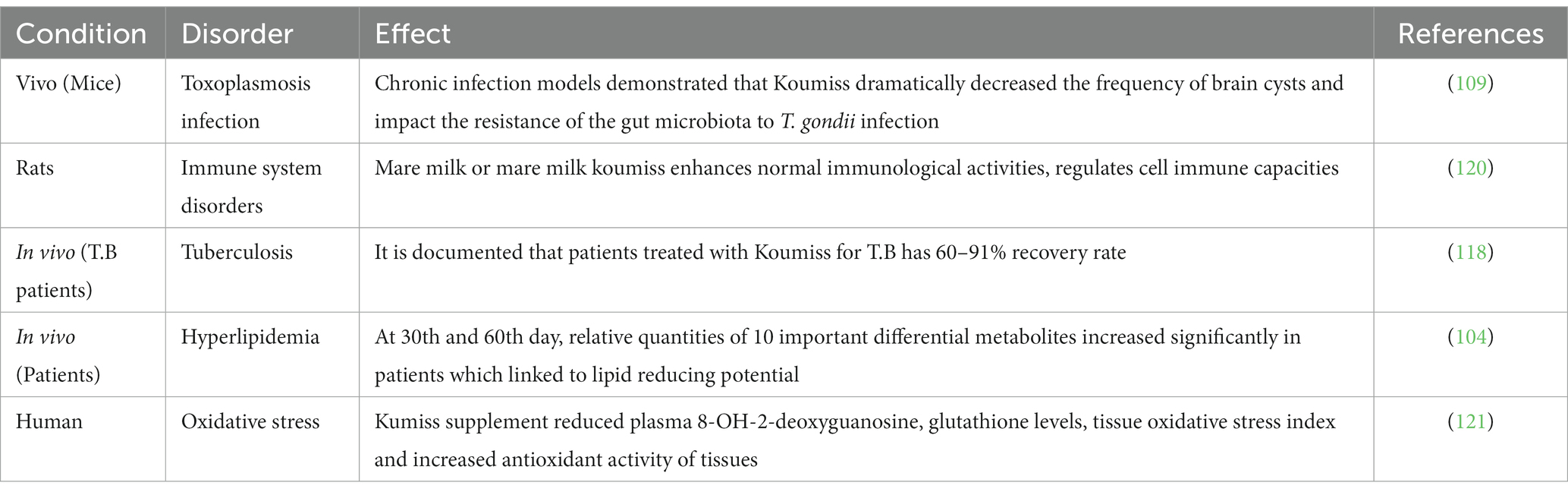
Table 3. Health promising therapeutic effects of Koumiss documented against some major pathological disorders.
The inhibitory impact of lactic acid antibodies on pathogenic microbes may be clearly shown when tested in vitro. Yeasts are the most prevalent microorganisms in Koumiss; they are essential for the fermentation process and are responsible for offering therapeutic advantages to the consumers. According to research carried out by Etienne-Mesmin et al. (107), certain strains of yeast can inhibit the growth of E. coli by producing antibacterial compounds, such as killer toxins and organic acids, during their metabolic processes. It has been determined that yeast strains and Koumiss have antibacterial substances that are efficient against E. coli. Four antibacterial substances have been identified from Koumiss yeasts (108). Three Gram-negative bacteria, three Gram-positive bacteria, and five pathogenic Escherichia coli strains were used to test the antibacterial properties of beneficial yeast (Kluyveromyces marxianus and Saccharomyces cerevisiae). The antibacterial chemicals produced by yeasts in Koumiss are found to be more effective against Gram-positive than Gram-negative bacteria. Koumiss considerably affected chronic T. gondii infection in mice and might ameliorate acute T. gondii infection signs. Surprisingly, chronic infection models demonstrated that Koumiss dramatically decreased the frequency of brain cysts in mice (p < 0.05), improved amyloid deposition in the hippocampus (p < 0.01), and decreased the levels of IFN- and TNF (p < 0.01, p < 0.05), also, Koumiss may impact the resistance of the gut microbiota to T. gondii infection. The research provides additional evidence for the development of safe and effective anti-T. Gondii strategies and expands our understanding of the potential use of Koumiss (109).
The World Health Organization (WHO) predicts that by 2030, heart disease will continue to be the leading cause of death, affecting around 23.6 million people. Hypercholesterolemia is one of the leading hazards of CHD (110). Hyperlipidemia is a condition of lipid metabolism characterized by high total cholesterol and triglycerides in the blood (TGs). The pharmaceutical industry has developed several treatments for these disorders. However, long-term use of hyplipidemic drugs is associated with several deleterious consequences (111).
Consequently, dietary modification has become an intriguing alternative treatment method for efficiently reducing blood lipids (112). Koumiss is excellent to consume due to its abundant minerals and probiotic microorganisms (113). Moreover, Dönmez et al. (102) found that drinking Koumiss decreased the levels of TGs and cholesterol in the blood of individuals with hyperlipidemia. Zhang et al. (114) also discovered that the probiotic strain derived from Koumiss could reduce dyslipidemia and hyperlipidemia. Another research (13 patients aged 43–57 treated with kumiss) revealed that frequent ingestion of koumiss regulated not only the blood cholesterol level but also the makeup of the gut flora in individuals with hyperlipidemia (104). Before and after koumiss medication, the fecal metabolomes of hyperlipidemia patients who ingested Koumiss daily were examined. At days 30 and 60, relative quantities of 10 important differential metabolites (ursolic acid, linoleic acid, stearic acid, −tocotrienol, −tocotrienol, alanine, tyrosine, sphingosine, acetate, and butyrate) increased significantly. These results showed that the 10 discovered metabolites were probably linked to the observed lipid-lowering effect (115).
Tuberculosis (TB) is one of the most prevalent infectious diseases globally, with 1, 2 million fatalities expected in 2019 (116). A Russian physician identified the efficacy of sour mare’s milk in treating 41 patients with pulmonary TB, observed remarkable patient improvement, and decided to include Koumiss in medical practices (117). Initially, Mongolian physicians used Koumiss to cure TB and included it in their therapeutic practices. At the Ximeng Mongolian Medical Research Institute, Koumiss effectively treats TB every summer and fall. In actual practice, treatment with Koumiss for TB patients has resulted in a 60–91% recovery rate, as validated by X-rays and tuberculosis tests. The lack of symptoms is indicative of therapeutic effectiveness (118). To avoid TB, the Cossacks have incorporated Koumiss into military meals (119).
About 80% of the body tissues are located in the intestines, and a regular meal of Koumiss boosts the immune system. Bacteria from fermented meals create substances that penetrate the intestinal wall and promote the immune system’s production of immune cells (25). Mare milk improves the weight of immune organs in rats, enhances normal immunological activities, regulates cell immune capacities, and regulates abnormal immune systems in bodily fluids (120) (Figure 3). The immunomodulatory and anti-inflammatory properties of kefir are very important and will be effective through the following mechanisms.
Many illnesses are connected to oxidative stress. It was discovered that Dimethylhydrazine (DMH) induced oxidative stress increases plasma 8-OH-2-deoxyguanosine, tissue oxidative stress index, and total oxidant capacity. It was discovered that the Kumiss supplement reduced these levels, increased the overall antioxidant capacity of the tissue, and decreased glutathione levels (121). The effect of lactobacilli on the suppression of lipid peroxidation was investigated. As various regions of the LAB are rooted in the duodenum, which is essential for releasing intracellular contents, the inhibitory effects of cell free intracellular extracts on lipid peroxidation have also been investigated. In all investigated strains, weak cells and internal CFE display antioxidative activity. However, intracellular CFE suppressed linoleic acid peroxidation at a considerably higher rate than weak lactobacilli cells recovered from Koumiss samples (122).
In conventional cheese production, a coagulating agent such as rennet, acid, heat and acid, or a combination thereof is utilized to transform aqueous milk into a semisolid state (123). Although cow’s milk is the typical ingredient in the production of cheese, sheep or goat milk is also utilized in the production of some varieties (124). Other agents may be incorporated alongside the starter, contingent upon the specific cheese variety and manufacturing conditions (125). In the context of maturation and storage, it is not necessary to mature fresh cheeses, including cream and cottage cheese, but it is necessary to mature hard cheeses, including Swiss and cheddar. In general, aged cheeses are produced using rennet curd. Unique flavors develop in a fresh clot during maturation due to the action of probiotics and enzymes (126).
The wide variety of coagulants that can be employed to coagulate milk into a gel structure is referred to as rennet. Bovine calf rennet, predominantly composed of chymosin, has conventionally been employed in the production of cheese (127). However, the expansion of the cheese industry and the constrained availability of calf rennet have prompted an exploration of alternative calf rennet. An additional source of rennet has been identified (128). Table 4 summarizes the different sources of rennet used for cheese production. The introduction of the bovine chymosin coding sequence into microorganisms has facilitated the widespread availability of fermentation-produced chymosin (FPC) (129). A number of researchers have been exploring rennet substitutes from plants and some of which have been applied to industrial-scale cheese production with control conditions (130, 131).
The use of rennet can impact the first breakdown of proteins. In a study conducted by Sheehan et al. (133), it was demonstrated that Mozzarella cheese with lower fat content, produced using R. pusillus proteinase, exhibited elevated amounts of pH 4.6 soluble nitrogen during the ripening process. This was in contrast to cheeses manufactured with either FPC or R. miehei proteinase. Prior studies (137, 138) have already documented the influence of FCC on the characteristics of Cheddar and Mozzarella. The C/P ratio of FCC is significantly higher than that of bovine chymosin, indicating a more excellent milk clotting action and overall proteolytic activity. According to Bansal et al. (139), after 150 days of ripening, FCC produces full-fat Cheddar cheese that is firmer and requires more chewing. This cheese has a lower level of initial breakdown of proteins and has reduced bitter and brothy flavors. Cheddar cheeses that are low in fat and created with FCC exhibit comparable characteristics of being firmer, more resistant to chewing, and having a reduced bitterness compared to cheeses made with FPC, as stated by Govindasamy-Lucey et al. (140). The cheeses manufactured with reduced rennet concentration exhibited a slower rate of increase in the concentration of 12% trichloroacetic acid soluble nitrogen during ripening. There was no significant impact on the concentration of αs-CN and the hardness or springiness of the cheese. A study conducted by Moynihan et al. (137) discovered that low-moisture part-skim Mozzarella cheese exhibited comparable hardness despite variations in rennet concentrations during its production. Plant rennets have become a subject of growing interest in cheese industry, due to their easy availability and simple purification processes. Plant based rennet sometime effects sensory attributes of final products but the selection of appropriate milk or its ultrafiltration, the mixture of coagulants as well as the increase of salting time of cheese during ripening could be efficient ways to improve texture and reduce bitterness (141).
Cheese is a globally popular dairy food product, particularly among young peoples. Historically, cheese has largely concentrated milk protein with the extended shelf life. It is very essential to the healthy American, Asian, and European diet (142). Numerous health benefits of cheese have been mentioned in Table 5. It is a very desirable food product due to its great nutritional content, the flexibility of its applications, and the organoleptic qualities (143). Cheese is a source of vital nutrients, including proteins, fats, minerals, and vitamins (144). The high fat cheese and protein content make it an energy-dense, nutrient-rich diet ideal for our laborious ancestors (145). It is widely proven that cheese supplies almost all necessary amino acids above the amounts recommended for infants or adults (146). Lactoferrin is the multifunctional glycoprotein that ranges from 672 μg g−1 (soft cheese) to 1,218 μg g−1 (semi-hard cheese) in the cheese (147). These proteins have several physiological functions, such as host defense against diverse range of pathogens, iron homeostasis, anticancer, antiviral, and anti-inflammatory activities (148). It is rich in fat-soluble vitamins such as vitamin K2 (149, 150), vitamin A, and vitamin E (151). Cheese is neutral food category that may be included in a balanced diet (152). Calcium intake from it, not only aids in maintaining strong bones but also significantly lowers blood pressure and aids in weight loss when combined with low-calorie meals (145).
Ripened cheese is lactose-free, rendering it is appropriate for the dietary needs of individuals who are lactose intolerant. A portion of the lactose is initially removed with the whey during the cheese maturation process; the remaining lactose is fermented into lactic acid, acetic acid, diacetyl, acetaldehyde, ethanol, and CO2 (153). The absence of lactose in matured cheese is a benefit for the majority of adults (154). Around 70% of adults worldwide suffer from lactose intolerance in maturity; milk consumption induces a variety of unpleasant symptoms, including abdominal pain, diarrhea, nausea, and flatulence (155). It is not, however, essential that these individuals abstain from dairy products. With the rare exception of soft cheese and fresh cheese, all other varieties of cheese do not contain lactose. Consequently, individuals with lactose intolerance are able to partake in the consumption of these cheeses, which support a nutritious diet by virtue of their essential components, including calcium (156).
The impact of the maturation process on the enhancement of the advantageous properties of cheese has been the subject of research utilizing animal models. One study administered diabetic db/db C57BL/J mice to various varieties of cheese that had ripened for 35 days; the effects were assessed using blood profiles, hepatic lipid content, and glucose tolerance (p < 0.05). Consumption of 35-day-ripened cheese enhanced glucose tolerance significantly without affecting insulin secretion, resulting in a substantial reduction in lipid peroxide markers (mRNA expression of TBARS and NADPH-oxidase) in fatty tissues, with no discernible impact on body weight, food intake, or fat mass. Furthermore, a substantial reduction was observed in the hepatic lipid content of rodents (157).
Cheese containing probiotic bacteria provides several health benefits, such as; improved oral hygiene due to less hypo salivation and dry mouth (158). It has been shown that acids produced by plaque bacteria during the fermentation of carbohydrates and starches because dental caries by dissolving tooth enamel. Caries is still the most prevalent dental disease (159), despite improving its incidence due to better prevention. Chewing a piece of cheese after consuming a sugary food quickly returns plaque pH to neutral (160) Cheese helps in management of hypo-salivation and dental caries through the stimulation of saliva production, neutralizing the oral pH, and providing calcium and phosphate minerals for tooth enamel remineralization. Its antibacterial properties stimulate anti cariogenic bacteria, while proteins reduce enamel demineralization through the formation of protective layer against acid and further dental decay (161, 162). According to the findings of Jensen et al. (163) not all cheeses are equally effective at preventing a fall in the pH of plaque. The preventive effects of fresh and young cheese appear lower than those of aged cheese.
However, cheese also includes a significant amount of saturated fatty acids (SFAs). Blood LDL-cholesterol levels are typically viewed as an indication of the risk of cardiovascular disease. SFAs have been related to elevate LDL-cholesterol levels (CVD) (164). Cheese has been linked to an elevated risk of cardiovascular disease due to the presence of saturated fatty acids in its composition. Regular consumption of whole milk, sour milk, or cheese (≥ 1 serving•d − 1) was found to be negatively correlated with weight gain and to have a reduced risk of cardiovascular disease (CVD) (165). Particularly pertinent in this circumstance appear to be calcium and bioactive peptides. Allender et al. conducted a meta-analysis of 28 studies and found that calcium supplementation significantly decreased systolic blood pressure in both hypertensive and non-hypertensive participants. Cheese contains an abundance of bioactive peptides. One of the most intriguing and extensively researched biological functions among these peptides is their ability to inhibit angiotensin-converting enzyme (ACE). By inactivating the hypotensive effect of bradykinin and facilitating the conversion of angiotensin I to the highly potent vasoconstrictor angiotensin II, ACE is a crucial enzyme in the regulation of blood pressure. Peptides that inhibit the activity of ACE have been found to exert a beneficial impact on hypertension (166). Numerous studies have demonstrated the ACE-inhibitory properties of diverse cheese variants, attributing this effect to a variety of bioactive peptides (167, 168). Within this collection of ACE-inhibiting peptides, the tripeptides valyl-prolyl-proline (VPP) and isoleucyl-prolyl-proline (IPP) are among the most efficacious. The intestine readily assimilates peptides containing a C-terminal Pro-Pro sequence, and research has demonstrated that such peptides are relatively resistant to additional degradation by digestive proteases and peptidases (169). IPP and VPP are encrypted within the milk protein β-casein. Proteinases derived from L. helveticus, a lactic acid bacteria, possess the capability to liberate the two peptides above from fermented milk. Rats with spontaneous hypertension that were fed sour milk fermented with specific strains of L. helveticus exhibited a hypotensive effect in numerous in vivo studies (170, 171), in addition to humans (172–174). On the basis of the prevalence of L. helveticus as a strain utilized in cheese production and the intensive proteolysis that occurs during ripening, it was hypothesized that cheese might also exhibit ACE-inhibiting activity via the formation of VPP and IPP. The concentrations of VPP and IPP in 44 traditional cheese varieties (Swiss and non-Swiss cheeses) were found to vary considerably, ranging from 0 to 224 mg/kg−1 and 0 to 95 mg/kg−1, respectively. Low concentrations were observed in soft cheese, followed by average concentrations in semi-hard and hard cheeses, and finally, high concentrations in extra-hard cheeses (175).
Rheumatoid arthritis (RA) is the inflammatory auto-immune disease that, when prolonged, can lead to joint abnormalities, cartilage, and bone damages (176). It targets comparatively more women than men (177). It has been observed that the disruption of gut microbiota may alter immune function by increasing pro-inflammatory substances, which may exacerbate the symptoms of rheumatoid arthritis (178, 179). Several research articles in the past decades have shown that rheumatoid patients have changed microflora in their digestive systems (RA). It has been established that probiotic-based treatments dramatically enhance patients’ ability to recover. In this context, patients with rheumatoid arthritis participated in a clinical trial to examine the effects of probiotic cheese on inflammatory and anti-inflammatory markers, disease severity, and symptoms. It has been demonstrated that consuming probiotic cheese can reduce inflammation and enhance the microbiota in the stomach, both of which can have a beneficial effect on the severity and symptoms of the disease (180). Also, it has been established that healthy elderly adults who consumed cheese containing both L. rhamnosus HN001 and L. acidophilus had enhanced their immunological response. Furthermore, consuming cheese can also mitigate some of the immune system declined associated with aging. By producing specific metabolites, cheese containing specific LAB species can also inhibit the growth of certain toxigenic microorganisms. Some cheese LAB species exhibit antimutagenic and antigenotoxic properties also (181). Thus, it may decrease the risk of cancer (182). Cheese act as anti-arthritis agent via acting for enhanced calcium and protein absorption and support bone health and tissue repair (183).
Several chronic diseases have been associated with oxidative stress (184). Food items have been fortified with synthetic antioxidants to prevent oxidative degradation, although they may represent possible health hazards (185). From this perspective, the present market needs more natural antioxidants with chemotherapeutic and preservation capabilities. The bioactive peptides extracted from fermented dairy products might be included in functional diets to lessen the risk of oxidative stress-related chronic illnesses (186). The antioxidant activity of bioactive peptides identified in Cheddar has been documented in multiple studies (187–190). In the study, assessed the antioxidant capacity of WSE in Cheddar cheese produced at various phases of ripening, both with and without adjunct cultures. The findings indicated that the antioxidant activity exhibited a dependence on the stage of maturation. The radical scavenging activity of 2,2′-casino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was found to be higher in Cheddar cheese produced with adjunct cultures. The activity peaked in the fourth month of ripening (16.61 and 9.76 μmol of TE/mg of protein, respectively, for Cheddar cheese produced with and without adjunct cultures). The Trolox-equivalent antioxidant capacity (TEAC) exhibited a consistent upward trend throughout the ripening process and reached its peak value of 9.81 μmol of TE/mg of protein (187). According to an in vitro study by Huma et al. (191), the WSPs (water-soluble peptides) extracted from Cheddar cheese can protect the intestinal epithelium from oxidative stress due to its antioxidant properties. Caco-2 cells were shown to be resistant to radical-mediated oxidation when exposed to a WSPs extract produced from Cheddar cheese. In addition, cheese has a low lactose content, making it an excellent choice for lactose-intolerant individuals (192). The inseparable tradition and enjoyment of cheese should not be forgotten amidst this scientific research. Cheese act as antioxidant agent effects through its vitamins (A, E, K), selenium, and minerals provision abilities (193).
Further research is required to identify the primary determinants of the ability to produce cheese with consistently high concentrations of the two bioactive peptides described Cheese consumption was found to have a causally inverse relationship with cardiovascular diseases, including type 2 diabetes, heart problems, coronary heart disease, hypertension, and ischemic stroke, as well as cardiovascular biomarkers, such as; body mass index (BMI), waist circumference (WC), triglycerides (TG), and fasting glucose (FG). Additionally, there were no effects have been reported for blood pressure or inflammatory indicators (195). There are significant evidences in the scientific literature that various probiotics supplemented through various dairy products including cheese are providing health promoting properties in the consumers (176, 186). An overview of the beneficial compounds produced by microorganisms in dairy products are presented in Table 6.
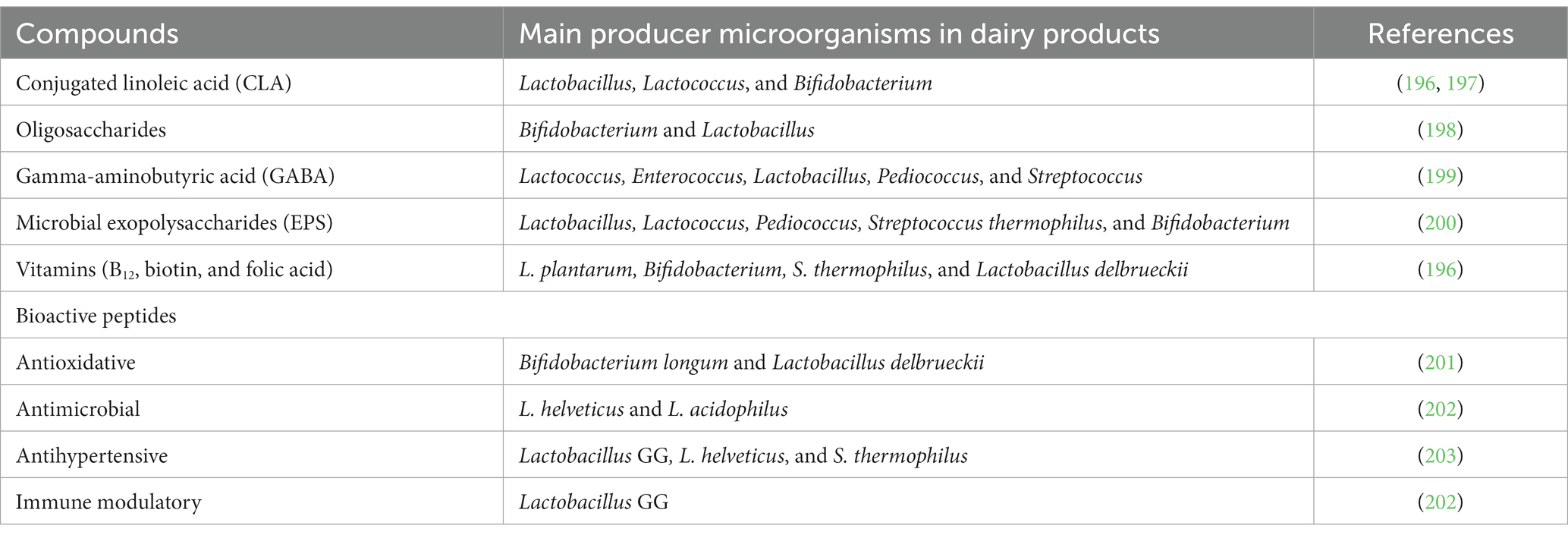
Table 6. Beneficial and detrimental microbial compounds that can be released in fermented dairy products during fermentation and the main producer microorganisms.
Fermented foods make up approximately 33% of diets in Asia, whereas they make up 60% of diets in developing countries. It has become abundantly obvious from an accumulation of studies that consuming fermented milk produces good health effects in several pathological disorders. Additionally, to improve gut health and modulate the immune system, these benefits also reduce the risk of osteoporosis, cardiovascular disease, and diabetes. Considering the growing interest showing that yogurt and fermented milk have health benefits, it may be wise to promote their frequent usage as nutritious additions to meals and tasty substitutions for other snack options. Furthermore, as fermented dairy foods, especially yogurt, kefir, cheese, and koumiss are generally accepted and consumed, they could serve as a perfect vehicle for delivering functional components, providing alternate methods for disease prevention, and promoting overall health. The stability of functional components and their interactions with the product matrix must be thoroughly investigated to ensure the functional potential of novel products throughout their commercial life. The growing body of supporting evidence from the published research is highly encouraging; it should serve as a driving force for the food industry to produce novel functional dairy products that are not currently available on the market.
GS: Conceptualization, Writing – original draft. RG: Writing – review & editing. HQ: Writing – review & editing. GB: Resources, Writing – original draft. IR: Resources, Writing – review & editing. MQ: Writing – review & editing. XC: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 31972094) and the National Key Research and Development Program of China (No. 2019YFF0217602).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Farag, MA, Jomaa, SA, Abd El-Wahed, A, and El-Seedi, The many faces of kefir fermented dairy products: quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients (2020) 12::346, doi: 10.3390/nu12020346
2. Marco, ML, Heeney, D, Binda, S, Cifelli, CJ, Cotter, PD, Foligné, B, et al. Effect of Arabian yogurt and white cheese on some physiological parameters of human. Curr Opin Biotechnol. (2017) 44:94–102. doi: 10.1016/j.copbio.2016.11.010
3. Gaba, K, and Anand, S. Incorporation of probiotics and other functional ingredients in dairy fat-rich products: benefits, challenges, and opportunities. Dairy. (2023) 4:630–49. doi: 10.3390/dairy4040044
4. Liu, A, Yang, X, Guo, Q, Li, B, Zheng, Y, Shi, Y, et al. Microbial communities and flavor compounds during the fermentation of traditional Hong Qu glutinous rice wine. Foods. (2022) 11:1097. doi: 10.3390/foods11081097
5. Şanlier, N, Gökcen, BB, and Sezgin, AC. Health benefits of fermented foods. Crit Rev Food Sci Nutr. (2019) 59:506–27. doi: 10.1080/10408398.2017.1383355
6. Jatmiko, YD, Howarth, GS, and Barton, MD. Naturally fermented milk and its therapeutic potential in the treatment of inflammatory intestinal disorders In: AIP Conference Proceedings. United Kingdom: AIP Publishing (2018).
7. Dimidi, E, Cox, SR, Rossi, M, and Whelan, K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. (2019) 11:1806. doi: 10.3390/nu11081806
8. Pilevar, Z, and Hosseini, H. Effects of starter cultures on the properties of meat products: a review. Annu Res Rev Biol. (2017) 17:1–17. doi: 10.9734/ARRB/2017/36330
9. Ravyts, F, De, VL, and Leroy, F. Bacterial diversity and functionalities in food fermentations. Eng Life Sci. (2012) 12:356–67. doi: 10.1002/elsc.201100119
10. Reuben, RC, Roy, PC, Sarkar, SL, Alam, ASMRU, and Jahid, IK. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J Dairy Sci. (2020) 103:1223–37. doi: 10.3168/jds.2019-17092
11. Shehata, MG, El Sohaimy, SA, El-Sahn, MA, and Youssef, MM. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann Agric Sci. (2016) 61:65–75. doi: 10.1016/j.aoas.2016.03.001
12. Khedid, K, Faid, M, Mokhtari, A, Soulaymani, A, and Zinedine, A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol Res. (2009) 164:81–91. doi: 10.1016/j.micres.2006.10.008
13. Markowiak, P, and Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. (2018) 10:21–17. doi: 10.1186/s13099-018-0250-0
14. Campagnollo, FB, Ganev, KC, Khaneghah, AM, Portela, JB, Cruz, AG, Granato, D, et al. The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: a review. Food Control. (2016) 68:310–29. doi: 10.1016/j.foodcont.2016.04.007
15. Deepthi, BV, Somashekaraiah, R, Poornachandra Rao, K, Deepa, N, Dharanesha, NK, Girish, KS, et al. Lactobacillus plantarum MYS6 ameliorates fumonisin B1-induced hepatorenal damage in broilers. Front Microbiol. (2017) 8:2317. doi: 10.3389/fmicb.2017.02317
16. Riane, K, Sifour, M, Ouled-Haddar, H, Idoui, T, Bounar, S, and Boussebt, S. Probiotic properties and antioxidant efficiency of Lactobacillus plantarum 15 isolated from milk. J Microbiol Biotechnol Food Sci. (2021) 9:516–20. doi: 10.15414/jmbfs.2019/20.9.3.516-520
17. Christoffersen, TE, Hult, LTO, Kuczkowska, K, Moe, KM, Skeie, S, Lea, T, et al. In vitro comparison of the effects of probiotic, commensal and pathogenic strains on macrophage polarization. Probiot Antimicrob Prot. (2014) 6:1–10. doi: 10.1007/s12602-013-9152-0
18. Guha, D, Banerjee, A, Mukherjee, R, Pradhan, B, Peneva, M, Aleksandrov, G, et al. A probiotic formulation containing Lactobacillus bulgaricus DWT1 inhibits tumor growth by activating pro-inflammatory responses in macrophages. J Funct Foods. (2019) 56:232–45. doi: 10.1016/j.jff.2019.03.030
19. Marsh, AJ, Hill, C, Ross, RP, and Cotter, PD. Fermented beverages with health-promoting potential: past and future perspectives. Trends Food Sci Technol. (2014) 38:113–24. doi: 10.1016/j.tifs.2014.05.002
20. Nazhand, A, Souto, EB, Lucarini, M, Souto, SB, Durazzo, A, and Santini, A. Ready to use therapeutical beverages: focus on functional beverages containing probiotics, prebiotics and synbiotics. Beverages. (2020) 6:26. doi: 10.3390/beverages6020026
21. Weerathilake, W, Rasika, DMD, Ruwanmali, JKU, and Munasinghe, M. The evolution, processing, varieties and health benefits of yogurt. Int J Sci Res Publ. (2014) 4:1–10.
22. McSweeney, PLH, Fox, PF, Cotter, PD, and Everett, DW. Cheese: chemistry, physics and microbiology. International Journal of Scientific and Research Publications. (2017) 4.
23. Azizi, NF, Kumar, MR, Yeap, SK, Abdullah, JO, Khalid, M, Omar, AR, et al. Kefir and its biological activities. Foods. (2021) 10:1210. doi: 10.3390/foods10061210
24. Danova, S, Petrov, K, Pavlov, P, and Petrova, P. Isolation and characterization of Lactobacillus strains involved in koumiss fermentation. Int J Dairy Technol. (2005) 58:100–5. doi: 10.1111/j.1471-0307.2005.00194.x
25. Kondybayev, A, Loiseau, G, Achir, N, Mestres, C, and Konuspayeva, G. Fermented mare milk product (Qymyz, koumiss). Int Dairy J. (2021) 119:105065. doi: 10.1016/j.idairyj.2021.105065
26. Salama, HH, and Bhattacharya, S. Advancement of yogurt production technology. in Advances in dairy microbial products. Eds. Joginder Singh, Ashish Vyas, India: Woodhead Publishing. (2022). 117–31.
27. Alhaj, OA, Altooq, NJ, Alenezi, AF, Janahi, AI, Janahi, MI, Humood, AM, et al. Camel milk composition by breed, season, publication year, and country: a global systematic review, meta-analysis, and meta-regression. Compr Rev Food Sci Food Saf. (2022) 21:2520–59. doi: 10.1111/1541-4337.12943
28. Masoumi, SJ, Mehrabani, D, Saberifiroozi, M, Fattahi, MR, Moradi, F, and Najafi, M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Sci Nutr. (2021) 9:1704–11. doi: 10.1002/fsn3.2145
29. Hadjimbei, E, Botsaris, G, and Chrysostomou, S. Beneficial effects of yoghurts and probiotic fermented milks and their functional food potential. Foods. (2022) 11:2691. doi: 10.3390/foods11172691
30. Pei, R, Martin, DA, DiMarco, DM, and Bolling, BW. Evidence for the effects of yogurt on gut health and obesity. Crit Rev Food Sci Nutr. (2017) 57:1569–83. doi: 10.1080/10408398.2014.883356
31. Ziaei, R, Ghavami, A, Khalesi, S, Ghiasvand, R, and Mokari Yamchi, The effect of probiotic fermented milk products on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis (2021) 31: 997–1015, doi: 10.1016/j.numecd.2020.12.023
32. Shlisky, JD, Durward, CM, Zack, MK, Gugger, CK, Campbell, JK, and Nickols-Richardson, SM. An energy-reduced dietary pattern, including moderate protein and increased nonfat dairy intake combined with walking promotes beneficial body composition and metabolic changes in women with excess adiposity: a randomized comparative trial. Food Sci Nutr. (2015) 3:376–93. doi: 10.1002/fsn3.231
33. Babio, N, Becerra-Tomás, N, Martínez-González, MÁ, Corella, D, Estruch, R, Ros, E, et al. Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J Nutr. (2015) 145:2308–16. doi: 10.3945/jn.115.214593
34. Mozaffarian, D, Hao, T, Rimm, EB, Willett, WC, and Hu, FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. (2011) 364:2392–404. doi: 10.1056/NEJMoa1014296
35. McFarland, LV, and Goh, S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. (2019) 27:11–9. doi: 10.1016/j.tmaid.2018.09.007
36. Allen, SJ, Martinez, EG, Gregorio, GV, and Dans, LF. Probiotics for treating acute infectious diarrhoea. São Paulo Med J. (2011) 129:185. doi: 10.1590/S1516-31802011000300012
37. Fox, MJ, Ahuja, KDK, Robertson, IK, Ball, MJ, and Eri, RD. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open. (2015) 5:e006474. doi: 10.1136/bmjopen-2014-006474
38. Pashapour, N, and Lou, SG. Evaluation of yogurt effect on acute diarrhea on 6-24 months old hospitalized infants. Turk J Pediatr. (2006) 48:115–8.
39. Sharif, A, Kheirkhah, D, Esfandabadi, PS, Masoudi, SB, Ajorpaz, NM, and Reza, SM. Comparison of regular and probiotic yogurts in treatment of acute watery diarrhea in children. J Probiotics Heal. (2017) 5:1–6. doi: 10.4172/2329-8901.1000164
40. Samir Ahmed El-husseiny, H, and Mohammed, AH. Effect of probiotic yogurt compared to traditional yogurt on management of antibiotic associated diarrhea among children. Egypt J Heal Care. (2023) 14:663–72. doi: 10.21608/ejhc.2023.301670
41. David, K, Narinx, N, Antonio, L, Evenepoel, P, Claessens, F, Decallonne, B, et al. Bone health in ageing men. Rev Endocr Metab Disord. (2022) 23:1173–208. doi: 10.1007/s11154-022-09738-5
42. Shevroja, E, Cafarelli, FP, Guglielmi, G, and Hans, D. DXA parameters, trabecular bone score (TBS) and bone mineral density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine. (2021) 74:20–8. doi: 10.1007/s12020-021-02806-x
43. Laird, E, Molloy, AM, McNulty, H, Ward, M, McCarroll, K, Hoey, L, et al. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos Int. (2017) 28:2409–19. doi: 10.1007/s00198-017-4049-5
44. Aryana, KJ, and Olson, DW. A 100-year review: yogurt and other cultured dairy products. J Dairy Sci. (2017) 100:9987–10013. doi: 10.3168/jds.2017-12981
45. Silva, BC, Costa, AG, Cusano, NE, Kousteni, S, and Bilezikian, JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Investig. (2011) 34:801–10. doi: 10.3275/7925
46. McGrail, L, Noel, S, Maldonado-Contreras, A, and Mangano, K. The effect of daily yogurt supplementation on inflammation and bone biomarkers. Curr Dev Nutr. (2021) 5:34. doi: 10.1093/cdn/nzab033_034
47. Sahni, S, Tucker, KL, Kiel, DP, Quach, L, Casey, VA, and Hannan, MT. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: the Framingham offspring study. Arch Osteoporos. (2013) 8:119–9. doi: 10.1007/s11657-013-0119-2
48. Deng, Y, Misselwitz, B, Dai, N, and Fox, M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. (2015) 7:8020–35. doi: 10.3390/nu7095380
49. Sen, M. Role of probiotics in health and disease–a review. Int J Adv Life Sci Res. (2019) 2:1–11. doi: 10.31632/ijalsr.2019v02i02.001
50. Oak, SJ, and Jha, R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr. (2019) 59:1675–83. doi: 10.1080/10408398.2018.1425977
51. Savaiano, DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. (2014) 99:1251S–5S. doi: 10.3945/ajcn.113.073023
52. Arrigoni, E, Marteau, P, Briet, F, Pochart, P, Rambaud, JC, and Messing, B. Tolerance and absorption of lactose from milk and yogurt during short-bowel syndrome in humans. Am J Clin Nutr. (1994) 60:926–9. doi: 10.1093/ajcn/60.6.926
54. Gijsbers, L, Ding, EL, Malik, VS, De Goede, J, Geleijnse, JM, and Soedamah-Muthu, SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr. (2016) 103:1111–24. doi: 10.3945/ajcn.115.123216
55. Ivey, KL, Lewis, JR, Hodgson, JM, Zhu, K, Dhaliwal, SS, Thompson, PL, et al. Association between yogurt, milk, and cheese consumption and common carotid artery intima-media thickness and cardiovascular disease risk factors in elderly women. Am J Clin Nutr. (2011) 94:234–9. doi: 10.3945/ajcn.111.014159
56. Margolis, KL, Wei, F, de Boer, IH, Howard, BV, Liu, S, Manson, JE, et al. A diet high in low-fat dairy products lowers diabetes risk in postmenopausal women. J Nutr. (2011) 141:1969–74. doi: 10.3945/jn.111.143339
57. Chen, M, Sun, Q, Giovannucci, E, Mozaffarian, D, Manson, JE, Willett, WC, et al. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. (2014) 12:215–4. doi: 10.1186/s12916-014-0215-1
58. Liu, J-E, Zhang, Y, Zhang, J, Dong, P-L, Chen, M, and Duan, Z-P. Probiotic yogurt effects on intestinal flora of patients with chronic liver disease. Nurs Res. (2010) 59:426–32. doi: 10.1097/NNR.0b013e3181fa4dc6
59. Salas-Salvadó, J, Guasch-Ferre, M, Díaz-López, A, and Babio, N. Yogurt and diabetes: overview of recent observational studies. J Nutr. (2017) 147:1452S–61S. doi: 10.3945/jn.117.248229
60. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
61. Gómez-Gallego, C, Gueimonde, M, and Salminen, S. The role of yogurt in food-based dietary guidelines. Nutr Rev. (2018) 76:29–39. doi: 10.1093/nutrit/nuy059
62. Pala, V, Sieri, S, Berrino, F, Vineis, P, Sacerdote, C, Palli, D, et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int J Cancer. (2011) 129:2712–9. doi: 10.1002/ijc.26193
63. Crichton, GE, Murphy, KJ, and Bryan, J. Dairy intake and cognitive health in middle-aged south Australians. Asia Pac J Clin Nutr. (2010) 19:161–71.
64. Mirghafourvand, M, Rad, AH, Charandabi, SMA, Fardiazar, Z, and Shokri, K. The effect of probiotic yogurt on constipation in pregnant women: a randomized controlled clinical trial. Iran Red Crescent Med J. (2016) 18:e39870. doi: 10.5812/ircmj.39870
65. Makino, S, Ikegami, S, Kume, A, Horiuchi, H, Sasaki, H, and Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br J Nutr. (2010) 104:998–1006. doi: 10.1017/S000711451000173X
66. Serafini, F, Turroni, F, Ruas-Madiedo, P, Lugli, GA, Milani, C, Duranti, S, et al. Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int J Food Microbiol. (2014) 178:50–9. doi: 10.1016/j.ijfoodmicro.2014.02.024
67. Rosa, DD, Dias, MMS, Grześkowiak, ŁM, Reis, SA, Conceição, LL, and Maria do Carmo, Milk kefir: nutritional, microbiological and health benefits. Nutr Res Rev (2017) 30: 82–96, doi: 10.1017/S0954422416000275
68. Jeon, H-Y, Kim, K-S, and Kim, S. Effects of yogurt containing probiotics on respiratory virus infections: influenza H1N1 and SARS-CoV-2. J Dairy Sci. (2023) 106:1549–61. doi: 10.3168/jds.2022-22198
69. Prado, MR, Blandón, LM, Vandenberghe, LPS, Rodrigues, C, Castro, GR, Thomaz-Soccol, V, et al. Milk kefir: composition, microbial cultures, biological activities, and related products. Microbiol. (2015) 6:10–3389. doi: 10.3389/fmicb.2015.01177
70. Sarkar, S. Biotechnological innovations in kefir production: a review. Br Food J. (2008) 110:283–95. doi: 10.1108/00070700810858691
71. Guzel-Seydim, ZB, Seydim, AC, and Greene, AK. Comparison of amino acid profiles of milk, yogurt and Turkish kefir. Milchwissenschaft. (2003) 58:158–60.
72. Liutkevičius, A, and Šarkinas, A. Studies on the growth conditions and composition of kefir grains–as a food and forage biomass. Vet Zootec. (2004) 25:64–70.
73. Otles, S, and Cagindi, O. Kefir: a probiotic dairy-composition, nutritional and therapeutic aspects. Pakistan J Nutr. (2003) 2:54–9. doi: 10.3923/pjn.2003.54.59
74. Golowczyc, MA, Gugliada, MJ, Hollmann, A, Delfederico, L, Garrote, GL, Abraham, AG, et al. Characterization of homofermentative lactobacilli isolated from kefir grains: potential use as probiotic. J Dairy Res. (2008) 75:211–7. doi: 10.1017/S0022029908003117
75. Silva, KR, Rodrigues, SA, Filho, LX, and Lima, ÁS. Antimicrobial activity of broth fermented with kefir grains. Appl Biochem Biotechnol. (2009) 152:316–25. doi: 10.1007/s12010-008-8303-3
76. Xie, N, Zhou, T, and Li, B. Kefir yeasts enhance probiotic potentials of Lactobacillus paracasei H9: the positive effects of coaggregation between the two strains. Food Res Int. (2012) 45:394–401. doi: 10.1016/j.foodres.2011.10.045
77. Hamet, MF, Medrano, M, Perez, PF, and Abraham, AG. Oral administration of kefiran exerts a bifidogenic effect on BALB/c mice intestinal microbiota. Benef Microbes. (2016) 7:237–46. doi: 10.3920/BM2015.0103
78. Kim, S, Lee, JY, Jeong, Y, and Kang, C-H. Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation. (2022) 8:29. doi: 10.3390/fermentation8010029
79. Schneedorf, J, and Anfiteatro, D. Fitoterapicos anti-inflamatorios. Asp Químicos. (2004) 33:443–62.
80. Cordeiro, MA, Souza, ELS, Arantes, RME, Balthazar, CF, Guimarães, JT, Scudino, H, et al. Fermented whey dairy beverage offers protection against Salmonella enterica ssp. enterica serovar typhimurium infection in mice. J Dairy Sci. (2019) 102:6756–65. doi: 10.3168/jds.2019-16340
81. Liu, J, Wang, S, Chen, M, Yueh, P, and Lin, C. The anti-allergenic properties of milk kefir and soymilk kefir and their beneficial effects on the intestinal microflora. J Sci Food Agric. (2006) 86:2527–33. doi: 10.1002/jsfa.2649
82. Oh, Y, Osato, MS, Han, X, Bennett, G, and Hong, WK. Folk yoghurt kills Helicobacter pylori. J Appl Microbiol. (2002) 93:1083–8. doi: 10.1046/j.1365-2672.2002.01779.x
83. Kwon, CS, Park, MY, Cho, JS, Choi, ST, and Chang, DS. Identification of effective microorganisms from kefir fermented milk. Food Sci Biotechnol. (2003) 12:476–9.
84. Odintsova, V, Klimenko, N, Tyakht, A, Volokh, O, Popov, V, Alexeev, D, et al. Yogurt fortified with vitamins and probiotics impacts the frequency of upper respiratory tract infections but not gut microbiome: a multicenter double-blind placebo controlled randomized study. J Funct Foods. (2021) 83:104572. doi: 10.1016/j.jff.2021.104572
85. Weir, TL, Trikha, SRJ, and Thompson, HJ. Diet and cancer risk reduction: the role of diet-microbiota interactions and microbial metabolites. Semin Cancer Biol. (2021) 70:53–60. doi: 10.1016/j.semcancer.2020.06.007
86. Liu, J-R, Wang, S-Y, Lin, Y-Y, and Lin, C-W. Antitumor activity of milk kefir and soy milk kefir in tumor-bearing mice. Nutr Cancer. (2002) 44:183–7. doi: 10.1207/S15327914NC4402_10
87. Jeong, CH, Cheng, WN, Kwon, HC, Kim, D-H, Seo, K-H, Choi, Y, et al. Effects of kefir on doxorubicin-induced multidrug resistance in human colorectal cancer cells. J Funct Foods. (2021) 78:104371. doi: 10.1016/j.jff.2021.104371
88. Hosono, A, Tanabe, T, and Otani, H. Binding properties of lactic acid bacteria isolated from kefir milk with mutagenic amino acid pyrolyzates. Milchwissenschaft. (1990) 45:647–51.
89. Khoury, N, El-Hayek, S, Tarras, O, El-Sabban, M, El-Sibai, M, and Rizk, S. Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Int J Oncol. (2014) 45:2117–27. doi: 10.3892/ijo.2014.2635
90. Chunchom, S, Talubmook, C, and Deeseenthum, S. Antioxidant activity, biochemical components and sub-chronic toxicity of different brown rice kefir powders. Pharm J. (2017) 9:388–94. doi: 10.5530/pj.2017.3.66
91. Yilmaz-Ersan, L, Ozcan, T, Akpinar-Bayizit, A, and Sahin, S. The antioxidative capacity of kefir produced from goat milk. Int J Chem Eng Appl. (2016) 7:22–6. doi: 10.7763/IJCEA.2016.V7.535
92. Güven, A, Güven, A, and Gülmez, M. The effect of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissues. J Veterinary Med Ser B. (2003) 50:412–6. doi: 10.1046/j.1439-0450.2003.00693.x
93. Liu, J-R, Lin, Y-Y, Chen, M-J, Chen, L-J, and Lin, C-W. Antioxidative activities of kefir. Asian-Aust J Anim Sci. (2005) 18:567–73. doi: 10.5713/ajas.2005.567
94. Sabokbar, N, and Khodaiyan, F. Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. J Food Sci Technol. (2016) 53:739–47. doi: 10.1007/s13197-015-2029-3
95. Likotrafiti, E, Valavani, P, Argiriou, A, and Rhoades, J. In vitro evaluation of potential antimicrobial synbiotics using Lactobacillus kefiri isolated from kefir grains. Int Dairy J. (2015) 45:23–30. doi: 10.1016/j.idairyj.2015.01.013
96. Sevencan, NO, Isler, M, Kapucuoglu, FN, Senol, A, Kayhan, B, Kiztanir, S, et al. Dose-dependent effects of kefir on colitis induced by trinitrobenzene sulfonic acid in rats. Food Sci Nutr. (2019) 7:3110–8. doi: 10.1002/fsn3.1174
98. Di Cagno, R, Tamborrino, A, Gallo, G, Leone, C, De Angelis, M, Faccia, M, et al. Uses of mares’ milk in manufacture of fermented milks. Int Dairy J. (2004) 14:767–75. doi: 10.1016/j.idairyj.2004.02.005
99. Kirdar, SS. Therapeutics effects and health benefits of the Caucasus koumiss: a review. Annu Res Rev Biol. (2021) 36:47–56. doi: 10.9734/arrb/2021/v36i1130450
100. Askarov, A, Kuznetsova, A, Gusmanov, R, Askarova, A, and Kovshov, V. Cost-effective horse breeding in the republic of Bashkortostan, Russia. Vet World. (2020) 13:2039–45. doi: 10.14202/vetworld.2020.2039-2045
101. Hongyu, C, Wenjun, L, Lingling, S, Jiao, W, and Heping, Z. Analysis of the structure of lactic acid Bacteria in Xinjiang fresh Mare Milk and koumiss using three generation sequencing technology. J Chinese Inst Food Sci Technol. (2022) 22:291. doi: 10.16429/j.1009-7848.2022.02.031
102. Dönmez, N, Kısadere, İ, Balaban, C, and Kadiralieva, N. Effects of traditional homemade koumiss on some hematological and biochemical characteristics in sedentary men exposed to exercise. Biotech Histochem. (2014) 89:558–63. doi: 10.3109/10520295.2014.915428
103. Ha, S, Leng, AMG, and Mang, L. Koumiss and its medicinal values. China J Chinese Mater Med. (2003) 28:11–4.
104. Hou, Q, Li, C, Liu, Y, Li, W, Chen, Y, Bao, Y, et al. Koumiss consumption modulates gut microbiota, increases plasma high density cholesterol, decreases immunoglobulin G and albumin. J Funct Foods. (2019) 52:469–78. doi: 10.1016/j.jff.2018.11.023
105. Tegin, RAA, and Gönülalan, Z. All aspects of koumiss, the natural fermented product. MANAS J Eng. (2014) 2:23–34.
106. Doreau, MM, and Wilhiam Martin-Rosset, W. Animals that produce dairy foods: horse. Elsevier. (2011).
107. Etienne-Mesmin, L, Livrelli, V, Privat, M, Denis, S, Cardot, J-M, Alric, M, et al. Effect of a new probiotic Saccharomyces cerevisiae strain on survival of Escherichia coli O157: H7 in a dynamic gastrointestinal model. Appl Environ Microbiol. (2011) 77:1127–31. doi: 10.1128/AEM.02130-10
108. Chen, Y, Aorigele, C, Wang, C, Hou, W, Zheng, Y, and Simujide, H. Effects of antibacterial compound of Saccharomyces cerevisiae from koumiss on immune function and caecal microflora of mice challenged with pathogenic Escherichia coli O8. Acta Vet Brno. (2019) 88:233–41. doi: 10.2754/avb201988020233
109. Yan, X, Sun, Y, Zhang, G, Han, W, Gao, J, Yu, X, et al. Study on the antagonistic effects of koumiss on toxoplasma gondii infection in mice. Front Nutr. (2022) 9:1014344. doi: 10.3389/fnut.2022.1014344
110. Fradi, I, Drissa, MA, Cheour, M, Meddeb, I, and Drissa, H. Coronary atherosclerosis and familial hypercholesterolemia: a case report. Tunis Med. (2008) 86:200–2.
111. Demyen, M, Alkhalloufi, K, and Pyrsopoulos, NT. Lipid-lowering agents and hepatotoxicity. Clin Liver Dis. (2013) 17:699–714. doi: 10.1016/j.cld.2013.07.016
112. Insull, W Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. (2006) 99:257–73. doi: 10.1097/01.smj.0000208120.73327.db
113. Yao, G, Yu, J, Hou, Q, Hui, W, Liu, W, Kwok, L-Y, et al. A perspective study of koumiss microbiome by metagenomics analysis based on single-cell amplification technique. Front Microbiol. (2017) 8:165. doi: 10.3389/fmicb.2017.00165
114. Zhang, Y, Du, R, Wang, L, and Zhang, H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur Food Res Technol. (2010) 231:151–8. doi: 10.1007/s00217-010-1255-1
115. Li, B, Hui, F, Yuan, Z, Shang, Q, Shuai, G, Bao, Y, et al. Untargeted fecal metabolomics revealed biochemical mechanisms of the blood lipid-lowering effect of koumiss treatment in patients with hyperlipidemia. J Funct Foods. (2021) 78:104355. doi: 10.1016/j.jff.2021.104355
116. World Health Organization. Global tuberculosis report 2013. Malang City, Indonesia: World Health Organization (2013).
117. Kudayarova, RR, Gilmutdinova, LT, Yamaletdinov, KS, Gilmutdinov, AR, Gabdelhakova, LT, and Zinnatullin, RK. Historical aspects of the use in medicine kumis. Bull Sib Med. (2010) 9:186–9. doi: 10.20538/1682-0363-2010-5-186-189
118. Dong, J, Zhang, Y, and Zhang, H. “Health properties of traditional fermented mongolian milk foods,” in Beneficial Microorganisms in Food and Nutraceuticals Microbiology Monographs (2015). 37–61.
119. Ishii, S, and Samejima, K. Feeding rats with kumiss suppresses the serum cholesterol and triglyceride levels. Milk Sci. (2001) 50.
120. Ya, T, Zhang, Q, Chu, F, Merritt, J, Bilige, M, Sun, T, et al. Immunological evaluation of Lactobacillus casei Zhang: a newly isolated strain from koumiss in Inner Mongolia, China. BMC Immunol. (2008) 9:1–9. doi: 10.1186/1471-2172-9-68
121. Gulmez, C, and Atakisi, O. Kumiss supplementation reduces oxidative stress and activates sirtuin deacetylases by regulating antioxidant system. Nutr Cancer. (2020) 72:495–503. doi: 10.1080/01635581.2019.1635628
122. Yilmaz, S, Emre, K, Yonar, H, and Mendil, AS. Doxorubicin-induced oxidative stress injury: the protective effect of kumiss on cardiotoxicity. J Hell Vet Med Soc. (2022) 73:4545–58. doi: 10.12681/jhvms.27822
123. Santiago-López, L, Aguilar-Toalá, JE, Hernández-Mendoza, A, Vallejo-Cordoba, B, Liceaga, AM, and González-Córdova, AF. Invited review: bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J Dairy Sci. (2018) 101:3742–57. doi: 10.3168/jds.2017-13465
124. Binetti, A, Carrasco, M, Reinheimer, J, and Suárez, V. Yeasts from autochthonal cheese starters: technological and functional properties. J Appl Microbiol. (2013) 115:434–44. doi: 10.1111/jam.12228
125. Upreti, P, and Metzger, LE. Influence of calcium and phosphorus, lactose, and salt-to-moisture ratio on Cheddar cheese quality: manufacture and composition. J Dairy Sci. (2006) 89:420–8. doi: 10.3168/jds.S0022-0302(06)72106-3
126. Moghaddas Kia, E, Alizadeh, M, and Esmaiili, M. Development and characterization of probiotic UF feta cheese containing Lactobacillus paracasei microencapsulated by enzyme based gelation method. J Food Sci Technol. (2018) 55:3657–64. doi: 10.1007/s13197-018-3294-8
127. Hayaloglu, AA, Karatekin, B, and Gurkan, H. Thermal stability of chymosin or microbial coagulant in the manufacture of Malatya, a halloumi type cheese: proteolysis, microstructure and functional properties. Int Dairy J. (2014) 38:136–44. doi: 10.1016/j.idairyj.2014.04.001
128. Sousa, MJ, Ardö, Y, and McSweeney, PLH. Advances in the study of proteolysis during cheese ripening. Int Dairy J. (2001) 11:327–45. doi: 10.1016/S0958-6946(01)00062-0
129. Kumar, A, Grover, S, Sharma, J, and Batish, VK. Chymosin and other milk coagulants: sources and biotechnological interventions. Crit Rev Biotechnol. (2010) 30:243–58. doi: 10.3109/07388551.2010.483459
130. Liburdi, K, Boselli, C, Giangolini, G, Amatiste, S, and Esti, M. An evaluation of the clotting properties of three plant rennets in the milks of different animal species. Foods. (2019) 8:600. doi: 10.3390/foods8120600
131. Mozzon, M, Foligni, R, Mannozzi, C, Zamporlini, F, Raffaelli, N, and Aquilanti, L. Clotting properties of Onopordum tauricum (Willd.) aqueous extract in milk of different species. Foods. (2020) 9:692. doi: 10.3390/foods9060692
132. Camin, F, Bontempo, L, Ziller, L, Franceschi, P, Molteni, A, Corbella, R, et al. Assessing the authenticity of animal rennet using δ15N analysis of chymosin. Food Chem. (2019) 293:545–9. doi: 10.1016/j.foodchem.2019.04.106
133. Sheehan, JJ, O’Sullivan, K, and Guinee, TP. Effect of coagulant type and storage temperature on the functionality of reduced-fat mozzarella cheese. Lait. (2004) 84:551–66. doi: 10.1051/lait:2004031
134. Cavalli, SV, Lufrano, D, Colombo, ML, and Priolo, N. Properties and applications of phytepsins from thistle flowers. Phytochemistry. (2013) 92:16–32. doi: 10.1016/j.phytochem.2013.04.013
135. Diouf, L, Mallaye, AN, Mbengue, M, Kane, A, and Diop, A. Carina papaya leaves: a substitute for animal rennet in cheese-making tradition. J Nat Prod Plant Resour. (2012) 2:517–23.
136. Roseiro, LB, Barbosa, M, Ames, JM, and Wilbey, RA. Cheesemaking with vegetable coagulants—the use of Cynara L. for the production of ovine milk cheeses. Int J Dairy Technol. (2003) 56:76–85. doi: 10.1046/j.1471-0307.2003.00080.x
137. Moynihan, AC, Govindasamy-Lucey, S, Jaeggi, JJ, Johnson, ME, Lucey, JA, and McSweeney, PLH. Effect of camel chymosin on the texture, functionality, and sensory properties of low-moisture, part-skim mozzarella cheese. J Dairy Sci. (2014) 97:85–96. doi: 10.3168/jds.2013-7081
138. Børsting, MW, Qvist, KB, Rasmussen, M, Vindeløv, J, Vogensen, FK, and Ardö, Y. Impact of selected coagulants and starters on primary proteolysis and amino acid release related to bitterness and structure of reduced-fat Cheddar cheese. Dairy Sci Technol. (2012) 92:593–612. doi: 10.1007/s13594-012-0080-7
139. Bansal, N, Drake, MA, Piraino, P, Broe, ML, Harboe, M, Fox, PF, et al. Suitability of recombinant camel (Camelus dromedarius) chymosin as a coagulant for Cheddar cheese. Int Dairy J. (2009) 19:510–7. doi: 10.1016/j.idairyj.2009.03.010
140. Govindasamy-Lucey, S, Lu, Y, Jaeggi, JJ, Johnson, ME, and Lucey, JA. Impact of camel chymosin on the texture and sensory properties of low-fat cheddar cheese. Aust J Dairy Technol. (2010) 65:139.
141. Ben Amira, A, Besbes, S, Attia, H, and Blecker, C. Milk-clotting properties of plant rennets and their enzymatic, rheological, and sensory role in cheese making: A review. Int J Food Prop. (2017) 20:S76–93. doi: 10.1080/10942912.2017.1289959
142. Moatsou, G. “Cheese: technology, compositional, physical and biofunctional properties:” a special issue. Foods. (2019) 8:512. doi: 10.3390/foods8100512
143. Papademas, P, and Bintsis, T. Preface: global cheesemaking technology: cheese quality and characteristics. Taylor & Francis (2017).
144. Ash, A, and Wilbey, A. The nutritional significance of cheese in the UK diet. Int J Dairy Technol. (2010) 63:305–19. doi: 10.1111/j.1471-0307.2010.00606.x
145. Walther, B, Schmid, A, Sieber, R, and Wehrmüller, K. Cheese in nutrition and health. Dairy Sci Technol. (2008) 88:389–405. doi: 10.1051/dst:2008012
146. Tome, D, Bos, C, Mariotti, F, and Gaudichon, C. Protein quality and FAO/WHO recommendations. Sci Aliments. (2002) 22:393–405. doi: 10.3166/sda.22.393-405
147. Dupont, D, Arnould, C, Rolet-Répécaud, O, Duboz, G, Faurie, F, Martin, B, et al. Determination of bovine lactoferrin concentrations in cheese with specific monoclonal antibodies. Int Dairy J. (2006) 16:1081–7. doi: 10.1016/j.idairyj.2005.09.012
148. Elbarbary, HA, Abdou, AM, Park, EY, Nakamura, Y, Mohamed, HA, and Sato, K. Novel antibacterial lactoferrin peptides generated by rennet digestion and autofocusing technique. Int Dairy J. (2010) 20:646–51. doi: 10.1016/j.idairyj.2009.12.019
149. Van Ballegooijen, AJ, and Beulens, JW. The role of vitamin K status in cardiovascular health: evidence from observational and clinical studies. Curr Nutr Rep. (2017) 6:197–205. doi: 10.1007/s13668-017-0208-8
150. Vermeer, C, Raes, J, Van’t Hoofd, C, MHJ, K, and Xanthoulea, S. Menaquinone content of cheese. Nutrients. (2018) 10:446. doi: 10.3390/nu10040446
151. Gaucheron, F. Milk and dairy products: a unique micronutrient combination. J Am Coll Nutr. (2011) 30:400S–9S. doi: 10.1080/07315724.2011.10719983
152. Dekker, LH, Vinke, PC, Riphagen, IJ, Minović, I, Eggersdorfer, ML, van den Heuvel, EGHM, et al. Cheese and healthy diet: associations with incident cardio-metabolic diseases and all-cause mortality in the general population. Front Nutr. (2019) 6:185. doi: 10.3389/fnut.2019.00185
153. Walther, B, Schmid, A, Sieber, R, and Wehrmuller, K. Cheese in nutrition and health. Méd Nutr. (2010) 46:38–51.
154. Sieber, R. Zusammensetzung von Milch und Milchprodukten schweizerischer Herkunft. Eidg. Forschungsanstalt für Milchwirtschaft: Liebefeld (2001).
155. Heyman, MBCommittee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. (2006) 118:1279–86. doi: 10.1542/peds.2006-1721
156. Facioni, MS, Dominici, S, Marescotti, F, Covucci, R, Taglieri, I, Venturi, F, et al. Lactose residual content in PDO cheeses: novel inclusions for consumers with lactose intolerance. Foods. (2021) 10:2236. doi: 10.3390/foods10092236
157. Geurts, L, Everard, A, Le Ruyet, P, Delzenne, NM, and Cani, PD. Ripened dairy products differentially affect hepatic lipid content and adipose tissue oxidative stress markers in obese and type 2 diabetic mice. J Agric Food Chem. (2012) 60:2063–8. doi: 10.1021/jf204916x
158. Hatakka, K, Ahola, AJ, Yli-Knuuttila, H, Richardson, M, Poussa, T, Meurman, JH, et al. Probiotics reduce the prevalence of oral Candida in the elderly—a randomized controlled trial. J Dent Res. (2007) 86:125–30. doi: 10.1177/154405910708600204
159. Dreizen, S, Dreizen, JG, and Stone, RE. The effect of cow’s milk on dental caries in the rat. J Dent Res. (1961) 40:1025–8. doi: 10.1177/00220345610400050301
160. Rugg-Gunn, AJ, Edgar, WM, Geddes, DA, and Jenkins, GN. The effect of different meal patterns upon plaque pH in human subjects. Br Dent J. (1975) 139:351–6. doi: 10.1038/sj.bdj.4803614
161. Singh, K, Kallali, B, Kumar, A, and Thaker, V. Probiotics: a review. Asian Pac J Trop Biomed. (2011) 1:S287–90. doi: 10.1016/S2221-1691(11)60174-3
162. Kumar, A, Chaubey, KK, Agarwal, K, and Kashyap, A. Probiotics: an adjunct to healthy human life. British Dental Journal. (2011). Springer Nature.
163. Jensen, ME, Harlander, SK, Schachtele, CF, Halambeck, SM, and Morris, HA. Evaluation of the acidogenic and antacid properties of cheeses by telemetric monitoring of human dental plaque pH. Foods Nutr Dent Heal. (1984) 4:31–47.
164. Ference, BA, Kastelein, JJP, Ray, KK, Ginsberg, HN, Chapman, MJ, Packard, CJ, et al. Association of triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants with risk of coronary heart disease. JAMA. (2019) 321:364–73. doi: 10.1001/jama.2018.20045
165. Rosell, M, Håkansson, NN, and Wolk, A. Association between dairy food consumption and weight change over 9 y in 19 352 perimenopausal women. Am J Clin Nutr. (2006) 84:1481–8. doi: 10.1093/ajcn/84.6.1481
166. Allender, PS, Cutler, JA, Follmann, D, Cappuccio, FP, Pryer, J, and Elliott, P. Dietary calcium and blood pressure: a meta-analysis of randomized clinical trials. Ann Intern Med. (1996) 124:825–31. doi: 10.7326/0003-4819-124-9-199605010-00007
167. Gómez-Ruiz, JÁ, Taborda, G, Amigo, L, Recio, I, and Ramos, M. Identification of ACE-inhibitory peptides in different Spanish cheeses by tandem mass spectrometry. Eur Food Res Technol. (2006) 223:595–601. doi: 10.1007/s00217-005-0238-0
168. Stepaniak, L, Jedrychowski, L, Wroblewska, B, and Sørhaug, T. Immunoreactivity and inhibition of angiotensin I converting enzyme and lactococcal oligopeptidase by peptides from cheese. Ital J food Sci. (2001) 13:373–81.
169. Foltz, M, Meynen, EE, Bianco, V, van Platerink, C, Koning, TMMG, and Kloek, J. Angiotensin converting enzyme inhibitory peptides from a lactotripeptide-enriched milk beverage are absorbed intact into the circulation. J Nutr. (2007) 137:953–8. doi: 10.1093/jn/137.4.953
170. Pan, D, Luo, Y, and Tanokura, M. Antihypertensive peptides from skimmed milk hydrolysate digested by cell-free extract of Lactobacillus helveticus JCM1004. Food Chem. (2005) 91:123–9. doi: 10.1016/j.foodchem.2004.05.055
171. Sipola, M, Finckenberg, P, Korpela, R, Vapaatalo, H, and Nurminen, M-L. Effect of long-term intake of milk products on blood pressure in hypertensive rats. J Dairy Res. (2002) 69:103–11. doi: 10.1017/S002202990100526X
172. Mizushima, S, Ohshige, K, Watanabe, J, Kimura, M, Kadowaki, T, Nakamura, Y, et al. Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am J Hypertens. (2004) 17:701–6. doi: 10.1016/j.amjhyper.2004.03.674
173. Nakamura, Y, Kajimoto, O, Kaneko, K, Aihara, K, Mizutani, J, Ikeda, N, et al. Effects of the liquid yogurts containing “lactotripeptide (VPP, IPP)” on high-normal blood pressure. J Nutr Food. (2004) 7:123–37.
174. Tuomilehto, J, Lindström, J, Hyyrynen, J, Korpela, R, Karhunen, ML, Mikkola, L, et al. Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. J Hum Hypertens. (2004) 18:795–802. doi: 10.1038/sj.jhh.1001745
175. Bütikofer, U, Meyer, J, Sieber, R, and Wechsler, D. Quantification of the angiotensin-converting enzyme-inhibiting tripeptides Val-pro-pro and Ile-pro-pro in hard, semi-hard and soft cheeses. Int Dairy J. (2007) 17:968–75. doi: 10.1016/j.idairyj.2006.11.003
176. Xu, B, and Lin, J. Characteristics and risk factors of rheumatoid arthritis in the United States: an NHANES analysis. PeerJ. (2017) 5:e4035. doi: 10.7717/peerj.4035
177. Kvien, TK, Uhlig, T, Ødegård, S, and Heiberg, MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. (2006) 1069:212–22. doi: 10.1196/annals.1351.019
178. Zechner, EL. Inflammatory disease caused by intestinal pathobionts. Curr Opin Microbiol. (2017) 35:64–9. doi: 10.1016/j.mib.2017.01.011
179. Lynch, SV, and Pedersen, O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
180. Asoudeh, F, Djafarian, K, Akhalghi, M, Mahmoudi, M, Jamshidi, AR, Farhadi, E, et al. The effect of probiotic cheese consumption on inflammatory and anti-inflammatory markers, disease severity, and symptoms in patients with rheumatoid arthritis: study protocol for a randomized, double-blind, placebo-controlled trial. Trials. (2022) 23:1–9. doi: 10.1186/s13063-022-06113-2
181. Hsieh, M-L, and Chou, C-C. Mutagenicity and antimutagenic effect of soymilk fermented with lactic acid bacteria and bifidobacteria. Int J Food Microbiol. (2006) 111:43–7. doi: 10.1016/j.ijfoodmicro.2006.04.034
182. Massi, M, Vitali, B, Federici, F, Matteuzzi, D, and Brigidi, P. Identification method based on PCR combined with automated ribotyping for tracking probiotic Lactobacillus strains colonizing the human gut and vagina. J Appl Microbiol. (2004) 96:777–86. doi: 10.1111/j.1365-2672.2004.02228.x
183. Morato-Martínez, M, López-Plaza, B, Santurino, C, Palma-Milla, S, and Gómez-Candela, C. A dairy product to reconstitute enriched with bioactive nutrients stops bone loss in high-risk menopausal women without pharmacological treatment. Nutrients. (2020) 12:2203. doi: 10.3390/nu12082203
184. Espey, MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med. (2013) 55:130–40. doi: 10.1016/j.freeradbiomed.2012.10.554
185. García-Nebot, MJ, Cilla, A, Alegría, A, and Barberá, R. Caseinophosphopeptides exert partial and site-specific cytoprotection against H2O2-induced oxidative stress in Caco-2 cells. Food Chem. (2011) 129:1495–503. doi: 10.1016/j.foodchem.2011.05.129
186. Carocho, M, and Ferreira, ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. (2013) 51:15–25. doi: 10.1016/j.fct.2012.09.021
187. Gupta, A, Mann, B, Kumar, R, and Sangwan, RB. Antioxidant activity of Cheddar cheeses at different stages of ripening. Int J Dairy Technol. (2009) 62:339–47. doi: 10.1111/j.1471-0307.2009.00509.x
188. Pritchard, SR, Phillips, M, and Kailasapathy, K. Identification of bioactive peptides in commercial Cheddar cheese. Food Res Int. (2010) 43:1545–8. doi: 10.1016/j.foodres.2010.03.007
189. Silva, RA, Lima, MSF, Viana, JBM, Bezerra, VS, Pimentel, MCB, Porto, ALF, et al. Can artisanal “Coalho” cheese from northeastern Brazil be used as a functional food? Food Chem. (2012) 135:1533–8. doi: 10.1016/j.foodchem.2012.06.058
190. Abadía-García, L, Cardador, A, del Campo, STM, Arvízu, SM, Castaño-Tostado, E, Regalado-González, C, et al. Influence of probiotic strains added to cottage cheese on generation of potentially antioxidant peptides, anti-listerial activity, and survival of probiotic microorganisms in simulated gastrointestinal conditions. Int Dairy J. (2013) 33:191–7. doi: 10.1016/j.idairyj.2013.04.005
191. Huma, N, Rafiq, S, Sameen, A, Pasha, I, and Khan, MI. Antioxidant potential of buffalo and cow milk Cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells. Asian-Aust J Anim Sci. (2018) 31:287–92. doi: 10.5713/ajas.17.0031
192. Silanikove, N, Leitner, G, and Merin, U. The interrelationships between lactose intolerance and the modern dairy industry: global perspectives in evolutional and historical backgrounds. Nutrients. (2015) 7:7312–31. doi: 10.3390/nu7095340
193. Wang, Q, Yu, H, Tian, B, Jiang, B, Xu, J, Li, D, et al. Novel edible coating with antioxidant and antimicrobial activities based on whey protein isolate nanofibrils and carvacrol and its application on fresh-cut cheese. Coatings. (2019) 9:583. doi: 10.3390/coatings9090583
194. Tayab, T, Rai, K, Kumari, V, and Thomas, E. Effect of chewing paneer and cheese on salivary acidogenicity: a comparative study. Int J Clin Pediatr Dent. (2012) 5:20–4. doi: 10.5005/jp-journals-10005-1128
195. Hu, M-J, Tan, J-S, Gao, X-J, Yang, J-G, and Yang, Y-J. Effect of cheese intake on cardiovascular diseases and cardiovascular biomarkers. Nutrients. (2022) 14:2936. doi: 10.3390/nu14142936
196. Hugenholtz, J, Hunik, J, Santos, H, and Smid, E. Nutraceutical production by propionibacteria. Lait. (2002) 82:103–12. doi: 10.1051/lait:2001009
197. Hennessy, AA, Barrett, E, Paul Ross, R, Fitzgerald, GF, Devery, R, and Stanton, C. The production of conjugated α-linolenic, γ-linolenic and stearidonic acids by strains of bifidobacteria and propionibacteria. Lipids. (2012) 47:313–27. doi: 10.1007/s11745-011-3636-z
198. Storhaug, CL, Fosse, SK, and Fadnes, LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2017) 2:738–46. doi: 10.1016/S2468-1253(17)30154-1
199. Beermann, C, and Hartung, J. Physiological properties of milk ingredients released by fermentation. Food Funct. (2013) 4:185–99. doi: 10.1039/C2FO30153A
200. Mozzi, F, Gerbino, E, Font de Valdez, G, and Torino, MI. Functionality of exopolysaccharides produced by lactic acid bacteria in an in vitro gastric system. J Appl Microbiol. (2009) 107:56–64. doi: 10.1111/j.1365-2672.2009.04182.x
201. Chinnadurai, K, Kanwal, HK, Tyagi, AK, Stanton, C, and Ross, P. High conjugated linoleic acid enriched ghee (clarified butter) increases the antioxidant and antiatherogenic potency in female Wistar rats. Lipids Health Dis. (2013) 12:1–9. doi: 10.1186/1476-511X-12-121
202. Parvez, S, Malik, KA, Ah Kang, S, and Kim, H. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. (2006) 100:1171–85. doi: 10.1111/j.1365-2672.2006.02963.x
Keywords: therapeutic, dairy food, milk, fermented products, probiotics, koumiss, human health
Citation: Saleem GN, Gu R, Qu H, Bahar Khaskheli G, Rashid Rajput I, Qasim M and Chen X (2024) Therapeutic potential of popular fermented dairy products and its benefits on human health. Front. Nutr. 11:1328620. doi: 10.3389/fnut.2024.1328620
Received: 27 October 2023; Accepted: 03 January 2024;
Published: 28 February 2024.
Edited by:
Christian Coelho, VetAgro Sup, FranceReviewed by:
Nasim Khorshidian, Shahid Beheshti University of Medical Sciences, IranCopyright © 2024 Saleem, Gu, Qu, Bahar Khaskheli, Rashid Rajput, Qasim and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruixia Gu, Z3VydWl4aWExOTYzQDE2My5jb20=; Xia Chen, Y2hlbnhpYUB5enUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.