
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 13 March 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1303822
This article is part of the Research Topic Women's Health in an Interdisciplinary Dimension – Determinants of Nutritional Disorders View all 16 articles
 Clark R. Sims1,2
Clark R. Sims1,2 Jessica L. Saben1,2,3,4
Jessica L. Saben1,2,3,4 Audrey Martinez2
Audrey Martinez2 Sarah R. Sobik1,2
Sarah R. Sobik1,2 Meghan R. Crimmins2,5
Meghan R. Crimmins2,5 Jessica E. Bulmanski2,6
Jessica E. Bulmanski2,6 Donald Turner2
Donald Turner2 Annalee Furst7
Annalee Furst7 Lisa T. Jansen2,8
Lisa T. Jansen2,8 Lars Bode7
Lars Bode7 Aline Andres1,2*
Aline Andres1,2*Introduction: Maternal obesity is associated with increased concentrations of human milk (HM) obesogenic hormones, pro-inflammatory cytokines, and oligosaccharides (HMOs) that have been associated with infant growth and adiposity. The objective of this pilot study was to determine if adherence to a Mediterranean meal plan during lactation modulates macronutrients and bioactive molecules in human milk from mothers with obesity.
Methods: Sixteen healthy, exclusively breastfeeding women with obesity (body mass index ≥30 kg/m2) enrolled between 4 and 5 months postpartum. The women followed a 4-week Mediterranean meal plan which was provided at no cost. Maternal and infant anthropometrics, HM composition, and infant intakes were measured at enrollment and at weeks 2 and 4 of the intervention. Thirteen mother-infant dyads completed the study. Additionally, participants from an adjacent, observational cohort who had obesity and who collected milk at 5 and 6 months postpartum were compared to this cohort.
Results: Participants’ healthy eating index scores improved (+27 units, p < 0.001), fat mass index decreased (−4.7%, p < 0.001), and daily energy and fat intake were lower (−423.5 kcal/day, p < 0.001 and-32.7 g/day, p < 0.001, respectively) following the intervention. While HM macronutrient concentrations did not change, HM leptin, total human milk oligosaccharides (HMOs), HMO-bound fucose, Lacto-N-fucopentaose (LNFP)-II, LNFP-III, and difucosyllacto-N-tetrose (DFLNT) concentrations were lower following the intervention. Infant intakes of leptin, tumor necrosis factor (TNF)-α, total HMOs, HMO-bound fucose, LNFP-III and DFLNT were lower following the intervention. Specific components of the maternal diet (protein and fat) and specific measures of maternal diet quality (protein, dairy, greens and beans, fruit and vegetables) were associated with infant intakes and growth.
Discussion: Adherence to a Mediterranean meal plan increases dietary quality while reducing total fat and caloric intake. In effect, body composition in women with obesity improved, HM composition and infants’ intakes were modulated. These findings provide, for the first time, evidence-based data that enhancing maternal dietary quality during lactation may promote both maternal and child health. Longer intervention studies examining the impact of maternal diet quality on HM composition, infant growth, and infant development are warranted.
In the United States, more than 50% of women enter pregnancy with either overweight or obesity (1). Pre-gravid obesity has been associated with changes in the macronutrient (2) and bioactive composition of human milk (HM) (2–4), which may impact infant health (5). HM from women with obesity has higher energy, fat, and protein content compared to milk from mothers with normal weight throughout lactation (2, 6, 7) and obesity-associated elevations in HM hormones (8, 9), pro-inflammatory cytokines (10, 11) and HM oligosaccharides (HMOs) (4), are positively associated with infant growth and adiposity (2, 4, 9). Therefore, obesity-associated alterations in HM composition may play a role in early-life nutritional programing of infant adiposity.
Dietary interventions during the postnatal period may provide a window to temper the effects of obesity on HM composition. It has been shown in observational studies that a higher Mediterranean diet score is associated with lower HM saturated fatty acid concentrations and with increased monounsaturated fatty acids and total antioxidant capacity (12, 13). However, very few dietary intervention studies have been conducted in breastfeeding women that have also analyzed components of HM. A crossover study, employing four different dietary paradigms (galactose vs. glucose and high carbohydrate vs. high fat) during lactation, showed an association between dietary energy source and HMO concentrations (14). Another study, aimed at decreasing maternal energy, fat, and sugar intake over 2 weeks postpartum, found that HM insulin, leptin and adiponectin were reduced by 10–25% following the dietary intervention (15). Together these studies suggest that dietary interventions can modulate HM composition. As such, the Mediterranean diet has shown efficacy in decreasing body mass index (BMI) (16, 17), circulating obesogenic hormones (17), adipokines (18), and systemic inflammation (16, 19) in non-pregnant/non-lactating women with obesity. However, it is yet unknown whether similar results can be attained in lactating women or if these changes may affect human milk content.
In this within-subject pilot intervention trial, we aimed to determine if adherence to a Mediterranean meal plan during lactation could modulate the macronutrient and bioactive (hormone, HMO, and cytokine) content of HM from women with obesity.
The within-subject intervention study took place at the Arkansas Children’s Nutrition Center in Little Rock, Arkansas between April 2019 and February 2020. Healthy women with obesity who were exclusively breastfeeding were recruited from the surrounding community. Of the 90 participants screened, 28 were eligible and of those, 16 enrolled between 4 and 5 months postpartum (Supplementary Figure S1). Three participants did not complete all study visits (19%), resulting in 13 participants for the current analysis. Inclusion criteria were: BMI = 30–50 kg/m2, ≥ 18 years of age, singleton pregnancy, intent to continue breastfeeding exclusively until at least 6 months postpartum, and child being able to be fed expressed milk from a bottle. Exclusion criteria included: pre-existing conditions (e.g., diabetes, hypertension, heart disease); use of recreational drugs, tobacco, or alcohol; food allergies, intolerances or preferences incompatible with meal plan; and the use of medications or supplements that are contraindicated for lactating mothers. Maternal age, race and ethnicity, and infant sex were self-reported. Assessments took place at enrollment (pre), 2 weeks and 4 weeks following the start of the dietary intervention (Wk2 and Wk4, respectively). To examine the impact of time on milk composition and infant intakes, participants from an adjacent, observational cohort from the same study center (2, 4, 20) were matched to the participants of the within-subject intervention study based on maternal BMI and HM sample availability at postpartum months 5 and 6. From the adjacent, observational study, there were only 10 participants that had a BMI above 30 and collected milk samples at both 5 and 6 months postpartum, therefore, all 10 were used to compare with the participants from this within-subject study. To learn about the observational study sample used, please refer to our group’s previous publications on this cohort (2, 4, 20).
Written, informed consent was obtained from all participants prior to study procedures. All study procedures were approved by the Institutional Review Board of the University of Arkansas for Medical Sciences (Protocol #: 228407). This trial was registered at clinicaltrials.gov (NCT03744429).
Habitual maternal dietary intake was assessed prior to the initiation of the dietary intervention using 3-day food records (two weekdays, one weekend day) and analyzed with the Nutrition Data System for Research (Nutrition Coordinating Center, University of Minnesota, MN) software by trained interviewers. Participants recorded all food, beverages, supplements, and medications that they consumed during the 3 day period.
Participants met with a registered dietitian at the initial study visit to receive education about the dietary intervention based on the Mediterranean diet (21) and weekly thereafter to monitor adherence to the meal plan. Motivational interviewing, active listening, and goal setting techniques were used to help participants comply with the intervention. The goals of the counseling sessions were to identify and resolve barriers to adherence as well as provide encouragement and support. The initial session educated on the study intervention and tracking dietary intake while subsequent sessions reviewed compliance to problem solve challenges and celebrate successes. The macronutrient distribution (20–35% of calories from fat, 45–65% carbohydrates, 10–35% protein) and provided caloric intake met the Dietary Guidelines for Americans recommendations (22). All lunches and dinners (2/day, in the form of fresh packaged meals) were provided weekly to the participants throughout the 4 weeks by Trifecta Nutrition (Sacramento, California). Breakfast (breakfast sandwiches and oatmeal, 1/day) and snacks (walnuts, granola bars, Greek yogurt, and fruits, 2/day) were provided by the research team. Participants were also provided with extra virgin olive oil to add to their meals and were instructed to buy 1% low fat milk to drink or combine with fruits as a smoothie. Participants recorded all food, beverages, supplements, and medications that they consumed and where they made substitutions in the meal plan for the entirety of the trial. Dietary intake was analyzed using the Nutrition Data System for Research. Healthy Eating Index (HEI) and Mediterranean Diet scores were derived from published guidelines (23–25). The overall intervention dietary composition is summarized in Supplementary Table S1 and an example of a week’s menu is shown in Supplementary Table S2. Intervention compliance was calculated as the participants HEI score of consumed meals divided by the HEI score of the prescribed meals multiplied by 100.
Maternal and infant anthropometrics and maternal body composition were measured at each visit. Maternal weight and height and infant weight and length were measured as previously described (2). Weight-for-length, weight-for-age and length-for-age z-scores were calculated based on the World Health Organization Child Growth Standards (26, 27). Maternal BMI was calculated as kg/m2. Maternal fat mass (FM) and fat free mass (FFM) were measured using air displacement plethysmography (Cosmed BodPod®, Concord, CA). Infant fat mass and lean mass were measured using quantitative nuclear magnetic resonance (EchoMRI-AH, Echo Medical Systems, Houston, TX). FM and FFM index (FMI and FFMI, respectively) were calculated as FM (kg)/m2 and FFM (kg)/m2.
Maternal blood was collected following an overnight fast at the pre-intervention and Wk4 visits only. Plasma was processed and stored at −80°C. Leptin, insulin, C-reactive protein (CRP), interleukin (IL)-6, IL-8, and TNF-α concentrations were measured using high-performance electrochemiluminescence immunoassays (Meso Scale Diagnostics, Rockville, MD). Cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) concentrations were measured using a clinical analyzer (Randox Laboratories, Kearneysville, WV).
Participants collected HM over 24-h, prior to each visit. Mothers were given the option to either feed their infant the expressed milk from a bottle or to feed baby from one breast and pump the other breast during the 24-h collection period. If only one breast was pumped, mothers were instructed to alternate the nursed breast and the pumped breast at each feed and record accordingly. At each feed, the mothers were asked to gently invert the expressed HM and aliquot 4 mL of HM into the provided polypropylene tubes. HM was stored at 4°C until the full 24-h collection was complete. Afterwards, the 24-h samples were pooled and stored intact at-80°C.
Macronutrients (fat, protein, and carbohydrates) were measured in milk from all visits using a Miris HM Analyzer (Miris, Uppsala, Sweden) according to manufacturer’s instructions, from which caloric content was derived. Leptin, insulin, CRP, IL-6, IL-8, and TNF-α concentrations were measured in milk from all visits using high-performance electrochemiluminescence immunoassays (Meso Scale Diagnostics, Rockville, MD). Concentrations of HMOs (nmol/mL) were measured in milk from the pre-intervention and Wk4 visits only.by high-performance liquid chromatography on an amide-80 column (2 μm particle size, 2 mm ID, 15 cm length) with fluorescent detection, as previously described (14). The absolute quantification of the 19 most abundant HMOs (4) was determined using the non-HMO oligosaccharide raffinose as an internal standard added to all milk samples at the beginning of analysis. Infant intakes were estimated using test weighing, which is considered a useful and precise method for assessing milk intake (28–30), at each visit to obtain a single-feed milk intake volume multiplied by regular, daily feeding frequency as reported by the mothers.
Demographic data was summarized using mean and standard deviation for continuous variables and counts (percentages) for categorical variables. Comparisons were made using linear mixed-effect models constructed with random intercepts for each participant followed by type 2 analysis of variance for measurements with no Wk2 values or using linear mixed-effects models constructed with random intercepts for each participant followed by contrasts of estimated marginal means using the lme4, car and modelbased R packages (31–33). Repeated measures correlations were performed to assess the relationship between dietary components and human milk content using the rmcorr R package (34) and were FDR-adjusted. Power analysis determined that n = 13 participants would allow consideration of an effect size greater than 1.6 g/100 mL for HM fat, 248 pg/mL for leptin, 0.16 pg/mL for TNF-α and 92 ng/mL for CRP. Significance was set at alpha ≤0.05. Data analyses were performed using R (version 4.1.0) (35). Extreme outliers were removed if they were 3 times above the upper quartile or 3 times below the lower quartile for all measurements.
Participants were on average 32.8 ± 3.8 years of age and 77% of participants were of non-Hispanic, White descent (Table 1). All participants had obesity at enrollment (mean BMI: 35.9 ± 5.0 kg/m2, FM: 46.1 ± 12.3 kg, and FMI: 17.1 ± 4.3 kg/m2). Infants showed expected growth with increases in weight and length parameters over the 4-week study (Supplementary Table S3). Of all the measured characteristics, only baseline plasma IL-8 was significantly different between the participants that completed the intervention (2.6 ± 1.1 pg/mL) and those that did not (4.3 ± 1.4 pg/mL).
The dietary intervention was started at 4.8 ± 0.28 months postpartum and ended at 5.7 ± months postpartum. The dietary intervention yielded 83.7 and 84.0% compliance to the meal plan at Wk2 and Wk4, respectively. Participants’ HEI increased by 70.5 and 71.0% (+27 and + 27.2 units, p < 0.001, respectively) and Mediterranean diet scores increased by 87.1 and 90.6% (+7.4 and + 7.7 units, p < 0.001, respectively) whereas daily energy intake was 26.5 and 17.9% and lower (−629 and-424 kcal/d, p < 0.001, respectively) at Wk2 and Wk4 of the intervention (Table 2). While on the meal plan, participants’ daily intake was significantly lower for total fat (−37.0% and − 33.0%, p < 0.001, respectively), saturated fat (−56.0% and − 52.0%, p < 0.001, respectively), polyunsaturated fat (−37.0% and − 34.0%, p = 0.01, respectively), ⍵6:⍵3 ratio (−48.3% and − 48.3%, p < 0.001), refined grains (−79.0% and − 75.0%, p < 0.001, respectively), and sodium (−62.0% and − 54.0%, p < 0.001, respectively). Intake of whole grains significantly increased (+60.0% and + 85.0%, p < 0.001, respectively). Daily protein intake was significantly different over the duration of the study (Table 2; p = 0.029), however, all post-hoc comparisons were non-significant (p > 0.05). Daily carbohydrate intake was significantly higher during the intervention at Wk2 compared to pre-intervention (p = 0.015).
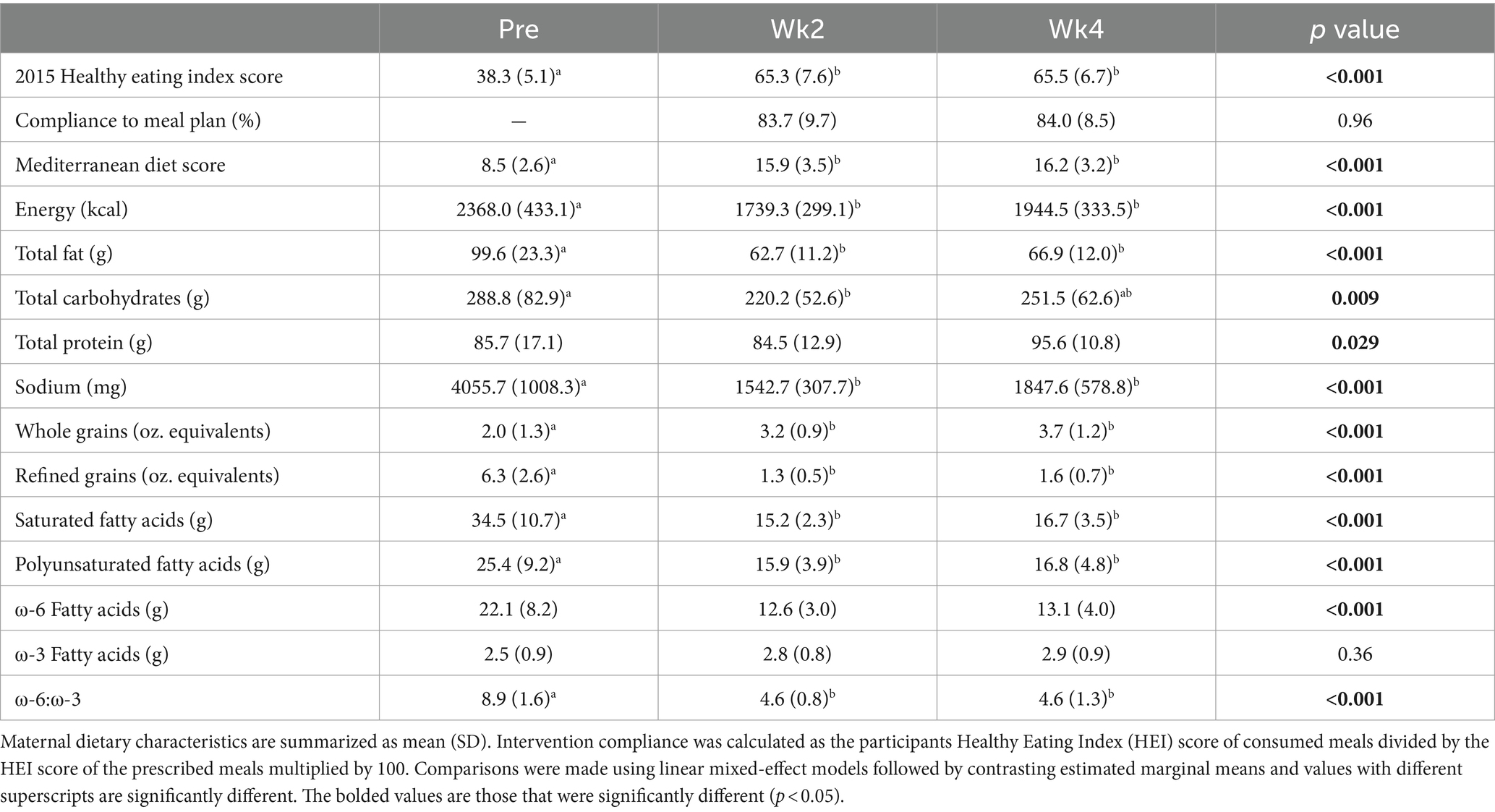
Table 2. Maternal dietary characteristics before (Pre) and during (Wk2 and Wk4) Mediterranean dietary intervention that was provided from 5 to 6 months postpartum to 13 lactating women with obesity.
Following the intervention, maternal weight (−2.7%, p < 0.001; Supplementary Table S3), BMI (−2.8%, p < 0.001; Figure 1A) and FMI (−5.0%, p < 0.001; Figure 1B) significantly decreased by Wk4. FFMI did not change by Wk4 (−0.5%, p = 0.39, Figure 1C). Maternal plasma cholesterol levels (total [−12.8%, p < 0.001; Figure 1D], HDL [−7.6%, p = 0.002; Figure 1E], and LDL [−17.2%, p < 0.001; Figure 1F]) were also significantly decreased, even after adjusting for maternal weight loss during the intervention. No significant differences were observed in plasma hormone or cytokine levels between visits (Supplementary Table S3). As expected during the 4-week period, infant length (4.5%, p < 0.001), weight (10.6%, p < 0.001), fat mass (16.7%, p < 0.001), lean mass (9.3%, p < 0.001) and FMI (4.3%, p < 0.001) increased; however, FFMI, WFA, LFA and WFL did not differ (p > 0.05; Supplementary Table S3).
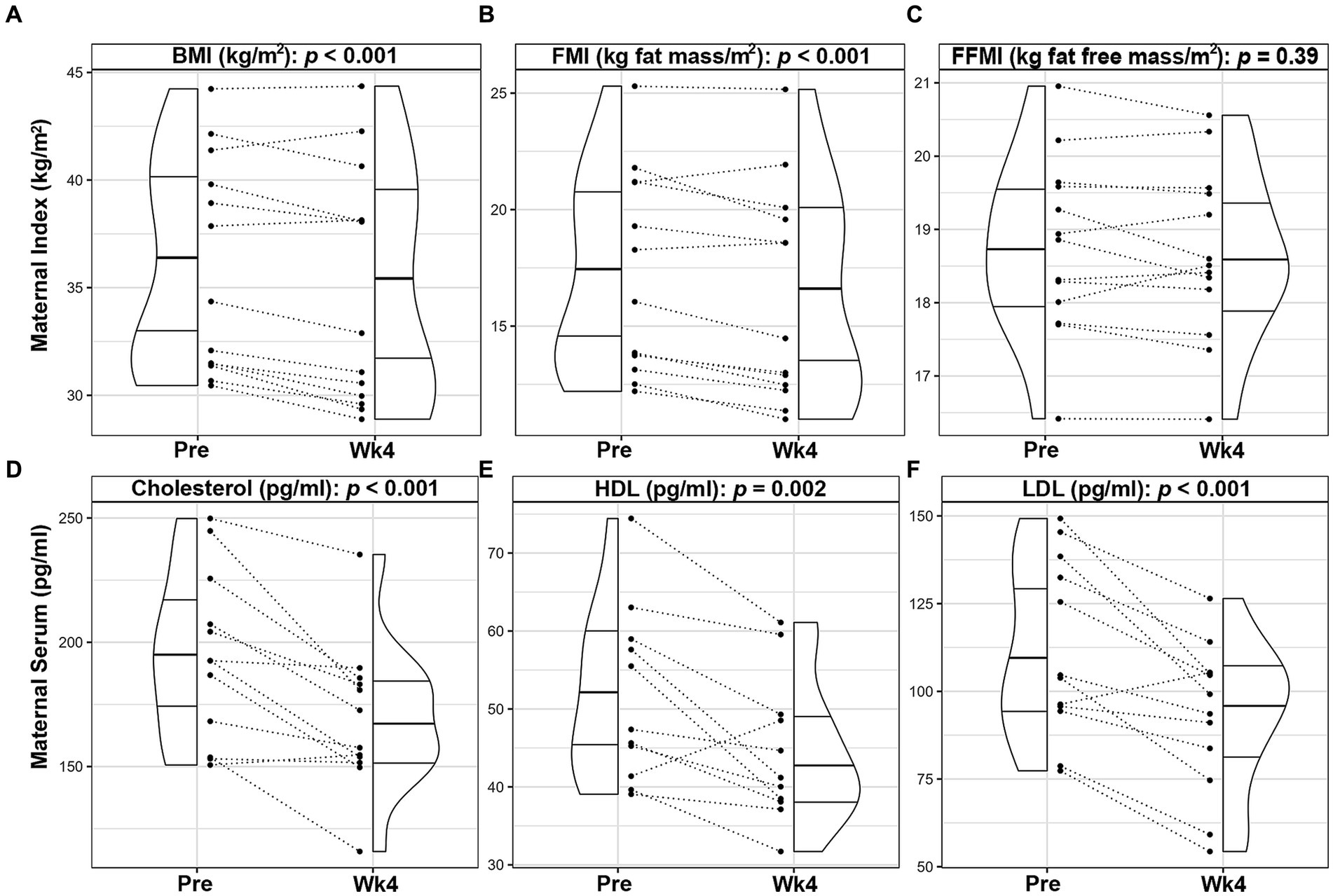
Figure 1. Changes in maternal outcomes of lactating women with obesity following a 4-week Mediterranean dietary intervention. Paired plots showing changes in maternal body mass index [BMI, (A)], fat mass index [FMI, (B)], fat free mass index [FFMI, (C)], cholesterol (D), high density lipoprotein [HDL, (E)], and low-density lipoprotein [LDL, (F)] between pre-intervention (Pre) and the end of the intervention (Wk4). Dotted lines connect the Pre and Wk4 measures for each participant. Quantiles within the density plots are indicated by the solid horizontal lines.
HM collections occurred 5.4 ± 4.5 days before the beginning of the dietary intervention, 0.77 ± 1.5 days before the Wk2 visit and 1.3 ± 2.5 days after finishing the dietary intervention. Following the 4-week intervention, mean HM leptin concentrations significantly decreased by 37.1% (p < 0.001, Figure 2; Supplementary Table S4), even after adjusting for maternal weight loss during the intervention. HM total energy (p = 0.77) and macronutrient levels (fat: p = 0.75, carbohydrate: p = 0.60, and protein: p = 0.78) did not change, nor did HM concentrations of insulin (p = 0.28), CRP (p = 0.78), IL-6 (p = 0.25), IL-8 (p = 0.37), as shown in Supplementary Table S4. HM concentrations of TNF-α (p = 0.11) were not significantly different between time points, albeit levels decreased in 9 out of the 13 participants (Figure 2).
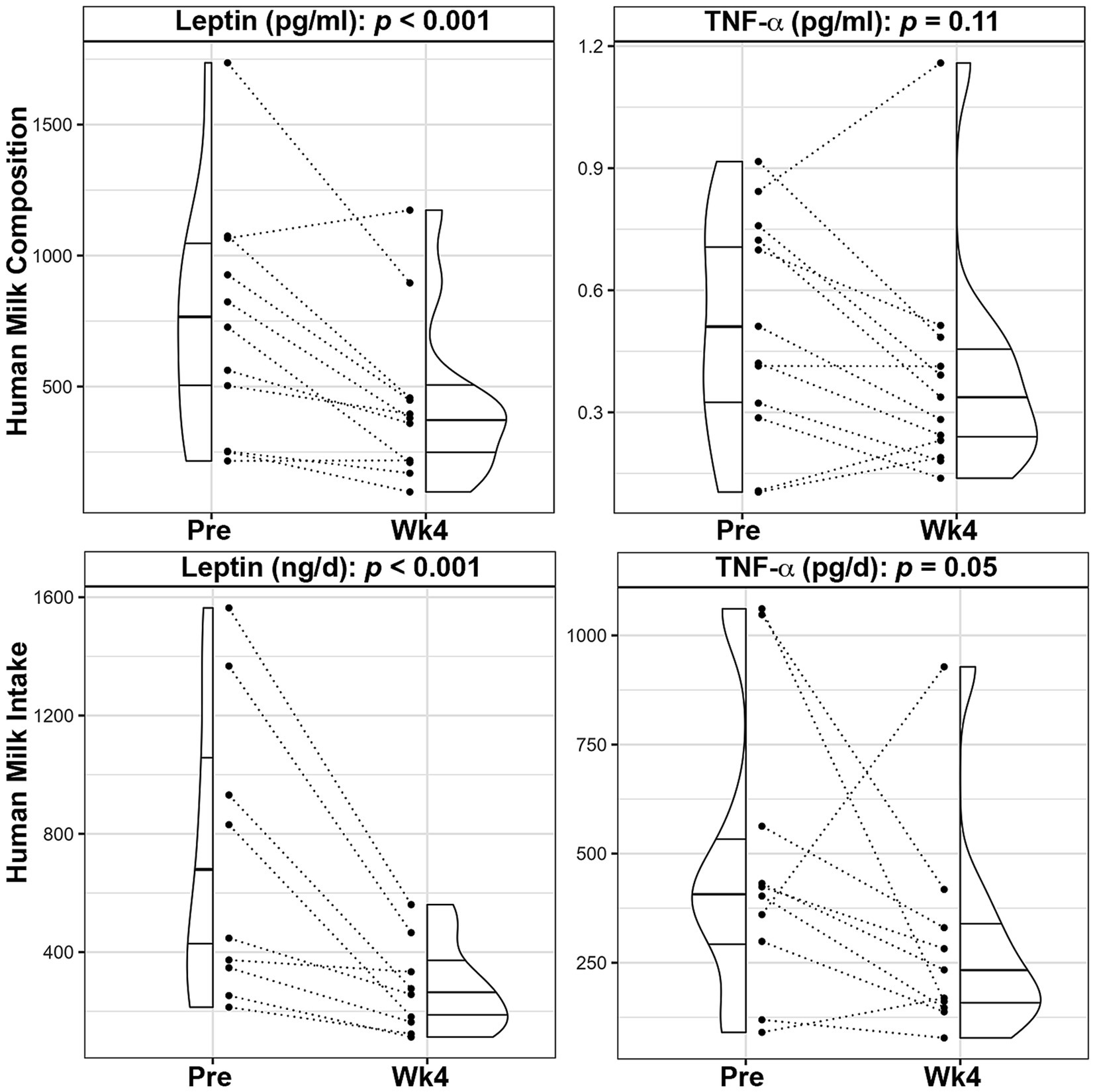
Figure 2. Changes in leptin and TNF-α composition and infant intake following a 4-week Mediterranean dietary intervention. Paired plots showing changes in human milk leptin and tumor necrosis factor α (TNF-α) between pre-intervention (Pre) and the end of the intervention (Wk4). Dotted lines connect the Pre and Wk4 measures for each participant. Quantiles within the density plots are indicated by the solid horizontal lines.
The mean total HMO concentrations decreased by 6.3% (p = 0.036) and mean concentrations of HMO-bound fucose (−8.7%, p = 0.001), lacto-N-fucopentaose-II (LNFP II, −10.7%, p = 0.048), lacto-N-fucopentaose-III (LNFP III, −38.7%, p = 0.041), and difucosyllacto-N-tetrose (DFLNT, −59.4%, p = 0.003) were significantly lower following the dietary intervention (Figure 3; Supplementary Table S4). After adjusting for maternal weight loss, concentrations of total HMOs (p = 0.075), HMO-bound fucose (p = 0.005), LNFP-III (p = 0.043), and DFLNT (p = 0.005) remained lower following the intervention.
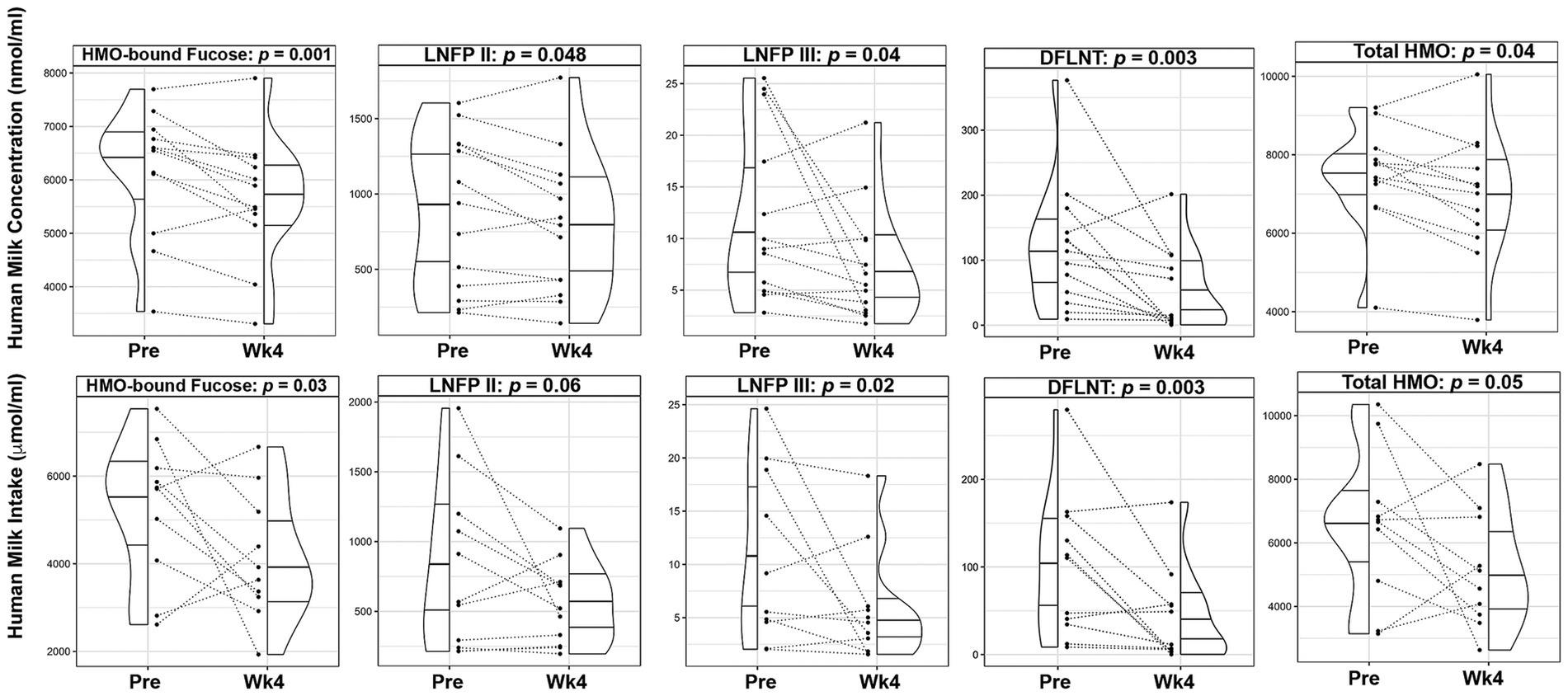
Figure 3. Changes in human milk oligosaccharide composition and infant intake following a 4-week Mediterranean dietary intervention. Paired plots showing changes in composition and intake of human milk oligosaccharide (HMO)-bound fucose, Lacto-N-fucopentaose (LNFP) II, LNFP III, Difucosyllacto-N-tetrose (DFLNT), and Total HMOs between pre-intervention (Pre) and the end of the intervention (Wk4). Dotted lines connect the Pre and Wk4 measures for each participant. Quantiles within the density plots are indicated by the solid horizontal lines.
Mean total HM volume intakes were not significantly different prior to and after the intervention (881.4 ± 310.9 mL vs. 708.0 ± 209.2 mL, p = 0.17; Supplementary Table S3). Consistent with lower concentrations, infants’ mean intake of HM leptin (−59.6%, p < 0.001) and TNF-α (−38.5%, p = 0.05), were significantly lower over the course of the intervention (Figure 2; Supplementary Table S4). Similarly, infants’ mean intake of total HMOs (−24.0%, p = 0.05), HMO-bound fucose (−25.5%, p = 0.029), LNFP III (−50.4%, p = 0.019) and DFLNT (−61.9%, p = 0.003) were decreased following the dietary intervention (Figure 3; Supplementary Table S4).
To understand the potential impact of time on HM composition and infant intakes, we examined HM parameters and infant intakes from the within-subject study compared with those of an observational cohort of lactating women with obesity at the same months postpartum. There were no demographic differences between the two cohorts (Supplementary Table S5). There were no differences between the studies in HM concentrations of fat, protein, energy, insulin, IL-6 or CRP, or infants’ total milk intake and daily intakes of carbohydrates, protein, energy, insulin, TNF-α, IL-6, or CRP (Supplementary Table S6). There were also no differences in infant weight-for-age, length-for-age or weight-for-length Z-scores or FFMI between cohorts nor did these parameters change with time (Supplementary Table S6). Infant fat mass index was also not different between cohorts, however we did observe that it increased with time, as would be expected in healthy growing infant cohorts.
We found that human milk carbohydrate concentrations were higher in the within subject study compared to the observational cohort (5 mo: 7.5 ± 0.29 g/100 mL vs. 7.2 ± 0.29 g/100 mL, 6 mo: 7.6 ± 0.22 g/100 mL vs. 7.1 ± 0.27 g/100 mL; p < 0.001) whereas concentrations of human milk leptin (5 mo: 694.4 ± 463.6 pg/mL vs. 1037.8 ± 532.4 pg/mL, 6 mo: 436.7 ± 324.1 pg/mL vs. 963.4 ± 669.5 pg/mL; p = 0.019), TNF- α (5 mo: 0.52 ± 0.27 pg/mL vs. 0.88 ± 0.72 pg/mL, 6 mo: 0.38 ± 0.27 pg/mL vs. 1.12 ± 0.89 pg/mL; p = 0.011), and IL-8 (trending - 5 mo: 177.7 ± 78.3 pg/mL vs. 176.7 ± 134.7 pg/mL, 6 mo: 146.8 ± 107.1 pg/mL vs. 365.8 ± 328.4 pg/mL; p = 0.06) were lower in the within subject study compared to the observation cohort (Figure 4; Supplementary Table S6). Interestingly, we found a significant interaction between time and cohorts for IL-8 HM concentrations (Supplementary Table S6; p = 0.012). This was likely a result of the observed increase over time in the observational cohort (delta = 161.3 pg/mL) compared to an observed decrease over time in the within subject cohort (delta = −30.9 pg/mL, Figure 4; Supplementary Table S6).
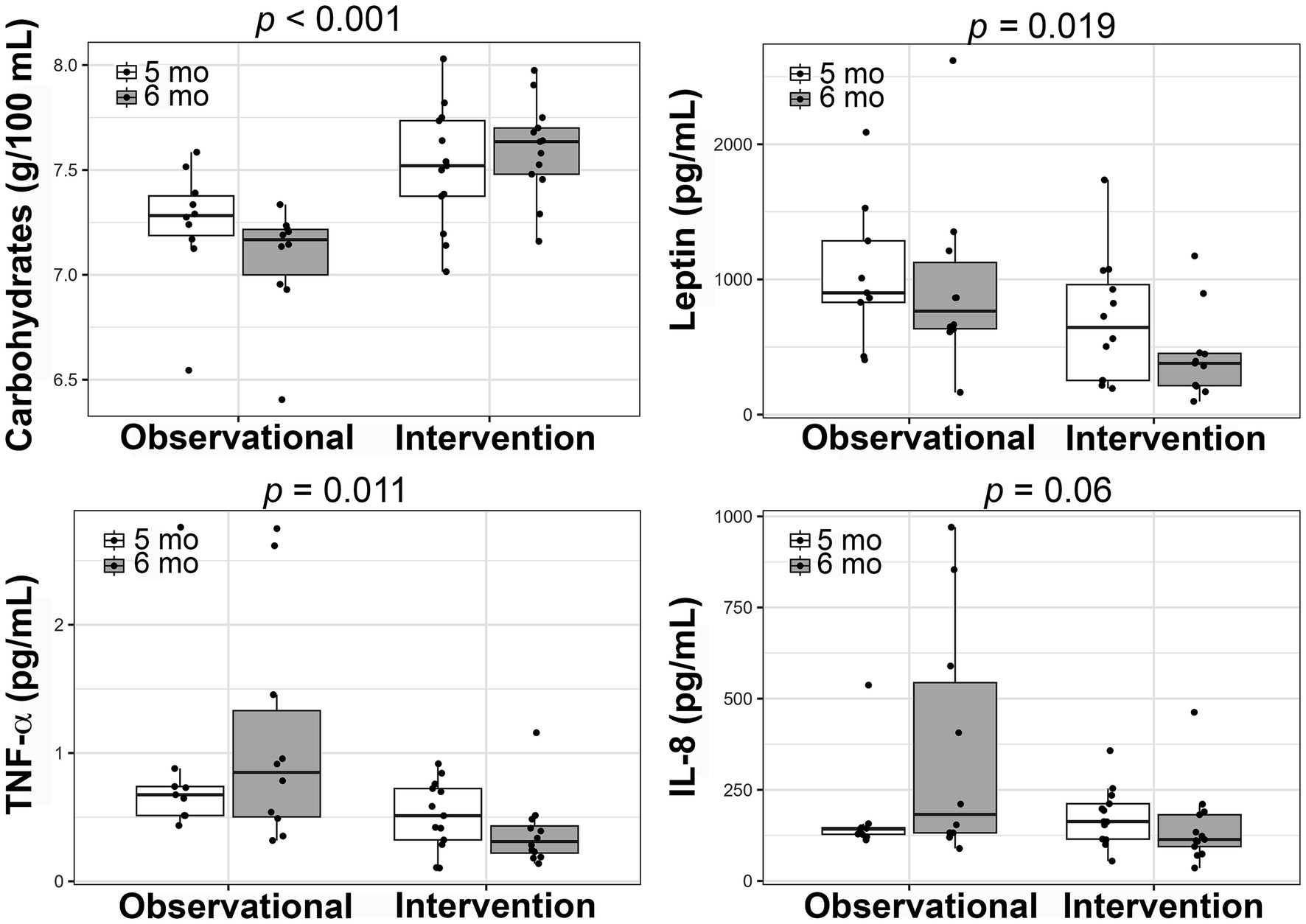
Figure 4. Comparison of changes in human milk composition between the within-subjects intervention and an adjacent, observational cohort. Boxplots showing the changes from 5 to 6 months postpartum in each of the studies for carbohydrate, leptin, tumor necrosis factor-α (TNF-α), and interleukin-8 (IL-8) concentrations. Linear mixed-effects models were used to compare the studies and the p-values are presented.
To determine if specific dietary components had a direct relationship with HM or infant outcomes, we performed repeated measures correlations (Figure 5). After FDR-adjustment, no components of maternal diet were associated with HM composition. Several dietary components showed significant, negative associations with infant daily intake of leptin (Figure 5) including maternal dairy HEI score (rrm = −0.76, prm adjusted = 0.004), total HEI score (rrm = −0.70, prm adjusted = 0.014), total fruit HEI score (rrm = −0.65, prm adjusted = 0.021), total vegetable HEI score (rrm = −0.68, prm adjusted = 0.019), and greens and beans HEI score (rrm = −0.72, prm adjusted = 0.01). Conversely, maternal fat intake as a percentage of daily calories was positively associated with infant daily intake of leptin (rrm = 0.65, prm adjusted = 0.021).
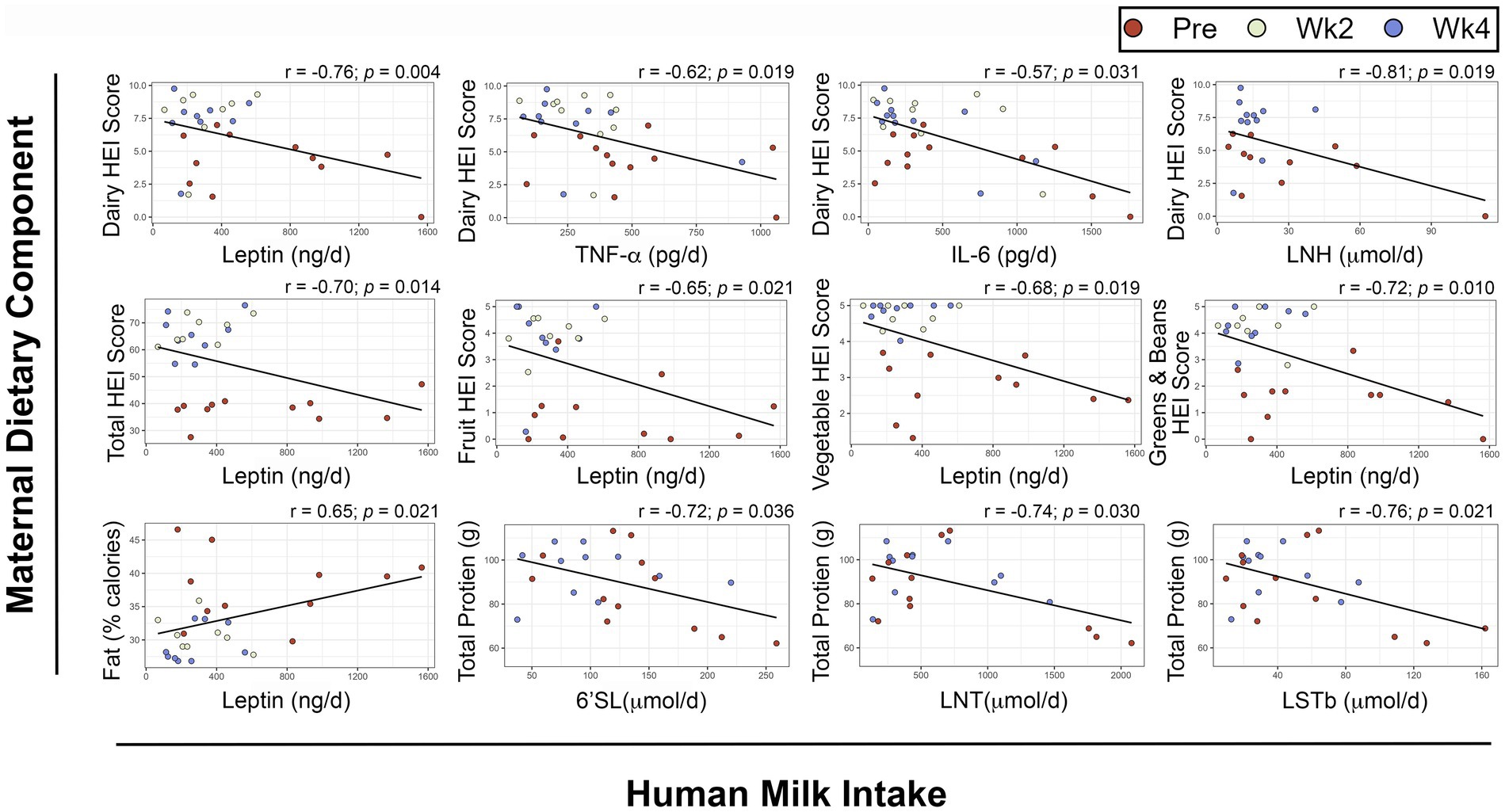
Figure 5. Repeated measures correlations between maternal diet components and infant intake of human milk components. Scatterplots showing relationships between maternal dietary components and infant intakes of human milk components. Maternal dietary intake was analyzed using the Nutrition Data System for Research. Infant intakes were estimated at each visit using test weighing and feeding frequency reported by the mothers. FDR-adjusted p-values are presented. HEI: Healthy Eating Index, 6’SL: 6’Sialyllactose, LNT: Lacto-N-tetrose, LSTb: Sialyl-lacto-N-tetraose b, IL: Interleukin LNH: Lacto-N-hexaose, TNF-α: Tumor Necrosis Factor α.
There were also negative associations between maternal dairy HEI score and infant intakes of TNF-α (rrm = −0.62, prm adjusted = 0.019), IL-6 (rrm = −0.57, prm adjusted = 0.031), and Lacto-N-hexaose (LNH, rrm = −0.81, prm adjusted = 0.019).
Maternal total protein intake was negatively associated with infant intakes of the HMOs 6′Sialyllactose (6’SL, rrm = −0.72, prm adjusted = 0.036), Lacto-N-tetrose (LNT, rrm = −0.74, prm adjusted = 0.03) and Sialyl-lacto-N-tetraose b (LSTb, rrm = −0.76, prm adjusted = 0.021).
Adjusted linear mixed effects models were used to investigate the relationships between HEI score components and infant intakes while adjusting for total HEI score (Table 3). The adjusted β coefficients were less than 25% different for the following models: dairy HEI score and daily infant intake of TNF-α (14.5%), and greens and beans HEI score and daily infant intake of leptin (16.5%). Together, these data suggest that the observed relationship between dietary components of maternal HEI scores and infant intakes of human milk components were not dependent on total HEI scores.
The growing evidence that having overweight and obesity modulates HM composition in ways that can promote infant adiposity (2, 4, 5, 9) warrants the development of interventions that may temper these effects. In this study, we tested the effect of a 4-week Mediterranean meal plan, implemented at 5 months postpartum in women with obesity and demonstrated for the first time that the intervention improved maternal HEI scores and plasma lipid profiles, reduced maternal BMI and fat mass, decreased HM concentrations and infants’ intakes of leptin, TNF-α, LNFP II, LNFP-III, DFLNT, total HMOs and HMO-bound fucose. While we observed differences in the concentrations of HM leptin, maternal circulating levels of leptin did not change, indicating other potential avenues for maternal dietary interventions to alter HM composition such as altering leptin production locally in the mammary gland (36). Additionally, we identified individual dietary components (e.g., protein, fat) and HEI score components (e.g., dairy, total fruit) that were significantly associated with intakes of bioactive molecules in HM. These findings provide compelling evidence that dietary interventions during lactation can mitigate obesity-associated alterations in HM composition that may ultimately affect early-life nutritional programing of infant health while promoting maternal health.
In non-pregnant/non-lactating women with obesity, adherence to a Mediterranean diet has been shown to decrease BMI (16, 17), circulating obesogenic hormones (17), adipokines (18), and systemic inflammation (16, 19). Findings from this study suggest that similar results can be attained in lactating women with obesity, which is of importance to prevent postpartum weight retention and optimize maternal and child health (37). Several randomized controlled trials have demonstrated the efficacy of caloric restriction and/or exercise on weight loss and body composition during the postnatal period, although they failed to evaluate their impact on HM composition or infant health (38). By proxy, replacing habitual post-partum maternal diet with a Mediterranean dietary pattern in our study population resulted in a reduced caloric intake while meeting dietary guideline recommendations. Consistent with our findings, caloric restriction resulted in significant weight loss and improved body composition that was sustained for up to 1 year in some studies (38, 39). Stendell-Hollis et al. also demonstrated that 4 months of a Mediterranean diet or a MyPyramid diet were effective in reducing postpartum maternal weight, fat mass, and plasma TNF-α levels (40). Low-fat diets, and diets that are high in fiber decreased HDL levels in adults with normal weight and overweight/obesity, similar to what we observed in lactating women (41, 42). Future studies will need to elucidate the unique contributions of energy deficit vs. maternal dietary quality to changes in HM composition and their benefits to the child.
Women with obesity have elevated pro-inflammatory chemokines (2, 11), leptin (2, 3), and insulin (2, 8) HM content compared to peers with normal weight. Importantly, infants’ intakes of HM insulin and CRP were significantly and positively associated with their fat mass index (2). It is believed that obesity-related systemic and local (mammary gland microenvironment) inflammation (43) may contribute to elevations in pro-inflammatory cytokines that have been observed in HM from women with obesity (2, 11). While much research has focused on how maternal obesity influences bioactives in HM (10), few studies exist describing the relationships between maternal dietary intake and HM bioactives. In animal models of obesity, caloric restriction has led to decreases in mammary gland inflammation (43, 44). Our study expands on these findings and demonstrates a reduction in HM pro-inflammatory cytokines (TNF-α and IL-8) from women with obesity who underwent a Mediterranean dietary intervention. In agreement with our current data, improved maternal dietary quality and reduced caloric intake led to lower HM insulin and leptin levels (17). Critically, infant intakes of these HM components were also reduced following the 4-week intervention. In accordance with our study, a previous investigation reported reduced caloric intake for 2 weeks did not result in changes in concentrations or infant intakes of HM macronutrients or in changes in infant weight-for-length, weight-for-age, or length-for-age z-scores, despite changes in infant intakes of leptin and insulin (15). With such an acute intervention, changes in infant growth that could be attributed to HM components would not necessarily be expected. Therefore, future studies that can implement dietary interventions throughout the postnatal period are critical to understanding the potential positive impact such dietary interventions may have on HM composition and subsequent offspring body composition.
We and others have also shown positive associations between maternal obesity and HMO content (4, 45). These associations are important because HMOs are among the most predominant bioactive components in HM, supporting infant gut development (46, 47) and the prevention of infectious diseases (47). However, recent evidence also suggests that some HMOs that are elevated in HM from women with obesity are associated with infant growth (greater weight-for-length Z-scores) and fat accretion (4, 48, 49). While LNFP III was not associated with maternal obesity in our previous study (4), it showed a strong positive association with infant fat mass at 2 months of age (4). This is important because in the current study, HM concentrations and infant intakes of LNFP III were significantly lower following the dietary intervention. Similarly, previous studies have found significant positive associations between HMO concentrations (disialyllacto-N-tetraose, LNFP II, total HMO concentrations, and total HMO-bound fucose) and infant fat mass at 5–6 months of age (48, 49), many of which were significantly reduced following the Mediterranean diet intervention in this study. It is important to recognize that HMO concentrations change over lactation (50, 51) and change in relation to maternal BMI (4). While these pilot data present compelling evidence for dietary influences on HMOs, our study did not include a prospective control group to test sufficiently the effect of dietary intervention vs. time on HMO concentrations nor did we have time-matched retrospective data on HMO concentrations in our observational cohort.
Previous studies have shown that maternal consumption of fruit, whole grains, and specific fatty acids have been associated with individual HMO concentrations (51–53). Our data are not completely aligned with these previous reports. It is possible that these discrepancies are related to differences in the analyses of dietary intake data (e.g., food frequency questionnaires vs. daily food records), in the timing of sample collection, or in the analytical approaches of the studies. Comparable to our data, Azad et al. reported no significant association between maternal HEI score and HMO concentrations (51). However, Azad et al. did find a weak but significant negative association between maternal total protein intake and LSTb concentrations consistent with the infant intake data presented herein. LSTb showed a strong, positive association with infant fat mass as well as weight-for-length and weight for age Z-scores in our previous study (4), suggesting that maternal protein intake may be a modifiable factor that can be used in future intervention studies to improve infant body composition. A recent short-term crossover study employing four different dietary paradigms (galactose vs. glucose and high carbohydrate vs. high fat) demonstrated a significant association between maternal dietary energy source and the concentrations of HMOs (14) further supporting the notion that interventions focused on specific dietary components may benefit infant health through alterations to HMOs.
Caution should be taken when interpreting these results because of the small sample size of mainly non-Hispanic White lactating women and the lack of a concurrent control group for comparison. Yet, given the US population-wide exclusive breastfeeding rates at 6 months of 24.9% (54), this study assesses the feasibility of Mediterranean diet pattern implementation in an exclusively breastfeeding cohort of women with obesity. Therefore, reporting data from 13 participants of this population provides foundational knowledge for future Mediterranean diet intervention designs. There is a clear need to conduct randomized control trials to confirm our pilot-study findings and to use standardized methodology to increase reproducibility and rigor of future research. A second limitation is the confounding effect of calorie restriction that occurred by replacing habitual dietary patterns with the Mediterranean diet plan in this study. While this prevents us from exclusively attributing the assessed effects to the change in diet quality, it provides insights to the prevailing nutrient poor, calorie dense habitual diets in the assessed cohort. Furthermore, participants consumed an average of 1841 kcal/d during the intervention, which is in-line with DGA for sedentary women ages 19-50y, as an additional 450–500 kcal/d intake during breastfeeding is only recommended for women aiming to maintain post-partum weight (22). Third, the effects of storage at 4°C of the HM during the 24-h collection at the participants’ homes were not investigated. Despite these limitations, this pilot study provides unique results that healthy dietary habits can influence maternal health, HM composition, and children’s HM intakes during the postpartum period in women with obesity. There were several significant strengths to this study, including: (1) greater than 80% adherence to the dietary intervention that resulted in significant improvements in maternal diet quality and body composition in only 4 weeks, (2) significant changes in human milk bioactive components and (3) measuring infant HM intakes and acquiring representative milk samples over 24-h to use best practices in estimating infants’ exposures. Future studies will need to evaluate a more diverse population, larger cohort, and a longer length of intervention while maintaining isocaloric intakes and body weight from baseline.
This study is the first to demonstrate the feasibility of implementing a Mediterranean meal plan in lactating women with obesity while examining its impact on human milk composition, infant intake, and infant anthropometrics. This in-depth investigation allows for a better understanding of the dynamic of the breastfeeding triad of mother/milk/infant and how a healthy diet could improve maternal and child health.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of Arkansas for Medical Sciences Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participant.
CS: Writing – review & editing, Writing – original draft, Visualization, Project administration, Investigation, Funding acquisition, Formal analysis, Conceptualization. JLS: Writing – review & editing, Writing – original draft. AM: Writing – review & editing, Investigation, Formal analysis, Conceptualization. SS: Writing – review & editing, Writing – original draft. MC: Writing – review & editing, Investigation. JB: Writing – review & editing, Investigation, Formal analysis. DT: Writing – review & editing, Investigation, Formal analysis. AF: Writing – review & editing, Investigation. LJ: Writing – review & editing, Writing – original draft. LB: Writing – review & editing, Investigation, Conceptualization. AA: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The United States Department of Agriculture Agricultural Research Service (USDA ARS) funded the Arkansas Children’s Nutrition Center through project plans #6026-51000-010-05S and #6026-51000-012-06S. Arkansas Children’s Research Institute/Arkansas Biosciences Institute provided funding through grant GR037121. The National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases partially supported CS and AA through grant R01DK107516. The funding agencies had no role in the design, analysis, interpretation, or presentation of the data and results.
We thank the participants and the clinical research team at ACNC for their dedication and hard work in producing and collecting the samples and data presented in this manuscript.
JLS is an editorial board member of Frontiers in Pediatrics – Pediatric Obesity.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1303822/full#supplementary-material
1. Fryar, CD , Carroll, MD , and Afful, J . Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats (2020).
2. Sims, CR , Lipsmeyer, ME , Turner, DE , and Andres, A . Human milk composition differs by maternal BMI in the first 9 months postpartum. Am J Clin Nutr. (2020) 112:548–57. doi: 10.1093/ajcn/nqaa098
3. Andreas, NJ , Hyde, MJ , Gale, C , Parkinson, JR , Jeffries, S , Holmes, E, et al. Effect of maternal body mass index on hormones in breast milk: a systematic review. PLoS One. (2014) 9:e115043. doi: 10.1371/journal.pone.0115043
4. Saben, JL , Sims, CR , Abraham, A , Bode, L , and Andres, A . Human Milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients. (2021) 13:446. doi: 10.3390/nu13020446
5. Lee, S , and Kelleher, SL . Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology. Am J Physiol Endocrinol Metab. (2016) 311:E405–22. doi: 10.1152/ajpendo.00495.2015
6. Bzikowska-Jura, A , Sobieraj, P , Szostak-Wegierek, D , and Wesolowska, A . Impact of infant and maternal factors on energy and macronutrient composition of human Milk. Nutrients. (2020) 12:2591. doi: 10.3390/nu12092591
7. Kugananthan, S , Gridneva, Z , Lai, CT , Hepworth, AR , Mark, PJ , Kakulas, F, et al. Associations between maternal body composition and appetite hormones and macronutrients in human Milk. Nutrients. (2017) 9:252. doi: 10.3390/nu9030252
8. Young, BE , Patinkin, Z , Palmer, C , De la Houssaye, B , Barbour, LA , Hernandez, T, et al. Human milk insulin is related to maternal plasma insulin and BMI: but other components of human milk do not differ by BMI. Eur J Clin Nutr. (2017) 71:1094–100. doi: 10.1038/ejcn.2017.75
9. Fields, DA , George, B , Williams, M , Whitaker, K , Allison, DB , Teague, A, et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. (2017) 12:78–85. doi: 10.1111/ijpo.12182
10. Erliana, UD , and Fly, AD . The function and alteration of immunological properties in human Milk of obese mothers. Nutrients. (2019) 11:1284. doi: 10.3390/nu11061284
11. Whitaker, KM , Marino, RC , Haapala, JL , Foster, L , Smith, KD , Teague, AM, et al. Associations of maternal weight status before, during, and after pregnancy with inflammatory markers in breast Milk. Obesity (Silver Spring). (2017) 25:2092–9. doi: 10.1002/oby.22025
12. Di Maso, M , Bravi, F , Ferraroni, M , Agostoni, C , Eussen, SRBM , Decsi, T, et al. Adherence to Mediterranean diet of breastfeeding mothers and fatty acids composition of their human Milk: results from the Italian MEDIDIET study. Front Nutr. (2022) 9:891376. doi: 10.3389/fnut.2022.891376
13. Karbasi, S , Mohamadian, M , Naseri, M , Khorasanchi, Z , Zarban, A , Bahrami, A, et al. A Mediterranean diet is associated with improved total antioxidant content of human breast milk and infant urine. Nutr J. (2023) 22:11. doi: 10.1186/s12937-023-00841-0
14. Seferovic, MD , Mohammad, M , Pace, RM , Engevik, M , Versalovic, J , Bode, L, et al. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci Rep. (2020) 10:22092. doi: 10.1038/s41598-020-79022-6
15. Leghi, GE , Netting, MJ , Lai, CT , Narayanan, A , Dymock, M , Rea, A, et al. Reduction in maternal energy intake during lactation decreased maternal body weight and concentrations of leptin, insulin and adiponectin in human Milk without affecting Milk production, Milk macronutrient composition or infant growth. Nutrients. (2021) 13:892. doi: 10.3390/nu13061892
16. Bendall, CL , Mayr, HL , Opie, RS , Bes-Rastrollo, M , Itsiopoulos, C , and Thomas, CJ . Central obesity and the Mediterranean diet: a systematic review of intervention trials. Crit Rev Food Sci Nutr. (2018) 58:3070–84. doi: 10.1080/10408398.2017.1351917
17. Greco, M , Chiefari, E , Montalcini, T , Accattato, F , Costanzo, FS , Pujia, A, et al. Early effects of a hypocaloric, Mediterranean diet on laboratory parameters in obese individuals. Mediat Inflamm. (2014) 2014:750860:1–8. doi: 10.1155/2014/750860
18. Rashad, NM , Sayed, SE , Sherif, MH , and Sitohy, MZ . Effect of a 24-week weight management program on serum leptin level in correlation to anthropometric measures in obese female: a randomized controlled clinical trial. Diabetes Metab Syndr. (2019) 13:2230–5. doi: 10.1016/j.dsx.2019.05.027
19. Nani, A , Murtaza, B , Sayed Khan, A , Khan, NA , and Hichami, A . Antioxidant and anti-inflammatory potential of polyphenols contained in Mediterranean diet in obesity: molecular mechanisms. Molecules. (2021) 26:985. doi: 10.3390/molecules26040985
20. Saben, JL , Sims, CR , Piccolo, BD , and Andres, A . Maternal adiposity alters the human milk metabolome: associations between nonglucose monosaccharides and infant adiposity. Am J Clin Nutr. (2020) 112:1228–39. doi: 10.1093/ajcn/nqaa216
21. Bach-Faig, A , Berry, EM , Lairon, D , Reguant, J , Trichopoulou, A , Dernini, S, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. (2011) 14:2274–84. doi: 10.1017/S1368980011002515
22. U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary guidelines for Americans, 2020-2025. (2020).
23. Krebs-Smith, SM , Pannucci, TE , Subar, AF , Kirkpatrick, SI , Lerman, JL , Tooze, JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
24. Reedy, J , Lerman, JL , Krebs-Smith, SM , Kirkpatrick, SI , Pannucci, TE , Wilson, MM, et al. Evaluation of the healthy eating Index-2015. J Acad Nutr Diet. (2018) 118:1622–33. doi: 10.1016/j.jand.2018.05.019
25. Goulet, J , Lamarche, B , Nadeau, G , and Lemieux, S . Effect of a nutritional intervention promoting the Mediterranean food pattern on plasma lipids, lipoproteins and body weight in healthy French-Canadian women. Atherosclerosis. (2003) 170:115–24. doi: 10.1016/S0021-9150(03)00243-0
26. World Health Organization . WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. (2006).
27. Myatt, M , and Guevarra, E . Zscorer: Child Anthropometry z-Score Calculator. R package version 0.3.1. (2019).
28. Scanlon, KS , Alexander, MP , Serdula, MK , Davis, MK , and Bowman, BA . Assessment of infant feeding: the validity of measuring milk intake. Nutr Rev. (2002) 60:235–51. doi: 10.1301/002966402320289368
29. Borschel, MW , Kirksey, A , and Hannemann, RE . Evaluation of test-weighing for the assessment of milk volume intake of formula-fed infants and its application to breast-fed infants. Am J Clin Nutr. (1986) 43:367–73. doi: 10.1093/ajcn/43.3.367
30. Neville, MC , and Keller, R . Accuracy of single- and two-feed test weighing in assessing 24 h breast milk production. Early Hum Dev. (1984) 9:275–81. doi: 10.1016/0378-3782(84)90038-0
31. Bates, D , Mächler, M , Bolker, B , and Walker, S . Fitting linear mixed-effects models Usinglme4. J Stat Softw. (2015) 67:1–48. doi: 10.18637/jss.v067.i01
32. Fox, J , and Weisberg, S . An {R} companion to applied regression, vol. 3. Thousand Oaks CA: Sage (2019).
33. Makowski, D , Ben-Shachar, MS , Patil, I , and Ludecke, D . Estimation of model-based predictions, contrasts and means. (2020).
34. Bakdash, JZ , and Marusich, LR . Repeated measures correlation. Front Psychol. (2017) 8:456. doi: 10.3389/fpsyg.2017.00456
35. R Core Team . R: a language and envrionment for statistical computing. R Foundation for Statistical Computing (2019).
36. Bonnet, M , Delavaud, C , Laud, K , Gourdou, I , Leroux, C , Djiane, J, et al. Mammary leptin synthesis, milk leptin and their putative physiological roles. Reprod Nutr Dev. (2002) 42:399–413. doi: 10.1051/rnd:2002034
37. Linne, Y . Effects of obesity on women's reproduction and complications during pregnancy. Obes Rev. (2004) 5:137–43. doi: 10.1111/j.1467-789X.2004.00147.x
38. Neville, CE , McKinley, MC , Holmes, VA , Spence, D , and Woodside, JV . The effectiveness of weight management interventions in breastfeeding women—a systematic review and critical evaluation. Birth. (2014) 41:223–36. doi: 10.1111/birt.12111
39. Bertz, F , Brekke, HK , Ellegard, L , Rasmussen, KM , Wennergren, M , and Winkvist, A . Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr. (2012) 96:698–705. doi: 10.3945/ajcn.112.040196
40. Stendell-Hollis, NR , Thompson, PA , West, JL , Wertheim, BC , and Thomson, CA . A comparison of Mediterranean-style and MyPyramid diets on weight loss and inflammatory biomarkers in postpartum breastfeeding women. J Womens Health (Larchmt). (2013) 22:48–57. doi: 10.1089/jwh.2012.3707
41. Galliano, LM , Del Vecchio, AHM , Silvani, J , Facanha, C , and Del Vecchio, FB . Physical activity level in women with gestational diabetes mellitus: lifestyle INtervention for diabetes prevention after pregnancy (LINDA-Brasil) study. J Diabetes. (2019) 11:457–65. doi: 10.1111/1753-0407.12872
42. Hartley, L , May, MD , Loveman, E , Colquitt, JL , and Rees, K . Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2016) 2016:CD011472. doi: 10.1002/14651858.CD011472.pub2
43. Kuziel, G , Moore, BN , Haugstad, GP , Xiong, Y , Williams, AE , and Arendt, LM . Alterations in the mammary gland and tumor microenvironment of formerly obese mice. BMC Cancer. (2023) 23:1183. doi: 10.1186/s12885-023-11688-3
44. Brown, KA . Impact of obesity on mammary gland inflammation and local estrogen production. J Mammary Gland Biol Neoplasia. (2014) 19:183–9. doi: 10.1007/s10911-014-9321-0
45. Samuel, TM , Binia, A , de Castro, CA , Thakkar, SK , Billeaud, C , Agosti, M, et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci Rep. (2019) 9:11767. doi: 10.1038/s41598-019-48337-4
46. Donovan, SM , and Comstock, SS . Human Milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab. (2016) 69:41–51. doi: 10.1159/000452818
47. Bode, L . Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. (2012) 22:1147–62. doi: 10.1093/glycob/cws074
48. Alderete, TL , Autran, C , Brekke, BE , Knight, R , Bode, L , Goran, MI, et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. (2015) 102:1381–8. doi: 10.3945/ajcn.115.115451
49. Larsson, MW , Lind, MV , Laursen, RP , Yonemitsu, C , Larnkjaer, A , Molgaard, C, et al. Human Milk oligosaccharide composition is associated with excessive weight gain during exclusive breastfeeding-an explorative study. Front Pediatr. (2019) 7:297. doi: 10.3389/fped.2019.00297
50. Plows, JF , Berger, PK , Jones, RB , Alderete, TL , Yonemitsu, C , Najera, JA, et al. Longitudinal changes in human Milk oligosaccharides (HMOs) over the course of 24 months of lactation. J Nutr. (2021) 151:876–82. doi: 10.1093/jn/nxaa427
51. Azad, MB , Robertson, B , Atakora, F , Becker, AB , Subbarao, P , Moraes, TJ, et al. Human Milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr. (2018) 148:1733–42. doi: 10.1093/jn/nxy175
52. Biddulph, C , Holmes, M , Kuballa, A , Davies, PSW , Koorts, P , Carter, RJ, et al. Human Milk oligosaccharide profiles and associations with maternal nutritional factors: a scoping review. Nutrients. (2021) 13:965. doi: 10.3390/nu13030965
53. Quin, C , Vicaretti, SD , Mohtarudin, NA , Garner, AM , Vollman, DM , Gibson, DL, et al. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J Biol Chem. (2020) 295:4035–48. doi: 10.1074/jbc.RA119.011351
54. ACOG . Barriers to breastfeeding: supporting initiation and continuation of breastfeeding: ACOG Committee opinion, number 821. Obstet Gynecol. (2021) 137:e54–62. doi: 10.1097/AOG.0000000000004249
Keywords: human milk, obesity, Mediterranean meal plan, human milk oligosaccharides, infant growth, healthy eating index, maternal diet
Citation: Sims CR, Saben JL, Martinez A, Sobik SR, Crimmins MR, Bulmanski JE, Turner D, Furst A, Jansen LT, Bode L and Andres A (2024) A Mediterranean diet plan in lactating women with obesity reduces maternal energy intake and modulates human milk composition – a feasibility study. Front. Nutr. 11:1303822. doi: 10.3389/fnut.2024.1303822
Received: 28 September 2023; Accepted: 29 February 2024;
Published: 13 March 2024.
Edited by:
Michał Czapla, Wroclaw Medical University, PolandReviewed by:
Tomás Cerdó, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), SpainCopyright © 2024 Sims, Saben, Martinez, Sobik, Crimmins, Bulmanski, Turner, Furst, Jansen, Bode and Andres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aline Andres, YW5kcmVzYWxpbmVAdWFtcy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.