
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr., 11 April 2024
Sec. Nutrition, Psychology and Brain Health
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1298807
 Fiona L. Dodd1*
Fiona L. Dodd1* David O. Kennedy1
David O. Kennedy1 Jodee Johnson2
Jodee Johnson2 Emily Haworth1,3
Emily Haworth1,3 Jessica P. Greener1
Jessica P. Greener1 Philippa A. Jackson1
Philippa A. Jackson1Introduction: Extracts made from the leaves of the edible mango plant (Mangifera indica L., Anacardiaceae) have a long history of medicinal usage, most likely due to the presence of high levels of mangiferin, a polyphenol compound. Previous research has demonstrated that mango leaf extract (MLE) can beneficially modulate cognitive function in both animals and humans. This study aimed to assess the effects of an acute dose of 300 mg MLE (standardised to contain ≥60% mangiferin) on cognitive performance and mood in healthy adults.
Methods: In this double-blind, placebo-controlled, crossover study, 114 healthy men and women (18–43 years) received either MLE or a matched placebo at each testing visit (separated by at least 7 days). Cognitive performance (including the cognitive demand battery) and mood were measured at 30, 180, and 300 min post-dose.
Results: The results showed that, compared to placebo, the group taking MLE displayed a significant increase in serial 3 s and serial 7 s subtraction errors overall. There were no other significant effects on cognitive performance.
Discussion: The results of the current study suggest that the consumption of 300 mg MLE in the absence of an observed multitasking psychological stressor does not improve cognitive performance or mood at up to 300 min post-dose. Due to the very limited nature of the effects and since they were observed among many analyses, these findings should be treated with caution.
Clinical trial registration: http://ClinicalTrials.gov, identifier [NCT05182450].
Mango leaf (Mangifera indica L., Anacardiaceae) has been utilised as a traditional medicine for its pharmacological effects in the treatment of diseases of the lungs, gallbladder, and kidney as well as bronchitis, diabetes, malaria, cancer, and inflammation (1, 2). The bioactivity of the mango leaf extract (MLE) is believed to be due to the presence of high levels of the polyphenol, xanthone, found in the extract, specifically mangiferin (1). Xanthones are only found in a small number of plant species, like Clusiaceae, but are seldom ingested by humans, with the exception of mango. Antibacterial, antifungal, and anti-inflammatory properties, among others, have all been found to result from mangiferin, a naturally occurring xanthone (1). It has been speculated that, although mangiferin is structurally different from other polyphenols, its mechanism of action may be similar (3).
Previous research has demonstrated that a single dose as well as chronic administration of polyphenols can beneficially modulate cognitive function (4–6), including during cognitively demanding task performance (7). A systematic review investigating the effects of doses ranging from 10 to 200 mg/kg of mangiferin over 12–154 days on memory impairment in animal models found that all studies reported an improvement in memory, specifically spatial recognition, episodic aversive events, and short- and long-term memories (8). Furthermore, a recent study in rats demonstrated a similar effect of MLE on electrophysiological (electroencephalopathy (EEG)) spectral power as that noticed following caffeine, with a synergistic effect of co-consumption of MLE and caffeine (9). M. indica has also been shown to enhance brain oxygenation and physical performance (10) and improve ergogenic parameters following ischaemia-reperfusion (11) in healthy humans when consumed alongside other polyphenols. Moreover, when consumed in isolation at a dose of 500 mg, MLE modulated brain electrical activity in humans (as measured by quantitative EEG) during cognitive challenge and led to reduced ratings of fatigue at 90 min and significant percentage improvement in reaction time as compared to placebo, at 60 min post-dose (12). Taken together, these findings raise the possibility that MLE, due to its high mangiferin content, may have beneficial effects on psychological parameters.
A recent study by our group (3) expanded on the above during an assessment of the effects of a single dose of MLE (>60% of polyphenol mangiferin) on performance across a number of cognitive domains and during laboratory-induced stress in humans. The results showed that a single dose of 300 mg MLE significantly improved performance accuracy across the tasks in the battery, with domain-specific effects observed in terms of enhanced performance on a global measure of “Accuracy of Performance”, the composite measures of “Accuracy of Attention”, and “Episodic Memory” as well as improved performance across the Cognitive Demand Battery sub-section (serial 3 s, serial 7 s and Rapid Visual Information Processing (RVIP) tasks) of the assessment. These findings were observed across assessments spanning 30 min to 5 h post-dose (measured at 30, 180, and 300 min). These results provide a robust demonstration of beneficial effects following consumption of MLE, reinforcing the findings of previous research that had shown that polyphenols and polyphenol-rich extracts can improve cognitive function.
The current study sought to confirm the previous findings on cognition by replicating only the cognitive procedures of the aforementioned study (3). For this reason, the observed multitasking stressor (OMS) included in the Wightman et al. study was not included here. The aim of the present study was to further assess the effect of a single dose (300 mg) of MLE on extended cognitive task performance and mood.
The study followed a randomised, double-blind, placebo-controlled, crossover design in which the acute effects of MLE on cognitive function and mood in healthy adults were observed. The study design included two in-lab testing visits, which were conducted at least 7 days apart, following a training/familiarisation session. The study was performed in accordance with the ethical principles enshrined in the Declaration of Helsinki (1996). The trial was conducted in compliance with protocol/Good Clinical Practice (GCP)applicable regulatory requirements and commenced only when a favourable ethical opinion was obtained from Northumbria University College of Reviewers (REF: 39521). Written informed consent was obtained from all the participants. The trial is registered at ClinicalTrials.gov (NCT05182450).
In the study by Wightman et al. (3), the required sample size for the study (n = 72) was calculated (GPower 3.0) on the basis of delivering adequate power (0.8) to detect a small effect size (f = 0.1). The study returned maximum effect sizes in the region of a medium effect size (f = 0.25). In the present study, a total sample size of n = 114 was therefore estimated to provide adequate power (0.8) to detect the small effect size apparent with respect to the primary outcome (Accuracy of Attention) at the first time point (30 min post-dose) and very good power (>0.98) to detect the larger effect sizes seen at later time points and on the primary outcome’s main effect.
A total of 191 participants aged 18–45 years, who self-reported as being in good health, were enrolled into the study. Participants were recruited via an opportunity sample from the students and staff of Northumbria University and the general population within Newcastle upon Tyne, as well as the surrounding areas. All participants reported being free from any relevant medical condition or disease including psychiatric disorders and neurodevelopmental differences. Blood pressure and body mass index (BMI) were measured at screening and participants were enrolled into the study if blood pressure measured was <159 mmHg (systolic) or < 99 mmHg (diastolic) and the measured BMI was within the range 18.5–35 kg/m2. Participants confirmed that they were not currently taking any relevant pharmaceuticals (or antibiotics in the last 4 weeks) and had not taken MLE or taken part in another clinical trial within the past 30 days. A full list of inclusion and exclusion criteria can be found in Supplementary File 1. All participants provided written informed consent to participate prior to any research-related procedures being performed.
Participants consumed one of two interventions at each testing visit (testing visits 1 and 2): 300 mg MLE (Zynamite®—standardised to contain ≥60% mangiferin) and a matched placebo, with intervention order counterbalanced across the sample. The full composition of the active intervention capsules and placebo capsules is listed in Supplementary File 2. All investigational products were prepared according to good manufacturing practice and delivered blind from the manufacturer (Merical, CA) in individual, sealed packets identified only by a three-digit code (365 or 972). Participants were randomly allocated to a computer-generated counterbalanced randomisation schedule (www.randomization.com), which was created by the research team and which dictated the order in which the participant received the two interventions. Randomisation numbers were allocated sequentially to the participants by the researcher during the first testing visit (testing visit 1) in the order in which they arrived. Participants consumed the interventions on each visit in the laboratory under the supervision of the researcher, allowing for 100% compliance.
This testing system delivers a bespoke collection of tasks, with fully randomised parallel versions of each task delivered at each assessment for each individual. It has previously been shown to be sensitive to a wide range of nutritional interventions (13–18). The selection of tasks employed in the present study comprised a number of standard and ‘classic’ tasks that assess aspects of memory (working, episodic, and spatial), attention and executive function, and the ‘Cognitive Demand Battery’ (CDB), which have been shown to be sensitive in numerous studies involving nutritional interventions, including the previous study that assessed the acute cognitive effects of MLE (3). Task outcomes were collapsed into composite scores to establish if the active intervention had a global effect on a given cognitive domain that might escape significance on the component tasks. These included the names of the cognitive domains in this section should capitalised. For example; Accuracy of Attention, Speed of Attention etc. All tasks administered are described in Supplementary File 3, and the task order and the contribution of the tasks to the composite scores is shown in Figure 1.
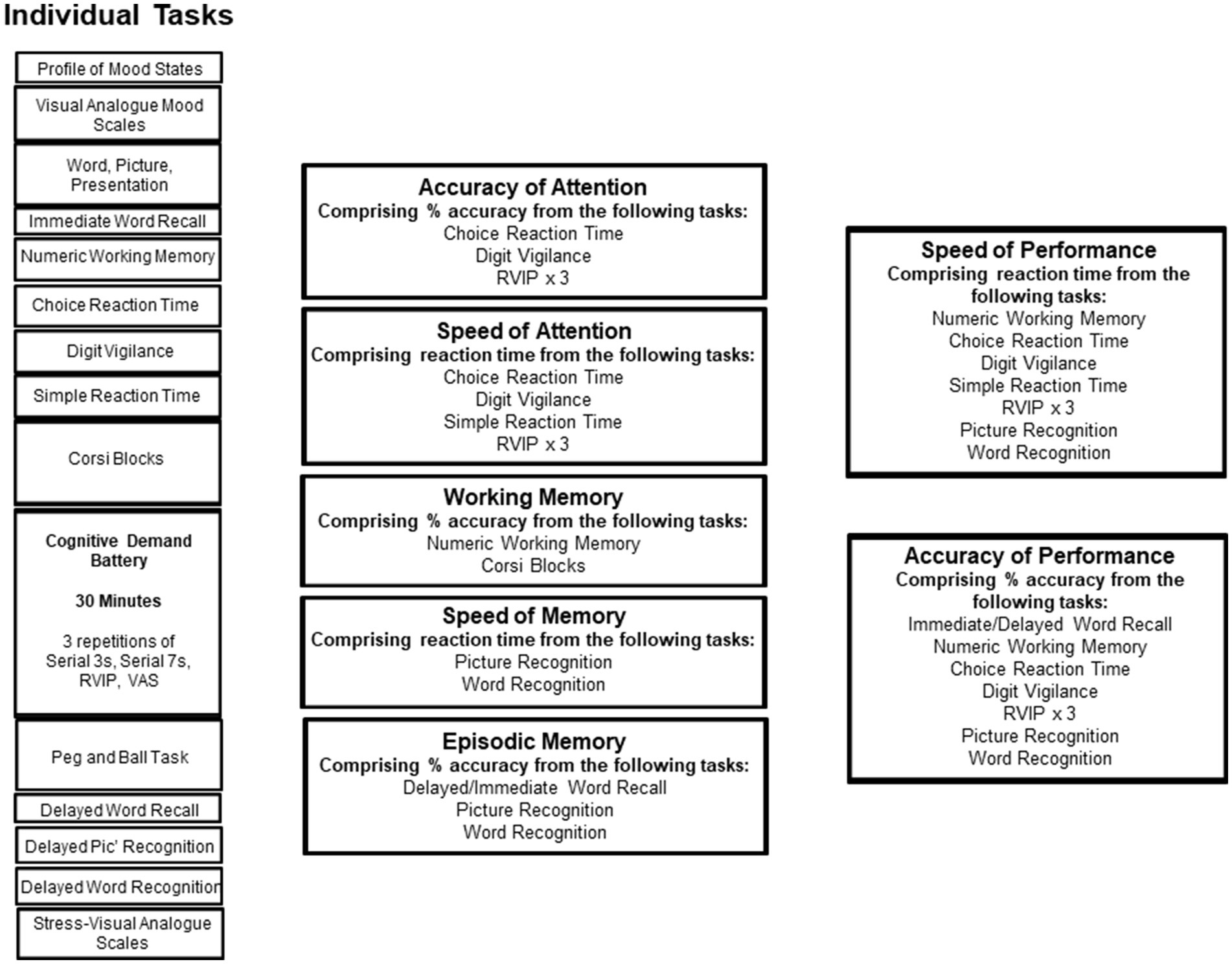
Figure 1. The running order of the individual tasks in the core cognitive assessment. Tasks are shown in order of completion on the left. On the right, the “cognitive domain” assessed by the tasks is shown, and the boxes to the far right show potential global measures into which data from several tasks are collapsed.
The POMS is a well-established, factor-analytically derived measure of psychological distress for which high levels of reliability and validity have been documented (19). The POMS consists of 65 adjectives rated on a 0–4 scale that can be consolidated into depression-dejection, tension-anxiety, anger-hostility, confusion-bewilderment, vigour-activity, and fatigue-inertia subscales. The latter two subscales can be interpreted as measures of fatigue and have been validated as separate factors in a number of studies. Norms have been published for a variety of patient and non-patient groups. The POMS was administered through Multi-Health Systems (MHS) Assessments Online Assessment Centre, prior to each cognitive assessment.
Prior to each cognitive assessment and as part of the COMPASS battery, participants completed a series of visual analogue scales anchored by 18 antonyms relating to mood and psychological state. Participants moved a marker along the line to describe how they currently feel. Each line was scored as a percentage along the line towards the more positive antonym. The factors were labelled Alertness (11 items: alert, inattentive; lethargic, energetic; clumsy, co-ordinated; lively, sluggish; quick-witted, slow-witted; sharp, dull; exhausted, refreshed; bored, engaged; focused, unfocused; drowsy, awake; motivated, unmotivated), Stress (4 items: tense, relaxed; fearful, fearless; stressed, carefree; peaceful, troubled), and Tranquillity (3 items: tranquil, agitated; contented, discontented; friendly, hostile).
Bespoke visual analogue scales were completed on COMPASS after each battery of cognitive tasks. The scales comprised 100 mm lines (anchored as 0 = not at all and 100 = extremely) and included the following questions:
How anxious do you feel? (Not at all—Extremely).
How stressed do you feel? (Not at all—Extremely).
How relaxed do you feel? (Not at all—Extremely).
How calm do you feel? (Not at all—Extremely).
All data were collected at the Brain, Performance and Nutrition Research Centre (BPNRC), located on Northumbria University’s Newcastle city centre campus. Participants were required to have an initial remote screening session followed by three separate visits to the laboratory: an introductory/training/familiarisation visit and two active testing visits.
The remote screening session was completed via video/telephone call and comprised a briefing on the requirements of the study, obtaining of informed consent via completion of an online consent form, medical history, collection of demographic data, and completion of the Caffeine Consumption Questionnaire (CCQ).
The introductory/training visit to the laboratory began with in-person consent and confirmation of eligibility. Physical eligibility measures of height, weight, blood pressure (BP), and waist-to-hip ratio (WHR) were obtained. Participants were then given the opportunity to ask questions about the study, familiarise themselves with the laboratory environment, and train on the cognitive/mood tasks they would complete at testing. An abbreviated version of the Pittsburgh Sleep Questionnaire (20) was also administered at this session in order to record the average number of hours of sleep per night. This information was then used to ensure the participants had experienced a typical night’s sleep prior to attending each testing visit.
The methodology at both testing visits was identical, with the exception of the intervention the participant consumed. Upon arrival, participants were asked to confirm that they had followed the pre-testing instructions, specifically (i) to eat a standardised breakfast of cereal and/or toast at home no later than 1 h prior to arrival at the lab, (ii) to avoid caffeine for 12 h prior to attending the lab, (iii) to avoid vigorous physical activity for 24 h prior to testing, (iv) to have a typical night’s sleep prior to each visit, and (v) to avoid alcohol 24 h prior to testing. Participants were advised they would be required to arrive in the same state for the second testing visit as their first testing visit, or they would need to reschedule. This included eating the same breakfast, having a typical night’s sleep and avoiding alcohol, strenuous physical exercise, and caffeine for the requisite amount of time prior to their attendance. Testing was conducted in a suite of dedicated temperature-controlled university laboratories with participants visually isolated from each other.
Upon arrival at each testing visit, the participants were screened for continued eligibility and then completed the POMS followed by a 60-min computerised cognitive/mood assessment (COMPASS—including the VAMS, individual cognitive tasks, CDB, and S-VAS). After the first set of cognitive/mood assessments, the participants took their intervention capsule with water under the supervision of a member of the research team. As this was a replication of Wightman et al. study and because they observed significant effects of the intervention within 30 min of administration, the same timeframe for absorption was applied here. Participants then underwent cognitive/mood assessments identical to the above at 30, 180, and 300 min post-dose. Following the completion of the 30-min post-dose assessment, the participants were given the option of a snack a decaffeinated hot tea or coffee and plain digestive biscuits (replicated at testing visit 2). Following completion of the 180-min post-dose assessment, the participants were provided with a standardised lunch comprising of a cheese sandwich on white bread with butter, crisps, and a custard pot (replicated at testing visit 2). No alternative snacks or lunches were offered. Upon completion of testing visit 2, participants completed a “treatment guess” form and were fully debriefed, thanked, and compensated for their time. See Figure 2 for a schematic depicting the procedure during testing visits 1 and 2.
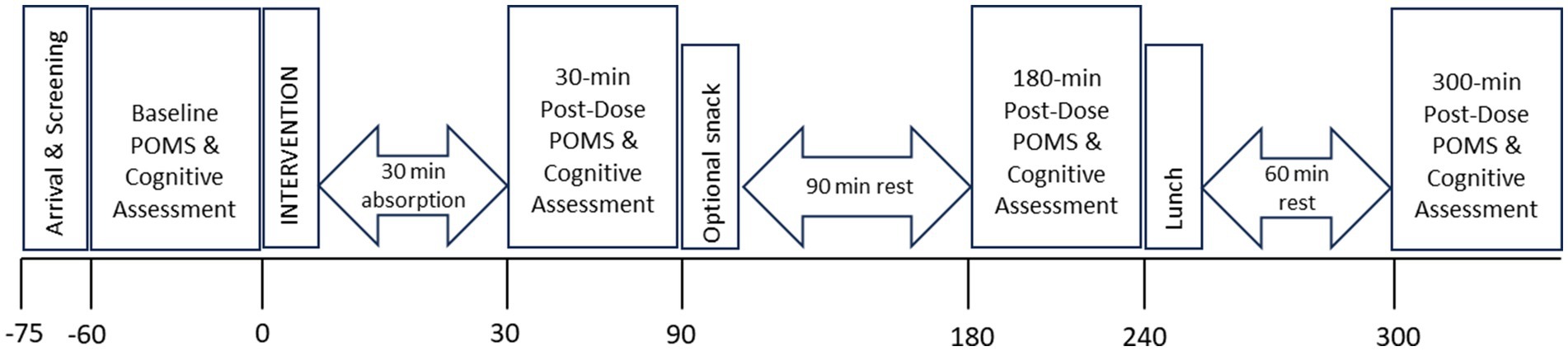
Figure 2. The timeline of the testing day for an individual participant, showing the cognitive assessment schedule.
All outcomes were analysed using SPSS (version 28.0, IBM corp.). Multilevel modelling was used to fit the composite or individual score for each outcome measure. Intervention (MLE, Placebo) and Time (30, 180, 300 min post-dose) were entered into the model as fixed effects along with their interaction and respective baseline score as a covariate. Repetition (1-3) was included as an additional fixed effect for the analysis of the CDB outcome measures. Participant was entered as a random effect where appropriate, as determined by Schwartz’s Bayesian Criteria. The covariance structures of the residuals were modelled as appropriate. Significant interaction effects were analysed further using pairwise comparisons and adjusted for multiple comparisons according to Sidak.
A total of 118 participants were randomised to the interventions. Four participants withdrew post-randomisation following testing visit 1, leaving a total of 114 complete datasets that were included in the analysis. See Figure 3. Missing data were presumed to be missing at random and were estimated within the analysis using the restricted maximum likelihood (REML) method.
Participant demographics and baseline characteristics are summarised in Tables 1, 2.
As participants consumed the interventions on each visit in the laboratory under the supervision of the researcher, compliance was at 100%. The success of blinding was confirmed via “treatment guess” at the end of the study (final testing visit). The chi-squared analysis confirmed that participants were not able to correctly identify whether they had received MLE or placebo [X2 (1, N = 114) = 0.04, p = 0.851] at their final visit. Specifically, 53% of participants who received placebo and 54% of participants who had received MLE at their final visit guessed that they had been given placebo.
No effect of the intervention was observed for the primary outcome measure Accuracy of Attention, nor any other of the composite or individual task scores.
A main effect of intervention was observed for serial subtractions errors for both serial 3 s and serial 7 s subtractions. Investigation revealed that there were significantly fewer errors following placebo (M = 2.50; SEM = 0.18) as compared to MLE (M = 2.86; SEM = 0.18), for serials 3 s errors [F (1, 561.94) = 7.77, p < 0.01], and significantly fewer errors following placebo (M = 2.75; SEM = 0.19) as compared to MLE (M = 3.10; SEM = 0.19), for serial 7 s errors [F (1, 475.73) = 7.37, p < 0.01]. See Figures 4, 5. No other cognitive performance effects were observed.
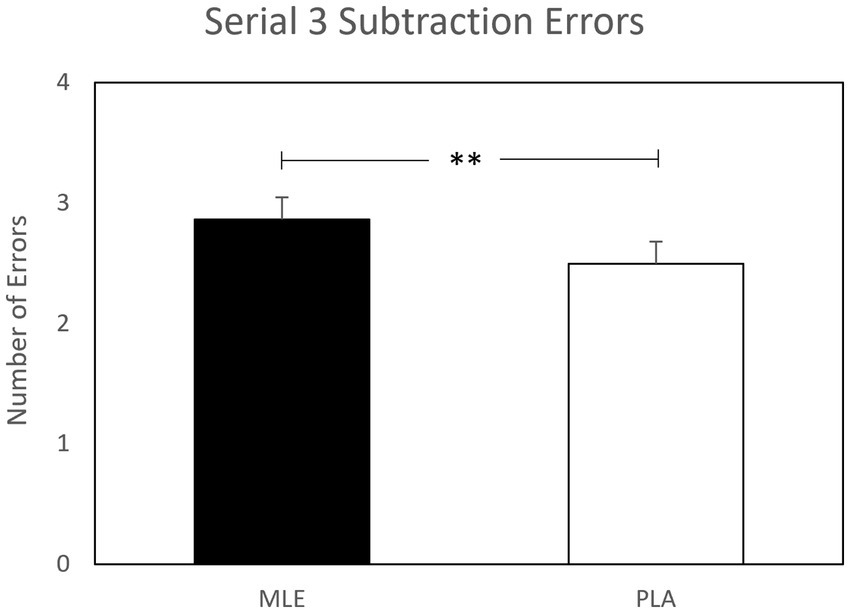
Figure 4. Effect of interventions on number of serial 3 subtractions errors during the cognitive demand battery. Data presented are estimated marginal means (±SE) derived from the linear mixed model for serial 3 subtraction errors. **p < 0.01. MLE, Mango leaf extract; PLA, Placebo.
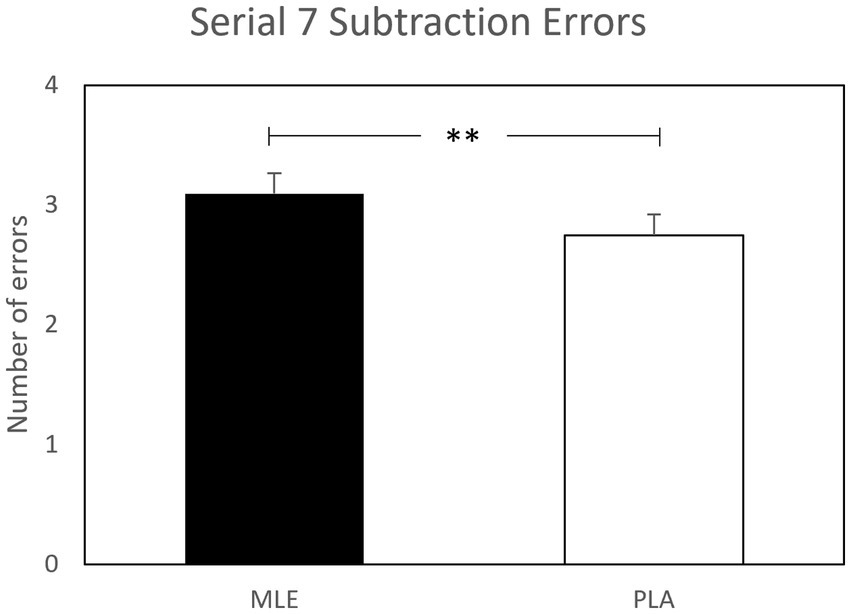
Figure 5. Effect of interventions on number of serial 7 subtractions errors during the cognitive demand battery. Data presented are estimated marginal means (±SE) derived from the linear mixed model for serial 7 subtraction errors. **p < 0.01. MLE, Mango leaf extract; PLA, Placebo.
No effect of intervention was observed for any of the mood outcome measures.
Tables of all outcome measures can be found in Supplementary File 4.
The results of the current study suggest that the consumption of 300 mg MLE does not improve cognitive performance or mood, at up to 300-min post-dose. The limited effects that were observed followed a pattern of worsening performance, with main effects across both serial 3 s and serial 7 s subtraction errors revealing that, compared to placebo, MLE led to an increase in subtraction errors overall.
The negative effects observed here for the consumption of 300 mg MLE are somewhat unexpected, particularly since previous research of this extract has demonstrated predominantly positive effects across physiological, cognitive, and mood parameters in humans and animals (3, 8, 9, 12). As a consequence and, given the small number of negative effects among many analyses, these results should be treated with caution.
The current study intended to confirm previous cognitive research on this mango extract and was a replication of the study by Wightman et al. (3), who administered the same extract and implemented similar study procedures to those described here. Their study demonstrated that 300 mg MLE led to improved Accuracy of Performance, Accuracy of Attention, Episodic Memory, and, specifically in relation to the findings here, improved performance on serial 3 s and serial 7 s subtractions tasks (demonstrated by an increase in the number of correct responses). The absence of extract-related cognitive effects in the present study in light of the series of positive effects observed previously is difficult to explain and may be as a result of inexorable methodological differences, specifically the sample of participants studied. It is pertinent to mention, however, that the participant criteria for inclusion and exclusion in the present study was matched to that of the Wightman study. Differences in batches may also provide some explanation for the disparate findings in this study. Here, MLE was standardised to contain ≥60% mangiferin, and variations in the composition of the extract as a consequence of the percentage of mangiferin (however small) have the potential to impact the results. However, previous research has demonstrated positive effects of this polyphenol, irrespective of differences in batch or composition of the extract investigated (3, 8, 12).
Of note here is a key methodological difference between the two studies; one possibility is that the observed multitasking stressor (OMS) included in the Wightman study (but not included here) led to an unexpected influence with regards to the impact of the extract on cognitive performance. Indeed, simple comparisons of the mood/stress levels observed here with the aforementioned study suggest a more pronounced effect upon these measures in the Wightman study (even when taking differences in the sample into consideration). In the study by Wightman et al., a 5-min OMS assessment was administered at baseline and again following the 30-min post-dose cognitive assessment. It has been demonstrated previously that the OMS procedure modulates subjective and physiological measures of stress for an extended period—during, after, and in anticipation of its administration. For example, cortisol levels have been shown to be elevated at 15 min before the OMS and staying elevated until their decline approximately 60 min post-OMS (21). Mangiferin is known to possess anti-inflammatory, immunomodulatory, antioxidant, and neuroprotective properties (22–26) and has been shown to alleviate corticosterone (27) and lipopolysaccharide-induced anxiety in mice (28), ameliorating neurobehavioural deficits (27). Taking this into consideration, it is possible that the additional demand placed on cognitive resources as a consequence of the OMS gave rise to the cognitive improvements observed following MLE previously and may provide some explanation as to why similar cognitive effects were not observed in the present study. It is important to note, however, that beneficial cognitive effects of MLE have been observed in healthy animal models when the demand experienced has been arguably lower than that experienced here (29).
There were, however, methodological limitations of the present research. Participants were not required to follow a low polyphenol diet in the days prior to their attendance at each of the study visits (as per Wightman et al., whose study this research sought to replicate). Since mangiferin (the compound considered to be active within MLE) is itself a polyphenol, variability in its levels at baseline has the potential to impact the results observed. Future research involving this extract would benefit from participants being advised to follow a low polyphenol diet 48 h prior to testing to control for this outcome. We also acknowledge that differences in food intake on testing days between participants may have influenced study findings; for example, breakfast items chosen on the morning of testing, as well as the optional snack chosen during the testing day. However, individuals were required to have the same food at testing visit 2 as they had at testing visit 1, so the food consumed at breakfast, during the optional snack, and at lunch remained consistent within the participants. Consequently, the impact is likely to be minimal.
Due to the sparse research into MLE and its cognitive effects in human participants, in addition to the disparate findings here with that of Wightman et al. (3), future studies would benefit from exploring this extract further with respect to cognition and mood. An investigation of the dose–response would be of interest since positive psychological effects of mangiferin have been observed in animals at doses as low 10 mg/kg and as high as 200 mg/kg. A similar exploration of the dose–response relationship in humans is warranted, since, at present, research is limited to the observation of positive cognitive and mood effects at doses of 300 and 500 mg (3, 12). Furthermore, an exploration of the chronic effects of MLE would be beneficial in order to determine if longer-term supplementation leads to a cumulative effect with regards to cognition.
Overall, the findings of the present study suggest that consumption of 300 mg MLE, in the absence of an observed multitasking psychological stressor, does not improve cognitive performance or mood, at 30, 180, or 300 min post-dose. The specific effects that were observed as a result of MLE followed a pattern of worsening performance. However, due to the very limited nature of the effects and since they were observed among many analyses, these findings should be treated with caution.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Northumbria University Ethical Approval System (ref: 39521). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FD: Writing – original draft, Writing – review & editing. DK: Writing – review & editing. JJ: Writing – review & editing. EH: Writing – review & editing, Writing – original draft. JG: Writing – original draft, Writing – review & editing. PJ: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by PepsiCo.
The authors would like to thank Rian Elcoate and Anna Small for their contribution to data collection.
JJ is an employee of PepsiCo, the sponsor of the study. JJ contributed to the design of the protocol and provided comments on the manuscript but had no role in collection, analysis, or interpretation of the results. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1298807/full#supplementary-material
1. Ediriweera, MK, Tennekoon, KH, and Samarakoon, SR. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (mango). Evid Based Complement Alternat Med. (2017) 2017:1–24. doi: 10.1155/2017/6949835
2. Kim, H-S, Quon, MJ, and Kim, J-a. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. (2014) 2:187–95. doi: 10.1016/j.redox.2013.12.022
3. Wightman, EL, Jackson, PA, Forster, J, Khan, J, Wiebe, JC, Gericke, N, et al. Acute effects of a polyphenol-rich leaf extract of Mangifera indica L. (Zynamite) on cognitive function in healthy adults: a double-blind, placebo-controlled crossover study. Nutrients. (2020) 12:2194. doi: 10.3390/nu12082194
4. Desideri, G, Kwik-Uribe, C, Grassi, D, Necozione, S, Ghiadoni, L, Mastroiacovo, D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the cocoa, cognition, and aging (CoCoA) study. Hypertension. (2012) 60:794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060
5. Mastroiacovo, D, Kwik-Uribe, C, Grassi, D, Necozione, S, Raffaele, A, Pistacchio, L, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the cocoa, cognition, and aging (CoCoA) study—a randomized controlled trial. Am J Clin Nutr. (2015) 101:538–48. doi: 10.3945/ajcn.114.092189
6. Haskell-Ramsay, CF, Stuart, RC, Okello, EJ, and Watson, AW. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur J Nutr. (2017) 56:2621–31. doi: 10.1007/s00394-017-1454-7
7. Scholey, AB, French, SJ, Morris, PJ, Kennedy, DO, Milne, AL, and Haskell, CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. (2010) 24:1505–14. doi: 10.1177/0269881109106923
8. Lum, PT, Sekar, M, Gan, SH, Pandy, V, and Bonam, SR. Protective effect of mangiferin on memory impairment: a systematic review. Saudi J Biol Sci. (2021) 28:917–27. doi: 10.1016/j.sjbs.2020.11.037
9. Dimpfel, W, Wiebe, J, Gericke, N, and Schombert, L. Zynamite (Mangifera indica leaf extract) and caffeine act in a synergistic manner on electrophysiological parameters of rat central nervous system. Food Nutr Sci. (2018) 9:502–18. doi: 10.4236/fns.2018.95039
10. Gelabert-Rebato, M, Wiebe, JC, Martin-Rincon, M, Galvan-Alvarez, V, Curtelin, D, Perez-Valera, M, et al. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients. (2019) 11:364. doi: 10.3390/nu11020344
11. Gelabert-Rebato, M, Wiebe, JC, Martin-Rincon, M, Gericke, N, Perez-Valera, M, Curtelin, D, et al. Leaf extract in combination with luteolin or quercetin enhances VO(2)peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front Physiol. (2018) 9:740. doi: 10.3389/fphys.2018.00740
12. López-Ríos, L, Wiebe, JC, Vega-Morales, T, and Gericke, N. Central nervous system activities of extract Mangifera indica L. J Ethnopharmacol. (2020) 260:112996. doi: 10.1016/j.jep.2020.112996
13. Stonehouse, W, Conlon, CA, Podd, J, Hill, SR, Minihane, AM, Haskell, C, et al. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr. (2013) 97:1134–43. doi: 10.3945/ajcn.112.053371
14. Kennedy, DO, Wightman, EL, Forster, J, Khan, J, Haskell-Ramsay, CF, and Jackson, PA. Cognitive and mood effects of a nutrient enriched breakfast bar in healthy adults: a randomised, double-blind, placebo-controlled, parallel groups study. Nutrients. (2017) 9:1332. doi: 10.3390/nu9121332
15. Kennedy, DO, Jackson, PA, Forster, J, Khan, J, Grothe, T, Perrinjaquet-Moccetti, T, et al. Acute effects of a wild green-oat (Avena sativa) extract on cognitive function in middle-aged adults: a double-blind, placebo-controlled, within-subjects trial. Nutr Neurosci. (2017) 20:135–51. doi: 10.1080/1028415X.2015.1101304
16. Kennedy, DO, Dodd, FL, Robertson, BC, Okello, EJ, Reay, JL, Scholey, AB, et al. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J Psychopharmacol. (2011) 25:1088–100. doi: 10.1177/0269881110385594
17. Haskell-Ramsay, CF, Jackson, PA, Forster, JS, Dodd, FL, Bowerbank, SL, and Kennedy, DO. The acute effects of caffeinated black coffee on cognition and mood in healthy young and older adults. Nutrients. (2018) 10:1386. doi: 10.3390/nu10101386
18. Kennedy, D, Okello, E, Chazot, P, Howes, MJ, Ohiomokhare, S, Jackson, P, et al. Volatile terpenes and brain function: investigation of the cognitive and mood effects of Mentha × piperita L. essential oil with in vitro properties relevant to central nervous system function. Nutrients. (2018) 10:1029. doi: 10.3390/nu10081029
19. Heuchert, JP, and McNair, DM. Profile of mood states second edition manual. Toronto, Canada: Multi-Health Systems Inc. (2012).
20. Buysse, DJ, Reynolds, CF 3rd, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
21. Dodd, F, Kennedy, D, Wightman, E, Khan, J, Patan, M, Elcoate, R, et al. The chronic effects of a combination of herbal extracts (Euphytose(®)) on psychological mood state and response to a laboratory stressor: a randomised, placebo-controlled, double blind study in healthy humans. J Psychopharmacol. (2022) 36:1243–56. doi: 10.1177/02698811221112933
22. Garrido, G, González, D, Delporte, C, Backhouse, N, Quintero, G, Núñez-Sellés, AJ, et al. Analgesic and anti-inflammatory effects of Mangifera indica L. extract (Vimang). Phytother Res. (2001) 15:18–21. doi: 10.1002/1099-1573(200102)15:1<18::AID-PTR676>3.0.CO;2-R
23. Guha, S, Ghosal, S, and Chattopadhyay, U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy. (1996) 42:443–51. doi: 10.1159/000239478
24. Kasbe, P, Jangra, A, and Lahkar, M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J Trace Elem Med Biol. (2015) 31:107–12. doi: 10.1016/j.jtemb.2015.04.002
25. Li, H-w, Lan, T-j, Yun, C-x, Yang, K-d, Du, Z-c, Luo, X-f, et al. Mangiferin exerts neuroprotective activity against lead-induced toxicity and oxidative stress via Nrf2 pathway. Chin Herb Med. (2020) 12:36–46. doi: 10.1016/j.chmed.2019.12.002
26. Rao, VS, Carvalho, AC, Trevisan, MT, Andrade, GM, Nobre-Júnior, HV, Moraes, MO, et al. Mangiferin ameliorates 6-hydroxydopamine-induced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Pharmacol Rep. (2012) 64:848–56. doi: 10.1016/S1734-1140(12)70879-4
27. Luo, GQ, Liu, L, Gao, QW, Wu, XN, Xiang, W, and Deng, WT. Mangiferin prevents corticosterone-induced behavioural deficits via alleviation of oxido-nitrosative stress and down-regulation of indoleamine 2,3-dioxygenase (IDO) activity. Neurol Res. (2017) 39:709–18. doi: 10.1080/01616412.2017.1310705
28. Jangra, A, Lukhi, MM, Sulakhiya, K, Baruah, CC, and Lahkar, M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur J Pharmacol. (2014) 740:337–45. doi: 10.1016/j.ejphar.2014.07.031
Keywords: mango leaf extract, cognition, cognitive demand, attention, stress, mood
Citation: Dodd FL, Kennedy DO, Johnson J, Haworth E, Greener JP and Jackson PA (2024) Acute effects of mango leaf extract on cognitive function in healthy adults: a randomised, double-blind, placebo-controlled crossover study. Front. Nutr. 11:1298807. doi: 10.3389/fnut.2024.1298807
Received: 22 September 2023; Accepted: 20 March 2024;
Published: 11 April 2024.
Edited by:
David Vauzour, University of East Anglia, United KingdomReviewed by:
Con Stough, Swinburne University of Technology, AustraliaCopyright © 2024 Dodd, Kennedy, Johnson, Haworth, Greener and Jackson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiona L. Dodd, Zi5kb2RkQG5vcnRodW1icmlhLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.