
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 27 May 2024
Sec. Nutrigenomics
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1292834
This article is part of the Research Topic Nutrition and Metabolism in Cancer: Role in Prevention and Prognosis View all 30 articles
Background: The causal association of sarcopenia with the incidence risk of hepatocellular carcinoma (HCC) in the European population, and the potential mediating role of C-reactive protein (CRP), remains unclear. This study employed a bidirectional two-sample, two-step Mendelian randomization (MR) analysis to investigate the causality and identify the mediator.
Methods: Summary statistics for HCC, CRP, and sarcopenia-related traits, including appendicular lean mass (ALM), hand grip strength (HGS), and walking pace (WP), were acquired from publicly available databases. We conducted bidirectional MR and Steiger tests of directionality to check the presence of reverse causality. Additionally, a two-step MR analysis was used to assess the mediating effect of CRP in the causality between sarcopenia and HCC. Tests for heterogeneity and horizontal pleiotropy were performed.
Results: As ALM increases, the risk of HCC occurrence decreases [odds ratio (OR), 95% confidence interval (CI): 0.703, 0.524–0.943; P = 0.019]. And, genetically predicted low-HGS (OR, 95%CI: 2.287, 1.013–5.164; P = 0.047) was associated with an increased incidence risk of HCC, with no reverse causality. However, we found no evidence supporting a causality between WP and HCC. CRP was identified as the mediator of the causal effect of ALM and low-HGS on HCC, with corresponding mediating effects of 9.1% and 7.4%.
Conclusions: This MR study effectively demonstrates that lower ALM and low-HGS are linked to an elevated risk of HCC within the European population, and the causality was not bidirectional. Furthermore, CRP serves as a mediator in the associations. These findings may help mitigate HCC risk among individuals with sarcopenia.
Liver cancer is one of the most prevalent malignancies globally, with hepatocellular carcinoma (HCC) accounting for more than 80% of liver cancers (1). Although the global fatality rate of HCC has exhibited a modest decline over the last decade, it remains alarmingly high (2). It is predicted that the annual incidence of HCC will exceed one million cases by 2025 (3).
Currently, the only widely accepted definition of sarcopenia originates from the European Working Group on Sarcopenia in Older People (EWGSOP) and updated to EWGSOP2 in 2019. In both clinical practice and scientific research, EWGSOP2 delineates sarcopenia as the concurrent presence of low muscle strength and low muscle mass or quality for diagnosis. Moreover, the severity of sarcopenia is ascertained if the patient exhibits concomitant low physical performance (4, 5). Sarcopenia, an age-related degenerative disorder affecting skeletal muscle quality and function, profoundly compromises mobility, nutritional status, and independence (4). The known risk factors associated with sarcopenia can be classified into five categories. Firstly, intrinsic factors encompass age, sex hormone deficiency, comorbidities (e.g., diabetes), and genetic factors. Secondly, body composition, including significant weight loss and sarcopenic obesity (6). Thirdly, lifestyle habits, comprising prolonged immobilization and low physical activity (7). Fourthly, dietary aspects, include low protein intake (8) and vitamin D deficiency (9). Lastly, pharmacological interventions, notably the utilization of ACEI or steroids (6).
At present, many observational studies have proved that sarcopenia is closely related to poor survival outcomes and prognosis of HCC subsequent to various therapeutic modalities, including hepatectomy (10–12), radiofrequency ablation therapy (13, 14), liver transplantation (15), trans-arterial chemoembolization (16), and systemic therapies (17–19). Nevertheless, a paucity of studies have investigated the association of sarcopenia with the incidence risk of HCC. Currently, merely two cohort studies based on Asian populations have proffered evidence that sarcopenia is an independent risk factor for HCC (20, 21). There has been a lack of research on the nexus between sarcopenia and HCC within the European population so far. Given the obvious difference in the impact of sarcopenia on HCC survival outcomes between Eastern and Western populations (11), it is necessary and important to investigate the causal influence of sarcopenia on the risk of HCC within the Western population.
C-reactive protein (CRP), a plasma protein, demonstrates escalated circulatory levels during inflammation and after tissue damage. A pioneering study as early as 1989 showed that serum CRP could be used as a diagnostic biomarker for HCC (22). Recently, many studies have indicated that the level of CRP holds predictive value concerning the prognosis and therapeutic effect of HCC (23–25). Meanwhile, some studies have shown that sarcopenia is closely related to CRP (26, 27). According to the above, CRP might be associated with both sarcopenia and HCC, suggesting that CRP could potentially serve as a risk factor for sarcopenia in people with HCC. Consequently, the exploration of the mediating role of CRP in the relationship between sarcopenia and HCC could enhance the comprehension of the pathogenesis of sarcopenia to HCC and provide potential targets for reducing sarcopenia-related HCC risk. So far, however, few researches have studied the mediating pathway from sarcopenia to HCC.
Mendelian randomization (MR) analysis effectively evaluates the causality between exposures and outcomes by utilizing genetic variations. Compared with traditional observational studies, MR can prevent the interference of unmeasured confounders. To the best of our knowledge, the application of the MR methodology to elucidate the causal impact of sarcopenia on HCC risk within Western populations remains an unexplored domain. Thus, we performed an MR study to investigate the genetic association between sarcopenia and HCC and to evaluate the extent of CRP's mediating role in these associations. This research aims to bridge the gap in the current investigation of the relationship between sarcopenia and HCC within Western populations utilizing MR analysis. Our study may offer a novel perspective for identifying individuals at high risk of HCC and preventing sarcopenia-related HCC.
This MR study mainly consists of two stages of analysis. Initially, we conducted bidirectional MR analyses to identify and estimate the causal associations of sarcopenia-related traits with HCC. Subsequently, we evaluated CRP's mediating role in the causal links between sarcopenia-related traits and HCC. As shown in Figure 1A, the MR analysis has three critical assumptions: (1) the relevance assumption: the instrumental variables (IVs) are associated with the exposures; (2) the independence assumption: the IVs cannot be connected to any known confounders; and (3) the exclusivity assumption: the IVs do not affect the outcome except through the exposures (28). This MR study employed publicly available GWAS datasets. Within the original GWAS, all study participants provided written informed consent.
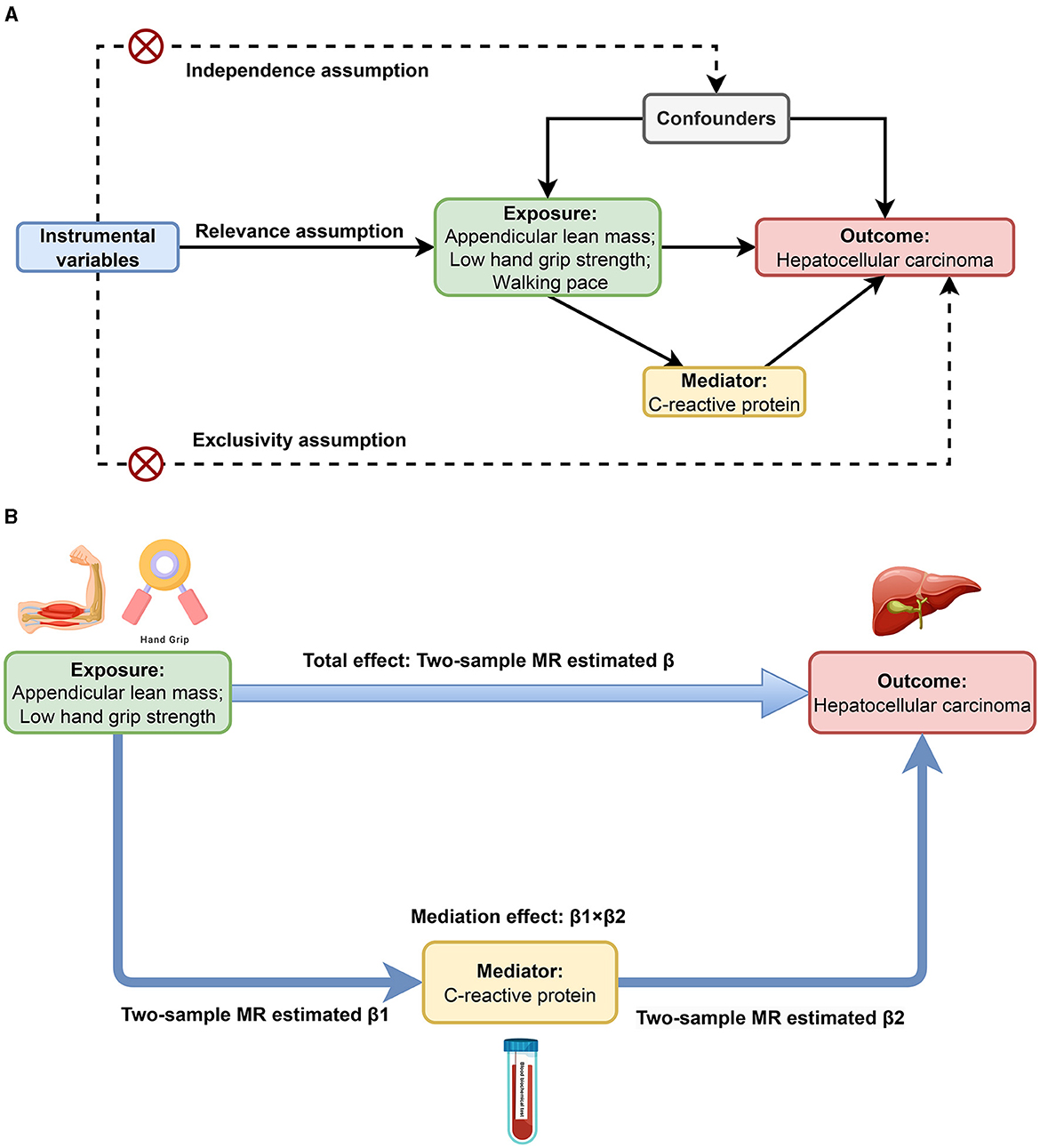
Figure 1. Study design overview. (A) Three critical assumptions of MR analysis. (B) Two-step MR framework.
EWGSOP2 delineates sarcopenia as the concurrent presence of low muscle strength and low muscle mass or quality for diagnosis. Moreover, low physical performance is used to ascertain the severity of sarcopenia. Muscle strength is assessed through hand grip strength (HGS) measurement or chair stand tests. According to the EWGSOP, HGS < 27 kg (male) or < 16 kg (female), or chair stand test >15 seconds for five rises, indicates low muscle strength. Evaluation of muscle mass or quality typically involves dual-energy X-ray absorptiometry, CT, MRI, or magnetic resonance spectroscopy. Appendicular lean mass (ALM) < 20 kg (male), < 15 kg (female) is defined as low muscle quantity. Physical performance is gauged by walking pace (WP), the timed-up-and-go (TUG) test, or completion of a 400-meter walk. And, WP ≤ 0.8 m/s, or TUG ≥20 s, or incomplete 400 m walking or completion exceeding 6 min, denotes low physical performance (5). Genetic instruments for ALM were derived from the UK Biobank, involving 450,243 individuals of European descent (29). Genetic instruments for low-HGS were derived from GWAS data encompassing 135,468 European individuals sourced from the Musculoskeletal Knowledge Portal (30). Genetic instruments for WP (n = 459,915) sourced from the MRC Integrative Epidemiology Unit (MRC-IEU). CRP in the blood is a biomarker of inflammation and is measured by blood biochemical tests. GWAS data for CRP were derived from the GWAS by UK Biobank based on 343,524 European individuals (31). To mitigate sample overlap with the exposure GWASs, summary statistics for HCC were from the FinnGen DF9 dataset (453 cases and 287,137 controls) (32). Supplementary Table S1 lists the detailed information of each GWAS summary data in this study.
The genetic IVs selection adhered to the following principles: Firstly, we incorporated SNPs demonstrating significant genome-wide associations with the exposure variables, adhering to stringent thresholds (P < 5 × 10−8; P < 5 × 10−6 for more SNP). Secondly, SNPs independent of each other (linkage disequilibrium [LD] r2 < 0.001 within 10,000 kb) were deemed suitable for inclusion (33). Thirdly, MR-PRESSO was used to remove outliers (34). Finally, the F-statistics were defined as F = /SE to quantify the potency of each SNP, and SNPs with F value < 10 were considered as weak instruments and excluded (35). Detailed IVs data included in this research are shown in Supplementary Tables S2–S9.
We conducted a bidirectional two-sample MR analysis to explore the relationship between sarcopenia-related traits and HCC, and the estimate was the total effect (β). As shown in Figure 1B, we further performed a two-step MR to investigate whether CRP mediates the causal associations between sarcopenia-related traits and HCC. The first step involved the application of a two-step MR approach to estimate the causal effect of each sarcopenia-related trait on CRP, and the estimate was denoted as β1. Meanwhile, we used Steiger test of directionality to examine whether there was a reverse causal relationship between CRP and each sarcopenia-related trait. In the second step, we estimated the causal impact of CRP on HCC employing two-sample MR in conjunction with multivariate MR (MVMR), adjusting for each sarcopenia-related trait. And the two-sample MR estimate was recorded as β2. Reverse MR was used to ensure no reverse causality between HCC and CRP. The mediation effect was calculated as β1 × β2, and the ratio of the mediation effect to the total effect was quantified as R = (β1 × β2)/β (36).
In two-sample MR, we primarily utilized the random effects inverse variance weighting (IVW) analysis method to ascertain the causal relationship between exposures and outcomes. At the same time, MR–Egger (37), weighted median (38), simple mode, and weighted mode (39) were used as supplementary analysis methods. IVW was the primary method of MVMR analysis, while MR–Egger was the auxiliary method.
For sensitivity analysis, we implemented three approaches: heterogeneity test, horizontal pleiotropy test, and “leave-one-out” method. Heterogeneity was assessed using Cochrane's Q-test, where a Q p value < 0.05 was considered indicative of heterogeneity (40). In the presence of heterogeneity, we applied the IVW method with random effects for our study. To identify the presence of horizontal pleiotropy, we scrutinized the statistical significance of the MR–Egger intercept term. In addition, we employed the global test within MR-PRESSO to examine the presence of pleiotropy in our study (34, 41). To evaluate the influence of a single SNP on the causal association, we conducted a “leave-one-out” analysis to eliminate each SNP in turn.
The two-sample MR analysis was carried out utilizing R (version 4.2.0) and the package “TwoSampleMR.” MVMR was performed using the “TwoSampleMR,” “MVMR,” and “Mendelian Randomization” R packages. In our MR analysis, SNPs with an F value < 10 were excluded from the study. IVW was the primary analysis to estimate the associations and statistical significance was indicated by a threshold of P < 0.05, signifying the presence of a significant causal relationship. We presented MR estimates as odds ratios (OR) with 95% confidence intervals (CI) for binary outcomes (HCC and low-HGS) and β coefficients with standard error (SE) for continuous outcomes (ALM, WP, and CRP). The calculation of standard errors and CIs was performed using the delta method (42). In order to evaluate the robustness of IVW results, we further performed sensitivity analysis.
Figure 2 shows the bidirectional MR results concerning the causal relationship between sarcopenia-related traits and HCC using the IVW method. The results indicate a negative correlation between ALM and HCC (OR = 0.703, 95% CI: 0.524–0.943, P = 0.019), as well as a positive correlation between low-HGS and HCC (OR = 2.287, CI: 1.013–5.164, P = 0.047). Notably, the scatter charts portraying the trend of fitting results reveal that as ALM increases, the risk of HCC decreases, whereas low-HGS is associated with an elevated risk of HCC (Figures 3A, B). However, according to the two-sample MR results, there was no significant causal relationship between WP and HCC. In reverse MR analysis, no causal effect of HCC on ALM, low-HGS or WP was observed, implying that the genetic predisposition to HCC did not exert an impact on any sarcopenia-related traits (Figure 2). The complete MR results using other methods, including MR Egger, weighted median, simple mode and weighted mode, are presented in Supplementary Table S10.
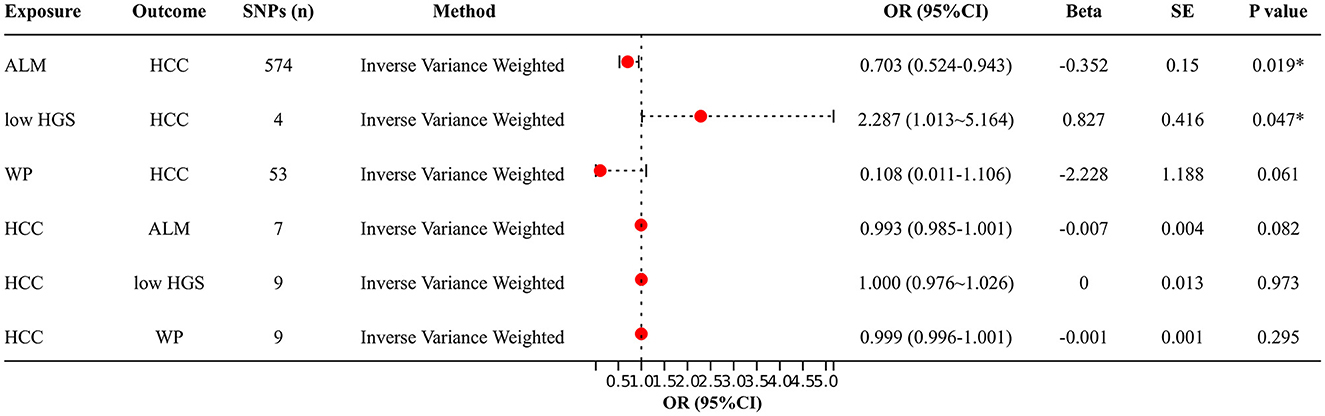
Figure 2. Two-sample MR analysis results. IVW method was used to evaluate the causal relationship between ALM, low-HGS, WP and HCC. OR > 1 signifies an increased risk associated with the exposure indicator, while OR < 1 indicates a decreased risk of the outcome. ALM, appendicular lean mass; low-HGS, low hand grip strength; WP, walking pace; HCC, hepatocellular carcinoma. The symbol * indicates statistical significance.
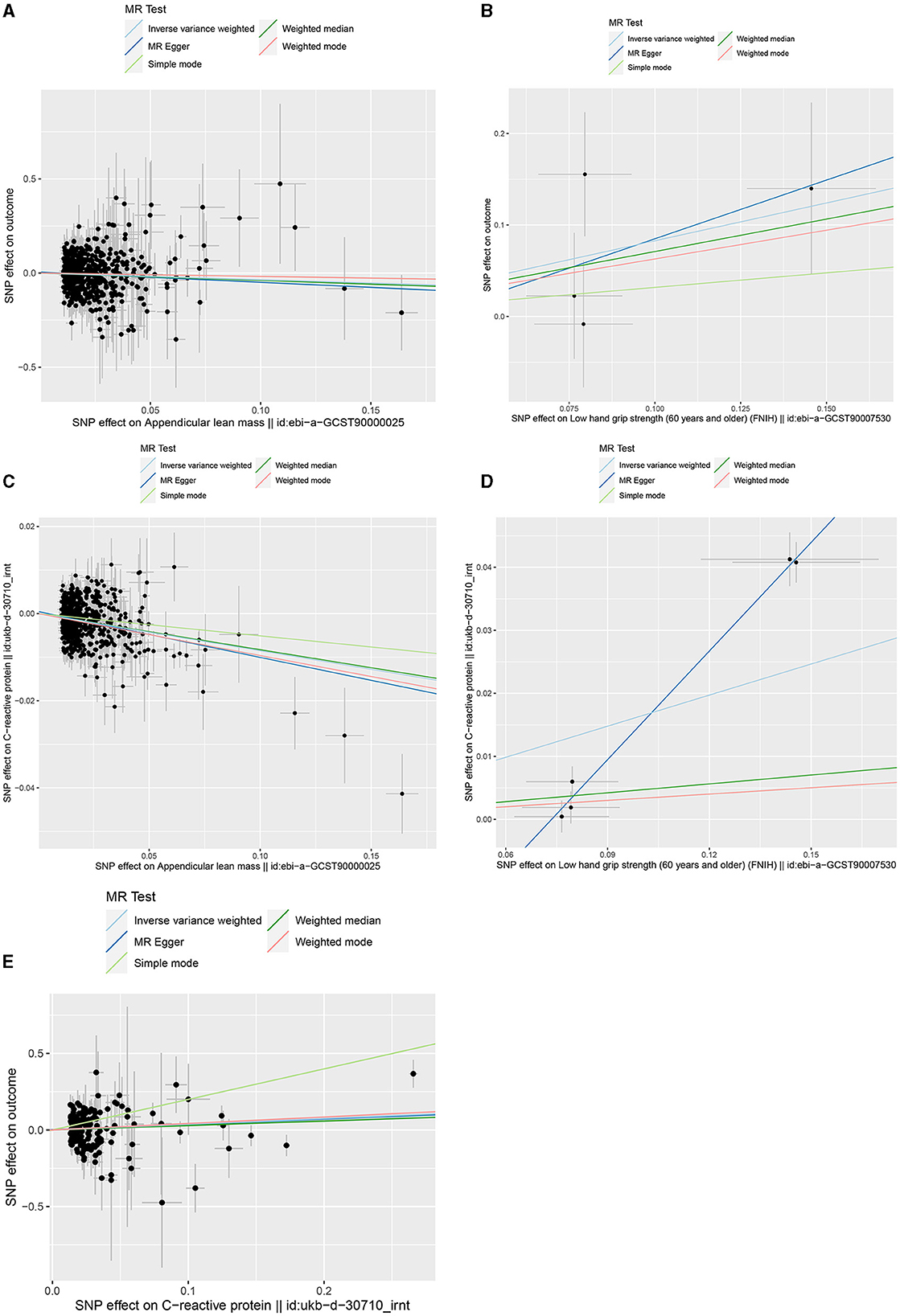
Figure 3. Scatter plots of genetic correlation by different MR analysis methods. (A) Scatter plot of genetic correlation between ALM and HCC. (B) Scatter plot of genetic correlation between low-HGS and HCC. (C) Scatter plot of genetic correlation between ALM and CRP. (D) Scatter plot of genetic correlation between low-HGS and CRP. (E) Scatter plot of genetic correlation between CRP and HCC.
Based on the aforementioned analysis, no causal relationship was observed between WP and HCC. Therefore, in the subsequent two-step MR analysis, our focus shifted exclusively to examining the mediating role of CRP in the causal associations of ALM and low-HGS with HCC. As detailed in Table 1, there was a significant negative causal relationship between ALM and CRP (βIVW = −0.085, 95%CI: −0.1 to −0.07, P = 2.59 × 10−29), while a significant positive causal relationship between low-HGS and CRP (βIVW = 0.164, 95%CI: 0.042 to 0.286, P = 0.008). To validate the causal direction, we conducted a Steiger test of directionality, affirming the correctness of the causal direction (P < 0.05) (Table 1). This result implies that CRP does not exert a reverse causal effect on ALM or low-HGS. As shown in Figures 3C, D, the scatter plots showed the trend of fitting results.
In two-sample MR analysis, it was discerned that elevated levels of CRP were significantly linked to an increased risk of HCC (OR = 1.455, 95%CI: 1.054–2.010, P = 0.023) (Figure 3E, Supplementary Table S10). In reverse MR analysis, no causal effect of HCC on CRP was observed (P = 0.437) (Supplementary Table S10). In MVMR, the causal association of CRP with HCC was diminished with adjustment for ALM, while low-HGS had no significant effect on it (Figure 4A). Detailed MVMR results can be found in Supplementary Table S11.
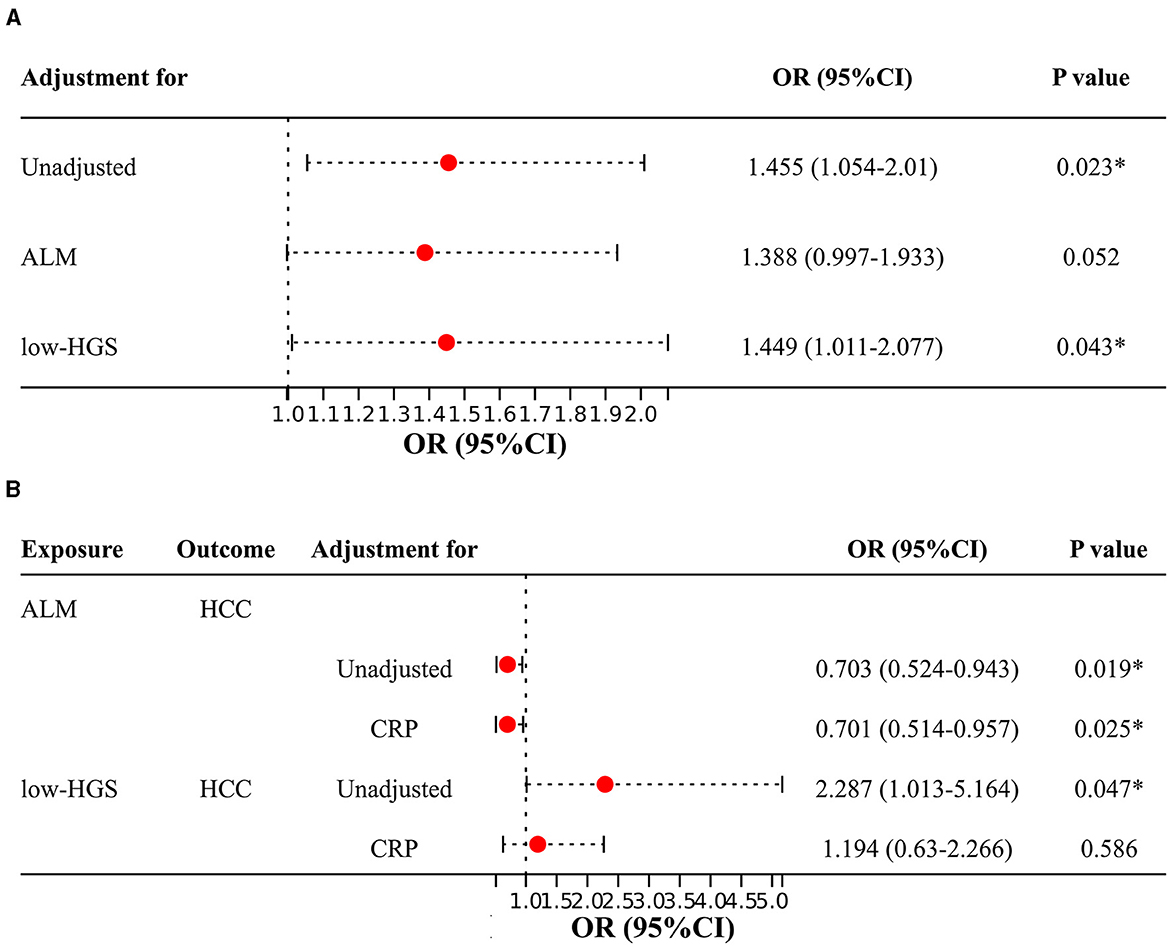
Figure 4. Two-sample MR and MVMR estimates for the causal associations of CRP, ALM and low-HGS with HCC. (A) Causal effects of CRP on HCC with adjustment for ALM or low-HGS. (B) Causal effects of ALM or low-HGS on HCC with adjustment for CRP. ORs (95% CIs) represent risks for HCC associated with CRP. ALM, appendicular lean mass; low-HGS, low hand grip strength; CRP, C-reactive protein. The symbol * indicates statistical significance.
The above two-step MR analysis results highlight the mediating role of CRP in the causal associations involving ALM or low-HGS with HCC. As shown in Figure 4B, in MVMR, the causal association of ALM with HCC remained relatively unchanged upon adjustment for CRP. In contrast, the causal association of low-HGS with HCC was notably diminished with adjustment for CRP, indicating that this association was largely affected by CRP. Specifically, CRP explained 9.1% (95% CI: 1.23%−16.95%) of the total effect of ALM on HCC (Table 2). The proportion mediated by CRP in the associations between low-HGS and HCC was 7.4% (95% CI: 3.11%−17.98%) (Table 2).
In our two-sample MR analysis, we employed IVW and MR–Egger methods to discern heterogeneity, while MR-PRESSO and MR–Egger analyses were harnessed to detect horizontal pleiotropy. As outlined in Table 3, MR analysis of ALM on CRP had heterogeneity, so IVW with random effects analysis was used in MR analysis. Except for this, there was no heterogeneity in other two-sample MR analyses (P > 0.05). Moreover, no evidence of horizontal pleiotropy was detected in any of our two-sample MR analyses (P > 0.05). The funnel plots illustrating the heterogeneity test results were presented in Supplementary Figure S1. Furthermore, the “leave-one-out” analysis revealed minimal fluctuations in the error line, indicating the robustness of the MR results (Supplementary Figure S2).
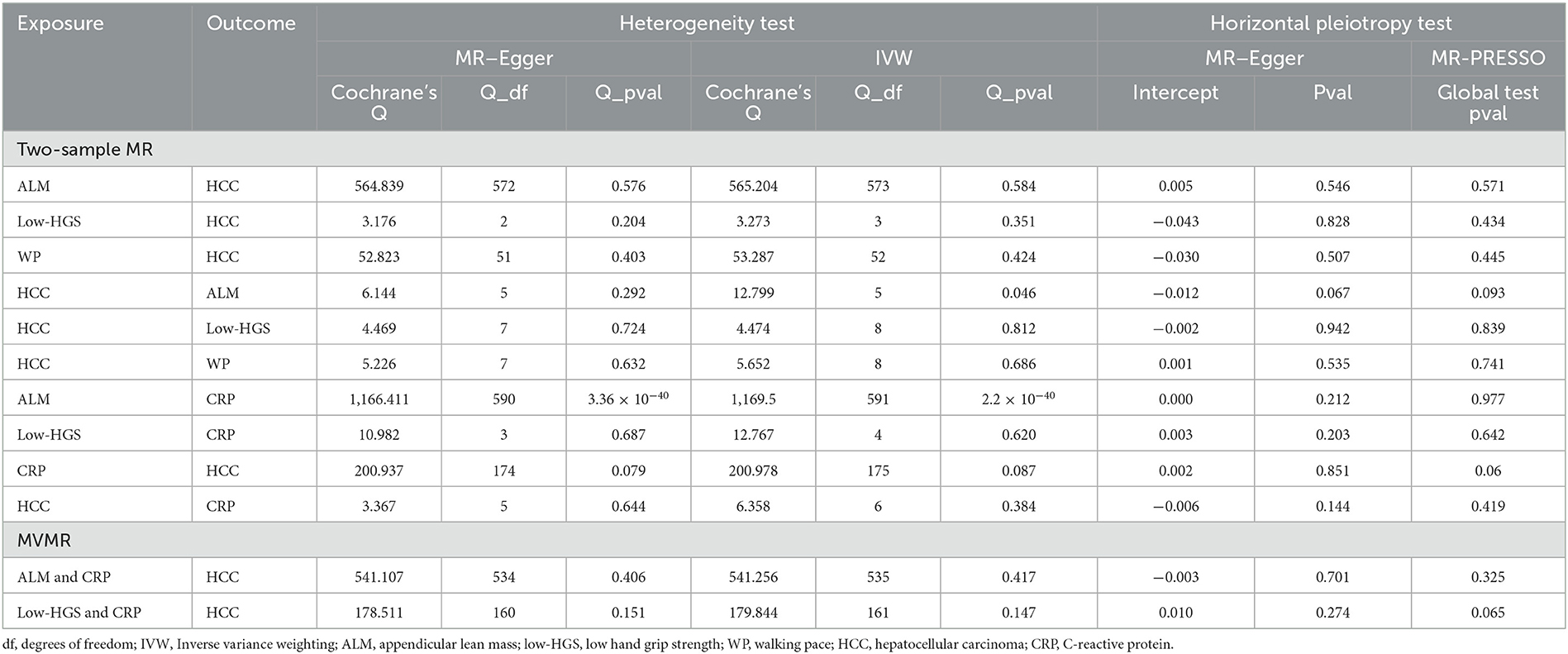
Table 3. Sensitivity analysis for two-sample MR and MVMR, including heterogeneity test and horizontal pleiotropy test.
In each MVMR analysis, both IVW and MR-Egger methods indicated the absence of heterogeneity, and the MR–Egger and MR-PRESSO methods confirmed the absence of horizontal pleiotropy (Table 3).
Our MR study systematically investigated the causal effects of sarcopenia-related traits, including ALM, low-HGS, and WP, on HCC. Simultaneously, the potential intermediation of CRP within these associations was explored. The results revealed that genetically determined lower ALM and low-HGS were causally associated with increased risk of HCC, but no significant causal relationship was discerned between WP and HCC. And there is no reverse causal relationship between HCC and sarcopenia-related traits. In addition, we assessed the mediating role of CRP in the causal connections between sarcopenia-related traits and HCC. And the mediating influence of CRP was quantified, revealing mediation proportions of 9.1% for the effect of ALM and 7.4% for the effect of low-HGS on HCC. It is noteworthy that the causal link between ALM and HCC remained substantively unchanged upon the adjustment for CRP. However, the causal association of low-HGS with HCC was diminished with CRP adjustment, suggesting this association was largely affected by CRP. Taken collectively, these findings support causal impacts of lower ALM and low-HGS on greater risks of HCC, demonstrating the substantial mediating role of CRP between sarcopenia and HCC. There is no horizontal pleiotropy in MR analyses, and the MR results are robust through “leave-one-out” analysis.
Sarcopenia significantly affected a series of adverse health-related outcomes, especially in patients with tumors and the geriatric population (43). EWGSOP used muscle mass and muscle strength to define sarcopenia, and physical performance was used to assess the severity (5). A previous systematic review and meta-analysis showed that in patients with HCC, skeletal muscle mass loss is associated with increased all-cause mortality and tumor recurrence (44). However, a cohort study of the Dutch population yielded contrasting findings, as no significant correlation emerged between sarcopenia and the overall survival of HCC (11). Such discrepancy in conclusions potentially stems from ethnic heterogeneity, considering the distinct ancestral backgrounds of the objects of these studies. HGS is the most robust indicator of muscle strength. A cohort study of the Japanese population showed that low HGS was a poor prognostic factor for unresectable HCC patients treated with lenvatinib (45). In addition, a retrospective study showed that reduced HGS in patients with chronic liver diseases was an independent adverse predictor of mortality (46). WP serves as an indicator of physical performance. According to the EWGSOP criteria, walking speed < 0.8 m/s is defined as slow WP (5). At present, investigations about the relationship between WP and HCC are sparse, with limited available data. A solitary study revealed that there was no significant difference in the prevalence of complications, delirium or falls after HCC treatment between the slow WP group and the normal group (47). Viral hepatitis and alcoholic hepatitis are significant causes of HCC, with sarcopenia being a very common comorbidity (48). The prevalence of sarcopenia is higher and prognosis poorer among patients with chronic viral hepatitis (49). Alcoholic hepatitis may exacerbate sarcopenia due to liver damage and malnutrition (50). Additionally, sarcopenia is believed to facilitate the progression of chronic hepatitis and the transformation into HCC (50). Hence, hepatitis and concurrent sarcopenia may jointly contribute to the occurrence and progression of HCC.
Our MR results unveiled a fresh perspective that genetically predicted lower muscle mass and muscle strength were strong risk factors for HCC within European population. Besides, we observed a more independent effect of ALM compared to HGS on HCC with adjustment for CRP. However, the MR investigation does not reveal a significant relationship between WP and HCC. Given that WP is not a necessary factor in the diagnostic criteria of sarcopenia according to the definition of EWGSOP, based on our MR results on the relationship between ALM and HGS and HCC, we can still conclude that there is a causal relationship between sarcopenia and HCC risk.
Notably, our study has shown the mediating role played by CRP and has quantified the proportions mediated by CRP between sarcopenia-related traits and HCC. CRP, an acute-phase reactant, exhibits its production under the regulation of proinflammatory cytokines, as delineated in the extant literature (51). Our MR results suggested that higher serum CRP levels increased the risk of HCC. This result harmoniously resonates with prior researches, wherein serum CRP has been extolled as a diagnostic marker for HCC and an independent marker for predicting poor prognosis and early recurrence (22, 52). A possible explanation for the effect of CRP on HCC is that prolonged inflammation leads to the infiltration of immunocytes into the liver to facilitate tissue remodeling, and additional investigations are necessary to clarify precise molecular mechanisms. Furthermore, our MR analysis unveiled a causal relationship between higher ALM and lower CRP, which is congruent with previous evidence that lean mass has a favorable effect on inflammation (53). Besides, a meta-analysis showed that HGS is negatively correlated with CRP (27), which supported our MR results. Subsidiary to this, our study identified relatively higher mediation proportions by CRP between ALM and HCC compared with low-HGS and HCC (ALM: 9.1%, low-HGS: 7.4%). This may be due to the endocrine function of skeletal muscle that affects systemic metabolism and inflammation, resulting in a more direct association of CRP with ALM than with HGS.
To the best of our knowledge, this study provided reliable causal evidence for the adverse impact of sarcopenia-related traits on HCC risk in European population for the first time, and elucidated a potential CRP-mediated pathway between sarcopenia and HCC. In a word, this study highlights the importance of monitoring muscle mass, strength, and serum CRP levels in individuals with sarcopenia, emphasizing their critical role in reducing the prevalence of HCC and diminishing the related disease burden.
This study boasts several strengths. Primarily, it offered a novel perspective on the causal associations and potential mediation effect between sarcopenia-related traits and HCC within the European population, as ascertained through the MR analysis. Furthermore, the deployment of bidirectional MR analysis and Steiger tests of directionality, has effectively mitigated the biases stemming from reverse causality. Moreover, comprehensive sensitivity analysis and “leave-one-out” analysis ensured the robustness of the results. These rigorous evaluations collectively buttress the robustness and reliability of our results, adding a layer of assurance to our conclusions.
Limitations warrant consideration. Firstly, despite the implementation of multiple sensitivity analyses, our study cannot entirely eliminate the potential interference of confounders, and thus we urge cautious interpretation of the causal associations elucidated herein. Secondly, the absence of individual-level data hindered our capacity to investigate the relationship between sarcopenia-related traits and HCC via subgroup analysis. Subsequent analyses are thus warranted to probe these associations stratified by age and sex. Thirdly, it is essential to emphasize that our study primarily relies on GWAS data from individuals of European descent. Consequently, circumspection is advised when extending our findings to other ethnic groups. Lastly, constraints imposed by the current GWAS database hindered the identification of more optimal mediators. These limitations include factors such as gene coverage in the database, measurement methods of phenotypes, and sample sizes. In the future, it will be necessary to analyze and study in broader GWAS datasets to explore more prominent mediators and to explore the potential mechanisms between sarcopenia and HCC.
In conclusion, this study shed light on the causal associations of lower ALM and low-HGS with increased HCC risk and the mediating role of CRP in the association pathways within the European population. Intervention of these influential factors may offer a promising avenue for preventive measures against HCC in individuals with sarcopenia.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JC: Conceptualization, Methodology, Writing—original draft. YH: Data curation, Writing—review & editing. MZ: Writing—review & editing, Formal analysis. ZW: Writing—review & editing, Investigation, Validation. ZJ: Writing—review & editing, Software, Visualization. ZX: Writing—review & editing, Funding acquisition, Resources, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2018YFC2002000), Fundamental Research Funds for the Central Universities under Grant (YCJJ202201051), and Scientific Research Fund of Liyuan Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology (2023LYYYCXTD002).
We thank Finngen, UK Biobank, Musculoskeletal Knowledge Portal and the MRC-IEU OPEN GWAS PROJECT databases, and all the researchers who share research data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1292834/full#supplementary-material
1. Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. (2023) 20:864–84. doi: 10.1038/s41571-023-00825-3
2. Lin L, Li Z, Yan L, Liu Y, Yang H, Li H. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990–2019. J Hematol Oncol. (2021) 14:197. doi: 10.1186/s13045-021-01213-z
3. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
6. Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. (2017) 13:340–7. doi: 10.1038/nrrheum.2017.60
7. Arnold P, Bautmans I. The influence of strength training on muscle activation in elderly persons: a systematic review and meta-analysis. Exp Gerontol. (2014) 58:58–68. doi: 10.1016/j.exger.2014.07.012
8. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. (2012) 302:E992–9. doi: 10.1152/ajpendo.00517.2011
9. Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. (2014) 810:500–25. doi: 10.1007/978-1-4939-0437-2_28
10. Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. (2013) 100:1523–30. doi: 10.1002/bjs.9258
11. Beumer BR, Takagi K, Buettner S, Umeda Y, Yagi T, Fujiwara T, et al. Impact of sarcopenia on clinical outcomes for patients with resected hepatocellular carcinoma: a retrospective comparison of eastern and western cohorts. Int J Surg. (2023) 109:2258–66. doi: 10.1097/JS9.0000000000000458
12. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. (2015) 261:1173–83. doi: 10.1097/SLA.0000000000000743
13. Yeh WS, Chiang PL, Kee KM, Chang CD, Lu SN, Chen CH, et al. Pre-sarcopenia is the prognostic factor of overall survival in early-stage hepatoma patients undergoing radiofrequency ablation. Medicine. (2020) 99:e20455. doi: 10.1097/MD.0000000000020455
14. Yuri Y, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, et al. Implication of psoas muscle index on survival for hepatocellular carcinoma undergoing radiofrequency ablation therapy. J Cancer. (2017) 8:1507–16. doi: 10.7150/jca.19175
15. Beumer BR, van Vugt JLA, Sapisochin G, Yoon P, Bongini M, Lu D, et al. Impact of muscle mass on survival of patients with hepatocellular carcinoma after liver transplantation beyond the milan criteria. J Cachexia Sarcopenia Muscle. (2022) 13:2373–82. doi: 10.1002/jcsm.13053
16. Chien TP, Huang SF, Chan WH, Pan KT Yu MC, Lee WC, et al. The combination of sarcopenia and biochemical factors can predict the survival of hepatocellular carcinoma patients receiving transarterial chemoembolization. Front Oncol. (2022) 12:1005571. doi: 10.3389/fonc.2022.1005571
17. Chen BB, Liang PC, Shih TT, Liu TH, Shen YC, Lu LC, et al. Sarcopenia and myosteatosis are associated with survival in patients receiving immunotherapy for advanced hepatocellular carcinoma. Eur Radiol. (2023) 33:512–22. doi: 10.1007/s00330-022-08980-4
18. Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, Kochi T, et al. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int J Mol Sci. (2015) 16:9612–24. doi: 10.3390/ijms16059612
19. Hiraoka A, Hirooka M, Koizumi Y, Izumoto H, Ueki H, Kaneto M, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. (2017) 47:558–65. doi: 10.1111/hepr.12780
20. Sun MY, Chang CL, Lu CY, Wu SY, Zhang JQ. Sarcopenia as an independent risk factor for specific cancers: a propensity score-matched asian population-based cohort study. Nutrients. (2022) 14:1910. doi: 10.3390/nu14091910
21. Feng Z, Zhao H, Jiang Y, He Z, Sun X, Rong P, et al. Sarcopenia associates with increased risk of hepatocellular carcinoma among male patients with cirrhosis. Clin Nutr. (2020) 39:3132–9. doi: 10.1016/j.clnu.2020.01.021
22. Lee FY, Lee SD, Tsai YT, Wu JC, Lai KH, Lo KJ. Serum C-reactive protein as a serum marker for the diagnosis of hepatocellular carcinoma. Cancer. (1989) 63:1567–71. doi: 10.1002/1097-0142(19890415)63:8<1567::AID-CNCR2820630820>3.0.CO;2-J
23. Zhang Y, Lu L, He Z, Xu Z, Xiang Z, Nie RC, et al. C-Reactive protein levels predict responses to pd-1 inhibitors in hepatocellular carcinoma patients. Front Immunol. (2022) 13:808101. doi: 10.3389/fimmu.2022.808101
24. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. (2015) 22:803–10. doi: 10.1245/s10434-014-4048-0
25. Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the crafity score. J Hepatol. (2022) 76:353–63. doi: 10.1055/s-0041-1734228
26. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. (2006) 119:526.e9–17. doi: 10.1016/j.amjmed.2005.10.049
27. Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev. (2020) 64:101185. doi: 10.1016/j.arr.2020.101185
28. Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
29. Pei YF, Liu YZ, Yang XL, Zhang H, Feng GJ, Wei XT, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the uk biobank study. Commun Biol. (2020) 3:608. doi: 10.1038/s42003-020-01334-0
30. Jones G, Trajanoska K, Santanasto AJ, Stringa N, Kuo CL, Atkins JL, et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun. (2021) 12:654. doi: 10.1038/s41467-021-20918-w
32. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. Finngen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
33. Sved JA, Hill WG. One hundred years of linkage disequilibrium. Genetics. (2018) 209:629–36. doi: 10.1534/genetics.118.300642
34. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
35. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
36. Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. (2021) 36:465–78. doi: 10.1007/s10654-021-00757-1
37. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using mr-egger regression: the role of the i2 statistic. Int J Epidemiol. (2016) 45:1961–74. doi: 10.1093/ije/dyw220
38. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
39. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
40. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
41. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
42. MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. (2002) 7:83–104. doi: 10.1037//1082-989X.7.1.83
43. Xia L, Zhao R, Wan Q, Wu Y, Zhou Y, Wang Y, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. (2020) 9:7964–78. doi: 10.1002/cam4.3428
44. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. (2018) 7:90–103. doi: 10.1159/000484950
45. Endo K, Kuroda H, Kanazawa J, Sato T, Fujiwara Y, Abe T, et al. Impact of grip strength in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Cancers. (2020) 12:2146. doi: 10.3390/cancers12082146
46. Nishikawa H, Shiraki M, Hiramatsu A, Hara N, Moriya K, Hino K, et al. Reduced handgrip strength predicts poorer survival in chronic liver diseases: a large multicenter study in Japan. Hepatol Res. (2021) 51:957–67. doi: 10.1111/hepr.13679
47. Nagamatsu A, Kawaguchi T, Hirota K, Koya S, Tomita M, Hashida R, et al. Slow walking speed overlapped with low handgrip strength in chronic liver disease patients with hepatocellular carcinoma. Hepatol Res. (2019) 49:1427–40. doi: 10.1111/hepr.13405
48. Cespiati A, Meroni M, Lombardi R, Oberti G, Dongiovanni P, Fracanzani AL. Impact of sarcopenia and myosteatosis in non-cirrhotic stages of liver diseases: similarities and differences across aetiologies and possible therapeutic strategies. Biomedicines. (2022) 10:182. doi: 10.3390/biomedicines10010182
49. Silva LD, Bering T, Rocha GA. The impact of nutrition on quality of life of patients with hepatitis C. Curr Opin Clin Nutr Metab Care. (2017) 20:420–5. doi: 10.1097/MCO.0000000000000396
50. Song DS, Chang UI, Choi S, Jung YD, Han K, Ko SH, et al. Heavy alcohol consumption with alcoholic liver disease accelerates sarcopenia in elderly korean males: the Korean national health and nutrition examination survey 2008-2010. PLoS ONE. (2016) 11:e0163222. doi: 10.1371/journal.pone.0163222
51. Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by Interleukin-6. Hepatology. (1990) 12:1179–86. doi: 10.1002/hep.1840120517
52. Sieghart W, Pinter M, Hucke F, Graziadei I, Schöniger-Hekele M, Müller C, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. HEPATOLOGY. (2013) 57:2224–34. doi: 10.1002/hep.26057
Keywords: sarcopenia, C-reactive protein, hepatocellular carcinoma, Mendelian randomization, appendicular lean mass, grip strength
Citation: Cao J, Huang Y, Zhu M, Wang Z, Jin Z and Xiong Z (2024) Causal association of sarcopenia with hepatocellular carcinoma risk in European population: a Mendelian randomization study. Front. Nutr. 11:1292834. doi: 10.3389/fnut.2024.1292834
Received: 14 September 2023; Accepted: 29 April 2024;
Published: 27 May 2024.
Edited by:
Robert Fred Clark, St. Jude Children's Research Hospital, United StatesReviewed by:
Owen Kelly, Sam Houston State University, United StatesCopyright © 2024 Cao, Huang, Zhu, Wang, Jin and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhifan Xiong, eGlvbmd6aGlmYW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.